Abstract
Background:
Biofilm can impair wound healing by maintaining an elevated, but ineffective, inflammatory state. This article describes interim results from the prospective RESPOND postmarketing registry evaluating the use of a native type 1, porcine collagen matrix with the embedded antimicrobial polyhexamethylene biguanide (PCMP) in the management of chronic wounds.
Methods:
Adults ≥18 years of age with ≥1 appropriate wound were eligible for inclusion. Data that were final on January 26, 2018 were included in this analysis. At week 0, wounds were cleaned, debrided, and prepared as necessary and PCMP was applied, with a dressing to fix it in place. Patients received standard wound care plus PCMP weekly, up to 24 weeks, at the investigator’s discretion. At each visit, wounds were assessed for area and quality of granulation tissue.
Results:
Most common wound types (N = 63) were venous ulcers (28.6%), trauma and lacerations (22.2%), postsurgical open wounds (15.9%), pressure injuries (12.7%), and diabetic ulcers (9.5%). Median baseline wound area was 6.5 cm2; mean wound duration at baseline was 4 months. Of the 63 wounds, 43 (68.3%) achieved complete wound closure, 41 of 43 (95.3%) closed after PCMP treatment, and 2 of 43 (4.7%) after bridging to other modalities and surgical closure. Twelve out of 63 wounds were bridged to other modalities after PCMP treatment. Mean time to closure for PCMP wounds was 5.0 weeks.
Conclusions:
PCMP appears to be a useful adjunct for treating various wound types. PCMP use should be considered when managing chronic or acute wounds.
INTRODUCTION
All wounds progress through a series of predictable stages before achieving complete wound closure.1 However, some wounds stall in the healing process and become chronic.2 The etiology of chronic nonhealing wounds is complex, but many occur as a result of increased bacterial bioburden and the formation of biofilm.3,4 Biofilm represents a stage of the normal growth cycle of bacteria, during which they are in a protected state.
A recent meta-analysis calculated the prevalence of biofilms in chronic wounds at 78.2%, and in some studies, the rates are even higher.5–9 Elevated bioburden and biofilm can also be found in acute wounds, such as burns and skin tears with tissue loss, with biofilm present in approximately 6% of acute wounds.8,10,11
Biofilm can impair wound healing by producing an elevated, but ineffective, inflammatory state.12 The formation of a biofilm results in an increase in proteolytic enzymes that manipulate the host response by inducing proinflammatory cytokines and proteases.13 This creates a cascade of changes that induce chronic inflammation and turn acute wounds into chronic wounds. Because of the extracellular polymeric substance secreted by the bacterial species in a biofilm, neutrophils and macrophages are unable to penetrate the biofilm and are rendered ineffective.
Debridement is an essential step in disrupting biofilm, but it is not sufficient by itself to reduce and maintain lower bacterial counts in wounds.14–16 A reduction in bacterial counts for up to 48 hours after debridement has been noted.17 After debridement, the use of topical antimicrobials is a treatment strategy that can be effective in reducing and maintaining lower bacterial counts and preventing biofilm reformation.18,19
Polyhexamethylene biguanide (PHMB) is a cationic topical antimicrobial that strongly binds to bacterial cell walls and membranes, disrupting the transport, biosynthesis, and catabolic functions of the bacteria.20,21 PHMB possesses broad antimicrobial activity against gram-positive and gram-negative bacteria, and plaque- and biofilm-forming bacteria.21 Furthermore, PHMB binds to biofilm matrix components, and the local PHMB concentration increases during application to cause an increasingly toxic environment for the bacteria.22 Multidrug efflux pumps or acquired resistance to an antimicrobial compound developed by the bacteria do not appear to affect PHMB efficacy.20,21 PHMB shows good biocompatibility and little to no toxicity or systemic uptake when applied on intact skin or wounds.21,23 PHMB also shows activity against a variety of other microorganisms including yeast and fungi.24,25 PHMB has been effective in wound care of leg ulcers and prevention of central-line–associated bloodstream infections.26,27
Collagen dressings are frequently used in wound care, owing to their properties that encourage cellular migration and new tissue formation, and to their ease of handling and conformability.28,29 Matrices made of native collagen can be beneficial in promoting wound healing.28 The native collagen matrix possesses many properties that make it attractive for wound management. The network provides a scaffold for constructive tissue remodeling, allowing fibroblasts and keratinocytes to attach, proliferate, and migrate.30,31 Native collagen matrices possess strength and flexibility, as well as growth factors that are released during matrix degradation.31,32 Another role that collagen matrices play is to act as a substrate to decrease the high levels of elastase and matrix metalloproteinases that are often present in chronic wounds.29,33
A recent development in wound healing is a skin substitute construct of a native type 1, porcine-derived collagen matrix with a 0.1% PHMB coating (porcine collagen matrix with PHMB, further referenced as PCMP). PCMP is a US Food and Drug Administration Class II medical device, 510(k)-cleared #K051647, and intended for the management of wounds. PCMP is supplied dry in sheet form and packaged as sterile, sealed single patches.34
PCMP is a native extracellular matrix (ECM) made from small intestine submucosa (SIS) that retains the native structure of collagen, with the addition of PHMB. SIS grafts provide structure for vascular and cellular ingrowth. Grafts with intact collagen structure are more resistant to enzymatic degradation and obtain better cell adhesion compared with those made from denatured collagen. The addition of PHMB to the SIS graft (or native ECM) provides all of the above attributes and antimicrobial action against a broad spectrum of bacteria.
The RESPOND Registry (Real-World Effectiveness Study of PuraPly AM on Wounds) is a postmarketing, open-label, prospective, observational multicenter study (ClinicalTrials.gov Registration #NCT03286452; registered September 18, 2017) examining the use of PCMP in treating wounds in real-world clinical settings. The aim of this study is to determine the impact of PCMP on wound environment and management of chronic wounds (eg, increase in healthy granulation tissue, decrease in bioburden, and decrease in amount of exudate) for up to 24 weeks.
This article reports an interim analysis of 63 patients in the RESPOND registry treated with PCMP (PuraPly Antimicrobial, Organogenesis Inc., Canton, Mass.) who had completed their participation and whose data were final as of January 26, 2018. Over 300 patients are enrolled in the registry to date.
METHODS
The registry protocol was reviewed and approved by Sterling Institutional Review Board (registration number IRB00001790) and was conducted in accordance with current International Council for Harmonisation Good Clinical Practice guidelines and 21 CFR Parts 11, 50, 56, and applicable regulations. All patients provided their written informed consent to participate in the registry.
One target wound per patient was identified for study inclusion and was followed for up to 24 weeks. The choice to initiate treatment of the wound with PCMP was made by the Investigator, independent of and before the patient’s decision to participate in the RESPOND registry. Patients received standard wound treatment and clinical assessments appropriate for their wound type, as determined by the Investigator.
Patients
Adults ≥18 years of age with at least 1 appropriate wound (partial and full-thickness wounds of various etiologies including pressure injuries, venous ulcers, diabetic ulcers, chronic vascular ulcers, open surgical wounds, or trauma wounds with tissue loss) were eligible for inclusion. The term “pressure injury” is interchangeable with “pressure ulcer” and describes localized damage to the skin and underlying soft tissue, usually over a bony prominence or related to a medical or other device. Individuals were excluded from participating if they were receiving concurrent treatment with other topical antimicrobials or skin substitute products, if they had received previous PCMP treatment for the study wound, or if they had a third-degree burn or a known sensitivity to any of the PCMP materials.
Objectives
The primary objective was to assess the effectiveness of PCMP in real-world clinical settings, as measured by percent reduction in wound area, time to complete wound closure, and improvement in wound bed condition (as indicated by an increase in healthy granulation tissue, reduction in biofilm, reduction in amount of exudate, and readiness for application of advanced therapy).
Procedures
Eligible and enrolled patients received their initial PCMP application at the baseline visit. Baseline assessments included patient demographics, medical and surgical history, target wound etiology, duration and recurrence information, and previous surgical interventions and treatments (ie, offloading, compression, skin substitutes, hyperbaric oxygen, and negative pressure wound therapy).
Before the first application, the target wound was cleansed, debrided as necessary, and measurements (surface area and depth) were taken. PCMP was applied and the patient returned to the clinic weekly for subsequent PCMP applications and wound care assessments. The investigator determined the frequency and type of assessments and standard wound care for each patient, but the clear majority of patients received a weekly wound surgical and/or sharp debridement, followed by wound brushing, cleansing with normal saline, and PCMP reapplication. Data were collected at each patient visit during which PCMP was applied, and at postapplication follow-up visits as appropriate for up to 24 weeks.
The registry protocol did not specify how the investigator was to perform normal wound care using PCMP. An example of a typical regimen at 2 of the study sites is as follows: Wounds were cleansed and debrided at the initial visit. After initial debridement, cultures would be taken if the patient’s wound was boggy, purulent, or cellulitic. PCMP was applied unless gross purulence was noted. After wound bed preparation, PCMP was applied, wetted with normal saline, affixed with Steri-Strips, and subsequently hydrogel and a nonstick layer were applied. Each PCMP sheet could remain on the study wound for 1 week. Dressings applied over PCMP were left to the treating physician; for example, a hydrogel and a low evaporative dressing (Adaptic or Xeroform) were often applied over PCMP. Patients would be placed in compression wraps if indicated (eg, in cases of venous leg ulcers). PCMP would then be reapplied weekly. After 4–6 weeks, the decision was made to switch to a cellular graft if there was no significant reduction in wound/ulcer size, or to continue with PCMP if the wound was reducing in size.
At one of the authors’ institutions, patients entering the study with lower extremity wounds and lower extremity edema received a venous duplex assessment for venous insufficiency and reflux, and all patients entering the study with lower extremity wounds with nonpalpable pulses had, at minimum, an ankle-brachial index assessment within the first week of enrollment. Patients with venous ulcers received endovenous procedures to correct venous insufficiency, and patients with arterial insufficiency received arterial interventions to augment wound healing, all within the first 2 weeks of entering the study. The use of vascular intervention in patients with venous or arterial insufficiency at enrollment is in concordance with clinical practice guidelines for the management of peripheral artery disease.35,36 In these patients, and all others in this study, PCMP was used as an adjunct to standard of care.
Assessments
At each follow-up clinic visit, wound characteristics, size (area and depth), and quality of granulation tissue were assessed and recorded. PCMP was applied weekly at the investigator’s discretion, and photographs were taken of the wound before and after PCMP application and debridement, if performed, and upon healing, if it occurred.
Any pertinent concomitant treatments, procedures, and medications, and target wound-related adverse events were assessed and recorded.
Statistical Analysis
Data from patients who received PCMP and completed the study by the interim analysis cutoff date (January 26, 2018) were analyzed. Descriptive statistics (mean, SD) and percentage wound closure at 4, 8, and 12 weeks and at the end of study were calculated with Microsoft Excel 2016 (version 16.0.9330.2124).
RESULTS
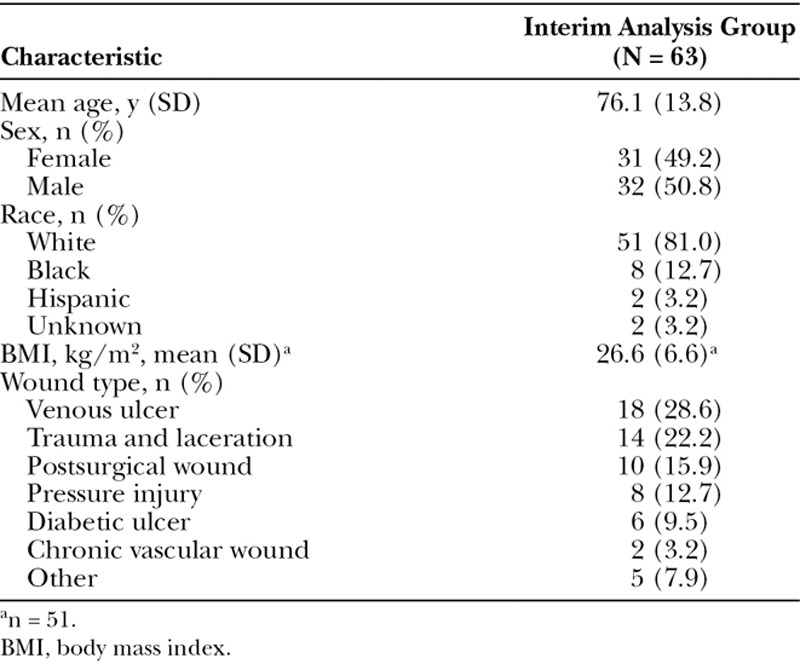
A total of 63 patients completed the study by the data cutoff. The mean (SD) age of patients was 76.1 (13.8) years and 32 (50.8%) were male (Table 1). At baseline, 18 of 63 (28.6%) of wounds were venous ulcers, 14 of 63 (22.2%) were trauma and lacerations (category includes skin tears [n = 2], and wound dehiscence [n = 1] 10 of 63 (15.9%) were postsurgical open wounds, 8 of 63 (12.7%) were pressure injuries, 6 of 63 (9.5%) were diabetic ulcers, 2 of 63 (3.2%) were chronic vascular wounds, and 5 of 63 (7.9%) were another type, including tick bite (n = 1) and vasculitis (n = 1) (data were unavailable for the 3 remaining wounds marked as “other”). The median (interquartile range) baseline wound area was 6.5 (11.8) cm2. The mean baseline wound duration was 4 months. The 63 wounds in this interim analysis received an average of 4 PCMP applications each. Some patients received adjunct therapy during their registry participation.
Table 1.
Demographic and Baseline Characteristics
Of the 63 wounds, 43 (68.3%) achieved complete wound closure by the interim analysis cutoff date (Fig. 1). Of the 43 wounds that achieved complete wound closure, 41 of 43 (95.3%) closed after PCMP treatment and 2 of 43 (4.7%) after bridging to other modalities and surgical closure. Overall, 12 of 63 wounds were bridged to other modalities after PCMP treatment. Two examples of wound healing are shown in Figure 3.
Fig. 1.
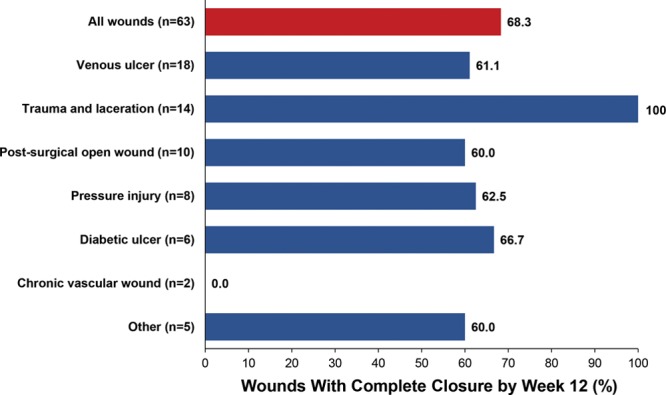
Complete wound closure after PCMP treatment at week 12, by baseline wound type.
Fig. 3.
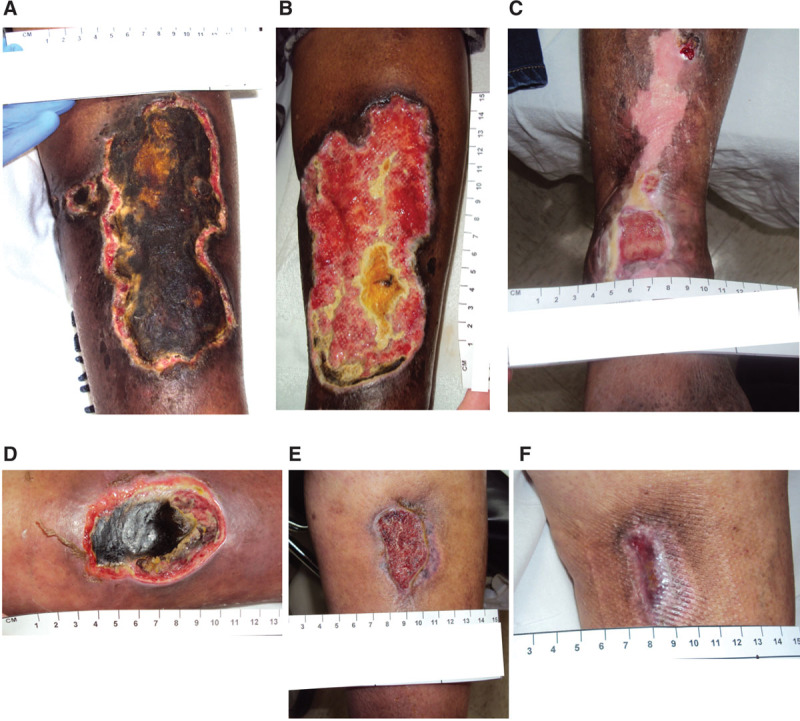
A, A 58-year-old female presented with a large arterial ischemic ulcer to her lower extremity, which had been present for 56 days prior to PCMP application. She received treatments including surgical debridement, 40 hyperbaric oxygen therapy treatments, negative pressure therapy, and 10 PCMP applications. The clinician’s goal of therapy was to progress the wound to be ready for a split-thickness skin graft. The patient was offered a split-thickness skin graft at week 24 but chose not to undergo grafting due to personal reasons, so she preferred to continue the existing course of treatment.
B, Wound at Week 6
C, At week 52 the patient was discharged with 95% epithelialization. The wound was completely healed at 22 months after initial presentation at the wound center.
D, A 55-year-old female presented with a history of a traumatic wound, which had been present for 1 week. She received surgical debridement and 4 PCMP applications.
E, Wound at Week 14.
F, Wound was completely healed at 28 weeks after PCMP applications began.
At week 4, 33.3% (21 of 63) of wounds had completely healed, and at week 8, 50.8% had completely healed. Wound closure by wound type at Weeks 4, 8, and 12 is shown in Figure 2. The mean and median time to wound closure was 5.0 weeks for those that closed after PCMP treatment. In the wounds that did not achieve complete closure, 14 decreased in area from baseline to the end of the study by an average of 54.1%, and 5 increased in size (data on the lesion area at the end of the study timepoint were missing for 1 patient). The 2 chronic vascular wounds did not heal. The 5 patients who had enlarged wounds ranged in age from 69 to 91 years, and the wounds had been present for 1–4 months (wound duration was unavailable for 1 of these 5 patients). The 5 wounds that increased in size were venous ulcers (n = 2), pressure ulcer (n = 1), postsurgery (n = 1), and post-Moh’s surgery (n = 1). Reasons or other factors given for increase in wound size were: congestive heart failure, renal failure, and chronic obstructive pulmonary disease led to death within 3 weeks (venous ulcer); worsening infection with contributing factors of polymyalgia rheumatica (treated with low-dose prednisone) and thrombocytosis (treated with hydroxyurea) (venous ulcer); myocardial infarction, peripheral vascular disease, and bladder cancer resulted in transfer to hospice 2 weeks into the study (postsurgical wound); after 4 weeks of treatment, patient dropped out of registry and transferred to different facility (post-Moh’s surgery), and noncompliance (pressure ulcer). There were 2 deaths during the study (1 due to natural causes; 1 due to exacerbations of chronic obstructive pulmonary disease). There were 5 registry discontinuations before healing or before reaching the end of the study (2 transfer to hospice; 1 Investigator decision; 2 lost to follow-up).
Fig. 2.
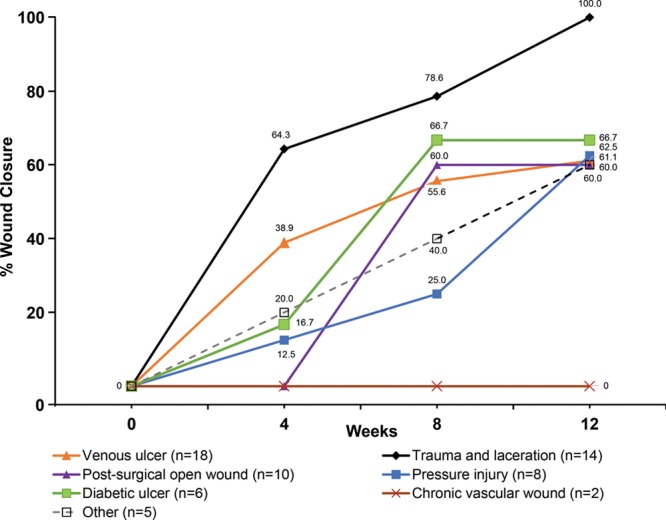
Healing trajectory of various wound types. Wound closure rates over time show an increase in the percentage of wounds achieving closure from the beginning of the study through Week 12. All wound types, except for chronic vascular wounds, exhibited some wound closure throughout the study.
Two adverse events of worsening infection and worsening of wound appearance were reported during the study, both of which were mild and were not attributed to PCMP. The patient reporting a worsening infection had swabs of the venous leg ulcer that were sent for culture, which returned positive. The patient was started on antibiotics and this event was reported as worsening infection. This patient also showed wound enlargement. The patient reporting worsening wound appearance had a diabetic foot ulcer that had been present for many years, which had healed a few times but continued to reopen. The wound was reported as showing a decline during this registry enrollment, which was reported as worsening in appearance.
DISCUSSION
Results of the interim analysis of the RESPOND Registry show that PCMP appeared to positively impact wound healing in a variety of lesion types. Many wounds (68%) achieved complete closure by 12 weeks, and most (90%) had reduced in area from baseline. Complete wound closure was achieved in an average of 5 weeks, which was less than the mean duration of the wounds (12 weeks) before PCMP treatment.
Complete wound closure was seen in many wound types, including trauma wounds and lacerations with tissue loss, venous ulcers, postsurgical wounds, and pressure injuries. Of the wounds that did not achieve complete closure in the study period, 2 were chronic vascular ulcers, which lack vascularity and tend not to heal regardless of treatment modality. PCMP was well tolerated, and no treatment-related adverse events were reported in this study. Results seen here were similar to an earlier case series of PCMP in chronic wounds of various etiology.37
The wound closure results may be attributed to the synergistic effect of the native collagen matrix, which provides a structural substrate and environmental conditions that support tissue ingrowth and reduce protease activity, and the PHMB antimicrobial, which inhibits bacterial growth. The collagen matrix by itself does not appear to possess antimicrobial properties.38
In this study, patients did not show any hypersensitivity to PCMP components, and the PHMB antimicrobial is known to be very biocompatible. PCMP was also used in conjunction with other treatments during this study, including oral and intravenous antibiotic therapy, and hyperbaric oxygen, with no detectable interactions or adverse effects.
As described by the authors representing a study site contributing to this interim data set, the use of PCMP had a positive influence on the healthcare costs associated with treating patients with appropriate wounds. With a streamlined application process for PCMP and the rates of healing and time to healing seen in the patients treated with PCMP, the medical center considered it a beneficial management plan.
PCMP was not compared with another treatment or to a standard-of-care group in this study, and the final results from the full registry enrollment of more than 300 patients may provide a broader perspective on the outcomes following the use of PCMP. In all patients enrolled in this registry, PCMP was used as an adjunct to standard of care. Wounds represented in this study were likely to be those that were amenable to healing without surgery (eg, ulcers that were stage 3 or less), and PCMP was useful to aid healing with these wounds. Skin substitutes, including PCMP, are intended to keep patients out of the operating room as much as possible and heal as many as possible without the need for surgery.
PCMP may be useful for wound management owing to its combination of native ECM and PHMB, ease of application, long shelf life, and the range of sizes available for managing different wound types. The success of the wound management strategy seen here demonstrates the importance of addressing bioburden, biofilm reformation, and high proteolytic activity to improve wound outcomes.
CONCLUSIONS
PCMP appears to be a useful adjunct in the treatment of various types of wounds. Its action against biofilm warrants further evaluation. It seems that the combined action of the PCMP components, native collagen matrix with addition of PHMB, may result in a faster time to wound closure than either component could achieve individually. In this case, the use of PCMP was associated with substantial reduction in wound area after 4 weeks and notable rates of wound closure at up to 12 weeks. PCMP use should be considered when managing chronic or acute wounds of various etiologies. We will await final data to determine if further study is needed to compare PCMP to other products on the outcomes measured.
ACKNOWLEDGMENTS
The authors would like to acknowledge medical writing and editorial support from Agnella Izzo Matic, PhD, CMPP (The Curry Rockefeller Group, LLC, Tarrytown, N.Y.). The authors would also like to acknowledge Santina Wendling, CCRA and Organogenesis Clinical Team for biostatistics support.
Footnotes
Published online 12 June 2019.
Poster presented at the 2018 Diabetic Limb Salvage Conference, April 5–7, 2018, Washington, DC; the 9th Annual Venous Symposium. April 5–7, 2018, New York, NY; and the 7th Meeting of the American College of Wound Healing and Tissue Repair. October 5–6, 2018, Chicago, IL.
Disclosure: M.A.B. serves on the speakers’ bureau for Organogenesis Inc. K.T.T. is a consultant for Acelity and is on the speakers’ bureau for Organogenesis Inc. M.S.S. is a consultant for Acelity. E.C. has nothing to declare. G.J.K. serves on the speakers’ bureau for Organogenesis Inc. This manuscript discusses the use of PuraPly AM Antimicrobial Wound Matrix (Organogenesis Inc., Canton, Mass.). Supported by Organogenesis Inc., Canton, MA. The article processing charge was paid for by Organogenesis Inc.
REFERENCES
- 1.Broughton G, II, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117(suppl 7):12S–34S. [DOI] [PubMed] [Google Scholar]
- 2.Mustoe TA, O’Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg. 2006;117(suppl 7):35S–41S. [DOI] [PubMed] [Google Scholar]
- 3.Hurlow J, Couch K, Laforet K, et al. Clinical biofilms: a challenging frontier in wound care. Adv Wound Care (New Rochelle). 2015;4:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schierle CF, De la Garza M, Mustoe TA, et al. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen. 2009;17:354–359. [DOI] [PubMed] [Google Scholar]
- 5.Malone M, Bjarnsholt T, McBain AJ, et al. The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. J Wound Care. 2017;26:20–25. [DOI] [PubMed] [Google Scholar]
- 6.Phillips P, Wolcott RD, Fletcher J, et al. Biofilms made easy. Wounds Int. 2010;1:1–5. [Google Scholar]
- 7.Schultz G, Bjarnsholt T, James GA, et al. ; Global Wound Biofilm Expert Panel. Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair Regen. 2017;25:744–757. [DOI] [PubMed] [Google Scholar]
- 8.James GA, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;16:37–44. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter S, Davis S, Fitzgerald R, et al. Expert recommendations for optimizing outcomes in the management of biofilm to promote healing of chronic wounds. Wounds. 2016;28(suppl 6):S1–S20.28682298 [Google Scholar]
- 10.Schaber JA, Triffo WJ, Suh SJ, et al. Pseudomonas aeruginosa forms biofilms in acute infection independent of cell-to-cell signaling. Infect Immun. 2007;75:3715–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taneja N, Chari P, Singh M, et al. Evolution of bacterial flora in burn wounds: key role of environmental disinfection in control of infection. Int J Burns Trauma. 2013;3:102–107. [PMC free article] [PubMed] [Google Scholar]
- 12.Seth AK, Geringer MR, Hong SJ, et al. In vivo modeling of biofilm-infected wounds: a review. J Surg Res. 2012;178:330–338. [DOI] [PubMed] [Google Scholar]
- 13.Barker JC, Khansa I, Gordillo GM. A formidable foe is sabotaging your results: what you should know about biofilms and wound healing. Plast Reconstr Surg. 2017;139:1184e–1194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Percival SL, Vuotto C, Donelli G, et al. Biofilms and wounds: an identification algorithm and potential treatment options. Adv Wound Care (New Rochelle). 2015;4:389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolcott RD, Rumbaugh KP, James G, et al. Biofilm maturity studies indicate sharp debridement opens a time-dependent therapeutic window. J Wound Care. 2010;19:320–328. [DOI] [PubMed] [Google Scholar]
- 16.Kim PJ, Attinger CE, Bigham T, et al. Clinic-based debridement of chronic ulcers has minimal impact on bacteria. Wounds. 2018;30:138–143. [PubMed] [Google Scholar]
- 17.Roy S, Elgharably H, Sinha M, et al. Mixed-species biofilm compromises wound healing by disrupting epidermal barrier function. J Pathol. 2014;233:331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Wound Infection Institute. Wound Infection in Clinical Practice. 2016London, UK: Wounds International. [Google Scholar]
- 19.Kramer A, Dissemond J, Kim S, et al. Consensus on wound antisepsis: update 2018. Skin Pharmacol Physiol. 2018;31:28–58. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert P, Moore LE. Cationic antiseptics: diversity of action under a common epithet. J Appl Microbiol. 2005;99:703–715. [DOI] [PubMed] [Google Scholar]
- 21.Hübner NO, Kramer A. Review on the efficacy, safety and clinical applications of polihexanide, a modern wound antiseptic. Skin Pharmacol Physiol. 2010;23(suppl):17–27. [DOI] [PubMed] [Google Scholar]
- 22.Kaehn K. Polihexanide: a safe and highly effective biocide. Skin Pharmacol Physiol. 2010;23(suppl):7–16. [DOI] [PubMed] [Google Scholar]
- 23.Müller G, Kramer A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother. 2008;61:1281–1287. [DOI] [PubMed] [Google Scholar]
- 24.COSMOCIL™ CQ Antimicrobial (Technical Information Bulletin). 2012Atlanta, Ga.: Lonza Microbial Control. [Google Scholar]
- 25.Woo KY, Alam T, Marin J. Topical antimicrobial toolkit for wound infection. Surg Technol Int. 2014;25:45–52. [PubMed] [Google Scholar]
- 26.Mancini S, Cuomo R, Poggialini M, et al. Autolytic debridement and management of bacterial load with an occlusive hydroactive deressing impregnated with polyhexamethylene biguanide. Acta Biomed. 2018;88:409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webster J, Larsen E, Marsh N, et al. Chlorhexidine gluconate or polyhexamethylene biguanide disc dressing to reduce the incidence of central-line-associated bloodstream infection: a feasibility randomized controlled trial (the CLABSI trial). J Hosp Infect. 2017;96:223–228. [DOI] [PubMed] [Google Scholar]
- 28.Chattopadhyay S, Raines RT. Review collagen-based biomaterials for wound healing. Biopolymers. 2014;101:821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleck CA, Simman R. Modern collagen wound dressings: function and purpose. J Am Col Certif Wound Spec. 2010;2:50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi L, Ronfard V. Biochemical and biomechanical characterization of porcine small intestinal submucosa (SIS): a mini review. Int J Burns Trauma. 2013;3:173–179. [PMC free article] [PubMed] [Google Scholar]
- 31.Chang J, DeLillo N, Jr, Khan M, et al. Review of small intestine submucosa extracellular matrix technology in multiple difficult-to-treat wound types. Wounds. 2013;25:113–120. [PubMed] [Google Scholar]
- 32.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–3593. [DOI] [PubMed] [Google Scholar]
- 33.Negron L, Lun S, May BC. Ovine forestomach matrix biomaterial is a broad spectrum inhibitor of matrix metalloproteinases and neutrophil elastase. Int Wound J. 2014;11:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.PuraPly™ Antimicrobial Wound Matrix [package insert]. 2015; http://www.puraplyam.com/wp-content/uploads/PuraPly-Antimicrobial-Package-Insert.pdf. Accessed May 10, 2019 [Google Scholar]
- 35.Conte MS, Pomposelli FB. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. Introduction. J Vasc Surg. 2015;61(Suppl 3):2S41S [DOI] [PubMed] [Google Scholar]
- 36.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69:1465–1508. [DOI] [PubMed] [Google Scholar]
- 37.Lintzeris D, Vernon K, Percise H, et al. Effect of a new purified collagen matrix with polyhexamethylene biguanide on recalcitrant wounds of various etiologies: a case series. Wounds. 2018;30:72–78. [PubMed] [Google Scholar]
- 38.Holtom PD, Shinar Z, Benna J, Patzakis MJ. Porcine small intestine submucosa does not show antimicrobial properties. Clin Orthop Relat Res. 2004;427:18–21. [DOI] [PubMed] [Google Scholar]


