Abstract
This study investigated the effect of sex hormones on 18F-fluorodeoxyglucose (FDG) uptake in normal breast tissue.
The retrospective study included 249 premenopausal women (median age, 45 years) who were diagnosed with unilateral breast cancer and underwent FDG positron emission tomography/computed tomography and hormone tests. The volume of interest was within the contralateral normal breast and the standardized uptake values (SUVs) were measured. The correlations of sex hormones (including estrogen, progesterone, testosterone, follicle-stimulating hormone [FSH] and luteinizing hormone [LH]) with the SUVs of the normal breast were analyzed.
There was a weak negative correlation between age and breast FDG uptake (P = .012, Spearman coefficient = −.16 for the maximum standardized uptake values [SUVmax]), especially in the luteal phase group (P = .005, Spearman coefficient = −.27 for SUVmax). The SUVs of normal breast tissue were increased when progesterone levels were higher (P = .043, Spearman coefficient = .13 for SUVmax). In the irregular menstrual cycle group, FDG uptake in the breast decreased as FSH (P = .027, Spearman coefficient = −.42 for SUVmax) and LH (P = .048, Spearman coefficient = −.44 for SUVmax) increased.
Glucose metabolism of normal breast tissue decreases with age, and progesterone weakly affects breast FDG uptake. Gonadotropins may affect breast FDG uptake in premenopausal women with irregular menstrual cycles.
Keywords: breast, fluorodeoxyglucose F18, gonadal steroid hormones, positron-emission tomography
1. Introduction
Although 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) is mainly used to evaluate malignant lesions, FDG uptake in normal tissues has also been investigated.[1,2] FDG uptake occurs in the brain, digestive tract, skeletal muscle, myocardium and genitourinary tract.[1] Physiological uptake also occurs in normal breast tissue and this uptake may be a limiting factor in the evaluation of malignant lesions.[3]
In clinical practice, FDG PET images have shown that that FDG uptake in normal breast tissue is variable. Previous studies on FDG uptake in normal breast tissue are limited,[4–7] as most have reported on the association between breast density and FDG uptake.
The menstrual cycle and sex hormones influence physiological changes in normal breast tissue[8,9] and these factors may be related to the physiological findings in breast imaging studies. The relationships of the menstrual cycle with the magnetic resonance imaging (MRI) parameters of the breast have been reported previously[10–12]; however, few studies used FDG PET. Lin et al[13] reported a significant correlation between the intensity of FDG uptake in normal breast tissues and the menstrual cycle, but the association with sex hormones remains unknown.
In this context, this study was carried out to investigate the effects of sex hormones on physiological FDG uptake in normal breast tissue.
2. Materials and methods
2.1. Subjects
A total of 249 premenopausal women (median age, 45 years) who were diagnosed with unilateral breast cancer from March 2015 to July 2017 in our institution, and who underwent FDG PET/computed tomography (CT) and hormone tests (including estradiol, progesterone, testosterone, follicle-stimulating hormone [FSH] and luteinizing hormone [LH]) for initial pretreatment staging, were included in this study. Hormone testing was performed on the same day as the PET. The date of the last normal menstrual period (LNMP) of the patients was also recorded. The menstrual phase on the day of the tests was divided into the follicular phase (from day 1 to 13) and luteal phase (from day 14 to 28) based on the LNMP.
The clinical design of this retrospective study was approved by the Institutional Review Board of Ajou University (AJIRB-MED-OBS-18–354). The need for informed consent was waived.
2.2. FDG PET/CT protocol
After fasting for at least 6 hours, the patients were administered 5 MBq/kg of FDG intravenously. The blood glucose level at the time of the FDG injection was <150 mg/dL in all patients. The patients were instructed to rest for 60 minutes and to urinate before being scanned. Whole-body PET/CT images were obtained with a Discovery ST 8-slice CT scanner or a Discovery STE 16-slice CT scanner (GE Healthcare, Milwaukee, WI, USA). Seven or eight frames (3 min/frame) of PET emission data were acquired in three-dimensional (3D) mode after a non-contrast CT scan from the base of the skull to the upper thigh (120 kV, 30–100 mA in the AutomA mode; section width = 3.75 mm). PET images were reconstructed using an iterative method (ordered-subsets expectation maximization with two iterations and 20 subsets, field of view = 600 mm, slice thickness = 3.27 mm) and attenuation-corrected non-contrast CT.
2.3. Image analysis
A specialist in nuclear medicine with 13 years of PET experience reviewed the FDG PET/CT images on an AW workstation (ver. 4.4; General Electric Healthcare, Chicago, IL), and was blinded to the clinical data. The volume of interest (VOI) was drawn manually on the CT images within the glandular tissue of the contralateral normal breast, with the nipple and areola area excluded. Delineated VOI were projected onto PET images to derive uptake values. The maximum and mean standardized uptake values (SUVmax and SUVmean, respectively) were calculated based on the injected dose and body weight.
2.4. Statistical analysis
The appropriate sample size for this study was calculated using MedCalc software (ver. 17.8.5; MedCalc Software bvba, Ostend, Belgium). Using a 2-sided test, at a 5% significance level (α = .05) and with 80% power (β = .2), the minimum required sample size to detect a simple correlation (r = .3) was 193. Therefore, the number of patients recruited to this study satisfied the minimum requirement.
All data were tested for a normal distribution by the Kolmogorov-Smirnov test; none showed such a distribution. Therefore, all continuous variables are provided as medians and interquartile range, and appropriate nonparametric statistical methods were used to analyze the data. The Kruskal–Wallis test was used to compare continuous variables among groups, based on the menstrual phase. If the Kruskal–Wallis test was positive, post-hoc analysis was performed for pairwise comparison of subgroups. For categorical variables, the chi-squared test was used to evaluate differences among the groups. Spearman correlation coefficient (r) was calculated to evaluate the correlations of sex hormone levels with SUVs. A correlation coefficient of .00 to .39 was considered weak, .40 to .59 as moderate, .60 to .79 as strong, and .80 to 1.00 as very strong.[14] All analyses were performed using MedCalc software and a value of P < .05 was considered statistically significant.
3. Results
3.1. Demographic characteristics
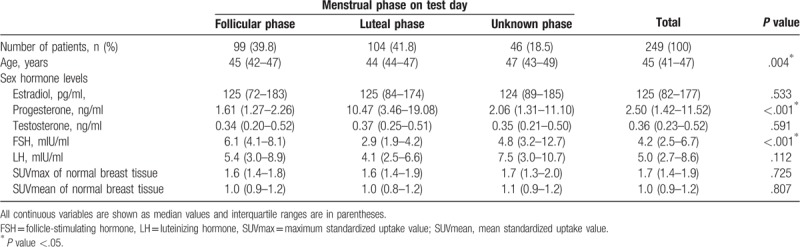
Of the total number of patients, 99 (39.8%) were in the follicular phase and 104 (41.8%) were in the luteal phase. The remaining 46 (18.5%) patients had an irregular menstrual cycle. The median age of the patients was 45 years and a group comparison revealed that the age of the irregular menstrual cycle group was significantly higher than that of the other groups (P = .004).
The progesterone level was significantly different among the groups (P < .001). FSH was significantly higher in the follicular group than in the other groups (P < .001). The levels of the other sex hormones did not differ significantly among the groups.
In all patients, the median SUVmax in normal breast tissue was 1.7 and the SUVmean was 1.0. FDG uptake in normal breast tissue was not significantly different among the groups according to the menstrual phase. The detailed patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics.
3.2. Correlation between FDG uptake and parameters
Older patients were more likely to show lower FDG uptake in normal breast tissue (for SUVmax, r = −.16, P = .012; for SUVmean, r = −.22, P = .006; Table 2). Among the menstrual cycle groups, only the luteal phase group showed a weak negative correlation between FDG uptake and age (for SUVmax, r = −.27, P = .005; for SUVmean, r = −.27, P = .006; Table 2).
Table 2.
Correlation between FDG uptake of normal breast tissue and parameters used.
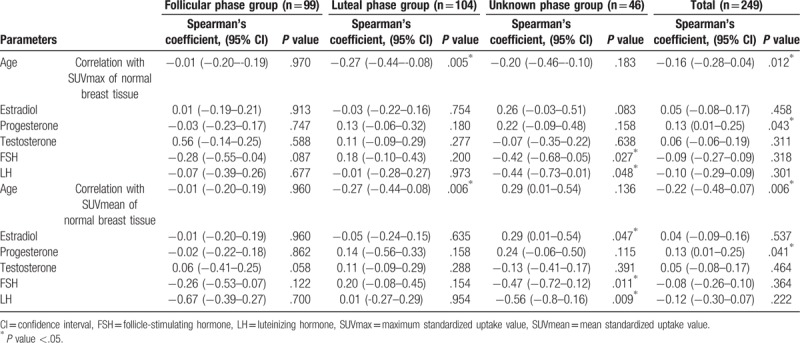
In the whole patient group, only progesterone showed a weak correlation with breast FDG uptake (for SUVmax, r = .13, P = .043; for SUVmean, r = .13, P = .041; Table 2, Fig. 1). FSH and LH showed a moderately negative correlation with breast FDG uptake in the groups with an irregular menstrual cycle (for correlation between FSH and SUVmax, r = −.42, P = .027; for correlation between FSH and SUVmean, r = −.47, P = .011; for correlation between LH and SUVmax, r = −.44, P = .048; for correlation between LH and SUVmean, r = −.56, P = .009; Table 2, Fig. 2). None of the hormones in the follicular or luteal groups showed a significant correlation with breast FDG uptake (Table 2).
Figure 1.
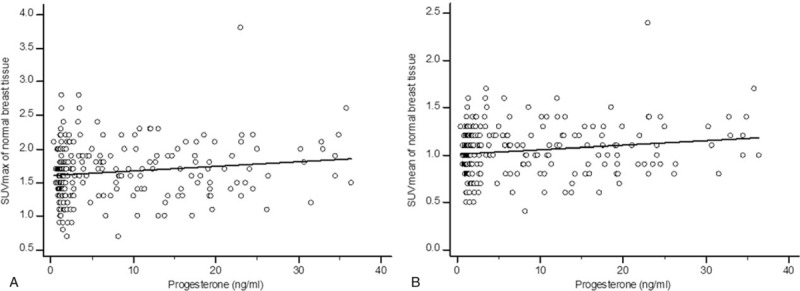
Scatter diagrams of the relationship between progesterone and 18F-fluorodeoxyglucose (FDG) uptake of normal breast tissue on whole-body scans. A. The progesterone level showed a weakly positive correlation with the maximum standardized uptake values (SUVmax) of breast tissue (r = .13, P = .043). B. There was a weak but significant positive correlation between progesterone and the mean standardized uptake values (SUVmean) of normal breast tissue (r = .13, P = .041).
Figure 2.
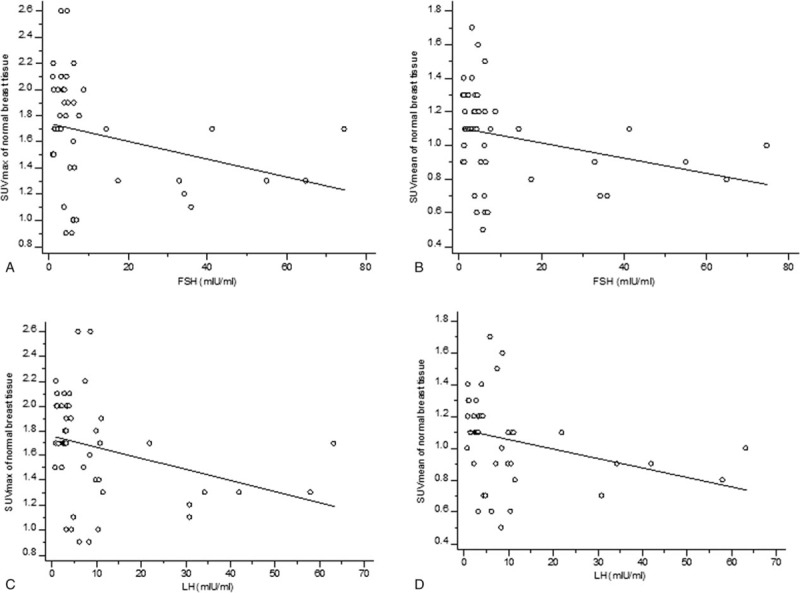
Scatter diagrams of the correlation between gonadotropins (follicle-stimulating hormone [FSH] and luteinizing hormone [LH]) and FDG uptake of normal breast tissue in groups with irregular menstrual cycles. A. The SUVmax of breast tissue was significantly negative correlated with FSH (r = −.42, P = .027). B. The higher the FSH level, the lower the SUVmean value of the breast tissue (r = −.47, P = .011). C. The SUVmax of normal breast and LH showed a significant negative correlation (r = −.44, P = .048). D. There was a significant negative correlation between LH and the SUVmean of normal breast tissue (r = −.56, P = .009).
4. Discussion
Studies on FDG uptake in normal breast tissue are limited. Here, we briefly summarize previous results and compared them to those of this study. Previous studies reported that breast density was a major factor affecting FDG, and that FDG uptake in dense breast tissues was significantly higher than in non-dense breast tissue.[4–7,13] Unfortunately, we did not analyze breast density because most of our patients (245/249, 98.4%) already had dense breast tissue visible on mammograms. Only four patients (1.6%) showed fatty breasts, and this number was too low for meaningful analyses. It is known that an extremely high proportion of premenopausal Korean women have high breast density[15,16] and the presence of this characteristic in our patients was therefore not surprising. Another common feature of previous studies was that age and FDG uptake in breast tissue were inversely related.[5,6] Our results also showed a statistically weak negative correlation between age and breast FDG uptake, consistent with previous results. It is possible that younger women show increased breast FDG uptake because their breast tissue is denser than that of older women.[5,6,17–20] However, considering that most of our subjects had dense breast tissue (98.4%), this was not a major finding of this study. We also found that age and breast FDG uptake was weakly negatively correlated only in the luteal phase group. It is known that during breast proliferation, lobule size increases and stromal edema are more apparent in the luteal phase than in the follicular phase.[21–23] Taking this into account, the negative correlation between age and breast FDG uptake in our results may indicate that proliferation of breast in luteal phase is greater in young woman than old.
Another important factor affecting breast FDG uptake reported in previous studies[4–6] was menopausal state. Zytoon[6] reported that breast FDG uptake was lower in postmenopausal women, but other studies[4,5] reported that menopausal status was not associated with breast FDG uptake. Unfortunately, as our study only included pre-menopausal women, we did not obtain any results relating to this: our study investigated the relationship between FDG uptake in breast tissue and sex hormones and, as such, postmenopausal women were not included because they could have confounded the results regarding the physiological effects of sex hormones on breast tissue. Additionally, if the postmenopausal women were taking hormone replacement drugs, this would not have been possible to evaluate the physiological effects of sex hormones. For this reason, this study only included premenopausal women.
Lin et al[13] reported a relationship between the menstrual cycle and breast FDG uptake. They showed that FDG uptake in normal breast tissue was significantly correlated with the menstrual cycle, and that breast FDG uptake in the secretory phase tended to be high. Lin results are inconsistent with this study, in which there was no significant difference in the FDG uptake of normal breast tissue between the follicular and luteal phase groups. It is difficult to determine whose results are correct. However, our study was sufficiently powered because we included a relatively large number of samples. Moreover, Lin et al[13] evaluated the qualitative grade of breast FDG uptake, whereas we analyzed breast FDG uptake by semi-quantitative evaluation using SUVs.
A key finding of our study was that the degree of FDG uptake in breast tissue and progesterone level had a weak but significant positive correlation in all patients. To the best of our knowledge, this has not been reported previously. Previous reports on the effects of progesterone on physiological changes of breast tissue suggested that mammary growth and mitotic activity of normal tissue may be increased by progesterone.[24,25] Furthermore, a significant positive correlation between mitosis and FDG uptake in breast cancer has been reported.[26] We hypothesized that FDG uptake in normal breast tissue increased when mitosis was active. Although it may be difficult to obtain samples of normal breast tissue, future studies on the pathology of normal tissue are needed to understand this correlation.
It is well-known that estrogen is associated with normal breast tissue proliferation,[27,28] and we thus expected that estrogen levels would be strongly associated with FDG uptake of normal breast tissue at the beginning of the study. However, the results did not show this. This was most likely due to the ‘estrogen plus progestin hypothesis’, in which estrogen is expected to cause physiological changes in breast tissue in conjunction with progesterone, rather than being involved in normal proliferation alone.[29] However, this remains unclear and further investigation to definitively determine the reason for our results will be needed in future.
An important finding of our study was that FSH and LH showed a significant negative correlation with the degree of FDG uptake in breast tissue in the group with an irregular menstrual cycle. It should be noted that the age of the group with an irregular menstrual cycle was significantly higher than that of the groups with regular menstrual cycles. Patients with irregular menstrual cycles may be presumed to be perimenopausal, such that our results were predictable. It is already known that FSH and LH gradually increase in perimenopausal women.[30,31] Our groups may have included perimenopausal women with irregular menstrual cycles; therefore, the negative correlation between gonadotropins (FSH, LH) and breast FDG uptake in this group can be interpreted in terms of decreased FDG uptake in breast tissue as the menopause approaches. The proliferation of breast tissue is also expected to be reduced in perimenopausal compared to premenopausal women.[32]
In our study, analysis of the differences in hormone levels among the groups revealed significantly higher progesterone levels in the luteal phase group than in the other groups. FSH levels were significantly higher in the follicular phase group than in the other groups. Sex hormone fluctuations during the menstrual cycle have been well-documented[33] and our results concerning the peak of progesterone in the luteal phase and high FSH levels in the follicular phase are consistent with previous findings. These results suggest that the LNMP data obtained from the self-report data of our study were validated by the actually recorded sex hormone levels, further proving the accuracy and objectivity of our study.
We correlated parameters of normal breast physiology with the degree of glucose metabolism, as assessed by FDG PET. Because the analysis of FDG uptake under the influence of sex hormones in normal breast tissue is novel, our data are difficult to interpret. Although the results were mostly predictable, the causal mechanisms are more difficult to identify. Therefore, further, larger studies are needed to clarify the factors affecting FDG uptake in normal breast tissue.
This study had some limitations. First, it was performed on breast cancer patients, and although data from the contralateral normal breast were obtained for patients with unilateral breast cancer, it is difficult to predict how our data would differ from those for normal breast tissue in women without a history of cancer. Therefore, it would be meaningful to conduct a similar study on women undergoing FDG PET for health screening purposes. Second, our results may differ from those for other ethnic groups, because of the particular racial characteristics of the patients included in this study. As previously described, this study was conducted on premenopausal Korean women who mostly presented with dense breast tissue, which is different from previous studies.[4–7,13] The final limitation was that we used body weight-corrected SUVs and did not obtain SUVs using lean body mass or body surface area. In the study by Vranjesevic et al,[7] SUVs corrected by lean body mass and body surface area were used, and showed strong correlations with the uncorrected SUVs. Subsequent studies[5,6] used weight-adjusted SUVs without any correction for lean body mass or body surface, as is commonly applied in clinical practice, without any noticeable effects on the results.
In conclusion, FDG uptake in normal breast tissue was positively correlated with serum progesterone concentrations in premenopausal women. Age is a significant factor affecting FDG uptake in normal breast tissue. In women approaching the menopause, gonadotropin concentration and breast FDG uptake showed a significant negative correlation.
Author contributions
Conceptualization: Tae Hee Kim, Young-Sil An.
Data curation: Yongsik Jung.
Formal analysis: Ji Young Kim.
Investigation: Yongsik Jung, Tae Hee Kim.
Methodology: Ji Young Kim.
Resources: Tae Hee Kim, Ji Young Kim, Sehwan Han.
Supervision: Young-Sil An.
Validation: Sehwan Han.
Visualization: Tae Hee Kim.
Writing – original draft: Yongsik Jung, Tae Hee Kim, Young-Sil An.
Writing – review & editing: Young-Sil An.
Footnotes
Abbreviations: CT = computed tomography, FDG = 18F-fluorodeoxyglucose, FSH = follicle-stimulating hormone, LH = luteinizing hormone, LNMP = last normal menstrual period, MRI = magnetic resonance imaging, PET = positron emission tomography, SUV = standardized uptake values, VOI = volume of interest.
YJ and THK contributed equally to this work.
The authors report no conflicts of interest.
References
- [1].Shreve PD, Anzai Y, Wahl RL. Pitfalls in oncologic diagnosis with FDG PET imaging: physiologic and benign variants. Radiographics 1999;19:61–77. quiz 150-151. [DOI] [PubMed] [Google Scholar]
- [2].Paquet N, Albert A, Foidart J, et al. Within-patient variability of (18)F-FDG: standardized uptake values in normal tissues. J Nucl Med 2004;45:784–8. [PubMed] [Google Scholar]
- [3].Lim HS, Yoon W, Chung TW, et al. FDG PET/CT for the detection and evaluation of breast diseases: usefulness and limitations. Radiographics 2007;27 Suppl 1:S197–213. [DOI] [PubMed] [Google Scholar]
- [4].Kumar R, Chauhan A, Zhuang H, et al. Standardized uptake values of normal breast tissue with 2-deoxy-2-[F-18]fluoro-D: -glucose positron emission tomography: variations with age, breast density, and menopausal status. Mol Imaging Biol 2006;8:355–62. [DOI] [PubMed] [Google Scholar]
- [5].Mavi A, Cermik TF, Urhan M, et al. The effect of age, menopausal state, and breast density on (18)F-FDG uptake in normal glandular breast tissue. J Nucl Med 2010;51:347–52. [DOI] [PubMed] [Google Scholar]
- [6].Zytoon AA. Standardized uptake value variations of normal glandular breast tissue at dual time point FDG-PET/CT imaging. Int J Med Imag 2014;1:56–65. [Google Scholar]
- [7].Vranjesevic D, Schiepers C, Silverman DH, et al. Relationship between 18F-FDG uptake and breast density in women with normal breast tissue. J Nucl Med 2003;44:1238–42. [PubMed] [Google Scholar]
- [8].Potten CS, Watson RJ, Williams GT, et al. The effect of age and menstrual cycle upon proliferative activity of the normal human breast. Br J Cancer 1988;58:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Battersby S, Robertson BJ, Anderson TJ, et al. Influence of menstrual cycle, parity and oral contraceptive use on steroid hormone receptors in normal breast. Br J Cancer 1992;65:601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Clendenen TV, Kim S, Moy L, et al. Magnetic resonance imaging (MRI) of hormone-induced breast changes in young premenopausal women. Magn Reson Imaging 2013;31:1–9. [DOI] [PubMed] [Google Scholar]
- [11].Fowler PA, Casey CE, Cameron GG, et al. Cyclic changes in composition and volume of the breast during the menstrual cycle, measured by magnetic resonance imaging. Br J Obstet Gynaecol 1990;97:595–602. [DOI] [PubMed] [Google Scholar]
- [12].Graham SJ, Stanchev PL, Lloyd-Smith JO, et al. Changes in fibroglandular volume and water content of breast tissue during the menstrual cycle observed by MR imaging at 1.5 T. J Magn Reson Imaging 1995;5:695–701. [DOI] [PubMed] [Google Scholar]
- [13].Lin CY, Ding HJ, Liu CS, et al. Correlation between the intensity of breast FDG uptake and menstrual cycle. Acad Radiol 2007;14:940–4. [DOI] [PubMed] [Google Scholar]
- [14].Weir I. Spearman's correlation. Statstutor, Mathematics Education Centre Loughborough University http://wwwstatstutor_ac_uk/resources/uploaded/spearmans.pdf [Accessed. 29, 2016]. [Google Scholar]
- [15].Bae JM, Shin SY, Kim EH, et al. Distribution of dense breasts using screening mammography in Korean women: a retrospective observational study. Epidemiol Health 2014;36:e2014027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Youn I, Choi S, Kook SH, et al. Mammographic breast density evaluation in korean women using fully automated volumetric assessment. J Korean Med Sci 2016;31:457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ciatto S, Zappa M. A prospective study of the value of mammographic patterns as indicators of breast cancer risk in a screening experience. Eur J Radiol 1993;17:122–5. [DOI] [PubMed] [Google Scholar]
- [18].Flook D, Gilhome RW, Harman J, et al. Changes in Wolfe mammographic patterns with aging. Br J Radiol 1987;60:455–6. [DOI] [PubMed] [Google Scholar]
- [19].Wolfe JN. Breast parenchymal patterns and their changes with age. Radiology 1976;121(3 Pt. 1):545–52. [DOI] [PubMed] [Google Scholar]
- [20].Kerlikowske K, Grady D, Barclay J, et al. Effect of age, breast density, and family history on the sensitivity of first screening mammography. JAMA 1996;276:33–8. [PubMed] [Google Scholar]
- [21].Ramakrishnan R, Khan SA, Badve S. Morphological changes in breast tissue with menstrual cycle. Mod Pathol 2002;15:1348–56. [DOI] [PubMed] [Google Scholar]
- [22].Longacre TA, Bartow SA. A correlative morphologic study of human breast and endometrium in the menstrual cycle. Am J Surg Pathol 1986;10:382–93. [DOI] [PubMed] [Google Scholar]
- [23].Pike MC, Spicer DV, Dahmoush L, et al. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev 1993;15:17–35. [DOI] [PubMed] [Google Scholar]
- [24].Speroff L. Role of progesterone in normal breast physiology. J Reprod Med 1999;442 Suppl:172–9. [PubMed] [Google Scholar]
- [25].Chang KJ, Lee TT, Linares-Cruz G, et al. Influences of percutaneous administration of estradiol and progesterone on human breast epithelial cell cycle in vivo. Fertil Steril 1995;63:785–91. [PubMed] [Google Scholar]
- [26].Bitencourt AG, Lima EN, Chojniak R, et al. Correlation between PET/CT results and histological and immunohistochemical findings in breast carcinomas. Radiol Bras 2014;47:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev 1997;18:502–19. [DOI] [PubMed] [Google Scholar]
- [28].Gompel A, Malet C, Spritzer P, et al. Progestin effect on cell proliferation and 17β-hydroxysteroid dehydrogenase activity in normal human breast cells in culture. J Clin Endocrinol Metab 1986;63:1174–80. [DOI] [PubMed] [Google Scholar]
- [29].Key TJ, Pike MC. The role of oestrogens and progestagens in the epidemiology and prevention of breast cancer. Eur J Cancer Clin Oncol 1988;24:29–43. [DOI] [PubMed] [Google Scholar]
- [30].Prior JC. Perimenopause: the complex endocrinology of the menopausal transition. Endocr Rev 1998;19:397–428. [DOI] [PubMed] [Google Scholar]
- [31].Ahmed Ebbiary NA, Lenton EA, Cooke ID. Hypothalamic-pituitary ageing: progressive increase in FSH and LH concentrations throughout the reproductive life in regularly menstruating women. Clin Endocrinol (Oxf) 1994;41:199–206. [DOI] [PubMed] [Google Scholar]
- [32].Hormonal Carcinogenesis III. Springer, Khan SA, Stickles S. Cell proliferation and apoptosis in the normal breast epithelium of pre, peri, and postmenopausal women. 2001;418-423. [Google Scholar]
- [33].Reed BG, Carr BR. The normal menstrual cycle and the control of ovulation. In: Endotext [Internet] MDText com, Inc 2015. [Google Scholar]


