Abstract
Pneumothorax is a common complication in computed tomography (CT)-guided percutaneous lung biopsy (CPLB). Whether the lobar location of lesions contributes to the incidence of pneumothorax should be further clarified.
A total of 1452 consecutive patients who underwent CPLB between January 2010 and March 2018 were retrospectively analyzed. The incidence of pneumothorax was compared among 5 different lobe biopsies. Minor pneumothorax was defined as pneumothorax without chest tube placement and major pneumothorax was defined as pneumothorax with chest tube placement.
The positive diagnosis rate of pathology for this cohort was approximately 84%, with 22.5% (326/1452) of the patients experiencing pneumothorax. The rates of pneumothorax were 19.5%, 24.5%, 33.9%, 21.4%, and 23.9% for the right upper lobe, right lower lobe, right middle lobe, left upper lobe, and left lower lobe, respectively (P = .09). Chest tube placement was necessary in 19.0% (62/326) of the patients with pneumothorax. The rates of major pneumothorax were 5.3%, 2.6%, 10.2%, 4.7%, and 2.6% for the right upper lobe, right lower lobe, right middle lobe, left upper lobe, and left lower lobe biopsies, respectively (P = .02). This result was further confirmed by the propensity score-matching method. Moreover, 8.7% (127/1452) of the patients experienced puncture of fissure, the rates of which were 13.5%, 5%, 10.2%, 9.1%, and 4.3% for the right upper lobe, right lower lobe, right middle lobe, left upper lobe, and left lower lobe, respectively (P < .001). Within the pneumothorax patient group, the rate of lobe fissure puncture (15.2%) was significantly lower in patients with minor pneumothorax than (51.6%) in those with major pneumothorax (P < .001).
Upper and middle lobe lesion biopsies show a significantly high rate of major pneumothorax, which may be due to more puncture of fissure. It is crucial to carefully distinguish the fissure around lesions and bypass it to avoid major pneumothorax.
Keywords: chest tube placement, computer tomography, lesion location, lung biopsy, pneumothorax
1. Introduction
Computed tomography (CT)-guided percutaneous lung biopsy (CPLB) is a well-established method for the differential diagnosis of lung lesions. In the era of targeted therapy, CPLB is important to obtain enough tumor tissue for detection of mutations in genes, such as EGFR and ALK, which can be used to guide the rational options of molecular targeted therapy in clinics. Current literature indicated that CPLB showed a high diagnostic yield with accepted complications, particularly for lung lesions that were not accessible for bronchoscopy.[1–3] Pneumothorax was the most frequent complication with reported rates of 29% to 54% for core biopsy, requiring chest drainage in 3.3% to 15% of the cases.[4–6] Recently, a study involving 4262 consecutive lung biopsies from the MD Anderson Cancer Center showed that the rates of pneumothorax and chest tube placement were 30.2% and 15%, respectively.[7] An obvious reduction in pneumothorax incidence has not occurred in the last 20 years.[8,9] Although CPLB is a widely accepted procedure with relatively few serious complications, precise planning and detailed knowledge of various aspects of the biopsy procedure is required to avoid complications.[8]
Many factors contribute to the occurrence of pneumothorax, such as the tumor size and location, patient position, needle size, needle pleura angle, presence of emphysema, needle path, and other technical factors.[8,10,11] However, how some of these parameters may influence pneumothorax complication remains controversial. For example, Hiraki et al demonstrated that lesions in the lower lobe were one significant independent risk factor for pneumothorax in CPLB (P < .001)[10]; however, 2 other studies demonstrated that pneumothorax incidence showed no significant difference between different lung lobe lesions following CPLB.[9,12] Thus, whether tumor location is related to distinct occurrence and/or severity of pneumothorax should be further clarified. The present study was retrospectively conducted to analyze the complications of different lobe lesion punctures, with the aim of more efficiently decreasing the rate of pneumothorax and chest tube placement.
2. Materials and methods
2.1. Patients
A total of 1452 consecutive patients who underwent CPLB between January 2010 and March 2018 at Hubei Cancer Hospital, China, were retrospectively analyzed. Before the procedure, all patients were required to undergo a routine blood test, complete coagulogram and thoracic contrast-enhanced CT, which helped radiologists to confirm any contraindications and acquire detailed knowledge about the tumor size, location, and vascularity, necrotic area and important structures located in the biopsy path. Before June 2017, only the noncoaxial technique was used. Following June 2017, the application of the coaxial technique was started, depending on radiologists’ preference. To reduce procedure-related complications, CPLB was performed only once with an 18G cutting needle for most patients; thus, only one piece of tissue was obtained for most patients undergoing biopsies without coaxial needles. However, 2 to 4 pieces of tissue were acquired from patients undergoing coaxial needle biopsy. Estazolam was orally administered to patients who were anxious 30 minutes before the procedure. Biopsies were performed by radiologists with more than 5 years of puncture experience (also listed as authors). Informed consent was obtained from each patient before the procedure. This study was approved by the Institutional Review Board of Hubei Cancer Hospital, China.
2.2. Biopsy
Procedures were performed under CT guidance with 64-section CT scanners (LightSpeed VCT-XT, GE Medical Systems; Somatom Definition AS, Siemens) and breathing instructions, using the step-and-shoot technique to assess needle angulation. First, appropriate positioning was chosen according to the baseline contrast-enhanced CT and patient's position tolerance. In rare cases, the planned positioning was changed, if it was not optimal during the procedure. The lateral position was considered based on the operator's suggestion as more convenient and safer than the regular prone and supine position, although it was less desirable due to lower stability. Subsequently, a radiopaque grid was placed on patient's skin over the area of interest to focus on the optimal access point, followed by a short spiral CT scan for this area. The needle entry point on the skin and angle were determined with the assistance of a gantry laser alignment light as well as comprehensive consideration of the shortest pathway from the skin to the lesion, avoiding critical structures (such as vessels, bronchi and fissures, and heart), and of the tumor characteristics. Local anesthesia was performed with lidocaine after confirmation of optimal biopsy access. Following sterile precautions, the core biopsy needle was advanced into the subcutaneous tissues prior to pleural puncture, and then punctured the pleura into the lung toward the lesions after proper needle angulation and direction. If repositioning was necessary, the needle was usually adjusted without exiting the lung. Once the needle tip was properly placed, biopsy was performed. Based on these principles and only 1-time biopsy, most patients underwent only 1 pleural pass during the procedure. In some procedures, the surface of tumor tissue sample was slightly smeared on a glass slide, which was immediately delivered to a cytopathologist before it was directly immersed in formalin for fixation.
After biopsy, an immediate CT scan for the whole thoracic area was performed to assess occurrence of procedure-related complications. Subsequently, patients were delivered to the unit, kept in bed without movement, and monitored for at least 24 hours. Patients with shortness of breath and serious cough due to the procedure were subjected to chest X-ray. Chest tube placement was based on the severity of symptoms (such as obvious shortness of breath) and/or the ratio of lung compression (>50%).
2.3. Definition of risk factors
Based on CT imaging characteristics, pleural effusion, emphysema, lung atelectasis, cavitary lesion, and lung fibrosis were assigned as concurrent abnormality of imaging. Minor pneumothorax was defined as pneumothorax without chest tube placement, and major pneumothorax was defined as pneumothorax with chest tube placement.
The severity of lung hemorrhage was divided into 4 grades according to Tai et al.[13] Grade 0 was defined as no pulmonary hemorrhage, grade 1 as needle tract hemorrhage ≤2 cm in width, grade 2 as hemorrhage >2 cm in width but sublobar, grade 3 as lobar hemorrhage or greater, and grade 4 as hemothorax.
2.4. Statistical analyses
Simple descriptive statistics were used to report general clinical information. Continuous data (age) in 5 different subgroups (right upper lobe, right lower lobe, right middle lobe, left upper lobe, left lower lobe) were described as mean value ± standard deviation (SD) and were compared using 1-way analysis of variance. Categorized variables (other clinical factors) were compared with the Chi-squared test. All statistical analyses were performed using SPSS 25.0. Two-sided P-value <.05 was considered indicative of a statistically significant difference.
3. Results
Lung biopsy was performed in 1452 patients with a mean age of 61.3 years (10–86 years) and a male to female ratio of 1.93:1. In this cohort, pathologic results from CPLB specimens showed that 1146 (78.9%) cases were malignant and 73 (5.0%) benign. A total of 233 results (approximately 16.0%) were nondiagnostic or suspicious for malignancies, thus the positive diagnosis rate of pathology was approximately 84%. Pathology findings are summarized in Figure 1.
Figure 1.
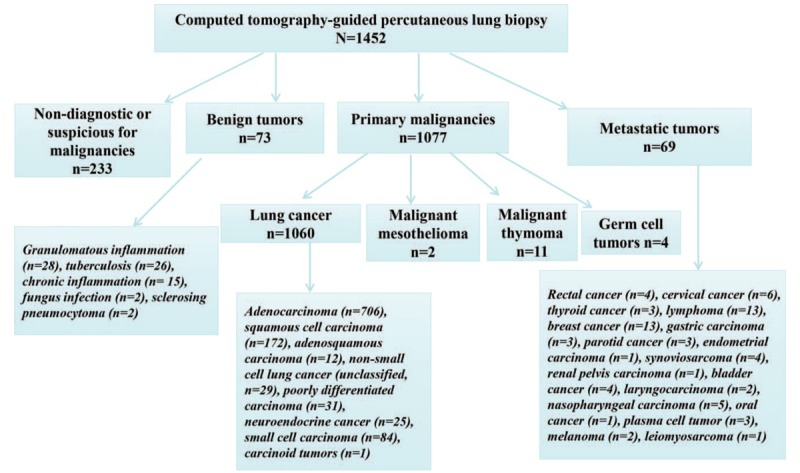
Final pathologic results in 1452 patients with computed tomography-guided percutaneous lung biopsy.
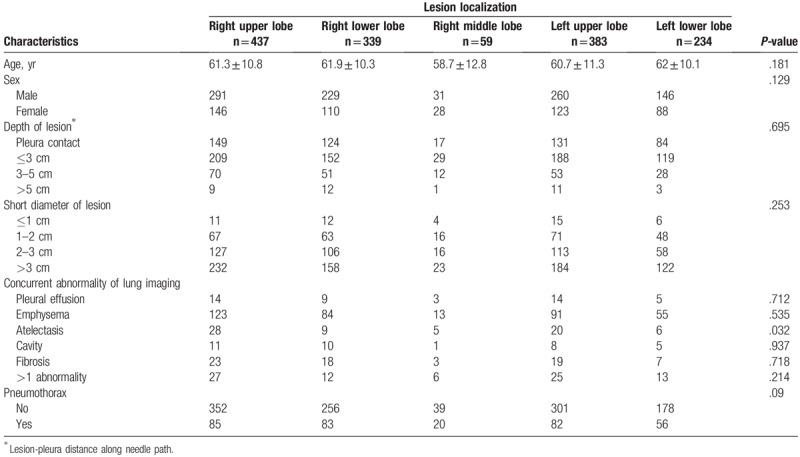
The rate of pneumothorax was 22.5% (326/1452) for all patients. To explore whether the lesion location was a crucial contributing factor to pneumothorax, a demographic comparison between patients with 5 different lobe lesions was performed and the results are summarized in Table 1. Age, sex, lesion-pleura distance along the needle path, short lesion diameter, and concurrent lung imaging characteristics (except for atelectasis) were well balanced between these 5 subgroups (all P-values >.05). Only 5.88% (4/68) of the patients with atelectasis experienced pneumothorax, which was significantly lower than the total pneumothorax rate (22.5%), suggesting that atelectasis was not a confounding factor for pneumothorax complication in these 5 lobe biopsy subgroups. The rates of pneumothorax were 19.5%, 24.5%, 33.9%, 21.4%, and 23.9% for the right upper lobe, right lower lobe, right middle lobe, left upper lobe, and left lower lobe, respectively. However, pneumothorax incidence failed to show a statistical difference between patients with different lung lobe lesions (P = .09), although puncture of the right middle lobe tended to have an obviously elevated pneumothorax rate.
Table 1.
Baseline characteristics of 1452 patients.
Conservative management was applied in 81% (264/326) of the patients with pneumothorax, but treatment of pneumothorax with chest drainage was necessary in 19.0% (62/326). Subsequently, the major pneumothorax rates of these 5 lung lobe categories were compared between patients with pneumothorax. Our analysis demonstrated a statistical difference for major pneumothorax events (P = .02, Table 2). The rates of major pneumothorax were 5.3% (23/437), 2.6% (9/339), 10.2% (6/59), 4.7% (18/383), and 2.6% (6/234) for the right upper lobe, right lower lobe, right middle lobe, left upper lobe, and left lower lobe, respectively. To minimize potential biases due to the retrospective nature of our study, the propensity score-matching method was applied in this cohort. Based on a relatively small sample size (n = 59) in the right middle lobe and a significantly high rate of major pneumothorax in the upper and middle lobe lesion biopsies, we categorized lesion localization as nonlower lobes vs lower lobes, which may help to generate a 1:1 matching. In these 2 subtypes, clinical variables that could potentially influence the development of pneumothorax were included in the analysis, such as short diameter of lesion, concurrent abnormality of lung imaging, patient position, puncture of fissure, age, and depth of lesion. After the propensity score matching, 263 pairs were matched. A total of 24 patients and 6 patients had major pneumothorax in nonlower lobe biopsies and lower lobe biopsies, respectively (P = .001), which further supported that CPLB induced a significantly higher major pneumothorax incidence in the upper and middle lobes than in the lower lobes.
Table 2.
Comparison of chest drainage events in patients with pneumothorax according to lesion localization.

Furthermore, 8.7% (127/1452) of the patients experienced puncture of a fissure, the rates of which were 13.5% (59/437), 5% (17/339), 10.2% (6/59), 9.1% (35/383), and 4.3% (10/234) in the right upper lobe, right lower lobe, middle lobe, left upper lobe, and left lower lobe, respectively (P < .001). The rate of fissure puncture was significantly higher in the upper and middle lobes than in the lower lobes for this cohort. Among patients with pneumothorax, the rate of lobe fissure puncture was as low as 15.2% (40/264) in the subgroup of patients with minor pneumothorax; however, it was as high as 51.6% (32/62) in the subgroup of patients with major pneumothorax (P < .001, Table 3), which presented a rational explanation for the higher rate of major pneumothorax observed in upper and middle lobe biopsies. One example regarding the risk of major pneumothorax by puncture of lobe fissure is illustrated in Figure 2.
Table 3.
Comparison of chest drainage events in the pneumothorax subgroup with or without puncture of lobe fissure.
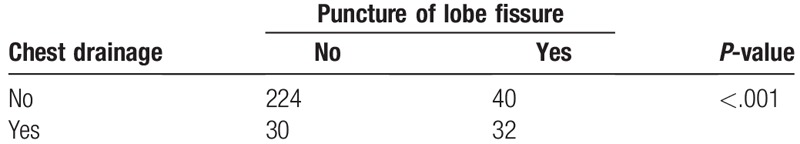
Figure 2.
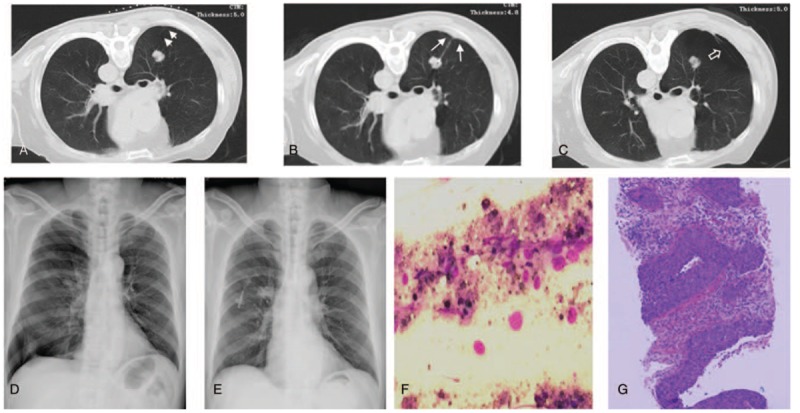
Lung squamous cell carcinoma in a 62-year-old man that was diagnosed with computed tomography (CT)-guided percutaneous lung biopsy. (A) Preprocedural CT scan showed a 2.0 cm (short diameter) solid nodule with a puncture depth of 4.1 cm in the right upper lobe in prone position. This nodule was adjacent to the oblique fissure (short arrows). (B) The puncture access point was on the posterior axillary line instead of the paravertebral area to avert the oblique fissure. The CT scan obtained during the biopsy showed that the needle tip punctured in the edge portion of the solid nodule, and 1 piece of tissue specimen was obtained with 1 biopsy. Unexpectedly, the puncture route still involved the oblique fissure (long arrows). (C) CT scan obtained after the biopsy showed obvious oblique fissure (hollow arrow) and pneumothorax. (D) The patient began to feel shortness of breath 1 hour after the procedure. Progressive pneumothorax was confirmed by about 60% compression of right lung volume on an emergent chest X-ray examination. (E) The chest tube was inserted into the right pleural cavity of this patient, significantly alleviating his symptoms. After 3 days of drainage, the 2nd X-ray showed that the right lung was completely re-expanded. (F) The squamous carcinoma cells were found in a cluster of necrotic cells with smear cytology of this biopsy tissue. (G) The squamous cell carcinoma of lung was diagnosed by the corresponding histopathology. IHC: CK5/6 (+), P40 (+), CK7 (+), TTF-1 (−), NapsinA (−), CD56 (−), Syn (−), CgA (+), Ki-67 (Li: 80%).
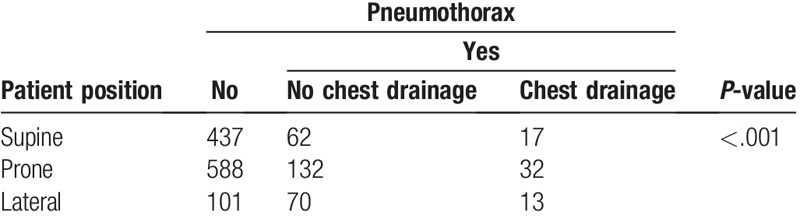
Although lesion location failed to establish a correlation with the occurrence of pneumothorax, it is indeed one of the determining parameters for patient position. Thus, we then analyzed the correlation between patient position and occurrence and severity of pneumothorax. Our analysis showed that lateral position was significantly related to a higher rate of pneumothorax (45.1%) than supine (15.3%) and prone (21.8%) positions, and also resulted in a higher percentage of major pneumothorax (7.1%) than prone (4.3%) and supine (3.3%) (P < .001, Table 4) positions.
Table 4.
Comparison of pneumothorax in patients with different puncture positions.
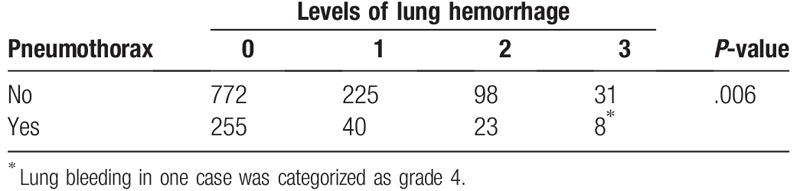
Lung hemorrhage was another common complication for CPLB. In our cohort, 29.3% (425/1452) of the patients experienced different grades of lung hemorrhage. The hemorrhage events for patients without or with pneumothorax are summarized in Table 5. Interestingly, the rate of lung hemorrhage was 27.8% and 31.4% for patients with or without pneumothorax (P = .006), respectively. Thus, pneumothorax tended to be negatively correlated with the occurrence and severity of lung hemorrhage.
Table 5.
Correlation between pneumothorax and lung hemorrhage complication.
4. Discussion
Minimally invasive biopsies, such as CPLB, are often used for pathologic diagnosis without subjecting patients to the risks associated with a surgical procedure.[14] In addition, the rate of diagnostic accuracy for CPLB was relatively high (85–90%) and acceptable.[15–17] In the present study, only 1-time biopsy was performed on most patients, and the positive diagnostic yield (about 84%) was comparable to the published results. Moreover, the same piece of core needle biopsy tissue was also used to detect EGFR and ALK gene mutations in some patients. Thus, 1-time biopsy may be feasible to obtain 1 piece of specimen for regular pathologic diagnosis, and even for gene mutation examination. However, it is better to obtain as much specimen as possible for driven gene detection by the coaxial technique.
The rate of pneumothorax in patients undergoing CPLB had been reported to be more than 30% in many studies.[8,18,19] In a recent meta-analysis of 8133 procedures from 32 publications, the pooled rate of pneumothorax was 25.3%, and 5.6% patients with pneumothorax required intervention for CT-guided core biopsy.[18] Our study showed that 22.5% of the patients (n = 1452) developed procedure-related pneumothorax, with <5% of the patients requiring drainage, which may be due to the reduced time of the needle being across the pleura. Many factors were considered to increase the risk of pneumothorax, such as age over 60 years old, diameter and depth of tumor, puncture of a fissure, chronic obstructive pulmonary disease and emphysema.[20–22] In fact, it is difficult to compare complication results of CPLB between published studies due to inconsistent or even contradictory results owing to various biopsy techniques, defined risk factors, statistical methods and baseline characteristics of lung imaging, and puncture experience, suggesting that the incidence of CPLB-induced complications is multivariate and complex.[23–25]
There has been an obvious controversy regarding the correlation between tumor location and pneumothorax. Hailemariam et al demonstrated that pneumothorax most commonly occurred in biopsies of middle lobe lesions (64.7%) as opposed to lower or upper lobe lesions (26.5% and 31.4%, respectively) (P = .007) in a retrospective analysis of 289 CT-guided lung biopsies.[26] However, another retrospective study (201 fine-needle aspiration biopsies and 68 core biopsies) failed to prove a significant correlation between increased risk of pneumothorax and lesion location.[9] Furthermore, univariate analysis in a study with 141 small nodules punctures showed that the risk of CPLB-induced pneumothorax was approximately 3 times higher for upper lobe tumors than for nonupper lobe tumors (P = .036), but multivariate analysis did not reveal a significant difference.[27] One possible explanation for these discrepancies was the unbalanced baseline characteristics, for example, the clinical information of emphysema was unknown for some cohorts, but the presence of emphysema correlated with the occurrence of pneumothorax and the need for drainage.[28]
To further clarify whether tumor location was a risk factor for pneumothorax for CPLB, we collected several crucial clinical characteristics (age, sex, tumor size, depth of tumor, and concurrent abnormality of lung imaging) and compared the difference of pneumothorax incidence between 5 different lobe biopsies. These factors were well balanced at baseline except for atelectasis, but atelectasis was not a confounding factor for pneumothorax rate in this cohort. Although the rates of pneumothorax showed no significant difference in the lobar location of the lesion, the rate of major pneumothorax was statistically correlated with tumor location. Right middle lobe and both upper lobes showed a significantly higher rate of chest tube placement than both lower lobes. Moreover, among patients with pneumothorax, our analysis showed that puncture of a fissure was statistically more common in patients with major pneumothorax than in those with minor pneumothorax. In particular, puncture of a fissure was more frequent in upper and middle lobe biopsies than in lower lobe biopsies. These results potentially explain why the rate of major pneumothorax was high in the upper and middle lobe biopsies. Thus, it is essential to avoid the puncture of a fissure, especially for lesions in these lobes. Combination of other manipulations, such as rapid needle-out patient-rollover approach and autologous blood patch techniques,[11,29] will make it possible to further reduce the occurrence/severity of pneumothorax.
This study demonstrated that supine position had the lowest incidence of pneumothorax and chest tube insertion, compared with the prone and lateral positions, which was different from the results of previous studies.[27,30] Generally, the prone position results in the lowest incidence of pneumothorax because of less posterior rib movement, wider posterior intercostal spaces, and reduction of patients’ anxiety, because patients do not see the needle during the procedure.[31] Among 127 patients experiencing puncture of fissure in this study, 99 were in prone position, which may reasonably explain this discrepancy.
Some studies indicated that intrapulmonary hemorrhage was protective against the development of pneumothorax, effectively sealing the biopsy tract,[32,33] which was similar with our results showing that lung hemorrhage complication was negatively correlated with pneumothorax. However, 1 patient in this cohort not only suffered from major pneumothorax but also hemothorax after CPLB. Nevertheless, it may be worth to further explore the correlation between CPLB-induced pneumothorax and lung hemorrhage.
In conclusion, the lobar location of lesions was found to correlate with the rate of major pneumothorax, but not the rate of pneumothorax in this large sample size study. Upper and middle lobe lesion biopsies tended to have a significantly high rate of major pneumothorax, potentially due to more puncture of fissure in these lobes. There are limitations of this study, such as its retrospective nature with unknown bias, and the small sample size for the middle lobe puncture group (n = 59). Nevertheless, it is necessary to carefully distinguish the fissure around the lesions and bypass it when operators decide on the puncture route before procedures.
Author contributions
Conceptualization: Yulin Liu.
Data curation: Weipeng Yan, Xiaofang Guo, Junfen Zhou, Changchun Chen, Manxiang Wang.
Formal analysis: Jing Zhang, Manxiang Wang, Zhaoxi Zhang.
Investigation: Weipeng Yan, Xiaofang Guo.
Methodology: Weipeng Yan, Xiaofang Guo, Junfen Zhou.
Resources: Zhaoxi Zhang, Yulin Liu.
Software: Weipeng Yan, Jing Zhang.
Supervision: Yulin Liu.
Validation: Weipeng Yan.
Writing – original draft: Weipeng Yan.
Writing – review & editing: Xiaofang Guo.
Footnotes
Abbreviations: CPLB = computed tomography-guided percutaneous lung biopsy, CT = computed tomography.
WY and XG contributed equally to this work.
This research was funded by the key project of the Health Commission of Hubei Province, China (No: WJ2019Z015), and the Middle-aged and Young Medical Core Talent Project of Wuhan, China (No: 59, 2016).
The authors have no conflicts of interest to disclose.
References
- [1].Moreland A, Novogrodsky E, Brody L, et al. Pneumothorax with prolonged chest tube requirement after CT-guided percutaneous lung biopsy: incidence and risk factors. Eur Radiol 2016;26:3483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yeow KM, Tsay PK, Cheung YC, et al. Factors affecting diagnostic accuracy of CT-guided coaxial cutting needle lung biopsy: retrospective analysis of 631 procedures. J Vasc Interv Radiol 2003;14:581–8. [DOI] [PubMed] [Google Scholar]
- [3].Fontaine-Delaruelle C, Souquet PJ, Gamondes D, et al. Negative predictive value of transthoracic core-needle biopsy: a multicenter study. Chest 2015;148:472–80. [DOI] [PubMed] [Google Scholar]
- [4].Klein JS, Salomon G, Stewart EA. Transthoracic needle biopsy with a coaxially placed 20-gauge automated cutting needle: results in 122 patients. Radiology 1996;198:715–20. [DOI] [PubMed] [Google Scholar]
- [5].Kazerooni EA, Lim FT, Mikhail A, et al. Risk of pneumothorax in CT-guided transthoracic needle aspiration biopsy of the lung. Radiology 1996;198:371–5. [DOI] [PubMed] [Google Scholar]
- [6].Charig MJ, Phillips AJ. CT-guided cutting needle biopsy of lung lesions--safety and efficacy of an out-patient service. Clin Radiol 2000;55:964–9. [DOI] [PubMed] [Google Scholar]
- [7].Kuban JD, Tam AL, Huang SY, et al. The effect of needle gauge on the risk of pneumothorax and chest tube placement after percutaneous computed tomographic (CT)-guided lung biopsy. Cardiovasc Intervent Radiol 2015;38:1595–602. [DOI] [PubMed] [Google Scholar]
- [8].Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6:S99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cruz JF, Rolo R, Iglesias L, et al. CT-guided transthoracic lung biopsy: predictive factors of pneumothorax. Rev Port Pneumol 2014;20:174–6. [DOI] [PubMed] [Google Scholar]
- [10].Hiraki T, Mimura H, Gobara H, et al. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy-guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9-year period. AJR Am J Roentgenol 2010;194:809–14. [DOI] [PubMed] [Google Scholar]
- [11].Neyaz Z, Mohindra N. Is the rapid needle-out patient-rollover approach after CT-guided lung biopsy really effective for pneumothorax prevention? J Thorac Dis 2015;7:E350–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kuriyama T, Masago K, Okada Y, et al. Computed tomography-guided lung biopsy: association between biopsy needle angle and pneumothorax development. Mol Clin Oncol 2018;8:336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tai R, Dunne RM, Trotman-Dickenson B, et al. Frequency and severity of pulmonary hemorrhage in patients undergoing percutaneous CT-guided transthoracic lung biopsy: single-institution experience of 1175 cases. Radiology 2016;279:287–96. [DOI] [PubMed] [Google Scholar]
- [14].Mukhopadhyay S. Utility of small biopsies for diagnosis of lung nodules: doing more with less. Mod Pathol 2012;25Suppl 1:S43–57. [DOI] [PubMed] [Google Scholar]
- [15].Sconfienza LM, Mauri G, Grossi F, et al. Pleural and peripheral lung lesions: comparison of US- and CT-guided biopsy. Radiology 2013;266:930–5. [DOI] [PubMed] [Google Scholar]
- [16].Loh SE, Wu DD, Venkatesh SK, et al. CT-guided thoracic biopsy: evaluating diagnostic yield and complications. Ann Acad Med Singapore 2013;42:285–90. [PubMed] [Google Scholar]
- [17].Yun S, Kang H, Park S, et al. Diagnostic accuracy and complications of CT-guided core needle lung biopsy of solid and part-solid lesions. Br J Radiol 2018;91:20170946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Heerink WJ, de Bock GH, de Jonge GJ, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 2017;27:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ng YL, Patsios D, Roberts H, et al. CT-guided percutaneous fine-needle aspiration biopsy of pulmonary nodules measuring 10 mm or less. Clin Radiol 2008;63:272–7. [DOI] [PubMed] [Google Scholar]
- [20].Wattanasatesiri T, Puntu W, Vithitsuvanakul N. Influencing factors of pneumothorax and parenchymal haemorrhage after CT-guided transthoracic needle biopsy: single-institution experience. Pol J Radiol 2018;83:e379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lim CS, Tan LE, Wang JY, et al. Risk factors of pneumothorax after CT-guided coaxial cutting needle lung biopsy through aerated versus nonaerated lung. J Vasc Interv Radiol 2014;25:1209–17. [DOI] [PubMed] [Google Scholar]
- [22].Vatrella A, Galderisi A, Nicoletta C, et al. Age as a risk factor in the occurrence of pneumothorax after transthoracic fine needle biopsy: our experience. Int J Surg 2014;12Suppl 2:S29–32. [DOI] [PubMed] [Google Scholar]
- [23].Yeow KM, Su IH, Pan KT, et al. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest 2004;126:748–54. [DOI] [PubMed] [Google Scholar]
- [24].Li H, Boiselle PM, Shepard JO, et al. Diagnostic accuracy and safety of CT-guided percutaneous needle aspiration biopsy of the lung: comparison of small and large pulmonary nodules. AJR Am J Roentgenol 1996;167:105–9. [DOI] [PubMed] [Google Scholar]
- [25].Heyer CM, Reichelt S, Peters SA, et al. Computed tomography-navigated transthoracic core biopsy of pulmonary lesions: which factors affect diagnostic yield and complication rates? Acad Radiol 2008;15:1017–26. [DOI] [PubMed] [Google Scholar]
- [26].Hailemariam M, Petrov D, Muzaffar N, et al. CT guided lung biopsy: a lesion's lobar location and distance from pleura as independent risk factors for pneumothorax. J Vasc Interv Radiol 2016;27:S168. [Google Scholar]
- [27].Li GC, Fu YF, Cao W, et al. Computed tomography-guided percutaneous cutting needle biopsy for small (</= 20 mm) lung nodules. Medicine (Baltimore) 2017;96:e8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Heck SL, Blom P, Berstad A. Accuracy and complications in computed tomography fluoroscopy-guided needle biopsies of lung masses. Eur Radiol 2006;16:1387–92. [DOI] [PubMed] [Google Scholar]
- [29].Wu CC, Maher MM, Shepard JA. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. AJR Am J Roentgenol 2011;196:W678–82. [DOI] [PubMed] [Google Scholar]
- [30].Covey AM, Gandhi R, Brody LA, et al. Factors associated with pneumothorax and pneumothorax requiring treatment after percutaneous lung biopsy in 443 consecutive patients. J Vasc Interv Radiol 2004;15:479–83. [DOI] [PubMed] [Google Scholar]
- [31].Winokur RS, Pua BB, Sullivan BW, et al. Percutaneous lung biopsy: technique, efficacy, and complications. Semin Intervent Radiol 2013;30:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Khan MF, Straub R, Moghaddam SR, et al. Variables affecting the risk of pneumothorax and intrapulmonal hemorrhage in CT-guided transthoracic biopsy. Eur Radiol 2008;18:1356–63. [DOI] [PubMed] [Google Scholar]
- [33].Topal U, Berkman YM. Effect of needle tract bleeding on occurrence of pneumothorax after transthoracic needle biopsy. Eur J Radiol 2005;53:495–9. [DOI] [PubMed] [Google Scholar]


