Abstract
Background:
Infantile lip hemangiomas are challenging to manage due to the functional and aesthetic importance of the lips. Hemangiomas in this region may lead to significant complications including ulceration, feeding difficulties, and lip contour distortion requiring surgical intervention.
Methods:
A retrospective chart review of children with focal lip hemangiomas treated at our institution between January 2000 and December 2016 was conducted. Patient demographics, lesion characteristics, complications, treatments, and outcomes were collected. Lesions were classified based on depth (superficial, deep, or mixed depth), vermillion border involvement, and location.
Results:
One hundred and two patients with focal lip hemangiomas were identified; 45.1% were managed expectantly, 43.1% were treated medically, and 18.6% required surgery. Residual lip contour deformity following involution was the most common complication (26.5% of patients). Ulceration during the proliferation phase was reported in 14.7% of patients, leading to significant feeding difficulties in 9.8% of patients. All ulcerations occurred in lesions with a superficial component. None of the patients with superficial lesions underwent surgery; 27.1% of patients with deep or mixed depth hemangiomas required surgical treatment to restore lip contour.
Conclusions:
Lip hemangiomas have high rates of complications that seem to be related to lesion morphology and phase of growth. Ulceration occurs during the early proliferative phase and is most frequently associated with mixed depth hemangiomas. Residual lip contour deformities are identified in the involution phase; presence of a deep component is the primary factor in predicting the need for surgical intervention in these patients.
INTRODUCTION
Infantile hemangiomas are benign vascular tumors that appear shortly after birth and undergo proliferation during the first year of life. Subsequently, growth will typically plateau and enter a prolonged, but often incomplete, involution phase. Although uncomplicated hemangiomas often resolve without intervention, lesions in anatomically sensitive locations require close follow-up and treatment.1
Hemangiomas located on the lips are at high risk of complications that can have significant functional and aesthetic impacts.2 Early ulceration of these lesions can cause pain and bleeding, leading to feeding difficulties for young infants. The lips are important landmarks of the face, and large hemangiomas can cause distortion of the complex lip architecture. If lip contour and symmetry are affected despite involution, surgical intervention may be required.3
It is unclear which patients are at highest risk of complication from lip hemangiomas. To address this question, a retrospective review was completed of all patients with lip hemangiomas at our institution over a 16-year period using a previously developed database.4 The primary objective was to identify patient and lesion factors that were associated with complications and need for surgical intervention.
METHODOLOGY
The Alberta Children’s Hospital multidisciplinary Vascular Birthmark Clinic is one of the busiest such clinics in Canada and includes representatives from plastic surgery, interventional radiology, pediatrics, and nursing.5 Other specialties are available for consultation as needed. A prospectively collected database of children with vascular birthmarks treated at the Alberta Children’s Hospital was used to identify all patients with infantile hemangiomas of the lips. Inclusion criteria: all patients with a diagnosis of focal infantile hemangioma of the lips treated at our institution between January 2000 and December 2016. Exclusion criteria: PHACE syndrome, patients with incomplete charts, and patients treated at other institutions.
Retrospective review of the patient cohort was conducted using both electronic and paper medical records. Photographs taken by the Vascular Birthmark Clinic photographer were available for all patients. The following information was extracted from the records: patient demographic data, characteristics of the hemangioma, treatment received, complications, and clinical outcomes. Hemangiomas were classified by location (upper lip, lower lip, or commissure), depth (superficial, deep, or mixed depth), vermillion border involvement, and oral mucosal involvement. Hemangioma morphology was determined by physical examination in clinic by the attending surgeon. Superficial lesions had cutaneous changes that were visible on inspection (red color, texture, and elevated) but did not have any palpable depth. Mixed lesions had visible cutaneous changes and were felt to have a subcutaneous component on examination. Deep lesions did not have cutaneous changes other than distortion of the surface contour, but did have a deeper palpable mass on examination, and often a bluish discoloration. Size and volume of the lesions could not be accurately assessed because those data were unavailable.
The data were compiled and analyzed using SPSS version 24.0 (IBM Corporation, Armonk, N.Y.). Two-tailed Student’s t tests and χ2 test were used to analyze continuous variables and categorical variables as appropriate.
This study was approved by the University of Calgary Conjoint Health Research Ethics Board.
RESULTS
Patient Demographics and Lesion Characteristics
There were 102 patients with infantile hemangiomas of the lips identified from our database. Complete records (including clinical photographs) were available for all patients. Baseline demographic data and hemangioma physical characteristics for the cohort are shown in Table 1. Females were more likely to have lip hemangiomas at an approximately 3:1 ratio. The vast majority (87.3%) of our patients were of white ethnicity. A family history of a first degree relative with a hemangioma was reported in 17.6% of the patients. Median age at initial presentation to clinic was 4.0 months.
Table 1.
General Demographics of Patient Population and Summary of Physical Characteristics of Documented Focal Infantile Hemangiomas of the Lips
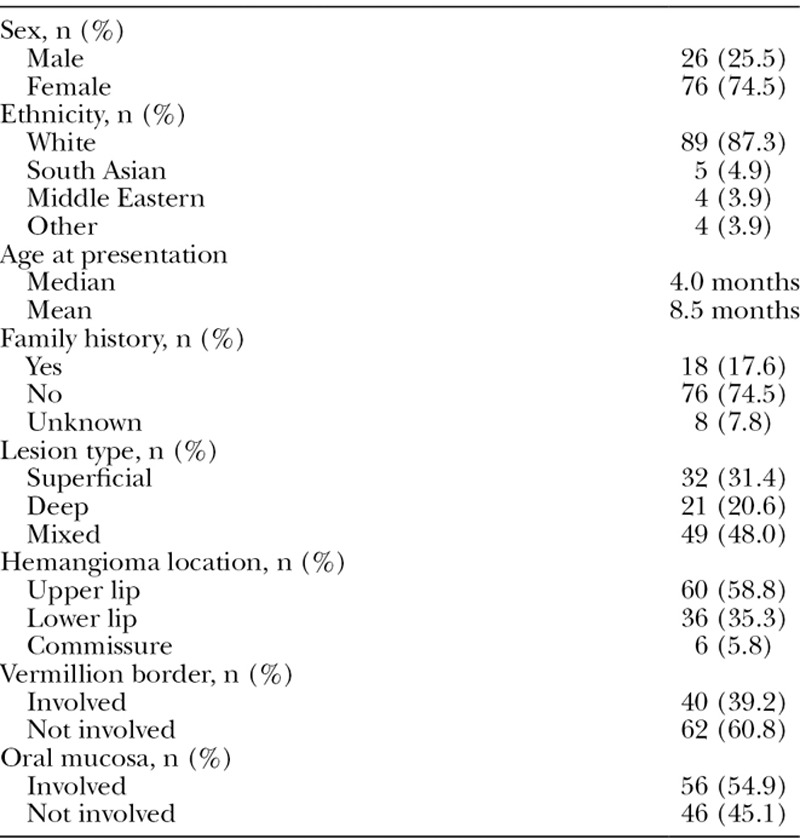
Approximately half (48.0%) of all lesions were of mixed depth (consisting of both a superficial and deep component); 31.4% were exclusively superficial, and 20.6% were exclusively deep (Fig. 1). Hemangiomas were most commonly located on the upper lip (58.8%), as compared to the lower lip (35.3%); a minority was found at the commissure (5.8%). Violation of the vermillion border occurred in 39.2% of cases, and the oral mucosa was involved in 54.9% of cases.
Fig. 1.
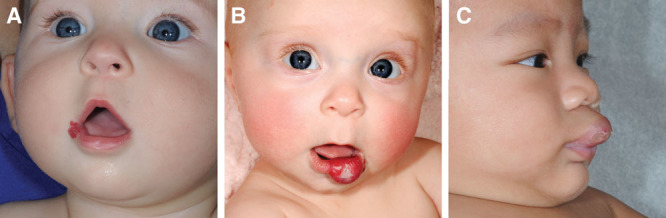
Comparison of 3 types of infantile lip hemangiomas based on depth: (A) superficial; (B) mixed; and (C) deep.
Complications
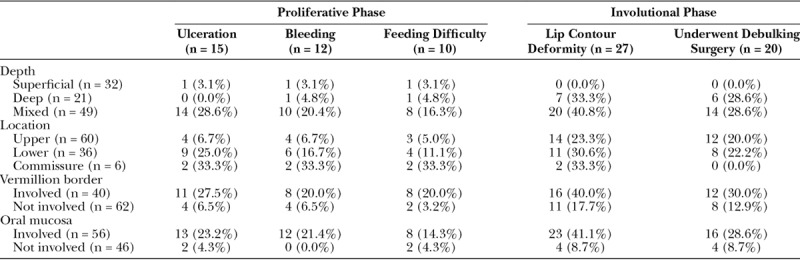
Some form of complication specific to the hemangioma occurred in 31.4% of the 102 patients (Table 2). Complications were analyzed according to the physical characteristics of the hemangioma to identify potential prognostic indicators (Table 3). Complications were divided into those occurring during the proliferative phase and those occurring during the involutional phase.
Table 2.
Summary of Complications Experienced by Patients with Focal Infantile Hemangiomas of the Lips (N = 102)

Table 3.
Comparison of Complications Arising in the Proliferative and Involution Phases and Association with Physical Characteristics of Hemangiomas of the Lip
Complications—Proliferative Phase
During the proliferative phase, the most common complication was ulceration of the hemangioma (14.7% of patients). Median age of ulceration was 6 weeks (range, 2–48 weeks). Fourteen of the 15 cases were treated with conservative wound care; 1 case required urgent operative intervention due to bleeding from the superior labial artery. Four patients with ulceration (26.7%) subsequently went on to require debulking surgery at a later stage. Hemangiomas with a cutaneous component (superficial and mixed types) had a significantly higher rate of ulceration as compared to lesions without a cutaneous component (deep type; 15/81 versus 0/21, respectively). Of the 15 cases that were complicated by ulceration, 14 (93.3%) involved mixed depth lesions (Fig. 2). Lesions that involved the vermillion border also had significantly higher rates of ulceration (11/40 versus 4/62, respectively).
Fig. 2.
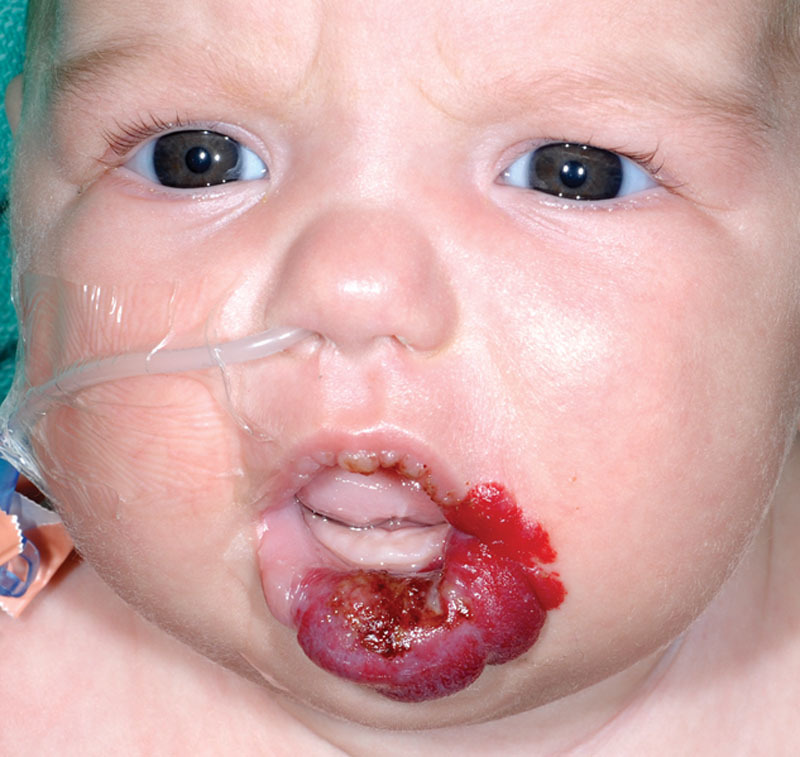
Patient with a mixed depth lip hemangioma, complicated by ulceration.
Ten patients (9.8%) reported feeding difficulties (with breast or bottle feeding), and 12 patients (11.8%) reported bleeding from the lesion; all of these cases involved ulcerated lesions. Two patients with feeding difficulties developed failure to thrive and required admission to hospital for nasogastric tube feeding while the ulcerated lesion healed. No other cases required hospital admission or nasogastric feeding. No patients had documented airway involvement or reported issues with speech delay.
Complications—Involutional Phase
During the involutional phase, the most common complication was residual lip contour deformity as identified by the attending surgeon during clinic follow-up visits (occurring in 26.5% of patients). All of these cases involved noticeable excess tissue bulk and residual fibrofatty tissue at the site of the involuted hemangioma. Residual lip contour deformity was associated with: hemangiomas having a deep component1 (either mixed depth or exclusively deep) and hemangiomas that involved the vermillion border2 (Table 3). No other lesion or patient characteristics were associated with residual lip contour deformity.
Hemangiomas that involved the vermillion border had significantly higher rates of residual lip contour deformity as compared with those that did not cross the vermillion border (40.0% versus 17.7%; χ2 = 6.19, P = 0.013). All patients with residual lip contour deformity had hemangiomas classified as having a deep component (mixed or exclusively deep); none of the patients with exclusively superficial lesions demonstrated this complication. A total of 29 patients had hemangiomas with both vermillion border involvement and a deep component; the majority (55%) of these patients experienced residual lip contour deformity.
Medical Treatment (Proliferative Phase)
A total of 45.1% of patients were treated expectantly with close observation by clinic pediatricians (Table 4). The most common medical treatment was administration of an oral beta-blocker (propranolol or atenolol), used in 37.2% of all cases (used in 51% of cases since 2008, when beta-blockers first became available for treatment of hemangiomas at our institution). Indications for beta-blocker treatment in the proliferative phase included: anatomically sensitive location (100% of cases), concern for growth (55% of cases), age of child (47% of cases), and evidence of ulceration (19% of cases). The median age of beta-blocker initiation was 3.8 months (range, 1–21 months). The average propranolol dose was 1.6 mg/kg/d for a mean duration of 13 months. There were no reported side effects to beta-blocker therapy.
Table 4.
Summary of Treatments Utilized for All Patients with Focal Hemangiomas of the Lips
Although it was not possible to objectively determine the effect of oral beta-blockers on hemangioma size, resolution of ulceration, or impact on cosmetic appearance, the prescribing pediatricians noted improvement in these domains for most patients. However, there was no difference in surgical rates between those treated and not treated with oral beta-blockers (32.1% versus 23.0%; χ2 = 2.27, P = 0.13).
Surgical Intervention (Involutional Phase)
All 27 patients with identified residual lip contour deformity were offered debulking/corrective surgery; 20 patients underwent surgery at some point during the involutional phase. Seven of the 27 patients (25.9%) who were offered surgical intervention for excess tissue bulk declined intervention. Specific reasons for declining surgery were not identified.
Lesion depth was the primary factor associated with the need for surgical intervention. None of the patients with exclusively superficial lesions were taken to the operating room (Fig. 3); however, 28.6% of patients with a deep component to their hemangiomas underwent surgical treatment. All of the patients who declined surgery also had a deep component to their lesion (6 mixed depth hemangiomas, and 1 exclusively deep lesion).
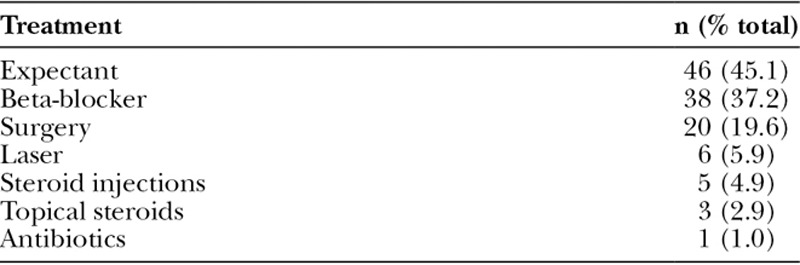
Fig. 3.
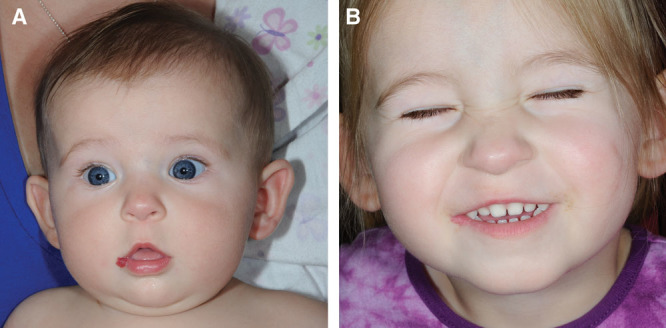
Example of an uncomplicated, superficial depth hemangioma of the lower lip and commissure (A) at 12 months of age and (B) during the involution at the age of 5 years.
All operations occurred during the involutional phase; the median age of first surgery was 52 months (range, 18–150 months). In all but one case, the primary indication for surgery was residual lip contour deformity requiring debulking of excess tissue (the single outlier was an ulcerated lesion that eroded into the labial artery, resulting in arterial bleeding). All surgical patients underwent debulking of the lesion with primary closure through advancement flaps as part of their operation (Fig. 4). Other than the single patient with a bleeding lesion requiring intervention, all operative cases were elective outpatient procedures. Six patients required more than 1 operation. Two patients had very large lesions that required multiple debulking procedures; 2 patients required scar revisions; and 2 patients required dehiscence repairs. The remaining 14 patients underwent a single operation.
Fig. 4.
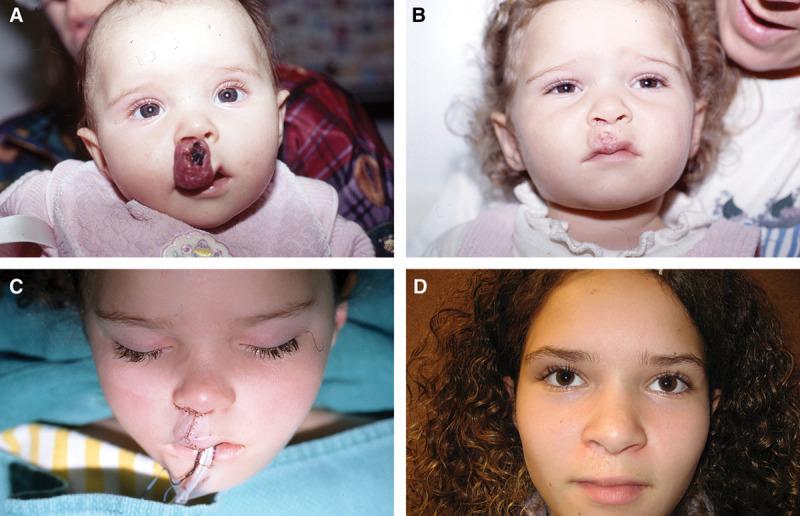
Example of a patient with large, mixed depth infantile hemangioma of the upper lip (A) at maximal proliferation; (B) at maximal involution with residual lip contour deformity; (C) immediately postoperatively from debulking and closure; and (D) 10 years postoperatively.
DISCUSSION
Infantile hemangiomas located in anatomically sensitive areas of the body pose a particular challenge for clinicians. Lesions involving the lip exemplify this issue, as there are both functional and cosmetic concerns to address. The 2 most common complications arising from these lesions are ulceration in the proliferative phase and residual lip contour deformity in the involutional phase. The morphology of individual hemangiomas seems to predispose lesions to particular complications. Consequently, the specific physical characteristics of lip hemangiomas may be used to predict which lesions will be at highest risk for ulceration or contour deformity.
During the proliferative phase, development of an ulceration is the most common and concerning complication.6 In our dataset, the age of initial ulceration was quite young (median age, 6 weeks), indicating that these infants are often presenting to care with an ulcerated hemangioma. Unfortunately, early development of an ulceration often precludes preventative treatment of this complication. Consequently, the majority of ulcerated hemangiomas are treated in a reactive manner. Only 1 patient required urgent surgical intervention (due to acute labial artery bleeding); the remaining 14 patients were managed nonoperatively with oral medication (steroids, beta-blockers, or both), wound care, analgesia, and nutritional support as needed.
Infants with ulcerated hemangiomas had high rates of feeding difficulties, and 2 patients developed clinically significant failure to thrive. These patients eventually required urgent admission to hospital for supplemental nasogastric tube feeding to regain weight and allow the lesion to heal. This highlights that ulcerated lip hemangiomas are poorly tolerated in young infants, and clinicians must be aware of the possibility of feeding difficulty. Given the high risk for failure to thrive, all patients with ulcerated hemangiomas were monitored closely by our clinic pediatricians for weight gain while the lesion healed.
All 15 hemangiomas that ulcerated were either superficial or mixed depth (having both superficial and deep components); none of the exclusively deep hemangiomas were complicated by ulceration. This seems to indicate that development of ulceration requires the presence a cutaneous component to the hemangioma. Mixed depth lesions were at the highest risk for ulceration, perhaps due to the combination of cutaneous involvement and displacement of deeper lip architecture, contributing to stress on the overlying skin and predisposing the lesion to incidental trauma.
The primary physical characteristic associated with residual lip contour deformity and need for surgery was the depth of the hemangioma. None of the exclusively superficial hemangiomas required surgical debulking; however, among patients with mixed depth or deep lesions, over one-quarter (28.6%) underwent debulking surgery. Furthermore, all 7 patients who were offered surgery (and subsequently declined) also had a deep component to their lesion. This stark difference highlights the impact of hemangioma depth on the risk of developing a contour deformity. Although hemangiomas with a deep component do involute significantly over time, there is often residual bulky tissue or excess skin/fibrofatty tissue.1 Thus, despite involution, deep lesions often require operative intervention to restore adequate lip contour.
Involvement of the vermillion border was also associated with high rates of lip contour deformity. Lesions that cross the vermillion are more likely to distort lip architecture and symmetry. This is significant, as even very small degrees of asymmetry are noticeable at conversational distance. Patients with hemangiomas, who had a deep component in conjunction with vermillion border involvement, were at the highest risk of developing significant contour deformity. Given their association with surgical intervention, these 2 lesion characteristics may be of considerable use to surgeons in providing parents with a prognosis with respect to the possibility of requiring surgery.
Optimal timing for surgical intervention for problematic lip hemangiomas is controversial. Several authors have suggested that early resection during the proliferative phase is beneficial.3,7,8 However, this approach may result in higher rates of unnecessary procedures and may increase the degree of lip resection required. Early intervention ameliorates the opportunity for further involution of the hemangioma and forces parents to make an earlier decision than necessary regarding surgery. Our data indicate that there is a subset of families who decline surgery despite the identification of lip contour distortion by their surgeon. Furthermore, ongoing research continues to raise concerns about the neurodevelopmental status of children below 3 years old undergoing general anesthesia for elective procedures.9,10 Delaying intervention until 4–6 years old reduces this risk in addition to allowing further involution (potentially reducing the size of required resection). Consequently, our practice is to delay surgery to allow involution of the lesion and potentially avoid the need to intervene completely.
Importantly, the primary indication for debulking surgery is aesthetic. There does not seem to be a functional limitation to excess lip bulk or tissue (none of the patients reported functional issues related to these findings). All parents of patients who underwent surgical debulking reported concerns with lip appearance. Although none of the patients reported mental health issues, the potential for teasing and social ridicule during the school years was a frequently cited concern among parents.
During the past decade, the nonselective beta-blocker propranolol has become standard treatment to induce involution in infantile hemangiomas.11–13 In this dataset, there were no differences in ulceration rate or surgical intervention rate based on treatment with propranolol. As noted above, ulceration seems to occur very early with lip hemangiomas, reducing the role for propranolol in preventing this complication. However, this drug may still have a role in limiting the extent of the ulceration (and was often prescribed with this indication). Regardless of ulceration, treatment with propranolol did not affect the rate of developing lip contour deformity. Although propranolol is postulated to initiate involution, the presence of a deep component often results in significant residual bulk after involution, necessitating surgical intervention.
This study has several limitations related to its retrospective nature. Lip hemangiomas are relatively rare, requiring a long period of study to collect a significant sample size. Consequently, several significant changes in management practice have occurred during this period that may have influenced the results, most importantly the introduction of propranolol in 2008. Loss to follow-up rate is unclear, as patients were not specifically contacted if they were not continuing to be seen in our clinic, leaving us to assume that they did not have unrecognized complications or require further intervention. Given that some of the younger patients in the study may yet go on to have surgery, we may be underestimating the true rate of surgical treatment for lip hemangiomas at our institution.
Although we were unable to quantitatively assess tumor size, we suspect that the size and volume of lip hemangiomas have an impact on prognosis. Hemangiomas with larger volumes may be at higher risk of having residual contour deformities following the involutional phase as such lesions likely have a greater degree of residual fibrofatty tissue. It is possible this may confound our results due to the inability to include this factor in our analysis. Further work may focus on elucidating the relationship between tumor volume and aesthetic outcomes in lip hemangiomas.
Based on our experience and data regarding infantile hemangiomas of the lips, we have made the following recommendations:
(1) Given the high risk of ulceration in the proliferative phase, all patients with hemangiomas of the lips should have early assessment in a specialized, multidisciplinary clinic with expertise in vascular birthmarks.
(2) Individualized treatment of lip hemangiomas using medical therapy should include early treatment of lip hemangiomas with deep components, feeding difficulties, or other interventions based on team/family preferences.
(3) All patients with ulcerated hemangiomas are at high risk for feeding difficulties and should be monitored closely for failure to thrive by a pediatrician.
(4) During the involutional phase, hemangiomas with a deep component are at the highest risk of persistent lip contour deformities. The surgeon should have iterative discussions with the patient and their family regarding the need for debulking procedures.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Surgery, University of Calgary, for funding support through the Calgary Surgical Research Development Fund.
Ethical approval for this study was obtained by the University of Calgary Conjoint Health Research Ethics Board.
Footnotes
Published online 19 June 2019.
Presented at the Canadian Society of Plastic Surgeons (CSPS) Annual Meeting, June 22, 2017, Winnipeg, Man.
No products, devices, or drugs were used in this article.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This work was supported by a monetary grant from Calgary Surgical Research Development Fund, administered by the Department of Surgery at the University of Calgary.
REFERENCES
- 1.Chang LC, Haggstrom AN, Drolet BA, et al. ; Hemangioma Investigator Group. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360–367. [DOI] [PubMed] [Google Scholar]
- 2.O TM, Scheuermann-Poley C, Tan M, et al. Distribution, clinical characteristics, and surgical treatment of lip infantile hemangiomas. JAMA Facial Plast Surg. 2013;15:292–304. [DOI] [PubMed] [Google Scholar]
- 3.Li WY, Chaudhry O, Reinisch JF. Guide to early surgical management of lip hemangiomas based on our experience of 214 cases. Plast Reconstr Surg. 2011;128:1117–1124. [DOI] [PubMed] [Google Scholar]
- 4.Sharma VK, Fraulin FO, Harrop AR, et al. The opportunities and obstacles in developing a vascular birthmark database for clinical and research use. Can J Plast Surg. 2011;19:122–124. [PMC free article] [PubMed] [Google Scholar]
- 5.Fraulin FO, Flannigan RK, Sharma VK, et al. The epidemiological profile of the Vascular Birthmark Clinic at the Alberta Children’s Hospital. Can J Plast Surg. 2012;20:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HJ, Colombo M, Frieden IJ. Ulcerated hemangiomas: clinical characteristics and response to therapy. J Am Acad Dermatol. 2001;44:962–972. [DOI] [PubMed] [Google Scholar]
- 7.Hynes S, Narasimhan K, Courtemanche DJ, et al. Complicated infantile hemangioma of the lip: outcomes of early versus late resection. Plast Reconstr Surg. 2013;131:373e–379e. [DOI] [PubMed] [Google Scholar]
- 8.Goldenberg DC, Hiraki PY, Marques TM, et al. Surgical treatment of facial infantile hemangiomas: an analysis based on tumor characteristics and outcomes. Plast Reconstr Surg. 2016;137:1221–1231. [DOI] [PubMed] [Google Scholar]
- 9.Andropoulos DB, Greene MF. Anesthesia and developing brains—implications of the FDA warning. N Engl J Med. 2017;376:905–907. [DOI] [PubMed] [Google Scholar]
- 10.Ho AM, Fleming ML, Mizubuti GB. Anesthetic neurotoxicity and the developing brain. CMAJ. 2017;189:E1028–E1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–2651. [DOI] [PubMed] [Google Scholar]
- 12.Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735–746. [DOI] [PubMed] [Google Scholar]
- 13.Chinnadurai S, Fonnesbeck C, Snyder KM, et al. Pharmacologic interventions for infantile hemangioma: a meta-analysis. Pediatrics. 2016;137:e20153896. [DOI] [PubMed] [Google Scholar]


