Supplemental Digital Content is available in the text.
Background:
Closed incision negative pressure therapy (ciNPT) is an emerging approach to managing closed incisions of patients at risk of postoperative complications. There are primarily 2 different commercially available ciNPT systems. Both systems consist of a single-use, battery-powered device and foam- or gauze-based peel-and-place dressing designed for closed incisions. These systems vary in design, and there are no data comparing outcomes between the 2 systems.
Methods:
We performed 2 separate meta-analyses to compare surgical site infection (SSI) rates postuse of (1) ciNPT with foam dressing (FOAM) versus conventional dressings and (2) ciNPT with multilayer absorbent dressing (MLA) versus conventional dressings.
Results:
Seven articles and 2 abstracts met inclusion criteria in the FOAM group (n = 489) versus the control group (n = 489) in meta-analysis 1; 7 articles and 1 abstract met inclusion criteria in the MLA group (n = 532) versus the control group (n = 540) in meta-analysis 2. Meta-analysis 1 showed that patients in the control group were 3.17 times more likely to develop an SSI compared with patients in the FOAM group [weighted mean odds ratios of FOAM group versus control group was 3.17 (P < 0.0001) with the 95% confidence intervals of 2.17–4.65]. Meta-analysis 2 showed no significant difference in SSI rates between patients in the MLA group and patients in the control group [weighted mean odds ratios of MLA group versus control group was 1.70 (P = 0.08) with the 95% confidence intervals of 0.94–3.08].
Conclusions:
Comparing outcomes of two different ciNPT systems with a common comparator (conventional dressings) may provide an interim basis for comparing ciNPT systems until further comparative evidence is available. More comparative research is required to determine outcomes in clinical practice.
INTRODUCTION
Surgical site infections (SSIs) are a high-priced complication of open surgical procedures and a dominant cause of unplanned 30-day hospital readmissions.1 They are the costliest of all hospital-acquired infections, increasing hospital stay by an average of 9.7 days and accounting for an estimated $3.5–10 billion annual expenditures in the United States. The mean cost of treating a patient who develops a deep wound SSI, superficial SSI, or wound disruption/dehiscence has been reported to be approximately 3.0, 1.6, or 3.6 times, respectively, the rate of treating a patient undergoing a similar operation but without complications.2 Overall, SSIs have been reported following an estimated 1%–3.1% of all surgical procedures, accounting for approximately 2.0% of deaths due to health care–associated infections.3–7 The true incidence of SSIs is likely even higher, as many SSIs are diagnosed in an outpatient setting or after discharge.6
SSIs may be caused by endogenous or exogenous microorganisms. Proper postoperative incision care is important to reduce exposure and susceptibility to pathogens that may cause infection. Historically, simple gauze dressings or steri-strips have been used over closed surgical incisions to provide limited barrier protection against exogenous bacteria, absorb drainage, and help hold incision edges together. Closed incision negative pressure therapy (ciNPT) is an emerging approach to managing closed incisions of patients at risk of postoperative complications, including infection, seroma, hematoma, and dehiscence. Initiating continuous negative pressure therapy (NPT) over closed incisions provides a barrier against external contamination while removing wound exudate, including infectious material. There is also evidence to suggest that ciNPT helps reduce edema, which may positively impact perfusion. Additionally, the therapy helps hold incision edges together and realign and reduce tensile forces across the suture line.8 Published studies have documented clinical experience using NPT over closed surgical incisions with successful outcomes in many fields of surgery including vascular, cardiac, gastrointestinal, gynecological, and orthopedic procedures.9–13
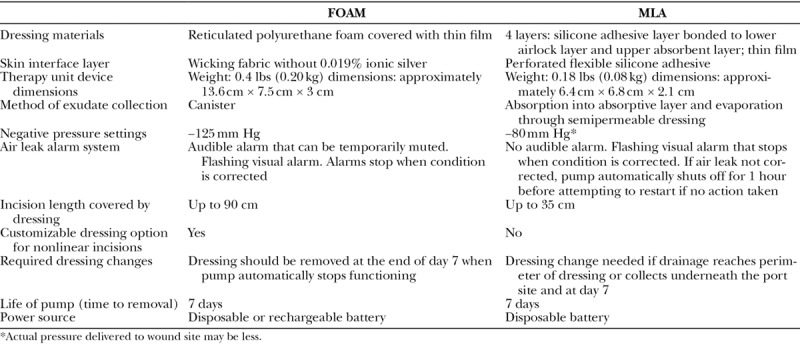
There are primarily two different portable, simplified ciNPT systems that have been commercially available since approximately 2010. Both systems consist of a single-use, battery powered device and a foam-based or absorbent layer-based peel-and-place dressing specifically designed for closed incisions. Otherwise, these systems vary in design and their major design characteristics are listed in Table 1. Although there are currently no data comparing the two systems, there are several published studies comparing outcomes of each of the ciNPT systems with conventional dressings. We performed two separate meta-analyses to compare SSI rates postuse of (1) ciNPT with foam dressing (FOAM; PREVENA Incision Management System; KCI, an Acelity Company, San Antonio, Tex.) versus conventional dressings and (2) ciNPT with a multilayer absorbent dressing (MLA; PICO; Smith & Nephew Ltd, Hull, UK) versus conventional dressings.
Table 1.
Characteristics of FOAM-based and MLA-based ciNPT Systems
METHODS
Literature Search
A comprehensive literature search was conducted to identify relevant published articles in PubMed, ScienceDirect, Embase, Ovid, and QUOSA databases from January 1, 2010, to June 30, 2018. The following combinations of terms were used in the search: (“negative pressure wound therapy” OR “vacuum assisted closure” OR “negative pressure therapy”) AND “incision management”; PREVENA AND (“negative pressure wound therapy” OR “vacuum assisted closure” OR “negative pressure therapy”); and PICO AND (“negative pressure wound therapy” OR “vacuum assisted closure” OR “negative pressure therapy”). The initial search yielded 408 citations after duplicates were eliminated. After screening titles and abstracts for English language, date range, clinical data, lack of subsequent publication, inclusion of FOAM or MLA ciNPT, and exclusion of preclinical studies, case studies, reviews, noncomparative studies, meta-analyses, and veterinary studies, 58 articles were retrieved for the examination of full text.
Eligibility for Inclusion
Review of the publications that met the eligibility criteria was performed by 2 reviewers. A clinical study was included for analysis if it had undergone peer review, was a randomized controlled trial comparing single-use ciNPT with standard care (any non-negative pressure wound therapy dressing) applied postoperatively on a closed surgical incision, contained at least 20 enrolled patients per study arm, and reported on SSI as an outcome measure. Study participants could be of any age and undergoing any type of operation, but all wounds in each study arm needed to be a closed surgical incision, not an empyema or open wound.
There were no restrictions on the inclusion or exclusion criteria with respect to risk factors for complications. When the study data were published in multiple sources, only the most complete and recent publication was included. Studies were excluded if the ciNPT treatment arm was a mix of ciNPT types/brands, and SSI rates were not reported by type/brand, as were studies that described the use of ciNPT with products other than the foam and MLA ciNPT. Figure 1 is a flow diagram showing steps in selecting articles for inclusion in the meta-analyses.
Figure 1.
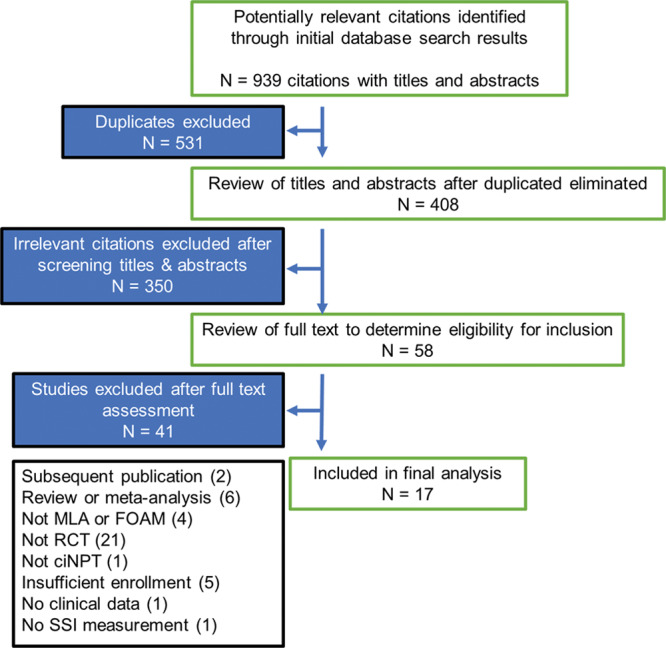
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of selection of articles for meta-analyses. RCT, randomized controlled trial; ciNPT, closed incision negative pressure therapy; SSI, surgical site infection.
Data Collection
The following data were extracted from each included study: number and characteristics of the participants, surgical procedure, type of NPT device used, type of dressing used in the control group, duration of treatment in the intervention and control groups, SSI incidence, system used to classify SSI, length of assessment for SSI, and time between assessments if there were multiple assessments.
Analysis of Results
Both meta-analyses were performed using the same methodology. For each analysis, the outcome was measured the presence (or absence) of an SSI using a binary variable. The chi-square test was used to statistically assess heterogeneity, and the I2 statistics was used to assess the magnitude of heterogeneity. Pooled and weighted odds ratios (OR) and 95% confidence intervals (CI) were calculated to pool study and control groups in each publication and compare for each meta-analysis. The treatment effects were combined using Mantel–Haenszel OR as the summary statistics. Choosing the more conservative analytic approach, a random effects model was used for each analysis performed, even when statistical heterogeneity was not evident. All analyses were performed using the RevMan Version 5.3 software (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
RESULTS
Study Selection
From 408 studies extracted for initial review, 350 studies were excluded after screening titles and abstracts and 58 studies were selected for full-text review. During text review, 41 studies were excluded, 21 of which were not prospective randomized controlled trials (RCTs). Four RCTs measured results of ciNPT versus control but included products other than FOAM or MLA ciNPT and were excluded. The literature review revealed no ciNPT studies with greater than 2 study arms. A complete rationale for exclusion and frequency is included in Figure 1. Lee et al14 met inclusion criteria with 35 consented, randomized patients in the FOAM group and 29 patients in the control group (dry gauze). However, only 27 patients in the FOAM group and 17 patients in the control group were included in the final analysis due to deaths (unrelated to therapy), withdrawal because of postoperative delirium, or lost to follow-up.
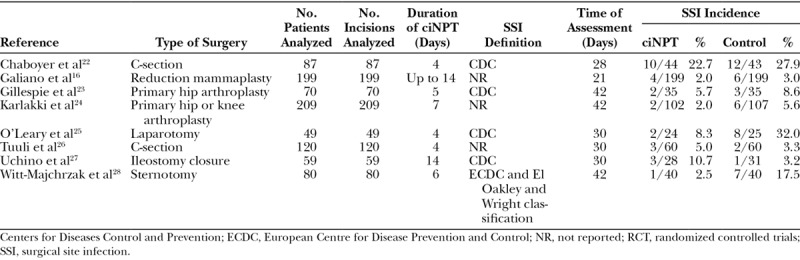
A total of 17 articles were selected for inclusion in the final analyses, comprising 1,689 patients with 1,851 wounds. Seven articles and 2 abstracts met all criteria for inclusion in the FOAM group (n = 489) versus the control group (n = 489) in meta-analysis 1, and 7 articles and 1 abstract met all criteria for inclusion in the MLA group (n = 532) versus the control group (n = 540) in meta-analysis 2. Two of the studies (Gombert et al15 and Galiano et al16) were multicenter RCTs, and the rest of the studies were single-center RCTs. The studies included in both meta-analyses and the extracted endpoints are listed in Tables 2 and 3.
Table 2.
RCTs Included in Meta-analysis 1: FOAM Versus Control
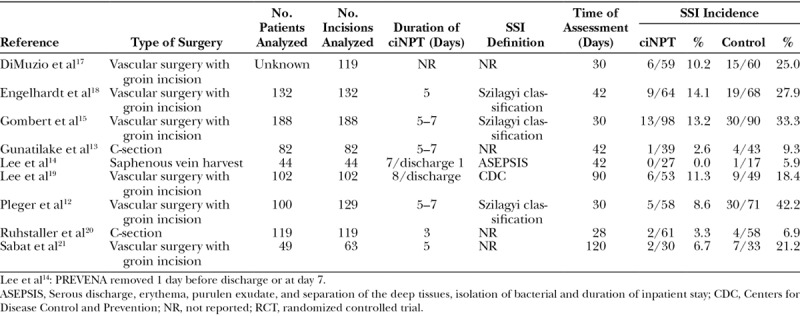
Table 3.
RCTs Included in Meta-analysis 2: MLA Versus Control
Meta-analyses Outcomes
Meta-analysis 1 showed that patients in the control group were 3.17 times more likely to develop an SSI compared with patients in the FOAM group [weighted mean OR of the FOAM group versus the control group was 3.17 (P < 0.0001) with the 95% CI of 2.17–4.65] (see figure, Supplemental Digital Content 1, which displays results from meta-analyses 1 and 2, http://links.lww.com/PRSGO/B67). Meta-analysis 2 showed no significant difference in SSI rates between patients in the MLA group and patients in the control group [weighted mean OR of the MLA group versus the control group was 1.70 (P = 0.08) with the 95% CI of 0.94–3.08] (see figure, Supplemental Digital Content 1, which displays results from meta-analyses 1 and 2, http://links.lww.com/PRSGO/B67).
DISCUSSION
Although the overall incidence of SSIs has been reduced by external improvements in operating room environments, instrument sterilization procedures, maximum barrier protection requirements, and the use of prophylactic antibiotics,29,30 there are still reported SSI increases along the spectrum for increasing wound classification (as defined by the Centers for Disease Control and Prevention [CDC]) and a number of risk factors. SSIs remain a considerable cause of morbidity and death due to multiple contributing factors, including larger numbers of older surgical patients, an increase in the variety of chronic and immunocompromising conditions, increased use of prosthetic implants and organ transplantation, and a growing incidence of antibiotic-resistant microorganisms.31 In a cross-sectional study using the American College of Surgeons National Surgical Quality Improvement Program dataset of 634,426 cases (between 2005 and 2008), the overall rates for all SSIs (superficial, deep incisional, and organ/space) were 2.6% in clean wounds, 6.7% in clean/contaminated wounds, 8.6% in contaminated wounds, and over 11.8% in dirty wounds.32
Based on our meta-analyses, analysis of FOAM versus control resulted in a statistically significant reduction in SSI rates, whereas the analysis of MLA versus control did not demonstrate a significant reduction in SSI rates. Possible factors that may have contributed to the differences in outcome of meta-analysis 1 versus meta-analysis 2 include differences in patient selection, type of surgery performed, patient and wound comorbidities, level of negative pressure delivered, dressing interface used, and duration of assessment between the 2 groups. Incidence of SSI after a surgical procedure is highly variable depending on the type of operation performed and underlying risk factors of the patient.33 Comorbidities such as diabetes, obesity, and poor vascular status and risk factors such as advanced age, chemotherapy, radiation therapy, and use of nicotine or steroids present challenges in maintaining incision closure after an open surgical procedure and can increase the risk of complications, including infection.34,35 Similarly, certain surgical procedures and conditions such as high-tension incision, repeated incisions, presence of preoperative open wound, longer operative times, extensive undermining, traumatized soft tissue, edema, contamination, and emergency procedure can create difficulties in optimal incision healing, which could lead to postoperative incision complications and/or additional surgeries.1,36 Due to the heterogeneous methodologies between the studies evaluated in these meta-analyses, neither comorbidities nor surgical procedures/conditions were tracked, thereby limiting our results.
Furthermore, types of surgery performed in the studies analyzed in meta-analysis 1 were different than the surgery types in the studies analyzed in meta-analysis 2. Whereas FOAM studies comprised patients who had C-section incisions (20.5%), incisions following saphenous vein harvest (4.5%), or groin incisions following vascular surgery (74.9%), the MLA groups had incisions resulting from a wider range of surgery types: C-section (23.7%), hip or knee arthroplasty (31.9%), reduction mammaplasty (22.8%), laparotomy (5.6%), ileostomy (6.8%), and sternotomy (9.2%). These major differences in heterogeneity between the 2 meta-analyses cannot be explained by differences in product indications because both ciNPT systems are indicated for closed surgical incisions. A more accurate comparison would be comprised patients who have undergone similar types of surgery. In addition, the number of days that patients were assessed for postsurgical complications in each of the studies varied from 28 to 120 days in meta-analysis 1 and from 21 to 42 days in meta-analysis 2 and these differences were not taken into account when analyzing the results. Longer lengths of postsurgical time, during which patients are assessed in studies, could have revealed a greater number of complications versus shorter assessment times, but the exact effect is unknown.
The outcomes of each of the studies within the MLA group of studies are also more variable than FOAM study outcomes. Of the MLA studies, one study shows a statistically significant difference28 and one study shows a marginally significant difference favoring MLA versus control,25 whereas 2 studies (Tuuli et al26 and Uchino et al27) show a higher rate of SSIs in the MLA group and the remaining 4 studies show a slightly lower rate of SSIs in the MLA group. All of the 9 FOAM studies favor FOAM versus control, 4 of which were statistically significant or marginally significant.
There are likely numerous reasons for these differences in outcomes of studies comprised in meta-analysis 2 (MLA versus control), including variances in wound type, bioburden levels, device negative pressure settings, and definitions of SSI. Different outcomes between Tuuli et al26 and Chaboyer et al22 studies, which evaluated ciNPT compared with standard incision care for obese women undergoing elective c-section, may be explained by different definitions of obesity. The Tuuli et al26 study included only obese patients with BMI ≥30 kg/m2, whereas Chaboyer et al22 defined obesity as BMI ≥25 kg/m2, which could somewhat account for differences in outcomes between the two studies in meta-analysis 2. For further comparison, in meta-analysis 1, Gunatilake et al13 defined obesity as BMI ≥35 kg/m2 and Ruhstaller et al20 defined obesity as BMI ≥30 kg/m2.
The Uchino et al27 study was the only study to explore use of ciNPT postdigestive surgery; patients with ulcerative colitis scheduled to undergo ileostomy closure with purse-string suture were randomly divided into groups with or without MLA ciNPT. To reduce the risk of potential complications such as enterocutaneous fistula and postoperative bleeding that could be affected by high negative pressure, pressure of the ciNPT device was set at −80 ± 20 mm Hg. However, this lower level of negative pressure (<125 mm Hg) could have reduced the effectiveness of ciNPT over contaminated closed incisions created during ileostomy closure. There is also the possibility that ciNPT may not be beneficial for these incision types, but considerably more research is required to determine the effects of ciNPT postdigestive surgery.
It should be noted that Uchino et al27 is a relatively small study (n = 59) with a large effect in this meta-analysis. Nevertheless, the study was included for analysis, because it fit the inclusion criteria. Although results of meta-analysis 2 do not significantly favor MLA over control groups, there is a trend toward significance. A future meta-analysis that would include a greater number of studies and/or larger studies with outcomes that favor MLA would likely yield results significantly in favor of MLA, but this analysis was limited to published studies that were available at the time of review.
Our results from meta-analysis 2 differ from a recently published meta-analysis by Strugala and Martin37 that analyzed complication rates of 1,839 patients with 2,154 incisions treated by MLA versus control in 16 studies evaluated. Their analysis showed a significant reduction in SSI of 58% from 12.5% to 5.2% with MLA [relative risk (RR) 0.43 (95% CI 0.32–0.57), P < 0.0001]. In their meta-analysis, RCTs and retrospective or prospective observational studies written between January 1, 2011, and March 31, 2017, of any sample size with no publication restrictions were eligible for inclusion. Published abstracts or PhD theses with sufficient information to extract mean and variance data were also included.37 Our meta-analysis included only those studies that have been published in a peer-reviewed journal and within a wider date range. These differences in article inclusion criteria led to outcomes’ differences between the 2 meta-analyses.
CONCLUSIONS
Our meta-analyses of 17 published RCTs comprising 1,689 patients with 1,851 wounds showed a significantly lower number of SSIs in the FOAM group compared with the control group, whereas there was no significant difference in SSI rates between the MLA group and the control group. Currently, there are no published head-to-head comparative studies of outcomes with MLA versus FOAM, both of which have been commercially available since approximately 2010. Comparing outcomes of two different ciNPT systems with a common comparator (conventional dressings) is meant to provide an interim basis for comparing ciNPT systems until further comparative evidence is available. However, limitations of this study, including differences in types of surgery performed between the MLA and FOAM study groups, require caution in the interpretation of these study results.
ACKNOWLEDGMENTS
The authors thank Karen Beach (Acelity) and Ricardo Martinez, MS (Acelity), for editorial assistance.
Supplementary Material
Footnotes
Published online 21 June 2019.
Some of the material in this article was presented at the Symposium on Advanced Wound Care (Spring 2018) held April 25–29, 2018, in Charlotte, NC.
Disclosure: Devinder P. Singh is a consultant for KCI, an Acelity Company, a consultant for Gore, and a consultant for Aeroform. Allen Gabriel is a consultant for KCI, an Acelity Company, and a consultant for Allergan. Ronald P. Silverman is an employee of KCI, an Acelity Company. Leah P. Griffin is an employee of KCI, an Acelity Company. Lucy D’Agostino McGowan is a contractor for KCI, an Acelity Company. Ralph B. D’Agostino Jr. is a contractor for KCI, an Acelity Company. The Article Processing Charge was paid for by KCI, an Acelity Company.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Hicks CW, Bronsert M, Hammermeister KE, et al. Operative variables are better predictors of postdischarge infections and unplanned readmissions in vascular surgery patients than patient characteristics. J Vasc Surg. 2017;65:1130.e9–1141.e9. [DOI] [PubMed] [Google Scholar]
- 2.Vaughan-Sarrazin M, Bayman L, Rosenthal G, et al. The business case for the reduction of surgical complications in VA hospitals. Surgery. 2011;149:474–483. [DOI] [PubMed] [Google Scholar]
- 3.Anderson DJ, Chen LF, Sexton DJ, et al. Complex surgical site infections and the devilish details of risk adjustment: important implications for public reporting. Infect Control Hosp Epidemiol. 2008;29:941–946. [DOI] [PubMed] [Google Scholar]
- 4.Klevens RM, Edwards JR, Richards CL, Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Lissovoy G, Fraeman K, Hutchins V, et al. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37:387–397. [DOI] [PubMed] [Google Scholar]
- 6.Barie PS, Wilson SE. Impact of evolving epidemiology on treatments for complicated skin and skin structure infections: the surgical perspective. J Am Coll Surg. 2015;220:105.e6–116.e6. [DOI] [PubMed] [Google Scholar]
- 7.Anderson DJ, Hartwig MG, Pappas T, et al. Surgical volume and the risk of surgical site infection in community hospitals: size matters. Ann Surg. 2008;247:343–349. [DOI] [PubMed] [Google Scholar]
- 8.Wilkes RP, Kilpad DV, Zhao Y, et al. Closed incision management with negative pressure wound therapy (CIM): biomechanics. Surg Innov. 2012;19:67–75. [DOI] [PubMed] [Google Scholar]
- 9.Bonds AM, Novick TK, Dietert JB, et al. Incisional negative pressure wound therapy significantly reduces surgical site infection in open colorectal surgery. Dis Colon Rectum. 2013;56:1403–1408. [DOI] [PubMed] [Google Scholar]
- 10.Stannard JP, Robinson JT, Anderson ER, et al. Negative pressure wound therapy to treat hematomas and surgical incisions following high-energy trauma. J Trauma. 2006;60:1301–1306. [DOI] [PubMed] [Google Scholar]
- 11.Reddy VS. Use of closed incision management with negative pressure therapy for complex cardiac patients. Cureus. 2016;8:e506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pleger SP, Nink N, Elzien M, et al. Reduction of groin wound complications in vascular surgery patients using closed incision negative pressure therapy (ciNPT): a prospective, randomised, single-institution study. Int Wound J. 2018;15:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunatilake RP, Swamy GK, Brancazio LR, et al. Closed-incision negative-pressure therapy in obese patients undergoing cesarean delivery: a randomized controlled trial. AJP Rep. 2017;7:e151–e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee AJ, Sheppard CE, Kent WD, et al. Safety and efficacy of prophylactic negative pressure wound therapy following open saphenous vein harvest in cardiac surgery: a feasibility study. Interact Cardiovasc Thorac Surg. 2017;24:324–328. [DOI] [PubMed] [Google Scholar]
- 15.Gombert A, Babilon M, Barbati ME, et al. Closed incision negative pressure therapy reduces surgical site infections in vascular surgery: a prospective randomised trial (AIMS trial). Eur J Vasc Endovasc Surg. 2018;56:442–448. [DOI] [PubMed] [Google Scholar]
- 16.Galiano RD, Hudson D, Shin J, et al. Incisional negative pressure wound therapy for prevention of wound healing complications following reduction mammaplasty. Plast Reconstr Surg Glob Open. 2018;6:e1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiMuzio P, Staley C, Reiter D, et al. A randomized study evaluating negative-pressure therapy to decrease vascular groin wound complications. J Vasc Surg. 2017;65:133S. [DOI] [PubMed] [Google Scholar]
- 18.Engelhardt M, Rashad NA, Willy C, et al. Closed-incision negative pressure therapy to reduce groin wound infections in vascular surgery: a randomised controlled trial. Int Wound J. 2018;15:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee K, Murphy PB, Ingves MV, et al. Randomized clinical trial of negative pressure wound therapy for high-risk groin wounds in lower extremity revascularization. J Vasc Surg. 2017;66:1814–1819. [DOI] [PubMed] [Google Scholar]
- 20.Ruhstaller K, Downes KL, Chandrasekaran S, et al. Prophylactic wound vacuum therapy after cesarean section to prevent wound complications in the obese population: a randomized controlled trial (the ProVac study). Am J Perinatol. 2017;34:1125–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabat J, Tyagi S, Srouji A, et al. Prophylactic negative-pressure therapy for femoral incision in vascular surgery: preliminary results of a prospective, randomized trial. J Vasc Surg. 2016;63:Paper presented at: 2016 Vascular Annual Meeting; June 8–11, 2016; National Harbor, MD 94S–95S. [Google Scholar]
- 22.Chaboyer W, Anderson V, Webster J, et al. Negative pressure wound therapy on surgical site infections in women undergoing elective caesarean sections: a Pilot RCT. Healthcare (Basel). 2014;2:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillespie BM, Rickard CM, Thalib L, et al. Use of negative-pressure wound dressings to prevent surgical site complications after primary hip arthroplasty: a Pilot RCT. Surg Innov. 2015;22:488–495. [DOI] [PubMed] [Google Scholar]
- 24.Karlakki SL, Hamad AK, Whittall C, et al. Incisional negative pressure wound therapy dressings (iNPWTd) in routine primary hip and knee arthroplasties: a randomised controlled trial. Bone Joint Res. 2016;5:328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Leary DP, Peirce C, Anglim B, et al. Prophylactic negative pressure dressing use in closed laparotomy wounds following abdominal operations: a randomized, controlled, open-label trial: the P.I.C.O. trial. Ann Surg. 2017;265:1082–1086. [DOI] [PubMed] [Google Scholar]
- 26.Tuuli MG, Martin S, Stout MJ, et al. Pilot randomized trial of prophylactic negative pressure wound therapy in obese women after cesarean delivery. Am J Obstet Gynecol. 2017;216:Paper presented at: 37th Annual Pregnancy Meeting; January 23–28, 2017; Las Vegas, NV. S245. [Google Scholar]
- 27.Uchino M, Hirose K, Bando T, et al. Randomized controlled trial of prophylactic negative-pressure wound therapy at ostomy closure for the prevention of delayed wound healing and surgical site infection in patients with ulcerative colitis. Dig Surg. 2016;33:449–454. [DOI] [PubMed] [Google Scholar]
- 28.Witt-Majchrzak A, Żelazny P, Snarska J. Preliminary outcome of treatment of postoperative primarily closed sternotomy wounds treated using negative pressure wound therapy. Pol Przegl Chir. 2015;86:456–465. [DOI] [PubMed] [Google Scholar]
- 29.Gottrup F, Replace with Melling A, Hollander DA. An overview of surgical site infections: aetiology, incidence and risk factors. EWMA J. 2005;5:11–15. [Google Scholar]
- 30.Spagnolo AM, Ottria G, Amicizia D, et al. Operating theatre quality and prevention of surgical site infections. J Prev Med Hyg. 2013;54:131–137. [PMC free article] [PubMed] [Google Scholar]
- 31.Hranjec T, Swenson BR, Sawyer RG. Surgical site infection prevention: how we do it. Surg Infect (Larchmt). 2010;11:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortega G, Rhee DS, Papandria DJ, et al. An evaluation of surgical site infections by wound classification system using the ACS-NSQIP. J Surg Res. 2012;174:33–38. [DOI] [PubMed] [Google Scholar]
- 33.Dellinger EP. Surgical site infection. In: Netter’s Infectious Diseases. 2012: Philadelphia, PA: Elsevier Saunders; 295–298. [Google Scholar]
- 34.Wilson JA, Clark JJ. Obesity: impediment to postsurgical wound healing. Adv Skin Wound Care. 2004;17:426–435. [DOI] [PubMed] [Google Scholar]
- 35.Abbas SM, Hill AG. Smoking is a major risk factor for wound dehiscence after midline abdominal incision; case–control study. ANZ J Surg. 2009;79:247–250. [DOI] [PubMed] [Google Scholar]
- 36.Willy C, Agarwal A, Andersen CA, et al. Closed incision negative pressure therapy: international multidisciplinary consensus recommendations. Int Wound J. 2017;14:385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strugala V, Martin R. Meta-analysis of comparative trials evaluating a prophylactic single-use negative pressure wound therapy system for the prevention of surgical site complications. Surg Infect (Larchmt). 2017;18:810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]


