Supplemental Digital Content is available in the text
Keywords: chemoradiation, non–small-cell lung cancer, objective response rate, overall survival, progression-free survival, radiotherapy
Abstract
Background:
This meta-analysis compared radiotherapy (RT) versus concurrent chemoradiotherapy (RT+CT) in treating patients with inoperable stage III non–small-cell lung cancer (NSCLC).
Methods:
Medline, Cochrane, EMBASE, Google Scholar databases were searched until July 28, 2015 using the following keywords non-small cell lung cancer, advanced cancer, incurable/inoperable/unresectable, chemotherapy, radiotherapy, chemoradiotherapy/chemoradiation. Randomized controlled trials (RCTs) and two-armed prospective studies that compared combined RT+CT with RT alone in patients with locally advanced (stage III) nonresectable NSCLC were eligible for inclusion. Treatment effect on overall survival, progression-free survival (PFS), and objective response rate (ORR) were evaluated.
Results:
Ultimately, 13 RCT studies were included in the systematic review and meta-analysis. The 13 studies included a total of 1936 patients with incurable/inoperable stage III NSCLC, of which 975 received RT alone and 961 received RT+CT combination therapy. The average age ranged from 54 to 77 years. At 1 and 2 years after treatment, the pooled data reveal that patients receiving CT+RT combination therapy had higher overall survival (pooled hazard ratio (HR), 0.72; 95% CI, 0.62–0.84; P < .001; 1-yr: HR, 0.67; 95% CI, 0.54–0.84; P < .001; 2-year: HR, 0.57; 95% CI, 0.45–0.73; P < .001), higher PFS (pooled HR, 0.73, 95% CI, 0.60–0.89; P = .002; 1-year: HR, 0.36; 95% CI, 0.24–0.53; P < .001; 2-year: HR, 0.38; 95% CI, 0.23–0.63; P < .001).
Conclusion:
Our findings show higher efficacy for concurrent CT+RT over RT alone in treating locally-advanced, unresectable stage III NSCLC.
1. Introduction
Non–small-cell lung cancer (NSCLC) represents greater than 80% of all lung tumors with about one-third of patients presenting with locally advanced stage III tumors that are not amenable to surgery.[1–3] For these patients post-surgical outcomes are poor.[4–8] The 5-year survival rate of surgical patients is highly variable, and surgery is associated with substantial morbidity and mortality.
Most patients who present with inoperable locally advanced NSCLC receive palliative care. The goals of treatment are to relieve pain and other symptoms and to improve or maintain the quality of life.[9] Until the 1990's radiotherapy alone was the standard treatment for patients with inoperable NSCLC, however the 5-year survival rate was poor (under 10%).[10–12] Over the last two decades, a number of studies have demonstrated that chemotherapy and radiotherapy can prolong survival and are recommended as treatment for locally advanced disease.[5,10,13–15] The combination of radiotherapy plus platinum-based chemotherapy for locally advanced NSCLC shows survival benefit compared with radiation therapy alone, and is considered the current standard of care.[4–8] The median survival time for the combined therapy ranges from 12 to 14 months while radiation alone ranges from 9 to 12 months.[4–8] The combination of radio- and chemotherapy have been recommended by guidelines for treating locally advanced disease.[16] The idea is that chemotherapy will reduce the risk of distant metastasis and radiotherapy will maintain loco-regional control.[17] The chemotherapeutic drug may also increase radio-sensitivity and increase the effectiveness of the radiation treatment.[18]
Combined chemotherapy and radiation therapy can be given concurrently or sequentially. While concurrent therapy is associated with higher toxicity, particularly acute esophageal toxicity,[16,19] several studies and meta-analyses indicate that concurrent therapy is superior to sequential therapy in treating this disease.[15,20,21] The use of chemoradiotherapy requires the total absence of negative clinical prognostic factors, including poor performance status and weight loss.[22]
Although a number of clinical studies have compared chemoradiotherapy with radiation or chemotherapy alone,[13–15] the role of chemoradiation therapy in patients with inoperable or unresectable stage III disease and who have a poor prognosis is not uniformly agreed upon. The purpose of this study is to evaluate whether the combination of radiotherapy and chemotherapy results in longer survival than radiotherapy alone in patients with inoperable or unresectable stage III NSCLC.
2. Methods
2.1. Search strategy
This study was performed in accordance with the PRISMA guidelines. Medline, Cochrane, EMBASE, Google Scholar databases were searched until July 128, 2018 using the following search terms: non-small cell lung cancer, advanced cancer, incurable/inoperable/unresectable, chemotherapy, radiotherapy, chemoradiotherapy/chemoradiation. Ethical approval and informed consent were not necessary as the meta-analyses did not involve human subjects and does not require internal review board review and approval. Randomized controlled trials (RCTs) that compared combined chemoradiotherapy (RT+CT) with radiotherapy (RT) alone in patients with histologically confirmed, locally advanced (stage III) nonresectable NSCLC were include. Cohort study; letters, comments, editorials, case report; proceeding, and personal communications were excluded. Studies that evaluated two types of chemotherapy (e.g., etoposide plus cisplatin or irinotecan plus cisplatin, docetaxel and cisplatin vs MVP, cisplatin/etoposide vs paclitaxel/carboplatin) were excluded. Also excluded were studies designed to compare dose and sequence of RT (e.g., sequential vs concurrent), or did not report quantitatively outcomes of interest. The list of prospective studies was reviewed by 2 independent reviewers, and where there was uncertainty regarding eligibility, a third reviewer was consulted. This study was approved by the Chang Gung Medical Foundation Institutional Review Board (IRB No. 201900509B0).
2.2. Data extraction and quality assessment
The following information/data were extracted from the studies that met the inclusion/exclusion criteria: the name of the first author, year of publication, study design, number of participants in each treatment group, age and gender, cancer type, primary, secondary outcomes, and time of follow-up.
The Cochrane Risk of Bias Tool was used to assess the included studies.[23] The quality assessment was performed by 2 independent reviewers, and a third reviewer was consulted to adjudicate any uncertainties.
2.3. Outcome measures
The primary outcome for this meta-analysis was the hazard ratio (HR) for overall survival (OS). The secondary outcomes were the HR of progression-free survival (PFS) and odds ratio (OR) of objective response rate (ORR). The additional OS rate and PFS rate at 1 and 2 years after treatment were also extracted from the reports.
2.4. Statistical analysis
The study characteristics are summarized according to the number of patients, mean age, % of males, and % of disease stage for patients receiving RT alone or RT+CT treatment. The clinical outcomes, OS rate, PFS rate, and ORR are presented as (%) for the given follow-up time points. Furthermore, the HR with corresponding 95% confidence intervals (95% CI) of RT+CT treatment compared to that of RT alone in OS times and PFS times are also presented for each of the studies.
For the major effect size, the HR of the OS time and PFS time and the HR with 95% CI was calculated for each individual study and for the studies combined. For the secondary effect size odds ratio, the OR with 95% CI was calculated for dichotomous outcomes, ORR, OS rate, and PFS rate for each individual study and for the studies combined. For data reported as Kaplan–Meier curves, we extracted the survival rates at specific times to reconstruct the estimated HR and its variance, under the assumption that the rate of patients censored was constant during the study follow-up.[24] HR < 1 indicates that CT+RT treatment resulted in longer survival than did combination therapy; HR > 1 indicates that RT alone resulted in longer survival than did the other treatments; and HR = 1 indicated similar survival between RT alone and CT+RT combination therapy. For the other effect size, OR < 1 indicates that CT+RT combination therapy resulted in higher OS, PFS or ORR; OR > 1, indicates that radiotherapy alone resulted in higher OS, PFS or ORR; and OR = 1 indicated that the OS rate, PFS rate, or ORR were similar between RT alone and CT+RT combination therapy. A χ2-based test of homogeneity was performed, and the inconsistency index (I2) and Q statistics were determined. Fixed-effects models were used unless I2 was > 50%, in which case a random-effects model was used. The combined effects were calculated, and a 2-sided P value < .05 was considered significance. Sensitivity analysis was prospectively planned for the major outcomes and carried out using the leave-one-out approach to investigate the validity and robustness of the results. This approach involves performing the meta-analysis on subsets of studies in which one study is left out in turn. Publication bias was not assessed because >10 studies are required to detect funnel plot asymmetry.[25] All analyses were performed using Comprehensive Meta-Analysis statistical software, version 2.0 (Biostat, Englewood, NJ).
3. Results
Of the 1029 studies initially identified, 974 were removed because they were irrelevant (Fig. 1 A). Fifty-five studies were fully reviewed, and 42 of these were eliminated because the study design did not meet the inclusion criteria, did not report outcomes of interest, was a one arm study or recruit patients with NSCLC stage I-II, or full text was not available. Thus, the final cohort included 13 studies.
Figure 1.
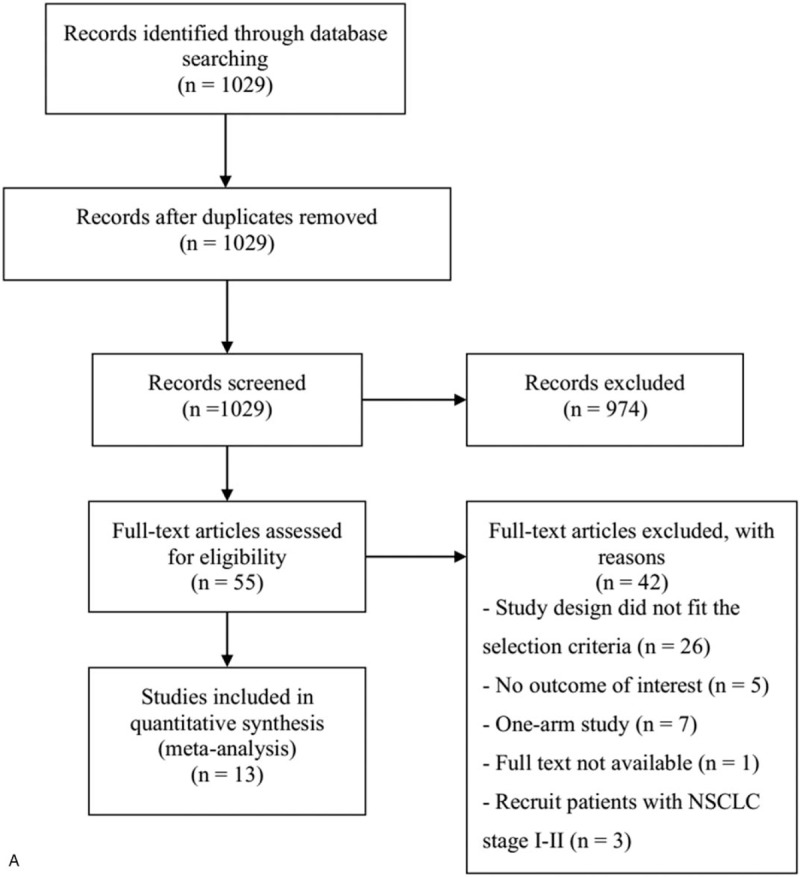
(A) Flow chart of study selection. Results of quality assessment using the Cochrane Risk of Bias Tool for (B) overall and (C) individual included studies.
3.1. Quality assessment
Quality assessment indicated a high risk for performance bias and detection bias and a low risk for selection bias, attrition bias, and reporting bias (Fig. 1 B and C). Less than half of the included studies performed an intent-to-treat analysis. Overall, these findings suggest the data are of medium to good quality.
3.2. Study characteristics
Thirteen RCT studies were included in the systematic review and meta-analysis (Fig. 1 and Table 1).[7,12,17,26–35] The 13 studies included a total of 1936 patients with incurable/inoperable stage III NSCLC, of which 975 received RT alone and 961 received RT+CT combination therapy. The average age ranged from 54 to 77 years. Overall, the patient characteristics were similar between the RT alone and RT+CT groups. For most of the included studies, squamous-cell NSCLC was the most common cancer type. The types of radiotherapy and chemoradiotherapy used differed across the studies and are summarized in Table 2.Clinical outcomes by interventions are summarized in Table S1.
Table 1.
Summary of characteristics of selected studies.
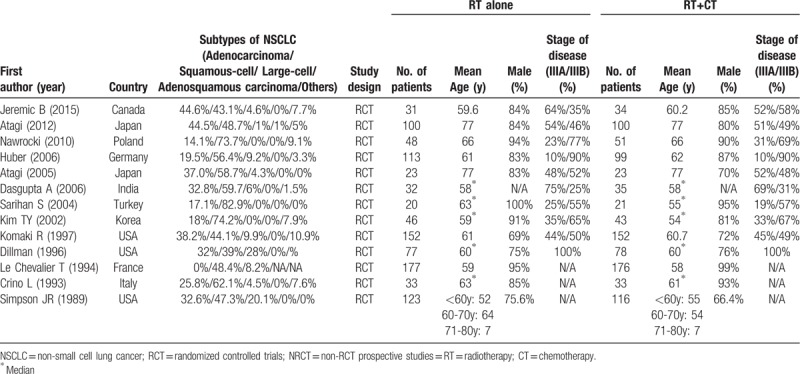
Table 2.
The protocol of radiotherapy and chemoradiotherapy.

3.3. Meta-analysis
3.3.1. Overall survival
All 5 studies were included in the analysis of OS. A fixed-effect model was used, as no heterogeneity was observed among the five studies (Q, 0.917; I2, 0%). The meta-analysis indicated that combined chemoradiotherapy reduced the risk of death in patients with stage III NSCLC compared with radiotherapy alone (pooled HR, 0.72; 95% CI, 0.62–0.84; P < .001) (Fig. 2A). Furthermore, the pooled data revealed that patients receiving CT+RT combination therapy had a higher OS at 1 and 2 years after treatment compared to those treated with RT alone (1-year: OR, 0.67; 95% CI, 0.54–0.84; P < .001; 2-year: OR, 0.57; 95% CI, 0.45–0.73; P < .001) (Supplementary Fig. S1A). With stratification of the pooled data for cohort location (North America, Europe, and East Asia), the difference between CT+RT and RT alone remained significant for all subgroups with respect OS and PFS except the 1- and 2-year OS of cohorts from East Asia (Table S2).
Figure 1 (Continued).
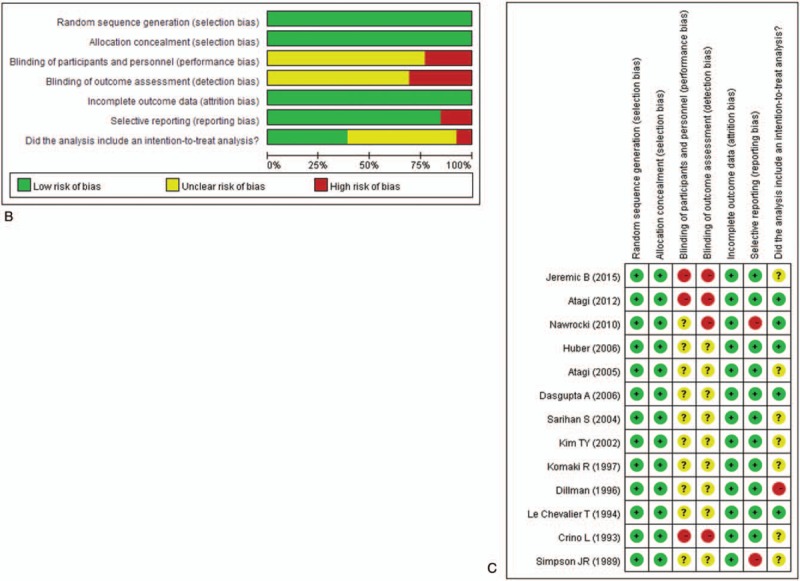
(A) Flow chart of study selection. Results of quality assessment using the Cochrane Risk of Bias Tool for (B) overall and (C) individual included studies.
Figure 2.
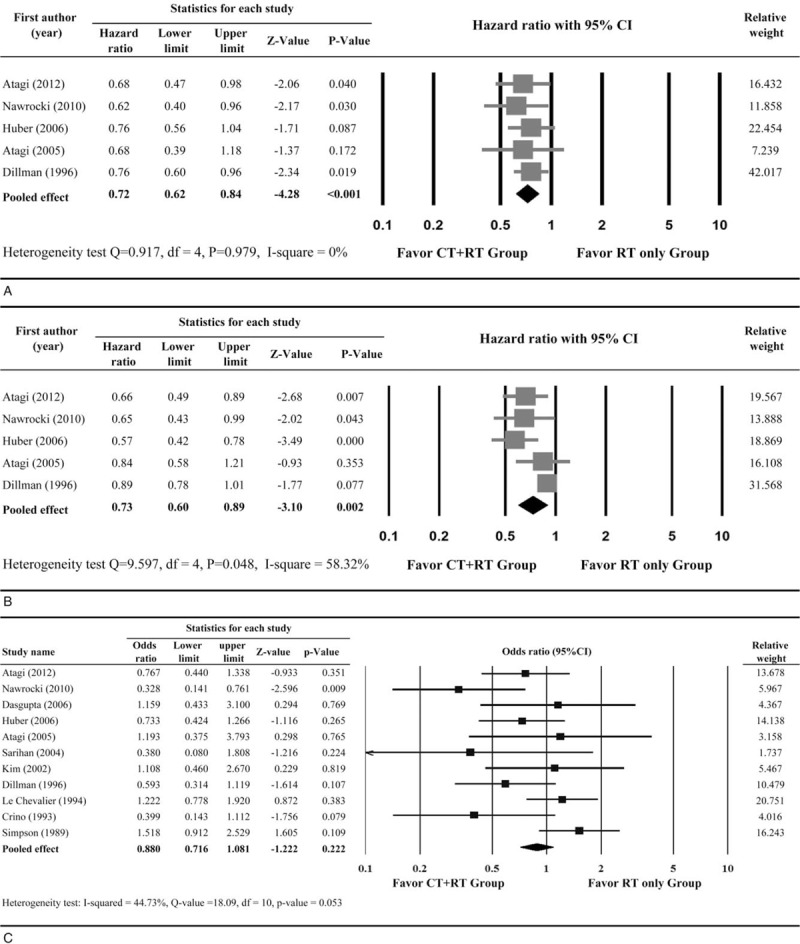
Results of meta-analysis for (A) overall survival; (B) progression-free survival and (C) objective response rate. CT = chemotherapy, RT = radiotherapy, CI = confidence interval, df = degree of freedom.
3.3.2. Progression-free survival
For PFS, 5 studies provided sufficient information for estimating the HRs. A significant heterogeneity was observed among the five studies (Q statistic = 9.597, I2 = 58.32%) (Fig. 2B); therefore, a random-effects model was used. The overall analysis revealed combined chemoradiotherapy provided a significant PFS benefit over radiotherapy alone (pooled HR, 0.73; 95% CI, 0.60–0.89; P = .002) (Fig. 2B). Furthermore, there were 4 studies with 1-year and three studies with 2-year PFS available. The pooled data also revealed that patients treated with CT+RT combination therapy had a higher PFS at 1 and 2 years after treatment compared to those treated with RT alone (1-year: HR, 0.36; 95% CI, 0.24–0.53; P < .001; 2-year: HR, 0.38; 95% CI, 0.23–0.63; P < .001) (Supplementary Fig. S1B).
3.3.3. Objective response rate
Eleven studies provided an ORR (complete response plus partial response) and were included in the meta-analysis. A fixed-effect model was used, as no heterogeneity was observed among the studies (Q statistic, 18.09; I2, 44.73%). The meta-analysis showed that patients treated with combined chemoradiotherapy had a higher ORR than did those treated with radiotherapy alone. However, this difference was not statistically significant (pooled OR, 0.88; 95% CI, 0.72–1.08; P = .222) (Fig. 2C).
3.4. Sensitivity analysis
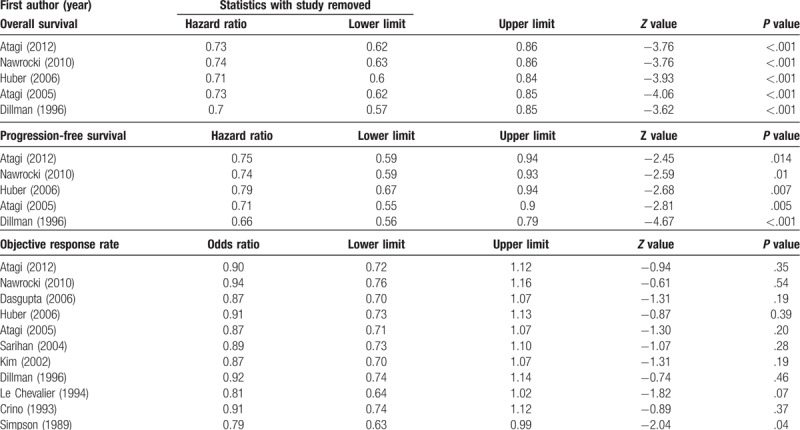
Sensitivity analysis was performed using the leave-one-out approach, in which the analysis was performed repeatedly with each study removed once (Table 3). The direction and magnitude of combined estimates for OS and PFS did not vary markedly with the removal of any one study, indicating that these findings were robust and the data were not overly influenced by any given study. For ORR, removal of Simpson et al[30] resulted in the difference between treatments becoming significant (P, .041); however removal of all other studies resulted in no change in magnitude or direction of the ORR. Overall, the sensitivity analysis indicates that no one study overly influenced the pooled estimates, demonstrating that the findings are robust.
Table 3.
Sensitivity analysis of included studies.
3.5. Overall quality of the meta-analysis findings
According to the GRADE approach, the quality of evidence for this meta-analysis was moderate (GRADE score, 3 points).
4. Discussion
Patients with locally advanced stage III NSCLC tumors are not amenable to surgery, and the best treatment and optimal management of these patients is not clear.[1,2] This meta-analysis compared RT versus concurrent RT+CT in treating patients with stage III NSCLC, and found that RT+CT showed greater benefit for OS, PFS, and ORR (P values ≤ .007). Sensitivity analysis indicated the data were robust for OS and PFS, but the study by Simpson et al[30] may have overly influenced the finding for ORR. Our results support the use of CT+RT compared with radiotherapy alone in treating locally advanced stage III NSCLC.
Two prior meta-analyses evaluated the efficacy of radiotherapy alone or combined with chemotherapy in patients with NSCLC.[14,15] Marino et al[14] included 14 trials comprising 1887 patients that assessed radiotherapy and chemoradiotherapy in patients with unresectable stage IIIa and IIIb NSCLC. In contrast to our study, their study also included trials that evaluated sequential use of radiotherapy and chemotherapy. They evaluated survival at one, two, three, and 5 years.[14] Marino et al found that in patients treated with cisplatin-based therapy plus radiation, the estimated pooled OR of death at 1 and 2 years was 0.76 and 0.70 (ORs of <1 show benefit for the combined therapy compared with radiotherapy alone), respectively, and the reduction in mortality was 24% and 30%. For patients who received non-cisplatin-based chemotherapy plus radiation, the pooled OR at 1 year was 1.05 and at 2 years was 0.82, with a reduction in mortality of 5% and 18%, respectively. Marino et al found no significant difference in survival at three and 5 years after receiving radiotherapy alone and combined radiotherapy with chemotherapy. Similar to our results, the findings of Marino et al favor the use chemotherapy (particularly cisplatin-based chemotherapy) plus radiation. We did not evaluate long-term survival. In addition, due to the small number of studies included in our meta-analysis, we were unable to perform subgroup analysis that evaluated the efficacy of different types of chemotherapy (e.g., carboplatin-based vs paclitaxel).
Another meta-analysis evaluated the effectiveness of concurrent chemoradiotherapy with radiotherapy alone in patients with NSCLC by evaluating OS, tumor control, and treatment-related morbidity.[15] Unlike our study, which focused on patients with unresectable stage III NSCLC, the study of O’Rourke et al[16] included RCTs in patients with stage I-III NSCLC undergoing radical radiotherapy and who were randomized to receive radiotherapy alone or chemoradiotherapy administered either concurrently or sequentially. Their study included 19 studies with 2728 patients. Consistent with our results, O’Rourke et al found chemoradiotherapy significantly reduced the overall risk of death (HR, 0.71; 95% CI, 0.64 to 0.88) and showed benefit for PFS (HR, 0.69; 95% CI, 0.58 to 0.81). O’Rourke et al did not evaluate ORR or long-term survival.
O’Rourke et al also found that the incidence of acute esophagitis, neutropenia, and anemia was greater with concurrent chemoradiotherapy compared with radiotherapy alone. Although we did not evaluate the difference in toxicity between radiation therapy and concurrent chemoradiotherapy, the included studies reported that the combination therapy had consistently a higher incidence of certain toxicities, such as grade 3–4 leukocytopenia, neutropenia, anemia, and esophagitis.[12,26–28] The findings of increased toxicity with concurrent chemoradiotherapy highlight the importance of patient selection when considering treatment choice.
One limitation of this study is that the age range of the participants in the included studies is broad (average age, 54–77 years). Elderly patients have more comorbidities and experience more frequent life-threatening toxicity from chemotherapy that can preclude treatment completion. Studies investigating outcomes of chemoradiotherapy for NSCLC in elderly patients report conflicting results. Elderly patients in one such study appeared to gain a survival advantage from combined RT and chemotherapy compared with RT alone.[36] However, the authors noted that, as is the case with younger patients, this benefit came at the cost of additional toxicity. Similarly, Davidoff et al concluded that survival benefits associated with chemoradiotherapy in clinical trials can extend to the elderly, but that gradual strategies are beneficial to reducing mortality risk.[37] In contrast, patients over 70 years of age were identified as a subgroup with significantly lower median survival times after chemoradiotherapy for NSCLC.[38] To further complicate this debate, data describing outcomes of chemoradiotherapy for NSCLC in the elderly is limited by their under-representation in such studies, likely due to physician biases regarding the tolerability of such treatment in older patients.[39] In light of this uncertainty, the results of our study should be interpreted with caution as they relate to elderly patients.
To our knowledge, only one other meta-analysis, that of Marino et al, which was performed over 10 years ago, has specifically evaluated the benefit of radiotherapy alone compared with chemoradiotherapy in treating patients with unresectable N2 stage III NSCLC. The strength or our study is that it included only RCTs. However, our findings are limited by the small number of studies included. Also, the small sample size and the data reported in the studies precluded us from performing subgroup analyses that evaluated different treatment regimens or histological subtypes of NSCLC.
Our study is also limited by the heterogeneity of treatment regimens used in the studies. However, despite particular heterogeneity in chemotherapy regimens, the heterogeneity of OS, PFS, and ORR was low to moderate. These results suggest that the heterogeneity in chemotherapy regimens did not have a large impact on survival analysis. Further studies are needed to evaluate the effects of different chemotherapy regimens on other outcome measures such as health-related quality of life, an accurate predictor of survival[40] for which data remain scarce for NSCLC.[41]
In summary, the combination of concurrent chemoradiotherapy offers greater benefit with regard to OS, PFS, and ORR than radiation therapy alone in patients with inoperable, locally advanced stage III NSCLC. However, potential toxicities associated with concurrent chemoradiotherapy warrant further investigation and more quality studies.
Acknowledgments
The authors would like to thank to Dr Meng Lee from the Department of Neurology, Chang Gung University College of Medicine, Chang Gung Memorial Hospital, Chiayi branch, Taiwan, for lecturing on the study methods and inspiring the authors to conduct the study.
Author contributions
Conceptualization: Ming-Szu Hung.
Data curation: Yi-Fang Wu.
Formal analysis: Ming-Szu Hung, Yi-Fang Wu, Yi-Chuan Chen.
Methodology: Ming-Szu Hung, Yi-Chuan Chen.
Software: Yi-Chuan Chen.
Supervision: Yi-Chuan Chen.
Validation: Yi-Fang Wu, Yi-Chuan Chen.
Writing – original draft: Ming-Szu Hung, Yi-Fang Wu, Yi-Chuan Chen.
Writing – review & editing: Ming-Szu Hung, Yi-Chuan Chen.
Supplementary Material
Footnotes
Abbreviations: RT+CT = chemoradiotherapy, CT = chemotherapy, CI = confidence interval, HR = hazard ratio, NSCLC = non–small-cell lung cancer, ORR = objective response rate, OR = odds ratio, OS = overall survival, PFS = progression-free survival, RT = radiotherapy, RCTs = randomized controlled trials.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374–403. [DOI] [PubMed] [Google Scholar]
- [2].Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [3].Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bayman N, Blackhall F, McCloskey P, et al. How can we optimise concurrent chemoradiotherapy for inoperable stage III non-small cell lung cancer? Lung Cancer 2014;83:117–25. [DOI] [PubMed] [Google Scholar]
- [5].Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical, trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- [6].Auperin A, Le Pechoux C, Pignon JP, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol 2006;17:473–83. [DOI] [PubMed] [Google Scholar]
- [7].Dillman RO, Herndon J, Seagren SL, et al. Improved survival in stage III non-small-cell lung cancer: seven-year follow-up of cancer and leukemia group B (CALGB) 8433 trial. J Natl Cancer Inst 1996;88:1210–5. [DOI] [PubMed] [Google Scholar]
- [8].Sause W, Kolesar P, Taylor SI, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest 2000;117:358–64. [DOI] [PubMed] [Google Scholar]
- [9].Ma JT, Zheng JH, Han CB, et al. Meta-analysis comparing higher and lower dose radiotherapy for palliation in locally advanced lung cancer. Cancer Sci 2014;105:1015–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Koning CC, Belderbos JS, Uitterhoeve AL. From cisplatin-containing sequential radiochemotherapy towards concurrent treatment for patients with inoperable locoregional non-small cell lung cancer: still unanswered questions. Chemother Res Pract 2010;2010:506047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Perez CA, Stanley K, Grundy G, et al. Impact of irradiation technique and tumor extent in tumor control and survival of patients with unresectable non-oat cell carcinoma of the lung: report by the Radiation Therapy Oncology Group. Cancer 1982;50:1091–9. [DOI] [PubMed] [Google Scholar]
- [12].Atagi S, Kawahara M, Tamura T, et al. Standard thoracic radiotherapy with or without concurrent daily low-dose carboplatin in elderly patients with locally advanced non-small cell lung cancer: a phase III trial of the Japan Clinical Oncology Group (JCOG9812). Jpn J Clin Oncol 2005;35:195–201. [DOI] [PubMed] [Google Scholar]
- [13].Strom HH, Bremnes RM, Sundstrom SH, et al. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer 2013;109:1467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Marino P, Preatoni A, Cantoni A. Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIb nonsmall cell lung cancer. A meta-analysis. Cancer 1995;76:593–601. [DOI] [PubMed] [Google Scholar]
- [15].O’Rourke N, Roque IFM, Farre Bernado N, et al. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev. 2010 (6):CD002140. [DOI] [PubMed] [Google Scholar]
- [16].O’Rourke N, Macbeth F. Is concurrent chemoradiation the standard of care for locally advanced non-small cell lung cancer? A review of guidelines and evidence. Clin Oncol (R Coll Radiol) 2010;22:347–55. [DOI] [PubMed] [Google Scholar]
- [17].Le Chevalier T, Arriagada R, Quoix E, et al. Radiotherapy alone versus combined chemotherapy and radiotherapy in unresectable non-small cell lung carcinoma. Lung Cancer 1994;10Suppl 1:S239–244. [DOI] [PubMed] [Google Scholar]
- [18].Blackstock AW, Govindan R. Definitive chemoradiation for the treatment of locally advanced non small-cell lung cancer. J Clin Oncol 2007;25:4146–52. [DOI] [PubMed] [Google Scholar]
- [19].Curran WJ, Jr, Paulus R, Langer CJ, et al. Sequential vs concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181–90. [DOI] [PubMed] [Google Scholar]
- [21].Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol 2005;23:5910–7. [DOI] [PubMed] [Google Scholar]
- [22].Crino L, Weder W, van Meerbeeck J, et al. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21Suppl 5:v:103–15. [DOI] [PubMed] [Google Scholar]
- [23].Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. In: Higgens JPT, Green LA (eds): The Cochrane Collaboration; 2011. Available at www.cochrane-handbook.org Accessed September 6, 2018. [Google Scholar]
- [24].Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- [25].Sutton AJ, Duval SJ, Tweedie RL, et al. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000;320:1574–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Atagi S, Kawahara M, Yokoyama A, et al. Thoracic radiotherapy with or without daily low-dose carboplatin in elderly patients with non-small-cell lung cancer: a randomised, controlled, phase 3 trial by the Japan Clinical Oncology Group (JCOG0301). Lancet Oncol 2012;13:671–8. [DOI] [PubMed] [Google Scholar]
- [27].Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized phase II study. J Thorac Oncol 2010;5:1255–62. [DOI] [PubMed] [Google Scholar]
- [28].Huber RM, Flentje M, Schmidt M, et al. Simultaneous chemoradiotherapy compared with radiotherapy alone after induction chemotherapy in inoperable stage IIIA or IIIB non-small-cell lung cancer: study CTRT99/97 by the Bronchial Carcinoma Therapy Group. J Clin Oncol 2006;24:4397–404. [DOI] [PubMed] [Google Scholar]
- [29].Crino L, Latini P, Meacci M, et al. Induction chemotherapy plus high-dose radiotherapy versus radiotherapy alone in locally advanced unresectable non-small-cell lung cancer. Ann Oncol 1993;4:847–51. [DOI] [PubMed] [Google Scholar]
- [30].Simpson JR, Bauer M, Perez CA, et al. Radiation therapy alone or combined with misonidazole in the treatment of locally advanced non-oat cell lung cancer: report of an RTOG prospective randomized trial. Int J Radiat Oncol Biol Phys 1989;16:1483–91. [DOI] [PubMed] [Google Scholar]
- [31].Komaki R, Scott CB, Sause WT, et al. Induction cisplatin/vinblastine and irradiation vs irradiation in unresectable squamous cell lung cancer: failure patterns by cell type in RTOG 88-08/ECOG 4588. Radiation Therapy Oncology Group. Eastern Cooperative Oncology Group. Int J Radiat Oncol Biol Phys 1997;39:537–44. [DOI] [PubMed] [Google Scholar]
- [32].Jeremic B, Fidarova E, Sharma V, et al. The International Atomic Energy Agency (IAEA) randomized trial of palliative treatment of incurable locally advanced non small cell lung cancer (NSCLC) using radiotherapy (RT) and chemotherapy (CHT) in limited resource setting. Radiother Oncol 2015;116:21–6. [DOI] [PubMed] [Google Scholar]
- [33].Dasgupta A, Dasgupta C, Basu S, et al. A prospective and randomized study of radiotherapy, sequential chemotherapy radiotherapy and concomitant chemotherapy-radiotherapy in unresectable non small cell carcinoma of the lung. J Cancer Res Ther 2006;2:47–51. [DOI] [PubMed] [Google Scholar]
- [34].Sarihan S, Kayisogullari U, Ercan I, et al. Randomized phase 2 study of radiotherapy alone versus radiotherapy with paclitaxel in non-small cell lung cancer. J Int Med Res 2004;32:375–83. [DOI] [PubMed] [Google Scholar]
- [35].Kim TY, Yang SH, Lee SH, et al. A phase III randomized trial of combined chemoradiotherapy versus radiotherapy alone in locally advanced non-small-cell lung cancer. Am J Clin Oncol 2002;25:238–43. [DOI] [PubMed] [Google Scholar]
- [36].Schild SE, Mandrekar SJ, Jatoi A, et al. The value of combined-modality therapy in elderly patients with stage III nonsmall cell lung cancer. Cancer 2007;110:363–8. [DOI] [PubMed] [Google Scholar]
- [37].Davidoff AJ, Gardner JF, Seal B, et al. Population-based estimates of survival benefit associated with combined modality therapy in elderly patients with locally advanced non-small cell lung cancer. J Thorac Oncol 2011;6:934–41. [DOI] [PubMed] [Google Scholar]
- [38].Werner-Wasik M, Scott C, Cox JD, et al. Recursive partitioning analysis of 1999 radiation therapy oncology group (RTOG) patients with locally-advanced non-small-cell lung cancer (LA-NSCLC): Identification of five groups with different survival. Int J Radiat Oncol Biol Phys 2000;48:1475–82. [DOI] [PubMed] [Google Scholar]
- [39].Firat S, Pleister A, Byhardt RW, et al. Age is independent of comorbidity influencing patient selection for combined modality therapy for treatment of stage III nonsmall cell lung cancer (NSCLC). Am J Clin Oncol 2006;29:252–7. [DOI] [PubMed] [Google Scholar]
- [40].Tanvetyanon T, Soares HP, Djulbegovic B, et al. A systematic review of quality of life associated with standard chemotherapy regimens for advanced non-small cell lung cancer. J Thorac Oncol 2007;2:1091–7. [DOI] [PubMed] [Google Scholar]
- [41].Fernández-López C, Expósito-Hernández J, Arrebola-Moreno JP, et al. Trends in phase III randomized controlled clinical trials on the treatment of advanced non-small-cell lung cancer. Cancer Med 2016;5:2190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


