Abstract
Aberrant expression of SRY-box 8 (SOX8) is closely correlated with the development and progression of many types of cancers in human. Limited studies report the relationship between SOX8 expression and overall survival in colorectal cancer (CRC). This study aimed to collect the pathological tissues and clinical data in order to analyze the relationship between SOX8 expression and clinicopathological parameters and prognosis of CRC patients. Tissue microarrays were constructed from 424 primary CRC patients with clinicopathological information and follow-up data. Immunohistochemistry (IHC) was performed on tissue microarrays to explore the relationship between SOX8 expression and clinicopathological information and patient's prognosis. The expression of SOX8 was higher in CRC tissues than that in non-tumor adjacent tissues (NATs, P <.001). High expression of SOX8 was associated with tumor stage (P = .04) and shorter overall survival (OS) after operation of patients (P = .004). Subsequently, univariate COX analysis identified that high expression of SOX8 (P = .004), differentiation (P = .006), distant metastasis (P <.001), tumor stage (P = .003), and higher rate of lymph node metastasis (P <.001), all significantly predicted decrease in OS. Multivariate analysis demonstrated that distant metastasis (P <.001), high SOX8 expression, (P = .013) and lymph node metastasis (P <.001) were independent poor prognostic factors in CRC patients. This study showed that SOX8 is over-expressed in patients with high T stage, which affects the outcome of prognosis in CRC patients. High expression of SOX8 usually has a poor independent prognostic factor for CRC.
Keywords: clinicopathologic, colorectal cancer, COX analysis, prognostic, SOX8
1. Introduction
Colorectal cancer (CRC) is the third most common malignant cancer.[1] Although improvements in early diagnosis and treatment have decreased the incidence and death rates, CRC remains a great threat to the human health.[2]
In recent years, studies showed that the expression of SRY-box (SOX) gene is closely related to the prognosis of patients with various tumors such as cerebellar cancer, non-small cell lung cancer, and hepatocellular cancer.[3–6] The SOX containing gene family is a part of the high mobility group (HMG) super-family, which is known to generate transcription factors.[7–9] Based on HMG sequence similarities, the SOX family can be divided into A-H groups.[10–13]SOX genes can regulate many developmental processes in the human body, including the lens, hair follicles, intestine, B cells, muscles, and blood vessels.[14–16] The SOX genes have also been found to play crucial roles as promoters in certain types and grades of tumors.[17,18]SRY-box 8 (SOX8) was a gene within group E, which facilitates chromatin loosening to promote the transcription of specific genes.[19,20] Increasing reports suggest that SOX8 can regulate cell proliferation via the WNT/Wingless signaling pathway, which regulates the transcriptional expression of β-catenin. SOX8 factors are found in all metazoans and regulate many processes similar to canonical WNT signaling, such as tissue specification, organ development, stem cell homeostasis, and the occurrences of cancer. Deregulation of SOX8 factors has been implicated in multiple diseases and cancers. However, the expression of SOX8 in CRC has not been studied; therefore, its role in the development of CRC remains unclear. In this study, we explore the expression of SOX8 in CRC patients and examine its relationship with clinicopathological features and prognosis.
2. Materials and methods
2.1. Patients
Total 424 primary CRC patients who had undergone surgical resection at The First Affiliated Hospital of Jinzhou Medical University between June 2008 and November 2011 were included in this study. All the patients were established in accordance with the American Joint Committee on Cancer (AJCC) staging system for tumor staging (TNM stage). There were 91 left colon cancer patients, 110 right colon cancer patients, and 223 rectal cancer patients. This research has been approved by the ethics of The First Affiliated Hospital of Jinzhou Medical University. In this study, informed consent was obtained from all the patients. These patients had detailed clinicopathological information and follow-up data. The follow-up information was provided to the patients by telephone, personal appointments, emails, and letters. The clinicopathological data included age, sex, tumor size, tumor location, differentiation, and tumor stage. The median age at the time of surgery was 62.5 years (range: 26.0–91.0 years) and the median tumor size was 4.5 cm (range: 1.0–15.0 cm).
2.2. Tissue microarrays
Tissue microarrays consisting of 424 primary CRC tissues and 196 matched non-tumor adjacent tissues (NATs) were assembled using a specialized manual arraying instrument. The optimal CRC tissue and NAT were obtained by screening the slides stained with hematoxylin and eosin. Instrument with 2.0 mm diameter punch was used for 3 cores, which collected from each formalin-fixed, paraffin-embedded tissue sample.
2.3. Immunohistochemistry
Streptomycin affinity was used as the method of immunostaining. The primary rabbit anti-SOX8 antibodies were purchased from Sigma, Saint Louis, USA and were diluted to 1:1000. The secondary antibody and the streptavidin-biotin conjugated with horseradish peroxidase were purchased from Zhongshanjinqiao Biological Technology (Beijing, China). Endogenous peroxidases were blocked using 3% diluted hydrogen peroxide. Antigen retrieval was done by autoclaving, and slices were then incubated overnight at 4°C with specific primary antibodies in a high humidity cabinet. Then, the slices were incubated with diaminobenzidine (Zhongshanjinqiao, Beijing, China) and counterstained with hematoxylin.
2.4. Scoring of Immunohistochemistry
Two pathologists, who were not given any clinical information, independently scored SOX8 staining intensity, and a third clinical pathologist evaluated the tissues if the scores were not consistent. Specific staining in the nucleus of the cancer cells was considered as antigen expression in these cells. The staining intensity score was 0, 1, 2, or 3 (negative, weak, medium, or strong). According to the percentage of positively stained cells, staining scores were 0, 1, 2, 3, or 4 (<5%, 6%–25%, 26%–50%, 51%–75%, and 76%–100%). The final staining scores were calculated by multiplying the staining intensity with the degree of staining. SOX8 staining was considered low or high using a cutoff value of 5 based on the analysis from the receiver operating characteristic (ROC) curve. A final score greater than 5 was defined as high expression of SOX8.
2.5. Statistical methods
All the statistical analyses of data were performed using GraphPad 7.0 (GraphPad Software) software. ROC curves were used to estimate the diagnostic accuracy of the cancer markers. The difference of SOX8 expression between CRC tissues and NATs were analyzed by Student t test. Bivariate analyses were assessed using the chi-square test. Kaplan–Meier method was used to calculate the survival rates, whereas the difference of survival curves was examined by the log-rank test. Survival analyses were performed to calculate hazard ratios (HRs) and associated 95% confidence intervals (CIs) by using Cox Regression model. To determine the independence of our classifier from clinicopathological variables in predicting an individual's risk of survival, we analyzed the validation set using univariate analysis and multivariate analysis in a Cox proportional-hazards model for prognostic predictors, and P <.05 (2-sided) was considered as statistically significant.
3. Results
3.1. The expression of SOX8 in CRC
SOX8 was stained in the nucleus of CRC and NATs cells (Fig. 1). The rate of positive expression in CRC tissues was 82.5%, which is higher than that in NATs (20.9%). Therefore, indicating that the expression of SOX8 was significantly higher in CRC tissues than that in NATs (P <.001, Table 1, Fig. 2).
Figure 1.
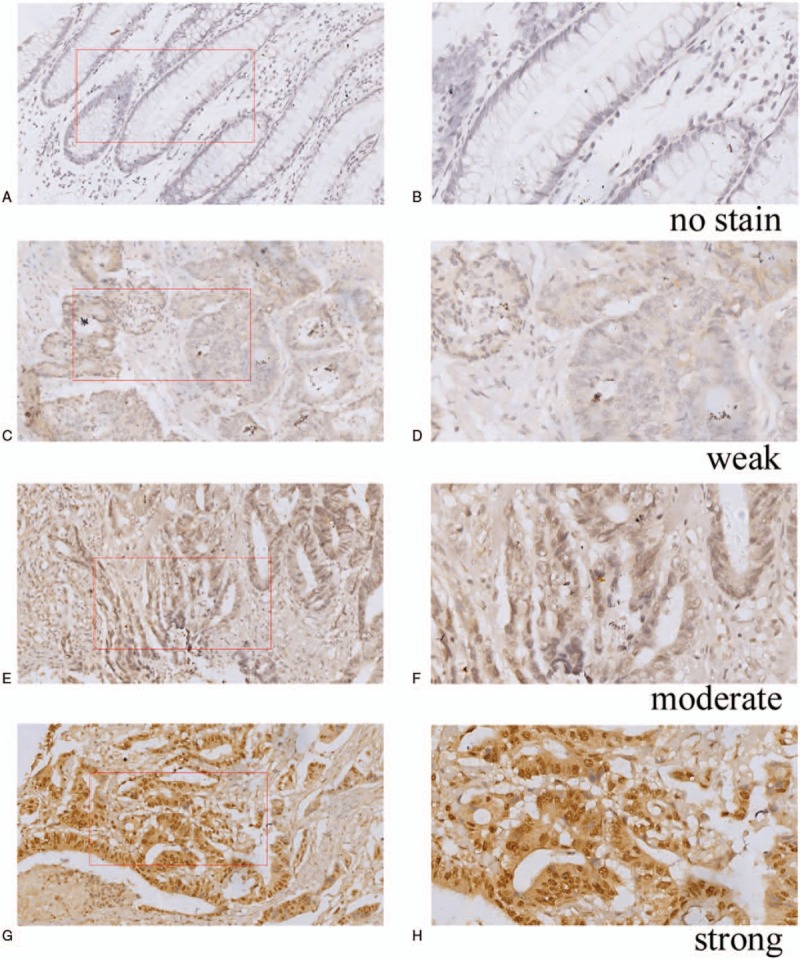
Immunohistochemical expression of SOX8 in CRC tissues and NATs. A Negative SOX8 expression is seen in NATs magnification (200×). B Negative SOX8 expression is seen in NATs magnification (400×). C Weak SOX8 expression is seen in CRC tissues magnification (200×). D Weak SOX8 expression is seen in CRC tissues magnification (400×). E Moderate SOX8 expression is seen in CRC tissues magnification (200×). F Moderate SOX8 expression is seen in CRC tissues magnification (400×). G Strong expression of SOX8 in CRC tissues magnification (200×). H Strong expression of SOX8 in CRC tissues magnification (400×).
Table 1.
The expression of SOX8 between in CRC tissues and NATs.

Figure 2.
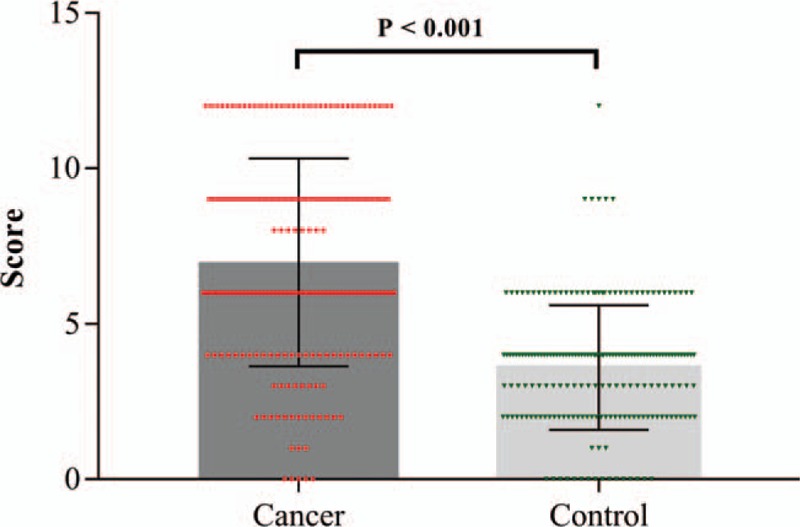
The scatter plot with bar show the scores of SOX8 expression in NATs and CRC.
3.2. The relationship between SOX8 expression and clinicopathological characteristics
The clinicopathological data of CRC patients are presented in Table 2. The results showed that high expression of SOX8 was significantly associated with tumor stage (P = .04). The mean staining score of SOX8 between the T3 and the T4 stage was significantly higher than that between the T1 and T2 stage (P <.05). There was no significant difference between SOX8 expression in 2 tissue groups based on tumor location, gender, age, differentiation, tumor size, lymph node metastasis, distant organ metastasis, and TNM stage (all P values >.05, Fig. 3).
Table 2.
The correlation of clinic-pathologic variables of CRC with SOX8.
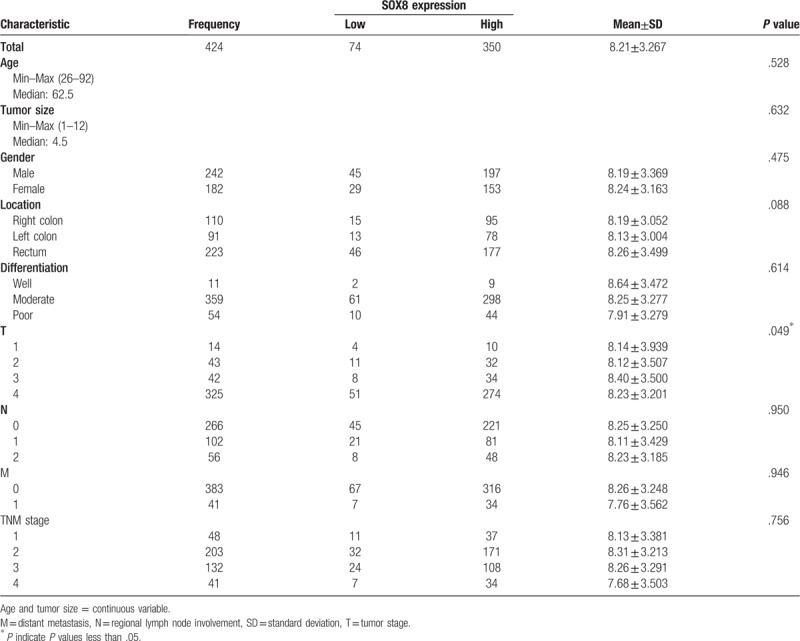
Figure 3.
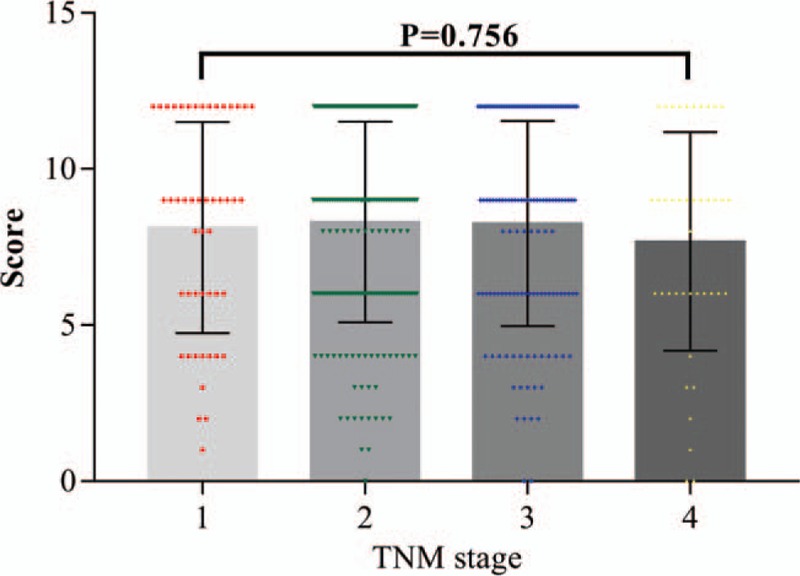
The scatter plot with bar show the scores of SOX8 expression in different TNM stages.
3.3. The relationship between SOX8 expression and prognosis of CRC patients
Results from Kaplan–Meier curve showed that patients with high SOX8 expression present poor OS following operation (P = .004, Fig. 4). We further conducted TNM stratification, and the results of subgroup analysis showed that the high expression of SOX8 was significantly correlated with poor OS between TNM I stage (P = .046) and TNM II stage (P = .028), while there was no statistical significance in case of TNM III stage (P = .152) and TNM IV stage (P = .454) (Fig. 5).
Figure 4.
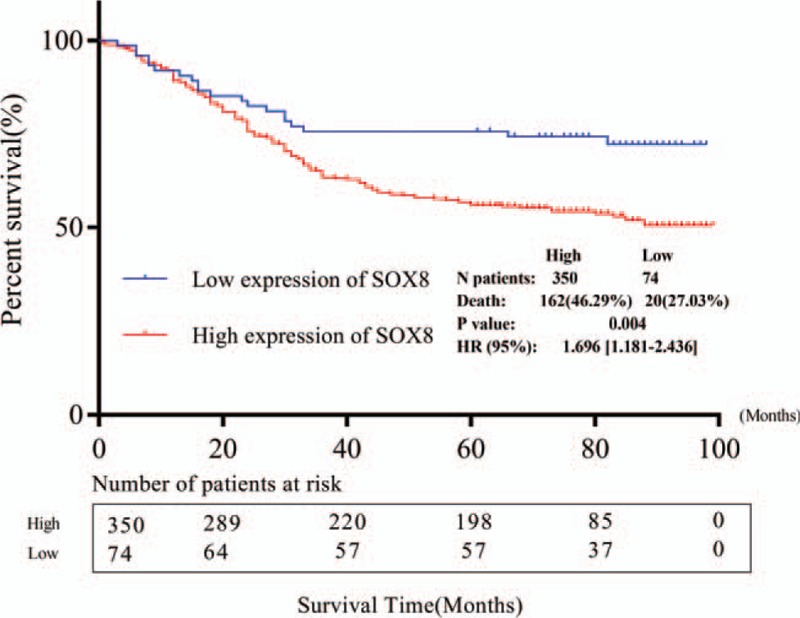
Kaplan–Meier curve for the correlation between the expression of SOX8 and overall survival after operation.
Figure 5.
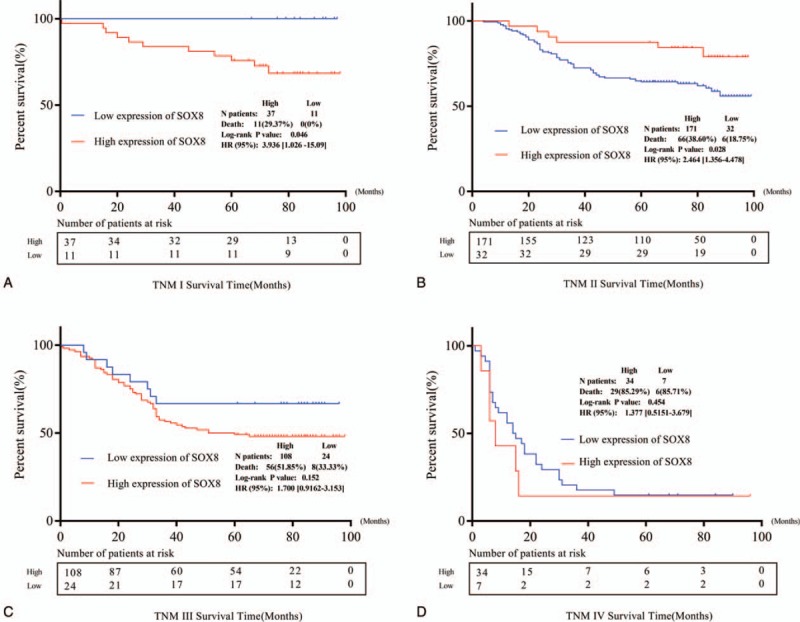
Kaplan–Meier curves of overall survival stratified according to the status of SOX8 expression. A and B Kaplan–Meier survival curves of overall survival among patients with SOX8 expression compared with patients TNM I stage and TNM II stage. C and D Kaplan–Meier survival curves of overall survival among patients with SOX8 expression compared with patients TNM III stage and TNM IV stage..
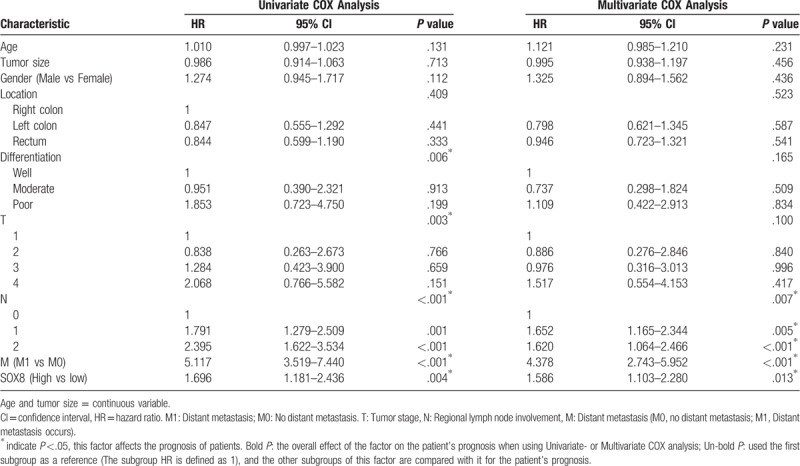
Subsequently, univariate analysis identified that the high expression of SOX8 (P = .004), poor differentiation (P = .006), distant metastasis (P <.001), tumor stage (P = .003), and high rates of lymph node metastasis (P <.001) presents poor OS (Table 3). Multivariate analysis demonstrated that distant metastasis (P <.001), high SOX8 expression (P = .013), and lymph node metastasis (P <.001) were independent poor prognostic factors in CRC patients.
Table 3.
Univariate and multivariate analysis of prognostic factors.
4. Discussion
At present, there are many studies on the expression of SOX8 in various cancers, which reveals its high expression in glial and medulloblastoma cells in cerebellar cancer, thereby providing strong evidence for its activity in cancer tissues.[6] Schlierf et al reported that high expression of the SOX8 transcript as well as high protein level in oligodendroglial tumors correlated with the loss of heterozygosity of 1p and 19q, suggesting that SOX8 expression might be useful in predicting therapeutic response and prognosis in patients with oligodendroglial neoplasm.[21] Zhang et al suggested that SOX8 was highly expressed in human hepatocellular cancer and colon cancer, which promoted cellular proliferation and enhanced tumor growth through WNT/β-catenin target gene expression.[5]
However, there was no study verifying the correlation between SOX8 expression and OS in CRC patients. Our results confirmed that SOX8 was higher in CRC tumor tissue than in NATs, which was consistent with the study results of hepatocellular carcinoma and non-small cell lung cancer (P <.05).[5] Our research indicates that high expression of SOX8 was significantly associated with malignancy TNM classification, indicating that SOX8 may be involved in CRC invasion. SOX8 regulates cell proliferation and invasion in CRC cells and hepatoma cancer cells through the activation of WNT/β-catenin signaling,[5] and up-regulation of β-catenin can promote invasion and migration of colon cancer cells in vitro.[22] Accordingly, we propose that SOX8 may affect T stage though WNT/β-catenin signaling pathway. However, functional experiments are needed to verify this mechanism.
Tumor invasion is a first and critical step in tumor metastasis, with increased invasive potential correlating with poor outcomes in patients with a variety of cancers.[23] In this study, we divided the TNM stage into different subgroups and performed a Kaplan–Meier stratified analysis, which showed that high expression of SOX8 was significantly correlated with poor OS in early TNM stage (Fig. 3). The stratified analysis indicates that SOX8 expression could be predicting the prognosis of early CRC patients. Therefore, we anticipate that SOX8 could be a tumorigenic factor in human malignant tumors. Our studies showed that SOX8 was associated with T stage and affects the prognosis of CRC patients. In the treatment of cancer, we expect that SOX8 could be a tumor molecular target, which could help in the clinical diagnosis and treatment for CRC patients.
In conclusion, high SOX8 expression was strongly associated with CRC invasion. The expression of SOX8 may be used to preoperatively predict prognosis of CRC patients.
Author contributions
Conceptualization: Yang Wang, Wei Wang.
Data curation: Yang Wang, Wangshuo Yang, Tianyi Liu.
Formal analysis: Wangshuo Yang, Mingxing Liu.
Investigation: Wangshuo Yang, Tianyi Liu, Mingxing Liu, Wei Wang.
Methodology: Tianyi Liu.
Resources: Guang Bai.
Software: Wangshuo Yang, Guang Bai.
Writing – original draft: Yang Wang.
Writing – review & editing: Wei Wang.
Footnotes
Abbreviations: CRC = colorectal cancer, CI = confidence interval, HR = hazard ratio, HMG = high mobility group, NATs = non-tumor adjacent tissues, SOX = SRY-box , SOX8 = SRY-box 8.
YW and WY contributed equally to the work.
This work was supported by the Natural Science Foundation of Liaoning Province, China (fund number: 20170540365).
All authors have contributed to read and approved the final manuscript for submission.
And the authors declare no conflict of interest.
References
- [1].Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. [DOI] [PubMed] [Google Scholar]
- [2].Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212–36. [DOI] [PubMed] [Google Scholar]
- [3].Schepers GE, Bullejos M, Hosking BM, et al. Cloning and characterisation of the Sry-related transcription factor gene Sox8. Nucleic Acids Res 2000;28:1473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xie C, Han Y, Liu Y, et al. miRNA-124 down-regulates SOX8 expression and suppresses cell proliferation in non-small cell lung cancer. Int J Clin Exp Pathol 2014;7:6534–42. [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang S, Zhu C, Zhu L, et al. Oncogenicity of the transcription factor SOX8 in hepatocellular carcinoma. Med Oncol 2014;31:918. [DOI] [PubMed] [Google Scholar]
- [6].Cheng YC, Lee CJ, Badge RM, et al. Sox8 gene expression identifies immature glial cells in developing cerebellum and cerebellar tumours. Brain Res Mol Brain Res 2001;92:193–200. [DOI] [PubMed] [Google Scholar]
- [7].Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol Cell Biol 1999;19:5237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res 1999;27:1409–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bianchi ME, Beltrame M. Upwardly mobile proteins. Workshop: the role of HMG proteins in chromatin structure, gene expression and neoplasia. EMBO Rep 2000;1:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Aksoy I, Jauch R, Eras V, et al. Sox transcription factors require selective interactions with Oct4 and specific transactivation functions to mediate reprogramming. Stem Cells 2013;31:2632–46. [DOI] [PubMed] [Google Scholar]
- [11].Hu S, Wu Z, Yan Y, et al. Sox31 is involved in central nervous system anteroposterior regionalization through regulating the organizer activity in zebrafish. Acta Biochim Biophys Sin (Shanghai) 2011;43:387–99. [DOI] [PubMed] [Google Scholar]
- [12].Jiang T, Hou CC, She ZY, et al. The SOX gene family: function and regulation in testis determination and male fertility maintenance. Mol Biol Rep 2013;40:2187–94. [DOI] [PubMed] [Google Scholar]
- [13].Goding CR. Melanocyte development and malignant melanoma. Forum (Genova) 2000;10:176–87. [PubMed] [Google Scholar]
- [14].Juarez M, Reyes M, Coleman T, et al. Characterization of the trunk neural crest in the bamboo shark, Chiloscyllium punctatum. J Comp Neurol 2013;521:3303–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lakiza O, Miller S, Bunce A, et al. SoxE gene duplication and development of the lamprey branchial skeleton: insights into development and evolution of the neural crest. Dev Biol 2011;359:149–61. [DOI] [PubMed] [Google Scholar]
- [16].Lavery R, Chassot AA, Pauper E, et al. Testicular differentiation occurs in absence of R-spondin1 and Sox9 in mouse sex reversals. PLoS Genet 2012;8:e1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schepers G, Wilson M, Wilhelm D, et al. SOX8 is expressed during testis differentiation in mice and synergizes with SF1 to activate the Amh promoter in vitro. J Biol Chem 2003;278:28101–8. [DOI] [PubMed] [Google Scholar]
- [18].Liu Q, Lu H, Zhang L, et al. Homologues of sox8 and sox10 in the orange-spotted grouper Epinephelus coioides: sequences, expression patterns, and their effects on cyp19a1a promoter activities in vitro. Comp Biochem Physiol B Biochem Mol Biol 2012;163:86–95. [DOI] [PubMed] [Google Scholar]
- [19].Barrionuevo F, Georg I, Scherthan H, et al. Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev Biol 2009;327:301–12. [DOI] [PubMed] [Google Scholar]
- [20].Postnikov YV, Bustin M. Functional interplay between histone H1 and HMG proteins in chromatin. Biochim Biophys Acta 2016;1859:462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schlierf B, Friedrich RP, Roerig P, et al. Expression of SoxE and SoxD genes in human gliomas. Neuropathol Appl Neurobiol 2007;33:621–30. [DOI] [PubMed] [Google Scholar]
- [22].Han J, Gao B, Jin X, et al. Small interfering RNA-mediated downregulation of beta-catenin inhibits invasion and migration of colon cancer cells in vitro. Med Sci Monit 2012;18:BR273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Curr Opin Cell Biol 2012;24:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


