Abstract
Plasmacytoma variant translocation 1 (PVT1) is highly expressed in a variety of cancer tissues and is related to the clinicopathological features and prognosis. However, the prognostic value of PVT1 is still controversial. Therefore, this systematic evaluation and meta-analysis were performed to evaluate the relationship between PVT1 expression and clinicopathological features.
PubMed, EMBASE, Web of science, and Cochrane library databases were searched for literature collection according to inclusion criteria and exclusion criteria. The pooled hazard ratios (HRs) or odds ratios (ORs) were used to evaluate the association between PVT1 expression and overall survival, tumor size, tumor–node–metastasis (TNM) stage, lymph node metastasis, and distant metastasis.
A total of 39 articles including 3974 patients were included in the study. The results showed that the expression of PVT1 was closely related to the overall survival rate of cancers (HR = 1.64, 95% confidence interval [CI]: 1.50–1.78, P < .000001). Subgroup analysis showed that the high expression of PVT1 was closely related to the low overall survival rate of patients with clear cell renal cell carcinoma, breast cancer, cervical cancer, colon cancer, epithelial ovarian cancer, gastric cancer, lung cancer, and osteosarcoma. In addition, the high expression of PVT1 was positively correlated with tumor size (OR = 1.50, 95% CI: 1.14–1.96, P = .004), TNM stage (OR = 3.39, 95% CI: 2.73–4.20, P < .00001), lymph node metastasis (OR = 2.60, 95% CI: 1.76–3.84, P < .00001), and distant metastasis (OR = 2.94, 95% CI: 1.90–4.56, P < .00001).
PVT1 could serve as a marker for the size, TNM stage, metastasis, and prognosis of different type of cancers.
Keywords: carcinoma, long non-coding RNA, meta-analysis, plasmacytoma variant translocation 1, prognosis, solid tumor
1. Introduction
Long non-coding RNAs are functionally defined as transcripts >200 bp in length with no protein-coding function. They are expressed by the tens of thousands in many differentiated tissues and cancer tissues. Long non-coding RNA (lncRNA) is an important component of many biological processes, including stem cell biology, development, and differentiation. Abnormal lncRNA expression is believed to be closely related to the occurrence of a variety of human diseases, including various cancers. Therefore, lncRNA is one of the hot spots in cancer research at present.
As a member of the LncRNA family, plasmacytoma variant translocation 1 (PVT1) is a long non-coding RNA with a length of 1.9 KB that can encode a variety of transcripts. It is located in the region 8q24.21 of human chromosome and close to the known oncogene MYC. PVT1 was originally identified as a retrovirus integration site in mouse leukemia virus-induced t-cell lymphoma. In recent years, PVT1 has become the focus of lncRNA research. More and more evidence showed that PVT1 is highly expressed in a variety of tumor tissues, including cervical cancer, multiple myeloma, prostate cancer, lung cancer, nasopharyngeal cancer, etc. PVT1 can inhibit apoptosis of tumor cells, promote cell proliferation by regulating cell cycle, and affect tumor invasion and metastasis. It is reported that the high expression of PVT1 increased the expression of autophagy related 7 (Atg7) and Beclin1 (BECN1) by targeting miRNA-186, thereby inducing the protective autophagy of glioma cells and promoting the proliferation, migration and angiogenesis of vascular endothelial cells.[1] In gastric cancer, PVT1 is highly expressed in gastric cancer tissues and interacts with fork head box M1 (FOXM1) to form a positive feedback loop pathway to promote the proliferation and invasion of gastric cancer cells and promote the development of gastric cancer.[2] Studies have also confirmed that PVT1 can interact with signal transducer and activator of transcription 3 (STAT3) signaling pathway and significantly induce angiogenesis in gastric cancer tissues by inducing high vascular endothelial growth factor A (VEGFA) expression.[3] These results indicate that PVT1 plays an important role in the occurrence, development, and recurrence of tumors.
In recent years, some literatures have also reported that PVT1 is related to the clinicopathological features and prognosis of various tumor patients, including lung cancer,[4,5] gastric cancer,[2,6] breast cancer,[7,8] cervical cancer,[9–12] colorectal cancer,[13,14] hepatocellular carcinoma.[15–18] However, most reports are limited by geographical, ethnic, and sample size limitations. Therefore, it is necessary to conduct a comprehensive meta-analysis of the existing data to evaluate the significance of PVT1 as a prognostic marker for the prognosis of various carcinomas.
2. Material and methods
2.1. Search strategy and literature selection
The public databases including PubMed, Web of science, Cochrane library database, and EMBASE database were retrieved independently by 2 researchers (KX and JL). The keywords were set as “((((((cancer) OR tumor) OR carcinoma) OR neoplasm)) AND ((((prognosis) OR survival) OR diagnosis) OR clinicopathological)) AND ((PVT1) OR plasmacytoma variant translocation 1).” The retrieval literature publication date was before November 22, 2018. The study was permitted by the Medical Ethical Committee of Liaocheng People's Hospital.
2.2. Inclusion and exclusion criteria
The inclusion criteria are as follows: the research object is human tumor tissues; the subjects were grouped according to the expression level of PVT1; the study assessed the relationship between PVT1 expression level and prognosis or clinicopathological features of tumors; the study provided enough data to extract hazard ratios (HRs) or odds ratios (ORs); English literature.
The exclusion criteria are as follows: the subjects were cell lines; reviews, editorials, expert opinions, letters, bioinformatics analysis articles, and case reports; duplicate publications; the article does not have enough data available.
2.3. Date extraction and quality assessment
Data extraction was completed independently by the 3 authors and consensus was reached. After the literature was determined according to the previously described criteria, the following information was extracted successively: author, Published year, cancer type, country, tumor size, follow-up period, detection method, cut-off value. the number of patients in each group was divided according to the presence or absence of lymph node metastasis, distant metastasis, tumor size, and tumor–node–metastasis (TNM) stage, as well as the number of patients with high and low PVT1 expression in each group, HRs as well as their 95% CIs. If Kaplan–Meier survival curve is only provided in paper, Engauge Digitizer V4.1 (Mark Mitchell) is used to estimate HR according to the previously reported method.[19] Newcastle-Ottawa quality assessment scale (NOS) was used to evaluate the quality of the literatures. NOS scores ranged from 0 to 9, and the higher the score, the higher the quality of the article.
2.4. Statistical analysis
RevMan5.3 (Cochrane community) software was used for meta-analysis to combine HR or OR values. The chi square-based Q test and I2 statistics were used to evaluate the heterogeneity. If the heterogeneity was obvious (I2 >50%, P < .05), the random-effects model was used for analysis; if the heterogeneity was absent (I2 <50%, P > .05), the fix-effects model was used to analyze the results. Begg funnel-plot was used to evaluate the potential publication bias. P < .05 was considered to be statistically significant.
3. Results
3.1. Included literature and their characteristics
A total of 202 literatures were preliminarily retrieved and 23 duplicated literatures were removed. After carefully reading the title, abstract, and full text, 39 literatures were included in this study according to the inclusion criteria and exclusion criteria (Fig. 1).
Figure 1.
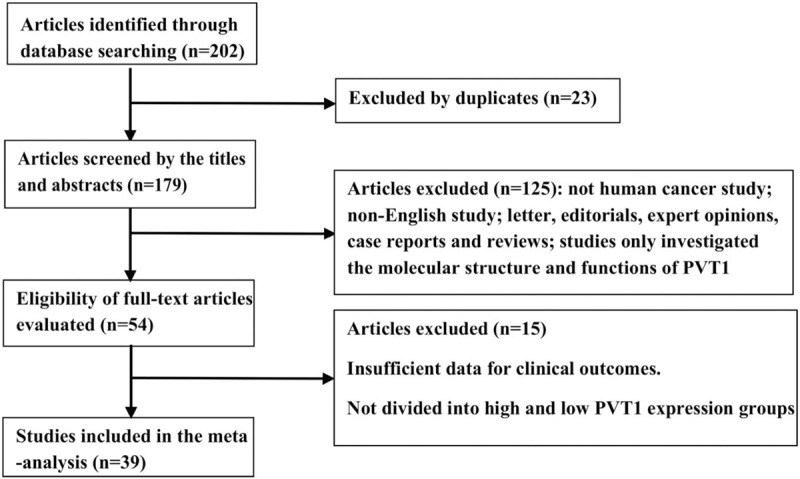
The flow diagram of this meta-analysis.
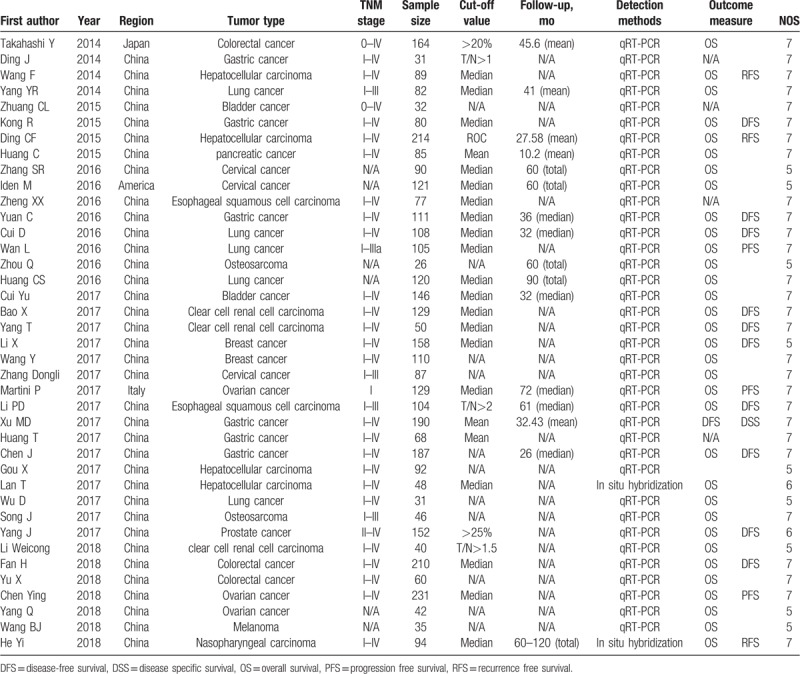
The 39 literatures were published between 2014 and 2018, including a total of 3974 patients, with the lowest sample size of 26 and the largest of 231, with an average of 102. The samples included in the article came from different regions, including China (3560), Japan (164), the United States (121), and Italy (129). The included studies involved 16 types of cancer, including bladder cancer,[20,21] clear cell renal cell carcinoma,[22–24] breast cancer,[7,8] cervical cancer,[9,11,12] colorectal cancer,[13,14,25] epithelial cancer,[26–28] esophageal squamous cell carcinoma,[29,30] gastric cancer,[2,6,31–34] hepatocellular carcinoma,[15–17] Melanoma,[35] nasopharyngeal carcinoma,[36] lung cancer,[37] Osteosarcoma,[38,39] pancreatic cancer,[40] and prostate cancer.[41] PVT1 expression level was detected by RT-PCR in all but 2 literatures. The clinical outcomes were also recoded including 33 studies for OS, 2 for RFS, 10 for DFS, 3 for PFS, and 1 for DSS. The basic information included in the article is summarized in Table 1.
Table 1.
The main characteristics of the included studies in the meta-analysis.
3.2. The expression of PVT1 was significantly correlated with survival
A total of 35 articles reported the relationship between PVT1 expression level and overall survival in various cancers. Due to the small heterogeneity (I2 = 19%, P = .17), the fixed-effect model was used for analysis. The result of merged HRs (HR = 1.64, 95% CI: 1.50–1.78, P < .000001) showed that increased PVT1 expression predicted decreased overall survival (Fig. 2). In addition, subgroup analysis was performed according to tumor types, the combined HR value showed that the expression of PVT was negatively correlated with the overall survival rate of patients with clear cell renal cell carcinoma (HR = 1.68, 95% CI: 1.25–2.24, P = .0005), breast cancer (HR = 1.96, 95% CI: 1.21–3.18, P = .007), cervical cancer (HR = 1.69, 95% CI: 1.27–2.26, P = .0004), colon cancer (HR = 2.03, 95% CI: 1.45–2.84, P < .0001), epithelial ovarian cancer (HR = 1.28, 95% CI: 1.10–1.48, P = .002), gastric cancer (HR = 1.82, 95% CI: 1.46–2.25, P < .00001), lung cancer (HR = 2.04, 95% CI: 1.58–2.65, P < .00001), osteosarcoma (HR = 2.11, 95% CI: 1.05–4.24, P = .04) and others (including: bladder cancer, nasopharyngeal carcinoma, pancreatic cancer, melanoma, prostate cancer) (HR = 2.23, 95% CI: 1.62–3.07, P < .00001). However, this negative correlation was not significant in the subgroup analysis of hepatocellular carcinoma (HR = 1.32, 95% CI: 0.92–1.90, P = .13).
Figure 2.
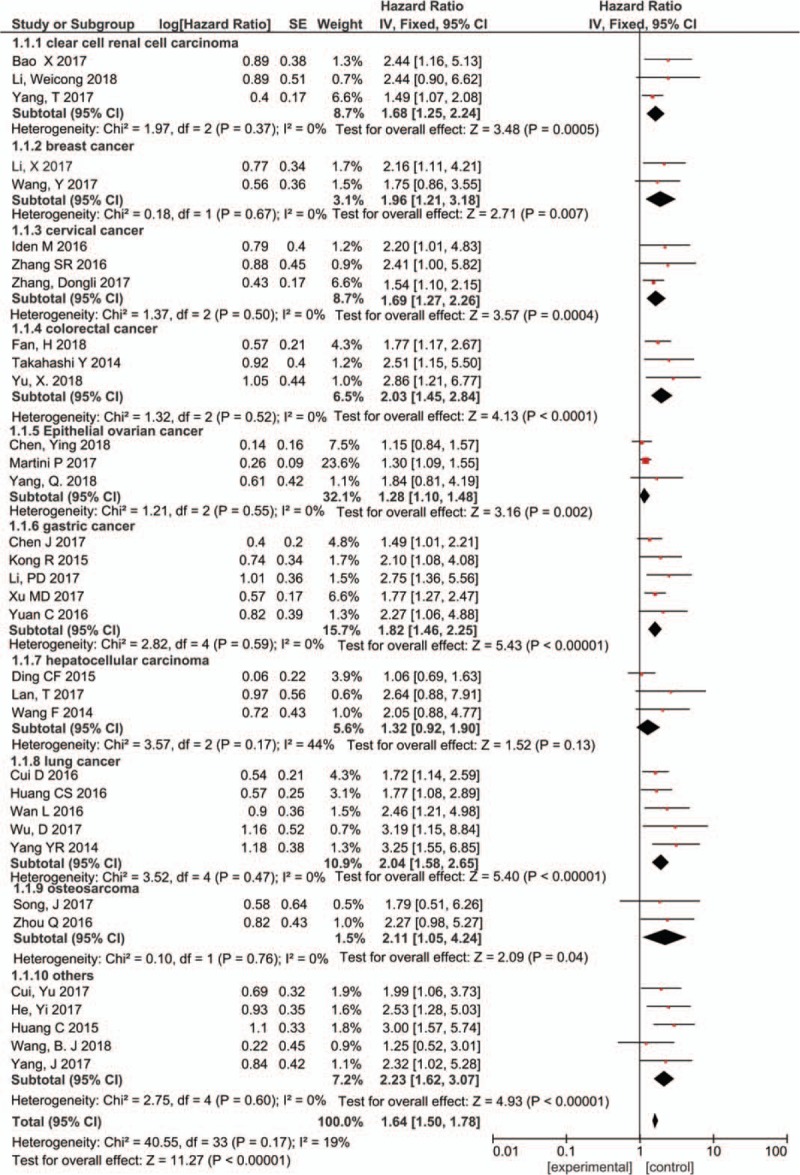
Forest plots of the included studies evaluating the HRs for PVT1 expression for OS by type of cancer. HR = hazard ratio, OS = overall survival, PVT1 = plasmacytoma variant translocation1.
3.3. The expression of PVT1 was significantly correlated with tumor size
A total of 22 literatures involving 2547 patients reported the expression levels of PVT1 in samples of different tumor sizes. Due to the heterogeneity (I2 = 57%, P = .0004), we used the random-effect model for analysis. The results showed that PVT1 expression was significantly associated with tumor size (OR = 1.50, 95% CI: 1.14–1.96, P = .004) (Fig. 3). The subgroup analysis was performed based on the type of cancers, including bladder cancer (n = 2), breast cancer (n = 2), colorectal cancer (n = 2), esophageal and gastric cancer (n = 4), hepatocellular carcinoma (n = 4), and lung cancer (n = 4). The results showed that the expression level of PVT1 was closely related to the tumor size of breast cancer (OR = 2.38, 95% CI: 1.45–3.91, P = .0006), hepatocellular carcinoma (OR = 2.04, 95% CI: 1.36–3.06, P = .0005), and lung cancer (OR = 1.60, 95% CI: 1.07–2.38, P = .02). Interestingly, in esophageal and gastric cancer, the expression of PVT1 seems to be elevated in tumors of small volumes (OR = 0.76, 95% CI: 0.51–1.15, P = .20), this requires more data validation.
Figure 3.
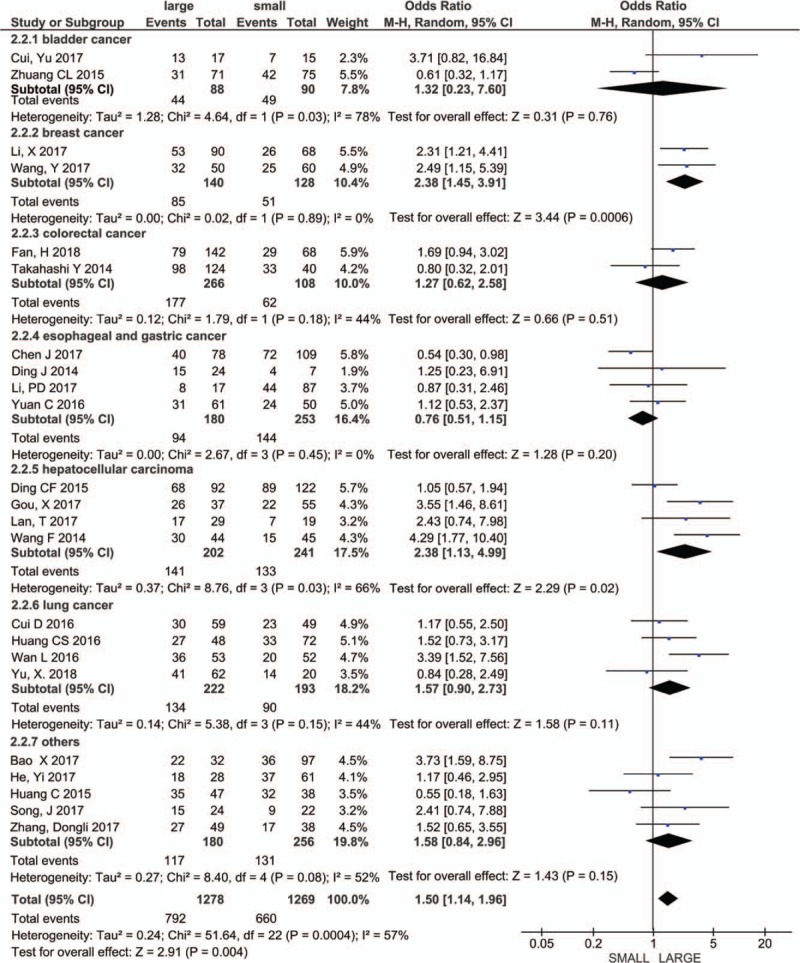
Forest plot for the association between PVT1 expression levels with tumor size. PVT1 = plasmacytoma variant translocation1.
3.4. The expression of PVT1 was significantly correlated with TNM stage
Twenty-eight studies including 2901 patients reported the relationship between PVT expression level and TNM stage. Due to the small heterogeneity (I2 = 38%, P = .02), we used the fixed-effect model for analysis. The merged OR value showed that the expression level of PVT1 was significantly associated with TNM stage (OR = 3.39, 95% CI: 2.73–4.20, P < .00001) (Fig. 4). The subgroup analysis was performed based on the type of cancers, including bladder cancer (n = 2), clear cell renal cell carcinoma (n = 2), breast cancer (n = 2), colorectal cancer (n = 2), esophageal and gastric cancer (n = 7), hepatocellular carcinoma (n = 4), and lung cancer (n = 3). Due to the heterogeneity of bladder cancer subgroup, we used a random model for subgroup analysis, the results showed that the expression level of PVT1 was closely related to the TNM stage of bladder cancer (OR = 2.81, 95% CI: 1.52–5.20, P = .001), clear cell renal cell carcinoma (OR = 5.79, 95% CI: 2.81–11.93, P < .00001), breast cancer (OR = 2.40, 95% CI: 1.45–3.98, P = .0007), colorectal cancer (OR = 5.54, 95% CI: 3.18–9.63, P < .00001), esophageal and gastric cancer (OR = 2.90, 95% CI: 2.07–4.05, P < .00001), hepatocellular carcinoma (OR = 2.20, 95% CI: 1.46–3.33, P = .0002), lung cancer (OR = 3.93, 95% CI: 2.26–6.85, P < .00001), and other cancers (OR = 3.98, 95% CI: 2.83–5.60, P < .00001).
Figure 4.
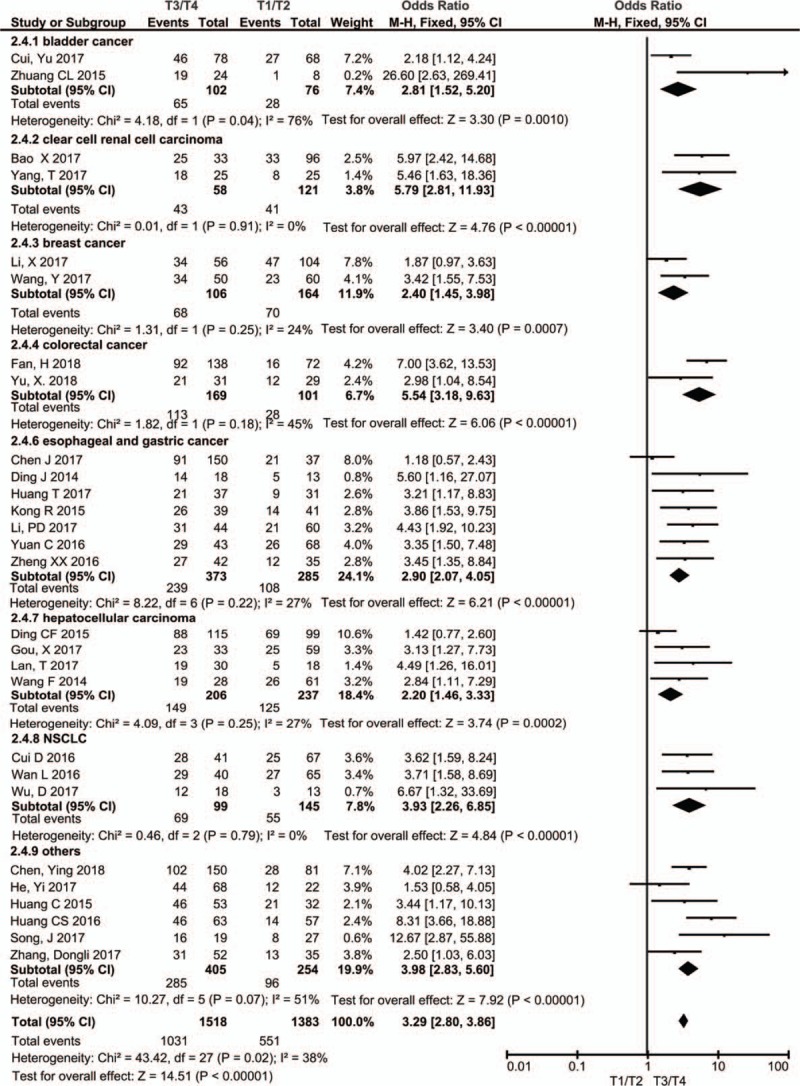
Forest plot for the association between PVT1 expression levels and clinical stage in different cancer patients. PVT1 = plasmacytoma variant translocation1.
3.5. The expression of PVT1 was closely related to lymph node metastasis
Twenty-two studies including 2329 patients reported the relationship between PVT expression level and lymph node metastasis. Due to the heterogeneity (I2 = 73%, P < .00001), we used the random-effect model for analysis. The results showed that PVT1 expression was significantly associated with lymph node metastasis (OR = 2.60, 95% CI: 1.76–3.84, P < .00001) (Fig. 5). The subgroup analysis was performed based on the type of cancers, including bladder cancer (n = 2), breast cancer (n = 2), colorectal cancer (n = 3), esophageal and gastric cancer (n = 5), lung cancer (n = 4). Due to the heterogeneity of bladder cancer and breast cancer subgroup, we used a random model for subgroup analysis, the results showed that the expression level of PVT1 was closely related to the lymph node metastasis of colorectal cancer (OR = 4.48, 95% CI: 2.81–7.16, P < .00001), esophageal and gastric cancer (OR = 2.04, 95% CI: 1.33–3.11, P = .001), lung cancer (OR = 3.34, 95% CI: 1.89–5.89, P < .0001), and other tumors (OR = 2.60, 95% CI: 1.76–3.84, P < .00001).
Figure 5.
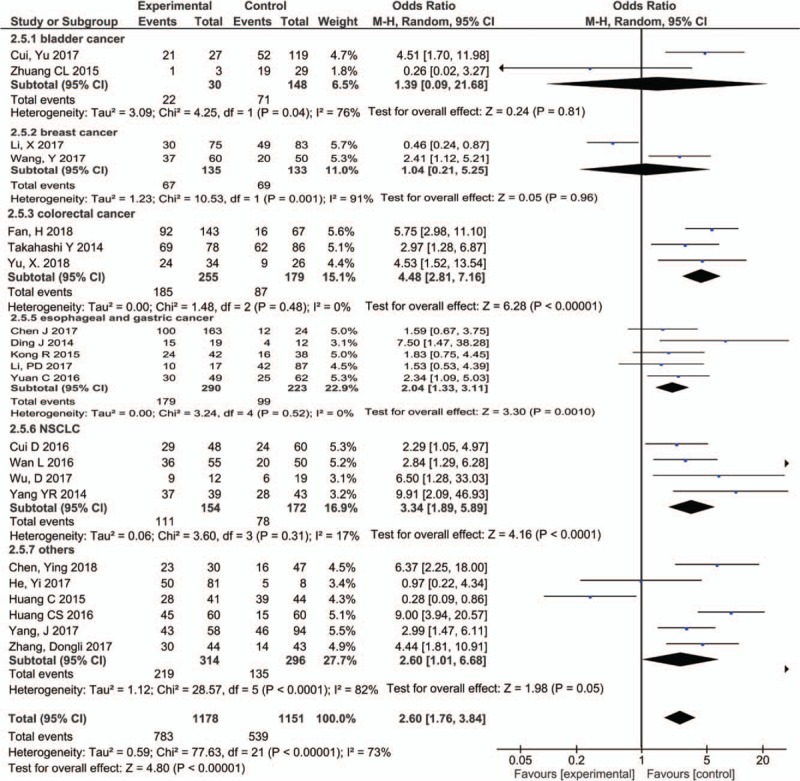
Forest plot for the association between PVT1 expression levels with LNM. LNM = lymph node metastasis, PVT1 = plasmacytoma variant translocation1
3.6. The expression of PVT1 was significantly correlated with distant metastasis
Nine studies including 996 patients reported the relationship between PVT expression level and distant metastasis. Due to the heterogeneity (I2 = 40%, P = .10), we used the fix-effect model for analysis. The results showed that PVT1 expression was significantly associated with distant metastasis (OR = 2.94, 95% CI: 1.90–4.56, P < .00001) (Fig. 6).
Figure 6.
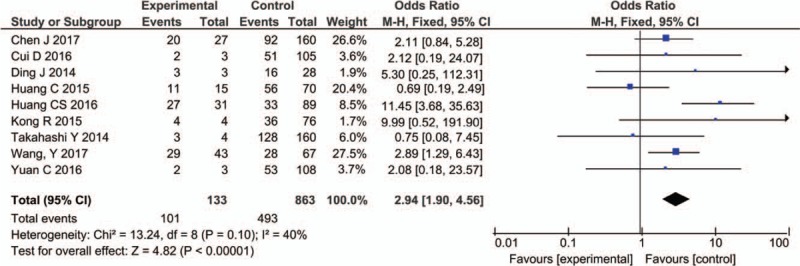
Forest plot for the association between PVT1 expression levels with DM. DM = distant metastasis, PVT1 = plasmacytoma variant translocation1.
3.7. Publication bias
The Begg test and funnel plot were used to assess the publication bias of this meta-analysis. Funnel plot showed uniform distribution of all studies, and no obvious asymmetry was observed among the studies investigating PVT1 expression on overall survival, tumor size, TNM stage, and distant metastasis (Fig. 7).
Figure 7.
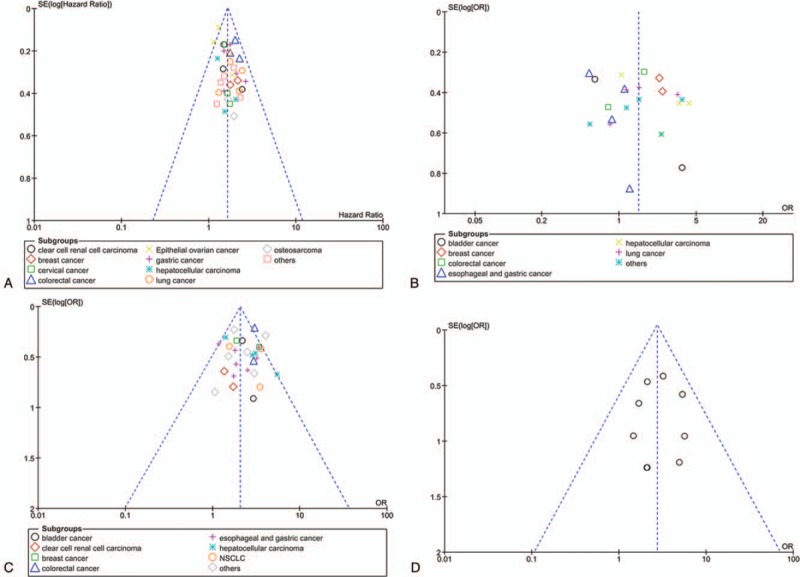
Funnel plot analysis of evaluating publication bias. (A) Begg funnel plot with pseudo 95% CIs for OS; (B) Begg funnel plot with pseudo 95% CIs for tumor size; (C) Begg funnel plot with pseudo 95% CIs for TNM stages; (D) Begg funnel plot with pseudo 95% CIs for distant metastases. CI = confidence interval, OS = overall survival, TNM = tumor–node–metastasis.
4. Discussion
PVT1, which is located at 8q24.21, was found to be upregulated in a diverse range of cancer types. In recent years, some literatures have also reported that PVT1 is related to the clinicopathological features and prognosis of various tumor patients, including lung cancer,[4,5] gastric cancer,[2,6] breast cancer,[7,8] cervical cancer,[9–12] colorectal cancer,[13,14] hepatocellular carcinoma.[15–18] However, most reports are limited by geographical, ethnic, and sample size limitations. Therefore, it is necessary to conduct a comprehensive meta-analysis of the existing data to evaluate the significance of PVT1 as a prognostic marker for the prognosis of various carcinomas. There have been some meta-analyses on PVT1 and tumor prognosis before,[42,43] but there have been many new literature reports in recent years. In order to improve the integrity and reliability of meta-analysis, we searched the literature published before November 22, 2018 on PVT1 and tumor prognosis for comprehensive analysis. A total of 39 literatures including 3974 patients were included in this study according to the inclusion and exclusion criteria.
To determine the relationship between PVT1 expression level and survival prognosis of patients, we conducted a comprehensive analysis of 35 literatures including various cancers. The results showed that increased PVT1 expression predicted decreased overall survival (HR = 1.64, 95% CI: 1.50–1.78, P < .000001). The subgroup analysis based on different tumor types showed that the high expression of PVT1 was related to the poor overall survival rate of patients with clear cell renal cell carcinoma, breast cancer, cervical cancer, colon cancer, epithelial ovarian cancer, gastric cancer, lung cancer, osteosarcoma and others (including: bladder cancer, nasopharyngeal carcinoma, pancreatic cancer, melanoma, prostate cancer) respectively. However, subgroup analysis of HCC showed that the high expression of PVT1 was not significantly correlated with the survival time of HCC patients. This may be because the number of cases included in Ding et al's[16] study (n = 214) was much higher than that in the other 2 literatures (n = 8948). Although no significant association was found, patients exhibiting high PVT1 expression levels demonstrated a trend for poor prognoses. Therefore, larger sample size data are needed to explore the relationship between PVT1 expression and overall survival of patients with liver cancer.
This study also analyzed the relationship between PVT1 expression and clinicopathological characteristics of different tumors. The results showed that the high expression of PVT1 was related to the tumor size and TNM stage of breast cancer, hepatocellular carcinoma, and lung cancer. Although it was found that the expression of PVT1 was negatively correlated with gastric cancer tumor size, the expression of PVT1 was significantly correlated with TNM stage and lymph node metastasis of gastric cancer. It was reported that PVT1 promotes hepatocellular carcinoma cell proliferation by stabilizing NOP2 protein.[18] It has also been reported that PVT1 inhibits miR-214 expression by interacting with EZH2 enhancer, thereby promoting the proliferation of hepatocellular carcinoma cells.[15] Therefore, pvt1-ezh2-mir-214-nop2 axis may be one of the mechanisms by which PVT1 regulates hepatocellular carcinoma cells. In gastric cancer, PVT1 interacts with FOXm1 to promote the proliferation and invasion of tumor cells.[2] In addition, PVT1 could directly interact with miR-186 and lead to the inhibition of HIF-1a expression, thereby promoting the proliferation of gastric carcinoma cells.[34]
In addition, meta-analysis showed that the increased expression of PVT1 was also significantly correlated with TNM staging of bladder cancer, clear cell renal cell carcinoma, and colorectal cancer, as well as lymph node metastasis of lung cancer and colon cancer. It has been reported that PVT1 may promote metastasis and proliferation of colon cancer via suppressing miR-30d-5p/RUNX2 axis.[25] PVT1 expression was also associated with distant metastasis of different cancers. Furthermore, no significant publication bias was found in our analysis.
Like other meta-analyses, this study also had its limitations. Firstly, there are more reports in Asia and less data in other regions. Secondly, the median value of PVT1 expression is inconsistent among different tumor types and literature reports, which may affect the heterogeneity of meta-analysis. Thirdly, the follow-up period of cancer patients may be inconsistent between different literature reports, which will also have a certain impact on the analysis results. Despite some disadvantages, this meta-analysis still has notable advantages. Firstly, we searched the literature before November 2018, and included 39 literatures including 3974 samples. The sample size was large enough to reduce the errors caused by insufficient sample size. Secondly, subgroup analysis was carried out for different types of tumors to reduce the heterogeneity caused by different types of tumors.
To sum up, although there are some limitations, our meta-analysis showed that high expression of PVT1 was significantly associated with tumor size, TNM stage, lymph node metastasis, distant metastasis, and overall survival time and so on, especially in breast cancer, liver cancer, lung cancer, indicating meaning is more apparent, but in bladder cancer, renal cell carcinoma and nasopharyngeal carcinoma, the indication is relatively weak as the fewer samples.
Author contributions
Conceptualization: Bin Zhang.
Data curation: Bin Zhang.
Formal analysis: Bin Zhang.
Funding acquisition: Dong Wang.
Investigation: Dong Wang.
Methodology: Dong Wang.
Project administration: Kai Xu.
Resources: Kai Xu, Jian-lin Liu.
Software: Kai Xu, Jian-lin Liu.
Supervision: Jian-lin Liu, Dao-ying Yuan.
Validation: Bo Zou, Dao-ying Yuan.
Visualization: Bo Zou, Dao-ying Yuan, Zhen Meng.
Writing – original draft: Zhen Meng.
Writing – review & editing: Bo Zou, Zhen Meng.
Footnotes
Abbreviations: ATG7 = autophagy related 7, DFS = disease-free survival, DSS = disease specific survival, FOXM1 = fork head box M1, HR = hazard ratio, lncRNA = long non-coding RNA, miRNA = micro RNA, OR = odds ratio, OS = overall survival, PFS = progression free survival, PVT1 = plasmacytoma variant translocation1, RFS = recurrence free survival, STAT3 = signal transducer and activator of transcription 3, TNM = tumor–node–metastasis, VEGFA = vascular endothelial growth factor A.
This work was supported by Medicine and Health Science Technology Development Plan Project (2017WSA15041), Shandong Province. This work was also supported by National Natural Science Foundation of China (81472530 and 81602374).
The authors declared no conflict of interests.
References
- [1].Ma Y, Wang P, Xue Y, et al. PVT1 affects growth of glioma microvascular endothelial cells by negatively regulating miR-186. Tumour Biol 2017;39:1010428317694326. [DOI] [PubMed] [Google Scholar]
- [2].Xu MD, Wang Y, Weng W, et al. A positive feedback loop of lncRNA-PVT1 and FOXM1 facilitates gastric cancer growth and invasion. Clin Cancer Res 2017;23:2071–80. [DOI] [PubMed] [Google Scholar]
- [3].Zhao J, Du P, Cui P, et al. LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene 2018;37:4094–109. [DOI] [PubMed] [Google Scholar]
- [4].Huang C, Liu S, Wang H, et al. LncRNA PVT1 overexpression is a poor prognostic biomarker and regulates migration and invasion in small cell lung cancer. Am J Transl Res 2016;8:5025–34. [PMC free article] [PubMed] [Google Scholar]
- [5].Cui D, Yu CH, Liu M, et al. Long non-coding RNA PVT1 as a novel biomarker for diagnosis and prognosis of non-small cell lung cancer. Tumour Biol 2016;37:4127–34. [DOI] [PubMed] [Google Scholar]
- [6].Chen J, Li Y, Zheng Q, et al. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer Lett 2017;388:208–19. [DOI] [PubMed] [Google Scholar]
- [7].Wang Y, Zhou J, Wang Z, et al. Upregulation of SOX2 activated LncRNA PVT1 expression promotes breast cancer cell growth and invasion. Biochem Biophys Res Commun 2017;493:429–36. [DOI] [PubMed] [Google Scholar]
- [8].Li X, Chen W, Wang H, et al. Amplification and the clinical significance of circulating cell-free DNA of PVT1 in breast cancer. Oncol Rep 2017;38:465–71. [DOI] [PubMed] [Google Scholar]
- [9].Iden M, Fye S, Li K, et al. The lncRNA PVT1 contributes to the cervical cancer phenotype and associates with poor patient prognosis. PLoS One 2016;11:e0156274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wang X, Wang G, Zhang L, et al. LncRNA PVT1 promotes the growth of HPV positive and negative cervical squamous cell carcinoma by inhibiting TGF-beta1. Cancer Cell Int 2018;18:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang S, Zhang G, Liu J. Long noncoding RNA PVT1 promotes cervical cancer progression through epigenetically silencing miR-200b. APMIS 2016;124:649–58. [DOI] [PubMed] [Google Scholar]
- [12].Zhang D, Sun G, Zhang H, et al. Long non-coding RNA PVT1 facilitates cervical cancer progression through negative modulation of miR-128-3p. Int J Clin Exp Pathol 2017;10:4522–9. [Google Scholar]
- [13].Takahashi Y, Sawada G, Kurashige J, et al. Amplification of PVT-1 is involved in poor prognosis via apoptosis inhibition in colorectal cancers. Br J Cancer 2014;110:164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fan H, Zhu JH, Yao XQ. Long non-coding RNA PVT1 as a novel potential biomarker for predicting the prognosis of colorectal cancer. Int J Biol Markers 2018;33:415–22. [DOI] [PubMed] [Google Scholar]
- [15].Gou X, Zhao X, Wang Z. Long noncoding RNA PVT1 promotes hepatocellular carcinoma progression through regulating miR-214. Cancer Biomark 2017;20:511–9. [DOI] [PubMed] [Google Scholar]
- [16].Ding C, Yang Z, Lv Z, et al. Long non-coding RNA PVT1 is associated with tumor progression and predicts recurrence in hepatocellular carcinoma patients. Oncol Lett 2015;9:955–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lan T, Yan X, Li Z, et al. Long non-coding RNA PVT1 serves as a competing endogenous RNA for miR-186-5p to promote the tumorigenesis and metastasis of hepatocellular carcinoma. Tumour Biol 2017;39:1010428317705338. [DOI] [PubMed] [Google Scholar]
- [18].Wang F, Yuan JH, Wang SB, et al. Oncofetal long noncoding RNA PVT1 promotes proliferation and stem cell-like property of hepatocellular carcinoma cells by stabilizing NOP2. Hepatology (Baltimore) 2014;60:1278–90. [DOI] [PubMed] [Google Scholar]
- [19].Wang Y, Zeng T. Response to: practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2013;14:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhuang C, Li J, Liu Y, et al. Tetracycline-inducible shRNA targeting long non-coding RNA PVT1 inhibits cell growth and induces apoptosis in bladder cancer cells. Oncotarget 2015;6:41194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cui Y, Liu L, Chen J, et al. Increased expression of long non-coding RNA PVT1 correlates with clinical progression and poor prognosis in bladder cancer. Int J Clin Exp Pathol 2017;10:3265–71. [Google Scholar]
- [22].Bao X, Duan J, Yan Y, et al. Upregulation of long noncoding RNA PVT1 predicts unfavorable prognosis in patients with clear cell renal cell carcinoma. Cancer Biomark 2017;21:55–63. [DOI] [PubMed] [Google Scholar]
- [23].Yang T, Zhou H, Liu P, et al. lncRNA PVT1 and its splicing variant function as competing endogenous RNA to regulate clear cell renal cell carcinoma progression. Oncotarget 2017;8:85353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Li W, Zheng Z, Chen H, et al. Knockdown of long non-coding RNA PVT1 induces apoptosis and cell cycle arrest in clear cell renal cell carcinoma through the epidermal growth factor receptor pathway. Oncol Lett 2018;15:7855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yu X, Zhao J, He Y. Long non-coding RNA PVT1 functions as an oncogene in human colon cancer through miR-30d-5p/RUNX2 axis. J BUON 2018;23:48–54. [PubMed] [Google Scholar]
- [26].Martini P, Paracchini L, Caratti G, et al. lncRNAs as novel indicators of patients’ prognosis in Stage I epithelial ovarian cancer: a retrospective and multicentric study. Clin Cancer Res 2017;23:2356–66. [DOI] [PubMed] [Google Scholar]
- [27].Chen Y, Du H, Bao L, et al. LncRNA PVT1 promotes ovarian cancer progression by silencing miR-214. Cancer Biol Med 2018;15:238–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yang Q, Yu Y, Sun Z, et al. Long non-coding RNA PVT1 promotes cell proliferation and invasion through regulating miR-133a in ovarian cancer. Biomed Pharmacother 2018;106:61–7. [DOI] [PubMed] [Google Scholar]
- [29].Zheng X, Hu H, Li S. High expression of lncRNA PVT1 promotes invasion by inducing epithelial-to-mesenchymal transition in esophageal cancer. Oncol Lett 2016;12:2357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li PD, Hu JL, Ma C, et al. Upregulation of the long non-coding RNA PVT1 promotes esophageal squamous cell carcinoma progression by acting as a molecular sponge of miR-203 and LASP1. Oncotarget 2017;8:34164–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ding J, Li D, Gong M, et al. Expression and clinical significance of the long non-coding RNA PVT1 in human gastric cancer. Onco Targets Ther 2014;7:1625–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kong R, Zhang EB, Yin DD, et al. Long noncoding RNA PVT1 indicates a poor prognosis of gastric cancer and promotes cell proliferation through epigenetically regulating p15 and p16. Mol Cancer 2015;14:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yuan CL, Li H, Zhu L, et al. Aberrant expression of long noncoding RNA PVT1 and its diagnostic and prognostic significance in patients with gastric cancer. Neoplasma 2016;63:442–9. [DOI] [PubMed] [Google Scholar]
- [34].Huang T, Liu HW, Chen JQ, et al. The long noncoding RNA PVT1 functions as a competing endogenous RNA by sponging miR-186 in gastric cancer. Biomed Pharmacother 2017;88:302–8. [DOI] [PubMed] [Google Scholar]
- [35].Wang BJ, Ding HW, Ma GA, et al. Noncoding RNA PVT1 promotes melanoma progression via endogenous sponging miR-26b. Oncol Res 2018;26:675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].He Y, Jing Y, Wei F, et al. Long non-coding RNA PVT1 predicts poor prognosis and induces radioresistance by regulating DNA repair and cell apoptosis in nasopharyngeal carcinoma. Cell Death Dis 2018;9:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yang YR, Zang SZ, Zhong CL, et al. Increased expression of the lncRNA PVT1 promotes tumorigenesis in non-small cell lung cancer. Int J Clin Exp Pathol 2014;7:6929–35. [PMC free article] [PubMed] [Google Scholar]
- [38].Zhou Q, Chen F, Zhao J, et al. Long non-coding RNA PVT1 promotes osteosarcoma development by acting as a molecular sponge to regulate miR-195. Oncotarget 2016;7:82620–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Song J, Wu X, Liu F, et al. Long non-coding RNA PVT1 promotes glycolysis and tumor progression by regulating miR-497/HK2 axis in osteosarcoma. Biochem Biophys Res Commun 2017;490:217–24. [DOI] [PubMed] [Google Scholar]
- [40].Huang C, Yu W, Wang Q, et al. Increased expression of the lncRNA PVT1 is associated with poor prognosis in pancreatic cancer patients. Minerva Med 2015;106:143–9. [PubMed] [Google Scholar]
- [41].Yang J, Li C, Mudd A, et al. LncRNA PVT1 predicts prognosis and regulates tumor growth in prostate cancer. Biosci Biotechnol Biochem 2017;81:2301–6. [DOI] [PubMed] [Google Scholar]
- [42].Lu D, Luo P, Wang Q, et al. lncRNA PVT1 in cancer: a review and meta-analysis. Clin Chim Acta 2017;474:1–7. [DOI] [PubMed] [Google Scholar]
- [43].Gao S, Zhao ZY, Wu R, et al. Prognostic value of long noncoding RNAs in gastric cancer: a meta-analysis. Onco Targets Ther 2018;11:4877–91. [DOI] [PMC free article] [PubMed] [Google Scholar]


