Supplemental Digital Content is available in the text
Keywords: exercise, gestational weight gain, meta-analysis, pregnancy
Abstract
Background:
Clinical evidence indicates that women will benefit from regular physical activity during pregnancy. This study aimed to summarize and update the evidence on the effect of exercise on maternal gestational weight gain (GWG).
Methods:
We conducted a systematic literature search of Pubmed, Embase, and Cochrane Library from inception until July, 2018 for randomized controlled trials (RCTs) that investigate the effect of physical exercises on the maternal GWG compared with that of no physical exercises or conventional medical care. We extracted data from eligible trials for study characteristics, interventions, patients’ baseline characteristics and outcomes for the study populations of interest. We conducted meta-analyses using random effects models.
Results:
From 844 citations, 23 RCTs including 4462 pregnant women met the inclusion criteria. Meta-analysis indicated that compared with that in women having conventional medical care, GWG was significantly decreased in pregnant women with physical exercise [weighted mean difference (WMD) −1.02, 95% CI −1.35 to −0.70; P < .01; I2 = 48.4%]. Women appeared to benefit more for gestational weight control for exercise frequency of 3 times per week (WMD −1.22, 95% CI −1.55 to −0.90; I2 = 40.3%) and exercise duration of 30 to 45 minutes each time (WMD −1.32, 95% CI −1.79 to −0.85; I2 = 1.5%).
Conclusion:
This meta-analysis provides indications that exercise intervention can reduce maternal GWG for pregnant women, especially for those with exercise frequency of 3 times per week and duration of 30 to 45 minutes each time.
1. Introduction
Excessive gestational weight gain (GWG) is a common issue among pregnant women, accounting for approximately 50% of all pregnant women in the USA.[1] Excessive GWG has also been reported to increase the risk of poor prognosis for pregnancy outcomes, including gestational diabetes, hypertension, preeclampsia, preterm birth and cesarean delivery, which could result in detrimental consequences for both mothers and infants’ health.[2–5] Nehring et al found that excessive GWG increased the risk of childhood overweight by nearly 30%.[6] A cohort study by Ensenauer et al also demonstrated that excessive GWG was associated with an increased risk of infant overweight as well as abdominal adiposity, and even offspring cognition.[7] However, few reports have found certain interventions with consistent results for the prevention of excessive GWG for both the mother and the infant.
Recent randomized controlled trials (RCTs) and meta-analyses summarized the effects of physical activity during pregnancy on maternal and infant prognosis with controversial findings. Streuling et al found that physical activity during pregnancy could significantly restrict GWG with 12 RCTs.[8] Moreover, a recent meta-analysis by Silva et al found that exercise programs during pregnancy could reduce the risk of excessive weight gain, gestational diabetes, delivering a preterm infant or a baby large for gestational age, while no effects of exercise during pregnancy was found on pre-eclampsia, preterm birth, or birth weight.[9] Nevertheless, some of the RCTs found no effects[10,11] and even harm at early stage of pregnancy with increased risk of miscarriage.[53] Price et al showed no significant difference on weight gain from 12-week gestation to the last prenatal visit between the exercise group and the control group.[12] In addition, there is a lack of high-quality evidence regarding the effects of exercise characteristics (including frequency, intensity, duration, type, and volume) on maternal GWG based on guidelines from all around the world mainly including European, American and Asian countries.[47,48] Therefore, based on high-quality RCTs, we aimed to collect and reassess all the evidence regarding whether physical exercise and its characteristics can have an effect on maternal GWG.
2. Methods
2.1. Search strategy
A systematic review and meta-analysis was conducted based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for meta-analyses of RCTs.[13] Both of the patient informed consent and the ethical approval were not required because this study was a meta-analysis based on previously published data. We searched relevant trials using the following terms: (exercise or exercise therapy or physical activity or cycling or swimming or dancing or walk or yoga or tai chi) and (pregnancy or pregnant) and (randomized controlled trial or controlled clinical trial or randomized or placebo or randomly or trial) as keywords or text words or the Medical Subject Headings (MeSH) terms published on Pubmed, Embase and Cochrane Library from initial to July, 2018. The initial search was conducted by 2 senior research authors and we applied no language restrictions. We restricted studies to RCT, controlled clinical trials or meta-analyses with original data. We also hand searched bibliographies of included trials. Detailed search strategies of the searched online databases are provided in Supplementary materials.
2.2. Study selection
Three authors (J.W., D.W., and X.L.) independently assessed trials for eligibility. Disagreements between the authors on the inclusion or exclusion of the trials were resolved by discussion, or when necessary, consultation with a senior author. The studies were eligible for inclusion if they satisfied the following criteria,
-
(1)
published as RCTs;
-
(2)
pregnant women having physical activity such as aerobic exercises, strength training, walking, cycling, or weight training compared with conventional medical care;
-
(3)
studies were included if they reported outcome of maternal GWG during pregnant period. We excluded non RCTs such as cohort studies.
2.3. Data extraction and quality assessment
For each included study, 3 authors (J.W., D.W., and X.L.) independently extracted data from the trials’ original texts and available supplementary materials using a predesigned data abstraction form. One author (Y.L.) entered the data into an excel datasheet, and the other authors checked these entries. The variables for abstraction were: first author of the study, the publication year, research country, sample size of the trial and control group, intervention applied, gestational period of the subjects, frequency, duration, and intensity of intervention.
The methodological quality of trials was assessed using the Jadad ranking score system to ascertain risk of trial bias.[14] The score system assesses the risk of bias in aspects of the method of randomization, double-blindness, the withdrawals, and dropouts of participants. A Jadad score of between 0 and 5 could be obtained based on these aspects, with higher score representing good methodological quality and lower score representing poor methodological quality.
2.4. Statistical analyses
We performed all meta-analyses using the Dersimonian–Laird random-effects model in Stata Software (version 12.0; StataCorp LP, College Station, TX). The Cochran chi-square test and the I2 statistic were used to measure inter-trial heterogeneity.[15] We defined an I2 statistic more than 50% as significant heterogeneity.[16] Summary data for continuous outcomes such as maternal GWG measured with the same scale were presented as weighted mean differences (WMDs) with 95% confidence intervals (CIs). Subgroup analyses were tested to further examine potential sources of between-trial heterogeneity in terms of some trial variables including trial region, sample size, gestational period, intervention frequency, duration and intensity, and Jadad score. Begg test and Egger linear regression method were quantified to assess publication bias.[17,18] When necessary, the trim-and-fill method was applied to adjust the pooled estimates to assess the possible effect of publication bias.[19] We also conducted sensitivity analysis to investigate the influence of each study on the pooled estimate. All statistical analyses were 2-sided. We considered a P value <.05 as significant difference.
3. Results
Our original search identified 844 original titles, of which 73 were considered potentially relevant for full-text review. After full text review, 23 primary RCTs published between 1999 and 2017 investigating exercise and maternal GWG contributed to the quantitative synthesis.[10–12,20–37]Figure 1 gives the detailed process for study selection of this meta-analysis.
Figure 1.
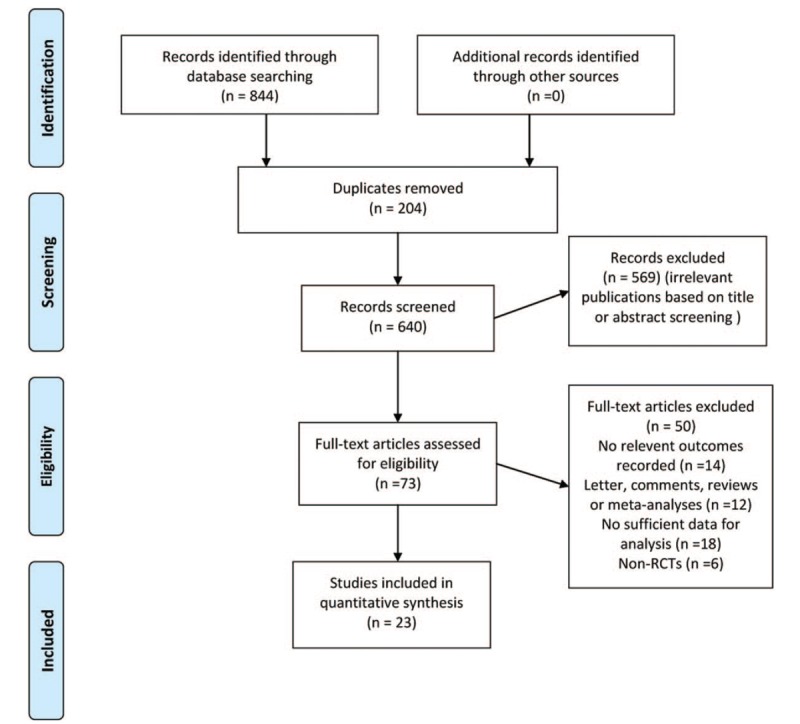
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for systematic review phases of exercise on maternal gestational weight gain.
3.1. Demographics
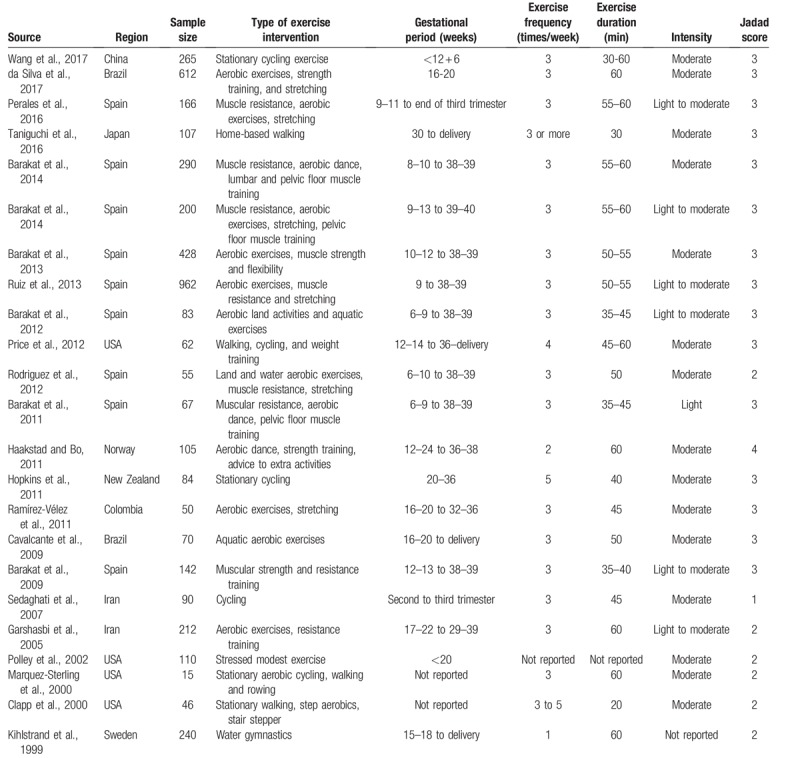
The 23 RCTs included a total of 4462 pregnant women, of whom 2128 had been randomized to certain physical exercise program and 2334 to standard antenatal care and normal daily activities. Eleven of the trials were conducted in Europe, 4 in the USA, 4 in Asia and 3 in South America. Physical exercise was administered from the 1st to the 3rd gestational trimester in 4 trials, from the 2nd to the 3rd gestational trimester in 12 trials and from the 2nd trimester to delivery in 3 trials. The frequency of physical exercise ranged from once to twice to 4 to 5 times per week. For risk of bias, 16 trials had higher Jadad score (3–4 points) and 7 had lower Jadad score (1–2 points). Table 1 summarizes the main characteristics of the trials included in the analysis of the main outcome.
Table 1.
Characteristics of studies satisfying search inclusion criteria.
3.2. GWG
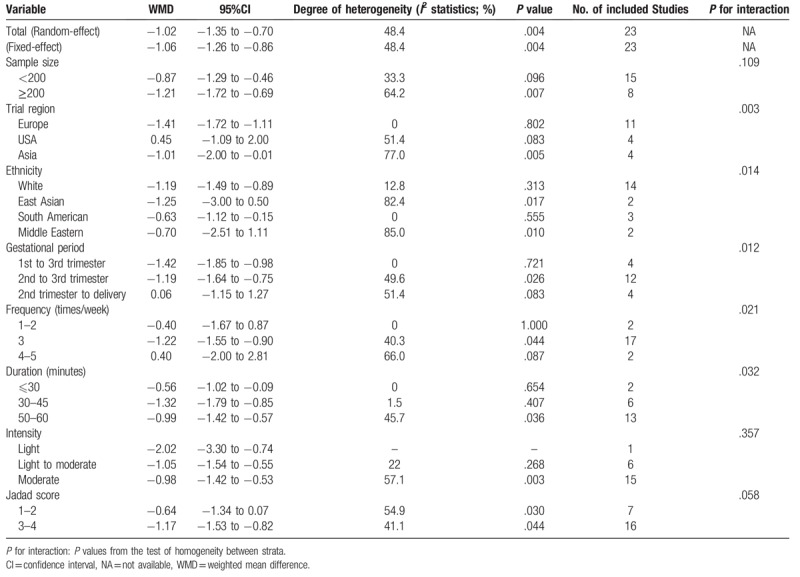
Meta-analysis from 23 RCTs indicated that compared with that in women having conventional medical care, GWG was significantly decreased in pregnant women with physical exercise (WMD −1.02, 95% CI −1.35 to −0.70), with moderate heterogeneity among trials (I2 = 48.4%) (Fig. 2). Subgroup analyses stratified by some baseline features such as trial region, sample size, gestational period, intervention frequency, duration and intensity, and Jadad score were also conducted. The results did not substantially alter compared with that of the primary analysis in most of the subgroups (Table 2).
Figure 2.
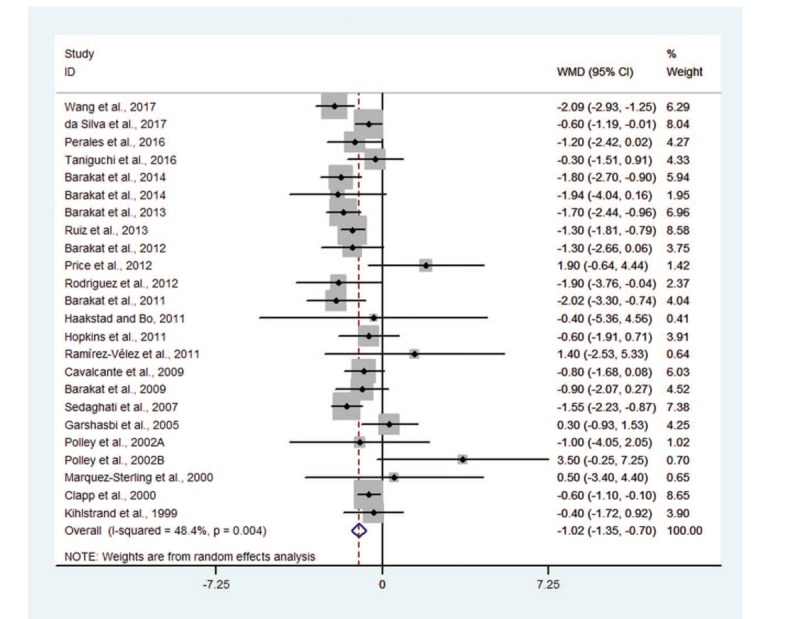
Forest plot for effect of exercise on maternal gestational weight gain.
Table 2.
Pooled WMD for the main effect estimates by subgroups of randomized controlled trials defined by characteristic of study participants and study design.
3.3. Sample size
Subgroup analysis by sample size showed that GWG was decreased moderately for trials with small sample size (<200) (WMD −0.87, 95% CI −1.29 to −0.46; I2 = 33.3%); similar finding was also obtained for trials with large sample size (≥200) (WMD −1.21, 95% CI −1.72 to −0.69; I2 = 64.2%). No statistically significant difference was found for inter-study heterogeneity (P = .109).
3.4. Trial region and ethnicity
Eleven, 4 and 4 trials were conducted in Europe, the USA, and Asia, respectively. Meta-analysis showed that GWG was decreased significantly for trials conducted in Europe (WMD −1.41, 95% CI −1.72 to −1.11; I2 = 0%), Asia (WMD −1.01, 95% CI −2.00 to −0.01; I2 = 77.0%), and South America (WMD −0.63, 95% CI −1.12 to −0.15; I2 = 0%). We did not find GWG significantly decreased for trials conducted in the USA (WMD 0.45, 95% CI −1.09 to 2.00; I2 = 51.4%). Significant difference for inter-study heterogeneity was indicated (P = .003). We also observed that GWG was decreased significantly across ethnic groups including white (WMD −1.19, 95% CI −1.49 to −0.89; I2 = 12.8%) and South American (WMD −0.63, 95% CI −1.12 to −0.15; I2 = 0) while not for East Asian (WMD −1.25, 95% CI −3.00 to 0.50; I2 = 82.4%) or Middle Eastern (WMD −0.70, 95% CI −2.51 to 1.11; I2 = 85.0%). There was statistically significant difference for inter-study heterogeneity (P = .014).
3.5. Gestational period
Three gestational periods were involved in conducting physical activity including the 1st to the 3rd gestational trimester, the 2nd to the 3rd gestational trimester and the 2nd trimester to delivery. Subgroup analyses showed that GWG was decreased significantly for women having physical activity from the 1st to the 3rd gestational trimester (WMD −1.42, 95% CI-1.85 to −0.98; I2 = 0%) and the 2nd to the 3rd gestational trimester (WMD −1.19, 95% CI −1.64 to −0.75; I2 = 49.6%). However, women who had physical activity from the 2nd trimester to delivery did not show decreased GWG (WMD 0.06, 95% CI −1.15 to 1.27; I2 = 51.4%). There was statistically significant difference for inter-study heterogeneity (P = .012).
3.6. Frequency of exercise
Women in 17 trials had physical exercise 3 time per week, showing significantly decreased GWG (WMD −1.22, 95% CI −1.55 to −0.90; I2 = 40.3%) compared with those having conventional medical care, while women having physical activity once to twice or 4 to 5 times per week did not show significant decreased GWG, with pooled WMD being −0.40 (95% CI −1.67 to 0.87) and 0.40 (95% CI −2.00 to 2.81), respectively (Fig. 4). Statistically significant difference for inter-study heterogeneity (P = .021) was noted.
Figure 4.
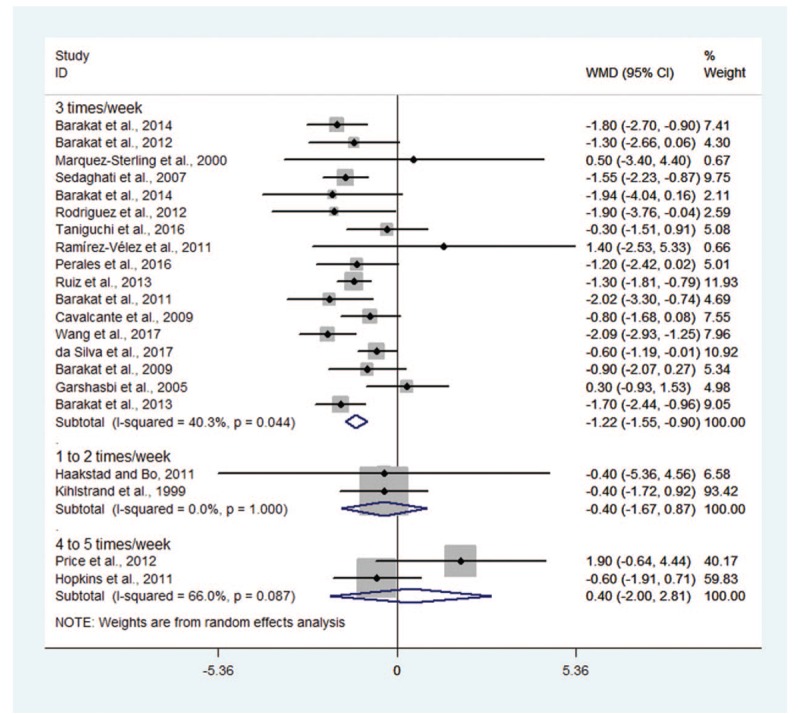
Forest plot for subgroup analyses by exercise frequency.
3.7. Duration of exercise
Meta-analysis stratified by duration of exercise indicated that GWG was decreased significantly in trials with women's duration of exercise ranging from 30 to 45 minutes (WMD −1.32, 95% CI −1.79 to −0.85; I2 = 1.5%) and 50 to 60 minutes (WMD −0.99, 95% CI −1.42 to −0.57; I2 = 45.7%); similar result was also noted for trial with women's duration of exercise ≤30 minutes (WMD −0.56, 95% CI −1.02 to −0.09; I2 = 1.5%) (Fig. 5). We found statistically significant difference for inter-study heterogeneity (P = .032).
Figure 5.
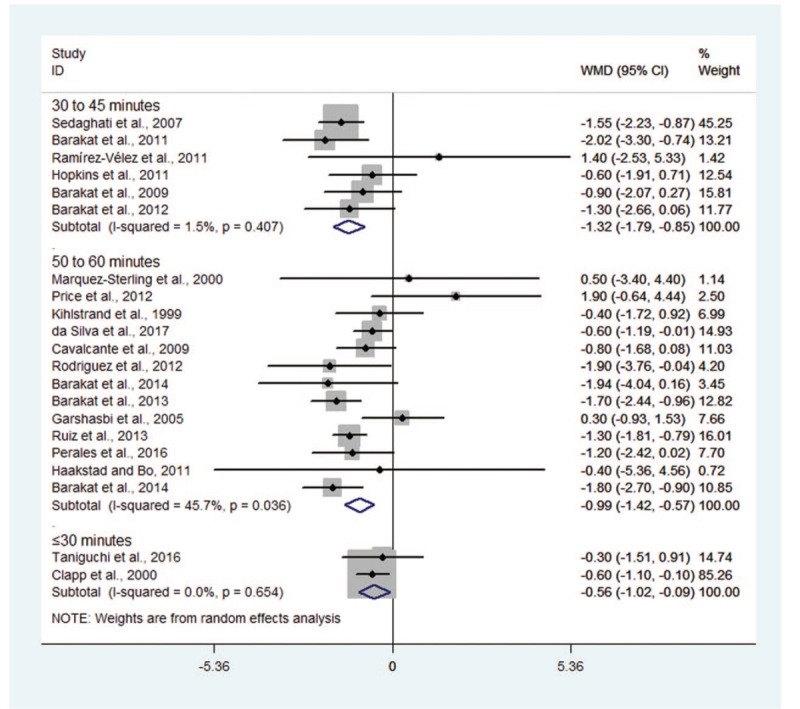
Forest plot for subgroup analyses by exercise duration.
3.8. Intensity of exercise
We conducted subgroup analysis stratified by intensity of exercise. The results showed that GWG was decreased significantly in trials with light (WMD −2.02, 95% CI −3.30 to −0.74), moderate to light (WMD −1.05, 95% CI −1.54 to −0.55; I2 = 22.0%) and moderate intensity of exercise (WMD −0.98, 95% CI −1.42 to −0.53; I2 = 57.1%). There was no statistically significant difference for inter-study heterogeneity (P = .357).
3.9. Methodological quality
Significant decreased GWG was observed in trials with high methodological quality (Jadad score 3 to 4) (WMD −1.17, 95% CI −1.53 to −0.82; I2 = 41.1%), while not in trial with low methodological quality (Jadad score 1–2) (WMD −0.64, 95% CI −1.34 to 0.07; I2 = 54.9%). No statistically significant difference for inter-study heterogeneity was indicated (P = .058).
3.10. Publication bias and sensitivity analyses
There seemed to be an asymmetry in the funnel plot (Fig. 3). However, the Begg (P = .189) and Egger test (P = .203) seemed to indicate no evident publication bias. Moreover, we also used the trim-and-fill method to further identify and adjust for funnel plot asymmetry. The adjusted random effects summary WMD of −1.13 (95% CI, −1.48 to −0.77) was consistent with the primary analysis, further confirming the robustness of the result. We also conducted sensitivity analysis by excluding one trial at each time and recalculating the summary estimate for the remaining trials to investigate the effect of each trial on the overall estimate. No significant alteration in the overall estimate when any one of the included trials was excluded. For subgroup analysis based on ethnicity, we noted that the summary WMD yielded −0.84, 95% CI −1.37 to −0.31 for other European countries except Spain, which was similar to that of the main analysis.
Figure 3.
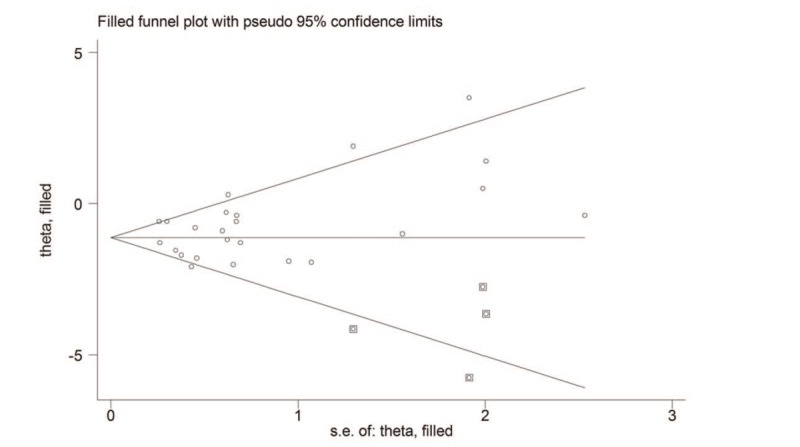
The trimmed funnel plot for effect of exercise on maternal gestational weight gain.
4. Discussion
Body weight is a significant concern for pregnant women across the spectrum of the gestational period.[38–41] Physical activity or regular exercise, one of the most generally recommended means to improve physical conditioning during pregnancy, has identified beneficial effects on both maternal and fetal health that are reported by numerous clinical trials.[42,43] Recent evidence has shown that pregnant women with regular exercise such as aerobic exercises, muscle strength, and flexibility are effective in reducing glycemic level in women with gestation diabetes mellitus (GDM) and other gestation-related conditions.[25,26,32,33] This systematic review sought to evaluate the effectiveness of regularly exercise in maternal GWG.
In this systematic review and meta-analysis, compared with conventional medical care, exercise or physical activity was associated with reduced maternal GWG. This effect seems consistent across different subgroups in terms of trial region, sample size, gestational period, intervention frequency, duration and intensity, and Jadad score. Though the real mechanisms have not been identified, it has been proposed that exercise not only attenuates the increase in insulin resistance in the population of overweight-obese pregnant women, but significantly lowers glucose concentrations in the fasted and postprandial state.[20,44] In the acute or chronic conditions of a body, exercise during pregnancy can suppress peripheral insulin resistance in the same way as the non-pregnant women, probably through exercise-induced increase in muscle insulin sensitivity.[54,55] Moreover, some hormones have also been proposed to play a pivotal role in nutrient partitioning during pregnancy, either directly through placental regulation of maternal metabolism or indirectly through regulation of maternal body composition changes, such as leptin and free fatty acids.[28]
Six previous systematic review and meta-analyses have reported the effect of exercises on maternal weight gain, among which 5 yielded beneficial effects, resulting in a significant reduction in GWG from 0.70 to 1.14 kg.[9,45,49–52] The other narrative review also concluded that exercise may help in the control of maternal weight gain.[45] Compared with these 6 studies, we included a larger number of studies with high-quality evidence (21 RCTs with a total of 4245 pregnant women) and conducted more thorough subgroup analyses. It provided more reliable estimates of effects on association between physical activity and maternal GWG, and subgroup analysis further investigated and raised the possibility of differential effects of different subgroup hypotheses, though these findings could have low credibility.[46]
Strengths of our study include a systematic and rigorous literature search to the identification of RCTs regarding this topic. We also conducted a number of preplanned subgroup analyses to explore for differences in effect estimates. Furthermore, we used the Jadad approach to assess the quality of evidence that indicated convincing evidence that physical activity could reduce GWG.
There are limitations to our study. First, studies might selectively report data regarding exercise on GWG in their full publications, which might have led to risk of selection bias; we tried to mitigate this risk by checking the registered records of the included studies on ClinicalTrials.gov for unreported data. Second, in order to assess the effects of different frequency, duration, and intensity of exercise on GWG, subgroup analyses based on patient-level data should preferably be performed. However, due to the nature of study-level data rather than patient-level data, we could not conduct more detailed analyses. For example, as the timing of exercise was unavailable in most of the included trials, we could not conduct further subgroup analysis based on this. Therefore, future trials are proposed on this aspect to further demonstrate the effect of the timing of exercise on GWG. Third, based on aggregate data, this meta-analysis shares the possible limitations of the original clinical trials. Differences in the enrolled populations (one trial enrolled exclusively overweight and obese pregnant women) and varied interventions of physical exercise might lead to statistical heterogeneity in the overall estimates. Finally, though sensitivity analyses indicated that exclusion of any one of the studies did not substantially change the pooled estimate, and the adjusted estimate yielded from the trim-and-fill model were in line with the initial findings, suggesting that the result of this meta-analysis was robust. However, we should interpret it cautiously considering the common existence of publication bias and incomplete statistical methods to test the publication bias.
In summary, the results of this meta-analysis suggest that exercise intervention can reduce maternal GWG for pregnant women. This effect is almost independent of trial region and other study characteristics. However, future trials should focus on the frequency, duration, intensity and type of physical exercise to further examine this effect.
Author contributions
Conceptualization: Danting Wen.
Data curation: Jianying Wang, Danting Wen.
Formal analysis: Jianying Wang, Danting Wen, Xiaofei Liu.
Investigation: Danting Wen, Xiaofei Liu.
Methodology: Danting Wen, Xiaofei Liu.
Project administration: Danting Wen, Yingjie Liu.
Software: Danting Wen, Yingjie Liu.
Supervision: Danting Wen.
Validation: Danting Wen, Xiaofei Liu, Yingjie Liu.
Visualization: Danting Wen, Xiaofei Liu.
Writing – original draft: Danting Wen.
Writing – review & editing: Danting Wen.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, GWG = gestational weight gain, MeSH = Medical Subject Heading, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RCT = randomized controlled trials, WMD = weighted mean difference.
JW, DW, and XL have contributed equally to this work.
This study is supported by Shaanxi Provincial Health and Family Planning Research Fund Project (grant no: 2016D055).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Chu S, Callaghan W, Bish C, et al. Gestational weight gain by body mass index among US women delivering live births, 2004–2005: fueling future obesity. Am J Obstet Gynecol 2009;200:271e1-7. [DOI] [PubMed] [Google Scholar]
- [2].Robinson H, O’Connell C, Joseph K, et al. Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol 2005;106:1357–64. [DOI] [PubMed] [Google Scholar]
- [3].Nohr E, Bech B, Davies M, et al. Prepregnancy obesity and fetal death: a study within the Danish National Birth Cohort. Obstet Gynecol 2005;106:250–9. [DOI] [PubMed] [Google Scholar]
- [4].Bodnar L, Ness R, Markovic N, et al. The risk of preeclampsia rises with increasing prepregnancy body mass index. Ann Epidemiol 2005;15:475–82. [DOI] [PubMed] [Google Scholar]
- [5].Baeten J, Bukusi E, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health 2001;91:436–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nehring I, Lehmann S, von Kries R. Gestational weight gain in accordance to the IOM/NRC criteria and the risk for childhood overweight: a meta-analysis. Pediatr Obes 2013;8:218–24. [DOI] [PubMed] [Google Scholar]
- [7].Ensenauer R, Chmitorz A, Riedel C, et al. Effects of suboptimal or excessive gestational weight gain on childhood overweight and abdominal adiposity: results from a retrospective cohort study. Int J Obes 2013;37:505–12. [DOI] [PubMed] [Google Scholar]
- [8].Streuling I, Beyerlein A, Rosenfeld E, et al. Physical activity and gestational weight gain: a meta-analysis of intervention trials. BJOG 2011;118:278–84. [DOI] [PubMed] [Google Scholar]
- [9].da Silva S, Ricardo L, Evenson K, et al. Leisure-time physical activity in pregnancy and maternal-child health: a systematic review and meta-analysis of randomized controlled trials and cohort studies. Sports Med (Auckland, NZ) 2017;47:295–317. [DOI] [PubMed] [Google Scholar]
- [10].Barakat R, Cordero Y, Coteron J, et al. Exercise during pregnancy improves maternal glucose screen at 24–28 weeks: a randomised controlled trial. Br J Sports Med 2012;46:656–61. [DOI] [PubMed] [Google Scholar]
- [11].Ramírez-Vélez R, Aguilar de Plata AC, Mosquera-Escudero M, BS, et al. The effect of aerobic exercise on oxygen consumption in healthy first-pregnancy females: a randomized clinical trial. Rev Colomb Obstet Ginecol 2011;62:15–23. [Google Scholar]
- [12].Price B, Amini S, Kappeler K. Exercise in pregnancy: effect on fitness and obstetric outcomes-a randomized trial. Med Sci Sports Exerc 2012;44:2263–9. [DOI] [PubMed] [Google Scholar]
- [13].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. W64. [DOI] [PubMed] [Google Scholar]
- [14].Jadad A, Moore R, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [15].Higgins J, Thompson S, Deeks J, et al. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Higgins J, Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [17].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Begg C, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [19].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [20].Wang C, Wei Y, Zhang X, et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am J Obstet Gynecol 2017;216:340–51. [DOI] [PubMed] [Google Scholar]
- [21].da Silva S, Hallal P, Domingues M, et al. A randomized controlled trial of exercise during pregnancy on maternal and neonatal outcomes: results from the PAMELA study. Int J Behav Nutr Phys Act 2017;14:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Perales M, Calabria I, Lopez C, et al. Regular exercise throughout pregnancy is associated with a shorter first stage of labor. Am J Health Promot 2016;30:149–54. [DOI] [PubMed] [Google Scholar]
- [23].Barakat R, Perales M, Bacchi M, et al. A program of exercise throughout pregnancy. Is it safe to mother and newborn? Am J Health Promot 2014;29:2–8. [DOI] [PubMed] [Google Scholar]
- [24].Barakat R, Pelaez M, Montejo R, et al. Exercise throughout pregnancy does not cause preterm delivery: a randomized, controlled trial. J Phys Act Health 2014;11:1012–7. [DOI] [PubMed] [Google Scholar]
- [25].Ruiz J, Perales M, Pelaez M, et al. Supervised exercise-based intervention to prevent excessive gestational weight gain: a randomized controlled trial. Mayo Clin Proc 2013;88:1388–97. [DOI] [PubMed] [Google Scholar]
- [26].Barakat R, Pelaez M, Lopez C, et al. Exercise during pregnancy and gestational diabetes-related adverse effects: a randomised controlled trial. Br J Sports Med 2013;47:630–6. [DOI] [PubMed] [Google Scholar]
- [27].Rodríguez YC, Puente MP, Abad MDM, RBC, et al. Can moderate physical exercise during pregnancy act as a factor in preventing Gestational Diabetes? Rev Int Cienc Deporte 2012;8:3–19. [Google Scholar]
- [28].Hopkins S, Baldi J, Cutfield W, et al. Effects of exercise training on maternal hormonal changes in pregnancy. Clin Endocrinol (Oxf) 2011;74:495–500. [DOI] [PubMed] [Google Scholar]
- [29].Haakstad L, Bø K. Exercise in pregnant women and birth weight: a randomized controlled trial. BMC Pregnancy Childbirth 2011;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Barakat R, Pelaez M, Montejo R, et al. Exercise during pregnancy improves maternal health perception: a randomized controlled trial. Am J Obstet Gynecol 2011;204:402e1-7. [DOI] [PubMed] [Google Scholar]
- [31].Cavalcante S, Cecatti J, Pereira R, et al. Water aerobics II: maternal body composition and perinatal outcomes after a program for low risk pregnant women. Reprod Health 2009;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Barakat R, Lucia A, Ruiz J. Resistance exercise training during pregnancy and newborn's birth size: a randomised controlled trial. Int J Obes V 33 20052009;1048–57. [DOI] [PubMed] [Google Scholar]
- [33].Sedaghati P, Ziaee V, Ardjmand AA. The effect of an ergometric training program on pregnants’ weight gain and low back pain. Gazz Med Ital 2007;166:209–13. [Google Scholar]
- [34].Garshasbi A, Faghih Zadeh S. The effect of exercise on the intensity of low back pain in pregnant women. Int J Gynaecol Obstet 2005;88:271–5. [DOI] [PubMed] [Google Scholar]
- [35].Marquez-Sterling S, Perry A, Kaplan T, et al. Physical and psychological changes with vigorous exercise in sedentary primigravidae. Med Sci Sports Exerc 2000;32:58–62. [DOI] [PubMed] [Google Scholar]
- [36].Clapp J, Kim H, Burciu B, et al. Beginning regular exercise in early pregnancy: effect on fetoplacental growth. Am J Obstet Gynecol 2000;183:1484–8. [DOI] [PubMed] [Google Scholar]
- [37].Kihlstrand M, Stenman B, Nilsson S, et al. Water-gymnastics reduced the intensity of back/low back pain in pregnant women. Acta Obstet Gynecol Scand 1999;78:180–5. [PubMed] [Google Scholar]
- [38].Yan J. Maternal pre-pregnancy BMI, gestational weight gain, and infant birth weight: a within-family analysis in the United States. Econ Hum Biol 2015;18:1–2. [DOI] [PubMed] [Google Scholar]
- [39].Lee K, Raja E, Lee A, et al. Maternal obesity during pregnancy associates with premature mortality and major cardiovascular events in later life. Hypertension 2015;66:938–44. [DOI] [PubMed] [Google Scholar]
- [40].Haugen M, Brantsæter A, Winkvist A, et al. Associations of pre-pregnancy body mass index and gestational weight gain with pregnancy outcome and postpartum weight retention: a prospective observational cohort study. BMC Pregnancy Childbirth 2014;14:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bhavadharini B, Anjana R, Deepa M, et al. Gestational weight gain and pregnancy outcomes in relation to body mass index in Asian Indian women. Indian J Endocrinol Metab 2017;21:588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rogozińska E, Marlin N, Jackson L, et al. Effects of antenatal diet and physical activity on maternal and fetal outcomes: individual patient data meta-analysis and health economic evaluation. Health Technol Assess 2017;21:1–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pastorino S, Bishop T, Crozier S, et al. Associations between maternal physical activity in early and late pregnancy and offspring birth size: remote federated individual level meta-analysis from eight cohort studies. BJOG 2018;126:459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Davenport M, Mottola M, McManus R, et al. A walking intervention improves capillary glucose control in women with gestational diabetes mellitus: a pilot study. Appl Physiol Nutr Metab 2008;33:511–7. [DOI] [PubMed] [Google Scholar]
- [45].Barakat R, Perales M, Garatachea N, et al. Exercise during pregnancy. A narrative review asking: what do we know? Br J Sports Med 2015;49:1377–81. [DOI] [PubMed] [Google Scholar]
- [46].Sun X, Briel M, Walter S, et al. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ (Clinical research ed) 2010;340:c117. [DOI] [PubMed] [Google Scholar]
- [47].Reid H, Smith R, Calderwood C, et al. Physical activity and pregnancy: time for guidance in the UK. Br J Sports Med 2017;51:1511–2. [DOI] [PubMed] [Google Scholar]
- [48].Evenson KR, Barakat R, Brown WJ, et al. Guidelines for physical activity during pregnancy: comparisons from around the world. Am J Lifestyle Med 2014;8:102–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].International Weight Management in Pregnancy (i-WIP) Collaborative Group. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials. BMJ 2017;358:j3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ruchat SM, Mottola MF, Skow RJ, et al. Effectiveness of exercise interventions in the prevention of excessive gestational weight gain and postpartum weight retention: a systematic review and meta-analysis. Br J Sports Med 2018;52:1347–56. [DOI] [PubMed] [Google Scholar]
- [51].Sanabria-Martínez G, García-Hermoso A, Poyatos-León R, et al. Effectiveness of physical activity interventions on preventing gestational diabetes mellitus and excessive maternal weight gain: a meta-analysis. BJOG 2015;122:1167–74. [DOI] [PubMed] [Google Scholar]
- [52].Du MC, Ouyang YQ, Nie XF, et al. Effects of physical exercise during pregnancy on maternal and infant outcomes in overweight and obese pregnant women: a meta-analysis. Birth 2019;46:211–21. [DOI] [PubMed] [Google Scholar]
- [53].Madsen M, Jørgensen T, Jensen ML, et al. Leisure time physical exercise during pregnancy and the risk of miscarriage: a study within the Danish National Birth Cohort. BJOG 2007;114:1419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Holloszy John O. Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol 2005;99:338–43. [DOI] [PubMed] [Google Scholar]
- [55].Clapp James F. Effects of diet and exercise on insulin resistance during pregnancy. Metab Syndr Relat Disord 2006;4:84–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


