Abstract
Background: Transcranial Doppler Ultrasonography (TCD) can be utilised to measure the tight coupling of cerebral blood flow velocity (CBFv) in response to cognitive demand by task activation, termed neurovascular coupling.
Aims: To investigate the differences in neurovascular coupling between healthy older (>50 years) and younger (18-49 years) adults in response to cognitive testing.
Methods: Fifty-four older (n=25) and younger (n=29) adults underwent continuous bilateral TCD, beat-to-beat blood pressure (MAP; Finapres), heart rate (HR; electrocardiogram), and end-tidal CO2 (ETCO2; capnography) monitoring. After a 5-min baseline period, memory (M1-4: recalling three learned words, learning a name and address, recalling US presidents and UK prime ministers, and recalling the previously learned name and address) and visuospatial (V1-4: drawing a cube and infinity diagram, drawing a clock face, counting dots, and recognising obscured letters) tasks from the Addenbrooke's Cognitive Examination (ACE-III) were performed. Data are mean (standard deviation).
Results: In the memory paradigms, the peak percentage change in CBFv differed significantly between younger and older groups only in the dominant hemisphere during the M1 task, (2.17 (9.16)% vs. 8.38 (9.27)%, respectively, p=0.017). In the visuospatial paradigm, there were also significant differences in peak percentage change in CBFv between younger and older groups in the V1 (5.87 (8.32)% vs. 11.89 (6.60)%, p=0.005) and V2 tasks (6.30 (8.72)% vs. 11.30 (7.77)%, p=0.032).
Conclusion: Healthy older adults demonstrate augmented cerebrovascular physiology in response to cognitive challenge compared to younger adults. The impact of abnormal ageing on cerebrovascular physiology, for example, related to cognitively impaired states, requires further investigation.
Keywords: Addenbrooke’s cognitive examination, transcranial doppler ultrasonography, neurovascular coupling, healthy ageing, cerebral blood flow, functional imaging
1. INTRODUCTION
As the population ages, the proportion of older adults within society is expected to nearly double from 12 to 22% between 2015 and 2050 [1]. Therefore, it is imperative to understand the physiology underpinning healthy ageing, and to explore differences associated with unhealthy ageing, for example in cognitive decline. Indeed, the world prevalence of dementia is expected to rise to 130 million by 2050 [2]. Interventions to delay or prevent the onset of cognitive decline in healthy older adults are therefore of increasing importance [2, 3]. The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) trial recently demonstrated sustained benefits to physical function in healthy older adults 10 years after undertaking a cognitive training program [4]. Delaying the onset of functional decline by just six years has the potential to reduce the number of people affected by a cognitive decline at 2050 by 38%, with significant benefits to individuals, society, and the economy [4].
Using physiological markers to identify individuals who might selectively benefit from such interventions would facilitate a person-centred approach and allow for efficient resource utilisation. Cerebral Blood Flow (CBF) has been implicated as a crucial pathological mechanism in early cognitive decline [5, 6], and therefore presents a potential early biomarker [7, 8]. The Rotterdam study demonstrated an important temporal relationship where hypoperfusion precedes cognitive decline. Moreover, hypoperfusion in healthy older adults was associated with an increased risk of dementia and Alzheimer’s disease [9]. Understanding the effects of ageing on cerebrovascular physiology, therefore, provides an opportunity to identify novel therapeutic targets at a stage preceding cognitive impairment.
CBF velocity (CBFv) can be measured non-invasively by Transcranial Doppler ultrasonography (TCD) in the intra- and extra-cranial arteries [10, 11]. TCD is advantageous in the measurement of important physiological processes in older adults, due to its acceptability, and availability to those with metal implants, claustrophobia, and pacemakers [10, 11]. Furthermore, TCD can be utilised to measure the tight coupling of CBF in response to cognitive demand by task activation, termed Neurovascular Coupling (NVC) [12-14].
To date, a number of studies have demonstrated important differences in the activation and perfusion patterns between healthy younger and older adults [15, 16]; healthy older adults consistently demonstrating increased responses to cognitive stimulation [17]. In addition, cognitive tasks, which usually lateralise to one hemisphere, often result in loss of hemispheric specialisation, demonstrating bilateral rises in CBFv or activation in older adults [15, 16]. The Hemispheric Asymmetry Reduction in Older Adults (HAROLD) model has previously been used to describe this phenomenon but has been superseded by the Compensation-Related Utilisation of Neural Circuits Hypothesis (CRUNCH) model [16]. The CRUNCH model postulates this loss of lateralisation signifies compensatory recruitment of additional neuronal circuits, thus older adults require greater neuronal activity at the same level of cognitive demand as healthy younger adults [16]. In a review by Van Beek et al., dynamic Cerebral Autoregulation (dCA) was found to be intact across studies of older adults (aged 50-75 years), however, few studies have investigated the differences in neurovascular coupling between healthy older and younger adults, and only then to a limited number of cognitive paradigms [10, 15, 17].
Therefore, in this study, we aimed to investigate differences in CBFv responses, as well as hemispheric lateralisation, to eight tasks from the Addenbrooke’s Cognitive Examination-III, a cognitive assessment tool used in routine clinical practice, with excellent sensitivity and specificity for the diagnosis of dementia [18, 19]. The memory and visuospatial domains were specifically selected for study, given their early involvement in mild cognitive impairment [20, 21], thus they may exhibit differences even in cognitively intact older adults.
2. METHODS
Data were extracted on healthy controls performing cognitive task activation studies from the Cerebral Haemodynamics in Ageing and Stroke Medicine (CHiASM) database at the University of Leicester. Data from these healthy control participants have been published previously in separate analyses [12, 13]. Written informed consent was obtained prior to the commencement of study procedures in accordance with local ethics committee protocols. Ethical approval was provided by the University of Leicester (5355) and Wales 7 Research Ethics Committee (REC 17/WA/0089). All study procedures were conducted in accordance with the Declaration of Helsinki (2008). Inclusion criteria stated adults of greater than 18 years of age, able to provide consent and without medical co-morbidity. Exclusion criteria included poor insonation of both temporal bone windows, any history of cardiovascular, neurological or respiratory disease, and pregnancy or currently lactating during the study period. Volunteers were requested to avoid caffeine, nicotine and alcohol >4 hours prior to attending for the study measurement. Study measurements were taken in the CHiASM research laboratory in controlled temperature conditions (24°C) and free from additional visual or auditory stimulation. Beat-to-beat Blood Pressure (BP) was continuously recorded using the Finometer (FMS, Finapres Measurement Systems, Arnhem, Netherlands). A 3-lead Electrocardiogram (ECG) was used to record the heart rate interval, and end-tidal CO2 (EtCO2) was measured via nasal prongs (Salter Labs) by a capnograph (Capnocheck Plus). Bilateral insonation of the Middle Cerebral Arteries (MCAs) was performed using TCD (Viasys Companion III; Viasys Healthcare and DWL Doppler Box 10.5.1 Software) with 2MHz probes, secured in place using a bespoke head frame. The MCAs were identified according to two main characteristics: signal depth and velocities.
At the outset of the protocol, a five-minute period of rest in silence was observed to facilitate baseline recordings of peripheral and central haemodynamic parameters, with subjects in the seated position. The memory and visuospatial tasks were then performed alongside haemodynamic assessments. The study measurements were conducted over a 90-minute period with 30 to 60 seconds of rest before and after each cognitive domain question. During resting periods, systolic and diastolic brachial BP readings (OMRON Model 705IT) were performed to permit calibration of the Finometer recordings. The Edinburgh Handedness Inventory [22] was used to assess handedness and thus dominant cerebral hemisphere. Task initiation was marked with an event recorder.
2.1. Data Analysis
Data were simultaneously recorded onto a data acquisition system (PHYSIDAS, Department of Medical Physics, University Hospitals of Leicester). All signals were visually inspected and removal of artefacts (noise and narrows spikes) occurred via linear interpolation. CBFv channels were processed using a median filter and all signals were passed through a low-pass filter. The recordings were separated into three relevant time frames. Two-time frames were selected for analysis, comparing the peak percentage change in CBFv from a 20-second baseline at T2 (25-30 seconds), and T3 (30-40 seconds). T2 represents the initial response occurring five to ten seconds after task initiation, and T3 represents the sustained response occurring at ten to 20 seconds after task initiation. As previously stated, in order to elicit CBFv responses, a number of tasks from the Addenbrooke’s Cognitive Examination-III encompassing memory and visuospatial cognitive domains were conducted (Table 1).
Table 1. List of the tasks used to elicit CBFv responses and their respective codes.
| Task Label | Task |
|---|---|
| Memory | |
| M1 | Recalling three words (lemon, key, ball) |
| M2 | Learning a name and address |
| M3 | Recalling current and past UK prime ministers and US presidents |
| M4 | Recalling the previously learned name and address |
| Visuospatial | |
| V1 | Drawing an infinity diagram and cube |
| V2 | Drawing a clock and orientating the time correctly |
| V3 | Counting dots |
| V4 | Recognising obscured letters |
2.2. Statistics
Data were tested for normality prior to statistical testing. Continuous, parametric data are presented as mean (standard deviation), or median [IQR] for non-parametric data. Nominal data are presented as number (percentage). Significance testing was by independent t-tests for parametric continuous data, Mann-Whitney U for non-parametric continuous data, and chi-square for nominal data. Data were considered significant where p<0.05. To assess the effect of hemispheric dominance, a two-way mixed ANOVA was used, with post hoc testing by Tukey. To assess for an interaction effect between age and gender, a two way ANOVA was carried out. All statistical analyses were performed using SPSS Version 24 for Windows, and graphs were prepared using GraphPad Prism Version 6.0 for Windows.
3. RESULTS
3.1. Demographics
Fifty-four healthy participants were divided into older (n=25) and younger (n=29) groups. Overall median age was 48 years [IQR: 22-61]; age being significantly different between groups (22.0 [13]) vs. (63.3 [19]) years, p<0.005) with a greater proportion of females (72% vs. 40%, p=0.03) in the younger cohort (Table 2). ACE-III scores were significantly lower in the older group (97.2 vs. 98.2, p=0.05), though all participants had a score above the threshold used to diagnose dementia (Table 2). Baseline systolic and diastolic blood pressure (BP) was also higher amongst the older cohort (p<0.05), but no participant had diagnosed hypertension (Table 2).
Table 2. Baseline demographic data for all study participants, and by older and younger cohorts. Data are mean (standard deviation) or median [IQR] unless stated. Significance testing was by independent t-tests or Mann-Whitney U testing. Significant differences are highlighted in bold. ACE-III, Addenbrooke’s cognitive examination-III; BMI, body mass index; BP, blood pressure. Non-Caucasian ethnicities were either south-east or south Asian.
| Demographic | All | Younger | Older | P value |
|---|---|---|---|---|
| - | 54 | 29 | 25 | - |
| Age, years | 48 [22-61] | 22 [22-34] | 64 [54-71]) | <0.005 |
| Female (n,%) | 31 (57.4) | 21 (72.4) | 10 (40) | 0.03 |
| Caucasian (n,%) | 48 (88.9) | 25 (86.2) | 23 (92) | 0.61 |
| Alcohol, >14 units/week (n,%) | 6 (11.1) | 2 (6.9) | 4 (16) | 0.30 |
| Non-smoker (n,%) Ex-smoker (n,%) |
46 (85.2) 8 (14.8) |
26 (89.7) 3 (10.3) |
20 (80) 5 (20) |
0.27 0.32 |
| BMI, kg/m2 | 25.1 (4.7) | 24.5 (5.2) | 25.7 (4.1) | 0.34 |
| ACE-III score | 97.76 (1.95) | 98.2 (1.7) | 97.2 (2.1) | 0.05 |
| Systolic BP, mmHg | 126.9 (15.9) | 120.9 (13.2) | 133.7 (16.2) | 0.002 |
| Diastolic BP, mmHg | 76.6 (10.2) | 73.3 (9.7) | 80.4 (9.6) | 0.009 |
| Right hand dominant (n,%) | 50 (92.6) | 28 (96.6) | 22 (88) | 0.41 |
3.2. Baseline (Resting) Data
In the 5 minute rest period, significant differences were seen in all peripheral and cerebral haemodynamic data, with lower CBFv in both dominant (younger: 52.2 (9.3), older: 49.1 (8.0), p=0.02) and non-dominant (younger: 52.9 (10.6), older: 47.5 (9.1), p=0.047) hemispheres, and higher Mean Arterial Pressure (MAP) (younger: 84.7 (11.1), older: 96.9 (12.9), p=0.001) and heart rate (HR) (younger: 72.7 (8.3), older: 66.9 (8.9), p=0.016) in older compared to younger participants (Table 3). Resting ETCO2 values were also significantly lower in older participants (younger: 37.0 (2.9), older: 38.8 (2.3), p=0.014).
Table 3. Peak percentage change in peripheral and cerebral haemodynamic parameters and ETCO2 at T2 (25-30 seconds), and T3 (30-40 seconds) for four memory tasks (M1-M4) from the ACE-III. Data are mean (standard deviation), with significance testing by independent t-tests.
| Parameter | Younger | Older | P value | |||
|---|---|---|---|---|---|---|
| N | 29 | 25 | ||||
| M1 | T2 | T3 | T2 | T3 | T2 | T3 |
| CBFv Non-dominant | 3.07 (9.03) | 0.49 (5.83) | 8.04 (10.73) | -0.01 (9.33) | 0.07 | 0.82 |
| CBFv dominant | 2.17 (9.16) | -0.03 (8.21) | 8.38 (9.27) | -1.01 (5.32) | 0.017 | 0.60 |
| MAP | 4.49 (6.13) | 3.76 (5.54) | 3.86 (4.55) | 0.72 (6.01) | 0.67 | 0.60 |
| HR | -0.95 (6.76) | -1.17 (8.48) | 2.82 (5.27) | -0.03 (7.24) | 0.028 | 0.06 |
| ETCO2 | 0.45 (4.62) | -0.43 (4.24) | -0.89 (3.79) | -1.67 (3.52) | 0.26 | 0.25 |
| M2 | ||||||
| CBFv Non-dominant | 2.60 (10.29) | 4.06 (7.90) | 8.07 (9.62) | 6.40 (11.16) | 0.05 | 0.37 |
| CBFv dominant | 4.60 (11.48) | 5.70 (7.45) | 7.71 (8.33) | 5.52 (9.18) | 0.27 | 0.94 |
| MAP | 3.61 (5.50) | 4.68 (9.79) | 4.67 (11.07) | 6.50 (9.29) | 0.45 | 0.49 |
| HR | 2.46 (10.05) | 1.62 (5.06) | 4.24 (5.11) | 5.18 (4.50) | 0.67 | 0.009 |
| ETCO2 | 0.86 (7.06) | 2.96 (5.60) | -2.77 (9.45) | -0.19 (10.84) | 0.11 | 0.18 |
| M3 | ||||||
| CBFv Non-dominant | 5.14 (8.58) | 3.35 (7.05) | 10.19 (8.44) | 5.57 (8.27) | 0.036 | 0.30 |
| CBFv dominant | 6.61 (8.94) | 5.45 (7.38) | 10.96 (7.99) | 6.98 (6.47) | 0.07 | 0.43 |
| MAP | 5.23 (4.69) | 2.74 (5.18) | 5.62 (5.92) | 6.05 (5.43) | 0.11 | 0.028 |
| HR | 2.02 (6.01) | 4.24 (4.98) | 4.79 (6.44) | 5.13 (5.24) | 0.79 | 0.53 |
| ETCO2 | 1.84 (5.67) | 0.34 (5.41) | 0.34 (8.47) | -0.47 (8.37) | 0.46 | 0.67 |
| M4 | ||||||
| CBFv Non-dominant | 8.16 (9.11) | 4.40 (8.65) | 13.26 (10.92) | 5.68 (7.99) | 0.07 | 0.58 |
| CBFv dominant | 8.42 (8.86) | 3.32 (8.41) | 15.93 (8.86) | 8.20 (7.85) | 0.003 | 0.03 |
| MAP | 4.24 (4.58) | 6.45 (6.59) | 6.00 (5.79) | 7.00 (5.52) | 0.17 | 0.74 |
| HR | 3.21 (8.51) | 1.89 (2.99) | 5.84 (4.96) | 3.20 (6.92) | 0.22 | 0.38 |
| ETCO2 | -0.69 (5.61) | -1.03 (6.98) | -1.86 (3.91) | -3.80 (6.63) | 0.39 | 0.14 |
3.3. Memory Tasks
3.3.1. Peripheral Haemodynamic and other Changes
At the T2 time-point (25 to 30 seconds after paradigm commencement) peak percentage change in HR was significantly greater in the older cohort for the M1 task (recalling three words [lemon, key, ball]) only (-0.95 (6.76)% vs. 2.82 (5.27)%, p=0.028). No other significant differences were seen in peripheral haemodynamic data between younger and older groups at the T2 time-point in response to memory tasks (Table 3). At the T3 time-point (30 to 40 seconds following paradigm commencement), peak percentage change in HR was significantly higher in the older cohort in the M2 task (learning a name and address) (1.62 (5.06)% vs. 5.18 (4.50)%, p=0.009), and in MAP for the M3 task (recalling current and past UK prime ministers and US presidents) (2.74 (5.18)% vs. 6.05 (5.43)%, p=0.028). Otherwise, there were no significant differences between the two cohorts in peak percentage changes (Table 3).
3.3.2. Cerebral Haemodynamic Changes
Peak percentage changes in CBFv in both dominant and non-dominant hemispheres were generally lower in the younger compared to older groups at the T2 time-point, though these differences were only significant for the dominant hemisphere for the M1 (2.17 (9.16)% vs. 8.38 (9.27)%, p=0.017) and M4 (recalling the previously learned name and address) (8.42 (8.86)% vs. 15.93 (8.86)%, p=0.003) tasks, and for the non-dominant hemisphere for the M2 (2.60 (10.29)% vs. 8.07 (9.62)%, p=0.05) and M3 (5.14 (8.58)% vs. 10.19 (8.44)%, p=0.036) tasks (Table 3, Fig. 1). At the T3 time-point, the only significant difference between younger and older groups was for the M4 task in the dominant hemisphere (3.32 (8.41)% vs. 8.20 (7.85)%, p=0.03) (Table 3, Fig. 1).
Fig. (1).
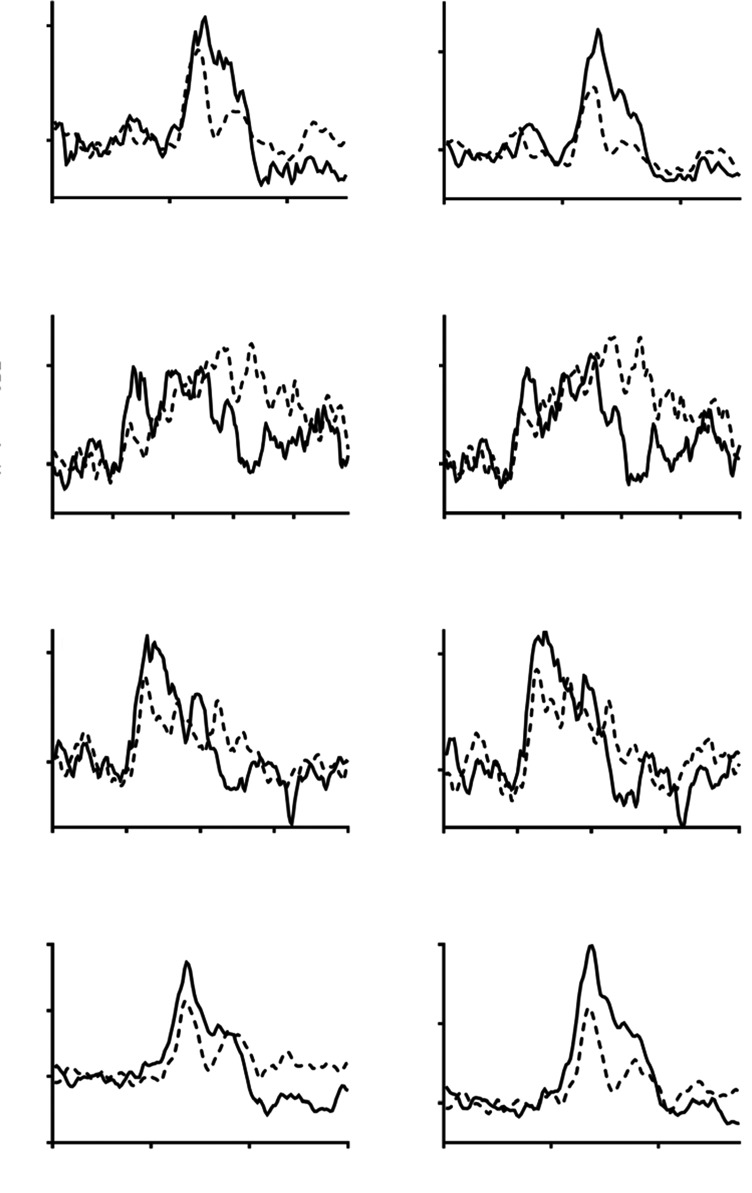
Percentage change in CBFv in older (continuous line), and younger (interrupted line) adults to four memory tasks (M1-M4). Task initiation occurred at 20 seconds. Left-hand panel = non-dominant hemisphere, right-hand panel = dominant hemisphere.
3.4. Visuospatial Tasks
3.4.1. Peripheral Haemodynamic and Other Changes
Peak percentage changes in HR were significantly greater in older participants for all visuospatial tasks at the T2 time-point (Table 4). However, with the exception of MAP for the V2 (drawing a clock and orientating the time correctly) (2.80 (3.92)% vs. 5.74 (6.29)%, p=0.039) and V3 (counting dots) (0.87 (3.62)% vs. (3.60 (5.53)%, p=0.034) tasks, no other significant differences were seen in peripheral haemodyna-mic and ETCO2 parameters between young and old groups at the T2 time-point (Table 4).
Table 4. Peak percentage change in peripheral and cerebral haemodynamic parameters and ETCO2 at T2 (25-30 seconds), and T3 (30-40 seconds) for four visuospatial tasks (V1-V4) from the ACE-III. Data are mean (standard deviation), with significance testing by independent t-tests.
| Parameter | Younger | Older | P value | |||
|---|---|---|---|---|---|---|
| N | 29 | 25 | ||||
| V1 | T2 | T3 | T2 | T3 | T2 | T3 |
| CBFv Non-dominant | 8.88 (9.24) | 5.87 (8.32) | 12.83 (5.69) | 11.89 (6.60) | 0.07 | 0.005 |
| CBFv dominant | 9.28 (8.92) | 6.38 (7.62) | 13.64 (7.47) | 12.93 (8.92) | 0.06 | 0.005 |
| MAP | 2.64 (3.82) | -1.07 (4.82) | 5.69 (6.50) | 3.56 (7.55) | 0.07 | 0.043 |
| HR | 4.82 (7.63) | 4.44 (8.29) | 8.02 (4.90) | 8.65 (6.30) | 0.046 | 0.009 |
| ETCO2 | 0.11 (3.76) | -0.50 (4.04) | -0.12 (4.07) | -1.32 (3.90) | 0.83 | 0.45 |
| V2 | ||||||
| CBFv Non-dominant | 6.30 (8.72) | 7.67 (8.79) | 11.30 (7.77) | 9.51 (8.67) | 0.032 | 0.45 |
| CBFv dominant | 7.52 (9.25) | 8.51 (9.80) | 13.20 (9.17) | 13.05 (10.14) | 0.028 | 0.10 |
| MAP | 2.80 (3.92) | 1.47 (4.71) | 5.74 (6.29) | 6.57 (6.96) | 0.039 | 0.003 |
| HR | 2.31 (5.78) | 1.82 (6.48) | 5.50 (5.17) | 7.30 (6.18) | 0.042 | 0.002 |
| ETCO2 | 0.68 (6.56) | 0.71 (8.57) | 1.01 (4.46) | -0.55 (4.25) | 0.83 | 0.51 |
| V3 | ||||||
| CBFv Non-dominant | 4.19 (8.75) | 5.20 (8.65) | 10.63 (7.67) | 11.72 (10.31) | 0.006 | 0.015 |
| CBFv dominant | 6.44 (9.62) | 4.42 (8.71) | 10.92 (8.45) | 10.57 (10.05) | 0.08 | 0.019 |
| MAP | 0.87 (3.62) | 1.25 (3.33) | 3.60 (5.53) | 2.57 (10.14) | 0.023 | 0.002 |
| HR | -0.35 (6.09) | -0.47 (5.25) | 3.27 (5.08) | 4.34 (5.53) | 0.034 | 0.54 |
| ETCO2 | 0.80 (9.19) | 1.67 (8.91) | 0.36 (3.46) | 0.06 (3.24) | 0.82 | 0.40 |
| V4 | ||||||
| CBFv Non-dominant | 5.21 (9.76) | 2.70 (7.15) | 9.02 (8.86) | 4.35 (7.15) | 0.14 | 0.40 |
| CBFv dominant | 5.83 (9.57) | 3.83 (8.48) | 9.54 (8.96) | 3.80 (7.96) | 0.15 | 0.99 |
| MAP | 0.43 (4.86) | 0.35 (3.64) | 4.19 (5.31) | -0.20 (3.30) | 0.08 | 0.56 |
| HR | -0.13 (6.52) | -0.01 (4.87) | 2.66 (4.57) | 1.97 (3.45) | 0.009 | 0.10 |
| ETCO2 | 2.47 (4.59) | 0.97 (6.36) | 0.22 (5.13) | -1.20 (5.90) | 0.09 | 0.20 |
With respect to differences in peripheral haemodynamic parameters at the T3 time-point, peak percentage changes were significantly greater in all visuospatial tasks, except V3 for HR and V4 (recognising obscured letters) for both HR and MAP (Table 4). No significant differences were seen in ETCO2 for all visuospatial tasks at the T3 time-point (Table 4).
3.4.2. Cerebral Haemodynamic Changes
In keeping with the memory tasks, peak percentage changes in CBFv were lower for all visuospatial tasks in the younger group at the T2 time-point, though these differences were only significant in the non-dominant hemisphere for the V2 (6.30 (8.72)% vs. 11.30 (7.77)%, p=0.032) and V3 (4.19 (8.75)% vs. 10.63 (7.67)%, p=0.006) tasks, and in the dominant hemisphere for V2 task (7.52 (9.25)% vs. 13.20 (9.17)%, p=0.028) (Table 4, Fig. 2).
Fig. (2).
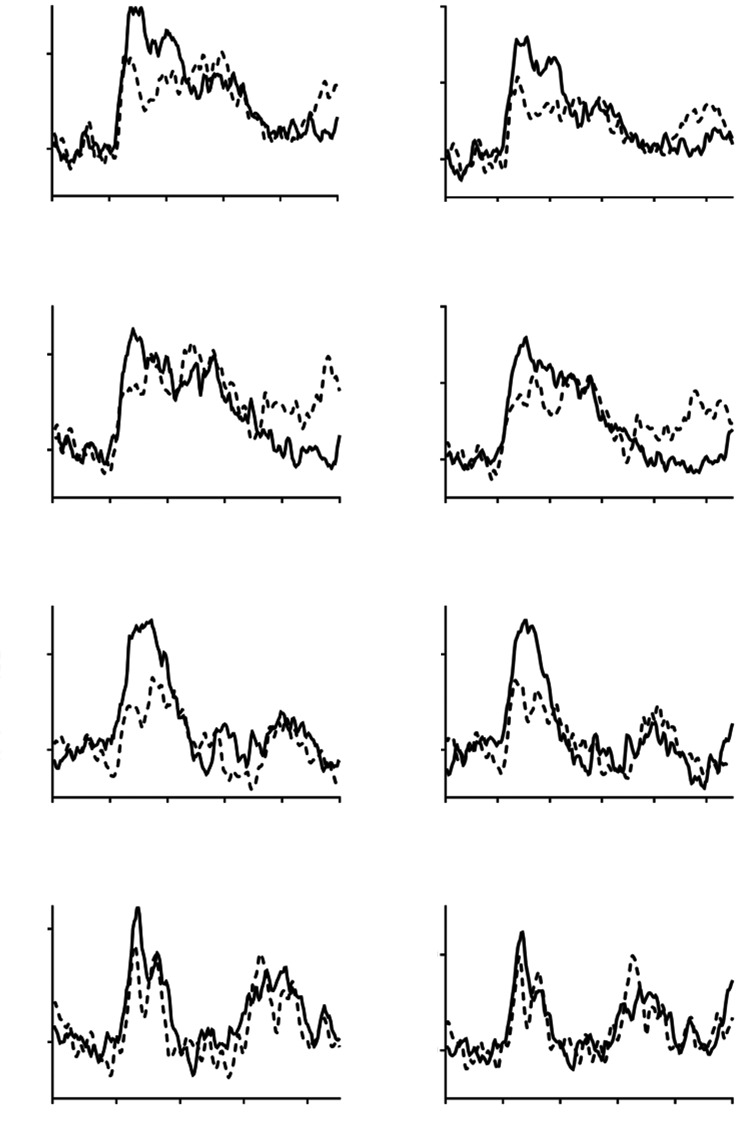
Percentage change in CBFv in older (continuous line), and younger (interrupted line) adults to four visuospatial tasks (V1-V4). Task initiation occurred at 20 seconds. Left-hand panel = non-dominant hemisphere, right-hand panel = dominant hemisphere.
At the T3 time-point, peak percentage changes in CBFv were significantly lower in the younger compared to older groups in both the non-dominant and dominant hemipsheres for the V1 (5.87 (8.32)% vs. 11.89 (6.60)%, p=0.005, and 6.38 (7.62)% vs. 12.93 (8.92)%, p=0.005, respectively) and V3 tasks (5.20 (8.65)% vs. 11.72 (10.31)%, p=0.015, and 4.42 (8.71)% vs. 10.57 (10.05)%, p=0.019, respectively) (Table 4, Fig. 2).
3.5. Hemispheric Dominance
3.5.1. Memory Tasks
Evidence of significant interaction between dominance and the effect of age group on CBFV response was observed for the following tasks at T3: M2 (p=0.03), and M4 (p=0.01). There were no significant effects on interaction at T2.
3.5.2. Visuospatial Tasks
There was no evidence of significant interaction between dominance and the effect of age group on CBFV response in any task at either time point.
3.6. Gender
There was no evidence of interaction between the effects of gender and age on statistically significant results from the primary analysis.
4. DISCUSSION
4.1. Summary of Results
In this study, we report significantly larger CBFv responses in healthy older adults to cognitive tasks taken from the clinical assessment tool (ACE-III). All four tasks from the memory section of the ACE-III demonstrated larger responses in healthy older adults, but this varied between hemispheres and time-points. These differences between groups do not appear to be attributable to differential changes in MAP or ETCO2, which remained constant between groups. This relationship was less clear for the visuospatial tasks, where tasks V1-V3 demonstrated larger CBFv responses in the healthy older group, varying between time-points and hemisphere, but with more tasks demonstrating concomitant BP rises in the healthy older group. Therefore, while memory tasks appear to exhibit a more selective rise in CBFv, visuospatial tasks appear to induce greater MAP changes in addition to CBFv responses.
Given that healthy older adults have previously demonstrated less lateralisation of the CBFv response, we assessed the effect of hemispheric dominance between the groups using a two-way mixed ANOVA. In this study, there was no significant interaction between dominance and group for the visuospatial tasks, but two tasks from the memory section demonstrated a significant interaction between dominance and group. This indicates differences in the lateralisation of the responses for these two tasks between healthy older and younger adults.
4.2. Comparison with the Literature
A number of studies have previously examined the effects of healthy ageing on cerebral autoregulation and neurovascular coupling. The study reported here is distinct for the large number of tasks used, spanning two cognitive domains, and taken from a clinical cognitive assessment tool. In a study by Harwood et al., older and younger adults performed a one-hour vigilance task, and they demonstrated loss of lateralisation of the CBFv response in older adults, with poorer cognitive performance, and bilateral rises in CBFv [15]. This is in keeping with the HAROLD hypothesis that loss of asymmetry and bilateral cortical activation can act to compensate for poorer cognitive functioning in ageing [16, 23]. The HAROLD model only partially explains these age-related changes, however, the CRUNCH hypothesis expands on this by suggesting that additional neuronal circuits, outside those normally used to execute a cognitive task, are recruited as part of age-related physiological compensation [16]. In the results reported here, we note that the majority of tasks demonstrate larger CBFv responses in the healthy older group, and this may be a reflection of the increased CBFv requirement through additional circuit recruitment, in order to meet those larger metabolic needs in older adults [16]. Thus, greater neuronal and metabolic activity is required to complete the same task, compared to younger adults. This phenomenon is also seen in the early stages of mild cognitive impairment, where there are hyperperfusion and larger stimulation seen initially, which becomes a hypoperfused state as cognitive decompensation occurs and progresses to dementia [8, 24, 25]. In a study by Sorond et al., they also noted larger increases in CBFv with two cognitive tasks (word stem completion and visual search) in the older group, with loss of asymmetry between frontal and posterior responses during the language task, but not a visual task in older adults [17]. However, these findings conflict with a number of previous studies detailed in a review by Stroobants and Vingerhoets, where blood flow responses were of lower magnitude, and slower to peak than younger adults. They postulated that this is due to reduced arteriole reactivity to stimulation, or lesser sympathetic response to cognitive stimulation [26]. In an fMRI study by Logan et al., they determined that older adults can demonstrate both under-recruitment of brain regions which are task-specific, in addition to over-recruitment of brain regions which are less specialised to the specific task [27]. This was consistent with a number of previous fMRI studies of task activation in healthy ageing [28-30]. Furthermore, Logan et al. demonstrated that under-recruitment to specialised areas was due to ineffective resource utilisation, rather than resource depletion [27].
In the study reported here, limited differences were noted in the lateralisation patterns of older and younger adults with only two of eight tasks demonstrating a significant interaction between hemispheric dominance and age. However, in a previous study, we did not demonstrate significant lateralisation in a healthy younger cohort using tasks to stimulate CBFv from the ACE-III [12], and thus the tasks here may not be sufficient to elicit distinct lateralisation between hemispheres. In this study, lateralisation was assessed by coding right-hand dominant individuals as left hemisphere dominant, and left-handed individuals as right hemisphere dominant. However, it is known that up to 25% of left-handed individuals show atypical lateralisation patterns [30], and, therefore this may explain the lack of distinct lateralisation patterns between the younger and older groups.
4.3. Limitations
Studies utilising TCD rely on CBFv as a proxy to CBF, assuming the insonated vessel diameter remains constant [11]. Furthermore, whilst TCD provides good temporal resolution, allowing real-time changes in CBFv responses to task activation to be measured, it lacks the spatial resolution of alternatives, such as magnetic resonance imaging [11]. In this study population, there was an unequal distribution of female participants between the two groups, with higher numbers amongst the younger cohort. Females have a tendency to less lateralisation and higher resting velocities, which could, therefore, affect lateralisation patterns [10, 26]. However, in a further analysis, there was no interaction effect between age and gender, and thus this is not likely to affect the results presented in this manuscript. In addition, older participants had significantly lower ACE-III scores, and higher resting BP. Although all were considered below thresholds for cognitive impairment and hypertension, this indicates that there are cognitive and physiological differences to be recognised in our populations, even in healthy individuals. Finally, data on years of education was insufficient across both groups. Thus, the differences in cerebral physiology could result from differential vascular adaption from greater exposure to learning and education in the younger cohort.
4.4. Future Directions
Future longitudinal studies would be useful to measure the changes in neurovascular coupling that occur with healthy ageing, and to see if early deficiencies can differentiate between those that will progress to cognitive impairment and those who will remain cognitively stable in later life. Some, but not all tasks in this study demonstrated concomitant BP changes with CBFv rises. Further work needs to be undertaken using a multivariate analysis that can account for changes in BP and ETCO2, to ensure that changes in CBFv are not confounded by these variables. This study examined a limited sample size and age range, ideally, a larger observational study which is able to categorise age across deciles would allow the natural history of age-related changes in cerebrovascular physiology to be investigated.
CONCLUSION
In conclusion, healthy older adults demonstrate augmented physiology in response to a cognitive challenge, when compared to healthy younger adults. This emphasises the need for a better understanding of the effect of ageing on normal physiology, and when this becomes pathological, resulting in cognitive decline. This will support the development of timely therapeutics to target early changes in aberrant physiology with the aim of preventing the development of cognitive impairment.
ACKNOWLEDGEMENTS
Declared none.
AUTHOR CONTRIBUTIONS
Lucy Beishon collected the data, analysed the data and drafted the manuscript.
Jatinder S. Minhas drafted the manuscript.
Kate Patrick analysed the data and contributed to drafting the manuscript.
Iswariya Shanmugam analysed the data and contributed to drafting the manuscript.
Claire A.L. Williams collected the data, contributed to drafting the manuscript.
Ronney B. Panerai contributed to drafting the manuscript.
Thompson G. Robinson contributed to drafting the manuscript.
Victoria J. Haunton contributed to drafting the manuscript.
DISCLOSURES
Lucy Beishon is a Dunhill Medical Trust Clinical Research Training Fellow (RTF1806\27), Jatinder S. Minhas is a Dunhill Medical Trust Clinical Research Training Fellow (RTF97/0117) and Thompson G. Robinson is an NIHR Senior Investigator.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical approval was provided by the University of Leicester (5355) and Wales 7 Research Ethics Committee (REC 17/WA/0089), UK.
HUMAN AND ANIMAL RIGHTS
No animals were used in this study, the reported experiments on humans were in accordance with the ethical standards of the committee responsible for human experimentation (institutional national), and with the Helsinki Declaration of 1975, as revised in 2008 (http://www.wma.net/).
CONSENT FOR PUBLICATION
Written informed consent was obtained prior to the commencement of study procedures in accordance with local ethics committee protocols.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
REFERENCES
- 1.Organisation W.H. Ageing and Health 2015 [cited 2018 23/03/2018]. Available from: http://www.who.int/mediacentre/factsheets/fs404/en/
- 2.Prince M., Wimo A., Guerchet M., et al. World Alzheimer Report 2015 The global impact of dementia Alzheimer's Disease Interna- tional. 2015. Contract No.: Report. [Google Scholar]
- 3.Rebok G.W., Ball K., Guey L.T., et al. Ten-year effects of the ACTIVE cognitive training trial on cognition and everyday functioning in older adults. J. Am. Geriatr. Soc. 2014;62(1):16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keage H.A., Churches O.F., Kohler M., et al. Cerebrovascular function in aging and dementia: A systematic review of transcranial Doppler studies. Dement. Geriatr. Cogn. Disord. Extra. 2012;2(1):258–270. doi: 10.1159/000339234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabayan B., Jansen S., Oleksik A.M., et al. Cerebrovascular hemodynamics in Alzheimer’s disease and vascular dementia: A meta-analysis of transcranial Doppler studies. Ageing Res. Rev. 2012;11(2):271–277. doi: 10.1016/j.arr.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Wierenga C.E., Hays C.C., Zlatar Z.Z. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. J. Alzheimers Dis. 2014;42(Suppl. 4):S411–S419. doi: 10.3233/JAD-141467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hays C.C., Zlatar Z.Z., Wierenga C.E. The utility of cerebral blood flow as a biomarker of preclinical Alzheimer’s disease. Cell. Mol. Neurobiol. 2016;36(2):167–179. doi: 10.1007/s10571-015-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolters F.J., Zonneveld H.I., Hofman A., et al. Cerebral perfusion and the risk of dementia: A population-based study. Circulation. 2017;136(8):719–728. doi: 10.1161/CIRCULATIONAHA.117.027448. [DOI] [PubMed] [Google Scholar]
- 9.Van Beek A.H., Claassen J.A., Rikkert M.G., et al. Cerebral autoregulation: An overview of current concepts and methodology with special focus on the elderly. J. Cereb. Blood Flow Metab. 2008;28(6):1071–1085. doi: 10.1038/jcbfm.2008.13. [DOI] [PubMed] [Google Scholar]
- 10.Panerai R.B. Transcranial doppler for evaluation of cerebral autoregulation. Clin. Auton. Res. 2009;19(4):197–211. doi: 10.1007/s10286-009-0011-8. [DOI] [PubMed] [Google Scholar]
- 11.Beishon L.C., Williams C.A.L., Panerai R.B., et al. The assessment of neurovascular coupling with the Addenbrooke’s cognitive examination: A functional transcranial doppler ultrasonographic study. J. Neurophysiol. 2018;119(3):1084–1094. doi: 10.1152/jn.00698.2017. [DOI] [PubMed] [Google Scholar]
- 12.Beishon L., Williams C.A.L., Panerai R.B., et al. Reproducibility of task activation using the Addenbrooke’s cognitive examination in healthy controls: A functional transcranial doppler ultrasonography study. J. Neurosci. Methods. 2017;291:131–140. doi: 10.1016/j.jneumeth.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Williams C.A.L., Panerai R.B., Robinson T.G., et al. Transcranial doppler ultrasonography in the assessment of neurovascular coupling responses to cognitive examination in healthy controls: A feasibility study. J. Neurosci. Methods. 2017;284:57–62. doi: 10.1016/j.jneumeth.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Harwood A.E., Greenwood P.M., Shaw T.H. Transcranial doppler sonography reveals reductions in hemispheric asymmetry in healthy older adults during vigilance. Front. Aging Neurosci. 2017;9:21. doi: 10.3389/fnagi.2017.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berlingeri M., Danelli L., Bottini G., et al. Reassessing the HAROLD model: Is the hemispheric asymmetry reduction in older adults a special case of compensatory-related utilisation of neural circuits? Exp. Brain Res. 2013;224(3):393–410. doi: 10.1007/s00221-012-3319-x. [DOI] [PubMed] [Google Scholar]
- 16.Sorond F.A., Schnyer D.M., Serrador J.M., et al. Cerebral blood flow regulation during cognitive tasks: Effects of healthy aging. Cortex. 2008;44(2):179–184. doi: 10.1016/j.cortex.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mioshi E., Dawson K., Mitchell J., et al. The Addenbrooke’s Cognitive Examination Revised (ACE-R): A brief cognitive test battery for dementia screening. Int. J. Geriatr. Psychiatry. 2006;21(11):1078–1085. doi: 10.1002/gps.1610. [DOI] [PubMed] [Google Scholar]
- 18.Velayudhan L., Ryu S.H., Raczek M., et al. Review of brief cognitive tests for patients with suspected dementia. Int. Psychogeriatr. 2014;26(8):1247–1262. doi: 10.1017/S1041610214000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 20.Iachini I., Iavarone A., Senese V.P., et al. Visuospatial memory in healthy elderly, AD and MCI: A review. Curr. Aging Sci. 2009;2(1):43–59. doi: 10.2174/1874609810902010043. [DOI] [PubMed] [Google Scholar]
- 21.Oldfield R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 22.Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol. Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 23.Beishon L., Haunton V.J., Panerai R.B., et al. Cerebral hemodynamics in mild cognitive impairment: A systematic review. J. Alzheimers Dis. 2017;59(1):369–385. doi: 10.3233/JAD-170181. [DOI] [PubMed] [Google Scholar]
- 24.Wierenga C.E., Bondi M.W. Use of functional magnetic resonance imaging in the early identification of Alzheimer’s disease. Neuropsychol. Rev. 2007;17(2):127–143. doi: 10.1007/s11065-007-9025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stroobant N., Vingerhoets G. Transcranial Doppler ultrasonography monitoring of cerebral hemodynamics during performance of cognitive tasks: A review. Neuropsychol. Rev. 2000;10(4):213–231. doi: 10.1023/a:1026412811036. [DOI] [PubMed] [Google Scholar]
- 26.Logan J.M., Sanders A.L., Snyder A.Z., et al. Under-recruitment and nonselective recruitment: Dissociable neural mechanisms associated with aging. Neuron. 2002;33(5):827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- 27.Cabeza R., Grady C.L., Nyberg L., et al. Age-related differences in neural activity during memory encoding and retrieval: A positron emission tomography study. J. Neurosci. 1997;17(1):391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reuter-Lorenz P.A., Jonides J., Smith E.E., et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J. Cogn. Neurosci. 2000;12(1):174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- 29.Grady C.L., Maisog J.M., Horwitz B., et al. Age-related changes in cortical blood flow activation during visual processing of faces and location. J. Neurosci. 1994;14(3 Pt 2):1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szaflarski J.P., Binder J.R., Possing E.T., et al. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59(2):238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]


