Abstract
Background:
Probiotics is a prevalence therapeutic method for irritable bowel syndrome (IBS), but there is lack of comparison in different protocols. We aim to differentiate the reasonable protocols by assessing the efficacy and safety through the combined way of traditional and network meta-analysis.
Method:
PubMed, Medline, EMBASE, Web of Science, and Cochrane Central Register of Controlled Trials databases were searched from January 2006 to April 2019. The relative risk (RR) with a 95% confidence interval (CI) was used to combine dichotomous data of responders.
Result:
Among 14 studies included 1695 patients were identified as suitable for inclusion. The proportion of responders was associated with the administration of multispecies probiotics (RR: 1.39; 95% CI: 1.19–1.61) and the dose of 109∼1010 (RR: 2.08; 95% CI: 1.59–2.71). In network meta-analysis, the protocol of DUO had a significant effect for diarrhea type of IBS compared with placebo (RR: 7.46; 95% CI: 2.00–32.23). In the rest of 4 protocols, no significant difference was found in each other except F19 which appears inferior when compared with Pro (RR: 0.16; 95% CI: 0.03–0.88). Meanwhile, Pro showed a superior effect for undifferentiated-type IBS compared with placebo (RR: 7.16; 95% CI: 1.72–29.89). No probiotics-associated severe adverse event was reported in included studies.
Conclusion:
Probiotics is a safety choice to improve the overall symptoms for IBS patient. The protocols with suitable dose combined of Lactobacillus and Bifidobacterium can have prepotent effects compared with single species or over-dosage protocols. Network meta-analysis shows that DUO may be the first recommendation for diarrhea-type IBS. In the remaining 4 regimes of this study, Pro has a high rank for undifferentiated-type IBS.
Keywords: intestinal microbiota, irritable bowel syndrome, network meta-analysis, probiotics, RCTs
1. Introduction
Irritable bowel syndrome (IBS) is a nonorganic bowel disorder characterized by recurrent abdominal pain or “discomfort" with stool irregularities.[1] The pooled prevalence of IBS in the general population is about 11.2%, which ranges from 1.1% to 45%.[2] High morbidity increases social expenditure including the economic and humanistic burden of disease.[3] Thus, the importance of effective treatment of IBS is self-evident.
However, the current drug options including antispasmodic, antidiarrheals, rifaximin, antidepressants, Laxatives and motility accelerants are limited by barely ideal efficacy or side-effect.[4] Probiotics, defined as live microorganisms, could change intestinal flora to regulate intestinal function such as to reduce visceral hypersensitivity,[5] improve the mucosal barrier function, modulate immunity and chronic inflammation,[6,7] communicate with central nervous system,[8] influence the gastrointestinal motility,[9,10] and so on. In addition, probiotics also could ameliorate hepatic steatosis of nonalcoholic fatty liver disease (NAFLD) which influences the intestinal function by gut–gut microbiota-liver axis.[11] According to evidence from numerous clinical trials of different probiotic protocols, medical scholars agree that specific strain could relieve gastrointestinal symptoms and recommend probiotics for IBS patients because of its trait of being inexpensive, safe, and potentially beneficial,[12–14] but its specific protocol is a pending issue.
Therefore, we performed a network meta-analysis to explore the potential protocol model.
2. Method
2.1. Ethics statement
As all analyses were based on previously published studies, no ethical approval or patient consent was required.
2.2. Literature search
The PubMed, Medline, EMBASE, Web Of Science, and Cochrane Central Register of Controlled Trials databases were searched from January 2006 to April 2019 through the strategy: (“probiotics” OR “probiotic”) AND (“irritable bowel syndrome” OR “IBS”) searched in [All Fields].
2.3. Inclusion and exclusion criteria
Inclusion criteria were: studies were randomized controlled trials (RCTs) with double-blind and parallel design; subjects should be adult patients (age ≥18 years); studies’ results were published in English, and the endpoint should meet the requirements in section of “Outcome assessment.” Exclusion criteria were: Studies not adhere to the inclusion criteria; studies with only an abstract or could not extract available data; studies include other functional gastrointestinal disease or pathologies other than IBS; studies concomitant of other drugs; studies absent of wash-time.
2.3.1. Outcome assessment
Dichotomous data are “responders” which reflect the global efficacy of probiotics, defined as reporting “adequate relief (AR)” or “satisfactory relief (SR)” of IBS symptoms for >50% of the time[15] or in the last week at those 4 weeks’ trials.[16] AR or SR is identified as a primary endpoint in Rome III. Investigators would ask the patient weekly, “In the last 7 days, have you had adequate (or satisfactory) relief of your IBS symptoms?”, then patient only give a subjective answer of “YES” or “NO.”[17]
Adverse events (AEs) are also recorded to assess the safety.
2.4. Data extraction
Two reviewers independently extracted and assessed the target data. Disagreements were resolved by discussion and an additional reviewer. The data included the first author, recruitment criteria, intervention, oral type, does of probiotics, treatment duration, sample size, and the number of responders. The dichotomous data are intent-to-treat data.
2.5. Assessment of quality
Quality was assessed as described in the Cochrane handbook.[18] Six items (selection bias, performance bias, detection bias, attrition bias, and reporting bias and other bias) were assessed by 2 dependent reviewers.
2.6. Statistical analysis
Review Manager Version 5.3 (The Cochrane Collaboration, Oxford, UK) was used for the traditional meta-analysis. Risk ratio (RR) with 95% confidence interval (CI) was used to evaluate global efficacy. Heterogeneity was examined with I2 statistics. A fixed-effects model was used in I2 <50%, if not random-effects model would be chosen.[17] Stata SE 15 (StataCorp. College Station, TX) was used for Begg or Egger test, network graph, and net weight graph. Gemtc (GitHub) was used for Bayesian network meta-analysis to compare the indirect treatment.[19] A P <.05 was judged as statistically significant. Convergence was assessed to calculate the Potential Scale Reduction Factor (PSRF), and values were limited to 1. Sensitivity analyses were performed by reassessing pooled outcomes after single study deletion.
For studies with >1 intervention arm, the addressing is that splitting the “shared" group into ≥2 groups with smaller sample size (reasonably independent comparisons) according to the Cochrane handbook.[17]
3. Result
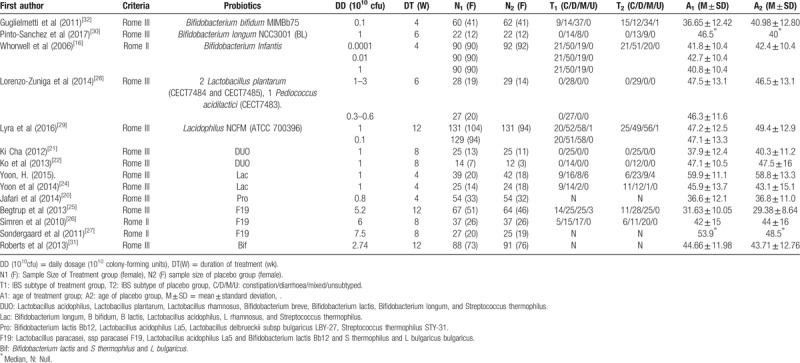
The search strategy generated a total of 4638 citations, of which 209 published articles appeared to be relevant, and were retrieved for further assessment (Fig. 1). Of these, 195 articles were excluded for various reasons, leaving 14 eligible articles which included 1695 patients to assess the efficacy. The 14 studies were all placebo-controlled RCTs.[16,20–32] The details were presented in Table 1.
Figure 1.
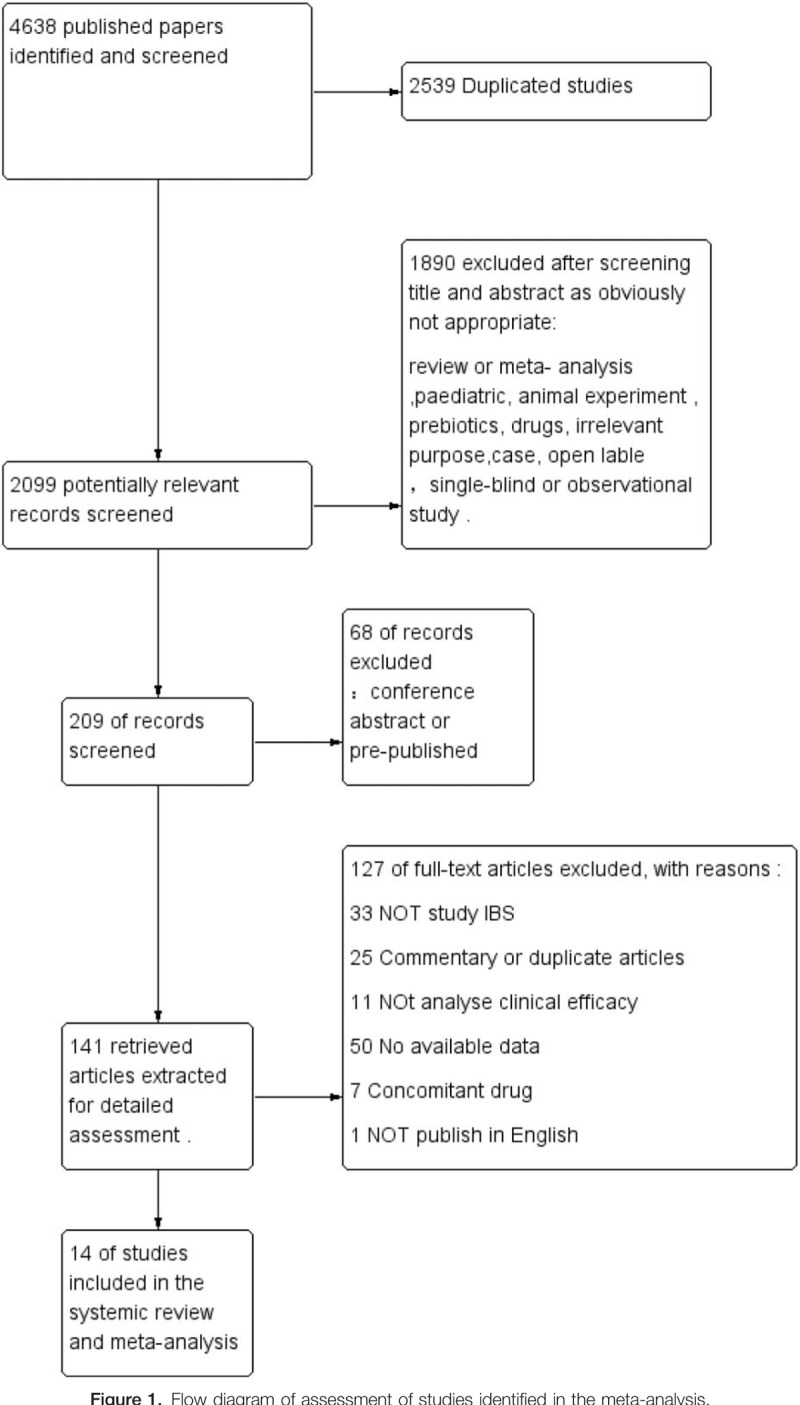
Flow diagram of assessment of studies identified in the meta-analysis.
Table 1.
Main characteristics of included studies.
3.1. Risk of bias in included studies
Majority of bias items showed low risk and some showed unclear risk. Only 1 study showed a high risk in the item of attrition bias because of the high level of drop-outs.[31] In the blinding method which is critical and more important than other bias for subjective outcomes, all studies exhibited low risk. See Figures 2 and 3 for more details.
Figure 2.
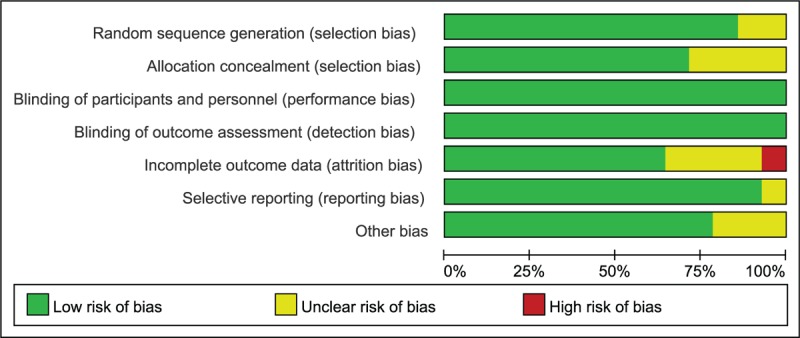
Risk of bias graph.
Figure 3.
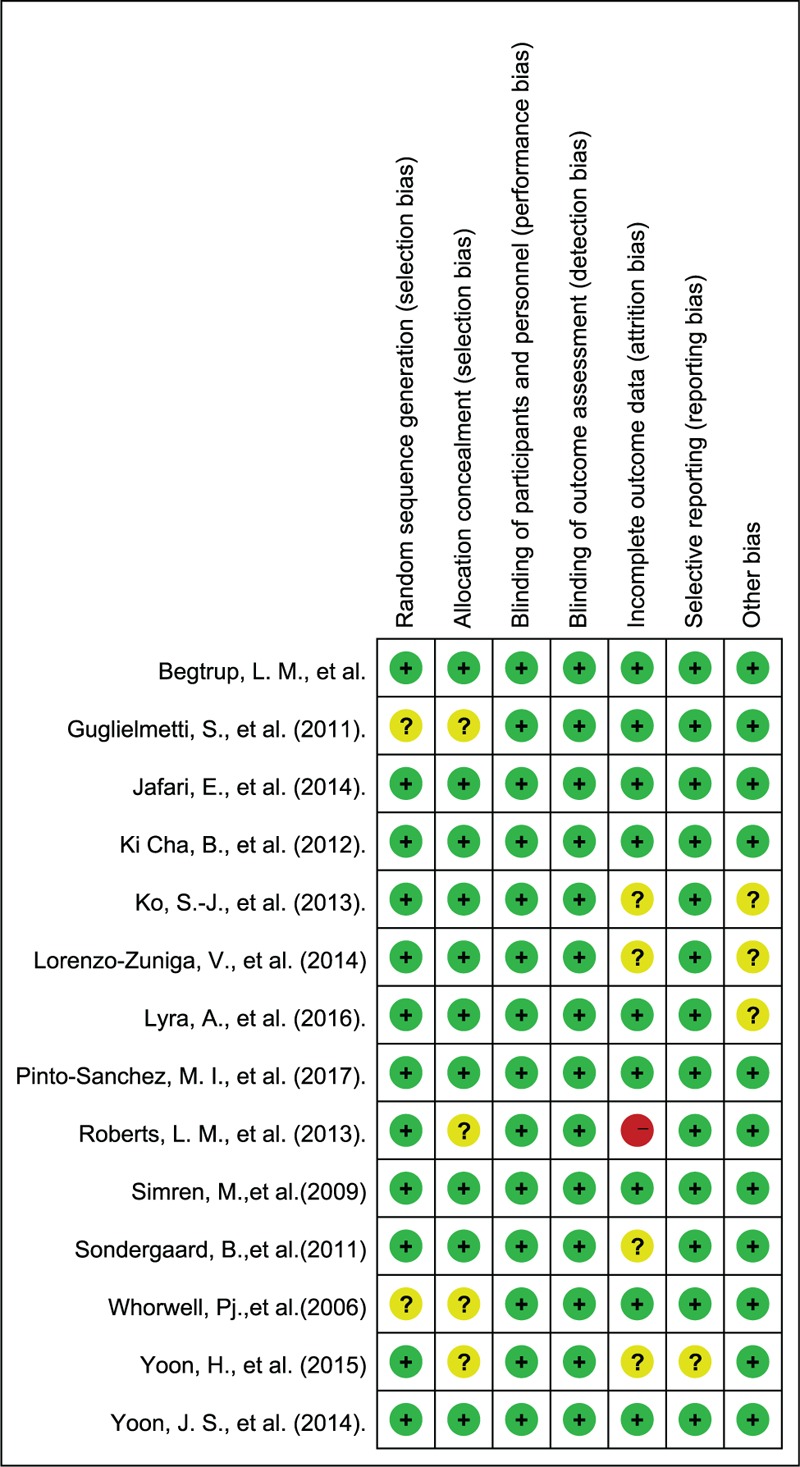
Risk of bias summary.
3.2. “Responders” in the meta-analysis
A total of 14 RCTs used the dichotomous data based on AR or SR for comparing probiotics with placebo. The sensitivity analysis found that the removal of any article did not have a significant impact on the final result, but suggested that the heterogeneity was mainly derived from the study of Guglielmetti et al.[32] Therefore, we analyzed the results of the remaining 13 studies.
The responder's proportion was 45.0% in the probiotics group and 37.5% in the control group. The RR of “responders” was significantly higher in the probiotics group (RR: 1.27; 95% CI: 1.13–1.44; P < .001; I2 = 34%) (Fig. 4).
Figure 4.
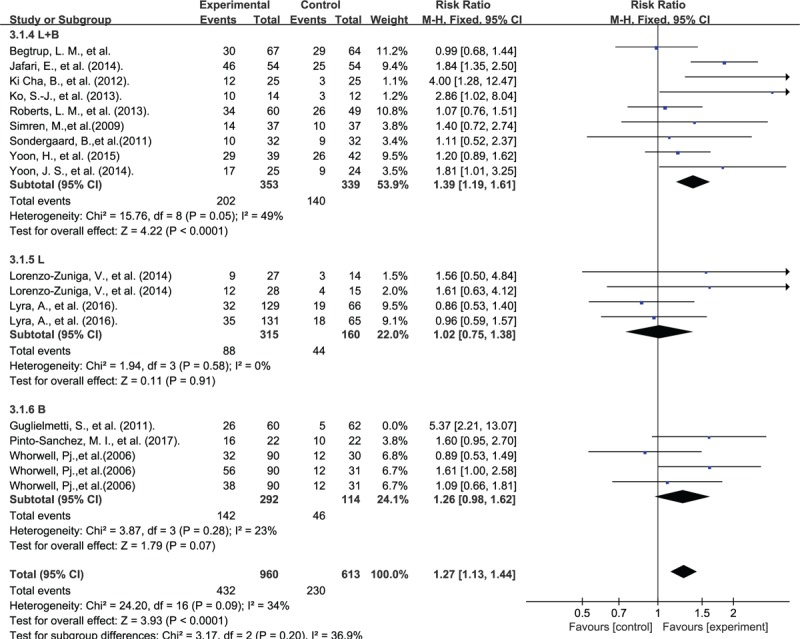
Forest plot of probiotic effect on overall symptoms: subgroup of probiotic species.
In subgroup analysis, the L+B (Lactobacillus+Bifidobacteriums) group had significantly benefit for IBS patient (RR: 1.39; 95% CI: 1.19–1.61; P < .001; I2 = 49%), but not in L (lactobacillus) group (RR: 1.02; 95% CI: 0.75–1.38; P = .91; I2 = 0%) or B (bifidobacteriums) group (RR: 1.26 95% CI: 0.98–1.62; P = .07; I2 = 23%) (Fig. 4).
3.3. Dosage analysis for L+B group
We divided the interventions in Group L+B into high-dose (probiotic intake >1010 cfu/day) and low-dose (probiotic intake = 109∼1010 cfu/day) groups for analysis. Sensitivity analysis suggested a good consistency, but heterogeneity was mainly derived from the study of Yoon et al.[24] For remaining studies, the average dose was 0.96 and 5.36 (1010 cfu/day) in low- and high-dose group, respectively. Low-dose group had significant effect for overall symptom (RR: 2.08; 95% CI: 1.59–2.71; P < .001; I2 = 0%), but high-dose group not (RR: 1.09; 95% CI: 0.86–1.37; P = .49; I2 = 0%) (Fig. 5).
Figure 5.
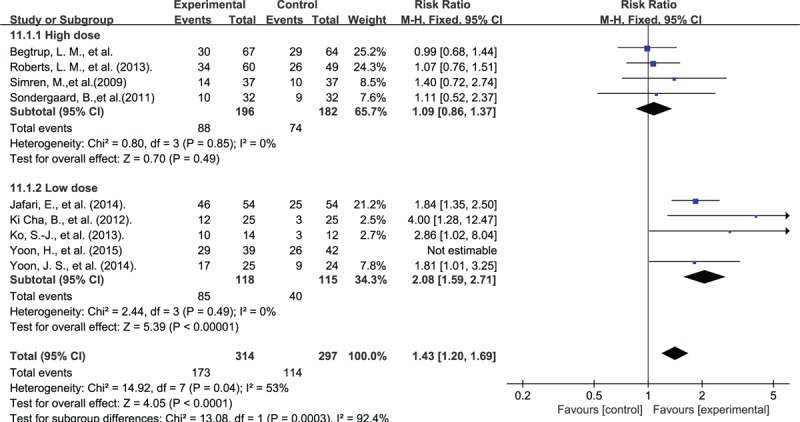
Forest plot of probiotic effect for L+B group: subgroup of dosage.
3.4. Network meta-analysis for L+B group
The L+B group composed of 5 probiotic programs: DUO, Lac, Pro, F19, and Bif. (details were shown in Table 1). A good convergence efficiency was proved by all PSRF values of the different parameters were limited to 1. The sample size of each protocol in the network meta-analysis was represented in the form of a network diagram (Fig. 6).
Figure 6.
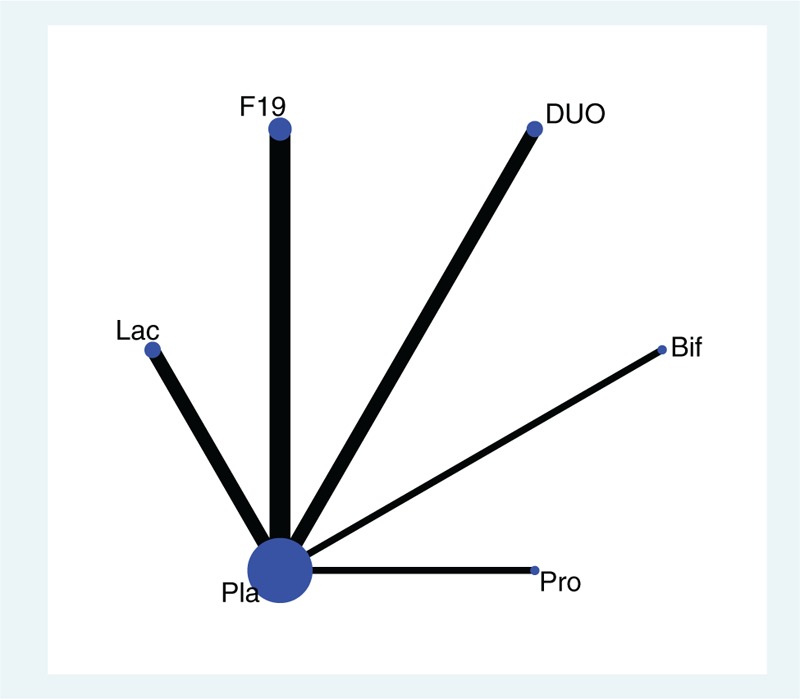
Star-shape network diagram.
The DUO and Pro showed superior efficacy compared with placebo (RR: 7.46; 95% CI: 2.00–32.23) and (RR: 7.16; 95% CI: 1.72–29.89), respectively. In the comparison of different protocols, DUO appears superior compared with F19 (RR: 6.37; 95% CI 1.34–32.59) and Bif (RR: 6.66; 95% CI 1.08–47.34). We also found inferior effect when F19 compared with Pro (RR: 0.16; 95% CI 0.03–0.88) (Table 2).
Table 2.
Network meta-analysis of responders.

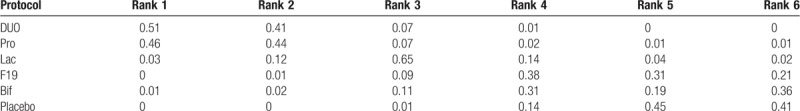
In the ranking table, DUO and Pro had a high probability of ranking top (Table 3).
Table 3.
Rank probability of probiotic protocols.
3.5. Assessment of published bias
The funnel plots seemingly suggest potential asymmetry (Fig. 7). To further assess whether there is a publication bias, we have taken the Begg and Egger test analysis and the results have suggested no evidence of publication bias (P >.1) (additional files).
Figure 7.
Funnel plot for publication bias. (A) Funnel plot for overall studies. (B) Funnel plot for studies of L+B group.
3.6. AEs with probiotics
Among the 14 studies, 12 reported the information about AEs. As the variance of definition or reported design to AEs, the quantitative assessment was not suitable. In general, the AEs were mainly concentrated in gastrointestinal symptoms and are often mild and transient. Moreover, AEs were at a low level and were not statistically different from the placebo groups.
4. Discussion
The therapeutic value of probiotics for IBS patients is increasingly being explored. It is unrealistic to find a final solution among many probiotics, but through statistical methods, we can find a closer idealized model.[33]
In the Pairwise comparisons, our meta-analysis proves that probiotics could improve the overall symptoms in patients with IBS. Moreover, L+B products exhibit superiority when we make a subgroup analysis based on Lactobacillus and Bifidobacterium which are the 2 major species of probiotics. This outcome should be reasonable that some studies have been indicated that Lactobacillus and Bifidobacterium are decreased in the patient with IBS.[34] Moreover, Lactobacillus and Bifidobacterium could produce a variety of beneficial effects because each species exerts a distinct action on the gastrointestinal from different mechanism like secrete bacteriocins, modulate the host immune system, and so on.[23,34] Those actions may be complementary or synergistic. The systematic review of Chapman et al[35] has indicated that probiotic mixtures appear to be effective against a wide range of endpoints. However, their study is a qualitative description mixed with animal and human study. Our research more clearly demonstrates that combined utilization of Lactobacillus and Bifidobacterium can have prepotent effects compared with single species probiotics.
To further explore the heterogeneous sources in the L+B group, we make subgroup analysis based on dose. We find that the heterogeneity was well eliminated after dose grouping and that “low dose” is associated with improvement of global symptom. The explanation for such an outcome is unclear, but some head-to-head studies have provided evidence that large doses of probiotics are not necessarily better than low doses, and may even be inferior to low doses.[16,28,29] Lorenzo-Zuniga et al[28] speculate that probiotics may not follow the saturation effect of typical pharmacological rules but involve more complex synergistic or antagonistic relationship. Fecal flora analysis before and after probiotics manifests that different bacterial strains have different survivability and overdosage may impair some probiotic living conditions through competition, especially in mixture probiotic products.[36,37] In addition, patient characteristics have an important influence on the efficacy of probiotics. Hod et al[38] find that responder have higher baseline proportions of Faecalibacterium, Leuconostoc, and Odoribacter compared to nonresponders. Meta-analysis find that Lactobacillus and Bifidobacterium would decrease in IBS-D but not in IBS-C.[39] Therefore, high-dose probiotics may aggravate dysbiosis rather than supplemental effects in some IBS patients. Meanwhile, high-dose probiotics may cause gastrointestinal discomfort in the short term by over-fermenting carbohydrates as Lactobacillus and Bifidobacterium have ability to digest carbohydrates.[40] In summary, our result suggests that reasonable total dosage and percentage of each component may be one of the research directions to improve the efficacy of probiotic products in patients with IBS and the dosage of 109∼1010 cfu/day may be a reference range.
To further analyze the effectiveness of multiple probiotics products, we make a network meta-analysis. In contrast to traditional meta-analyses, which make pairwise comparisons between 2 interventions, the network meta-analyses allow comparison of all interventions regardless of whether there have been direct comparisons in clinical trials.[41] Our study finds that the protocols of DUO have significant effect compared with placebo and might be the best method for improving overall symptoms from the outcome of the rank table. Maukonen et al.[42] have suggested that IBS patients have the characteristic of unstable composition of fecal flora. Analyzing patient feces after taking DUO, Ki Cha et al[21] found that probiotics in the experimental group have better stability than probiotics in the placebo group. This may be one of the mechanisms by which DUO effectively integrates various probiotics to improve the symptoms of patients. However, distinct with the mixture of 4 types patients of IBS, all of the enrolled patients are diarrheic type in DUO group.[21,22] Thus, we cannot fully predict whether the DUO protocols can still achieve a high role in undifferentiated types of IBS. In another 4 interventions, Pro has a higher ranking but no significant difference in each other except F19 showed inferior when compared with Pro. As mentioned above, the F19 protocol is in the high-dose group in this article, and it is inferior may be because of excessive doses. A ridiculous conjecture is that although Lactobacillus paracasei, ssp paracasei is usually considered safe and stable, it may play a “bad” or “nihility” role in the F19 protocol.[43]
Some limitations exist in our article which arose from the nature of the studies available for synthesis. A question cannot be ignored come from the difference of study included variations of baseline, characteristics, intervention, specific process, even though comparing the previous meta-analysis we have strict screen criteria and acceptable heterogeneity. Choosing “responders” as our endpoint makes it inevitable to remove some probiotic clinical trials. Thus, we only compare the effects of 5 different regimes. Meanwhile, for the natural rejection of repeated verification of the same regimes, the limited sample size weakens the statistical significance. Another limitation is that our study has variability in the duration of treatment (4–12 weeks; m ± sd = 7 ± 3.07 weeks) as some probiotic may need more time to take effect. In the network meta-analysis, we have planned to assess the consistency by loop-specific test and nodal analysis. However, no studies provide direct comparison in different probiotics. Consequently, we only make a simple Star-shaped network meta-analysis.
Strengths in our study design include trials with similar patient population and outcome assessments. Through our inclusion criteria, the 14 included RCTs based on Rome III or II have high quality from the risk outcome of bias graph, especially in the blinding method, all of which exhibited low risk. Moreover, we control heterogeneity within an acceptable range and even mild heterogeneity in some results. The low heterogeneity enhances the credibility of the results compared with previous meta-analysis.[44,45] Another strength is the strict control of the outcome indicators. Previous studies tend to use single symptom indicators to prove the partial efficacy of IBS, but patient may experience different chief complaints. To exclude the interference of symptoms variability in IBS, we choose “responders” as our endpoint based on AR or SR. This is an integrated index which could comprehensively demonstrate the patient's affirmation of his benefits.[46] Meanwhile, this endpoint could reduce deviation because of educational level and language background of patients. In our impression, this study is the first one to compare the efficacy in different probiotic protocols rather than the simple argument for probiotic benefits to IBS. Through the network meta-analysis, we find some improvement goals about the idealized probiotic pattern for IBS.
In conclusion, probiotics is a safety choice to improve the overall symptoms for IBS patient. The protocols with suitable dose combined of Lactobacillus and Bifidobacterium can have prepotent effects compared with single species or over-dosage protocols. Network meta-analysis shows that DUO may be the first recommendation for diarrhea-type IBS. In the remaining 4 regimes of this study, Pro has a high rank for undifferentiated type IBS.
Author contributions
Conceptualization: Xu Guoqiang.
Data curation: Ding Liang, Ning Longgui.
Formal analysis: Ding Liang, Ning Longgui.
Software: Ding Liang.
Writing – original draft: Ding Liang.
Writing – review & editing: Xu Guoqiang.
Footnotes
Abbreviations: AEs = Adverse events, AR = adequate relief, B = bifidobacteriums, cfu = Colony-Forming Units, CI = confidence interval, IBS = irritable bowel syndrome, L = lactobacillus, L+B = Lactobacillus+Bifidobacteriums, NAFLD = nonalcoholic fatty liver disease, PSRF = Potential Scale Reduction Factor, RCTs = randomized controlled trials, RR = relative risk, SR = satisfactory relief.
The authors report no conflicts of interest
References
- [1].Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Prime 2016;2:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012;10:712.e4–21.e4. [DOI] [PubMed] [Google Scholar]
- [3].Nellesen D, Yee K, Chawla A, et al. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J Manag Care Pharm 2013;19:755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu JC. Complementary and alternative medicine modalities for the treatment of irritable bowel syndrome: facts or myths? Gastroenterol Hepatol (N Y) 2010;6:705–11. [PMC free article] [PubMed] [Google Scholar]
- [5].Rousseaux C, Thuru X, Gelot A, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med 2007;13:35–7. [DOI] [PubMed] [Google Scholar]
- [6].Mangell P, Nejdfors P, Wang M, et al. Lactobacillus plantarum 299 v inhibits Escherichia coli-induced intestinal permeability. Dig Dis Sci 2002;47:511–6. [DOI] [PubMed] [Google Scholar]
- [7].Rachmilewitz D, Katakura K, Karmeli F, et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 2004;126:520–8. [DOI] [PubMed] [Google Scholar]
- [8].Quigley EMM. The Gut-Brain Axis and the Microbiome: Clues to Pathophysiology and Opportunities for Novel Management Strategies in Irritable Bowel Syndrome (IBS). J Clin Med 2018;7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Quigley EM. Microflora modulation of motility. J Neurogastroenterol Motil 2011;17:140–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Butel MJ. Probiotics, gut microbiota and health. Med Mal Infect 2014;44:1–8. [DOI] [PubMed] [Google Scholar]
- [11].Ahn SB, Jun DW, Kang BK, et al. Randomized, double-blind, placebo-controlled study of a multispecies probiotic mixture in nonalcoholic fatty liver disease. Sci Rep 2019;9:5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Song KH, Jung HK, Kim HJ, et al. Clinical Practice Guidelines for Irritable Bowel Syndrome in Korea, 2017 Revised Edition. J Neurogastroenterol Motil 2018;24:197–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology 2016;150:1262–79. [DOI] [PubMed] [Google Scholar]
- [14].Hungin APS, Mitchell CR, Whorwell P, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms - an updated evidence-based international consensus. Aliment Pharmacol Ther 2018;47:1054–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Irvine EJ, Tack J, Crowell MD, et al. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology 2016;150: 1469-1480.e1461. [DOI] [PubMed] [Google Scholar]
- [16].Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol 2006;101:1581–90. [DOI] [PubMed] [Google Scholar]
- [17].Irvine EJ, Whitehead WE, Chey WD, et al. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology 2006;130:1538–51. [DOI] [PubMed] [Google Scholar]
- [18].Higgins JPT, Green S. (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: www.cochrane-handbook.org. [Google Scholar]
- [19].John Wiley & Sons, Ltd, Valkenhoef GV, Kuiper J. gemtc: Network Meta-Analysis Using Bayesian Methods. 2016. [Google Scholar]
- [20].Jafari E, Vahedi H, Merat S, et al. Therapeutic effects, tolerability and safety of a multi-strain probiotic in Iranian adults with irritable bowel syndrome and bloating. Arch Iran Med 2014;17:466–70. [PubMed] [Google Scholar]
- [21].Ki Cha B, Mun Jung S, Hwan Choi C, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol 2012;46:220–7. [DOI] [PubMed] [Google Scholar]
- [22].Ko SJ, Han G, Kim SK, et al. Effect of korean herbal medicine combined with a probiotic mixture on diarrhea-dominant irritable bowel syndrome: a double-blind, randomized, placebo-controlled trial. Evid Based Complement Alternat Med 2013;2013:824605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yoon J, Sohn W, Lee O, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol 2014;29:52–9. [DOI] [PubMed] [Google Scholar]
- [24].Yoon H, Park YS, Lee DH, et al. Effect of administering a multi-species probiotic mixture on the changes in fecal microbiota and symptoms of irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Biochem Nutr 2015;57:129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Begtrup L, Muckadell O, Kjeldsen J, et al. Long-term treatment with probiotics in primary care patients with irritable bowel syndrome--a randomised, double-blind, placebo controlled trial. Scand J Gastroenterol 2013;48:1127–35. [DOI] [PubMed] [Google Scholar]
- [26].Simren M, Ohman L, Olsson J, et al. Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome - a randomized, double-blind, controlled study. Aliment Pharmacol Ther 2010;31:218–27. [DOI] [PubMed] [Google Scholar]
- [27].Sondergaard B, Olsson J, Ohlson K, et al. Effects of probiotic fermented milk on symptoms and intestinal flora in patients with irritable bowel syndrome: a randomized, placebo-controlled trial. Scand J Gastroenterol 2011;46:663–72. [DOI] [PubMed] [Google Scholar]
- [28].Lorenzo-Zuniga V, Llop E, Suarez C, et al. I.31, a new combination of probiotics, improves irritable bowel syndrome-related quality of life. World J Gastroenterol 2014;20:8709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lyra A, Hillila M, Huttunen T, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol 2016;22:10631–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pinto-Sanchez M, Hall G, Ghajar K, et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology 2017;153: 448-459.e448. [DOI] [PubMed] [Google Scholar]
- [31].Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ’functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol 2013;13:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—a double-blind, placebo-controlled study. Aliment Pharmacol Ther 2011;33:1123–32. [DOI] [PubMed] [Google Scholar]
- [33].Whelan K. The importance of systematic reviews and meta-analyses of probiotics and prebiotics. Am J Gastroenterol 2014;109:1563–5. [DOI] [PubMed] [Google Scholar]
- [34].Rodino-Janeiro BK, Vicario M, Alonso-Cotoner C, et al. A review of microbiota and irritable bowel syndrome: future in therapies. Adv Ther 2018;35:289–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chapman CM, Gibson GR, Rowland I. Health benefits of probiotics: are mixtures more effective than single strains? Eur J Nutr 2011;50:1–7. [DOI] [PubMed] [Google Scholar]
- [36].Bogovic Matijasic B, Obermajer T, Lipoglavsek L, et al. Effects of synbiotic fermented milk containing Lactobacillus acidophilus La-5 and Bifidobacterium animalis ssp. lactis BB-12 on the fecal microbiota of adults with irritable bowel syndrome: a randomized double-blind, placebo-controlled trial. J Dairy Sci 2016;99:5008–21. [DOI] [PubMed] [Google Scholar]
- [37].Ludidi S, Jonkers DM, Koning CJ, et al. Randomized clinical trial on the effect of a multispecies probiotic on visceroperception in hypersensitive IBS patients. Neurogastroenterol Motil 2014;26:705–14. [DOI] [PubMed] [Google Scholar]
- [38].Hod K, Dekel R, Aviv Cohen N, et al. The effect of a multispecies probiotic on microbiota composition in a clinical trial of patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil 2018;30:e13456. [DOI] [PubMed] [Google Scholar]
- [39].Liu HN, Wu H, Chen YZ, et al. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: a systematic review and meta-analysis. Dig Liver Dis 2017;49:331–7. [DOI] [PubMed] [Google Scholar]
- [40].Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017;66:1517–27. [DOI] [PubMed] [Google Scholar]
- [41].Kiefer C, Sturtz S, Bender R. Indirect comparisons and network meta-analyses. Dtsch Arztebl Int 2015;112:803–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Maukonen J, Satokari R, Matto J, et al. Prevalence and temporal stability of selected clostridial groups in irritable bowel syndrome in relation to predominant faecal bacteria. J Med Microbiol 2006;55(pt 5):625–33. [DOI] [PubMed] [Google Scholar]
- [43].West NP, Pyne DB, Cripps AW, et al. Gut Balance, a synbiotic supplement, increases fecal Lactobacillus paracasei but has little effect on immunity in healthy physically active individuals. Gut Microbes 2012;3:221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhang Y, Li L, Guo C, et al. Effects of probiotic type, dose and treatment duration on irritable bowel syndrome diagnosed by Rome III criteria: a meta-analysis. BMC Gastroenterol 2016;16:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol 2014;109:1547–61. quiz 1546, 1562. [DOI] [PubMed] [Google Scholar]
- [46].Mangel AW. Personal view: adequate relief as a primary endpoint in irritable bowel syndrome. Aliment Pharmacol Ther 2006;23:879–81. [DOI] [PubMed] [Google Scholar]


