Abstract
The prognostic utility of histologic features in patients with diabetic nephropathy (DN) classified according to the Renal Pathology Society (RPS) classification is controversial. Therefore, we aimed to evaluate the relationship between histologic changes and renal outcome in DN patients.
We examined the renal outcome at November 30, 2017 of 74 adult patients (median age of 54.6 years, 69% male, 81% diabetes mellitus (DM) type 2, estimated GFR (eGFR) 29.6 mL/min) with biopsy proven DN between 2010 and 2015. The primary endpoint was renal replacement therapy (RRT) initiation.
Half of the patients progressed to end stage renal disease (ESRD) during follow-up; they had lower eGFR, increased proteinuria, hematuria and serum cholesterol. Regarding the pathologic features, they were more frequently in class III and IV, had higher interstitial fibrosis and tubular atrophy score (IFTA), increased interstitial inflammation, more frequent arteriolar hyalinosis and higher glomerular basement membrane (GBM) thickness. The mean kidney survival time was 2.7 (95%CI 2.1, 3.3) years. In univariate time-dependent analyses, higher RPS DN class, increased IFTA, the presence of arteriolar hyalinosis and arteriosclerosis were associated with RRT initiation.
In the fully adjusted model, the clinical characteristics associated with poor renal survival were longer duration of DM, lower eGFR, increased proteinuria and higher hematuria and the only pathologic lesions to remain significant were the GBM thickness and the IFTA.
In conclusion, in this European cohort, the severity of glomerular lesions evaluated with the RPS DN classification had limited utility in predicting RRT initiation. However, IFTA and GBM thickness were significantly associated with renal survival.
Keywords: diabetic nephropathy, renal outcome, renal pathology
1. Introduction
Diabetic nephropathy (DN) is one of the leading causes of end stage renal disease (ESRD) worldwide, which develops in nearly 10 to 30% of patients with diabetes mellitus (DM).[1] Methods to predict chronic kidney disease progression to ESRD in DN patients currently rely upon clinical factors like diabetes duration, blood pressure, estimated glomerular filtration rate (eGFR), proteinuria, and hemoglobin A1c level.[2–4] Data regarding the pathologic features with prognostic utility in DN patients are limited, mostly because kidney biopsy is performed only if an alternative diagnosis that would impose specific therapy is suspected.
In order to standardize the identification and scoring of diabetic kidney lesions, the Renal Pathology Society (RPS) conceived a DN classification in 2010.[5] However, whether this pathologic classification can be used for predicting renal outcome is controversial.
The largest study to date reported that the severity of glomerular and interstitial lesions is significantly associated with renal outcomes in patients with DN.[6] In a smaller study, interstitial lesions—but not glomerular lesions—were found to be a significant predictor of renal prognosis.[7] However, these data have been obtained in Southeast Asian cohorts, in whom the natural history of DN may be distinct from European derived cohorts. Moreover, in a study on a broad ethnic diverse cohort, RPS DN classification was not predictive of time to ESRD.[8] Therefore, challenges remain in the evaluation of the role of the pathologic features in prediction renal prognosis in patients with DM.
In this study, we aimed to evaluate the relationship between histologic changes evaluated with RPS DN and renal outcome in European patients (i.e., Caucasian) with biopsy-proven DN who were followed up for more than one year after kidney biopsy.
2. Materials and methods
2.1. Study design and population
This is a longitudinal, retrospective study of patients who underwent native kidney biopsy at “Dr Carol Davila” Teaching Hospital of Nephrology between January 1, 2010 and December 31, 2015 and had a pathologic diagnosis of DN. Patients with superimposed glomerular diseases were excluded. If patients had more than one kidney biopsy performed during the follow-up period, only the first biopsy was included in the study. Biopsy cores with inadequate tissue (i.e., <8 glomeruli) were excluded.
The electronic medical records were reviewed from the time of kidney biopsy to one of four endpoints: ESRD, death, loss to follow-up, or until November 30, 2017. Renal survival was the primary end point of our study and was defined by the progression to ESRD (the need for chronic renal replacement therapy - RRT).
The following data at diagnostic kidney biopsy were available: age and duration of diabetes at the time of biopsy, mean arterial pressure (MAP defined as diastolic blood pressure plus 1/3 of pulse pressure), presence of hypertension (defined as a blood pressure >140/90 mmHg or the use of antihypertensive agents), fasting blood glucose, inflammatory status (serum hemoglobin, erythrocyte sedimentation rate, C-reactive protein), lipid profile (serum cholesterol and triglycerides), serum albumin, eGFR using the four variable CKD-EPI formula, proteinuria, hematuria (Stansfeld–Webb method) and treatment history. Proteinuria was quantified by either the spot urine protein-to-creatinine ratio or the 24-hour urine protein-to-creatinine ratio, depending on which was available.
Diabetes type was unclear in medical records in 7 patients. In these cases, a physician (GS) deduced diabetes type on a combination of age of onset, history of oral hypoglycemic use, and/or insulin regimen.
We used the hypertension-augmented Charlson comorbidity score (hCCS), adding hypertension as a comorbid condition to the CCS (i.e., hCCS was constructed by assigning the weight of 1 point to hypertension and adding to the CCS), in order to quantify the comorbidity burden.[9]
The study was conducted with the provisions of the Declaration of Helsinki and the protocol was approved by the local Ethics Committee.
2.2. Pathologic classification
For each biopsy specimen, light microscopy, immunofluorescence and electron microscopy were routinely performed. All the biopsies were categorized based on the pathologic classification of the Renal Pathology Society Diabetic Nephropathy Classification. The glomerular classifications were as follows - class I: glomerular basement membrane (GBM) thickening; class IIa: mild mesangial expansion; class IIb: severe mesangial expansion; class III: nodular sclerosis and class IV: global glomerulosclerosis in >50% of glomeruli.[5]
In addition, we also evaluated the presence of several common glomerular changes, such as endotheliosis, extracapillary hypercellularity and glomerular basement membrane thickness.
Interstitial fibrosis and tubular atrophy (IFTA) scores were classified as follows: 0, absent; 1, less than 25%; 2, 25% to 50%; and 3, greater than 50% of the total area. Interstitial inflammation was scored as follows: 0, absent; 1, inflammation only in relation to IFTA; 2, inflammation in areas without IFTA.[5]
We also assessed the arteriolar hyalinosis and arteriosclerosis defined as non-hyaline thickening of the vascular wall with reduction of the lumen and grouped the lesions as present or absent.
All the specimens were scored by the same pathologist (ME) who was blinded to the clinical findings.
2.3. Statistical analysis
Continuous variables are presented as mean or median according to distribution with 95% confidence interval, and categorical variables as percentage. Survival analyses were conducted with the Kaplan–Meier method and the log-rank test was used for comparisons. Variables related to kidney survival were further evaluated in univariate and multivariate Cox proportional hazard models; logarithmic transformation of skewed variables in order to normalize distribution was performed. Two multivariable risk models were created, both of which included clinical characteristics: age, gender, DM duration, hCCS, baseline eGFR, urinary protein and hematuria. The Glomerular Model incorporated only those glomerular characteristics significant to P < .1 in the univariate models. The Fully Adjusted Model included glomerular characteristics significant to P < .05 in the Glomerular Model, as well as interstitial and vascular characteristics significant to P < .1 in univariate models. We used two methods in order to test for collinearity among our predictor variables: i) the variance inflation factor (VIF), where VIF <10 is desirable; (ii) the absolute value of correlation coefficients, where |r| or |rs| <0.7 is desirable. There was no significant colinearity between the variables used in Cox proportional hazard models. A P value of less than .05 was considered statistically significant.
Statistical analyses were performed with SPSS (SPSS Inc., Chicago, IL) and Analyse-it (Analyse-it Software, Ltd., Leeds, UK) packages.
3. Results
3.1. Patients characteristics
In the study period, 90 renal biopsies of DN were found in our database. Exclusion criteria dropped out 16 cases; the main reasons were the presence of less than eight glomeruli or incomplete clinical data. The study population included 74 patients, with a median age of 54.6 years at the time of the renal biopsy and with a male gender predominance (69%). Most of the patients had DM type 2 (81%). The median duration of DM was 10 years and diabetic retinopathy was present in half of the patients. Insulin was the most frequent treatment, followed by oral antidiabetic drugs and diet only (Table 1).
Table 1.
Baseline (at the time of kidney biopsy) characteristics of 74 patients with biopsy proven diabetic nephropathy, stratified by renal replacement therapy initiation.
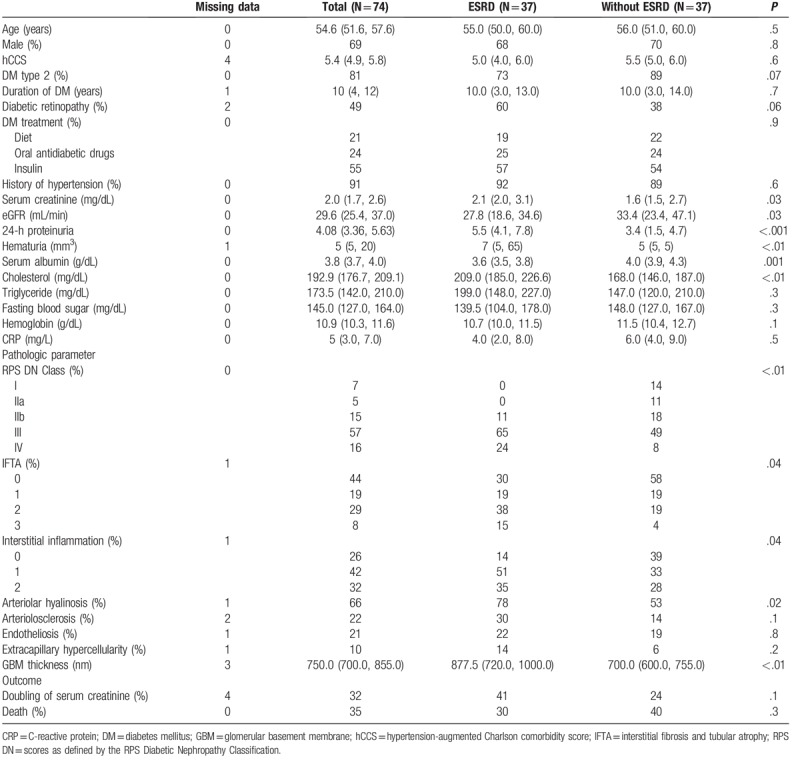
Significant renal impairment was present in our cohort, with median serum creatinine of 2 mg/dL and eGFR 29.6 mL/min at time of biopsy; just over half of patients had eGFR <30 mL/min. Median 24-h proteinuria was in the nephrotic range (4.0 g/day), but only 27% had nephrotic syndrome at time of biopsy (Table 1).
According to the RPS DN glomerular classification, more than half of the patients were in class III (57%), followed by class IV (16%) and IIb (15%). Ten percent of the patients had extracapillary hypercellularity. IFTA evaluated with the IFTA score were present in 56% of the studied patients and interstitial inflammation in relation to IFTA was present in 42% of the patients. Arteriolar hyalinosis was the most frequent vascular lesion (Table 1).
The median follow-up period was 32 months (95%CI; 26, 38). Half of the patients progressed to ESRD during follow-up; they had lower eGFR, increased proteinuria, higher hematuria and higher serum cholesterol. Regarding the pathologic features, patients who started RRT were classified more frequent as class III and IV; moreover, they had higher IFTA score, increased interstitial inflammation, more frequent arteriolar hyalinosis and higher GBM thickness (Table 1).
3.2. The renal outcome according to pathologic groups
A total of 26 (35%) patients died during the period of the study; cardiovascular disease was the leading cause of death (n = 16), followed by infectious disease (n = 8). However, 11 patients died after RRT initiation, thus the total number of patients included for multivariate renal survival analysis was 59. The mean kidney survival time for this cohort was 2.7 (95%CI 2.1, 3.3) years. Renal survivals at 12, 24, 36, 48, 60, and 72 months were 62, 52, 43, 34, 26, and 26%, respectively.
In univariate time-dependent analyses of kidney survival, higher RPS DN class, increased IFTA score, the presence of arteriolar hyalinosis and arteriosclerosis were associated with poor renal survival (Fig. 1).
Figure 1.
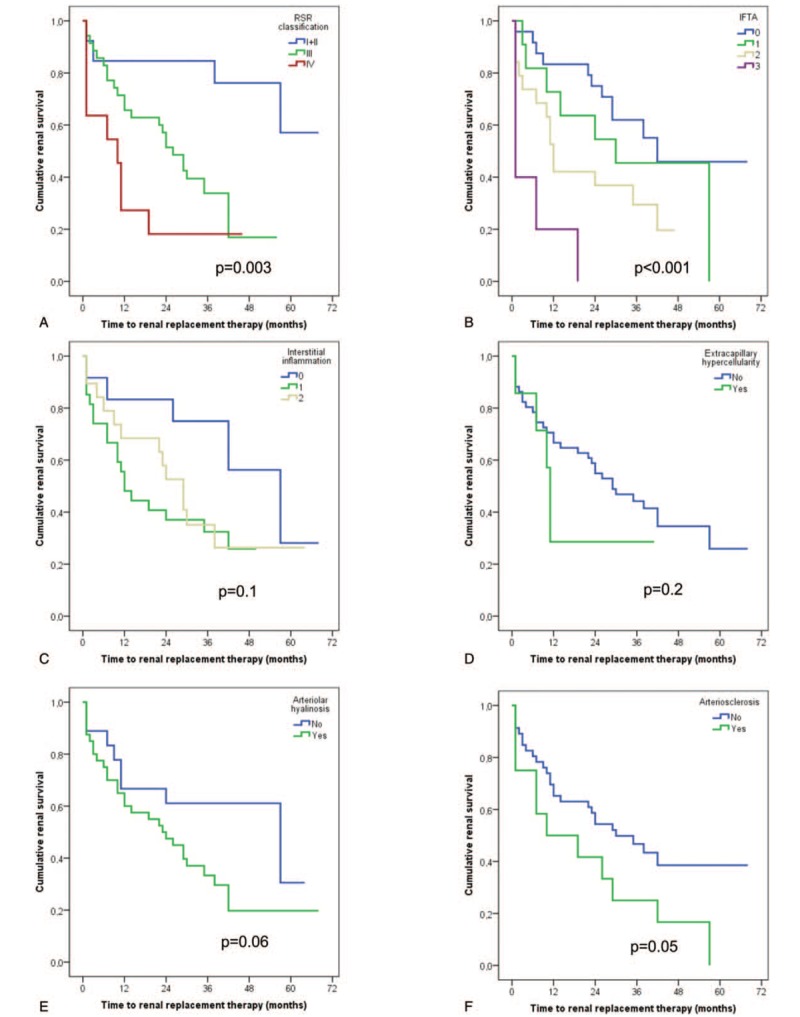
Kaplan–Meier curves of renal survival rate in patients with diabetic nephropathy. (A) Renal survival rates in glomerular pathologic classes. (B) Renal survival rates in IFTA. (C) Renal survival rates in interstitial inflammation. (D) Renal survival rates in extracapillary hypercellularity. (E) Renal survival rates in arteriolar hyalinosis. (F) Renal survival rates in arteriosclerosis.
Results from univariate and multivariable Cox proportional sub-distribution hazards models of ESRD are displayed in Table 2. The clinical characteristics associated with poor renal survival in the fully adjusted model were longer duration of DM, lower eGFR, increased proteinuria and higher hematuria and the only pathologic lesions to remain significant were the GBM thickness and the IFTA score.
Table 2.
Univariate and multivariate Cox proportional hazards models for ESRD of 59 patients. The glomerular model included clinical covariates (regardless of P value) and glomerular variables significant to P < .1 in univariate models. The fully adjusted model included clinical covariates (regardless of P value), glomerular variables significant to P < .1 in the glomerular model, and interstitial and vascular variables significant to P < .1 in univariate models.

Of note, RPS DN classification introduced as a continuous variable was significantly associated with the renal outcome in the glomerular model and had a significant trend in the fully adjusted model (Table 2). However, when RPS DN classification was introduced as a categorical variable there was no relationship with the renal outcome in the fully adjusted model. In contrast, higher IFTA score remained significantly associated with the renal outcome (Fig. 2).
Figure 2.

Multivariate Cox proportional hazards models for ESRD of 59 diabetic nephropathy patients with RPS DN and IFTA introduced as categorical variables: glomerular model (left) and fully adjusted model (right).
4. Discussion
In the present study, we evaluated the relationship between histologic changes and renal outcome in patients with biopsy proven DN. Interstitial fibrosis and tubular atrophy evaluated with IFTA score and the GBM thickness, but not the RPS DN classes were significantly associated with renal survival, independent of clinical and other pathologic features.
In the fully adjusted model, the RPS DN classes introduced as categorical variables were not predictive for ESRD initiation. This finding is contrary to previous studies, which have supported the prognostic utility of the RPS DN classification.[6,7,10,11] However, these data have been obtained from Southeast Asian cohorts, in whom the natural history of DN may be distinct from European populations. In line with this observation, Mottl et al on a broad ethnic diverse cohort (49% black race, 51% other races) showed that RPS DN is not predictive of time to ESRD.[8] To the best of our knowledge, our study is the first European report on RPS DN classification. Moreover, care practices may vary between countries, so the findings of clinical biopsies performed elsewhere may not be applicable in Europe.
In the largest study to date on histologic changes and renal outcome in DN (396 patients, mean baseline eGFR of 73±33 mL/min, 24-hour urine protein 1.5 g/d), glomerular and interstitial lesions were independently predictive of the time to ESRD. However, the authors used the RPS DN classification as a continuous variable in the multivariate analysis and adjusted only for eGFR, proteinuria and mean arterial pressure.[6] In order to overcome this limitation, we adjusted for the full spectrum of DN progression risk factors and reported the models with RPS DN classification as continuous and categorical variable. In neither fully adjusted models, the glomerular lesions classified according to RPS were not predictive for renal survival.
Several studies evaluated the relationship between interstitial lesions and the renal outcome in patients with diabetic kidney disease. Ruggenenti et al reported that interstitial fibrosis significantly predicted renal survival only in univariate analysis.[12] Moreover, Christensen et al found no relationship between focal IFTA of cortical area and rate of decline in GFR in patients with typical diabetic glomerulopathy.[13] Nevertheless, these results are limited by a small number of selected patients and different assessment methodology. An et al showed that IFTA as well as glomerular lesions (RPS DN classification) constituted an independent risk factor for renal outcome. However, when patients in classes I and IIa were excluded in the analysis, IFTA became the only independent predictor of renal outcomes.[6] Furthermore, Okada et al reported that interstitial lesions, but not glomerular lesions were a significant predictor for renal outcome in patients with type 2 DM and overt proteinuria.[7] Similarly, we found that IFTA score remained an independent determinant of progression to ESRD when adjusting for clinical, glomerular, and vascular features. Taken together, this data suggests that renal outcome correlate more strongly with the presence of progressive deterioration in tubular and interstitial architecture than with changes in glomerular integrity. Therefore, IFTA seems to be a final common pathway to end-stage renal disease.
GBM thickening is a characteristic early change in type 1 and type 2 DN and, more importantly, increases with duration of disease.[14–17] GBM thickening is a consequence of an imbalance between extracellular matrix synthesis and degradation, nonenzymatic glycosylation, change in spatial distribution of collagen type IV, and nonspecific trapping of serum proteins.[18–21] Interestingly, in our data GBM width was a predictor of renal outcome independently of the clinical, glomerular, interstitial and vascular features.
Recently, Caramori et al reported a structural–functional study on 94 patients with long-standing type 1 diabetes and normoalbuminuria who volunteered for research kidney biopsy and had more than 5 years of follow-up.[22] In this cohort, higher GBM thickness and higher level of hemoglobin A1c were independent predictors of progression to proteinuria, ESRD, or both.[22] Moreover, Zhang et al showed that type 2 diabetes patients with greater width of GBM had relatively poorer renal prognosis, although GBM width did not emerge as an independent indicator of disease progression.[23]
Attached to the underlying GBM via transmembrane cell receptors, the podocyte is likely the key culprit in GBM thickening in diabetes, because it is responsible for the matrix turnover.[24] Therefore, GBM thickening in DN may be an injured podocyte response to stress, which could appear even before cell detachment, apoptosis, and albuminuria.
In contrast to the pathological findings, the clinical variables associated with renal survival in the current study were consistent with those in previous reports: male gender, longer duration of DM, lower eGFR and increased proteinuria.[25]
The urine sediment in diabetic kidney disease is usually bland, but several studies have shown that microscopic hematuria can occur in patients with biopsy proven DN with a rate of 15% to 35%.[26,27] Moreover, previous studies have demonstrated an association between the severity of proteinuria, reduced renal function, and an increase in the prevalence of hematuria in patients with DN.[28–31] In line with these studies, we found that hematuria was associated with poor renal survival in the fully adjusted model. Mesangiolysis, loss of matrix and detachment of mesangium from the peripheral capillary loop lead to capillary microaneurysms formation, usually found around the expanded Kimmelstiel–Wilson nodules.[32] It has been suggested that hematuria in DN might result from the rupture of these capillary microaneurysms.[33]
Our study has several limitations, including the retrospective design and the sample of small size, from a single institution. Also, most of the patients had an eGFR lower 60 mL/min (86%) and over 70% were in RPS DN classes III and IV. Therefore, the skewed distribution towards the higher RPS DN classes might have concealed the prognostic significance of earlier glomerular lesions (I and II RPS DN classes), which is further suggested by the fact that GBM, the initial lesion in DN was an independent predictor of ESRD in both models. Moreover, studies on kidney biopsies in diabetic patients may be subjected to biases related to indication and timing of the biopsy. Of note, due to the retrospective nature of the study - data regarding DM control like hemoglobin A1C and glycated albumin were absent at the moment of kidney biopsy; also, in 7 patients the type of DM was deduced form the medical records.
In conclusion, in this European cohort, the severity of glomerular lesions evaluated with the RPS DN classification had a limited utility in predicting renal replacement therapy initiation. However, interstitial fibrosis and tubular atrophy evaluated with IFTA score and the GBM thickness were significantly associated with renal survival.
Acknowledgment
We would like to acknowledge Dr Anne-Marie Crăciun, a talented and insightful nephrologist who cared for many of the patients with DN included in the present study. Dr Anne-Marie Crăciun passed away last year. She was an inspiration to all of us and she will be missed deeply.
Author contributions
Conceptualization: Gabriel Stefan, Gabriel Mircescu.
Data curation: Simona Stancu, Adrian Zugravu, Nicoleta Petre, Eugen Mandache.
Formal analysis: Gabriel Stefan.
Investigation: Simona Stancu, Adrian Zugravu, Nicoleta Petre, Eugen Mandache.
Methodology: Gabriel Stefan, Gabriel Mircescu.
Supervision: Gabriel Mircescu.
Validation: Gabriel Stefan, Simona Stancu, Adrian Zugravu, Nicoleta Petre, Eugen Mandache, Gabriel Mircescu.
Writing – original draft: Gabriel Stefan.
Writing – review & editing: Simona Stancu, Adrian Zugravu, Nicoleta Petre, Eugen Mandache, Gabriel Mircescu.
Footnotes
Abbreviations: DM = diabetes mellitus, DN = diabetic nephropathy, eGFR = estimated GFR, ESRD = end stage renal disease, GBM = glomerular basement membrane, hCCS = hypertension-augmented Charlson comorbidity score, IFTA = interstitial fibrosis and tubular atrophy, RPS = renal pathology society, RRT = renal replacement therapy, VIF = variance inflation factor.
The authors have no conflicting interests that are relevant to this article.
References
- [1].Guariguata L, Whiting DR, Hambleton I, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014;103:137–49. [DOI] [PubMed] [Google Scholar]
- [2].Ivory SE, Packham DK, Reutens AT, et al. Residual proteinuria and eGFR predict progression of renal impairment within 2 years in type 2 diabetic patients with nephropathy who are receiving optimal treatment with angiotensin receptor blockers. Nephrology (Carlton) 2013;18:516–24. [DOI] [PubMed] [Google Scholar]
- [3].Zoppini G, Targher G, Chonchol M, et al. Predictors of estimated GFR decline in patients with type 2 diabetes and preserved kidney function. Clin J Am Soc Nephrol 2012;7:401–8. [DOI] [PubMed] [Google Scholar]
- [4].Bakris GL, Weir MR, Shanifar S, et al. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med 2003;163:1555–65. [DOI] [PubMed] [Google Scholar]
- [5].Tervaert TW, Mooyaart AL, Amann K, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 2010;21:556–63. [DOI] [PubMed] [Google Scholar]
- [6].An Y, Xu F, Le W, et al. Renal histologic changes and the outcome in patients with diabetic nephropathy. Nephrol Dial Transplant 2015;30:257–66. [DOI] [PubMed] [Google Scholar]
- [7].Okada T, Nagao T, Matsumoto H, et al. Histological predictors for renal prognosis in diabetic nephropathy in diabetes mellitus type 2 patients with overt proteinuria. Nephrology (Carlton) 2012;17:68–75. [DOI] [PubMed] [Google Scholar]
- [8].Mottl AK, Gasim A, Schober FP, et al. Segmental sclerosis and extracapillary hypercellularity predict diabetic ESRD. J Am Soc Nephrol 2018;29:694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jung SY, Rosenzweig M, Linkov F, et al. Comorbidity as a mediator of survival disparity between younger and older women diagnosed with metastatic breast cancer. Hypertension 2012;59:205–11. [DOI] [PubMed] [Google Scholar]
- [10].Oh SW, Kim S, Na KY, et al. Clinical implications of pathologic diagnosis and classification for diabetic nephropathy. Diabetes Res Clin Pract 2012;97:418–24. [DOI] [PubMed] [Google Scholar]
- [11].Mise K, Hoshino J, Ueno T, et al. Clinical and pathological predictors of estimated GFR decline in patients with type 2 diabetes and overt proteinuric diabetic nephropathy. Diabetes Metab Res Rev 2015;31:572–81. [DOI] [PubMed] [Google Scholar]
- [12].Ruggenenti P, Gambara V, Perna A, et al. The nephropathy of non-insulin-dependent diabetes: predictors of outcome relative to diverse patterns of renal injury. J Am Soc Nephrol 1998;9:2336–43. [DOI] [PubMed] [Google Scholar]
- [13].Christensen PK, Larsen S, Horn T, et al. Renal function and structure in albuminuric type 2 diabetic patients without retinopathy. Nephrol Dial Transplant 2001;16:2337–47. [DOI] [PubMed] [Google Scholar]
- [14].Mauer SM, Steffes MW, Ellis EN, et al. Structural-functional relationships in diabetic nephropathy. J Clin Invest 1984;74:1143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Drummond K, Mauer M. International Diabetic Nephropathy Study Group. The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes 2002;51:1580–7. [DOI] [PubMed] [Google Scholar]
- [16].White KE, Bilous RW. Type 2 diabetic patients with nephropathy show structural-functional relationships that are similar to type 1 disease. J Am Soc Nephrol 2000;11:1667–73. [DOI] [PubMed] [Google Scholar]
- [17].Perrin NE, Torbjornsdotter TB, Jaremko GA, et al. The course of diabetic glomerulopathy in patients with type I diabetes: a 6-year follow-up with serial biopsies. Kidney Int 2006;69:699–705. [DOI] [PubMed] [Google Scholar]
- [18].Bendayan M. Alteration in the distribution of type IV collagen in glomerular basal laminae in diabetic rats as revealed by immunocytochemistry and morphometrical approach. Diabetologia 1985;28:373–8. [DOI] [PubMed] [Google Scholar]
- [19].Desjardins M, Bendayan M. Ultrastructural distribution of glomerular basement membrane components in experimental diabetes. Diabetes Res 1990;14:65–73. [PubMed] [Google Scholar]
- [20].Adler S. Structure-function relationships associated with extracellular matrix alterations in diabetic glomerulopathy. J Am Soc Nephrol 1994;5:1165–72. [DOI] [PubMed] [Google Scholar]
- [21].Inoue S, Bendayan M. High-resolution ultrastructural study of the rat glomerular basement membrane in aminonucleoside nephrosis. Ultrastruct Pathol 1996;20:409–16. [DOI] [PubMed] [Google Scholar]
- [22].Caramori ML, Parks A, Mauer M. Renal lesions predict progression of diabetic nephropathy in type 1 diabetes. J Am Soc Nephrol 2013;24:1175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang J, Wang Y, Gurung P, et al. The relationship between the thickness of glomerular basement membrane and renal outcomes in patients with diabetic nephropathy. Acta Diabetol 2018;55:669–79. [DOI] [PubMed] [Google Scholar]
- [24].Marshall CB. Rethinking glomerular basement membrane thickening in diabetic nephropathy: adaptive or pathogenic? Am J Physiol Renal Physiol 2016;311:F831–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tziomalos K, Athyros VG. Diabetic nephropathy: new risk factors and improvements in diagnosis. Rev Diabet Stud 2015;12:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Parving HH, Gall MA, Skott P, et al. Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int 1992;41:758–62. [DOI] [PubMed] [Google Scholar]
- [27].Wong TY, Choi PC, Szeto CC, et al. Renal outcome in type 2 diabetic patients with or without coexisting nondiabetic nephropathies. Diabetes Care 2002;25:900–5. [DOI] [PubMed] [Google Scholar]
- [28].Lopes de Faria JB, Moura LA, Lopes de Faria SR, et al. Glomerular hematuria in diabetics. Clin Nephrol 1988;30:117–21. [PubMed] [Google Scholar]
- [29].Matsumura N, Hanatani M, Nishino T, et al. The clinico-pathological significance of hematuria in diabetics. Nihon Jinzo Gakkai Shi 1994;36:1036–45. [PubMed] [Google Scholar]
- [30].Akimoto T, Ito C, Saito O, et al. Microscopic hematuria and diabetic glomerulosclerosis--clinicopathological analysis of type 2 diabetic patients associated with overt proteinuria. Nephron Clin Pract 2008;109:c119–126. [DOI] [PubMed] [Google Scholar]
- [31].Shen FC, Lee CT, Sun CK, et al. Prevalence of haematuria positively associated with urine albumin excretion in Type 2 diabetes. Diabet Med 2012;29:1178–83. [DOI] [PubMed] [Google Scholar]
- [32].Alsaad KO, Herzenberg AM. Distinguishing diabetic nephropathy from other causes of glomerulosclerosis: an update. J Clin Pathol 2007;60:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Okada T, Nagao T, Matsumoto H, et al. Clinical significance of microscopic haematuria in diabetic nephropathy in type 2 diabetes patients with overt proteinuria. Nephrology (Carlton) 2013;18:563–8. [DOI] [PubMed] [Google Scholar]


