Abstract
Kaposi sarcoma (KS) is an endothelial tumor etiologically related to Kaposi sarcoma herpesvirus (KSHV) infection. The aim of our study was to screen out candidate genes of KSHV infected endothelial cells and to elucidate the underlying molecular mechanisms by bioinformatics methods. Microarray datasets GSE16354 and GSE22522 were downloaded from Gene Expression Omnibus (GEO) database. the differentially expressed genes (DEGs) between endothelial cells and KSHV infected endothelial cells were identified. And then, functional enrichment analyses of gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) pathway analysis were performed. After that, Search Tool for the Retrieval of Interacting Genes (STRING) was used to investigate the potential protein–protein interaction (PPI) network between DEGs, Cytoscape software was used to visualize the interaction network of DEGs and to screen out the hub genes. A total of 113 DEGs and 11 hub genes were identified from the 2 datasets. GO enrichment analysis revealed that most of the DEGs were enrichen in regulation of cell proliferation, extracellular region part and sequence-specific DNA binding; KEGG pathway enrichments analysis displayed that DEGs were mostly enrichen in cell cycle, Jak-STAT signaling pathway, pathways in cancer, and Insulin signaling pathway. In conclusion, the present study identified a host of DEGs and hub genes in KSHV infected endothelial cells which may serve as potential key biomarkers and therapeutic targets, helping us to have a better understanding of the molecular mechanism of KS.
Keywords: bioinformatics, biomarker, differentially expressed genes, Kaposi sarcoma, KSHV
1. Introduction
Kaposi sarcoma herpesvirus (KSHV), taxonomical name human gamma herpesvirus 8 (HHV-8) was first discovered in 1994 by Chang.[1] It is a linear double-stranded DNA virus belongs to Gamma Herpesviridae subfamily.[2] KSHV was identified as a class I carcinogen by International Agency for Research on Cancer (IARC) in 2009 for its linkage with tumorigenesis of several cancer types.[3,4] During the last score years after KSHV was first discovered, a great deal of experimental and epidemiological evidence has been made to demonstrate that KSHV is the etiological agent of three tumor types including Kaposi sarcoma (KS), Primary Effusion Lymphoma (PEL), and Multi-centric Castleman Disease (MCD).
Observations indicated that the continued presence of KSHV is needed during the development of KS. the main cell type of KS lesions which were called Spindle cells (SCs). However, whether the original precursor of SCs was lymphatic endothelial cells (LECs) or blood endothelial cells (BECs) is yet an unanswered question. Several studies have indicated that SCs may be closely related to LECs more than BECs.[5,6] LECs can be more successfully infected by KSHV and keep a higher viral copy number than BECs. In addition, the overall gene expression profile analysis showed that Kaposi sarcoma is more similar to LECs, than to that of BECs.[7] But the existence of KSHV-infected BECs during KS development cannot be ignored, some studies hypothesize that KSHV infects both BECs and LECs at the early stages of KS development.[8] And then, KSHV-infected LECs become predominant in advanced KS lesions.
Dishearteningly, after almost 25 years of discovery of KSHV, Mechanism(s) underlying the development of KS remains unclear. In recent years, microarray technology and bioinformatic analysis enabled us to investigate the molecular mechanism(s) and identify tumor-related genes in a new way. Thus, the purpose of this work is to have a better understanding of the exact mechanism(s) of KSHV-associated cancer and to identify potential novel diagnostic and prognostic markers or therapeutic targets by bioinformatic analysis.
2. Materials and methods
2.1. Microarray data
We searched Gene Expression Omnibus database[9] (http://www.ncbi.nlm.nih.gov/geo/) to obtain microarray data by using searching strategy: ((((KSHV) OR Kaposi sarcoma herpesvirus)) AND endothelial cells). There were 201 results in PubMed GEO Datasets. After we have read all these results. We found only 2 gene expression Datasets of GSE16354[10] and GSE22522[11] can be used for further analyzing by synthesis. The platforms of both datasets were all based on GPL570, [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array. There were 24 samples in GSE16354, 6 blood vessel endothelial cells (BECs) control samples compared with KSHV 72 hours post infection of blood vessel endothelial cells (BEC); and 6 LECs control samples compared with KSHV 72 hours post infection of LECs. GSE22522 contained 6 samples including 3 LECs controls and 3 KSHV infected LECs.
2.2. Identification of DEGs
The candidate differentially expressed genes between LECs (BECs) and KSHV infected LECs (BECs) were screened using GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r). GEO2R is an online web tool for users to identify DEGs between experimental samples and control samples. |logFC| (fold change) > 1.0, and adjusted P values <.01 were considered statistically significant. We used the online software Venn diagram (http://bioinfogp.cnb.csic.es/tools/venny/) to obtain the overlapping genes in 3 different experiments.
2.3. GO and KEGG pathway enrichment analysis for DEGs
The DAVID database[12] (https://david.ncifcrf.gov/) (version 6.7) was used for GO annotation and Kyoto encyclopedia of genes and genomes (KEGG) pathway analysis, it is an online analysis tool for grouping a list of related genes into certain organized classes or related networks. KEGG database is a database that systematically analyzes the metabolic pathways of gene products and their functions in cells, it helps to study genes and their expression as a whole network.[13]The GO database provides a standardized description of gene products from the perspectives of function, biological pathways involved and location in cells, namely, simple annotation of gene products.[14]
2.4. PPI network construction and module analyzing
The Search Tool for the Retrieval of Interacting Genes (STRING) database (http://string-db.org/) was used to identify the interactions between known proteins and predicted proteins. We set confidence score >0.4 as the cut-off criterion. Then, we used Cytoscape software[15] (version 3.6.1) to conduct the protein–protein interaction network visualization. The APP Molecular Complex Detection (MCODE)[16] (version 1.5.1) was used to identify the most significant module in the networks. The selection criteria were chosen as follows: MCODE scores >5, node score cut-off = 0.2, degree cut-off = 2, Max depth = 100 and k-score = 2. After that, we used DAVID to perform GO and KEGG analyses for the most significant module.
2.5. Central gene screening and analysis
We identified the hub genes using degrees ≥10. Biological Networks gene Oncology tool (BiNGO)[17] (version 3.0.3) is an a open-source Java tool which used for analyzing the GO terms of hub genes.[17]P < .01 was regarded as statistically significant. Database Oncomine (P ≤ .01, fold change ≥2) (https://www.oncomine.org) and Serial Analysis of Gene Expression (SAGE) (https://cgap.nci.nih.gov/SAGE) were used to analyze the expression profiles of TOP2A and DEPDC1.
3. Results
3.1. Identification of DEGs in KSHV infected endothelial cells
A total of 2363 DEGs (593 in GSE 16354 BECs, 677 in GSE 16354 LECs, 1093 in GSE22522 LECs) were identified, among them, there were 113 overlapping DEGs (Fig. 1A) including 61 upregulated genes and 33 down regulated genes, while 19 of the DEGs have inconsistent gene regulatory trends between different databases.
Figure 1.
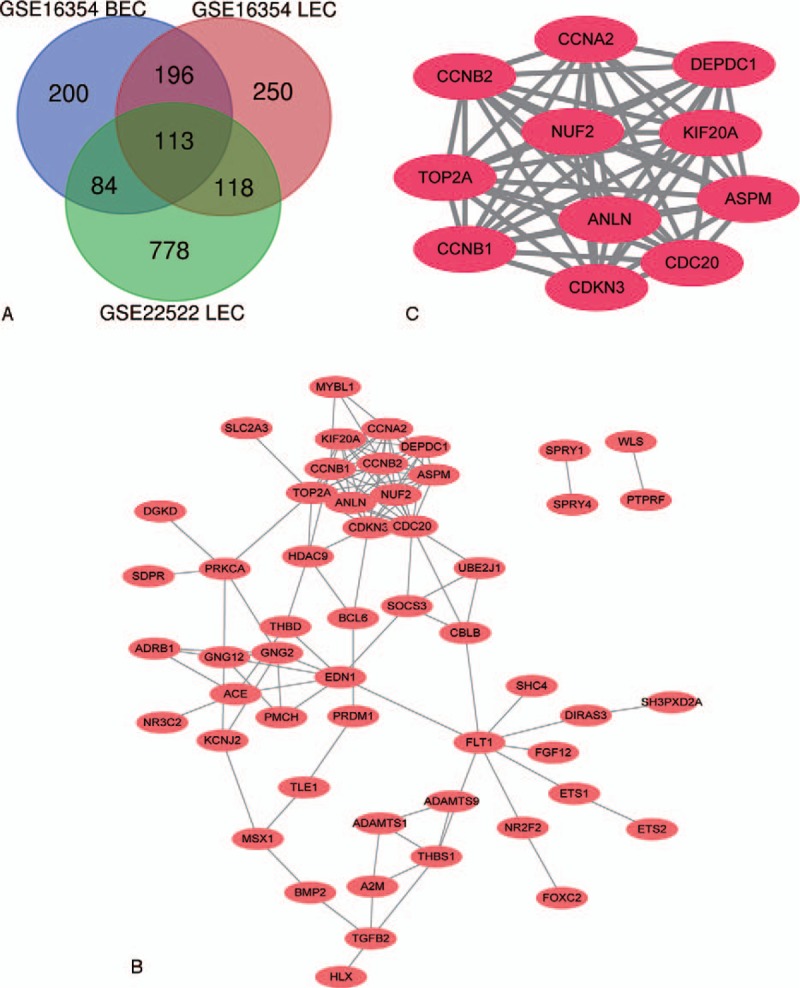
Venn diagram, PPI network and the most significant module of DEGs. (A) DEGs were selected with |logFC| (fold change) ≥1 and P value <.01 among the mRNA expression profiling sets GSE16354 and GSE22522. The 2 datasets showed an overlap of 113 genes. (B) The PPI network of DEGs was performed using Cytoscape. (C) The most significant module was obtained from PPI network with 11 nodes and 55 edges.
3.2. KEGG and GO enrichment analysis of DEGs
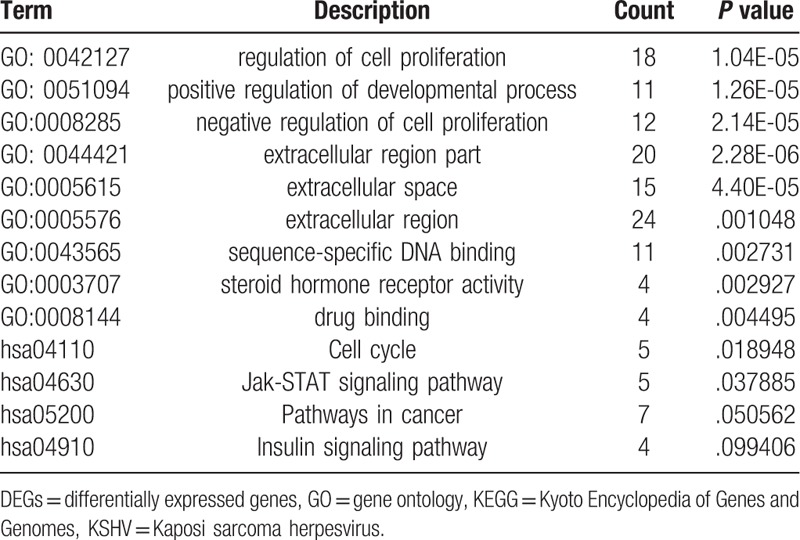
We used DAVID to analyze the biological processes (BP), cellular components (CC), molecular functions (MFs) and KEGG pathways of the 113 overlapping DEGs. GO enrichments showed that BP mainly enriched in regulation of cell proliferation, positive regulation of developmental process, and negative regulation of cell proliferation; CC was significantly enriched in extracellular region part, extracellular space, and extracellular region; MF main focused on sequence-specific DNA binding, steroid hormone receptor activity, and drug binding (Table 1). KEGG pathway enrichment displayed that Cell cycle, Jak-STAT signaling pathway, Pathways in cancer, and Insulin signaling pathway (Table 1).
Table 1.
GO and KEGG pathway enrichment analysis of DEGs in KSHV infected endothelial cells.
3.3. PPI network construction and hub gene analyzing
We download the PPI network of the 113 overlap genes from STRING, and then we used Cytoscape to construct the whole network (Fig. 1B). The most significant module of the whole network was obtained with 11 nodes and 55 edges (Fig. 1C). A total of 11 hub genes were identified from the whole network. The results of GO and KEGG enrichments of hub genes were listed in Table 2, the Biological Networks of 11 hub genes using BiNGO was shown in Figure 2. The full name and functional role of hub genes are shown in Table 3. Among these hub genes, the results of MCODE analysis showed that DEP Domain Containing 1 (DEPDC1) was the only seed gene of this cluster, and DNA Topoisomerase II Alpha (TOP2A) is a protein coding gene which is widely recognized having strong associations with a lot of different cancer types. Hence, we selected DEPDC1 and TOP2A to conduct further analysis using online database Oncomine and SAGE. The results are shown in Figures 3 and 4. Data of Oncomine showed that DEPDC1 and TOP2A were significantly upregulated when compared with normal tissues in various cancer types. And the results of SAGE analysis displayed that the expression profile of DEPDC1 and TOP2A were higher in different caner tissues compared with matched normal tissues.
Table 2.
GO and KEGG pathway enrichment analysis of hub genes in KSHV infected endothelial cells.
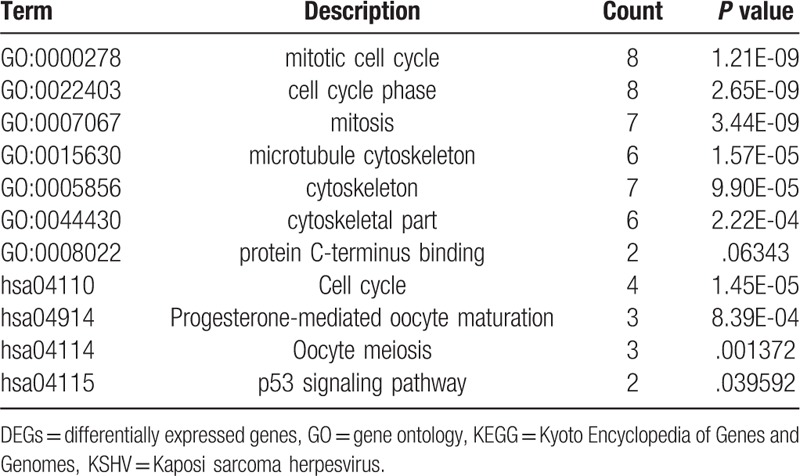
Figure 2.
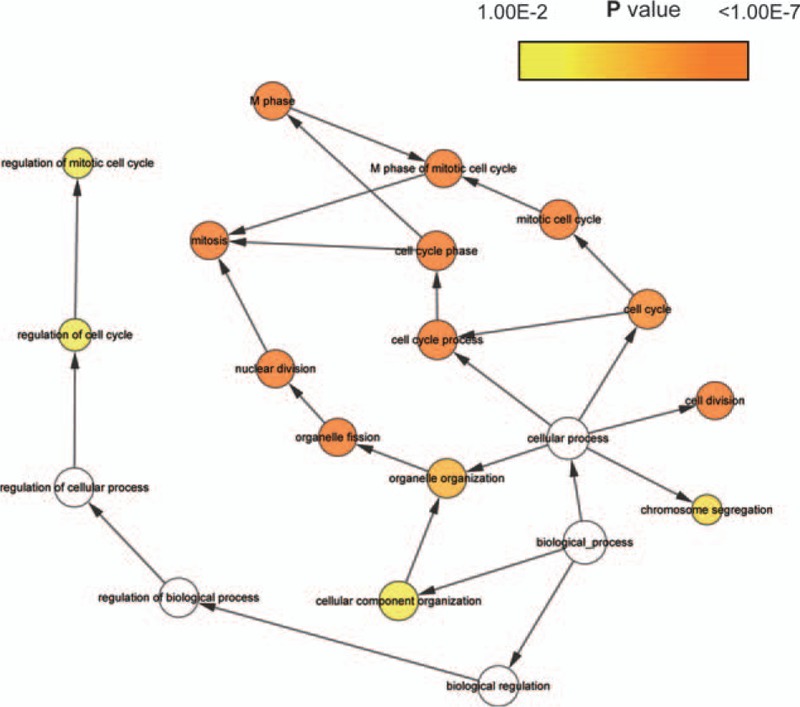
The biological process analysis of hub genes was conducted using BiNGO. The color depth of nodes refers to the corrected P value of ontologies. The size of nodes refers to the numbers of genes that are involved in the ontologies. P < .01 was considered statistically significant.
Table 3.
Functional roles of 11 hub genes.
Figure 3.
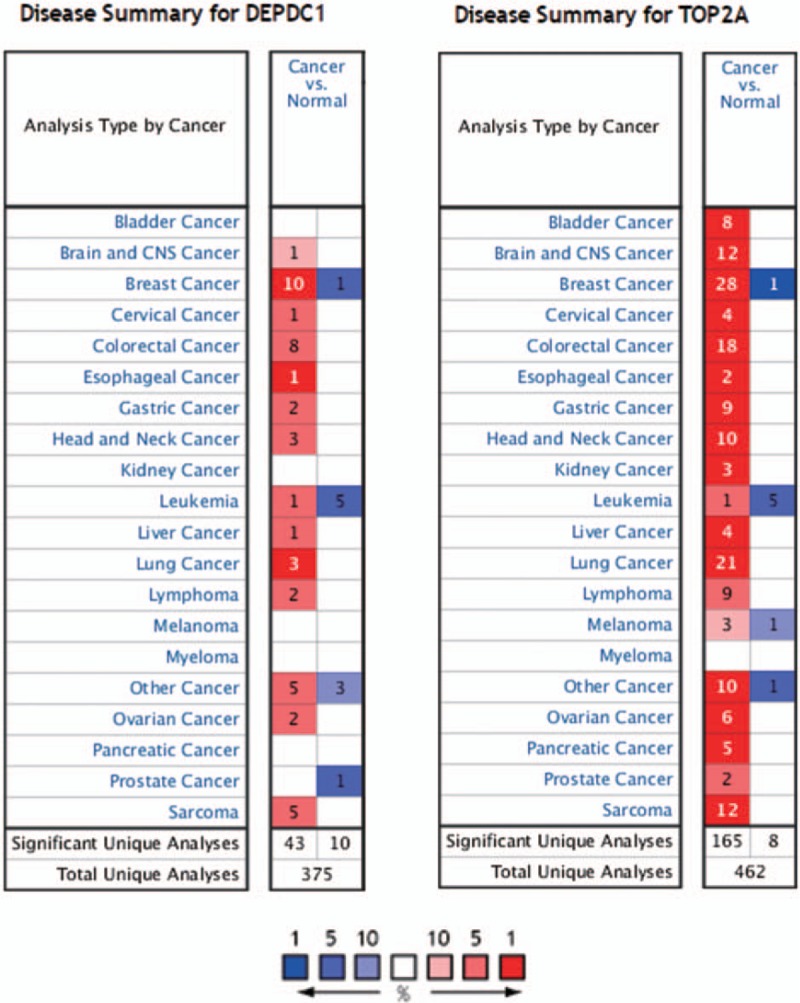
Disease summary for DEPDC1 and TOP2A in Oncomine database, P < .01 and fold change threshold ≥2 were chosen as selection criteria.
Figure 4.
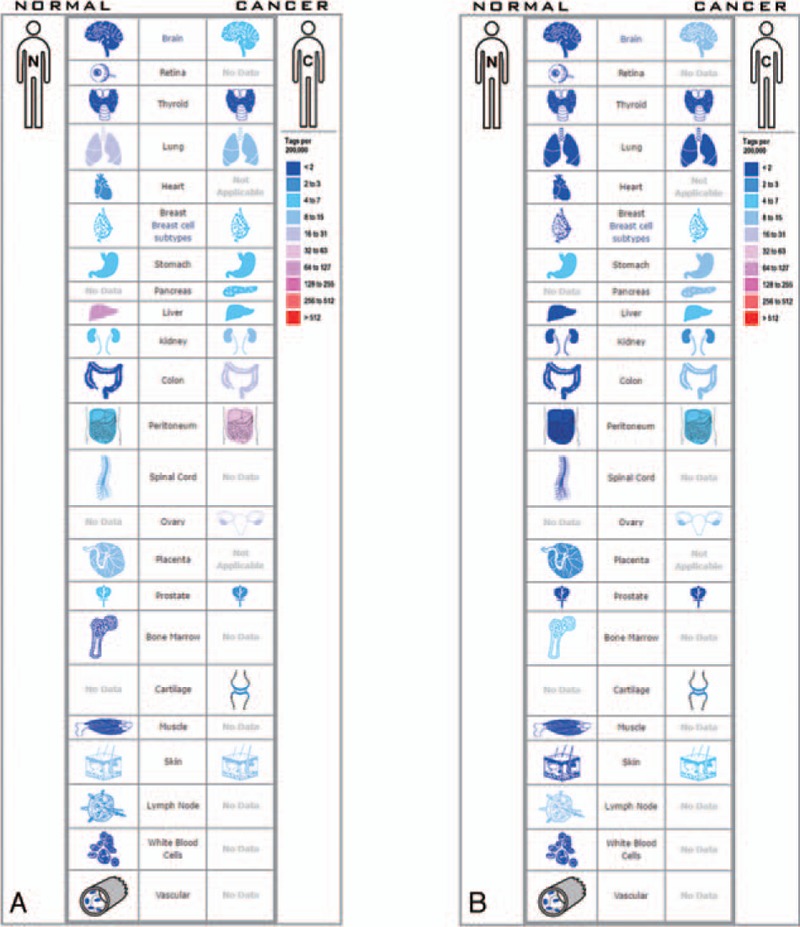
Expression profiles for (A) DEPDC1 and (B) TOP2A in human cancers analyzed with SAGE.
4. Discussion
KSHV is a tumor-producing virus which is believed to have the largest number of potential oncogenes. Its oncogenicity derived from inducing and encoding a lot of cellular and viral oncogenes after infecting different human cell types.[18] One of the most studied disease associated with KSHV is KS which is believed an endothelial cell origin tumor. Our study focuses on the bioinformatic analysis to identify DEGs between LECs (BECs) and KSHV infected LECs (BECs).
In our present work, we conducted the analysis by extracting data from the 2 datasets, a total of 113 DEGs were obtained. 61 upregulated genes and 33 down regulated genes. What puzzled us was that 19 of the DEGs have inconsistent gene regulatory trends between different databases. One possible explanation for this phenomenon is that KSHV infected BECs and KSHV infected LECs may play different roles at different stages of KS.[8] Among the 113 DEGs, 11 hub genes were identified by using Cytoscape software. The GO enrichment results of hub genes revealed that they were mostly enriched in mitotic cell cycle, cell cycle phase, and mitosis, and KEGG enrichment results displayed that they were mainly enriched in Cell cycle, Oocyte meiosis, p53 signaling pathway. Evidences in previous studies showed that these GO terms and KEGG pathways may play important roles in the occurrence and developments of tumors.[19–21]
All these 11 hub genes might serve as key biomarkers in the process of KSHV induced tumor development. Among them, we show great interest in DEPDC1, CCNB1, CCNB2, and TOP2A. For as the results of MCODE analysis, DEPDC1 is the only seed gene of this hub gene cluster. More importantly, DEPDC1, CCNB1, and CCNB2 has been proved to have a significant impact on NF-κB signaling pathway, and NF-κB signaling pathway is widely recognized as having a strong correlation of angiogenesis. As for TOP2A, we did a survey on the databases of PubMed and GeneCards. TOP2A has the biggest number of articles among the 11 hub genes when searching PubMed, and it has been proved to have strong correlations with lots of cancer types. The detail information of these hub genes is as following.
DEPDC1 is a Protein Coding gene which was first reported aberrantly overexpressed and plays important roles in bladder cancer.[22] After that, DEPDC1 was found to be involved in the process of tumorigenesis in many other cancer types.[23,24] As is known to all, Cancer is commonly regarded as a cell cycle dysregulation disease. In Mi et al's study,[25] DEPDC1 was found have a higher expression in the mitotic phase during the cell cycle, and siRNA-mediated knockdown of DEPDC1 would make an effect of mitotic arrest and defects. Moreover, the downregulated DEPDC1 would make an upregulated of A20 – a protein which negatively regulate the NF-κB signaling pathway as well as another two NF-κB regulated cell cycle genes of CCNB1 (Cyclin B1) and CCNB2 (Cyclin B2), and the activity of NF-κB pathway is believed to have a significant correlation with cell cycle regulation.[26] CCNB1 and CCNB2 are two hub genes we have screened out in our present work. They are also cell cycle-related genes regulated by NF-κB signaling pathway.[27] More importantly, NF-κB is a known signaling pathway activated by KSHV to facilitate host cell proliferation and angiogenesis.[28] A lot of KSHV-encoded proteins including viral FLICE inhibitory protein (vFLIP), KSHV G protein-coupled receptor (vGPCR), KS-associated herpesvirus K1, together with viral miRNAs activate NF-κB signaling pathway by decreasing the expression level of IκBα.[29–31] Thus, DEPDC1 might be a target gene of KSHV in the process of infecting host cells, Further studies are needed to test this hypothesis.
Evidences suggested that persistent activation of Wnt signaling could also confer risk to cancer,[32,33] TOP2A and ASPM (Abnormal Spindle Microtubule Assembly) were found to be involved in regulating Wnt signaling pathway. In Pei et al's study,[34] TOP2A together with β-catenin worked as co-activator to promote the process of epithelial-mesenchymal transition in pancreatic cancer cells via activating Wnt signaling pathway. ASPM is another novel Wnt co-activator of Wnt signaling pathway in prostate cancer. Evidence from Pai et al's study[35] indicated that the ASPM expression level is upregulated in prostate cancer samples and the downregulation of ASPM could inhibit the biological behaviors such as proliferation, invasion, and colony formation in prostate cancer cells. In addition, A gastric cancer research highlighted that ANLN (Anillin Actin Binding Protein) expression was significantly associated with Wnt/β-catenin signaling pathway.[36] Wnt Signal Transduction Pathways also plays a key role in KSHV-related Tumors, latency-associated nuclear antigen (LANA) is an KSHV encoded protein that plays a vital role during the process of infection. The interaction of glycogen synthase kinase-3β (GSK-3β) and LANA could make a dysregulation of β-catenin and thus regulating Wnt signaling pathway.[37] Therefore, TOP2A, ASPM, and ANLN might be possible functional targets in the formation and development of KS and other KSHV-related tumors.
As for other hub genes, such as Cyclin Dependent Kinase Inhibitor 3 (CDKN3), CCNA2 (Cyclin A2), CDC20 (Cell Division Cycle 20), NUF2 (NUF2, NDC80 Kinetochore Complex Component), Kinesin Family Member 20A (KIF20A), the literature retrieval results indicated that their relationship between KSHV-induced tumors and KSHV regulated pathways has not been widely reported. However, there are plenty of studies on other tumor types, elevated CDKN3 expression level has been reported in various types of human cancer, including lung cancer, cervical cancer, and gastric cancer.[38–40] And CDKN3 also plays a role of regulating cell division in the formation of these cancers.[41] In addition, CDKN3 seems to be regulated by another virus human papilloma virus (HPV) the same way KSHV to host genes.[42]
Taken together, our analysis identified the crucial genes which may serve as key diagnostic biomarkers and therapeutic targets for KS and other KSHV induced tumors by comprehensive bioinformatics analysis. However, Molecular and cell biology studies are required to validate the functional role of these genes.
5. Conclusion
In conclusion, the present study identified a host of DEGs and hub genes in KSHV infected endothelial cells which may serve as potential key biomarkers and therapeutic targets, helping us to have a better understanding of the molecular mechanism of KS. Further studies are strongly encouraged in the future to validate these biomarkers.
Author contributions
Conceptualization: Hai-Bo Gong.
Supervision: Xiao-Jing Kang, Xiong-ming Pu.
Validation: Xiong-Ming Pu.
Writing – original draft: Hai-Bo Gong.
Writing – review & editing: Xiu-Juan Wu, Xiao-Jing Kang, Xiong-Ming Pu.
Footnotes
Abbreviations: ANLN = anillin actin binding protein, ASPM = abnormal spindle microtubule assembly, BECs = blood endothelial cells, BiNGO = biological networks gene oncology, BP = biological processes, CC = cellular components, CCNA2 = CyclinA2, CCNB1 = Cyclin B1, CCNB2 = Cyclin B2, CDC20 = Cell Division Cycle 20, CDKN3 = Cyclin Dependent Kinase Inhibitor 3, DEGs = differentially expressed genes, DEPDC1 = DEP domain containing 1, GEO = gene expression omnibus, GO = gene ontology, GSK-3β = glycogen synthase kinase-3β, HPV = human papilloma virus, KEGG = Kyoto encyclopedia of genes and genomes, KIF20A = kinesin family member 20A, KS = Kaposi sarcoma, KSHV = Kaposi sarcoma herpesvirus, LANA = latency-associated nuclear antigen, LECs = lymphatic endothelial cells, MCD = multi-centric Castleman disease, MCODE = molecular complex detection, MF = molecular function, NUF2 = NUF2 Component Of NDC80 Kinetochore Complex, PEL = primary effusion lymphoma, PPI = protein–protein interaction, SAGE = serial analysis of gene expression, SCs = spindle cells, STRING = search tool for the retrieval of interacting genes, TOP2A = DNA Topoisomerase II Alpha, vFLIP = viral FLICE inhibitory protein, vGPCR = KSHV G protein-coupled receptor.
All analyses of this article were based on previous published literatures and public databases, no human participants were included in our study. Therefore, this work does not need any ethical approval and patient consent.
This work was supported by grants from the National Natural Science Foundation of China (grant no. 81660454).
The authors have no conflicts of interests to disclose.
References
- [1].Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 1994;266:1865–9. [DOI] [PubMed] [Google Scholar]
- [2].Li S, Bai L, Dong J, et al. Kaposi's sarcoma-associated herpesvirus: epidemiology and molecular biology. Adv Exp Med Biol 2017;1018:91–127. [DOI] [PubMed] [Google Scholar]
- [3].Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum 2012;100(Pt B):1–441. [PMC free article] [PubMed] [Google Scholar]
- [4].Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol 2009;10:321–2. [DOI] [PubMed] [Google Scholar]
- [5].Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer 2008;8:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med 2013;137:289–94. [DOI] [PubMed] [Google Scholar]
- [7].Wang HW, Trotter MW, Lagos D, et al. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat Genet 2004;36:687–93. [DOI] [PubMed] [Google Scholar]
- [8].Gramolelli S, Ojala PM. Kaposi's sarcoma herpesvirus-induced endothelial cell reprogramming supports viral persistence and contributes to Kaposi's sarcoma tumorigenesis. Curr Opin Virol 2017;26:156–62. [DOI] [PubMed] [Google Scholar]
- [9].Clough E, Barrett T. The gene expression omnibus database. Methods Mol Biol 2016;1418:93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hansen A, Henderson S, Lagos D, et al. KSHV-encoded miRNAs target MAF to induce endothelial cell reprogramming. Genes Dev 2010;24:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cheng F, Pekkonen P, Laurinavicius S, et al. KSHV-initiated notch activation leads to membrane-type-1 matrix metalloproteinase-dependent lymphatic endothelial-to-mesenchymal transition. Cell Host Microbe 2011;10:577–90. [DOI] [PubMed] [Google Scholar]
- [12].Huang DW, Sherman BT, Tan Q, et al. The DAVID gene functional classification tool: a novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol 2007;8:R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kanehisa M. The KEGG database. Novartis Found Symp 2002;247:91–101. discussion 101–103, 119–128, 244–152. [PubMed] [Google Scholar]
- [14].Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. the gene ontology consortium. Nat Genet 2000;25:25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003;13:2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bader GD, Hogue CW. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics 2003;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Maere S, Heymans K, Kuiper M. BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics (Oxford, England) 2005;21:3448–9. [DOI] [PubMed] [Google Scholar]
- [18].Hussein HAM, Okafor IB, Walker LR, et al. Cellular and viral oncogenes: the key to unlocking unknowns of Kaposi's sarcoma-associated herpesvirus pathogenesis. Arch Virol 2018;163:2633–43. [DOI] [PubMed] [Google Scholar]
- [19].Williams GH, Stoeber K. The cell cycle and cancer. J Pathol 2012;226:352–64. [DOI] [PubMed] [Google Scholar]
- [20].Topham CH, Taylor SS. Mitosis and apoptosis: how is the balance set? Curr Opin Cell Biol 2013;25:780–5. [DOI] [PubMed] [Google Scholar]
- [21].Joerger AC, Fersht AR. The p53 pathway: origins, inactivation in cancer, and emerging therapeutic approaches. Annu Rev Biochem 2016;85:375–404. [DOI] [PubMed] [Google Scholar]
- [22].Kanehira M, Harada Y, Takata R, et al. Involvement of upregulation of DEPDC1 (DEP domain containing 1) in bladder carcinogenesis. Oncogene 2007;26:6448–55. [DOI] [PubMed] [Google Scholar]
- [23].Miyata Y, Kumagai K, Nagaoka T, et al. Clinicopathological significance and prognostic value of Wilms’ tumor gene expression in colorectal cancer. Cancer Biomark: section A of Disease markers 2015;15:789–97. [DOI] [PubMed] [Google Scholar]
- [24].Yuan SG, Liao WJ, Yang JJ, et al. DEP domain containing 1 is a novel diagnostic marker and prognostic predictor for hepatocellular carcinoma. Asian Pac J Cancer Prev 2014;15:10917–22. [DOI] [PubMed] [Google Scholar]
- [25].Mi Y, Zhang C, Bu Y, et al. DEPDC1 is a novel cell cycle related gene that regulates mitotic progression. BMB Rep 2015;48:413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ledoux AC, Perkins ND. NF-kappaB and the cell cycle. Biochem Soc Trans 2014;42:76–81. [DOI] [PubMed] [Google Scholar]
- [27].Prajapati S, Tu Z, Yamamoto Y, et al. IKKalpha regulates the mitotic phase of the cell cycle by modulating Aurora A phosphorylation. Cell Cycle 2006;5:2371–80. [DOI] [PubMed] [Google Scholar]
- [28].Brinkmann MM, Schulz TF. Regulation of intracellular signalling by the terminal membrane proteins of members of the Gammaherpesvirinae. J Gen Virol 2006;87(Pt 5):1047–74. [DOI] [PubMed] [Google Scholar]
- [29].Bottero V, Kerur N, Sadagopan S, et al. Phosphorylation and polyubiquitination of transforming growth factor beta-activated kinase 1 are necessary for activation of NF-kappaB by the Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor. J Virol 2011;85:1980–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chugh P, Matta H, Schamus S, et al. Constitutive NF-kappaB activation, normal Fas-induced apoptosis, and increased incidence of lymphoma in human herpes virus 8 K13 transgenic mice. Proc Natl Acad Sci U S A 2005;102:12885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Moody R, Zhu Y, Huang Y, et al. KSHV microRNAs mediate cellular transformation and tumorigenesis by redundantly targeting cell growth and survival pathways. PLoS Pathog 2013;9:e1003857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene 2017;36:1461–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat Rev 2018;62:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pei YF, Yin XM, Liu XQ. TOP2A induces malignant character of pancreatic cancer through activating beta-catenin signaling pathway. Biochim Biophys Acta Mol Basis Dis 2018;1864:197–207. [DOI] [PubMed] [Google Scholar]
- [35].Pai VC, Hsu CC, Chan TS, et al. ASPM promotes prostate cancer stemness and progression by augmenting Wnt-Dvl-3-beta-catenin signaling. Oncogene 2019;38:1340–53. [DOI] [PubMed] [Google Scholar]
- [36].Pandi NS, Manimuthu M, Harunipriya P, et al. In silico analysis of expression pattern of a Wnt/beta-catenin responsive gene ANLN in gastric cancer. Gene 2014;545:23–9. [DOI] [PubMed] [Google Scholar]
- [37].Fujimuro M, Liu J, Zhu J, et al. Regulation of the interaction between glycogen synthase kinase 3 and the Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen. J Virol 2005;79:10429–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fan C, Chen L, Huang Q, et al. Overexpression of major CDKN3 transcripts is associated with poor survival in lung adenocarcinoma. Br J Cancer 2015;113:1735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang LP, Li WJ, Zhu YF, et al. CDKN3 knockdown reduces cell proliferation, invasion and promotes apoptosis in human ovarian cancer. Int J Clin Exp Pathol 2015;8:4535–44. [PMC free article] [PubMed] [Google Scholar]
- [40].Li Y, Ji S, Fu LY, et al. Knockdown of cyclin-dependent kinase inhibitor 3 inhibits proliferation and invasion in human gastric cancer cells. Oncol Res 2017;25:721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Srinivas V, Kitagawa M, Wong J, et al. The tumor suppressor Cdkn3 is required for maintaining the proper number of centrosomes by regulating the centrosomal stability of Mps1. Cell Rep 2015;13:1569–77. [DOI] [PubMed] [Google Scholar]
- [42].Berumen J, Espinosa AM, Medina I. Targeting CDKN3 in cervical cancer. Expert Opin Ther Targets 2014;18:1149–62. [DOI] [PubMed] [Google Scholar]


