Abstract
Aim:
To evaluate the efficacy and safety of celecoxib combined with chemotherapy in the treatment of metastatic or postoperative recurrent gastric cancer.
Methods:
This preliminary, three-center, clinical trial study was conducted between September 2010 and December 2016. In the experimental group (n = 100), patients were treated with celecoxib combined with chemotherapy, and chemotherapy alone was used in the control group. Progression-free survival (PFS) was considered as the primary efficacy parameter. Overall survival (OS), remission rate (RR), quality of life (QOL) and drug safety were considered as the secondary efficacy parameters.
Results:
The PFS of the experimental group was 6 months, which was not significantly longer than that of the control group (5 months, P = .73). The average OS was not significantly different between the experimental group (12 months) and the control group (10 months, P = .59). The average OS of the COX-2 positive patients in the experimental group was 14 months and it was significantly longer than the 10-month OS in the control group (P = .01). The PFS of the COX-2 positive patients in the experimental group was 7.5 months, significantly longer than the 5-month PFS of patients in the control group (P < .001). No statistical significance was identified in the incidence of nausea, neutropenia, anorexia, peripheral neurotoxicity, diarrhea, vomiting, asthenia and thrombocytopenia. The EORTC QLQ-C30 questionnaire revealed that the overall QOL of the experimental group was significantly higher than that of the control group (P < .05). No statistical significance was found in the scores of functioning scale between the 2 groups. However, the scores of the symptom scale, especially for pain and fatigue in the experimental group was remarkably higher than that in the control group (P < .05). The overall score of EORTC QLQ-STO22 for the experimental group was considerably higher compared to that for the control group (P < .05). No statistical significance was identified in term of the domains of restrictions on feeding, dysphagia, anxiety, reflux, sense of taste, dry mouth, hair loss and body shape between the 2 groups (P > .05 for all mentioned outcomes).
Conclusion:
Celecoxib combined with chemotherapy offers more clinical benefits for COX-2 positive advanced gastric cancer patients.
Keywords: celecoxib, chemotherapy, gastric cancer, overall survival, progression-free survival
1. Introduction
Advanced gastric cancer was often found already invaded surrounding tissues such as blood vessels, liver, pancreas, and peritoneum, or missed the opportunity of radical mastectomy due to the distant metastasis. Therefore, chemotherapy and molecular targeting therapy are the main treatment methods for advanced gastric cancer, which can relieve the symptoms, improve the quality of life and the prognosis. Adjuvant chemotherapy plays an increasingly important role in the treatment of advanced gastric cancer. Consequently, evaluation of clinical efficacy and safety of new adjuvant chemotherapeutic drugs and testing the targeting specificity and sensitivity of drugs still remain one of the important contents of clinical researches. In our previous study,[1] it has been proved that celecoxib combined with chemotherapy is effective and safe in patients undergoing radical gastrectomy, especially in patients with positive expression of COX-2. In this study, we evaluate the efficacy and safety of celecoxib combined with basic first-line chemotherapy platinum and fluorouracil on patients with metastatic or postoperative recurrent advanced gastric cancer to explore whether the combination therapy still has equivalent effect.
2. Materials and methods
2.1. Study subjects
Patients with metastatic or postoperative recurrent advanced gastric cancer from three medical centers including the First Affiliated Hospital of Lanzhou University, Gansu Wuwei Tumor Hospital and the General Hospital of Lanzhou Military Command between September 2010 and December 2016 were enrolled in this clinical trial. Written informed consents were obtained from all participants. The study procedures were approved by the ethics committee of the First Affiliated Hospital of Lanzhou University.
All patients were randomly assigned into experimental group or control group. In control group, Patients assigned to adjuvant treatment received six 3-week cycles of capecitabine (1000 mg/m2 twice daily on days 1–14 of each cycle) plus or tegafur (1000 mg once daily on days 1–5 of each cycle) plus intravenous oxaliplatin (130 mg/m2 once daily on day 1 of each cycle) starting within 7 days of randomisation. In experimental group, the chemotherapy regime was referred to that of control group. Celecoxib capsule (200 mg) was administered twice daily for approximate 5 months continuously until the day of final chemotherapy. According to the compliance and tolerance of gastric cancer patients, 6 cycles of adjuvant chemotherapy were delivered. The clinical efficacy and safety of adjuvant chemotherapy were assessed every 2 cycles.
Toxicity assessments were made according to the National Cancer Institutes common toxicity scale (Version 1.0). Dose interruptions or reductions were allowed to manage potentially serious or life-threatening adverse events. In cases of oxaliplatin-related neurological adverse events, capecitabine could be continued as monotherapy, and oxaliplatin was temporarily stopped and subsequently dose reduced by 50% for a serum creatinine > 2.0 mg/dL, grade 3 to 4 ototoxicity, and grade 3 to 4 neuropathy. Oxaliplatin monotherapy was not allowed. Palliative and supportive care was offered as needed for disease-related symptoms.
2.2. Inclusion and exclusion criteria
Inclusion criteria were as follows: patients aged between 18 and 70; patients with metastatic or postoperative recurrent advanced gastric cancer; patients without chemotherapy before, or the last adjuvant chemotherapy was done over 1 year ago; patients with RECIST standard measurable indicators; ECOG PS 0-2; estimated survival >12 weeks; no vital organ dysfunction; normal outcomes for liver, kidney heart function tests; routine blood test: neutrophilic granulocyte count ≥1.5 × 109/L, hemoglobin ≥90 g/L and platelet count ≥85 × 109/L. Liver function: total bilirubin < 1.5 times of 3. 1 μmol range; AST and ALT < 2.5 times of 49 U/L range, respectively (patients without liver metastases), AST and ALT < 5 times of 49 U/L range, respectively (patients whit liver metastases); kidney function: serum creatinine < 1.25 times of 108 μmol range; electrocardiograph revealed no abnormality.
Exclusion criteria were as the following: uncontrollable hypertension, diabetes mellitus and digestive tract ulcer; serious allergic history; uncontrollable mental diseases; pregnant or lactating female patients; Patients with symptomatic brain metastases.
2.3. Immunohistochemical staining of COX-2
Immunohistochemical SP method: 5 μm-paraffin embedded sections were subject to the following steps in order: deparaffinization in xylene for 10 minutes, gradient ethanol dehydration, 3% hydrogen peroxide incubation at 37°C for 10 minutes, phosphate buffer solution (PBS) rinsing for 5 minutes, antigen retrieval in .01 M citric acid buffer solution (pH = 6.0) at 95°C for 20 minutes, PBS wash for 5 minutes, normal goat serum working solution incubation at 37°C for 10 minutes, primary antibody (mouse anti-human COX-2 monoclonal antibody (SGB-bio company, Beijing, China)) incubation overnight at 4°C, PBS wash for 5 minutes, incubation with biotin-labeled secondary antibody at 37°C for 30 minutes, PBS wash for 5 minutes, incubation in horseradish peroxide enzyme-labeled streptomycin avidin working solution at 37°C for 30 minutes, diaminobenzidine (DAB) incubation, counterstained with hematoxylin for 3 minutes, washing under running water until it is clear. Then, the staining sections were observed under microscope. Substantially, no staining was graded as 0, slight staining as 1 and dark staining as 2. The percentage of positive cells ≤5% was considered as 0, 6% to 25% as 1, 26% to 50% as 2 and ≥51% as 3. The result of section staining density score and the percentage of positive cells was calculated as the final score. A score of 0 to 1 was graded as negative (−), 2 to 3 as weakly positive (+), 4 to 6 as positive (++), >6 as strongly positive (+++) and >=4 represented highest expression of COX-2.
2.4. Clinical efficacy assessment
The time they signed consent form was enrollment time. Progression-free survival (PFS) was defined as the time duration from patient enrollment to the disease progression or death. PFS was assessed every 2 cycles during the treatment (every three weeks is a chemotherapy cycle and the total cycle was 6). Overall survival (OS) referred to the time from patient enrollment to the death. The follow-up was initiated from the beginning of the chemotherapy and during the treatment; it was performed every 2 months until the death of patients. After the treatment, the follow-up was delivered every 3 months until the death of patients. Remission rate (RR) referred to the percentage of patients who were fully relieved or partially relieved. Quality of life (QOL) was assessed by EORTC QLQ-C30 and EORTC QLQ-STO22 questionnaires.
2.5. Therapeutic safety assessment
Common adverse events included nausea, neutropenia, anorexia, peripheral, neurotoxicity, diarrhea, vomiting, fatigue, thrombocytopenia, hand-foot syndrome, abdominal pain, constipation, dizziness, oral inflammation, and emaciation. The severity of these symptoms was classified and compared between the 2 groups by statistical analysis.
2.6. Statistical methods
2.6.1. Sample size
The sample size was calculated from our previous study performed.[1] Based on the results of this previous study, a sample size of 100 patients per group was derived. The study is powered for a 2-sided test with a Type I error rate of 5% and 95% power, that is, Z a = 1.96 and Z b = 1.64, adjusted for a 20% drop-out rate. Therefore, a total sample size of 240 is used in this study to evaluate the efficacy and safety of celecoxib combined with chemotherapy in the treatment of metastatic or postoperative recurrent gastric cancer.
2.6.2. Statistical analyses
All of the statistical analyses were performed by using PRISM statistical analysis software (GrafPad Software, Inc., San Diego, CA). The primary efficacy parameter was PFS and the secondary efficacy parameters consisted of OS, RR, QOL, and drug safety. The values of α = .05 and β = .10 were considered as a level of significance. The ratio comparison was performed by using chi-square test. Measurement data were statistically analyzed by analysis of variance, and the method of proportional hazards model (COX proportional hazard regression model) was used to estimate the baseline data. Survival data were statistically analyzed by log-rank test, Kaplan–Meier test. Both PFS and OS were analyzed with an intention to treat analysis. A series of multivariable analyses using logistic regression model also was carried out to identify and control for several possible confounders of the primary and secondary outcome variables. Statistical significance will be defined as P < .05.
3. Results
3.1. Baseline data
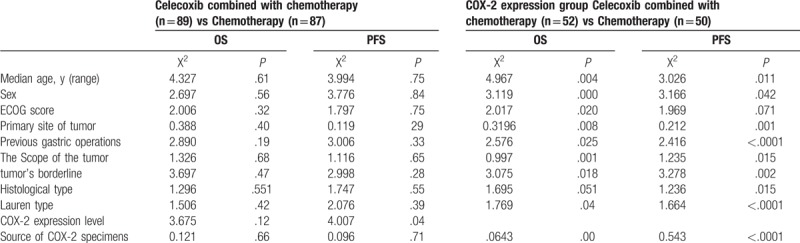
A total of 200 patients with metastatic or postoperative recurrent advanced gastric cancer were enrolled in this study. Eleven patients in the experimental group and 13 in control group rejected to receive treatment. Finally, 176 cases were eligible for subsequent investigation (Fig. 1). Among them, 130 cases were male and 46 were female with an age range from 28 to 70 years old. One hundred thirty-three patients got an ECOG score of 0 to 1, 43 patients got an ECOG score of 2. Ninety-two patients had locally advanced tumor, 84 patients suffered from metastatic tumor. The borderline of tumor was measurable in 78 patients (41 in experimental group and 37 in control group), the other 98 patients had borderline immeasurable tumors (48 in the experimental group and 50 in control group). The subtypes of tumors according to histology include: differentiated tubular adenocarcinoma in 108 patients; mucinous adenocarcinoma in 43 patients and signet-ring cell carcinoma in 25 patients. Tumors were intestinal type in 90 patients, diffuse type in 42 patients and mixed type in 44 patients. Among all the patients, 102 patients had COX-2 positive tumors and 72 patients had COX-2 negative tumor. Tumor tissues were sampled from gastroscope in 86 patients, from surgery in 66 patients and from metastatic lesions in 22 patients. All patients were screened strictly according to the inclusion criteria and exclusion criteria and were randomly assigned into the experimental group (n = 89) or control group (n = 87). No statistical significance was found between the experimental group and the control group regarding the demographic and baseline data, as illustrated in Table 1.
Figure 1.
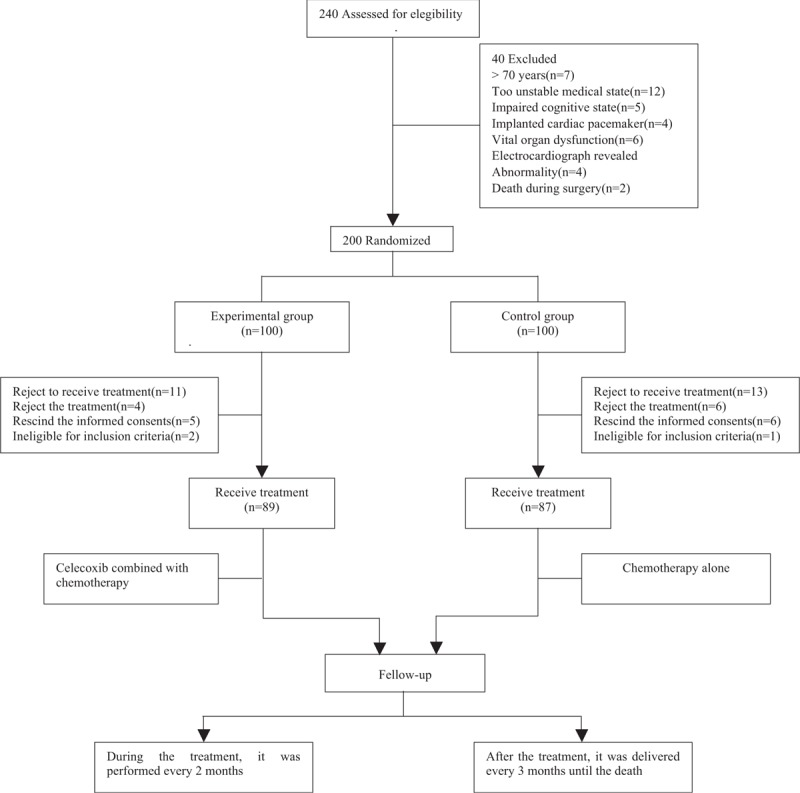
Flow chart of patients screening, grouping, and follow-up.
Table 1.
Demographic characteristics and illness history of the participants at randomization, according to prespecified subgroups and trial group.
3.2. COX-2 expression level
Immunohistochemical staining of COX-2 was performed in tumor specimen for each patient in both groups and the staining scores were calculated according to the method described. The result revealed that the positive rate of COX-2 expression in tumor was 58% (n = 52) in the experimental group and 57% (n = 50) in the control group. Representative images showing COX-2 positive and COX-2 negative were shown in Fig. 2.
Figure 2.
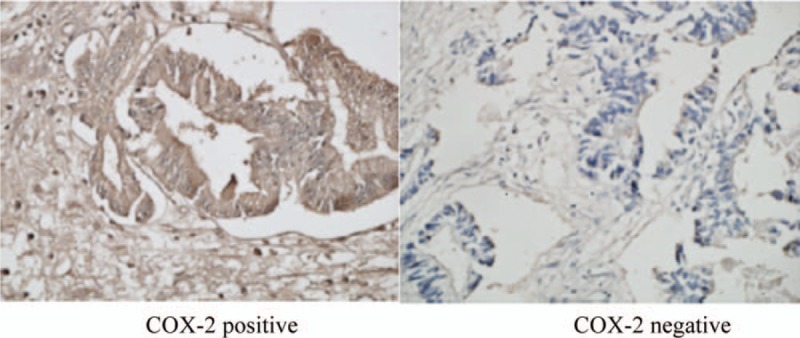
Immunohistochemical staining of COX-2 in gastric cancer tissues.
3.3. Survival after chemotherapy
3.3.1. Short-term effects analysis
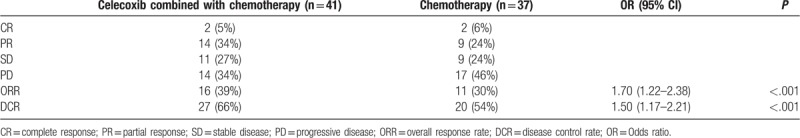
Forty-one patients with measurable lesions in the experimental group and 37 patients with measurable lesions in the control group were analyzed following the RECIST guideline (version 1.1); PFS was defined as the time from the beginning of intervention treatment to PD or death from any cause; ORR = (CR + PR)/total number of cases × 100%; DCR = (CR + PR + SD)/total number of cases × 100%. Results were listed below: In the experimental group, there were complete responses (CR) in 2 patients, partial response (PR) in 14 patients, stable disease (SD) in 11 patients, progressive disease (PD) in 14 patients. The total effective rate was 39% and the disease control rate was 66%; in the control group, there were CR in 2 patients, PR in 9 patients, SD in 9 patients, PD in 17 patients. The total effective rate was 30% and the disease control rate was 54%. The overall response rate (ORR) and the disease control rate (DCR) in the experimental group were significantly higher than those in the control group (P < .05), as illustrated in Table 2.
Table 2.
Comparison of short-term effects of the treatments in patients between 2 groups.
3.3.2. Comparison of survival analysis of patients
Data for patients in this study were censored at clinical cut-off. The Cox regression model used for the primary analysis included median age, sex, ECOG score and COX-2 expression level as factors. The results for OS and PFS in the celecoxib combined with chemotherapy population were consistent with those in the chemotherapy population except for COX-2 expression level (Table 3).
Table 3.
The multivariate analysis for overall survival and disease free survival.
Kaplan–Meier survival analysis was performed to compare the OS and PFS of the patients between the 2 groups. As shown in Fig. 3, there was no statistically significant difference between the 2 groups regarding the OS and PFS (hazard ratio [HR] = 0.49, 95% confidence interval [CI] = 0.17–1.00, P = .59). By the clinical cutoff date, the average OS was 12 months and 10 months for the experimental group and the control group, respectively (Table 4). Similar result was achieved in terms of PFS. PFS was 6 months and 5 months for the experimental group and the control group, respectively (Table 4). No statistically significant difference was found between the 2 groups (HR = 0.74, 95% CI = 0.41–1.23, P = .73). However, the average OS was 14 months for COX-2 positive patients from the experimental group, which was significantly higher than the 10-month OS for COX-2 positive patients from the control group (HR = 0.56, 95% CI = 0.28–0.93, P = .01). The PFS for COX-2 positive patients were 7.5 months and 5 months for the experimental group and the control group, respectively. The difference between the 2 group was statistically significant regarding the PFS (HR = 0.27, 95% CI = 0.10–0.65, P < .001) (Table 4 and Fig. 4).
Figure 3.
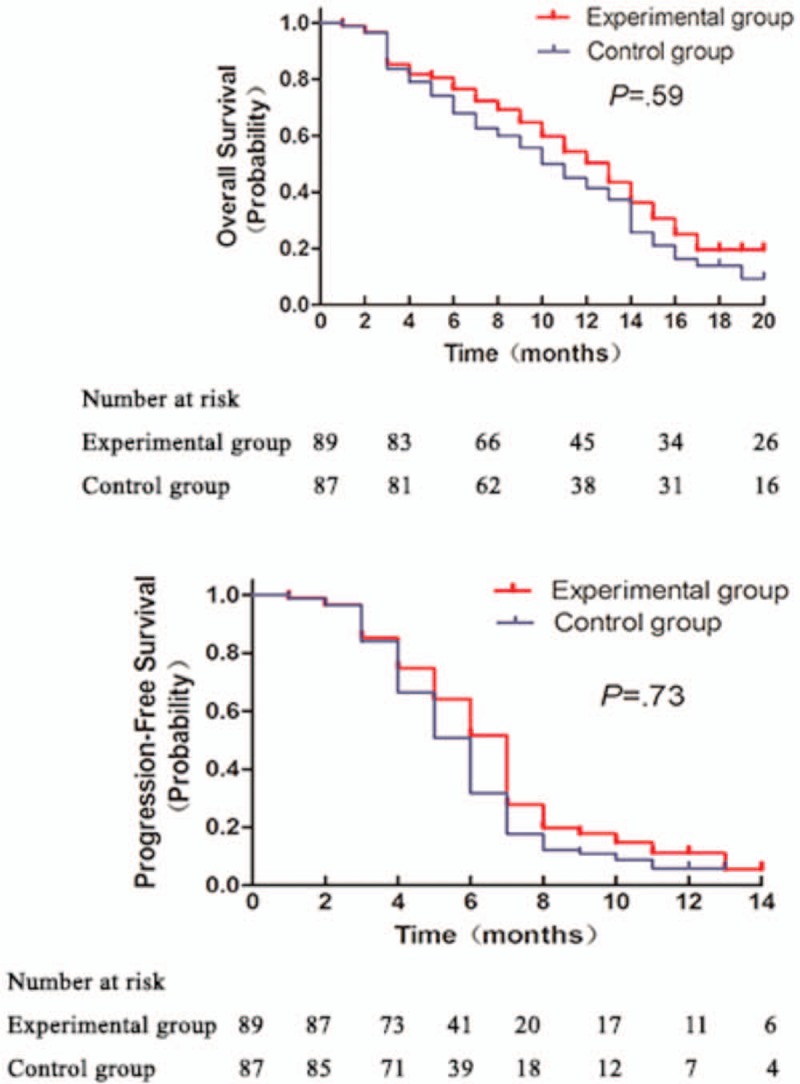
Comparison of OS (A) and PFS (B) in gastric cancer patients between the 2 groups.
Table 4.
Survival analysis of patients between the 2 groups.

Figure 4.
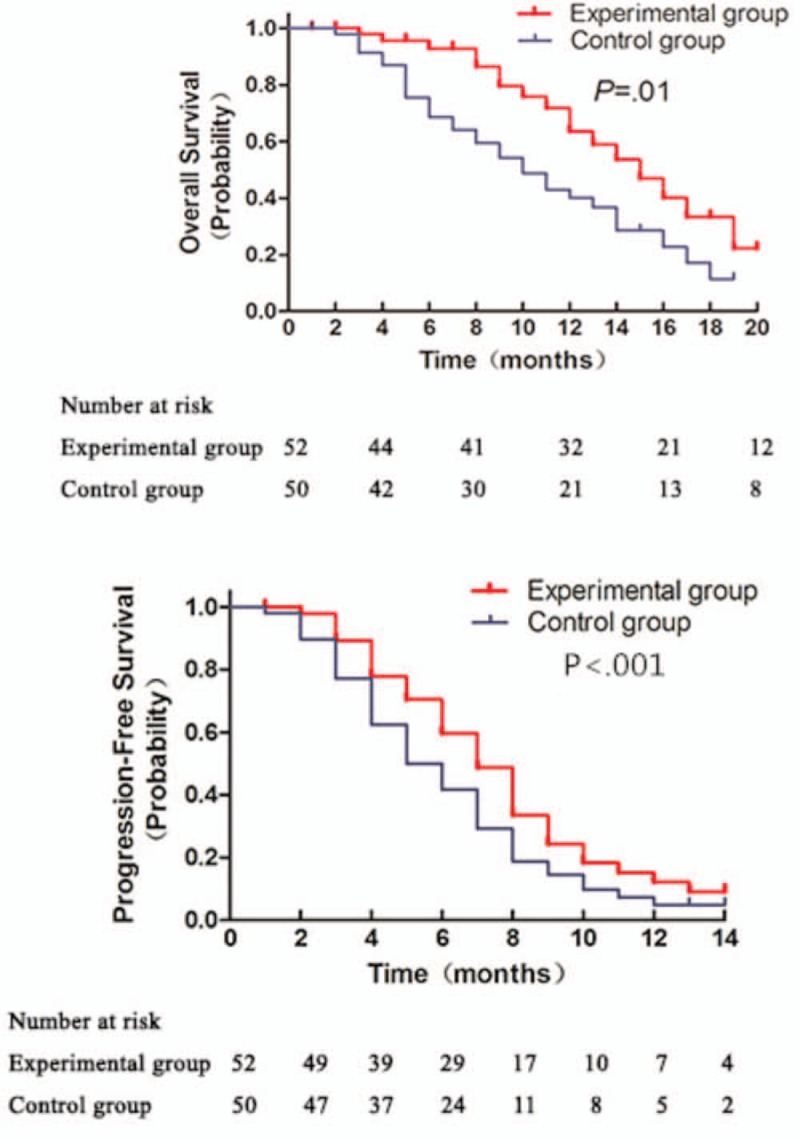
Comparison of OS (A) and PFS (B) in gastric cancer patients whit positive COX-2 between 2 groups.
3.4. Therapeutic safety evaluation
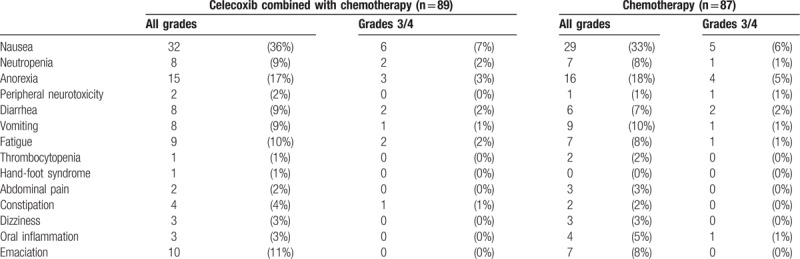
Observed adverse events induced by chemotherapy included nausea, appetite loss, vomiting, diarrhea, granulocytopenia, abdominal pain and emaciation, etc. In the experimental group, the most common adverse event was nausea. 32 out of 89 patients (36%) suffered nausea, including 6 cases (7%, 6/89) of severe nausea (grading at 3–4). In the control group, 29 out of 87 patients (33%) suffered nausea, including 5 cases nausea grading 3 to 4 (6%, 5/87). No statistical significant difference was found in the incidence of nausea between the 2 groups (Table 5). In addition, the incidence of anorexia, emaciation, fatigue, vomiting, diarrhea, peripheral and neutropenia did not significantly differ between the 2 groups, as illustrated in Table 5.
Table 5.
Comparison of incidence of adverse events after treatment between the 2 groups.
3.5. QOL assessment
In both groups, QOL was evaluated by QLQ-C30 and QLQ-STO22 questionnaires. Prior to the treatment, no statistically significant difference was found regarding the scores of each scale of QOL between the 2 groups (P > .05 for each scale). The scores of each scale in QLQ-C30 and QLQ-STO22 questionnaires did not show significant difference before and after chemotherapy in the control group (P > .05). After chemotherapy, the global QOL of EORTC QLQ-C30 questionnaire in the experimental group was significantly higher compared to that in the control group (P < .05). No statistical significance was found regarding the scores of functioning scales between the 2 groups (P > .05). Regarding the symptom scales, scores of pain scale was higher in the experimental group than that in the control group (P < .05). In the experimental group, the global score of EORTC QLQ-STO22 questionnaire was significantly higher than that in the control group (P < .05), whereas no statistical significant difference was identified regarding the scores of the domains of restrictions on feeding, dysphagia, anxiety, reflux, sense of taste, dry mouth, hair loss and body shape between the 2 groups (P > .05).
4. Discussion
Gastric cancer is one of the most common malignant tumors. The prognosis of patients with early stage gastric cancer is significantly different from that of patients with advanced gastric cancer. The 5-year postoperative survival rate isover 90%[2] and only 11% to 40%[3] for early stage and advanced gastric cancer patients, respectively. The average survival time for patients with untreated advanced gastric cancer is about 3 months, while the average survival time for patients treated with surgery, radiotherapy and chemotherapy is only 9 to 16 months. Except a few countries, many countries do not conduct mass screening for early gastric cancer among their population.[4] Gastric cancer is often diagnosed at an advanced stage or even with distant metastasis due to the lack of specific clinical manifestations and screening indicators. It is very often that patients with advanced gastric cancer have missed the surgery treatment opportunities when diagnosed because of the surrounding large blood vessels, tumor peritoneum invasion or distant organ metastasis. For those patients who have undergone a successful surgery, they still risk a recurrence rate as high as 50%.[5] Therefore, chemotherapy and molecular targeted therapy have become the main treatments for advanced gastric cancer.
In recent years, due to the development of new biological agents, molecular targeting therapy has demonstrated high efficiency and low toxicity in lymphoma,[6] breast cancer,[7] gastrointestinal stromal tumors,[8] colorectal cancer[9] and non-small cell lung cancer (NSCLC)[10] etc. A new therapy regimen comprising chemotherapeutic drugs and molecular targeting drugs has become a hot spot of research for the treatment of advanced gastric cancer. COX-2 is one of those molecular targeting drugs that have been involved in a lot of basic and clinical studies.
Studies[11] have shown that COX-2 may be involved in the proliferation, invasion and metastasis of gastric cancer via different signal transduction pathways, providing a theoretical basis for the targeted molecular therapy of gastric cancer. In addition, other studies showed that COX-2 was highly expressed in gastric cancer tissues, especially in gastric epithelial dysplasia.[12–14]
COX-2 inhibitor can inhibit the expression of multidrug resistance protein (MDR) caused by high expression of COX-2, therefore it is speculated that COX-2 inhibitor may enhance the antitumor effect of chemotherapeutic drugs by reducing the drug resistance of tumor.[15] It has been reported that celecoxib, a selective COX-2 inhibitor, could increase the expression of p21 protein, block the cell cycle progression, inhibit the growth of cancer cells, induce the expression of Fas protein and promote apoptosis of cancer cells in BGC-823 gastric cancer cells line.[16] Other researchers[17] demonstrated that celecoxib could inhibit multidrug resistance of human gastric cancer cell SGC7901/ VCR by inhibiting the drug pump of P-gP and partially reverse the multidrug resistance of gastric cancer cells. Celecoxib combined with different concentrations of 5-FU, DDP or VP16 were used to treat gastric cancer BGC-823 cell line, and the results showed that it could work with the chemotherapeutic drugs in a synergistic way, and could be used as a good sensitizer for chemotherapy in patients with advanced gastric cancer to improve the chemotherapeutic effect.[18] Relevant researches[19–20] showed that celecoxib regulated apoptosis and autophagy via the PI3K/Akt signaling pathway in SGC-7901 gastric cancer cells and showed anti-gastric cancer effects through inhibiting Akt signaling cascade. In animal experiments, celecoxib can also be used as a radiosensitizer to enhance the effect of radiotherapy.[21] In conclusion, targeting COX-2 is expected to be a new way to treat gastric cancer and has a good clinical potential.
Overall, the clinical study of COX-2 inhibitor in preventing and treating gastric cancer is still at the early stage throughout the world. At present, it has been found that a combination of celecoxib and octreotide can inhibit the preoperative growth of human gastric cancer.[22] The combination of celecoxib and octreotide before gastric cancer surgery can increase the expression of E-cadherin and NAG-1 (transforming growth factor superfamily member), promote cancer cell apoptosis, decrease the microvessel density in cancer tissue, promote the proliferation of fibrous tissue and increase the necrosis of cancer tissue. At the same time, patients showed good drug tolerance and no side effects.[23] We also carried out a clinical trial in which patients with gastric cancer were given celecoxib for a week before operation, and the results showed that celecoxib could significantly up-regulate the expression of E-cadherin, down-regulate the expression of COX-2, VEGF and MVD, inhibit tumor angiogenesis and promote apoptosis of tumor cells.[24]
In this study, patients with metastatic or postoperative recurrent advanced gastric cancer from Gansu Province were treated with celecoxib combined with chemotherapy or chemotherapy alone to evaluate the clinical efficacy and safety of the combination of celecoxib and chemotherapy in the treatment of advanced gastric cancer. In this multi-center randomized case-control study, 200 patients diagnosed with metastatic or postoperative recurrent advanced gastric cancer were recruited and randomly assigned into the experimental group and control group with 100 cases in each group. In the experimental group (n = 100), patients were administered with celecoxib in combination with chemotherapy for almost 5 months until the final chemotherapy. In the control group, chemotherapy regime consisting of fluorouracil in combination with oxaliplatin (5-Fu, capecitabine, and tegafur) alone was adopted. According to the therapeutic compliance and tolerance, the adjuvant chemotherapy was delivered for 6 cycles.
In this study, the average overall survival was 12 months in the experimental group and 10 months in the control group with no statistical significant difference (P = .59). The progression-free survival was 6 months and 5 months for experimental and control group, respectively, and there was no significant difference between the 2 groups (P = .73). In the experimental group, overall response rate was 39%, significantly higher than that of control group (30%, P < .001). The disease control rate of the former was 66% and the latter's was 54%. There was also a significant difference between the 2 groups (P < .001). In COX-2 positive subgroup, the average overall survival in the experimental group was 14 months, significantly higher than the 10-month average OS for the control group (P = .01). The PFS in COX-2 positive patients from the experimental group was 7.5 months, significantly higher than the 5-month average PFS for the control group (P < .001).
The above data showed that the overall OS and PFS for patients were not significantly improved by combination of celecoxib and chemotherapy compared to chemotherapy alone. However, ORR and DCR were improved to a small extent. In patients with positive COX-2 expression, the OS and PFS were also improved. These results showed that celecoxib could benefit the patients with metastatic or postoperative recurrent gastric cancer as a whole, especially in patients with positive COX-2.
Prior to the treatment, the scores of QLQ-C30 and QLQ-STO22 questionnaires did not significantly differ between 2 groups. In addition, no statistical significance was identified in the QLQ-C30 and QLQ-STO22 questionnaires before and after chemotherapy in the control group. Nevertheless, the global QOL score, the scores of the pain domain of QLQ-C30 questionnaire, and the score of the pain domain of QLQ-STO22 questionnaire in the experimental group were significantly higher after chemotherapy. No statistical significant difference was noted in terms of other domains between the 2 groups. The results showed that administration of celecoxib combined with chemotherapy could significantly enhance the QOL by mitigating the pain symptom of gastric cancer patients. In terms of the side effects during chemotherapy, 32 patients suffered from nausea in the experimental group, and 29 in the control group; 15 patients suffered from anorexia, and 16 in the control group; 10 patients suffered from emaciation, and 7 in the control group (Table 3). However, the incidence of grade 3 to 4 adverse events was lower. These results also indicate that celecoxib in combination with chemotherapy is a relatively safe treatment for metastatic or postoperative recurrent gastric cancer without more side effects.
In summary, celecoxib in combination with the first-line chemotherapy is an effective and safe treatment for metastatic or postoperative recurrent gastric cancer and has a good clinical application potential, especially in patients with positive COX-2, as evidenced by our study.
A weakness of this paper is that the number of patients studied was relatively small, and all specimens were collected from only 3 medical centers in Gansu province. An additional weakness of this study is that the distinction between experimental group and control group is possibly due to the local COX-2 inhibition or the general analgesic effect of the celecoxib. Celecoxib was also held for any grade 4 toxicity, grade 3 gastric or duodenal ulcers, bleeding or vomiting, which will affect the results. At the same time, the limitations of this study include the absence of data suggesting a possible mechanism of action of celecoxib during therapy, which was beyond the scope of this study. So, we hope that the conclusion in this clinical trial should be further validated by multi-center, large sample-size investigations, and it can get more convincing conclusions through a larger sample of clinical, randomized controlled study.
Acknowledgments
The authors thank all the medical staff of the First Affiliated Hospital of Lanzhou University, Gansu Wuwei Tumor Hospital and the General Hospital of Lanzhou Military Command for their strong support on the data collection.
Author contributions
Data curation: Qinghong Guo, Qiang Li, Min Liu.
Formal analysis: Yongning Zhou, Min Liu.
Funding acquisition: Yongning Zhou.
Investigation: Yuping Wang, Zhaofeng Chen.
Methodology: Qinghong Guo, Yuping Wang.
Project administration: Qinghong Guo, Quanlin Guan, Yongning Zhou.
Resources: Yongning Zhou.
Software: Zhaofeng Chen.
Supervision: Qinghong Guo, Qiang Li.
Validation: Yongning Zhou, Quanlin Guan.
Writing – original draft: Qinghong Guo, Jiong Wang.
Writing – review & editing: Yongning Zhou, Qinghong Guo, Jiong Wang.
Footnotes
Abbreviations: CI = confidence interval, COX-2 = cyclooxygenase-2, CR = complete response, DAB = diaminobenzidine, DCR = disease control rate, HR = hazard ratio, OR = Odds ratio, ORR = overall response rate, OS = overall survival, PBS = phosphate buffer solution, PFS = progression-free survival, PR = partial response, PD = progressive disease, QOL = quality of life, RR = remission rate, SD = stable disease.
This study was funded by the National key research and development plan, digital diagnosis and treatment equipment research and development special project (2016YFC0107006); Science and Technology Foundation of Gansu (17JR5RA190).
The authors have no conflicts of interest to disclose.
References
- [1].Guo QH, Liu XJ, Lu LZ, et al. Comprehensive evaluation of clinical efficacy and safety of celecoxib combined with chemotherapy in management of gastric cancer. Medicine 2017;96:e8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Saito H, Miyatani K, Takaya S, et al. Clinicopathologic characteristics and prognosis of advanced gastric cancer simulating early gastric cancer. Yonago Acta Med 2013;56:73–8. [PMC free article] [PubMed] [Google Scholar]
- [3].Chen XZ, Wen L, Rui YY, et al. Long-term survival outcomes of laparoscopic versus open gastrectomy for gastric cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Suh YS, Yang HK. Screening and early detection of gastric cancer: East versus West. Surg Clin North Am 2015;95:1053–66. [DOI] [PubMed] [Google Scholar]
- [5].Kashihara H, Shimada M, Yoshikawa K, et al. Risk factors for recurrence of gastric cancer after curative laparoscopic gastrectomy. J Med Invest 2017;64:79–84. [DOI] [PubMed] [Google Scholar]
- [6].Mondello P, Brea EJ, De Stanchina E, et al. Panobinostat acts synergistically with ibrutinib in diffuse large B cell lymphoma cells with MyD88 L265 mutations. JCI Insight 2018;3:125568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jackisch C, Lammers P, Jacobs I. Evolving landscape of human epidermal growth factor receptor 2-positive breast cancer treatment and the future of biosimilars. Breast 2017;32:199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cameron S, Beham A, Schildhaus HU. Current standard and future perspectives in the treatment of gastrointestinal stromal tumors. Digestion 2017;95:262–8. [DOI] [PubMed] [Google Scholar]
- [9].Miyamoto Y, Suyama K, Baba H. Recent advances in targeting the EGFR signaling pathway for the treatment of metastatic colorectal cancer. Int J Mol Sci 2017;18:e752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kumarakulasinghe NB, Van Zanwijk N, Soo RA. Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC). Respirology 2015;20:370–8. [DOI] [PubMed] [Google Scholar]
- [11].Ye YW, Liu M, Yuan H, et al. COX-2 regulates Snail expression in gastric cancer via the Notch1 signaling pathway. Int J Mol Med 2017;40:512–22. [DOI] [PubMed] [Google Scholar]
- [12].Van Rees B, Saukkonen P, Ristimäki K, al, et al. Cyclooxygenase-2 expression during carcinogenesis in the human stomach. J Pathol 2002;196:171–9. [DOI] [PubMed] [Google Scholar]
- [13].Lim HY, Joo HJ, Choi JH, et al. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res 2000;6:519–25. [PubMed] [Google Scholar]
- [14].Saukkonen K, Nieminen O, van Rees B, et al. Expression of cyclooxygenase-2 in dysplasia of the stomach and in intestinal-type gastric adenocarcinoma. Clin Cancer Res 2001;7:1923–31. [PubMed] [Google Scholar]
- [15].Liu J, Zhu HH, Pu P, et al. Study on the expression of cyclooxygenase-2 protein in gastric carcinoma and correlation with P-glyco-protein. China Oncol 2004;14:230–3. [Google Scholar]
- [16].Li Q, Peng J, Zhang GY. Effect of a selective COX-2 inhibitor on cell proliferation and apoptosis in human gastric cancer cell line BGC-823. J Central South Univ (Med Sci) 2008;33:1123–8. [PubMed] [Google Scholar]
- [17].Huang L, Wang C, Zheng W, et al. Effects of celecoxibon the reversal of multidrug resistance in human gastric carcinoma by downregulation of the expression and activity of P-glycoprotein. Anticancer Drugs 2007;18:1075–80. [DOI] [PubMed] [Google Scholar]
- [18].Zhu FS, Chen XM, Wang YJ, et al. Antitumor effects of specific cyclooxygenase inhibitors combined with chemotherapeutic agents on gastric cancer cells in vitro. Chin J Oncol 2007;29:186–8. [PubMed] [Google Scholar]
- [19].Liu M, Li CM, Zhou YN, et al. Celecoxib regulates apoptosis and autophagy via the PI3K/Akt signaling pathway in SGC-7901 gastric cancer cells. Int J Mol Med 2014;33:1451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kim N, Kim CH, Ahn DW, et al. Anti-gastric cancer effects of celecoxib, a selective COX-2 inhibitor, through inhibition of Akt signaling. J Gastroenterol Hepatol 2009;24:480–7. [DOI] [PubMed] [Google Scholar]
- [21].Nakata E, Mason KA, Hunter N, et al. Potentiation of tumor response to radiation or chemoradiation by selective cyclooxygenase-2 enzyme inhibitors. Int J Radiat Oncol Biol Phys 2004;58:369–75. [DOI] [PubMed] [Google Scholar]
- [22].Huang MT, Chen ZX, Wei B, et al. Preoperative growth inhibition of human gastric adenocarcinoma treated with a combination of celecoxib and octreotide. Acta Pharmacol Sin 2007;28:1842–5. [DOI] [PubMed] [Google Scholar]
- [23].Tang CW. Advances in the treatment of gastric cancer with non-cytotoxic drugs. Chin J Pract Intern Med 2015;12:1062–3. [Google Scholar]
- [24].Ran JT, Zhou YN, Tang CW, et al. Short-term preoperative treatment of celecoxib, a selective cyclooxygenase-2 inhibitor, on E-cadherin expression in gastric carcinoma tissues. Chin J Cancer 2009;8:361–5. [PubMed] [Google Scholar]


