Supplemental Digital Content is available in the text
Keywords: adolescent idiopathic scoliosis, ladybird homeobox 1, meta-analysis, polymorphisms, systematic review
Abstract
The Ladybird Homeobox 1 (LBX1) gene has been implicated in the etiology of adolescent idiopathic scoliosis (AIS). The association between LBX1 gene polymorphisms and AIS has been investigated in several studies. However, these findings have yield contradictory results rather than conclusive evidence.
This study is to provide a meta-analysis of the published case-control studies on the association between LBX1 gene polymorphisms and AIS in Asian and Caucasian populations.
This meta-analysis conformed to the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines. We conducted a literature research on PubMed, Embase, Web of Science, and Cochrane Library until February 10, 2018. We included all case-control or cohort studies about association between LBX1 gene polymorphisms and AIS. The Risk Of Bias In Non-randomised Studies-of Interventions and Critical Appraisal Skills Programme were used to evaluate the risk of bias and study quality. We assessed the strength of association by pooled odds ratios (ORs) and 95% confidence intervals (CIs) in all genetic models under a fixed-effect model or random-effect model. We further performed subgroup analysis by ethnicity and sex. Sensitivity analysis and publication bias were also undertaken.
A total of 10 studies (11,411 cases and 26,609 controls) were included in this meta-analysis. The pooled results showed a statistically significant association between LBX1 gene polymorphisms and AIS (for rs11190870, T vs C, OR = 1.54, 95% CI = 1.48–1.61, P < .00001; for rs625039, G vs A, OR = 1.50, 95% CI: 1.38–1.62; P < .00001; for rs678741, G vs A, OR = 0.74, 95% CI: 0.63–0.86; P < .0001; for rs11598564, G vs A, OR = 1.41, 95% CI: 1.31–1.51; P < .0001). For stratified analyses by ethnicity and sex, robust significant associations were detected in Asian and Caucasian populations, and in women and men under all genetic models.
T allele of rs11190870 and G alleles of rs625039 and rs11598564 represent risk factors for AIS, but G allele of rs678741 may play a protective role in the occurrence of AIS. Further research is needed to confirm this finding and to understand its implications.
1. Introduction
Adolescent idiopathic scoliosis (AIS) is a common structural spine deformity characterized by lateral curvature of the spine >10°.[1] It affects approximately 1% to 3% of children aged between 10 and 16 years. Despite decades of researches, the exact etiology of AIS remains unknown. Many hypotheses have been proposed, including genetic factors, skeletal growth, nervous system, and metabolic dysfunction.[2–4] It is widely accepted that genetic factors play a crucial role in the occurrence and development of AIS.[5]
The development of genome-wide association study (GWAS) provides a more comprehensive picture on the possible genes involved in the etiology of AIS. A GWAS study in a Japanese cohort reported an association between ladybird homeobox 1 (LBX1) gene polymorphisms and AIS.[6] They reported several common single nucleotide polymorphisms (SNPs) in the vicinity of the LBX1 gene including rs11190870, rs625039, rs678741, and rs11598564. A number of studies were undertaken to replicate these findings in multiple independent populations.[7–15] Two systematic reviews confirmed that LBX1 gene polymorphisms were the susceptibility loci for AIS in Asian populations.[16,17] With more new studies involving non-Asian populations,[10–12,15] updated evidence is necessary. Therefore, we performed a meta-analysis of the published case-control studies to validate the association between LBX1 gene polymorphisms and AIS risk in Asian and Caucasian populations, and to examine this association by odds ratios (OR) and 95% confidential intervals (CI).
2. Methods
Our meta-analysis was conducted in accordance with a prespecified protocol registered with PROSPERO International Prospective Register of Systematic Reviews (CRD42019124118), and conformed to the Meta-Analysis of Observational Studies in Epidemiology (MOOSE)[18] guidelines. As this meta-analysis based on published studies, the special ethic review and the ethical approval were not necessary.
2.1. Search strategy and selection criteria
Two authors (HJ and QY) independently conducted a comprehensive search of PubMed, Embase, Web of Science, and Cochrane Library to identify suitable studies published until February 10, 2018. The following keywords were used for searching: (“adolescent idiopathic scoliosis” OR “AIS” OR “scoliosis” OR “spinal deformity”) AND (“single nucleotide polymorphism” OR “polymorphism” OR “SNP” OR “variant”) AND (“LBX1” OR “ladybird homeobox 1” OR “HPX6” OR “homeobox”). No language or publication date restrictions were applied. Additional studies were identified through a hand search of references listed in the reports and reviews, and through personal contact with the authors if necessary. We transferred all relevant titles and abstracts from the databases to EndNote X7.0 (USACO Corporation, Tokyo, Japan).
2.2. Inclusion and exclusion criteria
The eligible studies were included for this meta-analysis if they met the following inclusion criteria: experimental subjects were diagnosed as AIS by clinical and radiological examination; the study evaluated the precise association between LBX1 gene polymorphisms and risk of AIS; genotype of control group conformed to the Hardy-Weinberg balance; the study provided available data for calculating an OR with 95% CI; the study design was cohort based or case control. Moreover, studies were excluded according to the following criteria: repeated publications; reviews, meta-analysis, and unpublished studies; unavailable data for extraction. Based on inclusion and exclusion criteria, 2 authors (HJ and QY) independently screened the titles and abstracts of references and obtained the full text for reference. For disagreements, a consensus was reached by a third author (QW). When we found potentially relevant studies in languages other than English, we had them translated. To limit publication bias, we considered meeting abstracts and unpublished data if there were sufficient results to analyze. If required, we contacted study investigators to obtain the information, and reported the results of these contacts.
2.3. Data extraction
We included studies assessing whether the LBX1 gene polymorphisms was associated with AIS risk. The extracted information included the following data: first author; publication year; study population (country, ethnicity); study design; number of participants; characteristics of participants (age and sex); Cobb angle of patients with AIS; outcome measure (SNP Genotyping); allele and/or genotype frequencies; results of Hardy-Weinberg equilibrium (HWE) test. HWE was checked in study controls using the χ2 goodness-of-fit test as a quantitative assessment for potential selection bias and confounding. According to a standardized and pilot-tested form, 2 authors (HJ and QY) independently extract data on outcomes for each study. Disagreements between 2 authors were resolved by discussion, and if necessary, a third author (QW) was consulted.
2.4. Assessment of risk of bias and study quality
We evaluated risk of bias (RoB) in included studies using the Risk Of Bias In Non- randomised Studies-of Interventions (ROBINS-I).[19] The ROBINS-I contains 7 bias distinct domains confounding; selection of participants into the study; classification of interventions; deviations from intended interventions; missing data; measurement of outcomes; and selective reporting Overall RoB judgment for each study are divided into 4 grades: low RoB, moderate RoB, serious RoB, and critical RoB. Moreover, we evaluated the methodological quality of studies according to the Critical Appraisal Skills Program (CASP).[20] The assessment of CASP contains 3 sections (10 questions), including the validity of results, appropriate reporting of results, and significance of results. The maximum score is 20, and the minimum score is 0. Studies could be divided into 3 grades: grade A (high quality, scored 15–22), grade B (medium quality, scored 8–14), and grade C (low quality, scored 0–7). Two authors (HJ and QY) independently evaluated the RoB and methodological quality of included studies.
2.5. Assessment of heterogeneity
The pooled OR was calculated by a fixed-effect model or a random-effect model according to the heterogeneity. Heterogeneity was checked by a χ2-based Q statistic and P < .10 was considered statistically significant. To calculate the summary OR, the fixed effect model was performed if no heterogeneity existed (P > .10, I2 < 50%). Otherwise, the random-effect model was used.[21,22] A pooled OR was determined by Z test and a P value of .05 was considered statistically significant.
2.6. Statistical analyses
ORs and 95% confidence intervals (CIs) used to assess the strength of association between LBX1 gene polymorphisms and AIS predisposition. Measures of treatment effect are considered statistically significant when P is <.05. Sensitivity analysis was performed to check the robustness of meta-analysis by assessing the influence of individual studies. Furthermore, subgroup analysis was stratified based on ethnicity and sex. Funnel plots were used to evaluate the potential publication bias assess the in meta-analyses in which at least 10 studies were included. Data analysis was conducted using STATA version 11.0 (Stata Corporation, College Station, TX) and Review Manager 5.31 (Nordic Cochrane Center: http://ims.cochrane.org/revman/download).
3. Results
3.1. Characteristics of the selected studies
A total of 182 abstracts that met the inclusion criteria were retrieved through the selected electronic databases. One hundred thirty articles were excluded after reading the titles and abstracts. Thirty-one potential articles were subsequently included for full-text review. Finally, 10 studies were included in our meta-analysis according to our strict inclusion criteria. The flow chart for the identification of the studies is presented in Figure 1.
Figure 1.
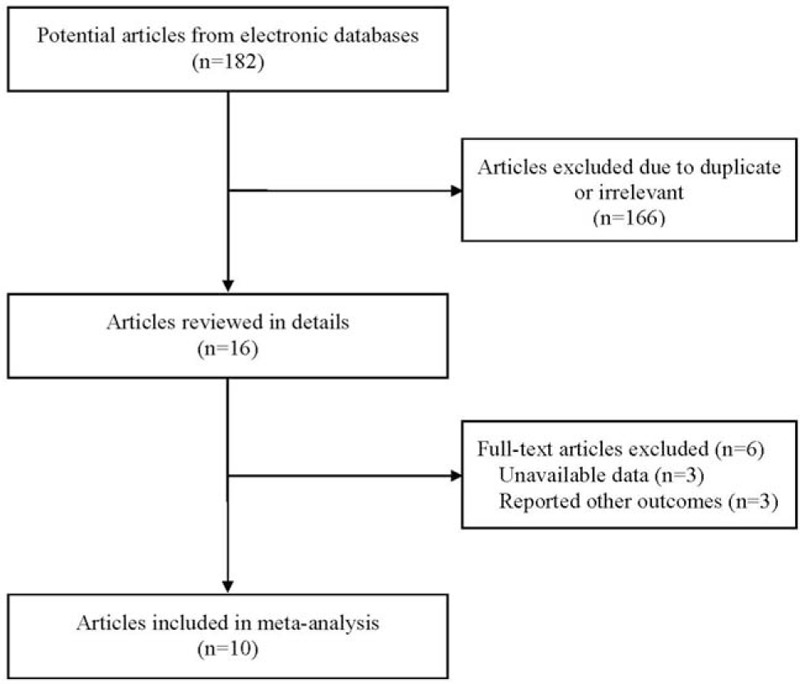
Flow diagram of the results of the search strategy.
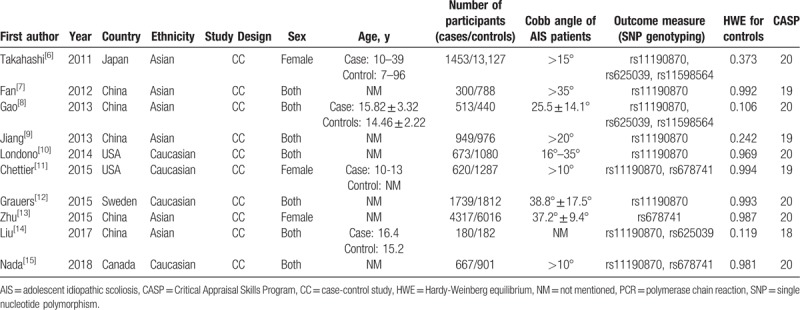
A total of 10 case-control studies published from 2011 to 2018 were identified in the meta-analysis, including 11,411 patients with AIS and 26,609 normal controls. The sample size of all eligible studies ranged from 362 to 14,580. The races of the participants were Asian (n = 6) and Caucasian (n = 4). For rs11190870, rs625039, rs678741, and rs11598564, 9 studies, 3 studies, 3 studies, and 2 studies were included in the pooled analysis, respectively. The control subjects in 10 included studies comprised 3 sources: healthy volunteers without spine deformity were recruited from the general population; young students were recruited after scoliosis screening at elementary and middle schools; normal controls were included from the population-based cohorts. All of these studies were case-control studies which provided the allele and/or genotype frequencies in case and control groups. The main characteristics of the included studies are listed in Table 1 and Supplemental Table 1.
Table 1.
Characteristics of the included studies.
3.2. Risk of bias in included studies
Based on ROBINS-I, 8 studies[6–11,13,15] were rated as moderate RoB, and 2 studies[12,14] were deemed to have serious RoB. None of the studies received the critical RoB rating. Moreover, the results of quality assessment are shown in Table 1. All included studies were categorized as grade A, with scores ranging from 18 to 20, which indicated a good quality.
3.3. Main results and subgroup analyses
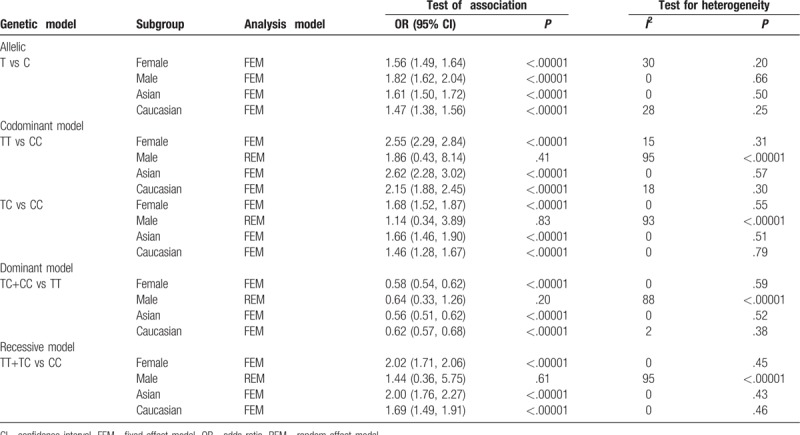
The pooled results suggested a highly statistically significant association between the rs11190870 polymorphism and AIS in all genetic models (Table 2). In the stratified analysis by ethnicity, significant associations were found when all studies were pooled with fixed-effects models for the allelic and genotypic comparisons (for allelic comparison in Asian population: OR = 1.61, 95% CI = 1.50–1.72, P < .00001; for allelic comparison in Caucasian population: OR = 1.45, 95% CI = 1.31–1.60, P < .00001) (Fig. 2). In addition, significant associations were found when all studies were pooled in fixed-effects models for all genetic models in females (for allelic comparison in female: OR = 1.62, 95% CI = 1.54–1.71, P < .00001) (Fig. 3). For men, significant association was only found in allelic comparison (OR = 1.79, 95% CI = 1.58–2.02, P < .00001); no statistical significant association was detected in other genetic models (Table 3). For male subgroup, significant heterogeneity was found in all genetic models except allelic comparison. The remaining 3 gene polymorphisms, including rs625039, rs678741, and rs11598564, were also found to be related to the risk of AIS (rs625039, G vs A, OR = 1.50, 95% CI: 1.38–1.62; P < .00001; rs678741, G vs A, OR = 0.74, 95% CI: 0.63–0.86; P < .0001; rs11598564, G vs A, OR = 1.40, 95% CI: 1.31–1.51; P < .0001) (Table 2). The G allele of rs625039 and rs11598564 increased the risk of AIS. In contrast, G allele of rs678741 may be a protective factor for AIS predisposition.
Table 2.
Meta-analysis of association between LBX1 gene polymorphisms and adolescent idiopathic scoliosis susceptibility.
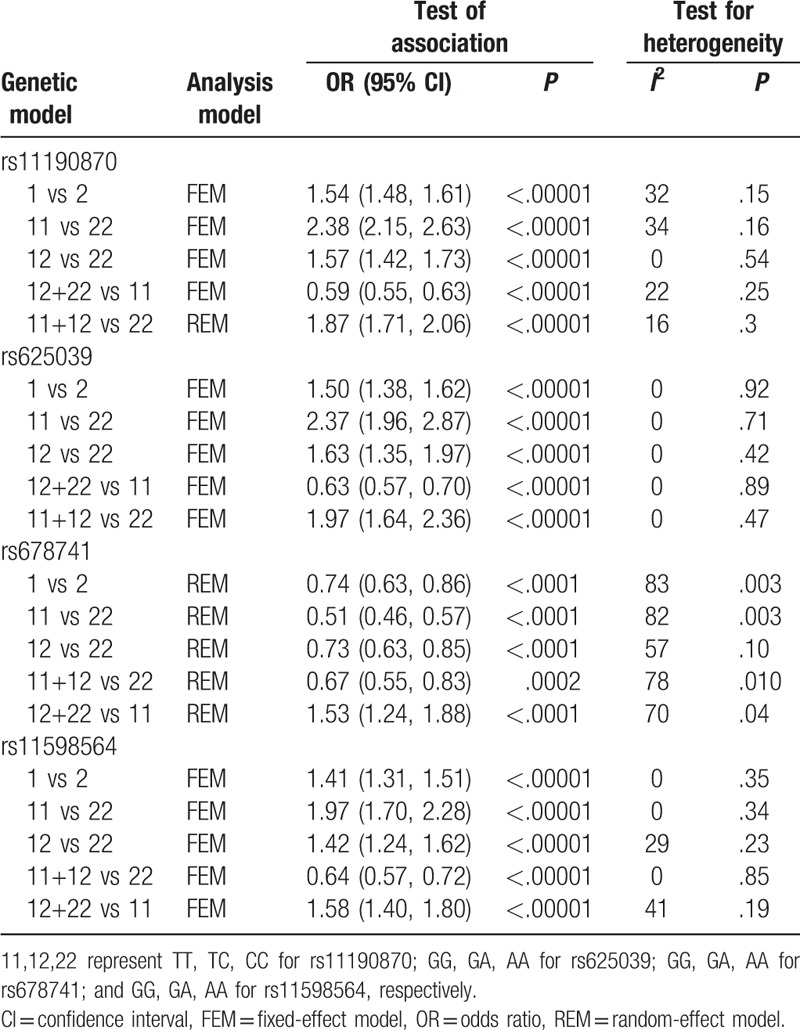
Figure 2.
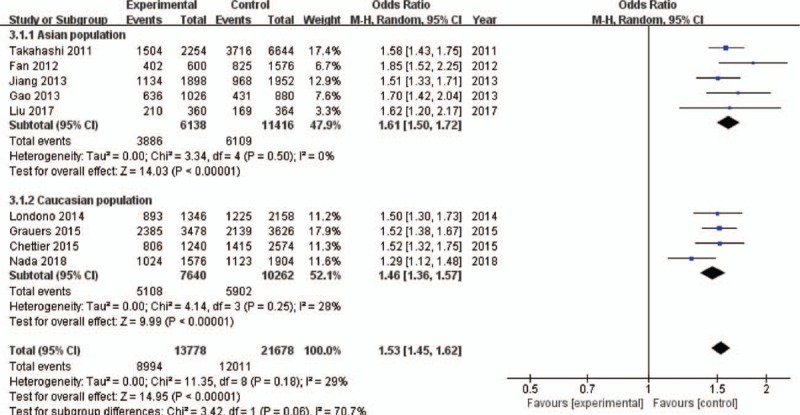
Forest plot of rs11190870 polymorphism and adolescent idiopathic scoliosis (AIS) predisposition in Asian and Caucasian populations under allelic contrast model. CI = confidence interval.
Figure 3.
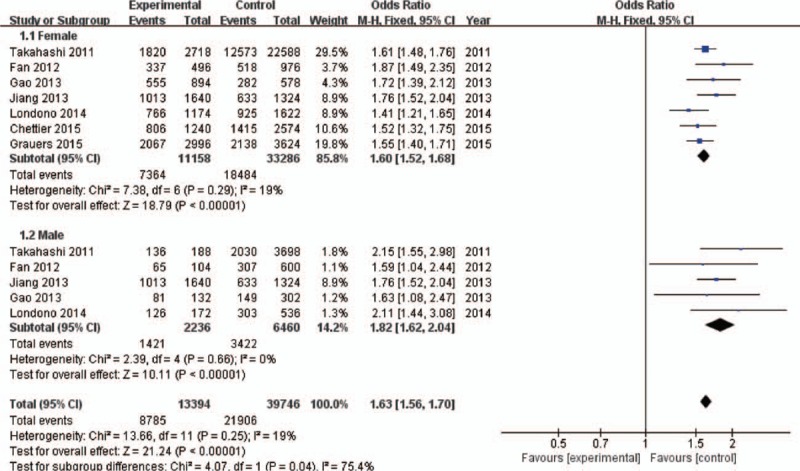
Forest plot of rs11190870 polymorphism and adolescent idiopathic scoliosis (AIS) predisposition in women and men under allelic contrast model.
Table 3.
Subgroup analysis on the association between rs11190870 polymorphism and adolescent idiopathic scoliosis susceptibility.
3.4. Sensitivity analyses
A sensitivity analysis was conducted to explore the source of the heterogeneity. We performed sensitivity analysis to assess the influence of each study on the abovementioned 4 polymorphisms under all comparison genetic models. By the sequential omission of individual studies in these models, we found that none of the individual studies significantly affected the pooled ORs, and that the results were not substantially altered (all P > .05). This finding supports the reliability of the meta-analysis.
3.5. Publication bias
Begg funnel plot was performed to assess the publication bias of the literature. We detected publication biases for rs11190870 under all genetic models. The results suggest no evidence of obvious asymmetry in all comparison models (z = 0.83, P = .404) (Fig. 4).
Figure 4.
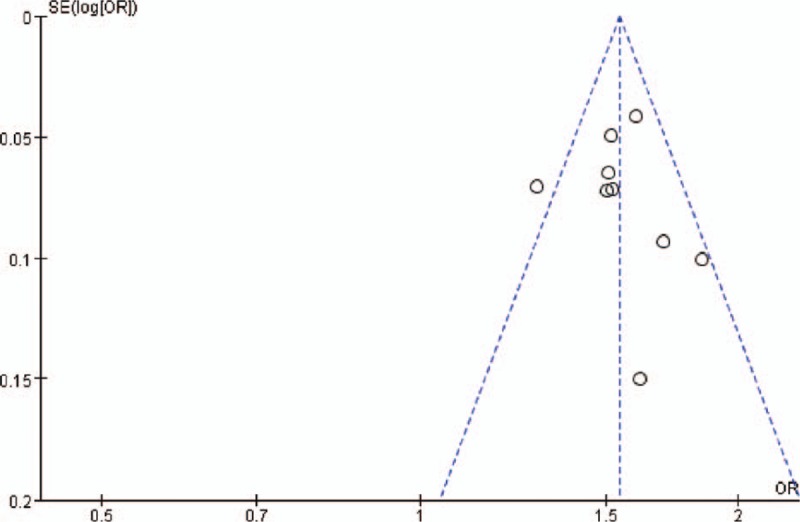
Funnel plot of rs11190870 polymorphism and adolescent idiopathic scoliosis (AIS) predisposition (allelic comparison) in the overall population (z = 0.83, P = .404).
4. Discussion
Our meta-analysis of 10 studies, involving 11,411 cases and 26,609 controls, found a statistically significant association between LBX1 gene polymorphisms (rs11190870, rs625039, rs678741, and rs11598564) and AIS. The results indicated that T allele of rs11190870 is significant associated with increased risk of AIS in overall population. The significance and consistency of results is across the sex and ethnicity. In remaining 3 variants (rs625039, rs11598564, and rs678741), the G allele represented an approximately 1.50-fold, 1.40-fold increased risk factor, and 0.74-fold decreased risk factor for AIS predisposition, respectively. Similar effects were found in the subgroup analysis of sex, compared with the meta-analysis of overall population. It was partly in accordance with the results of previous studies.[16]
In 2011, Takahashi et al[6] first reported that LBX1 gene polymorphisms (rs11190870, rs625039, and rs11598564) are correlated with an increased risk of AIS, using a GWAS to compare 1376 Japanese female patients with AIS with 11,297 female controls. Subsequently, a number of studies were investigated the association between LBX1 genotypes and the risk of AIS.[7–15] However, the results are inconclusive. Three meta-analyses have been conducted on this topic.[16,17,23] There are some major limitations in prior meta-analyses. By reviewing all studies published before November 2013, Liang et al[17] and Chen et al[16] performed the meta-analyses regarding East Asian population, without Caucasian population. It may weaken the validity of the results in variant ethnicities. In another meta-analysis, 3 recent studies were not included, which may lead to inaccuracy of conclusion.[23] The meta-analysis should provide the best current evidence for clinical decision making. Cochrane Back Review Group recommended that meta-analysis require timely updates as upcoming new studies. To the best of our knowledge, the current study is the largest sample size of meta-analysis to investigate the association between LBX1 polymorphisms and AIS.
Clinical heterogeneity is a problem when interpreting the results of meta-analysis. Among 10 included studies, there is an obvious difference in clinical phenotype of AIS patients. Cobb angle indicated the curve severity of AIS. The inclusion criteria of Cobb angle of patients with AIS in 10 studies ranged from 10° to 35°. It is becoming more apparent that the nature of AIS genetic susceptibility is likely to vary between different severities of scoliosis.[8,9] In view of the clinical heterogeneity, the results of the meta-analysis should be interpreted with caution. Therefore, the implications for future research are that a large patient population with uniform clinical phenotype should be studied by several international groups to enhance the power and significance of these findings. According to the ROBINS-I, 8 studies were rated as moderate RoB, and 2 studies were rated as serious RoB. The high RoB in included studies may be related to the inherent problem of case-control design, such as selection bias. We also evaluated the methodological quality of studies by CASP. There was high quality in all the included studies. Although no language restrictions were applied, only English-language articles were included in this study. There were mainly 2 reasons: first, non-English language publications may be missed as many electronic databases are English-language resource; second, some studies published in other languages may be not rigorous and are often of low quality. This may lead to publication bias. We used Begg funnel plots to evaluate the potential publication bias. Figure 4 indicates that no obvious asymmetry in Begg funnel plot (z = 0.83, P = .404). Therefore, no evidence of publication bias was observed in our study. Findings were consistent and precise across the studies. The overall quality of evidence is high and imparts confidence in the contribution of the LBX1 gene polymorphisms to risk of AIS.
Our meta-analysis included more original studies, and explored more stratification factors by subgroups, and used sensitivity analysis to find heterogeneity, which could make the results more reliable and more accurate. Nevertheless, several limitations of this meta-analysis should be acknowledged. First, publication biases may still exist as some studies were excluded due to unavailable information, and studies with negative results often have less chance for publication. It may affect the stability of positive results. Second, the lack of the original data limited the further analysis regarding the correlation of LBX1 gene polymorphisms and scoliotic curve severity. Thus it is still not sure whether the genetic variants in LBX gene is an independent predictive factor for development of AIS.
In conclusion, our results indicate that T allele of rs11190870, G alleles of rs625039, and rs11598564 represent risk factors for AIS, but G allele of rs678741 may play a protective role in the occurrence of AIS. The function studies are necessary to assess the mechanisms underlying the association between LBX1 gene polymorphisms and AIS risk.
Author contributions
Data curation: Qinghua Yang, Yewen Guan.
Formal analysis: Qinghua Yang, Yewen Guan.
Investigation: Hua Jiang, Yang Liu.
Methodology: Yang Liu, Zengming Xiao.
Software: Yewen Guan.
Supervision: Zengming Xiao, Qingjun Wei.
Validation: Hua Jiang, Xinli Zhan, Zengming Xiao.
Writing – original draft: Hua Jiang.
Writing – review and editing: Hua Jiang, Qingjun Wei.
Supplementary Material
Footnotes
Abbreviations: AIS = adolescent idiopathic scoliosis, CASP = Critical Appraisal Skills Program, CI = confidence interval, GWAS = genome-wide association study, HWE = Hardy-Weinberg equilibrium, LBX1 = ladybird homeobox 1, OR = odds ratio, RoB = risk of bias, ROBINS-I = Risk Of Bias In Non- randomised Studies-of Interventions, SNP = single nucleotide polymorphism.
This work was supported by the Natural Science Foundation of China (81460353/81560371), Guangxi Natural Science Foundation (2015GXNSFBA139167) and Youth Science Foundation of Guangxi Medical University (GXMUYSF201329).
Supplemental Digital Content is available for this article.
The authors report no conflicts of interest.
References
- [1].Weinstein SL, Dolan LA, Cheng JC, et al. Adolescent idiopathic scoliosis. Lancet 2008;371:1527–37. [DOI] [PubMed] [Google Scholar]
- [2].Wang WJ, Yeung HY, Chu WC, et al. Top theories for the etiopathogenesis of adolescent idiopathic scoliosis. J Pediatr Orthop 2011;311 suppl:S14–27. [DOI] [PubMed] [Google Scholar]
- [3].Acaroglu E, Bobe R, Enouf J, et al. The metabolic basis of adolescent idiopathic scoliosis: 2011 report of the “metabolic” workgroup of the Fondation Yves Cotrel. Eur Spine J 2012;21:1033–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stetkarova I, Zamecnik J, Bocek V, et al. Electrophysiological and histological changes of paraspinal muscles in adolescent idiopathic scoliosis. Eur Spine J 2016;25:3146–53. [DOI] [PubMed] [Google Scholar]
- [5].Tang NL, Yeung HY, Hung VW, et al. Genetic epidemiology and heritability of AIS: a study of 415 Chinese female patients. J Orthop Res 2012;30:1464–9. [DOI] [PubMed] [Google Scholar]
- [6].Takahashi Y, Kou I, Takahashi A, et al. A genome-wide association study identifies common variants near LBX1 associated with adolescent idiopathic scoliosis. Nat Genet 2011;43:1237–40. [DOI] [PubMed] [Google Scholar]
- [7].Fan YH, Song YQ, Chan D, et al. SNP rs11190870 near LBX1 is associated with adolescent idiopathic scoliosis in southern Chinese. J Hum Genet 2012;57:244–6. [DOI] [PubMed] [Google Scholar]
- [8].Gao W, Peng Y, Liang G, et al. Association between common variants near LBX1 and adolescent idiopathic scoliosis replicated in the Chinese Han population. PLoS One 2013;8:e53234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jiang H, Qiu X, Dai J, et al. Association of rs11190870 near LBX1 with adolescent idiopathic scoliosis susceptibility in a Han Chinese population. Eur Spine J 2013;22:282–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Londono D, Kou I, Johnson TA, et al. A meta-analysis identifies adolescent idiopathic scoliosis association with LBX1 locus in multiple ethnic groups. J Med Genet 2014;51:401–6. [DOI] [PubMed] [Google Scholar]
- [11].Chettier R, Nelson L, Ogilvie JW, et al. Haplotypes at LBX1 have distinct inheritance patterns with opposite effects in adolescent idiopathic scoliosis. PLoS One 2015;10:e0117708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Grauers A, Wang J, Einarsdottir E, et al. Candidate gene analysis and exome sequencing confirm LBX1 as a susceptibility gene for idiopathic scoliosis. Spine J 2015;15:2239–46. [DOI] [PubMed] [Google Scholar]
- [13].Zhu Z, Tang NL, Xu L, et al. Genome-wide association study identifies new susceptibility loci for adolescent idiopathic scoliosis in Chinese girls. Nat Commun 2015;6:8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu S, Wu N, Zuo Y, et al. Genetic polymorphism of LBX1 is associated with adolescent idiopathic scoliosis in northern Chinese Han population. Spine (Phila Pa 1976) 2017;42:1125–9. [DOI] [PubMed] [Google Scholar]
- [15].Nada D, Julien C, Samuels ME, et al. A replication study for association of LBX1 locus with adolescent idiopathic scoliosis in French-Canadian Population. Spine (Phila Pa 1976) 2018;43:172–8. [DOI] [PubMed] [Google Scholar]
- [16].Chen S, Zhao L, Roffey DM, et al. Association of rs11190870 near LBX1 with adolescent idiopathic scoliosis in East Asians: a systematic review and meta-analysis. Spine J 2014;14:2968–75. [DOI] [PubMed] [Google Scholar]
- [17].Liang J, Xing D, Li Z, et al. Association between rs11190870 polymorphism near LBX1 and susceptibility to adolescent idiopathic scoliosis in East Asian population: a genetic meta-analysis. Spine (Phila Pa 1976) 2014;39:862–9. [DOI] [PubMed] [Google Scholar]
- [18].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [19].Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Egerton T, Diamond L, Buchbinder R, et al. Barriers and enablers in primary care clinicians’ management of osteoarthritis: protocol for a systematic review and qualitative evidence synthesis. BMJ Open 2016;6:e011618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chang KV, Hsu TH, Wu WT, et al. Association between sarcopenia and cognitive impairment: a systematic review and meta-analysis. J Am Med Dir Assoc 2016;17:1164e1167-1164 e1115. [DOI] [PubMed] [Google Scholar]
- [22].Chang KV, Hsu TH, Wu WT, et al. Is sarcopenia associated with depression? A systematic review and meta-analysis of observational studies. Age Ageing 2017;46:738–46. [DOI] [PubMed] [Google Scholar]
- [23].Cao Y, Min J, Zhang Q, et al. Associations of LBX1 gene and adolescent idiopathic scoliosis susceptibility: a meta-analysis based on 34,626 subjects. BMC Musculoskelet Disord 2016;17:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


