Abstract
Background and Objectives:
Microbial communities residing in the gut play a major role in the communication between the gut and the brain through neural, immune, and hormonal routes. Changes in abundance of beneficial intestinal bacteria can affect health of individuals. Conversely, drugs, disease, diet and other factors can alter the gut microbiome. However, there is limited information on the effect of exogenous factors on gut microbiota. In this study, we investigated whether a beneficial bacterium, the probiotic Lactobacillus plantarum IS-10506, can stimulate the gut–brain axis using Wistar rats.
Materials and Methods:
The animals were divided into two groups: one received L. plantarum IS strain 10506 supplementation, while the control group received no treatment. Activation of the gut–brain axis was evaluated by immunohistochemical analysis of intestinal and brain serotonin (5-HT) and brain neurotrophin (NT), serotonin transporter (5-HTT), and brain-derived neurotrophic factor (BDNF) levels.
Results:
The results showed that BDNF (p< 0.000), NT (p< 0.000007), and 5-HTT (p< 0.000007) expression was upregulated in the brain along with intestinal 5-HT (p< 0.000) level in rats treated with L. plantarum strain IS-10506 relative to the control group.
Conclusion:
The probiotic L. plantarum IS-10506 stimulates the gut–brain axis and can potentially promote brain development and function.
Keywords: Gut–brain axis, Brain-derived neurotrophic factor, Serotonin, Probiotic Lactobacillus plantarum IS 10506
INTRODUCTION
The digestive tract and brain are intimately connected (1). Microbial communities residing in the gut play a major role in the communication between the gut and the brain (1–3). In humans, the 200–600 million neurons of the enteric nervous system respond to chemical and mechanical stimuli from the gastrointestinal tract and combine this information with that transmitted by the brain via the vagus nerve (i.e., cranial nerve X) (4, 5). Signals between these two organs are bidirectional but most (90%) are retrograde and keep the brain informed of gut activity. The communication between the brain's emotional and cognitive centres and peripheral intestinal function is known as the gut–brain axis (1, 2, 6, 7).
Data from mice and humans have shown that gut microbiota influence the gut–brain axis; disruption of gut microbiota communities alters the expression of the neuromodulator brain-derived neurotrophic factor (BDNF), the growth factor neurotrophin (NT), and the serotonin (5-HT) transporter (5-HTT) in the hippocampus and amygdala (8–10). Additionally, clinical studies have reported that changes in gut microbiota lead to observable changes in mood or behaviour; conversely, ingestion of probiotics can positively affect brain function in healthy individuals (6, 11–13).
In the present study, we investigated whether the probiotic bacterium Lactobaccillus plantarum strain IS-10506, which is prevalent in Indonesia and is a typical resident of the intestine, can influence the gut–brain axis.
MATERIALS AND METHODS
Animals.
Male Wistar rats (8–12 weeks old; 100–120 g) were divided into two groups (n = 10 each): animals in the first served as untreated controls, and the second group received probiotic supplementation (L. plantarum IS-10506) for 7 days at 2.67 × 109 CFU/day in 0.25 ml saline. At the end of the experiment, rats were sacrificed by cervical dislocation and the brain and small intestine (ileum) were immediately dissected. The intestinal contents were removed, and the tissues were washed with phosphate-buffered saline (PBS) and prepared for immunohistochemistry. This study was approved by the Animal Care and Use Committee of Ethics Committee of the Veterinary Faculty of Universitas Airlangga, Surabaya, Indonesia.
Probiotic supplementation.
Microencapsulated L. plantarum strain IS-10506 (GenBank accession no. DQ860148) was packed in an aluminium foil sachet at the Pharmacy Installation of Dr. Soetomo Hospital (Surabaya, Indonesia) and dissolved in 1.5 ml sterile water, and administered to rats via a gastric tube once daily for 7 days at a dose of 2.67 × 109 CFU/day. Probiotic viability was assessed 1 week prior to the treatment.
Immunohistochemistry.
Ileum and brain tissues were fixed in 10% formalin solution, followed by dehydration and paraffin embedding. Serial sections of the tissues were done by cleaned and fixed in 1n 10% formallin buffer solution. Then, this procedure followed by dehydration, clearing, and embedding. Tissue sections were probed with antibodies against 5-HT (YC5/45 sc-58031, Santacruz Biotech, USA), BDNF ((n20) sc-546, Santacruz Biotech, USA), 5-HTT (SR2A antibody (A-4) sc-166775, Santacruz Biotech, USA) and NT (sc-365444, Santacruz Biotech, USA). The sections were observed under a light microscope (CX21; Olympus, Tokyo, Japan) and photographed with an ILCE6000 camera (Sony, Tokyo, Japan). The number of immunopositive cells in 20 random fields at 1000× magnification was counted.
Statistical analysis.
Differences between groups were evaluated with a paired sample t test and one-way analysis of variance for normally distributed data. Significance was set at p< 0.05.
RESULTS
Rats that received L. plantarum IS-10506 supplementation showed an increased number of 5-HT-positive cells in the ileum relative to the control group ( p< 0.0001; Figs. 1–3). Similar trends were observed in the brain levels of BDNF ( p< 0.0001; Figs. 4–6), 5-HTT ( p< 0.000007; Figs. 7–9), and NT ( p< 0.000007; Figs. 10–12): in each case, the number of immunopositive cells was higher in the treatment group than in control rats.
Fig. 1.
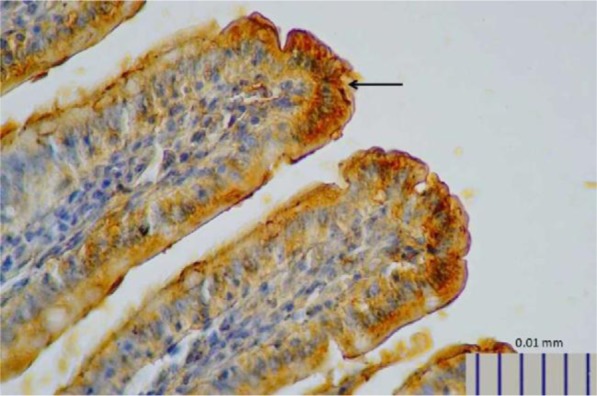
Representative image of intestinal 5-HT expression in control rats, as detected by immunohistochemistry (400× magnification).
Fig. 3.
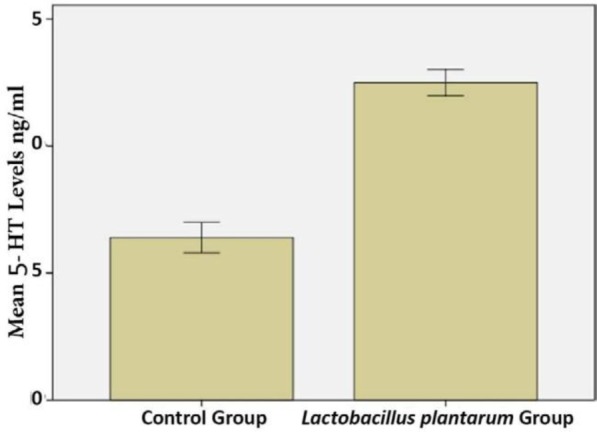
Quantitative analysis of intestinal 5-HT levels in L. plantarum IS-10506 treated and control rats. Data represent mean ± SE (n = 10/group).
Fig. 4.
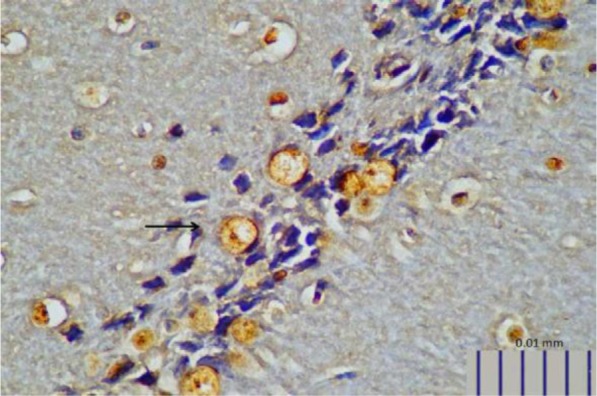
Representative image of brain BDNF expression in control rats, as detected by immunohistochemistry (400× magnification).
Fig. 6.
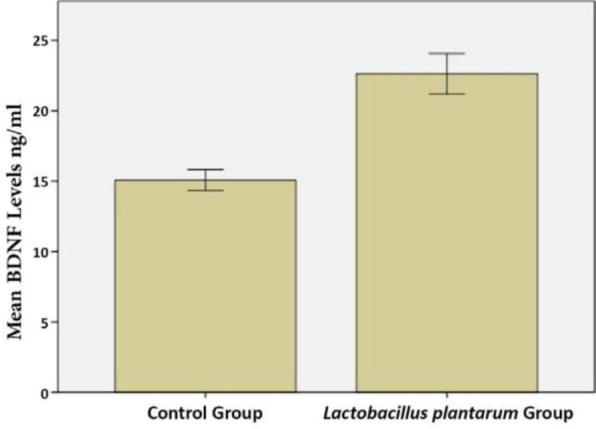
Quantitative analysis of intestinal BDNF levels in L. plantarum IS-10506 treated and control rats. Data represent mean ± SE (n = 10/group).
Fig. 7.
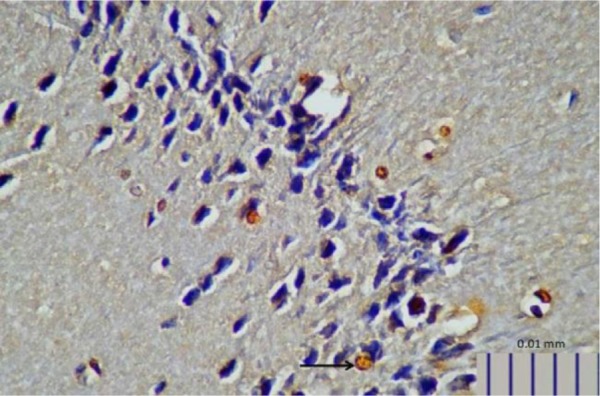
Representative image of brain 5-HTT expression in control rats, as detected by immunohistochemistry (400× magnification).
Fig. 9.
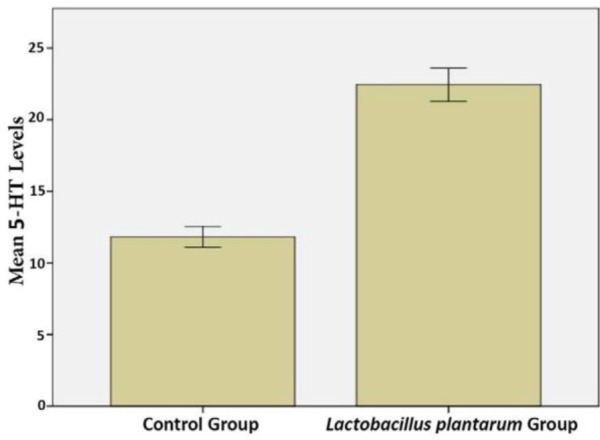
Quantitative analysis of brain 5-HTT levels in L. plantarum IS-10506 treated and control rats. Data represent mean ± SE (n = 10/group).
Fig. 10.
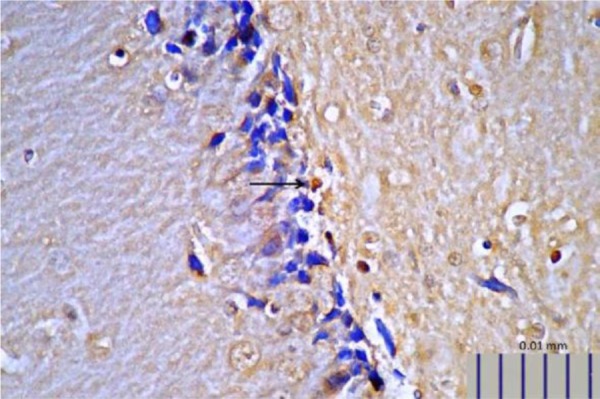
Representative image of brain NT expression in control rats, as detected by immunohistochemistry (400× magnification).
Fig. 12.
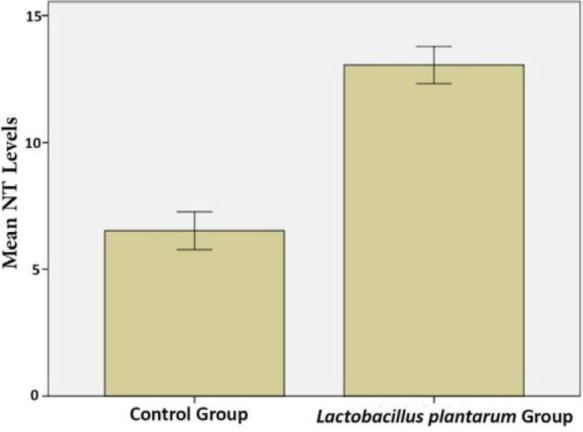
Quantitative analysis of brain NT levels in L. plantarum IS-10506 treated and control rats. Data represent mean ± SE (n = 10/group).
Fig. 2.
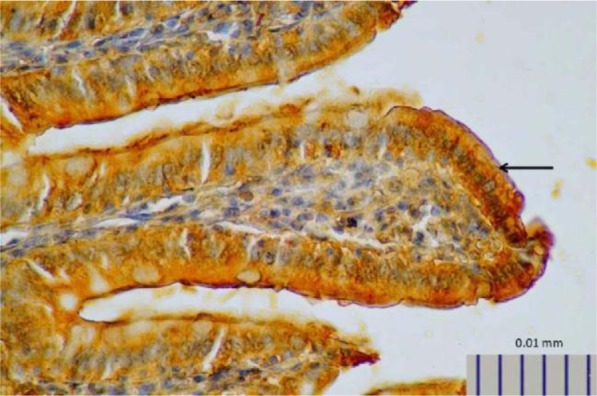
Representative image of intestinal 5-HT in rats treated with L. plantarum IS-19596 (400× magnification).
Fig. 5.
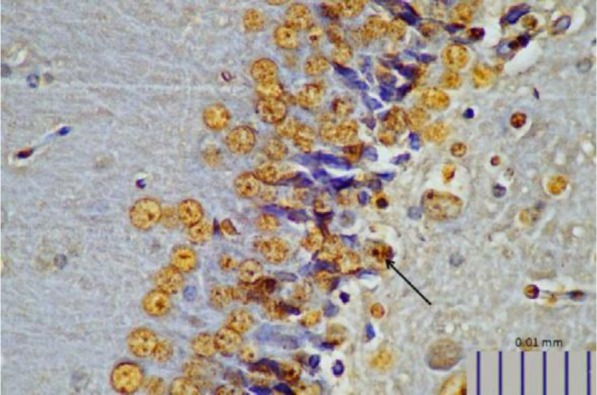
Representative image of brain BDNF expression in rats treated with L. plantarum IS-10506 (400× magnification).
Fig. 8.
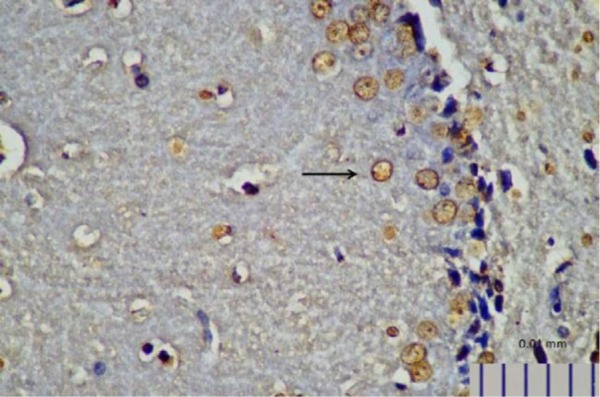
Representative image of brain 5-HTT expression in rats treated with L. plantarum IS-10506 (400× magnification).
Fig. 11.
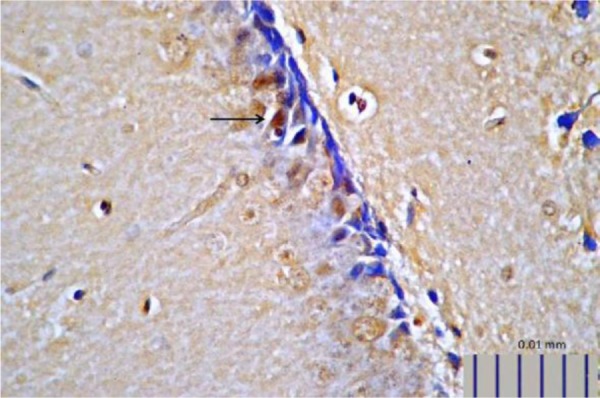
Representative image of brain NT expression in rats treated with L. plantarum IS-10506 (400× magnification).
DISCUSSION
A growing body of evidence indicates that gut–brain communication is influenced by the gut microbiome (5, 12, 14). The results of the present study demonstrate that a probiotic bacterium, which is a typical gut microbe, influences neurotransmitter and neurotrophin expression in the brain and intestine.
5-HT is released from enterochromaffin cells of the gastric mucosa in response to various stimuli including signals from gut microbiota (15–17). Although 5-HT is a well-known neurotransmitter that is associated with emotion and behaviour, about 90% of 5-HT in the body is produced in the digestive tract where it is involved in the detection of resource availability and regulation of bowel movement. In platelets, 5-HT contributes to haemostasis and in the central nervous system (CNS), it regulates mood, cognition, appetite, and sleep. 5-HT is also associated with bone metabolism and organ development (8, 9, 17). Gut microbiota stimulates host intestinal cells to produce 5-HT. An imbalance of 5-HT in the brain can lead to depression, whereas peripheral dysregulation of this neurotransmitter has been linked to a variety of diseases (1, 2, 7, 12, 15, 16, 18–21). In this study we found that treatment with L. plantarum IS-10506 caused an increase in brain 5-HT level in rats relative to the control group.
BDNF is the most abundant and widely distributed growth factor in the CNS and is important for neuronal survival, migration, and differentiation; axonal and dendritic growth; synapse formation; regulation of synaptic plasticity and behaviour (1, 10, 14, 16, 22); and hippocampal neurogenesis (23). We found here that brain BDNF expression in the hippocampus was enhanced by L. plantarum IS-10506 treatment relative to untreated rats, implying that probiotic supplementation can enhance brain development, especially memory and brain plasticity.
5-HTT may contribute to the maintenance of BDNF equilibrium and brain function. Gut microbes are thought to regulate 5-HTT expression (24). Previous studies have shown that the supernatant of Lactobacillus rhamnosus GG (LGG) cultures increases 5-HTT expression in mouse intestine (25), whereas Lactobacillus acidophilus and Bifidobacterium longum culture supernatants have the same effect in intestinal epithelial cells (2). In this study, the number of hippocampal neurons expressing 5-HTT was higher in rats that received L. plantarum IS-10506 supplementation than in those without treatment, suggesting that probiotics enhance serotonergic function in the brain.
NTs constitute a family of proteins that promote the survival, development, and function of neurons, in part by inducing BDNF release from hippocampal neurons (26). We observed that L. plantarum IS-10506 treatment increased the level of NT in the brain, which corresponded to the elevation in BNDF level. Thus, L. plantarum IS-10506 supplementation may enhance neuronal survival, differentiation, and growth.
CONCLUSION
The results of this study indicate that L. plantarum IS-10506 treatment can increase intestinal 5-HT and brain 5-HTT, BDNF, and NT expression in adult rats. These findings suggest that probiotics can promote brain development and function and offer a model for investigating the effects of exogenous factors on the gut–brain axis.
ACKNOWLEDGEMENTS
All authors declare that they have no conflict of interest.
REFERENCES
- 1.Carabotti M, Scirocco A, Antonietta M, Severi C. The gut-brain axis : interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 2015; 28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 2.Cao H, Liu X, An Y, Zhou G, Liu Y, Xu M. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci Rep 2017;7:10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter : Abnormal intestinal motility and the expression of cation transporters. J Neurosci 2001;21:6348–6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress 2017;7:124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farzi A, Fröhlich EE, Holzer P. Gut microbiota and the neuroendocrine system. Neurotherapeutics 2018;15:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am 2017;46:77–89. [DOI] [PubMed] [Google Scholar]
- 7.Cerdó T, Ruíz A, Suárez A, Campoy C. Probiotic, pebiotic, and brain development. Nutrients 2017;9(11):E1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Popova NK, Naumenko VS. 5-HT 1A receptor as a key player in the brain 5-HT system. Rev Neurosci 2013;24:191–204. [DOI] [PubMed] [Google Scholar]
- 9.Colucci R, Gambaccini D, Ghisu N, Rossi G, Costa F, Tuccori M, et al. Influence of the serotonin transporter 5HTTLPR polymorphism on symptom severity in irritable bowel syndrome. PLoS One 2013;8(2):e54831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morse JK, Wiegand SJ, Anderson K, You Y, Cai N, Carnahan J, et al. Brain-derived neurotrophic factor (BDNF) prevents the degeneration of medial septal cholinergic neurons following fimbria transection. J Neurosci 1993;13:4146–4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montiel-Castro AJ, González-Cervantes RM, Bravo-Ruiseco G, Pacheco-López G. The microbiota-gut-brain axis: neurobehavioral correlates, health and sociality. Front Integr Neurosci 2013;7:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Brain, behavior, and immunity altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 2015;48:186–194. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, Burnet PWJ. Psychobiotics and the manipulation of bacteria–gut–brain signals. Trends Neurosci 2016;39:763–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hata T, Asano Y, Yoshihara K, Kimura-Todani T, Miyata N, Zhang XT, et al. Regulation of gut luminal serotonin by commensal microbiota in mice. PLoS One 2017;12(7):e0180745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malinova TS, Dijkstra CD, de Vries HE. Serotonin: A mediator of the gut-brain axis in multiple sclerosis. Mult Scler 2018;24:1144–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology 2008;33:73–83. [DOI] [PubMed] [Google Scholar]
- 17.Pittalà V, Salerno L, Modica M, Siracusa MA, Romeo G. 5-HT 7 receptor ligands: recent developments and potential therapeutic applications. Mini Rev Med Chem 2007;7:945–960. [DOI] [PubMed] [Google Scholar]
- 18.Articles I, Academy N. Neurotrophin release by neurotrophins: Implications for activity-dependent neuronal plasticity. Proc Natl Acad Sci U S A 1997;94:13279–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015;18:965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evrensel A, Ceylan ME. Gut-microbiota-brain axis: the missing link in depression. Clin Psychopharmacol Neurosci 2015;13:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George R, Kumar J, Gouda S, Park Y, Shin H, Das G. Science direct benefaction of probiotics for human health: A review. J Food Drug Anal 2018;26:927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer EA. Gut feelings: the emerging biology of gut–brain communication. Nat Rev Neurosci 2011;12:453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci 2006;24: 1850–1856. [DOI] [PubMed] [Google Scholar]
- 24.Aidy Sahar El, Ramsteijn Anouschka S., Dini-Andreote Francisco, van Eijk Roel, Houwing Danielle J., Salles Joana F., et al. Serotonin transporter genotype modulates the gut microbiota composition in young rats, an effect augmented by early life stress. Front Cell Neurosci 2017; 11: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang YM, Ge XZ, Wang WQ, Wang T, Cao HL, Wang BL, et al. Lactobacillus rhamnosus GG supernatant up-regulates serotonin trasporter expression in intestinal epithelial cells and mice intestinal tissues. Neurogastroenterol Motil 2015; 27: 1239–1248. [DOI] [PubMed] [Google Scholar]
- 26.Canossa M, Giordano E, Cappello S, Guarnieri C, Frri S. Nitric oxide down-regulates brain-derived neurotrophoc factor secretion in culture hippocampal neurons. Proc Natl Acad Sci U S A 2002;99:3282–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]


