Abstract
Background:
The Stemmer sign is a physical examination finding used to diagnose lymphedema. If the examiner cannot pinch the skin of the dorsum of the foot or hand then this positive finding is associated with lymphedema. The purpose of the study was to determine the accuracy of the Stemmer sign to predict lymphedema.
Methods:
All patients referred to our Lymphedema Program between 2016 and 2018 were tested for the Stemmer sign and underwent lymphoscintigraphy to define the patient’s lymphatic function. Patient age, lymphedema type (primary and secondary), disease location (arm and leg), lymphoscintigraphy findings, stage, severity, and body mass index were recorded. Comparison of predictive variables and Stemmer sign result was performed using Fisher’s exact test and Student’s t test.
Results:
One hundred ten patients were studied: patients with a positive Stemmer sign (n = 87) exhibited abnormal (n = 80) or normal (n = 7) lymphatic function by lymphoscintigraphy (sensitivity = 92%). False-positive Stemmer signs included individuals with obesity (n = 6) or spinal muscle atrophy (n = 1). Subjects with a negative Stemmer sign (n = 23) had normal (n = 13) or abnormal (n = 10) lymphatic function by imaging (specificity = 57%). Patients with a false-negative Stemmer sign were more likely to have a normal body mass index (P = 0.02) and Stage 1 disease (P = 0.01).
Conclusions:
A positive Stemmer sign is a sensitive predictor for primary and secondary lymphedema of the arms or legs and, thus, is a useful part of the physical examination. Because the test exhibits moderate specificity, lymphoscintigraphy should be considered for patients with a high suspicion of lymphedema that have a negative Stemmer sign.
INTRODUCTION
In 1976, Stemmer described the inability to pinch the skin of the proximal phalanx of the second or third toe in patients with lymphedema.1 If the examiner is unable to grab the dorsal skin between his/her thumb and index finger, then the “Stemmer sign” is positive suggesting lymphedema.2 Since the description of the Stemmer sign, lymphoscintigraphy has become the gold-standard diagnostic test for lymphedema.3–5 It is unclear whether the Stemmer sign accurately predicts lymphedema and should be used as part of the physical examination. The purpose of this study was to determine the sensitivity and specificity of the Stemmer sign for lymphedema by comparing it with the patient’s lymphatic function by lymphoscintigraphy.
METHODS
Patients referred to our Lymphedema Program between 2016 and 2018 were studied. All individuals were tested for the Stemmer sign by the senior author and underwent lymphoscintigraphy to document the patient’s lymphatic function. Subjects were diagnosed with lymphedema based on their lymphoscintigram result, which is 96% sensitive and 100% specific for lymphedema.5 Abnormal lymphoscintigraphy findings confirming lymphedema were delayed transit of radiolabeled tracer to inguinal or axillary lymph nodes or dermal backflow.3–5 Patient age, sex, lymphedema type (primary and secondary), disease location (arm and leg), body mass index (BMI), lymphoscintigram findings (delayed transit of radiolabeled colloid to axillary or inguinal nodes and presence of dermal backflow of tracer), lymphedema stage, and severity were recorded. Lymphedema stage and clinical severity were documented based on the International Society of Lymphology criteria.6 Stage 0 corresponds to abnormal lymphatic function before the appearance of clinical swelling; stage 1 corresponds to early edema which improves with elevation; stage 2 corresponds to pitting edema that does not resolve with elevation; and stage 3 corresponds to fibroadipose deposition and skin changes. Severity was defined by the increase in limb volume: mild <20%, moderate 20%–40%, and severe >40%.6 Analysis of the data was performed using Fisher’s exact test and Student’s t test (Stata version 15.0, StataCorp, College Station, Tex.). All 2-tailed P < 0.05 were considered statistically significant.
RESULTS
In total, 110 patients were included; 74 females and 36 males. Mean age was 35 years (range 1–88 years). Edema location included the lower extremity (n = 90) or upper limb (n = 20). Eighty-seven patients had a positive Stemmer sign and exhibited abnormal (n = 80) or normal (n = 7) lymphatic function by lymphoscintigraphy (sensitivity = 92%; Fig. 1). False-positive Stemmer signs included individuals with obesity (n = 6) or spinal muscle atrophy [n = 1; mean BMI 45 (range 23–70); Table 1, Fig. 2). Individuals with a negative Stemmer sign (n = 23) had normal (n = 13) or abnormal (n = 10) lymphatic function by imaging (specificity = 57%; Table 2, Fig. 3). Subjects with a false-negative Stemmer sign were likely to have a normal BMI [mean BMI 24 (range 16–34)] compared to the individuals with a true-positive test [mean BMI 38 (range 19–90)], P = 0.02.
Fig. 1.
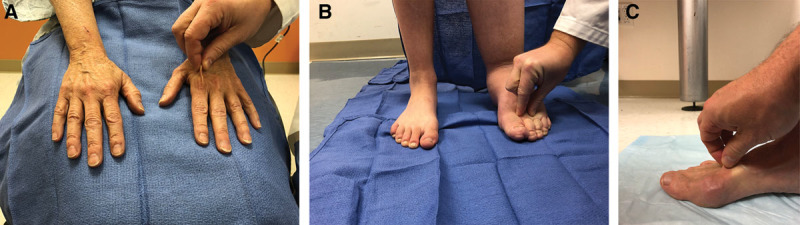
Stemmer sign result examples. A, True-negative Stemmer sign (examiner is able to pinch the skin in a patient without lymphedema). B, True-positive Stemmer sign (skin is unable to be pinched in an individual with lymphedema). C, False-negative Stemmer sign (the skin is able to be pinched in a subject with lymphedema).
Table 1.
Patients with a False-positive Stemmer Sign
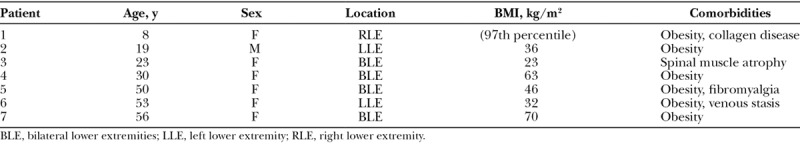
Fig. 2.
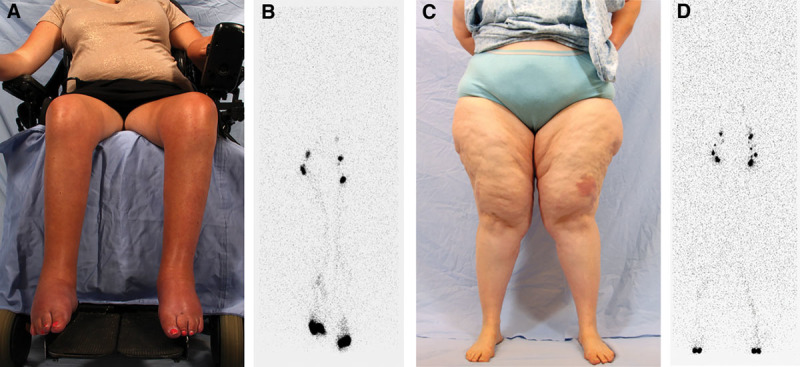
Patients with a false-positive Stemmer sign. A and B, 23-year-old nonambulatory woman with spinal muscle atrophy and bilateral lower extremity edema (Table 1, patient #3). C and D, 50-year-old woman with a BMI of 46 (Table 1, patient #5). Both patients exhibit normal lymphatic function on their 45-minute lymphoscintigram image (normal transit of radiolabeled tracer to the inguinal nodes without dermal backflow).
Table 2.
Patients with a False-negative Stemmer Sign
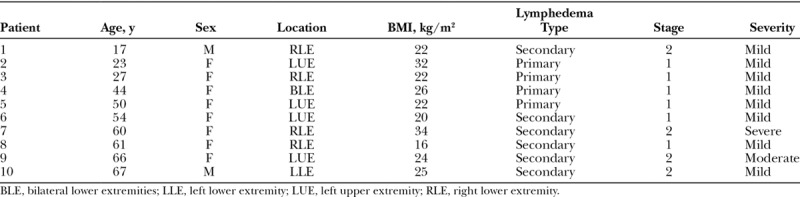
Fig. 3.

Patients with a false-negative Stemmer sign. A and B, 50-year-old woman with primary left upper extremity lymphedema (BMI, 22). Her lymphoscintigram image at 45 minutes shows reduced flow of radiolabeled tracer to her left axillary nodes (Table 2, patient #5). C and D, 61-year-old woman with a secondary right lower extremity lymphedema (BMI 16; Table 2, patient #8). Her lymphoscintigram image at 45 minutes illustrates absence of right inguinal node uptake of radiolabeled tracer and dermal backflow.
Analysis of patients with lymphedema confirmed by lymphoscintigraphy (n = 90 total; 84 with stage/severity data) showed that Stemmer sign outcome (positive or negative) was not associated with lymphedema type (59 primary and 31 secondary), location (leg 75 and arm 15), severity (mild 34, moderate 20, and severe 30), or lymphoscintigram result (delayed transit 80 and dermal backflow 40); P = 0.06–0.50. Patients with stage 1 lymphedema (n = 14) were more likely to have a false-negative Stemmer sign compared to individuals with stage 2 (n = 56; P = 0.01) or stage 3 (n = 14; P = 0.04) disease.
DISCUSSION
One fourth of patients referred to a Lymphedema Program with “lymphedema” do not have the condition.7,8 “Lymphedema” often is used as a generic term to describe limb overgrowth regardless of the underlying etiology. Lymphedema usually can be diagnosed based on the patient’s medical history and physical examination. The Stemmer sign was originally described to differentiate lower extremity lymphedema from other causes of swelling.1,9 Stemmer correlated the inability to pinch the skin over the proximal second or third toe with patients who also exhibited abnormal resorption and backflow after the injection of patent blue dye.1
Lymphedema generally affects the distal extremity and the senior author has used the Stemmer sign to help differentiate lymphedema from other conditions affecting not only the lower limb, but also the upper extremity. Although Stemmer described pinching the dorsal skin over the proximal phalanx of the second toe, the senior author pinches the skin immediately proximal to the metatarsophalangeal joint because it is technically easier to perform, especially in the pediatric population. In addition, the senior author also translates the test to the upper extremity pinching the skin proximal to the metacarpalphalangeal joint of the index finger. The skin is pinched between the examiner’s index finger and thumb, rather than using a forceps, to reduce the risk of pain and skin injury. The upper extremity test is performed with the patient sitting up and their hands resting on their proximal thighs, whereas the lower extremity is examined while the patient is standing.
False-negative and false-positive findings have been observed by the senior author when compared with the patient’s medical history and lymphoscintigram findings. False-negative exams have been reported in the literature as well.10 We aimed to determine the accuracy of the Stemmer sign for lymphedema to determine if the test should continue to be used in clinical practice. The pathophysiology that prevents the pinching of the dorsal skin of the extremity in patients with lymphedema likely is thickened skin and excess subcutaneous fibroadipose tissue with edema. Lymphedema results in the accumulation of high-protein fluid in the subcutaneous tissues. This fluid causes inflammation, adipose deposition, and fibrosis.11–13 In contrast, other causes of swelling or limb overgrowth do not result in enough inflammatory fibroadipose formation to prevent the pinching of the dorsal skin of the hand or foot: for example, venous stasis, heart disease, liver failure, renal insufficiency, rheumatologic disease, lipedema, hemihypertrophy, posttraumatic swelling, and vascular anomalies.
BMI was associated with both false-negative and false-positive Stemmer signs. Patients with lymphedema and a normal or below normal BMI could exhibit minimal swelling and a normal sign, whereas obese patients without lymphedema could have a positive sign. Obesity negatively affects lymphatic function by causing inflammation, fibrosis, and destruction of lymphatics.14–16 Consequently, normal weighted individuals would have less inflammation of their distal extremity causing skin thickening, fibrosis, and edema. Obese patients with greater subcutaneous adipose, in contrast, would be more likely to have inflammation, edema, and thicker skin/subcutis leading to a positive sign.
Our results show that the Stemmer sign has a sensitivity of 92% to predict lymphedema in patients who have the disease and a specificity of 57% to exclude lymphedema in patients who do not have the condition. Thus, we conclude that the test is a useful component of the physical examination in patients with suspected lymphedema. It is easy to perform and adds minimal effort when also evaluating the patient for pitting edema. Subjects with a positive finding are likely to have lymphedema, although obese individuals can exhibit the sign and have normal lymphatic function. A negative Stemmer sign does not rule out lymphedema, typically in patients with a normal BMI and stage 1 disease.
Although the Stemmer sign is a useful method to differentiate lymphedema from other diseases, we obtain a lymphoscintigram on almost all patients, because the test is more sensitive and specific for the condition. Lymphoscintigraphy is the most accurate method to determine whether a patient has lymphedema if the diagnosis is equivocal. The test also rules out the condition if the clinical suspicion is low, which provides comfort to the patient. We also typically perform the study for patients with a high clinical suspicion of lymphedema because it confirms the diagnosis, provides objective measurement of lymphatic dysfunction, and serves as a baseline evaluation to be compared to if the test is repeated in the future.
ACKNOWLEDGEMENT
The authors would like to thank Steven J. Staffa, MS for his statistical expertise.
Footnotes
Published online 19 June 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Stemmer R. A clinical symptom for the early and differential diagnosis of lymphedema. Vasa. 1976;5:261–262. [PubMed] [Google Scholar]
- 2.Fries R. Differential diagnosis of leg edema. MMW Fortschr Med. 2004;146:39–41. [PubMed] [Google Scholar]
- 3.Gloviczki P, Calcagno D, Schirger A, et al. Noninvasive evaluation of the swollen extremity: experiences with 190 lymphoscintigraphic examinations. J Vasc Surg. 1989;9:683–689; discussion 690. [DOI] [PubMed] [Google Scholar]
- 4.Szuba A, Shin WS, Strauss HW, et al. The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med. 2003;44:43–57. [PubMed] [Google Scholar]
- 5.Hassanein AH, Maclellan RA, Grant FD, et al. Diagnostic accuracy of lymphoscintigraphy for lymphedema and analysis of false-negative tests. Plast Reconstr Surg Glob Open. 2017;5:e1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Executive Committee. The diagnosis and treatment of peripheral lymphedema: 2016 Consensus document of the International Society of Lymphology. Lymphology. 2016;49:170–184. [PubMed] [Google Scholar]
- 7.Schook CC, Mulliken JB, Fishman SJ, et al. Differential diagnosis of lower extremity enlargement in pediatric patients referred with a diagnosis of lymphedema. Plast Reconstr Surg. 2011;127:1571–1581. [DOI] [PubMed] [Google Scholar]
- 8.Maclellan RA, Couto RA, Sullivan JE, et al. Management of primary and secondary lymphedema: analysis of 225 referrals to a center. Ann Plast Surg. 2015;75:197–200. [DOI] [PubMed] [Google Scholar]
- 9.Stemmer RA. Stemmer’s sign: possibilities and limits of clinical diagnosis of lymphedema [in German]. Wien Med Wochenschr. 1999;149:85–86. [PubMed] [Google Scholar]
- 10.Földi M, Földi E, Strößenreuther C. Földi’s Textbook of Lymphology: for Physicians and Lymphedema Therapists. 2012München, Germany: Urban & Fischer. [Google Scholar]
- 11.Brorson H, Ohlin K, Olsson G, et al. Adipose tissue dominates chronic arm lymphedema following breast cancer: an analysis using volume rendered CT images. Lymphat Res Biol. 2006;4:199–210. [DOI] [PubMed] [Google Scholar]
- 12.Ghanta S, Cuzzone DA, Torrisi JS, et al. Regulation of inflammation and fibrosis by macrophages in lymphedema. Am J Physiol Heart Circ Physiol. 2015;308:H1065–H1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ly CL, Kataru RP, Mehrara BJ. Inflammatory manifestations of lymphedema. Int J Mol Sci. 2017;18:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greene AK, Grant FD, Slavin SA. Lower-extremity lymphedema and elevated body-mass index. N Engl J Med. 2012;366:2136–2137. [DOI] [PubMed] [Google Scholar]
- 15.Weitman ES, Aschen SZ, Farias-Eisner G, et al. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One. 2013;8:e70703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savetsky IL, Torrisi JS, Cuzzone DA, et al. Obesity increases inflammation and impairs lymphatic function in a mouse model of lymphedema. Am J Physiol Heart Circ Physiol. 2014;307:H165–H172. [DOI] [PMC free article] [PubMed] [Google Scholar]


