Abstract
Background:
Mastectomy is a commonly requested procedure in the transmasculine population and has been shown to improve quality of life, although there is limited research on safety. The aim of this study was to provide a nationwide assessment of epidemiology and postoperative outcomes following masculinizing mastectomy and compare them with outcomes following mastectomy for cancer prophylaxis and gynecomastia correction in cisgender patients.
Methods:
The American College of Surgeons National Surgical Quality Improvement Program database from 2005 to 2017 was queried using International Classification of Diseases and Current Procedural Terminology codes to create cohorts of mastectomies for 3 indications: transmasculine chest reconstruction, cancer risk-reduction (CRRM), and gynecomastia treatment (GM). Demographic characteristics, comorbidities, and postoperative complications were compared between the 3 cohorts. Multivariable regression analysis was used to control for confounders.
Results:
A total of 4,170 mastectomies were identified, of which 14.8% (n = 591) were transmasculine, 17.6% (n = 701) were CRRM, and 67.6% (n = 2,692) were GM. Plastic surgeons performed the majority of transmasculine cases (85.3%), compared with the general surgeons in the CRRM (97.9%) and GM (73.7%) cohorts. All-cause complication rates in the transmasculine, CRRM, and GM cohorts were 4.7%, 10.4%, and 3.7%, respectively. After controlling for confounding variables, transgender males were not at an increased risk for all-cause or wound complications. Multivariable regression identified BMI as a predictor of all-cause and wound complications.
Conclusion:
Mastectomy is a safe and efficacious procedure for treating gender dysphoria in the transgender male, with an acceptable and reassuring complication profile similar to that seen in cisgender patients who approximate either the natal sex characteristics or the new hormonal environment.
INTRODUCTION
The term transgender is used to describe a diverse group of individuals who transcend culturally defined categories of gender.1 Compared with the general population, transgender individuals are disproportionately subject to harassment, social stigma, and physical and sexual abuse.2,3 Additionally, a substantial portion of the transgender population experiences gender dysphoria (GD), defined as clinically significant distress or functional impairment that arises from a marked incongruence between a person’s experienced/expressed gender and their birth-assigned biological sex.4 GD may be effectively addressed through a combination of medical and surgical treatments.1 For patients undergoing transmasculine transition, surgical options include masculinizing chest reconstruction (“top surgery”), hysterectomy, phalloplasty or metoidioplasty (“bottom surgery”), and facial masculinization procedures.
Chest wall contouring, typically via mastectomy, is often the first and the only surgical intervention that transgender males will undergo.5 Importantly, masculinizing chest reconstruction has been shown to significantly improve psychosocial functioning and quality of life.6–8 Expanded access to care and improvements in social stigma have led to a substantial rise in the number of gender-affirming surgeries (GAS) performed in the United States over the past 5 years, opening up new avenues for quality improvement.9 Ongoing evaluation and review of epidemiologic and postoperative complications data are imperative to maintaining high standards of care and identifying demographic disparities.
Numerous studies have been published on the technical considerations and aesthetic outcomes following mastectomy in the transmasculine population.10 However, the majority of this literature comprises single-institution studies with relatively small sample sizes,5,11–17 thus limiting generalizability. Furthermore, much of the transgender epidemiologic data arise from survey-based studies,18,19 thereby precluding a direct assessment of surgical patient demographics and outcomes. Comparatively, mastectomy for other indications, such as cancer risk-reduction (CRRM) in cisgender females20 and correction of gynecomastia (GM) in cisgender males,21 has been well described in the literature.
This study used the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) to provide a nationwide assessment of the demographic characteristics and postoperative outcomes associated with masculinizing mastectomy in comparison to mastectomy for other indications in cisgender males and females to determine whether it is practical to apply safety and outcomes research from cisgender mastectomy to transmasculine patients. To our knowledge, this is the first study to examine demographics and complication profiles for transgender patients at the national level and to compare these results with their cisgender counterparts.
METHODS
Dataset
The ACS NSQIP is a nationally validated, risk-adjusted, surgical outcomes program that collects information on approximately 240 Health Insurance Portability and Accountability Act of 1996 compliant variables, including demographics, preoperative comorbidities, and 30-day postoperative outcomes from over 400 institutions nationwide.22 The ACS NSQIP database from 2005 to 2017 was used to perform this retrospective analysis. The information contained within this database is deidentified and is available to all institutions complying with the ACS NSQIP data use agreement. Methods of data collection have been previously detailed.23
Transgender Cohort Selection
To establish our transgender cohort, we first selected patients with a diagnosis of gender dysphoria and/or related conditions (eg, transsexualism) using codes from the International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10; Table 1). Within this cohort, we selected patients undergoing mastectomy using Current Procedural Terminology (CPT) codes (Table 2). We excluded subjects that underwent other or concurrent operations unrelated to the mastectomy, such as hysterectomy, bottom surgery, or abdominoplasty to restrict outcomes to those related to the primary procedure.
Table 1.
ICD-9 and ICD-10 Codes
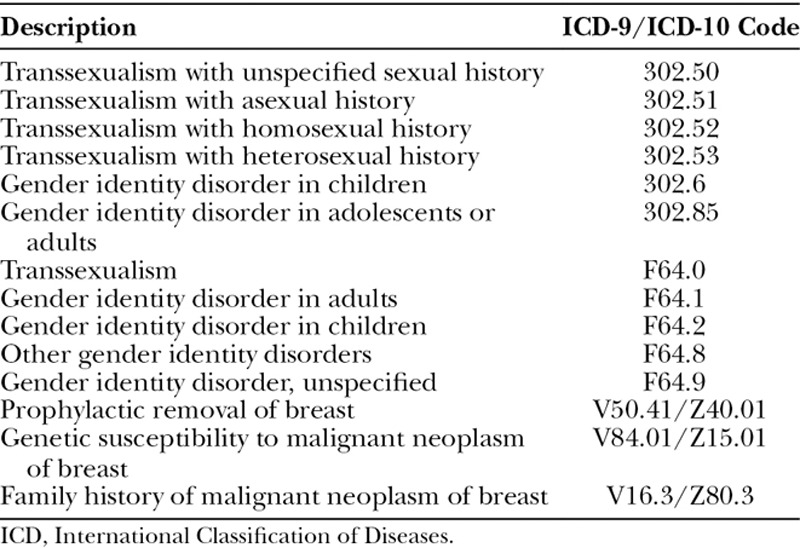
Table 2.
Current Procedural Terminology Codes

Cisgender Cohort Selection
To establish a cisgender cohort of healthy patients of the same natal sex seeking breast removal, patients with a surgical encounter for prophylactic breast removal, genetic susceptibility to breast cancer, or family history of breast cancer were identified using ICD-9/ICD-10 codes (Table 1). From this dataset, patients undergoing mastectomy were selected via CPT codes (Table 2). Careful review of all 21 potential CPT codes was performed so as to identify and exclude all patients undergoing postmastectomy breast reconstruction and any other or concurrent procedure unrelated to the mastectomy.
To establish a cisgender cohort of healthy patients with the same hormonal environment as our transmasculine cohort, we identified cisgender male patients undergoing surgical correction of gynecomastia using CPT codes (Table 2). As with the initial 2 cohorts, thorough review of all 21 potential CPT codes was performed so as to identify and exclude any cases involving other or concurrent procedures unrelated to the mastectomy.
Variables
Demographic information, including age, race, baseline health characteristics, past medical and surgical history, and American Society of Anesthesiologists physical status (ASA Class) were collected and analyzed. The complete list of variables contained within this dataset, and their corresponding definitions, can be found on the National Surgical Quality Improvement Program website (http://site.acsnsqip.org/).
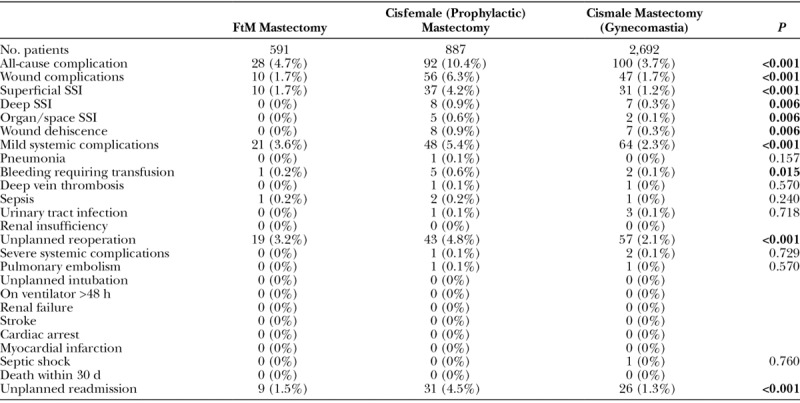
The NSQIP also collects more than 20 variables related to 30-day postoperative outcomes. These variables were used for univariate comparison between cohorts and were aggregated to define several additional outcome measures. These composite variables are wound complications, mild systemic complications, and severe systemic complications with all-cause complications representing the sum of all groups. A complete list of the variables used to define aggregate outcome measures can be found in Table 5.
Table 5.
Postoperative Outcomes
Statistical Analysis
All statistical analyses were performed using IBM SPSS version 24 for Windows (IBM Corp, Armonk, N.Y.). We performed a univariate analysis to assess for unadjusted differences between our 3 cohorts in terms of demographic features, clinical characteristics, perioperative comorbidities, and risk factors, and individual and aggregate postoperative outcomes measures. The 2-sided unpaired t test was used to assess the difference in means of continuous variables, whereas categorical data were compared using the Chi-square test. Statistical significance was reported as P< 0.05. To identify independent predictors of all-cause and wound complications, we performed a multivariable binary logistic regression that included the indication for mastectomy and variables with unadjusted P < 0.05 on univariate analysis, including body mass index (BMI), smoking, hypertension, and diabetes.
The patient information in this study is de-identified and available to all institutions complying with the ACS NSQIP Data Use Agreement.
RESULTS
General
The ACS NSQIP database contained 6,637,415 entries from 2005 to 2017. From this dataset, 8,932 mastectomy cases of interest were initially identified (Fig. 1). All cases involving concurrent breast reconstruction or other procedures unrelated to the mastectomy were excluded from analysis (n = 4,762) to maximize the comparability of the outcomes. Ultimately, 4,170 mastectomy cases were selected, of which 14.2% (n = 591) were transmasculine, 21.3% (n = 887) were (prophylactic) CRRM in cisgender females, and 64.6% (n = 2,692) were for treatment of gynecomastia (GM) in cisgender males.
Fig. 1.
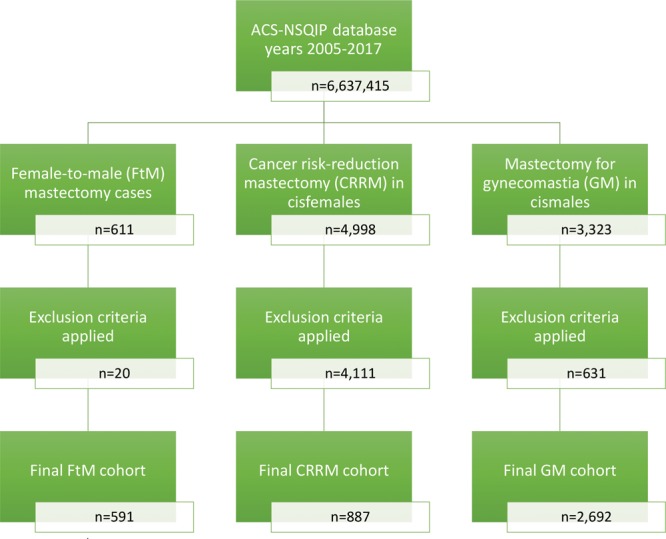
Data extraction strategy.
Patient Demographics and Surgical Specialty
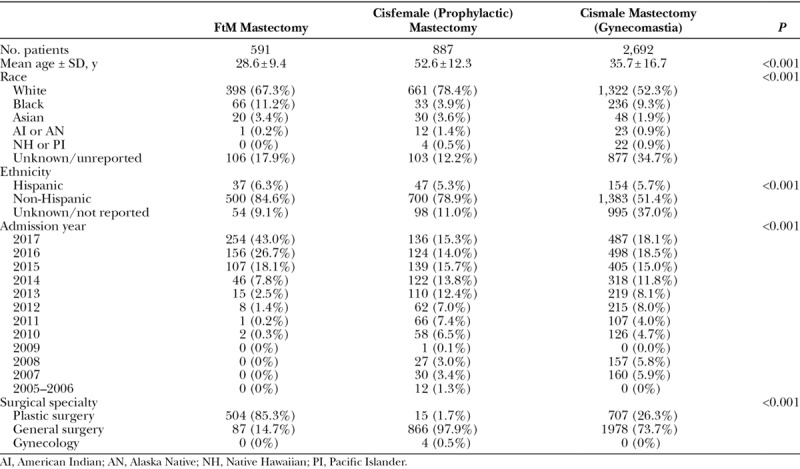
The transmasculine cohort was significantly younger (average age 28.6 ± 9.4 years) than the CRRM (53.2 ± 12.2 years) and GM (35.7 ± 16.7 years) cohorts (P < 0.001). As shown in Figure 2, the average age of patients seeking masculinizing mastectomy significantly decreased from 2010 to 2017 (P = 0.048). The majority of patients in the full study population were white [57.1% (n = 2,381)] and non-Hispanic [61.9% (n = 2,583)]. Plastic surgeons performed the majority of chest masculinization cases [85.3% (n = 504)], whereas general surgery was the predominant specialty for CRRM [97.9% (n = 866)] and GM [73.7% (n = 1,978)] cases (P < 0.001). Demographic characteristics for each cohort are summarized in Table 3.
Fig. 2.
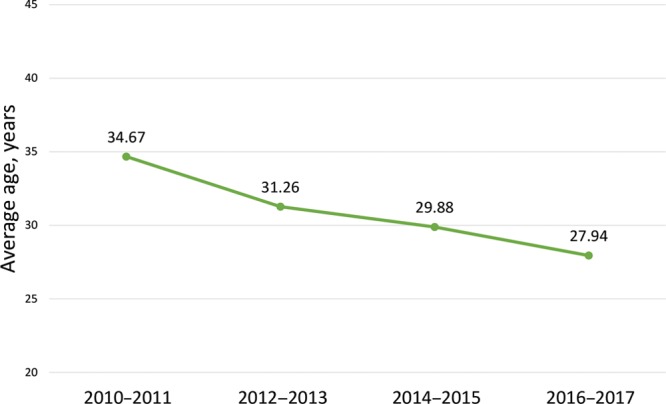
Average age of patients undergoing mastectomy for female-to-male chest reconstruction from 2010–2017.
Table 3.
Demographic Characteristics
Comorbidities and Operative Characteristics
Mean body mass index (BMI) was significantly different between the 3 cohorts (Table 4), with GM patients having the lowest average BMI (28.0 ± 5.3 kg/m2), followed by the transmasculine (28.6 ± 7.0 kg/m2) and CRRM (30.5 ± 8.1 kg/m2) cohorts (P < 0.001). The distribution of ASA physical status classification, a subjective assessment of a patients’ physiological functioning before surgery,24 was significantly different between the 3 cohorts (P < 0.001), but the majority of patients in all groups were ASA class 2 or greater. Patients in the CRRM cohort had the highest rates of diabetes [8.6% (n = 76)], hypertension [28.4% (n = 252)], and steroid use [1.8% (n = 16)]. Smoking was most prevalent in the transmasculine cohort [19.1% (n = 113), P < 0.001].
Table 4.
Comorbidities and Intraoperative Characteristics
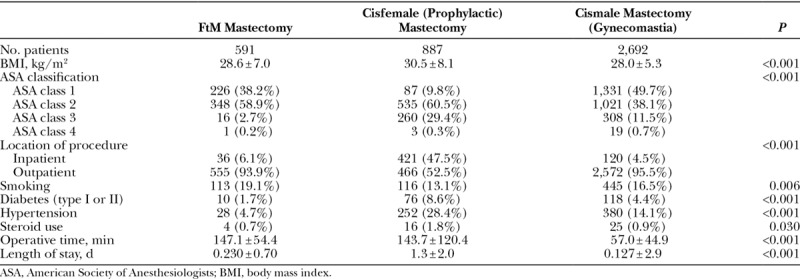
The vast majority of transmasculine [93.9% (n = 555)] and GM [95.5% (n = 2,572)] cases were performed in the outpatient setting, whereas 47.5% (n = 421) of risk-reduction mastectomies were inpatient procedures (P < 0.001). Operative time was significantly shorter in the GM cohort (57.0 ± 44.9 minutes) compared with the transmasculine (147.1 ± 54.4 minutes) and CRRM (143.7 ± 120.4 minutes) cohorts (P < 0.001).
Postoperative Complications and Multivariable Regression
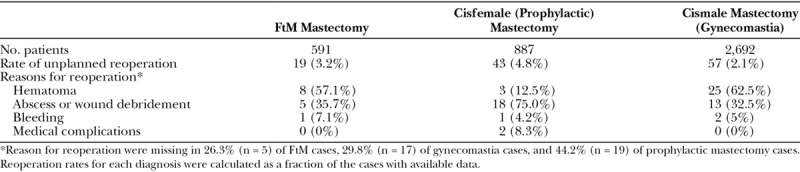
Overall, 220 (5.3%) patients experienced at least 1 all-cause complication, with the highest rate noted in the CRRM cohort [10.4% (n = 92), P < 0.001]. The CRRM cohort also had the highest rates of wound [6.3% (n = 56), P < 0.001] and mild systemic [5.4% (n = 48), P < 0.001] complications (Table 5). Unplanned reoperation was the most common complication in the transmasculine [3.2% (n = 19)] and GM [2.1% (n = 57)] cohorts, although these rates were lower than those seen in the CRRM cohort [4.8% (n = 43)]. In comparison, wound complications [6.3% (n = 56)] were the most common adverse event in the CRRM cohort, followed by mild systemic complications [5.4% (n = 48)]. Table 6 summarizes the reasons for reoperation in each of the 3 cohorts.
Table 6.
Reason for Reoperation
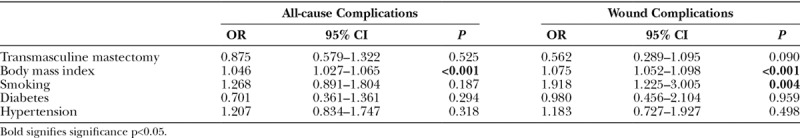
To control for confounders, a multivariable binary regression analysis was performed (Table 7). BMI was identified as an independent risk factor for all-cause (OR 1.046, P < 0.001) and wound (OR 1.075, P < 0.001) complications. Smoking status was also associated with an increased risk of wound complications (OR 1.918, P = 0.004). Being in the transmasculine cohort did not increase patient risk for all-cause (OR 0.875, P = 0.525) or wound (OR 0.562, P = 0.090) complications in the multivariate analysis.
Table 7.
Multivariable Regression Analysis
DISCUSSION
Gender dysphoria is associated with significant psychosocial, health-related, and fiscal burdens.19 The degree of psychological comorbidity in this population is difficult to understate, with rates of depression reported at 2–3.6 times that of the general population and rates of suicide attempts up to 25 times as high.19,25 Furthermore, compared with national averages, transgender individuals have double the rates of unemployment and homelessness, triple the rates of illicit drug use, and four times the rate of HIV infection.19,26 Moreover, there is a considerable financial burden unique to the healthcare needs of this population, as evidenced by costly hormonal and surgical therapies, and the medical and behavioral health appointments needed to access them.27
Clinically significant reductions in psychopathology and substance abuse following GAS have been documented in the literature,28,29 including studies specific to masculinizing mastectomy.6,8 As such, continued investigation into the safety, efficacy, and epidemiology of this procedure is essential to facilitate the development of optimal and ethical management strategies. However, there are numerous challenges associated with research into this population and procedure. To begin, the first case series of masculinizing mastectomy was not published until 1995;30 despite the recent increase in similar studies, the vast majority are single institution in nature.5,11,12,14,30,31 Furthermore, these projects are largely related to technique and aesthetic outcome, with fewer reporting on epidemiology and complication rates. In contrast, there is an abundance of literature pertaining to mastectomy for indications other than masculinizing chest reconstruction, including cancer prophylaxis20 and correction of gynecomastia.21 To address this aim, we have used the ACS NSQIP database to provide an assessment of nationally reported epidemiologic characteristics and postoperative complication rates of mastectomies performed for FtM chest contouring when compared with mastectomies performed for CRRM and surgical correction of gynecomastia.
Recent improvements in social acceptance, legislative regulations,32 and depth of the transgender workforce providing affirmative care have resulted in a number of important epidemiologic changes, which were also present in our analysis. Increased number of masculinizing mastectomies were noted for each year in our dataset. Similarly, annual procedural statistics from the American Society of Plastic Surgeons noted a 328% increase in transmasculine procedures between 2015 and 2017.33,34 Furthermore, Lane et al. analyzed trends in GAS between 2009 and 2015 and identified mastectomy as the most common operation performed for gender affirmation.35
Between 2010 and 2017, the average age of transmasculine patients in our study consistently decreased, which likely reflects both expanded access to care and an increased awareness of gender dysphoria in younger populations. A recent study published in JAMA Pediatrics found significant improvements in body satisfaction following transmasculine chest reconstruction in patients aged 13–25 years, further highlighting the ongoing changes in transgender patient management.6
Surgical approaches to chest masculinization by means of mastectomy can be dichotomized into those historically used for cosmetic, ablative, and reconstructive breast surgery,10,36 and those more commonly employed in the correction of gynecomastia,13,15,37 albeit with technical modifications reflecting the inherently different goals. A 2018 review of transgender chest surgery described the anatomical considerations of mastectomy in the transgender male patient as “virtually identical” to that of the prophylactic mastectomy in cisgender females, while also noting the significant overlap with techniques used for gynecomastia treatment.38 Colić and Colić published a retrospective series detailing their experience with a circumareolar mastectomy technique used in both masculinizing chest reconstruction and gynecomastia treatment.37
Although exogenous testosterone is not expected to contribute to surgical risk,39 juxtaposition of the transmasculine subjects with 2 different control cohorts that approximate either the natal sex characteristics or the new hormonal environment allows for an interesting comparison of postoperative outcomes. Overall, the results of this study illustrate that transmasculine mastectomy is a safe procedure, with an all-cause complication rate of 4.7%, and a similar risk profile to mastectomy in cisgender men and women after adjusting for differences in demographic characteristics and comorbid risk factors. This is consistent with the findings from prior studies, including a systematic review of masculinizing chest reconstruction by Wilson et al., which noted rates of acute complications ranging from 2.1% to 9.2%.10,40
Unplanned return to the operating room was the most common complication in our transmasculine cohort. Overall, reoperation was noted in 3.2% of masculinizing mastectomy cases, most commonly due to hematoma formation (1.5% of all cases). In comparison, only 0.4% of CRRM and 0.9% of GM patients experienced a hematoma within the 30-day postoperative period. Of note, a 2018 Continuing Medical Education article on various techniques in masculinizing chest reconstruction reported rates of hematoma formation in masculinizing chest reconstruction that ranged between 4.5% and 33%.41 It is possible that, given larger numbers, this difference in rates would be statistically, if not necessarily clinically, significant. If so, it may be due to the differences in technique used for transmasculine chest reconstruction. Numerous authors have suggested that the risk of hematoma is increased with techniques that offer poor exposure, such as the limited incision semicircular or transareolar approaches.10,13,17
Postoperative outcomes were also favorable in the gynecomastia cohort, with an overall complication rate of 3.7%. These rates were lower than expected based on several retrospective studies which reported postoperative complication rates between 1.9% and 33%.42–47 In contrast, postoperative complication rates were considerably higher in the prophylactic mastectomy cohort, with 10.4% of subjects experiencing at least 1 all-cause complication. These results are consistent with a recent Cochrane systematic review of risk-reducing mastectomy, which reported postoperative complication rates of 4%–22% among those that did not undergo postmastectomy reconstruction.20 Given that CRRM is directly related to the amount of tissue removed during mastectomy,48 the express goal of mastectomy in this population is the removal of as much breast tissue as possible. Aggressive dissection and removal of breast tissue may jeopardize the mastectomy skin flaps and could explain the higher complication rate in this cohort.
As expected, BMI and smoking were identified as independent predictors of postoperative complications in our study. The association between BMI and postoperative complications has been extensively documented, including studies on mastectomy for transgender chest reconstruction,30,41 cancer prophylaxis,49 and gynecomastia.44 Smoking has also been shown to adversely impact outcomes following risk-reduction mastectomy.20
Importantly, after controlling for confounding variables, transmasculine mastectomy did not have an increased risk of complications compared with the other 2 cohorts. Thus, despite a potentially challenging learning curve, this study shows that surgeons are able to perform masculinizing chest surgery safely, and that the transgender patient is not at an increased risk of complications when compared with cisgender patients undergoing the same procedure. That said, although the nature of the ACS NSQIP dataset effectively precludes an assessment of revisions due to poor cosmesis, it is an important consideration when evaluating postoperative outcomes in this population. Data from retrospective studies show that secondary aesthetic revisions occur in 9%–40.4% of cases.12
Despite the advantages of a robust, multi-institute dataset, there are important limitations associated with the ACS NSQIP database. To begin, evaluation of a given procedure depends on the precision with which the CPT code is defined. In this study, inherent limitations in the rigorousness of CPT coding prohibits the granularity necessary to more thoroughly assess surgical technique. Furthermore, all studies utilizing the ACS NSQIP database are bound by the variables contained within the dataset. Therefore, evaluation of aesthetic and patient-reported outcomes is not possible in this setting. Additionally, postoperative outcomes are only collected for a period of 30 days and thus fail to capture potential long-term complications. Other limitations include the inability to assess perioperative medication use, such as hormone replacement therapy, which is particularly relevant in this study. Furthermore, data entry is susceptible to human error, and reporting practices likely differ between and even within institutions. Finally, it should be noted that the number and composition of hospitals enrolled in the ACS NSQIP often changes from year-to-year, and in the absence of statistical weighting of the dataset, trend analyses should not be extrapolated onto a population level.
Nonetheless, this study benchmarks the epidemiologic characteristics of patients undergoing transmasculine mastectomy nationwide and provides context for assessing the complication profile of this procedure in comparison with other, more common indications for mastectomy. Further research is needed to correlate this data with aesthetic and patient-reported outcomes data. Other important future directions for this study include a thorough assessment of the socioeconomic, geographic, and financial aspects of mastectomy in the transgender population.
CONCLUSIONS
Mastectomy is an integral component in the management of gender dysphoria in transgender males. This study suggests that mastectomy is a safe procedure overall, as evidenced by low rates of postoperative complications and readmissions. When compared with cisgender male and female counterparts undergoing mastectomy for other indications, transgender males were not at an increased risk of adverse outcomes. These favorable results should encourage surgeons to expand their offerings to transgender patients and reassure them as to the safety of chest masculinization as a component of gender affirmation.
Footnotes
Published online 12 June 2019.
Portions of this work will be presented as an oral podium presentation at the New England Society of Plastic and Reconstructive Surgeons Annual Meeting in Newport, R.I., May 31, 2019–June 2, 2019.
The ACS NSQIP databases are the source of information used in this study. Data extrapolated, statistical analysis performed, and conclusions reached have not been verified by the ACS NSQIP but rather are the result of the work done by the authors of this study.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Coleman E, Bockting W, Botzer M, et al. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int J Transgend. 2012;13:165–232. [Google Scholar]
- 2.Hughto JMW, Reisner SL, Pachankis JE. Transgender stigma and health: a critical review of stigma determinants, mechanisms, and interventions. Soc Sci Med. 2015;147:222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winter S, Diamond M, Green J, et al. Transgender people: health at the margins of society. Lancet. 2016;388:390–400. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Gender dysphoria. Diagnostic and Statistical Manual of Mental Disorders. 2013Washington, DC: American Psychiatric Publishing. [Google Scholar]
- 5.McEvenue G, Xu FZ, Cai R, et al. Female-to-male gender affirming top surgery: a single surgeon’s 15-year retrospective review and treatment algorithm. Aesthet Surg J. 2017;38:49–57. [DOI] [PubMed] [Google Scholar]
- 6.Olson-Kennedy J, Warus J, Okonta V, et al. Chest reconstruction and chest dysphoria in transmasculine minors and young adults: comparisons of nonsurgical and postsurgical cohorts. JAMA Pediatr. 2018;172:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal CA, Scheefer MF, Wright LN, et al. Quality of life improvement after chest wall masculinization in female-to-male transgender patients: a prospective study using the BREAST-Q and Body Uneasiness Test. J Plast Reconstr Aesthet Surg. 2018;71:651–657. [DOI] [PubMed] [Google Scholar]
- 8.van de Grift TC, Elfering L, Greijdanus M, et al. Subcutaneous mastectomy improves satisfaction with body and psychosocial function in trans men: findings of a cross-sectional study using the BODY-Q chest module. Plast Reconstr Surg 2018;142:1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Society of Plastic Surgeons. Gender confirmation surgeries rise 20% in first ever report [press releases]. 2017ASPS. [Google Scholar]
- 10.Wilson SC, Morrison SD, Anzai L, et al. Masculinizing top surgery: a systematic review of techniques and outcomes. Ann Plast Surg. 2018;80:679–683. [DOI] [PubMed] [Google Scholar]
- 11.Frederick MJ, Berhanu AE, Bartlett R. Chest surgery in female to male transgender individuals. Ann Plast Surg. 2017;78:249–253. [DOI] [PubMed] [Google Scholar]
- 12.Kääriäinen M, Salonen K, Helminen M, et al. Chest-wall contouring surgery in female-to-male transgender patients: a one-center retrospective analysis of applied surgical techniques and results. Scand J Surg. 2017;106:74–79. [DOI] [PubMed] [Google Scholar]
- 13.Monstrey S, Selvaggi G, Ceulemans P, et al. Chest-wall contouring surgery in female-to-male transsexuals: a new algorithm. Plast Reconstr Surg. 2008;121:849–859. [DOI] [PubMed] [Google Scholar]
- 14.Berry MG, Curtis R, Davies D. Female-to-male transgender chest reconstruction: a large consecutive, single-surgeon experience. J Plast Reconstr Aesthet Surg. 2012;65:711–719. [DOI] [PubMed] [Google Scholar]
- 15.Knox ADC, Ho AL, Leung L, et al. A Review of 101 consecutive subcutaneous mastectomies and male chest contouring using the concentric circular and free nipple graft techniques in female-to-male transgender patients. Plast Reconstr Surg. 2017;139:1260e–1272e. [DOI] [PubMed] [Google Scholar]
- 16.Tong W, Guinness R, Simonds R, et al. A review of our experience on gender affirmation top surgeries. Plas Reconstr Surg Glob Open 2018;6(8S):170–171. [Google Scholar]
- 17.Cregten-Escobar P, Bouman MB, Buncamper ME, et al. Subcutaneous mastectomy in female-to-male transsexuals: a retrospective cohort-analysis of 202 patients. J Sex Med. 2012;9:3148–3153. [DOI] [PubMed] [Google Scholar]
- 18.Flores AR, Brown TN, Herman J. Race and Ethnicity of Adults Who Identify as Transgender in the United States. 2016Los Angeles, CA: Williams Institute, UCLA School of Law. [Google Scholar]
- 19.James SE, Herman J. The report of the 2015 US transgender survey: executive summary. 2017Washington, DC: National Center For Transgender Equality. [Google Scholar]
- 20.Carbine NE, Lostumbo L, Wallace J, et al. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst Rev. 2018;4:CD002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waltho D, Hatchell A, Thoma A. Gynecomastia classification for surgical management: a systematic review and novel classification system. Plast Reconstr Surg. 2017;139:638e–648e. [DOI] [PubMed] [Google Scholar]
- 22.ACS NYSQIP User guide for the 2012 ACS NSQIP procedure targeted participant use data file (PUF). 2017
- 23.Birkmeyer JD, Shahian DM, Dimick JB, et al. Blueprint for a new American College of Surgeons: National Surgical Quality Improvement Program. J Am Coll Surg. 2008;207:777–782. [DOI] [PubMed] [Google Scholar]
- 24.Doyle DJ, Garmon EH. American Society of Anesthesiologists Classification (ASA Class). In: StatPearls. 2018Treasure Island, FL: StatPearls Publishing LLC. [PubMed] [Google Scholar]
- 25.McDowell MJ, Hughto JMW, Reisner SL. Risk and protective factors for mental health morbidity in a community sample of female-to-male trans-masculine adults. BMC Psychiatry. 2019;19:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant JM, Mottet LA, Tanis J, et al. Injustice at Every Turn: A Report of the National Transgender Discrimination Survey. 2011Washington: National Center for Transgender Equality and National Gay and Lesbian Task Force. [Google Scholar]
- 27.Padula WV, Heru S, Campbell JD. Societal implications of health insurance coverage for medically necessary services in the U.S. transgender population: a cost-effectiveness analysis. J Gen Intern Med. 2016;31:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heylens G, Verroken C, De Cock S, et al. Effects of different steps in gender reassignment therapy on psychopathology: a prospective study of persons with a gender identity disorder. J Sex Med. 2014;11:119–126. [DOI] [PubMed] [Google Scholar]
- 29.Murad MH, Elamin MB, Garcia MZ, et al. Hormonal therapy and sex reassignment: a systematic review and meta-analysis of quality of life and psychosocial outcomes. Clin Endocrinol (Oxf). 2010;72:214–231. [DOI] [PubMed] [Google Scholar]
- 30.Hage JJ, van Kesteren PJ. Chest-wall contouring in female-to-male transsexuals: basic considerations and review of the literature. Plast Reconstr Surg. 1995;96:386–391. [DOI] [PubMed] [Google Scholar]
- 31.Lo Russo G, Tanini S, Innocenti M. Masculine chest-wall contouring in FtM transgender: a personal approach. Aesthetic Plast Surg. 2017;41:369–374. [DOI] [PubMed] [Google Scholar]
- 32.Department of Health and Human Services. Transsexual Surgery, Docket No. A-13–87, Decision No. 2576. 2014Washington, DC: Departmental Appeals Board Appellate Division. [Google Scholar]
- 33.American Society of Plastic Surgeons. 2017 Plastic surgery statistics report. Available at https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-full-report-2017.pdf. Accessed January 5, 2019.
- 34.American Society of Plastic Surgeons. 2016 Plastic surgery statistics report. Available at https://www.plasticsurgery.org/documents/News/Statistics/2016/plastic-surgery-statistics-full-report-2016.pdf. Accessed January 5, 2019.
- 35.Lane M, Ives GC, Sluiter EC, et al. Trends in gender-affirming surgery in insured patients in the United States. Plast Reconstr Surg Glob Open. 2018;6:e1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Top H, Balta S. Transsexual mastectomy: selection of appropriate technique according to breast characteristics. Balkan Med J. 2017;34:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colić MM, Colić MM. Circumareolar mastectomy in female-to-male transsexuals and large gynecomastias: a personal approach. Aesthetic Plast Surg. 2000;24:450–454. [DOI] [PubMed] [Google Scholar]
- 38.Claes KEY, D’Arpa S, Monstrey SJ. Chest surgery for transgender and gender nonconforming individuals. Clin Plast Surg. 2018;45:369–380. [DOI] [PubMed] [Google Scholar]
- 39.Boskey ER, Taghinia AH, Ganor O. Association of surgical risk with exogenous hormone use in transgender patients: a systematic review. JAMA Surg. 2019154159–169 [DOI] [PubMed] [Google Scholar]
- 40.Bjerrome Ahlin H, Kölby L, Elander A, et al. Improved results after implementation of the Ghent algorithm for subcutaneous mastectomy in female-to-male transsexuals. J Plast Surg Hand Surg. 2014;48:362–367. [DOI] [PubMed] [Google Scholar]
- 41.Ammari T, Sluiter EC, Gast K, et al. Female-to-male gender-affirming chest reconstruction surgery. Aesthet Surg J. 2019;39:150–163. [DOI] [PubMed] [Google Scholar]
- 42.Zavlin D, Jubbal KT, Friedman JD, et al. Complications and outcomes after gynecomastia surgery: analysis of 204 pediatric and 1583 adult cases from a National Multi-center Database. Aesthetic Plast Surg. 2017;41:761–767. [DOI] [PubMed] [Google Scholar]
- 43.Steele SR, Martin MJ, Place RJ. Gynecomastia: complications of the subcutaneous mastectomy. Am Surg. 2002;68:210–213. [PubMed] [Google Scholar]
- 44.Handschin AE, Bietry D, Hüsler R, et al. Surgical management of gynecomastia–a 10-year analysis. World J Surg. 2008;32:38–44. [DOI] [PubMed] [Google Scholar]
- 45.Kasielska A, Antoszewski B. Surgical management of gynecomastia: an outcome analysis. Ann Plast Surg. 2013;71:471–475. [DOI] [PubMed] [Google Scholar]
- 46.Brafa A, Campana M, Grimaldi L, et al. Management of gynecomastia: an outcome analysis in a multicentric study. Minerva Chir. 2011;66:375–384. [PubMed] [Google Scholar]
- 47.Gioffrè Florio MA, Alfio AR, Famà F, et al. [Evaluation of complications and long-term results after surgery for gynaecomastia]. Chir Ital. 2004;56:113–116. [PubMed] [Google Scholar]
- 48.Brinton LA, Persson I, Boice JD, Jr, et al. Breast cancer risk in relation to amount of tissue removed during breast reduction operations in Sweden. Cancer. 2001;91:478–483. [PubMed] [Google Scholar]
- 49.Osman F, Saleh F, Jackson TD, et al. Increased postoperative complications in bilateral mastectomy patients compared to unilateral mastectomy: an analysis of the NSQIP database. Ann Surg Oncol. 2013;20:3212–3217. [DOI] [PubMed] [Google Scholar]


