Supplemental Digital Content is available in the text
Keywords: clear cell renal cell carcinoma, meta-analysis., nephrectomy, papillary renal cell carcinoma, prognosis
Abstract
Objective:
To compare the prognosis of papillary and clear cell renal cell carcinoma (RCC) in order to determine the optimal follow-up and therapy for patients with RCC.
Methods:
A systematic search of Web of Science, EMBASE, Cochrane Library, and PubMed databases was conducted for articles published through July 30, 2018, reporting on a comparison of the prognosis of papillary RCC and clear cell RCC using the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.
Results:
Of 1896 studies, 11 were considered for the evidence synthesis. A total of 35,832 patients were included. Of these patients, 6907 patients were diagnosed with papillary renal cell carcinoma, and 28,925 patients were diagnosed with clear cell renal cell carcinoma. The prognosis of papillary RCC was better than that of clear cell RCC (hazard ratio (HR) = 0.50; 95% confidence interval (CI) 0.45 to 0.56; P < .001; I2 = 91.9%). A subgroup analysis indicated that papillary RCC was associated with better outcomes (HR = 0.76, 95% CI 0.50–1.16), and a trend toward a higher risk of mortality was observed in patients with metastatic RCC presenting with papillary histology, but the difference was not statistically significant (HR = 1.12, 95% CI 0.71–1.76, P = .085). Pooled data suggested a lack of a significant difference between papillary RCC (p-RCC) type 1 and clear cell RCC (cc-RCC) (HR = 0.30, 95% CI 0.12–0.73, P = .085). The pooled HR for the prognosis of p-RCC type 2 compared to cc-RCC was 1.69 (95% CI 0.93–3.08; P = .032).
Conclusion:
Papillary RCC is associated with better outcomes than clear cell RCC in patients without metastases, but not in patients with metastases. Optimal follow-up or therapy for patients with RCC should be assigned according to the tumor stage and subtype.
Key points
Papillary renal cell carcinoma was associated with a better outcome than clear cell renal cell carcinoma in patients without metastases, but not in patients with metastases.
Type 1 p-RCC was not significantly associated with a better outcome than cc-RCC, whereas type 2 p-RCC resulted in a worse prognosis.
Optimal follow-up or therapy for patients with RCC should be assigned according to the tumor stage and subtype.
1. Introduction
Approximately 65,340 new cases of renal cell carcinoma (RCC) and 14,970 RCC-related deaths occurred in 2018 in the United States.[1] Generally, RCC is classified into conventional RCC; papillary RCC; collecting duct carcinoma, with medullary carcinoma of the kidney; chromophobe RCC; and unclassified RCC.[2] Each subtype of RCC manifests unique and specific properties. Papillary RCC (p-RCC) accounts for 15% to 20% of RCC cases and is heterogeneous, consisting of various types of RCC, including a multifocal, indolent presentation, and solitary tumors with aggressive, highly lethal phenotypes.[3] Traditionally, p-RCC is divided into 2 types: type 1 is characterized by a basophilic cytoplasm and is classified as a low-grade tumor, whereas type 2 displays a bulky eosinophilic cytoplasm and pseudostratified tumor cell nuclei and is a high-grade tumor.[4]
Several studies have consistently reported that a p-RCC histology is associated with a favorable prognosis compared with clear cell RCC (cc-RCC).[5–8] However, p-RCC was a significant risk factor for overall survival in other studies.[9,10] In this regard, different p-RCC types and tumor stages may result in distinct outcomes. A type 2 p-RCC histology is considered to predict worse outcomes,[11,12] whereas type 1 p-RCC may result in a reduced risk of death compared with cc-RCC.[5] Some research was performed on patients with non-metastatic p-RCC,[5,9,13] and the papillary subtype was identified as a positive prognostic factor in patients with localized disease but a negative prognostic factor in patients with metastatic tumor stages.[7]
Thus, we conducted this systemic review and meta-analysis to improve our understanding of the oncological outcomes associated with p-RCC, p-RCC subtypes, and cc-RCC and to assign the appropriate follow-up or therapy to patients with RCC. We also suggest optimal surgical strategies according to the p-RCC subtype (nephron-sparing partial nephrectomy vs radical nephrectomy) in patients with nonmetastatic RCC or patients with metastatic RCC (radical nephrectomy vs cytoreductive nephrectomy).
2. Materials and methods
2.1. Search strategy
The meta-analysis and systemic review were performed by searching the Web of Science, PubMed, Cochrane Library, and EMBASE databases for studies published through July 29, 2018. Additional records identified through searchers of other sources (by screening the reference in the identified studies and other handbooks). Searches included the terms “clear cell renal cell carcinoma” AND “papillary renal cell carcinoma” AND “prognosis OR survival” AND “nephrectomy”. The citations listed in the retrieved articles were reviewed to identify other potentially relevant studies.
2.2. Inclusion and exclusion criteria
Two investigators (JD and JPH) independently extracted the data, and an agreement was reached by discussion. Studies that met the following criteria were included in this meta-analysis:
-
1.
all patients were pathologically diagnosed with RCC;
-
2.
patients were regularly treated and followed;
-
3.
sufficient data were available for examining cancer-specific survival or overall survival, and the hazard ratio (HR) with its 95% confidence interval (95% CI) were reported.
Major criteria for the exclusion of studies were:
-
1.
incomplete data for the analysis;
-
2.
conference abstracts, reviews, letters to editors/commentaries/editorials, and articles published in a language that cannot be translated; and
-
3.
duplicate data.
2.3. Data extraction
Authors’ names, publication year, country, patient number, cancer stage, papillary type, risk ratio or HR and the upper confidence interval (UCI), and lower confidence interval (LCI) of each comparison were collected from the included publication. For articles that only provided survival data in a Kaplan–Meier curve, formulas designed by Jayne et al were used to extract the HR and its 95% CI.[14] The quality of the selected articles was evaluated using the Newcastle–Ottawa Scale.[15]
2.4. Statistical analysis
The HR with its 95% CI were extracted from the included studies. The heterogeneity of the studies was evaluated using I2 statistics (values ranged from 0–100%).[16] We pooled the information with a random or fixed effect model according to the I2 value. The fixed effects model method was used when I2 < 50%, indicating a lack of heterogeneity among studies. When heterogeneity was observed, the random effects model was applied.[17] Publication bias was evaluated using Begg and Egger tests. A sensitivity analysis was performed to assess the stability of the results. Funnel plots were drawn to estimate publication bias, and the symmetry of the funnel plot was assessed using Egger test.[18,19] When using Egger test to assess the publication bias, P < .05 indicated statistically significant publication bias. The statistical analysis was performed using STATA 12.0 software (StataCorp., College Station, TX, USA).
3. Results
3.1. Study selection and characteristics
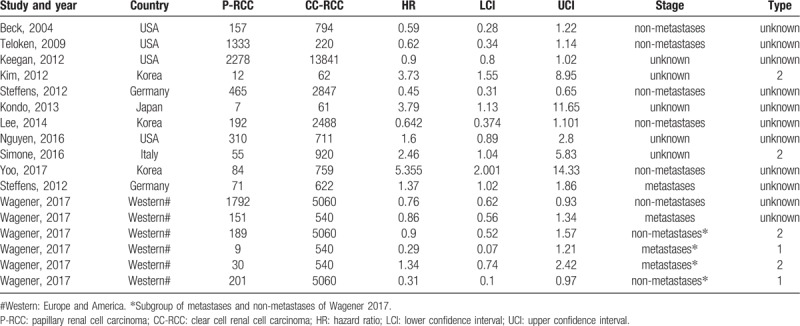
One thousand eight hundred ninety potentially relevant studies were identified through the literature search. Six other studies were added through searches of other sources (by screening the references of the identified studies and other handbooks). After excluding 934 unrelated articles, 183 articles were further assessed. Finally, 11 studies met the inclusion criteria and were included in our meta-analysis[5–13,20,21] (Fig. 1). A total of 35,832 patients were included. Of these individuals, 6907 patients were diagnosed with p-RCC and 28,925 patients were diagnosed with cc-RCC. Six studies including 4245 patients with p-RCC and 13,330 patients with cc-RCC described known and definite tumor stages (nonmetastatic or metastatic).[5,7–9,13,21] Only 3 studies evaluated the relationship between the papillary subtype and prognosis.[5,11,12] Two hundred ten and 286 patients with p-RCC were diagnosed with type 1 and 2 diseases, respectively. The control groups included 5600 and 6582 patients with type 1 and 2 cc-RCC, respectively. Some data were extracted from only 1 study[5] (Table 1, noted by an asterisk).
Figure 1.
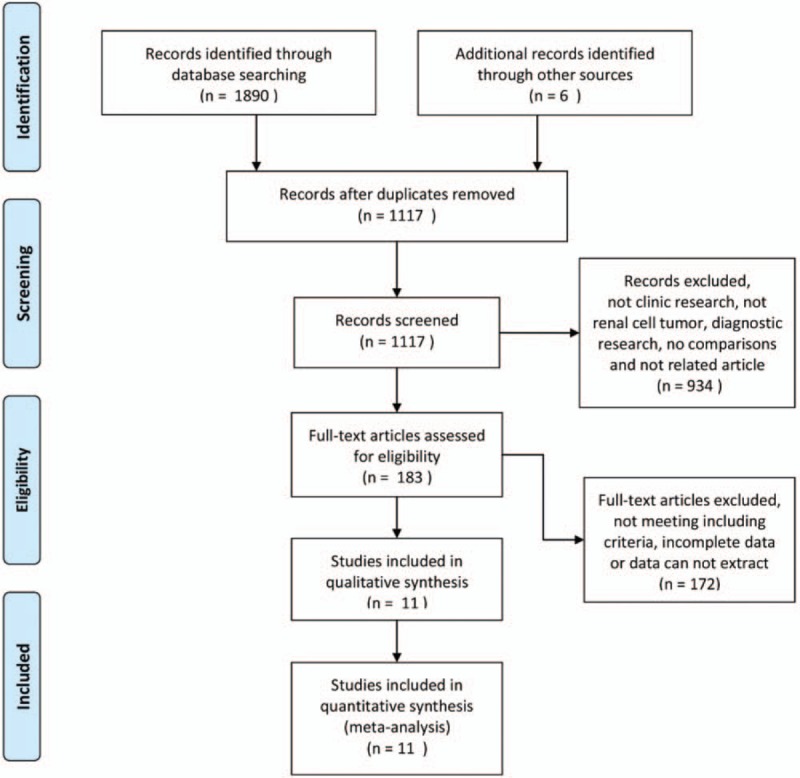
Flow diagram of the details of the study.
Table 1.
Characteristics of enrolled studies.
3.2. The prognosis of p-RCC compared to cc-RCC
Of the 11 studies, 5 studies with 32,158 patients indicated a better prognosis for patients with p-RCC compared to patients with cc-RCC.[5,8,13,20,21] These studies included 6368 patients with p-RCC and 25,790 patients with cc-RCC. Another 5 studies with 3674 patients showed that the p-RCC histology was an independent predictor of worse outcomes.[6,9–12] In our meta-analysis, I2 was 81.7%; therefore, the random effect model was used for the analysis, and the pooled HR was 1.07 (95% CI 0.82–1.40). Thus, p-RCC resulted in a significantly increased risk of death of 7% (Fig. 2).
Figure 2.
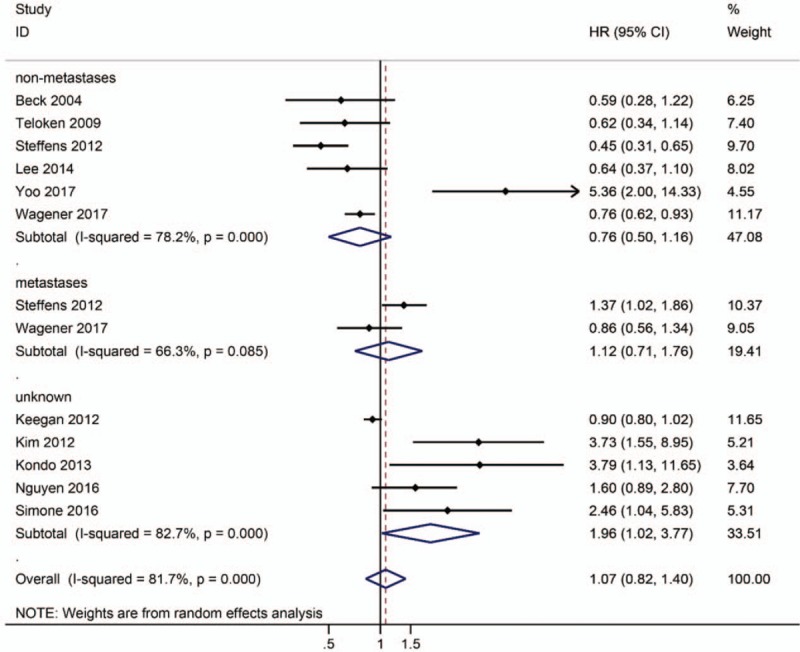
Forest plot HR for prognosis of p-RCC compared to cc-RCC according to tumor stage (non-metastases/ metastases).
3.3. Subgroup analysis
Steffens et al proposed the division of p-RCC into 2 subgroups: an organ-confined/localized subgroup with a good prognosis and an advanced/metastatic subgroup with a worse prognosis compared to cc-RCC.[7] A subgroup analysis was necessary, as 1 study divided p-RCC into 2 subgroups according to tumor stage.[7] For patients with nonmetastatic RCC, the subgroup analysis indicated that p-RCC was associated with good outcomes (HR = 0.76, 95% CI 0.50–1.16; Fig. 2). On the other hand, a trend toward a higher risk of mortality in was observed in patients with metastatic RCC with papillary histology, but the difference was not statistically significant (HR = 1.12, 95% CI 0.71–1.76, P = .085; Fig. 2). Three studies[5,11,12] evaluated the prognosis of p-RCC type 2 compared to cc-RCC. The pooled HR was 1.69 (95% CI 0.93–3.08; P = .032; Fig. 3), indicating that p-RCC type 2 increased the risk of death by 69%. One study [5] reported a comparison of the prognosis of p-RCC type 1 with cc-RCC, and the pooled data did not reveal a significant difference between p-RCC type 1 and cc-RCC (HR = 0.30, 95% CI 0.12–0.73, P = .085; Fig. 3).
Figure 3.
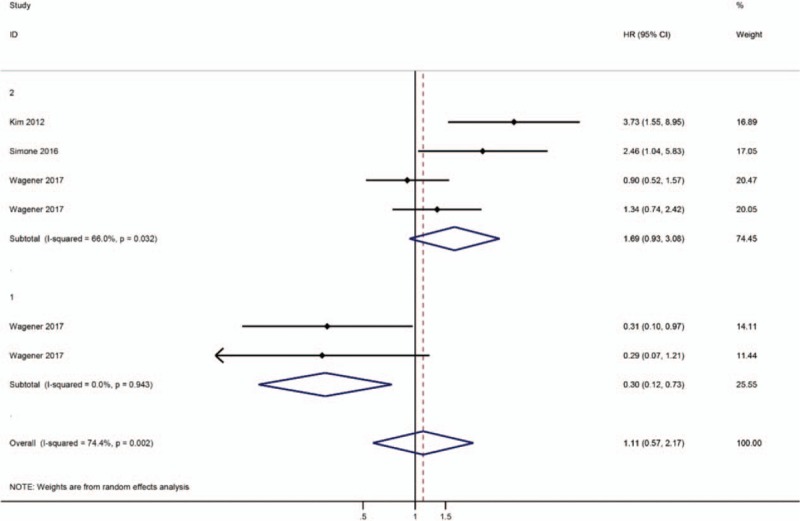
Forest plot HR for prognosis of p-RCC subtype compared to cc-RCC.
3.4. Publication bias
Egger test and Begg funnel plots were used to assess the publication bias in this meta-analysis. Egger funnel plot test (P = .195) verified that publication bias did not exist among the included studies; however, Begg test (P = .044; Fig. 4) did not support this conclusion.
Figure 4.
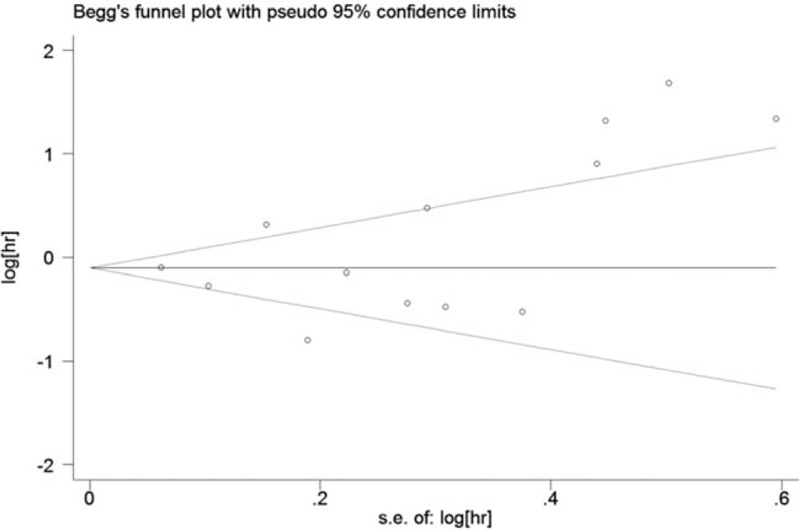
Funnel plots evaluating possible publication bias.
3.5. Sensitivity analysis
Studies were sequentially removed to investigate whether any study affected the pooled results. The pooled result did not exhibit alterations when an individual study was excluded (Fig. 5).
Figure 5.
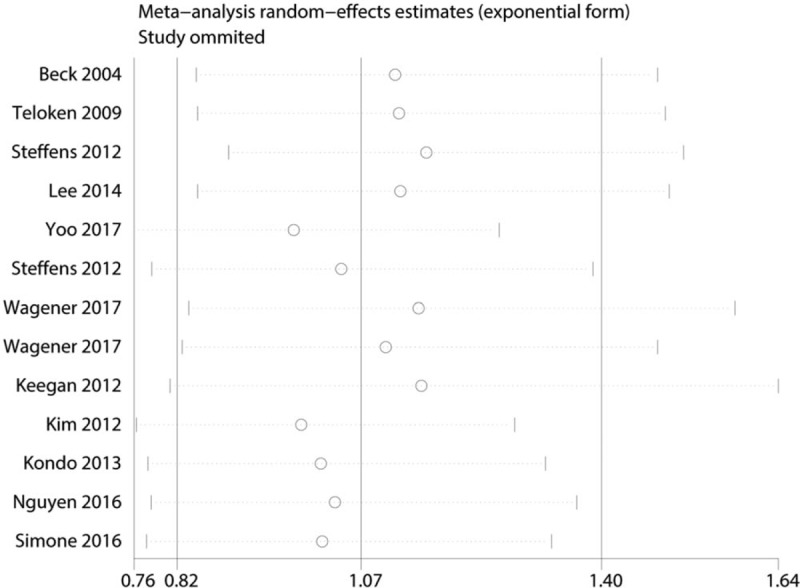
Sensitivity analysis for the p-RCC with OS.
4. Discussion
p-RCC is the second most common type of RCC. The common classification system for p-RCC divides it into 2 distinct subtypes based on histological features, including type 1 for basophilic p-RCC and type 2 for eosinophilic p-RCC.[22] Type 1 p-RCC is characterized by small round cells with a minimal cytoplasm distributed on the surface of papillae and basophilic nuclei, and type 2 p-RCC is characterized by cells with an abundant cytoplasm and large nuclei with prominent nucleoli arranged in a pseudostratified manner.[23] However, a recent study suggested that the p-RCC subtype alone is not suitable for estimating survival; the only independent predictors of overall survival are proliferation, tumor stage, metastasis, and age.[24] We performed this meta-analysis to re-evaluate the prognosis of type 1 and 2 p-RCC.
According to our meta-analysis, p-RCC was associated with a worse outcome than cc-RCC. However, the data must be interpreted with caution, as Begg funnel plot (P = .044; Fig. 4) suggested the potential for publication bias within the included cohorts. By examining the source of bias, we identified 2 studies with a small population of patients with p-RCC (Table 1).[10,12] Thus, we excluded these studies and repeated the analysis. Both Begg funnel plot (P = .213; Fig. 4S) and Egger test (P = .560) verified a lack of publication bias within the included cohorts. The pooled HR was 0.94 (95% CI 0.73–1.21), suggesting that p-RCC was associated with a significantly decreased risk of death of 6% (Fig. 2S). Nevertheless, a subgroup analysis evaluating the prognosis of patients with p-RCC compared to patients with cc-RCC in groups stratified by nonmetastatic and metastatic stages (HR = 0.76 and 1.12, respectively; Fig. 2S) yielded the same results as the initial analysis (Fig. 2).
Based on the findings from this meta-analysis, the papillary histological type was associated with better outcomes (HR = 0.76, P = .000; Fig. 2). Two studies[5,7] assessed the prognosis of p-RCC in patients with metastatic disease, and the pooled data suggested a lack of a significant difference between patients with p-RCC and cc-RCC (HR = 1.12, P = .085; Fig. 2). Although the papillary histological subtype manifests with less invasiveness, metastatic p-RCC is not associated with a better prognosis.[25] Wells et al characterized the outcomes of 466 patients with metastatic p-RCC and found that the overall survival of patients with cc-RCC was 8 months longer than patients with p-RCC presenting with metastatic tumors.[26] Unfortunately, sufficient data were unable to be extracted for our meta-analysis. Our pooled HR for metastatic p-RCC was calculated based on 222 patients with p-RCC and 1162 patients with cc-RCC. Additional research is needed to verify the results of this meta-analysis.
Based on the present data, type 2 p-RCC results in a worse outcome than cc-RCC; in contrast, type 1 p-RCC showed no significant difference in prognosis (Fig. 3). Previous basic research suggested the presence of cytogenetic abnormalities in patients with p-RCC, as both subtypes displayed a gain of at least 1 of the primary chromosomes at 7, 16q and 17q, and type 2 tumors had moderately lower frequencies of gains of chromosome 17p.[27] A recent study suggested a third subtype based on genomic analysis; p-RCC type 3 has a distinct molecular signature, but it behaves in a similar manner to type 2 p-RCC in the clinic.[28] Notably, cc-RCC is a recently recognized subtype of the RCC entity.[29,30] These findings challenged the classification of p-RCC. Based on our findings, type 2 p-RCC predicted a poor prognosis.
The optimal treatment option for p-RCC has not been established.[31] To date, no therapy has been specifically approved for the treatment of p-RCC or other nonclear cell cancers of the kidney. Surgery is recommended to achieve a cure in patients with localized RCC, and cytoreductive nephrectomy combined with targeted therapies may improve the survival of patients with mRCC.[32,33] Conventionally, p-RCC has been treated with the same therapies as cc-RCC.[34] Our research indicated that p-RCC resulted in a better outcome than cc-RCC for patients with nonmetastatic RCC; therefore, partial or radical nephrectomy to remove the tumor may be sufficient to address nonmetastatic RCC. For patients with metastatic RCC, p-RCC appears to be more aggressive (see Fig. 2, HR = 1.12, 95% CI 0.71–1.76, P = .085), and the molecular characterization of tumors to stratify patients may improve outcomes [35] The regular follow-up employed for patients with cc-RCC is sufficient for patients with nonmetastatic p-RCC; additional, more frequent radiographic screening may improve the survival of patients with metastatic p-RCC and should be further investigated.[36]
RCC is characterized by gene mutations involved in metabolic pathways.[37] VHL, MET, FLCN, TSC1, TSC2, FH, NDUFA4L2, and SDH were known as kidney cancer genes.[38,39] These mutations were involved with aerobic glycolysis, tryptophan, glutamine, arginine, and fatty acids metabolism. These changes allow tumor cells to survive in conditions of energy deprivation and hypoxia, which could serve as a marker of RCC aggressiveness and as a prognostic factor for CSS and PFS.[40–42] Thus, RCC is fundamentally a metabolic disease whose hallmarks are genetic abnormalities involved especially in metabolic reprogramming.
Several improvements should be implemented in the future. First, studies of larger populations are required to confirm the findings of this meta-analysis, as some previous studies included small sample sizes.[11,12] Second, laparoscopic partial nephrectomy (PN) is associated with improvements in 5- and 10-year overall survival and cancer-specific survival compared to laparoscopic radial nephrectomy (RN), as well as better preservation of renal function than RN.[43] When PN is used, the oncological outcomes of patients with p-RCC may be worse than expected due to tumor multifocality and vulnerability.[44,45] Studies comparing the effects of PN and RN on patients with p-RCC are necessary. Third, the median time to recurrence of p-RCC may exceed 5 years[9]; currently, the long-term prognosis of patients with p-RCC after PN has not been clearly determined.
5. Conclusions
Based on our meta-analysis, p-RCC was associated with a better overall survival than cc-RCC in patients with nonmetastatic disease, but not in patients with metastatic disease. Type 1 p-RCC was not significantly associated with better survival than cc-RCC, whereas type 2 p-RCC resulted in a worse prognosis. Optimal follow-up or therapy for patients with RCC should implemented according to the tumor stage and subtype.
Author contributions
Project development: Jieping Hu; data collection or management: Jun Deng and Lei Li; data analysis and interpretation: Haimei Xia, Ju Guo, and Xin Wu; manuscript writing: Jun Deng; manuscript editing: Xiaorong Yang, Yanyan Hong, and Qingke Chen; and study supervision: Jieping Hu.
Conceptualization: Jieping Hu.
Data curation: Jun Deng, Xin Wu, Jieping Hu.
Formal analysis: Lei Li, Haimei Xia, Ju Guo, Jieping Hu.
Funding acquisition: Jun Deng, Jieping Hu.
Investigation: Jieping Hu.
Methodology: Lei Li, Xin Wu, Yanyan Hong, Jieping Hu.
Project administration: Jieping Hu.
Resources: Yanyan Hong, Jieping Hu.
Software: Ju Guo, Xiaorong Yang, Jieping Hu.
Supervision: Xiaorong Yang, Jieping Hu.
Validation: Qingke Chen, Jieping Hu.
Visualization: Jieping Hu.
Writing – original draft: Jun Deng.
Writing – review & editing: Lei Li, Haimei Xia, Qingke Chen, Jieping Hu.
Jieping Hu orcid: 0000-0001-5136-2958.
Supplementary Material
Footnotes
Abbreviations: RCC = renal cell carcinoma, cc-RCC = clear cell renal cell carcinoma, p-RCC = papillary renal cell carcinoma, UCI = upper confidence interval, LCI = lower confidence interval.
JD, LL, and HX Contributed equally.
This study was funded by the Project of the Education Department of Jiangxi Province (GJJ160036, GJJ180007 and GJJ180031).
All procedures performed in the included studies involving human participants were conducted in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Declaration of Helsinki and its subsequent amendments or comparable ethical standards. This article does not contain any studies with human participants performed by any of the authors.
Informed consent was obtained from all individual participants in the included studies. For this study, formal consent was not required.
The authors have no conflicts of interest to declare.
Supplemental Digital Content is available for this article.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Kovacs G, Akhtar M, Beckwith BJ, et al. The Heidelberg classification of renal cell tumours. J Pathol 1997;183:131–3. [DOI] [PubMed] [Google Scholar]
- [3].Linehan WM, Spellman PT, et al. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med 2016;374:135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Delahunt B, Eble JN, McCredie MR, et al. Morphologic typing of papillary renal cell carcinoma: comparison of growth kinetics and patient survival in 66 cases. Hum Pathol 2001;32:590–5. [DOI] [PubMed] [Google Scholar]
- [5].Wagener N, Edelmann D, Benner A, et al. Outcome of papillary versus clear cell renal cell carcinoma varies significantly in non-metastatic disease. PLoS One 2017;12:e0184173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nguyen DP, Vertosick EA, Corradi RB, et al. Histological subtype of renal cell carcinoma significantly affects survival in the era of partial nephrectomy. Urol Oncol 2016;34:259e1–8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Steffens S, Janssen M, Roos FC, et al. Incidence and long-term prognosis of papillary compared to clear cell renal cell carcinoma--a multicentre study. Eur J Cancer 2012;48:2347–52. [DOI] [PubMed] [Google Scholar]
- [8].Teloken PE, Thompson RH, Tickoo SK, et al. Prognostic impact of histological subtype on surgically treated localized renal cell carcinoma. J Urol 2009;182:2132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yoo S, You D, Jeong IG, et al. Histologic subtype needs to be considered after partial nephrectomy in patients with pathologic T1a renal cell carcinoma: papillary vs. clear cell renal cell carcinoma. J Cancer Res Clin Oncol 2017;143:1845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kondo T, Ikezawa E, Takagi T, et al. Negative impact of papillary histological subtype in patients with renal cell carcinoma extending into the inferior vena cava: single-center experience. Int J Urol 2013;20:1072–7. [DOI] [PubMed] [Google Scholar]
- [11].Simone G, Tuderti G, Ferriero M, et al. Papillary type 2 versus clear cell renal cell carcinoma: Survival outcomes. Eur J Surg Oncol 2016;42:1744–50. [DOI] [PubMed] [Google Scholar]
- [12].Kim KH, You D, Jeong IG, et al. Type II papillary histology predicts poor outcome in patients with renal cell carcinoma and vena cava thrombus. BJU Int 2012;110(11 Pt B):E673–8. [DOI] [PubMed] [Google Scholar]
- [13].Lee WK, Lee SE, Hong SK, et al. Characteristics and prognostic value of papillary histologic subtype in nonmetastatic renal cell carcinoma in Korea: a multicenter study. Urol J 2014;11:1884–90. [PubMed] [Google Scholar]
- [14].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [16].Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol 2005;28:123–37. [DOI] [PubMed] [Google Scholar]
- [17].Sacks HS, Berrier J, Reitman D, et al. Meta-analyses of randomized controlled trials. N Engl J Med 1987;316:450–5. [DOI] [PubMed] [Google Scholar]
- [18].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [20].Keegan KA, Schupp CW, Chamie K, et al. Histopathology of surgically treated renal cell carcinoma: survival differences by subtype and stage. J Urol 2012;188:391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Beck SD, Patel MI, Snyder ME, et al. Effect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinoma. Ann Surg Oncol 2004;11:71–7. [DOI] [PubMed] [Google Scholar]
- [22].Delahunt B, Eble JN. Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod Pathol 1997;10:537–44. [PubMed] [Google Scholar]
- [23].Sukov WR, Lohse CM, Leibovich BC, et al. Clinical and pathological features associated with prognosis in patients with papillary renal cell carcinoma. J Urol 2012;187:54–9. [DOI] [PubMed] [Google Scholar]
- [24].Polifka I, Agaimy A, Herrmann E, et al. High proliferation rate and TNM-stage but not histomorphological subtype are independent prognostic markers for overall survival in papillary renal cell carcinoma. Hum Pathol 2019;83:212–23. [DOI] [PubMed] [Google Scholar]
- [25].Daste A, Gross-Goupil M, Roca S, et al. Prolonged efficacy of mTOR inhibitors in papillary renal cell carcinoma: progression-free survival lasting for over 3 years, a case report and review of the literature. Target Oncol 2014;9:81–4. [DOI] [PubMed] [Google Scholar]
- [26].Connor Wells J, Donskov F, Fraccon AP, et al. Characterizing the outcomes of metastatic papillary renal cell carcinoma. Cancer Med 2017;6:902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gunawan B, von Heydebreck A, Fritsch T, et al. Cytogenetic and morphologic typing of 58 papillary renal cell carcinomas: evidence for a cytogenetic evolution of type 2 from type 1 tumors. Cancer Res 2003;63:6200–5. [PubMed] [Google Scholar]
- [28].Saleeb RM, Brimo F, Farag M, et al. Toward biological subtyping of papillary renal cell carcinoma with clinical implications through histologic, immunohistochemical, and molecular analysis. Am J Surg Pathol 2017;41:1618–29. [DOI] [PubMed] [Google Scholar]
- [29].Wang Y, Ding Y, Wang J, et al. Clinical features and survival analysis of clear cell papillary renal cell carcinoma: A 10-year retrospective study from two institutions. Oncol Lett 2018;16:1010–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang K, Zarzour J, Rais-Bahrami S, et al. Clear cell papillary renal cell carcinoma: new clinical and imaging characteristics. Urology 2017;103:136–41. [DOI] [PubMed] [Google Scholar]
- [31].Park I, Lee SH, Lee JL. A multicenter phase ii trial of axitinib in patients with recurrent or metastatic non-clear-cell renal cell carcinoma who had failed prior treatment with temsirolimus. Clin Genitourin Cancer 2018. [DOI] [PubMed] [Google Scholar]
- [32].Bamias A, Escudier B, Sternberg CN, et al. Current clinical practice guidelines for the treatment of renal cell carcinoma: a systematic review and critical evaluation. Oncologist 2017;22:667–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hanna N, Sun M, Meyer CP, et al. Survival analyses of patients with metastatic renal cancer treated with targeted therapy with or without cytoreductive nephrectomy: a national cancer data base study. J Clin Oncol 2016;34: 3267-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vaishampayan U. Evolving treatment paradigms in non-clear cell kidney cancer. Curr Treat Options Oncol 2018;19:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Giles RH, Choueiri TK, Heng DY, et al. Recommendations for the management of rare kidney cancers. Eur Urol 2017;72:974–83. [DOI] [PubMed] [Google Scholar]
- [36].Song C, Hong SH, Chung JS, et al. Renal cell carcinoma in end-stage renal disease: multi-institutional comparative analysis of survival. Int J Urol 2016;23:465–71. [DOI] [PubMed] [Google Scholar]
- [37].Lucarelli G, Loizzo D, Franzin R, et al. Metabolomic insights into pathophysiological mechanisms and biomarker discovery in clear cell renal cell carcinoma. Expert Rev Mol Diagn 2019;19:397–407. [DOI] [PubMed] [Google Scholar]
- [38].Linehan WM, Srinivasan R, Schmidt LS. The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol 2010;7:277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lucarelli G, Rutigliano M, Sallustio F, et al. Integrated multi-omics characterization reveals a distinctive metabolic signature and the role of NDUFA4L2 in promoting angiogenesis, chemoresistance, and mitochondrial dysfunction in clear cell renal cell carcinoma. Aging (Albany NY) 2018;10:3957–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lucarelli G, Rutigliano M, Ferro M, et al. Activation of the kynurenine pathway predicts poor outcome in patients with clear cell renal cell carcinoma. Urol Oncol 2017;35: 461 e15-61e27. [DOI] [PubMed] [Google Scholar]
- [41].Bianchi C, Meregalli C, Bombelli S, et al. The glucose and lipid metabolism reprogramming is grade-dependent in clear cell renal cell carcinoma primary cultures and is targetable to modulate cell viability and proliferation. Oncotarget 2017;8:113502–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lucarelli G, Rutigliano M, Sanguedolce F, et al. Increased expression of the autocrine motility factor is associated with poor prognosis in patients with clear cell-renal cell carcinoma. Medicine (Baltimore) 2015;94:e2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cai Y, Li HZ, Zhang YS. Comparison of partial and radical laparascopic nephrectomy: long-term outcomes for clinical T1b renal cell carcinoma. Urol J 2018;15:16–20. [DOI] [PubMed] [Google Scholar]
- [44].Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015;67:913–24. [DOI] [PubMed] [Google Scholar]
- [45].Mejean A, Hopirtean V, Bazin JP, et al. Prognostic factors for the survival of patients with papillary renal cell carcinoma: meaning of histological typing and multifocality. J Urol 2003;170:764–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


