Abstract
Background
Financial incentives, monetary or vouchers, are widely used in an attempt to precipitate, reinforce and sustain behaviour change, including smoking cessation. They have been used in workplaces, in clinics and hospitals, and within community programmes.
Objectives
To determine the long‐term effect of incentives and contingency management programmes for smoking cessation.
Search methods
For this update, we searched the Cochrane Tobacco Addiction Group Specialised Register, clinicaltrials.gov, and the International Clinical Trials Registry Platform (ICTRP). The most recent searches were conducted in July 2018.
Selection criteria
We considered only randomised controlled trials, allocating individuals, workplaces, groups within workplaces, or communities to smoking cessation incentive schemes or control conditions. We included studies in a mixed‐population setting (e.g. community, work‐, clinic‐ or institution‐based), and also studies in pregnant smokers.
Data collection and analysis
We used standard Cochrane methods. The primary outcome measure in the mixed‐population studies was abstinence from smoking at longest follow‐up (at least six months from the start of the intervention). In the trials of pregnant women we used abstinence measured at the longest follow‐up, and at least to the end of the pregnancy. Where available, we pooled outcome data using a Mantel‐Haenzel random‐effects model, with results reported as risk ratios (RRs) and 95% confidence intervals (CIs), using adjusted estimates for cluster‐randomised trials. We analysed studies carried out in mixed populations separately from those carried out in pregnant populations.
Main results
Thirty‐three mixed‐population studies met our inclusion criteria, covering more than 21,600 participants; 16 of these are new to this version of the review. Studies were set in varying locations, including community settings, clinics or health centres, workplaces, and outpatient drug clinics. We judged eight studies to be at low risk of bias, and 10 to be at high risk of bias, with the rest at unclear risk. Twenty‐four of the trials were run in the USA, two in Thailand and one in the Phillipines. The rest were European. Incentives offered included cash payments or vouchers for goods and groceries, offered directly or collected and redeemable online. The pooled RR for quitting with incentives at longest follow‐up (six months or more) compared with controls was 1.49 (95% CI 1.28 to 1.73; 31 RCTs, adjusted N = 20,097; I2 = 33%). Results were not sensitive to the exclusion of six studies where an incentive for cessation was offered at long‐term follow up (result excluding those studies: RR 1.40, 95% CI 1.16 to 1.69; 25 RCTs; adjusted N = 17,058; I2 = 36%), suggesting the impact of incentives continues for at least some time after incentives cease.
Although not always clearly reported, the total financial amount of incentives varied considerably between trials, from zero (self‐deposits), to a range of between USD 45 and USD 1185. There was no clear direction of effect between trials offering low or high total value of incentives, nor those encouraging redeemable self‐deposits.
We included 10 studies of 2571 pregnant women. We judged two studies to be at low risk of bias, one at high risk of bias, and seven at unclear risk. When pooled, the nine trials with usable data (eight conducted in the USA and one in the UK), delivered an RR at longest follow‐up (up to 24 weeks post‐partum) of 2.38 (95% CI 1.54 to 3.69; N = 2273; I2 = 41%), in favour of incentives.
Authors' conclusions
Overall there is high‐certainty evidence that incentives improve smoking cessation rates at long‐term follow‐up in mixed population studies. The effectiveness of incentives appears to be sustained even when the last follow‐up occurs after the withdrawal of incentives. There is also moderate‐certainty evidence, limited by some concerns about risks of bias, that incentive schemes conducted among pregnant smokers improve smoking cessation rates, both at the end of pregnancy and post‐partum. Current and future research might explore more precisely differences between trials offering low or high cash incentives and self‐incentives (deposits), within a variety of smoking populations.
Keywords: Female, Humans, Male, Pregnancy, Motivation, Behavior Therapy, Health Facilities, Randomized Controlled Trials as Topic, Reward, Smoking Cessation, Smoking Cessation/methods, Smoking Cessation/psychology, Smoking Prevention, Workplace
Plain language summary
Can rewards help smokers to quit in the long term?
Background
Smoking is the leading cause of disease and death worldwide. Most smokers want to quit, but stopping smoking can be very challenging. Quitting smoking can greatly improve people's health. Rewards, such as money or vouchers, can be used to encourage smokers to quit, and to reward them if they stay stopped. Such schemes can be run in workplaces, in clinics, and sometimes as community programmes.
Study types
We conducted our most recent search for studies in July 2018.
General trials: We found 33 trials, covering more than 21,600 people, that tested different rewards schemes to help smokers to quit. Two studies included smokers from mental health clinics, two from primary care clinics, two from head‐and‐neck cancer treatment clinics, two from colleges or universities, and one in Thai villages. Twenty‐four of the trials were run in the USA. All the trials followed up participants for at least six months. Those who had quit were checked by testing their breath or bodily fluids. Rewards were cash payments, vouchers, or the return of money deposited by those taking part.
Pregnancy trials: We looked at studies in pregnant women separately. We found ten trials, nine based in the USA and one in the UK, covering 2571 pregnant women who smoked. Rewards were vouchers that were sometimes increased in value, depending on how long the woman had managed to stay quit.
Key results General trials: Six months or more after the beginning of the trial, people receiving rewards were more likely to have stopped smoking than those in the control groups. Success rates continued beyond when the incentives had ended. Studies varied in the total amounts of rewards that were paid. There was no noticeable difference between trials paying smaller amounts (less than USD 100 (US dollars)) compared to those paying larger amounts (more than USD 700).
Pregnancy trials: Combining data from nine trials showed that women in the rewards groups were more likely to stop smoking than those in the control groups, both at the end of the pregnancy and after the birth of the baby.
Quality of the studies Some of the studies did not provide enough data for us to fully assess their quality. Taking out the lowest‐quality trials from the analysis did not change the results. Our certainty in our main findings is high. Our certainty in our findings in pregnant women is moderate, as some studies were of lower quality.
Summary of findings
Summary of findings for the main comparison. Incentives vs no incentives for smoking cessation in mixed populations.
| Smoking cessation: incentives compared to no incentives in mixed populations | ||||||
| Patient or population: Adult smokers Setting: Mixed Intervention: Incentives for smoking cessation Comparison: No incentives | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with incentives: mixed populations | |||||
|
Smoking cessation in mixed populations ‐ Longest follow‐up Follow‐up: 6 months to 24 months) |
71 per 1000 | 106 per 1000 (91 to 123) | RR 1.49 (1.28 to 1.73) | 21,627 (adjusted n = 20,097) (30 studies, 33 comparisons) | ⊕⊕⊕⊕ HIGHa | For 1 included study extractable data were available but did not contribute anything to the analysis as no events (episodes of smoking cessation) occurred in either arm; we excluded a further two studies from the formal analysis, since no extractable data were available on programme participants at follow‐up. More recent studies were higher quality and routinely included longer‐term follow up beyond 6 months assessment |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aOverall we rate the quality of evidence as high, despite some of the included studies being considered at high risk of bias. This is because when analyses were restricted to only those studies at low risk of overall bias there was still a significant effect in favour of the intervention. Similarly, when we removed studies at high risk of bias from analyses, leaving only those at low and unclear risk of bias, there remained an effect estimate clearly in favour of the intervention. We are therefore very confident that the true effect lies close to that of the estimate of the effect.
Summary of findings 2. Incentives vs no incentives for smoking cessation in pregnant women at longest follow‐up.
| Smoking cessation: incentives compared to no incentives in pregnant women | ||||||
|
Patient or population: Pregnant women who smoke
Setting: Antenatal clinics
Intervention: Incentives for smoking cessation Comparison: No incentives | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with incentives: pregnancy | |||||
|
Smoking cessation in pregnancy at longest follow‐up Follow‐up: 10 to 24 weeks post‐partum |
72 per 1000 | 170 per 1000 (110 to 264) | RR 2.38 (1.54 to 3.69) | 2273 (9 RCTs) | ⊕⊕⊕⊕ MODERATEa | 1 included study did not contribute to the analysis because of lack of usable data |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aOverall we rate the quality of evidence as moderate, as we judged only two of the included studies to be at low risk of bias (one study at high risk; the rest at unclear risk). When we restricted analyses to only those studies at low risk of overall bias, there was still a significant effect in favour of the intervention, but this represents only two studies with fewer than 100 events overall.
Background
Description of the condition
Smoking is the leading cause of preventable death and disease worldwide. Most adults who smoke wish to quit, but quitting is challenging and despite the presence of effective evidence‐based cessation methods, quit rates remain low. Quitting smoking can lead to substantial health gains, even later in life. The earlier someone quits smoking, the more they reduce their risk of developing smoking‐related diseases (WHO 2018).
Description of the intervention
There is interest and support for incentive‐based programmes to change unhealthy behaviours, including smoking, weight loss, and alcohol consumption, and to increase levels of physical activity (Giles 2014; NICE 2010). However, financial incentives to promote behaviour change are controversial. Qualitative research demonstrates that public acceptability of incentives varies (Giles 2015), perhaps due to misinformation or a lack of education (Robertson 2018), and a concern about commissioning (funding of) incentive‐based schemes. There has also been a concern that incentive schemes may only be effective for the duration of time that incentives are offered. There may be cultural variation in acceptability, such that implementation of incentive‐based programmes may prove more difficult in some settings (Berlin 2018).
Many developing countries, particularly in Latin America, operate conditional national or regional cash transfer programmes of monetary rewards for behaviour change or compliance, often targeting improvements in child and maternal health (Lagarde 2009; Paes‐Sousa 2011; Powell‐Jackson 2011). In the UK, incentive schemes often focus on encouraging pregnant women to quit smoking, with well‐established programmes such as 'Give It Up For Baby' (Ballard 2009; Radley 2013), conducted in Tayside (Scotland) and awarding grocery vouchers for verified abstinence. A series of studies included in the last update of this review, conducted in the USA (Donatelle 2000a; Donatelle 2000b; Donatelle 2002; Heil 2008; Higgins 2004; Higgins 2014) and a large randomised trial in the UK (Tappin 2015a) also attest to the tobacco control community's interest in the feasibility of rewarding pregnant women who smoke for achieved abstinence.
How the intervention might work
Incentives and rewards (terms used interchangeably in studies contributing to this review) routinely feature in smoking cessation programmes. Theory suggests they might work according to behavioural processes of operant conditioning (positively rewarding the desired behaviour), or by providing short‐term gain for behaviour change that ultimately results in long‐term gain, but is perceived as less proximal to the individual (delay discounting) (Gneezy 2011; Miglin 2017). Incentives can be used to encourage recruitment into the programme, to reward compliance with the process, and to reward cessation achieved at predefined stages, usually contingent on production of a biochemically‐confirmed cessation outcome. A variety of rewards have been used for these purposes, including cash payments, vouchers exchangeable for goods (excluding alcohol and cigarettes) or leisure activities, salary bonuses, or promotional items such as T‐shirts, pens and bags.
Rewards can be given for attendance at the programme and at follow‐up appointments, irrespective of subsequent smoking status (i.e. guaranteed or non‐contingent), or can be paid and scaled relative to the participant's success in smoking cessation (i.e. contingent) (Higgins 2002). Recent trials and systematic reviews have explored variations in the type, the scale, and the scheduling of rewards (Adams 2014; Crossland 2015; Giles 2014; Jochelson 2007; Leeks 2010; Sigmon 2012b), and in their acceptability as a mechanism for behaviour change (Hoddinott 2014; Thomson 2014). This review focuses on rewards for abstinence (as opposed to attendance, etc.).
Why it is important to do this review
This updated review is a modified version of our previous review (Cahill 2015). Over the thirteen‐year lifetime of this review, the debate about incentive‐based smoking cessation programmes has shifted from their feasibility (i.e. can they work?) to their effectiveness (i.e. do they work?), relative success or limitations of the mechanisms deployed (Higgins 2012; Promberger 2012), the merits of rewards ('carrots') versus penalties ('sticks') (Adams 2014; Lynagh 2013; Volpp 2014), the extent to which achieved changes can be maintained (Jochelson 2007; Strickland 2014), the possibilities of unintended consequences (Marteau 2009; Thomson 2014), and the acceptability and implementation of incentive‐based programmes (Berlin 2018). Although many of the older included studies may not address these issues, our review contributes to a growing evidence base that defines the rationale for incentive‐based programmes and identifies areas for further investigation. In this update we also explore the use of incentives in sub‐populations of participants, consider the longevity of effects of incentives, and the cumulative value of incentives optimal for cessation outcomes.
Objectives
To determine the long‐term effect of incentives and contingency management programmes for smoking cessation. We address the following questions:
1. Do incentives reduce the prevalence of smoking at longest follow‐up?
2. What is the optimal amount and type of incentives that might be offered to impact on cessation outcomes?
3. What are the cost implications of incentives, to employers and to the community?
4. How great is the risk of disbenefits arising from the use of incentives, e.g. false claims, ineligible applicants?
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or cluster‐RCTs allocating individuals, communities, workplaces or groups within workplaces to intervention or to control conditions.
Types of participants
Adult smokers, of any gender, in any setting, including trials conducted in pregnant women who smoke. We have not included trials aimed exclusively at adolescents, as they are covered by a separate Cochrane Review (Fanshawe 2017).
Types of interventions
Incentive schemes to reward participants for validated cessation and abstinence in smoking cessation programmes. We have not included reports of the effectiveness of incentives or rewards to healthcare workers (physicians, nurses) for the delivery of smoking cessation interventions, or of reimbursement to participants for smoking cessation treatment costs, as these are covered in another Cochrane Review (Van den Brand 2017). We include in this review studies which offered entry into prize draws alongside other guaranteed incentives, but studies which offer only non‐guaranteed rewards (e.g. raffle only) are covered by a separate review of 'Competitions for smoking cessation' (Fanshawe 2019).
Control groups could be usual care or a smoking cessation intervention similar to that provided in the experimental group, but without incentives. Studies comparing two interventions providing incentives, but which varied by the amount or type of incentive, were also eligible.
Types of outcome measures
The primary outcome for this review is long‐term smoking cessation. This could be measured as point prevalence, sustained or continuous abstinence; however, where multiple measures were used in one study we took the most stringent measure. For trials in mixed populations abstinence had to be assessed at a minimum of six months from the start of the intervention. For trials in pregnant women, we extracted smoking cessation outcomes at the closest follow‐up to end of pregnancy, and also at longest follow‐up post‐partum if reported. We did not require the minimum six‐month follow‐up period for pregnant smokers because of the time‐limited nature of pregnancy. Abstinence could be self‐reported or biochemically validated, but we preferred biochemically validated over self‐reported rates.
We also looked at disbenefits and costs, where reported.
Search methods for identification of studies
We ran the most recent literature searches on 30th July 2018. For this update we searched the Cochrane Tobacco Addiction Group Specialised Register, using the search strategy in Appendix 1. The Specialised Register includes studies identified by systematic electronic searches of multiple databases, handsearching of specialist journals, and 'grey' literature, i.e. conference proceedings and unpublished reports not normally covered by most electronic indexing systems. At the time of the search the Register included the results of searches of the Cochrane Central Register of Controlled Trials (CENTRAL), issue 1, 2018; MEDLINE (via OVID) to update 20180726; Embase (via OVID) to week 201836; PsycINFO (via OVID) to update 201800820. See the Cochrane Tobacco Addiction Group website for full search strategies and a list of other resources searched.
We also conducted searches of the trial registers, clinicaltrials.gov, and the WHO International Clinical Trials Registry Platform (ICTRP). We checked reference lists of eligible papers, and consulted with experts in the field to identify any relevant forthcoming or unpublished research. We have contacted the authors of ongoing and included studies where necessary, and have recorded their co‐operation in the Acknowledgements section.
Data collection and analysis
Selection of studies
Two review authors (CN and SG) independently screened all search results (titles and abstracts) for possible inclusion, resolving any discrepancies through discussion. The same two review authors then independently assessed the full text of potentially relevant studies, again resolving discrepancies through discussion or through referral to a third review author (JHB). We noted reasons for the non‐inclusion of key studies, and report these in the Characteristics of excluded studies tables.
Data extraction and management
Two review authors independently extracted and summarised study data for each study, using a tailored data extraction form (CN, JHB, SG, CM). We resolved any discrepancies through discussion or referral to a third review author. Where available, we recorded the following information in the Characteristics of included studies table:
Methods: study design, study name (if applicable), study recruitment period, country, number of study centres, study setting, study recruitment procedure.
Participants: N (intervention/control), definition of smoker used, specific demographic characteristics (e.g. age, gender), mean cigarettes per day, mean Fagerström Test for Nicotine Dependence (FTND), inclusion criteria, and any relevant exclusion criteria.
Interventions: Description of intervention(s) (treatment, dosage, regimen, behavioural support, duration of intervention, monetary value of incentives), description of control (treatment, dosage, regimen, behavioural support); what comparisons were constructed between which groups, and any concomitant interventions received by intervention and control groups.
Outcomes: primary and secondary outcomes specified and collected, time points reported, biochemical validation, definitions of abstinence, adverse events, costs.
Notes: we recorded trial funding and declarations of interest of trial authors where reported.
Assessment of risk of bias in included studies
We evaluated each included study for risks of bias, using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Cochrane Handbook, Higgins 2017, Chapter 8). The domains examined for this review include:
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Biochemical validation of abstinence (detection bias)
Incomplete outcome data (attrition bias)
Other potential risks of bias
Two review authors independently rated each domain as being at low, unclear, or high risk of bias, with quotations from the study report and reasons to justify our judgements. We have summarised the consensus‐agreed 'Risk of bias' judgements across different studies for each of the domains listed, and display the summary results in a 'Risk of bias' figure. As blinding of participants is not feasible due to the nature of intervention, we do not assess performance bias, as in the standard methods of the Cochrane Tobacco Addiction Review Group.
Measures of treatment effect
We report results as risk ratios (RRs) with 95% confidence intervals (CIs), calculated as (number quit in intervention group/number randomised to intervention group)/(number quit in control group/number randomised to control group).
Unit of analysis issues
Several mixed‐population studies were cluster‐randomised, i.e. allocated by group, community, or workplace. We have used the intraclass correlation coefficient (ICC) reported by Martinson 1999 (unadjusted ICC for percentage quit smoking in a worksite) to obtain an adjusted estimate of the effect size for the studies that were cluster‐randomised and that contributed to our analyses.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and to obtain missing numerical outcome data where possible (e.g. when a study is reported as abstract only).
Where possible, we conducted an intention‐to‐treat analysis, including all smokers randomised. Where possible we have treated participants who dropped out or who were lost to follow‐up after randomisation as being continuing smokers. We note the proportion of participants for whom the outcome was imputed in this way, and whether there was either high or differential loss to follow‐up between the groups.
In trials of pregnant women, we have followed the convention observed in most of the trials, and not included in the denominator women whose pregnancies were uncompleted because of termination or foetal death.
Assessment of reporting biases
As there are a sufficient number of included studies (10 or more contributing to the outcome), we have created a funnel plot for the analysis in mixed‐population studies to assist in identifying possible publication bias, methodological flaws, or small‐study effects. We have searched for and report on studies we know to have been completed, but for which results are unavailable.
Data synthesis
For our primary outcome of smoking cessation, we have combined eligible studies using a Mantel‐Haenzel random‐effects model. We have combined studies carried out in mixed populations separately from those carried out in pregnant women. In both cases we include an analysis with smoking cessation at longest follow‐up as the outcome. For the pregnancy studies we also include an analysis with smoking cessation at end of pregnancy as an outcome.
We have not combined data on costs or disbenefits, as this information was sparsely and heterogeneously reported. Where reported, we summarise results narratively in the text.
Subgroup analysis and investigation of heterogeneity
We have used the I2 statistic to assess statistical heterogeneity, given by the formula ((Q ‐ df)/Q) x 100%, where Q is the Chi2 statistic and df is its degrees of freedom (Higgins 2003). This describes the percentage of the variability in effect estimates that is due to heterogeneity rather than to sampling error (chance). A value greater than 50% may be considered to indicate substantial heterogeneity.
Nine included studies involved participants who misused substances. We included this group in the mixed‐population analyses, but we also investigated them separately through subgroup analysis, new to this version of the review, as they have been shown to have different barriers and facilitators to smoking cessation from the general population (Gentry 2017).
We analysed nine pregnancy trials separately from the studies in mixed populations, due to different outcome data. These analyses did not require six‐month follow‐up and explored smoking cessation at longest follow‐up, and at least until the end of pregnancy.
For this update, we also ran an exploratory meta‐regression comparing incentive amount to effect estimate.
We conducted sensitivity analyses removing studies at high risk of bias and removing studies where incentives were provided at longest follow‐up.
'Summary of findings' tables
We have created 'Summary of findings' tables using the following outcomes:
Mixed‐population studies: smoking cessation at longest follow‐up (Table 1).
Pregnancy trials: smoking cessation at longest follow‐up (post‐partum where available) (Table 2).
We have used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence as it relates to the studies which contribute data to the prespecified outcomes. We have used methods and recommendations described in Chapter 11 of the Cochrane Handbook (Schünemann 2017), using GRADEpro software. We justify all decisions to down‐ or upgrade the quality of the evidence using footnotes, and have made comments to aid readers' understanding of the review where necessary.
Results
Description of studies
We included RCTs, allocating individuals, workplaces, groups within workplaces, or communities to experimental or control conditions. Included trials recruited from diverse populations, internationally, using a broad range of incentive interventions, from self‐incentives/deposits to modest or large‐value financial incentives.
Results of the search
For this update we screened the titles/abstracts of 279 studies, and 129 full texts. We included 19 new studies in this update, giving a total of 43 studies, across all populations. We excluded four studies included in the previous review update because they were not randomised (three mixed population studies: Paxton 1980; Paxton 1981; Paxton 1983, and one pregnancy study: Higgins 2004), and one because it did not evaluate guaranteed incentives (Crowley 1995, which is now covered in Fanshawe 2019). We identified 27 ongoing trials. The flow of studies for this update is recorded in Figure 1.
1.
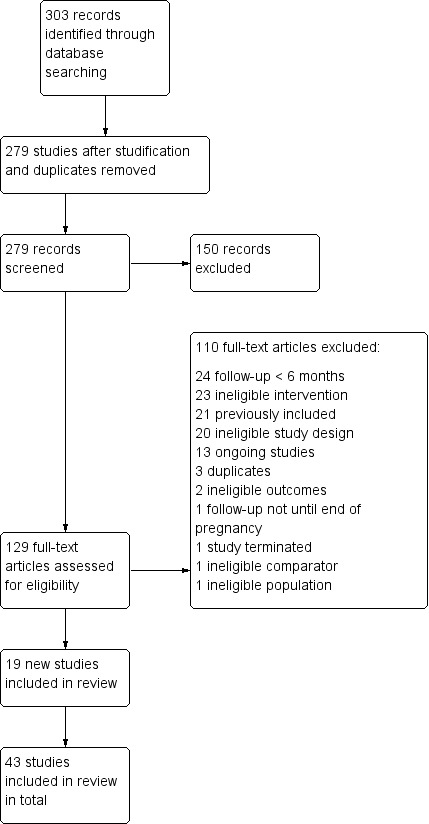
Study flow diagram for 2019 update
Included studies
Interventions in mixed populations
We retain 17 studies which had met our inclusion criteria and were included in the 2015 version of this review. The previous version of the review included non‐randomised studies, which we exclude for this update of the review (Paxton 1980;Paxton 1981;Paxton 1983). From our latest searches, we included 16 new trials recruiting mixed populations. These include four further community‐based studies (Cheung 2017; Etter 2016; Fraser 2017; White 2018), four trials recruiting from substance misusing populations (community or residential settings) (Ainscough 2017;Cooney 2017; Rohsenow 2015; Rohsenow 2017), three workplace‐based studies (Halpern 2018; Romanowich 2015; Van den Brand 2018), four recruiting via clinics (mental health, head and neck cancer or primary care) (Brunette 2017; Ghosh 2016; Lasser 2017; Rettig 2018), and one trial recruiting an online community population (Dallery 2016). We include White 2018, which was not published at the time of conducting our searches, because the authors kindly provided data. Romanowich 2015 was borderline include, as the trial initially recruited smokers willing to quit, but randomisation did not take place until a brief incentivised five‐day abstinence phase had been completed ('Early success' participants were randomised to the trial). As this may have skewed results, we excluded the study from our meta‐analysis in a sensitivity analysis, which did not substantially alter the findings, hence our decision to include the study despite the strict inclusion criteria of 'smokers' not being met. In total, we included 33 mixed‐population studies (21,627 participants) for this update. We identified 78 excluded studies (from all versions of the review), three ongoing studies in published protocols and 19 ongoing studies from trials registries.
Settings
Five studies were set in community settings (Cheung 2017; Etter 2016; Fraser 2017; Giné 2010; White 2013), with one additional study recruiting a community sample but delivering online‐only support (Dallery 2016). Six studies delivered smoking cessation support in clinics (mental health, head and neck cancer, or primary care) (Brunette 2017; Gallagher 2007; Ghosh 2016; Lasser 2017; Rettig 2018; Volpp 2006), and eight delivered interventions in substance misuse clinics, representing a large subgroup (Ainscough 2017; Alessi 2014; Cooney 2017; Drummond 2014; Rohsenow 2015; Rohsenow 2017; Secades‐Villa 2014; Shoptaw 2002). Type of substance misuse was mixed where specified. Three of the older studies delivered the intervention in an academic institution (Ledgerwood 2014; Tevyaw 2009; Windsor 1988), and the rest were delivered in worksites, including White 2018. Twenty‐four of the trials were run in the USA, two in Thailand (White 2013; White 2018), one in the Phillipines (Giné 2010) and one in Hong Kong (Cheung 2017). Five were European.
Incentives
Approximately half of studies (16 in total) offered cash for abstinence (contingent rewards), or monetary incentives in the form of vouchers (seven studies). Four studies used entry into a prize draw alongside a guaranteed reward (Cheung 2017; Glasgow 1993; Hennrikus 2002; Ledgerwood 2014). Two studies used self‐deposited money as the reward incentive (Dallery 2016; Giné 2010) and a further four studies used a combination of deposit arms with cash rewards or mixed‐rewards arms for abstinence at fixed time points (Halpern 2015; Halpern 2018; White 2013; White 2018). Seven studies included more complex payment schedules, especially with a 'reset' option, meaning that a non‐abstinent biochemically‐confirmed outcome at any time point would reset the escalating schedule of reinforcement to a lower level, thus reinforcing continued abstinence (Ainscough 2017; Cooney 2017; Drummond 2014; Rohsenow 2017; Secades‐Villa 2014; Shoptaw 2002; Tevyaw 2009).
Most of the studies (Ainscough 2017; Alessi 2014; Cooney 2017; De Paul 1994; Drummond 2014; Etter 2016; Gallagher 2007; Ghosh 2016; Giné 2010; Glasgow 1993; Hennrikus 2002; Lasser 2017; Rettig 2018; Secades‐Villa 2014; Shoptaw 2002; Van den Brand 2018; Volpp 2006; Windsor 1988) compared the incentive intervention arm to 'usual care', or to another intervention arm with different support options (non‐incentives). We combined these controls in our analyses. White 2013 and White 2018 examined different arms offering deposits and varying schedules of bonus payments (individual and team bonuses).
Brunette 2017 compared 'usual care' to quitline support or cognitive behavioural therapy (CBT). Approximately half within each experimental group received incentives. As exact numbers could not be calculated from reported results, we excluded this study from our analysis.
Nine studies (Dallery 2016; Fraser 2017; Ledgerwood 2014; Rand 1989; Rohsenow 2015; Rohsenow 2017; Romanowich 2015; Tevyaw 2009; Volpp 2009) compared non‐contingent incentives against contingent (outcome‐related) incentives.
Cheung 2017 compared 'usual care' with two incentive groups – those who were ‘early informed’ about the incentive intervention, and those who were ‘late informed', so were not initially aware they would receive rewards for abstinence.
Halpern 2015 compared 'usual care', including non‐contingent rewards, to individual rewards, as well as to collaborative awards (where rewards were given for peer/buddy abstinence in addition to individual abstinence) and to deposits and team deposits. Halpern 2018 compared 'usual care' and text message support to rewards and redeemable deposits.
Cessation methods
Only one trial did not deploy any kind of cessation support programme (Glasgow 1993). Most of the trials included self‐help support of brief advice at a minimum for the usual‐care control group. Eleven trials included nicotine replacement therapy or pharmacotherapy to support their participants (Ainscough 2017; Brunette 2017; Cooney 2017; Gallagher 2007; Halpern 2015; Halpern 2018; Rohsenow 2015; Rohsenow 2017; Romanowich 2015Shoptaw 2002; Volpp 2006). The most recent published trial also offered an electronic cigarette option to some participants as part of the smoking cessation intervention (Halpern 2018).
Most of the included studies used some form of multicomponent support programme, by combining, for example, self‐help and brief advice, with pharmacotherapy. Dallery 2016 and Etter 2016 offered online support, and Halpern 2018 used motivational text messages to offer digital support to trial participants. De Paul 1994 combined self‐help with a buddy system. Drummond 2014 provided motivational feedback on 'lung age' to promote cessation. Van den Brand 2018, White 2013, and White 2018, which were workplace or community‐based studies, used group intervention including group‐based 'pledges' for abstinence or peer pairing, thus employing peer pressure/motivation as part of the intervention. However, White 2018 reported that the size of the worksites did not lend itself to the strategy for pairing teammates. Many teammates did not know each other, and did not interact during the study period.
Outcomes
All the included studies rewarded smoking cessation, either alone or in combination with recruitment, participation or both (see the Characteristics of included studies table for full details).
As reported in the previous review update, raw outcome data, particularly in the older studies, were often difficult to extract. For this update we found the new included trials to be more clearly reported. Fifteen trials followed up participants for a maximum of six months (Ainscough 2017; Alessi 2014; Cheung 2017; Cooney 2017; Dallery 2016; Drummond 2014; Fraser 2017; Ghosh 2016; Ledgerwood 2014; Rand 1989; Romanowich 2015; Secades‐Villa 2014; Tevyaw 2009; Volpp 2006; White 2013), one for nine months (Gallagher 2007), 12 for 12 months (Brunette 2017; Giné 2010; Halpern 2015; Halpern 2018; Lasser 2017; Rettig 2018; Rohsenow 2015; Rohsenow 2017; Shoptaw 2002; Van den Brand 2018; Windsor 1988; White 2018), two for 18 months (Etter 2016; Volpp 2009), and three for 24 months (De Paul 1994; Glasgow 1993; Hennrikus 2002). Most of the more recent studies included 12‐month follow‐up as the standard primary outcome time point.
Few studies formally reported on harms or costs; where reported, we present them narratively below.
Interventions in pregnancy
We include trials conducted in pregnant women as a separate group. We retain eight of the nine studies included in the last update (Cahill 2015), with Higgins 2004 now excluded as it was not randomised. We identified 10 excluded studies (from all versions of the review), two ongoing studies in published protocols and three ongoing studies from trials registries. IN our updated searches we found two new completed pregnancy trials that met our inclusion criteria (Baker 2018; Harris 2015). Baker 2018 is the largest pregnancy trial of incentives for cessation in pregnancy yet reported, recruiting 1014 US pregnant women, and so considerably contributes to the growing evidence base. We include a total of 10 trials recruiting pregnant smokers (2273 women) in this update.
Settings
Nine studies were conducted in the USA, mostly in public or private antenatal clinics, obstetric practices, and community antenatal programmes. One trial (Tuten 2012) in methadone‐maintained pregnant women, was conducted in the Center for Addiction and Pregnancy in Baltimore. The only included UK‐based study (Tappin 2015a; the Cessation in Pregnancy Incentives Trial) with 612 participants, was mediated through the pregnancy referral pathway to the UK NHS stop‐smoking service.
Incentives
The largest pregnancy trial (Baker 2018) provided cash payments as the incentive. In all other cases the rewards were vouchers for goods or services. Three trials (Donatelle 2000a; Donatelle 2000b; Donatelle 2002) delivered monthly rewards contingent upon proven abstinence. Four trials evaluated the allocation of incremental rewards, with the voucher reset to baseline value in the case of relapse or missed visits, but restored to previous levels if abstinence was re‐established (Harris 2015; Heil 2008; Higgins 2014; Tuten 2012). Ondersma 2012, using a computer‐based intervention, shifted the onus of testing to the participants, who could present themselves as often as they wished for verification of abstinence, and could win up to five USD 50 gift cards over the course of the programme. Harris 2015 also offered the option of web‐based confirmation of biochemical validation of abstinence. Tappin 2015a awarded vouchers up to a value of GBP 350 (pounds sterling) for achieving staged cessation targets, and a further GBP 50 for engaging with the programme and setting a quit date. Donatelle 2000a also rewarded a social supporter, in tandem with the participant smoker. Non‐contingent rewards, roughly equivalent to the value available to the intervention group, were given to control participants in three trials (Baker 2018; Heil 2008; Higgins 2014), while Tuten 2012 incorporated a group on a schedule of non‐contingent rewards generated from an earlier pilot study. Donatelle 2000a gave a USD 5 voucher to all participants for each of three attendances during the trial. Tappin 2015a gave all participants in both arms of the trial a GBP 25 shopping voucher for supplying primary outcome information (34 to 38 weeks gestation) and a biological sample for those who self‐reported as quitters.
Cessation methods
All the trials offered a programme of practical cessation support, in addition to the routine care delivered by the host clinics. Three trials (Donatelle 2000b; Donatelle 2002; Ondersma 2012) used the 5As approach (Ask, Advise, Assess, Assist, Arange), while five trials offered self‐help materials. Tuten 2012 also included a brief motivational interviewing feedback session for all participants. Harris 2015 offered a web‐based smoking cessation programme in addition to telephone support. The UK trial (Tappin 2015a) referred all participants to UK stop smoking services, that routinely conducted a one‐hour cessation session, four weekly phone calls, and provided free NRT if the women chose to use it.
Outcomes
All the included studies reported abstinence at the end of pregnancy, with seven of the 10 tracking participants into the post‐partum stage. Two trials (Donatelle 2000b; Donatelle 2002) referred simply to "abstinence", without further definition of the type or duration. In all cases, rewards were available only for biochemically‐verified abstinence. Two trials rewarded smoking reduction as well as complete abstinence, with Tuten 2012 setting percentage reduction targets to be met for rewards, while Higgins 2014 allocated higher‐value vouchers for breath samples below 4 ppm rather than 6 ppm in the early stages of the trial. Our primary outcome of interest for this group is abstinence at the longest available assessment point (which allows us to be the most inclusive in terms of studies included in the analysis); we also report abstinence rates at or around the end of pregnancy for all the trials which had these data.
Few studies formally reported on harms or costs; where reported, we describe these below.
Excluded studies
We list 90 excluded studies in the Characteristics of excluded studies table. The main reasons for exclusion were ineligible study design, not meeting our definition of the intervention, or not following up participants for at least six months.
Risk of bias in included studies
Overall, we judged eight studies to be at low risk of bias (low risk of bias across all domains) and 10 studies to be at high risk of bias (high risk of bias in at least one domain), with the remaining studies at unclear risk of bias. Assessments of the risk of bias domains for each study are shown in Figure 2.
2.
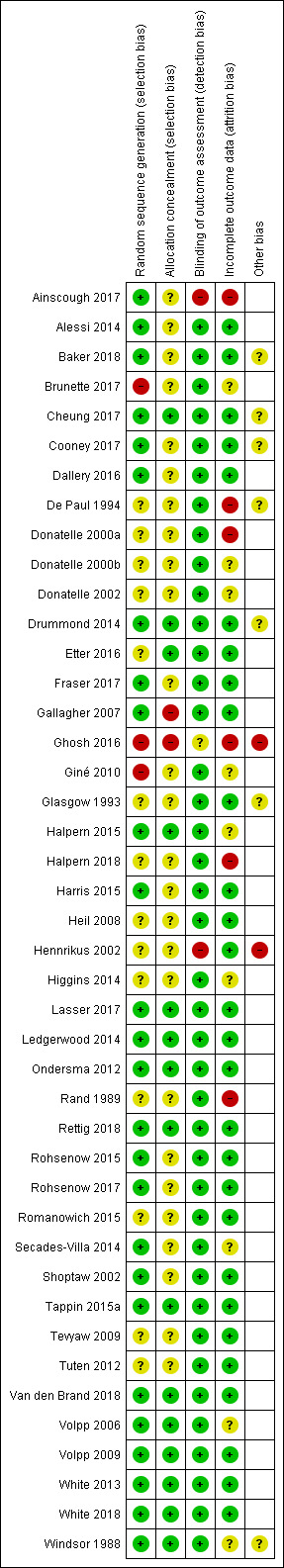
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Mixed‐populations studies
In the mixed‐population studies, we judged eight studies to be at low risk of bias and seven to be at high risk of bias, with the remaining 18 at unclear risk of bias.
Selection bias
Of the 33 mixed‐population studies, we judged 13 to be at low risk of selection bias (low risk of both random sequence generation and allocation concealment). We judged four to be at high risk of selection bias, due to issues with either random sequence generation, allocation concealment, or both (Brunette 2017; Gallagher 2007; Ghosh 2016; Giné 2010). We judged the remainder to be at unclear risk of selection bias, due to insufficient information on which to judge.
Detection bias
Of the mixed‐population studies we rated 30 at low risk of detection bias, because biochemical measures were used to verify abstinence. Because of the explicit mechanism of rewards, most of the reported trials did not attempt to blind participants, trialists or assessors. In Ainscough 2017, major study problems were encountered and no participants were followed up at the primary endpoint. Hennrikus 2002 did not validate the abstinence of all participants claiming abstinence. We judged these two trials to be at high risk of bias for this domain. We judged Ghosh 2016 to be at unclear risk, as limited detail was provided on the method of validation used.
Incomplete outcome data
In our analysis of all of the included studies, we treated programme dropouts and losses to follow‐up as continuing smokers, whether or not the trial reported results in this way, and conducted the analyses on an intention‐to‐treat basis, i.e. the denominator included all persons randomised at the start of the trial in their original groups. Of the mixed‐population studies, we considered 22 to be at low risk of bias for this domain.
In accordance with standard Cochrane Tobacco Addiction Group methods for assessing attrition bias, we rated five studies at high risk of bias in this domain, due to high or differential rates of dropout (Ainscough 2017; De Paul 1994; Ghosh 2016; Halpern 2018; Rand 1989), and six as unclear, as there were not sufficient details available on which to make a judgement for this domain (Brunette 2017; Giné 2010; Halpern 2015; Secades‐Villa 2014; Volpp 2006; Windsor 1988).
Other risk of bias
We judged two studies to be at high risk of other biases. We rated Ghosh 2016 at high risk of bias due to inconsistent reporting of length of follow‐up, and Hennrikus 2002 at high risk of bias as group dropouts were not followed up.
In order to test the robustness of the cessation interventions we have included in our review only those studies which followed up participants for at least six months from the beginning of the intervention. Six of the trials, however, (Drummond 2014; Fraser 2017; Gallagher 2007; Ghosh 2016; Lasser 2017; Van den Brand 2018) delivered their final cessation rewards at the same time point as the end of the designated follow‐up period, thereby potentially confounding the intervention rewards with testing at the longest follow‐up. A sensitivity analysis considering these trials separately made no relevant difference to the overall combined outcomes.
Pregnancy studies
In the pregnancy studies, we judged two studies to be at low risk of bias, one at high risk of bias, and seven at unclear risk of bias.
Selection bias
Of the included pregnancy studies, we judged two to be at low risk of selection bias (low risk for both random sequence generation and allocation concealment). We judged the remainder to be at unclear risk due to insufficient detail reported.
Detection bias
We judged all of the studies of pregnant women to be at low risk of detection bias, because each study used biochemical validation of abstinence.
Incomplete outcome data
Of the included pregnancy studies, we rated six at low risk of bias for incomplete outcome data. Two were unclear due to insufficient detail. We ranked Donatelle 2000a at high risk of attrition bias, as it had lost 36% of the intervention group by two months post‐partum, and 52% of the control group, although the authors report that this level of depletion was not unusual for the antenatal clinic in question.
Although we routinely prefer to conduct an intention‐to‐treat analysis (including all participants randomised), for these trials we have excluded from the denominators any predefined withdrawals due to termination or foetal demise, where these were reported. Tappin 2015a excluded three control participants from the denominator, as they had withdrawn immediately after randomisation and had withheld their data from inclusion in analyses; we have adjusted our calculations accordingly.
Other risk of bias
We found no other risks of bias in the included pregnancy studies.
Effects of interventions
Mixed populations
Cessation
Details of the results for the 33 mixed‐population included studies in this review are tabulated in Table 3, and are displayed graphically where data were available in Analysis 1.1. In our analyses results for the two intervention arms (early‐ and late‐informed incentives) of Cheung 2017 are collapsed and compared with the control arm. Results of two incentive groups in Halpern 2018 are collapsed and compared to a control group. Results of incentives arms in Romanowich 2015 are also collapsed and compared to a control group.
1. Results of included studies: mixed‐populations.
| Study ID | Denominator | Abstinence | Time point | Biological criterion | Quit rate | Stat sig? | Other outcomes | Comment |
| Ainscough 2017 | 19 (IG) 18 (CG) |
PPA | 6 months | CO < 10 ppm | 1 (IG) | N.S. | N of participants completing the smoking cessation intervention; opioid treatment outcomes (opioid treatment adherence, drug types, treatment schedule); illicit drug use. | Only 1 participant followed up, not CO verified |
| Alessi 2014 | 24 (CM) 21 (control) |
7‐day PPA | 24 weeks | CO < 6 ppm cotinine < 30 ng/ml |
12.5% (I) 23.8% (C) |
N.S. | % reduction in cpd; self‐efficacy | Raw data supplied by the author |
| Brunette 2017 | "approximately half of 146" (CG) "approximately half of 146" (PV+incentives) "approximately half of 303" (PV+Q) "approximately half of 303" (PV+Q+incentives) "approximately half of 212" (PV+CBT) "approximately half of 212" (PV+CBT+incentives) |
PPA | 12 months | CO < 4 ppm; cotinine 100 ng/mL | 8% (PV) 6% (PV + incentives) 3.5% (PV+Q) 14% (PV+Q+incentives) 5% (PV+CBT) 12.5% (PV+CBT+incentives) |
N.S. | Treatment programme participation, medications | ‐ |
| Cheung 2017 | 379 (early infomed) 385 (Late informed) 379 (Control) |
PPA | 6 months | CO < 4 ppm cotinine < 10 ng/ml |
19 (5%) EI 11 (2.9%) LI 17 (4.5%) CG |
N.S. | Quit attempts (longest duration and number of quit attempts , mean number of quit attempts, no smoking for at least 24 hours); cessation aids | ‐ |
| Cooney 2017 | 42 CM 41 CG |
PPA | 6 months | CO ≤ 5 ppm | 5 (12%) CM 2 (5%) CG |
P = 0.004 | Smoking at 1.5 weeks after quit date, 1 month. Alcohol use, drug use | ‐ |
| Dallery 2016 | 48 AC 46 SC (CG) |
PPA | 6 months | CO ≤ 4 ppm | 11 (22.9%) AC 6 (13%) SC |
N.S. | PP at week 4 and 3‐month follow‐up. Treatment acceptability, behavioural change | CO results were video recorded and submitted remotely |
| De Paul 1994 | 281 (I) 280 (SH) | PPA | 24 months | CO < 9 ppm | 13.2% (I) 10.3 %(SH) | N.S. | PP, ITT and continuous quit rates reported at all time points | Comparison confined to I and SH groups in this review. Cluster‐randomised so adjusted in main analyses; unadjusted data presented here |
| Drummond 2014 | 50 (UC/LA) 50 (CM x 2) |
7‐day PPA | 6 months | cotinine, eCO | UC/LA 1/50 CM 3/50 |
N.S. | CO values, Fagerström score, N of visits wanting to quit, trying to quit, reporting cessation, eCO‐confirmed quitting | Groupings collapsed, as lung age alone or combined with CM produced no quitters |
| Etter 2016 | 401 (IG) 404 (CG) |
Continuouse abstinence from months 6 ‐ 18 verified by PPA | 18 months | CO to 3 ppm; cotinine < 10 ng/ml | 39 (9.7%) IG 19 (4.7) CG |
P = 0.001 | Quit attempts during the intervention phase (number, duration and dates) Cigarette consumption, motivation to quit, confidence in ability to quit Use of the online smoking cessation programme | ‐ |
| Fraser 2017 | 948 (IG) 952 (CG) |
PPA | 6 months | CO ≥ 7 ppm contine |
205 (21.62%) IG 131 (13.76%) CG |
P < 0.001 | Treatment engagement, medications | Continine testing: value that exceeded either 50 ng/mL, 100 ng/mL, or 200 ng/mL, depending on the clinic. 4 clinics used 300 ng/mL as the smoking cut‐score |
| Gallagher 2007 | 60 (CR) 60 (CR+NRT) 60 (Cont) | PPA | 36 weeks | CO ≤ 10 ppm SCN < 15 ng/mL | 7% (CR) 5% (Cont) (based on SCN) | N.S. | CO‐validated rates higher, i.e. 37% (CR), 8% (Cont). Reduction, psychiatric symptoms | CR+NRT group not used in our comparison |
| Ghosh 2016 | 6 (IG) 8 (CG) |
PPA | 6 months | Not defined | 2 (IG) 0 (CG) |
N.S. | Quality of life (SF12) | 6‐month follow‐up but methods state 12 months. Attempted to contact author to clarify but no reply |
| Giné 2010 | 781 (CARES) 603 (Cards) 616 (Control) |
PPA | 12 months | NicCheck strip (urinary cotinine) = 0 | 11% (CARES) 9.3% (Cards) 8.9% (Cont) |
P = 0.05 | 6‐month PPA: CARES 9.7%, Cards 10%, Control 8.3%. Cost effectiveness: USD 700 per quitter |
12‐month assessment was 'sprung' on participants |
| Glasgow 1993 | 344 (I) 426 (C) | 7‐day abstinence | 2 years | CO ≤ 9 ppm Cotinine ≤ 25 ng/mL | 14.2% (I) 11.5% (C) | N.S. | Incentives had a sig. effect (P < 0.03) on less educated participants (18.6% vs 8.8% at 2 years 'probably chance'). Compared participants with non‐participants (22.1% vs 9.4% at 1 year, P < 0.005; 21.3% vs 16.8% at 2 years, N.S.) | 27% of all abstinent claims could not be biochemically verified. Cluster‐randomised so adjusted in main analyses; unadjusted data presented here |
| Halpern 2015 | 498 (Ind R) 519 (Coll R) 582 (Ind D) 471 (Com D) 468 (UC) |
sustained | 12 months | Cotinine < 10 ng/ml anabasine/anabitine < 3 ng/ml |
7.4% (Ind R) 8.7% (Coll R) 3.6% (Ind D) 6.2% (Com D) 3.4% (UC) |
vs UC: 0.007 0.001 0.94 0.052 |
Sustained verified abstinence @ 14 days, 30 days, 6m; Self‐reported abstinence at 12m; per protocol analyses; Uptake rates of assigned intervention |
No differences between individual and group interventions, so both reward arms versus both deposit arms combined for analysis |
| Halpern 2018 | 1198 (rewards) 1208 (redeemable) 1599 (Control) |
PPA | 12 months | Cotinine < 20 ng per milliliter, anabasine level of less than 3 ng per milliliter or CO less than 4%. | 13 (rewards) 16 (redeemable) 5 (control) |
Deposits: P ≥ 0.001 Rewards P ≥ 0.006 |
Point prevalence for quitting at 1 month and sustained abstinence rates at 3 months and 6 months | ‐ |
| Hennrikus 2002 | 407 | 7‐day PPA | 24 months | Saliva from 149 random sample of quitters at 24 months | 19.4% (cohort survey) | Not stated | Cohort prevalence and cessation rates (PP and continuous) Recruitment rate Programme format | Programme registrants' outcomes not available. Unadjusted data presented here |
| Lasser 2017 | 177 IG 175 CG |
Continuous verified at 6 and 12 months | 12 months | Saliva or urine cotinine (≤ 10 ng/ml) or anabasine < 3 ng/mL) | 21 (12%) IG 4 (2%) |
P ≤ 0.001 | Receipt of counselling, medications | ‐ |
| Ledgerwood 2014 | ECM: 36 TCM: 28 SC (Control): 17 |
PPA | 6 months | Urinary cotinine ≤ 100 ng/mL CO ≤ 6 ppm |
4/64 (TCM+ECM) 1/17 |
N.S. | Prize money won; 81% CM participants earned prizes (median USD 120.56); Differences between TCM and ECM in week 1 non‐significant | Both CM arms combined for analysis |
| Rand 1989 | 17 contingent 16 non‐cont 14 control | Continuous | 6 months | CO ≤ 11 ppm | 1/17 contingent 1/16 non‐contingent 0/14 control | N.S. | Numbers of abstinent CO samples and missed samples | Pairwise comparisons gave sig diffs at 11 ppm, but not at 8 ppm |
| Rettig 2018 | 8 (CG) 13 (IG) |
PPA | 12 months | 8 ppm | 0 (CG) 4 (31%) (!G) |
P = 0.05 | Smoking abstinence at 1, 2, 3,4,5,6,7 and 8 weeks, and at 3 and 6 months. Smoking intensity (total cigarettes per previous 7 days), the reduction from baseline, and total cigarettes smoked | ‐ |
| Rohsenow 2015 | 44 Control (BA/CV) 42 Control (BA/NCV) 53 Intervention (MI/CV) 44 Intervention (MI/NCV) |
PPA | 12 months | CO ≤ 4 ppm and salivary cotinine ≤ 15 ng/ml | 0 Control (BA/CV) 2 Control (BA/NCV) (4.8) 4 (7.5) Intervention (MI/CV) 2 (4.5) Intervention (MI/NCV) |
N.S. | Cigarette reduction (CPD), number of heavy drinking days, number of drug use days, relapse to any heavy drinking or drug use over the 12 months | ‐ |
| Rohsenow 2017 | 166 CG (NV) 163 IG (CV) |
PPA | 12 months | CO l ≤ 4 ppm and salivary cotinine ≤ 15 ng/ml | 3 (1.8%) CG (NV) 6 (3.7%) IG (CV) |
N.S. | CPD at 1, 3, 6 months. Number of heavy drinking days Smoking Self‐Efficacy Questionnaire pretreatment and at 1 month. |
‐ |
| Romanowich 2015 | 32 HTT percentile criterion 27 HTT fixed criterion 14 HTT random payments 44 ES escalating payments 43 ES fixed payments 23 ES random payments |
Continuous | 6 months | CO < 4 ppm. Cotinine < 20 ng/ml | 3 (8.3%) HTT percentile criterion 2 (5.0%) HTT fixed criterion 1 (5.6%) HTT random payments 4 (6.8%) ES escalating payments 6 (10.3%) ES fixed payments 5 (17.2%) ES random payments |
‐ | Use of smoking cessation medication. CPD in past 6 weeks at 6 months |
Results confirmed by authors by email. CO < 3 ppm Stated in NCT entry but < 4 ppm stated in email correspondence. HTT are participants who did not deliver a breath CO level < 4 ppm during the first 5 study days when they could earn USD 5 for doing so and were randomised to 1 set of conditions. ES did deliver at least 1 CO sample < 4, and were randomised to another set of conditions |
| Secades‐Villa 2014 | 43 CBT + CM 49 CBT |
Continuous | 6 months | CO < 4 ppm; Cotinine < 80 ng/ml | 17/43 CM 13/49 CBT |
N.S. | Treatment retention; % attending all sessions for 6 months | ‐ |
| Shoptaw 2002 | 42 (P) 42 (RP) 43 (P+CM) 47 (P+RP+CM) | PPA | 12 months | CO ≤ 8 ppm Cotinine < 30 ng/mL | 4/36 (P) 2/33 (P+RP) 2/35 (P+CM) 1/38 (P+RP+CM) | N.S. | Treatment group and cocaine and opiate abuse | Quit rates supplied by authors. P group relapsed more slowly than other groups (P = 0.0017) |
| Tevyaw 2009 | 28 (CM+MET) 27 (CM+REL) 27 (NR+MET) 28 (NR+REL) | 7‐day PPA | 6 months | CO < 5 ppm Cotinine < 15 ng/mL | 1/55 (CM) 3/55 (NR) | N.S. | Attendance, sample returns. | ‐ |
| Tuten 2012 | ||||||||
| Van den Brand 2018 | 319 (IG) 284 (CG) |
Continuous | 12 months | CO 9 ppm | 131 (41%) IG 75 (26%) CG |
P < 0.001 | 3‐ and 6‐month biochemically validated abstinence, and self‐reported abstinence | Cluster‐randomised so adjusted in main analyses; unadjusted data presented here |
| Volpp 2006 | 92 (I) 87 (C) | 7‐day PPA | 6 months post‐completion (˜7.5 months) post‐quit date | Urinary cotinine < 500 ng/mL | 6/92 (I) 4/87 (C) | N.S. | Enrolment attendance programme completion | Denominators could be Ns enrolled (I:38, C:17). No quitters outside the enrollers |
| Volpp 2009 | 436 (I) 442 (C) | Prolonged | 15 or 18 months | Salivary cotinine < 15 ng/ml or urinary cotinine < 2 ng/ml | 41/436 (I) 16/442 (C) |
P < 0.001 | Enrolment in SC course, completion of SC course | 15 to 18 months results shown in 12‐month forest plot |
| White 2013 | 131 (I) 69 (C) |
7‐day PPA | 6 months | Urinary cotinine | 58/131 (I) 13/69 (C) |
P < 0.001 | PPA at 3 months (verified), 14 months (self‐report). Relative success of teams vs individuals? Yes Choosing team partner vs random assignment? No Did text messages help? No Cost effectiveness; No figures given |
‐ |
| White 2018 | 508 (USD 20 individual bonus) 481 (USD 40 individual bonus) 491 (team bonus) 396 (deposits) 363 (deposits plus teammate (no bonus)) 514 (deposits plus $20 individual bonus) 489 (deposits plus USD 40 individual bonus) 496 (deposits plus team bonus) 444 (CG) |
7‐day PPA | 12 months | Cotinine cut‐off level of 200 ng/mL | 74 (14.6%) (USD 20 individual bonus) 104 (21.6%) (USD 40 individual bonus) 60 (12.2%) (team bonus) 57 (14.4%) (deposits) 49 (13.5%) (deposits plus teammate (no bonus)) 72 (14%) (deposits plus USD 20 individual bonus) 91 (18.6%) (deposits plus USD 40 individual bonus) 67 (13.5%) (deposits plus team bonus) 42 (9.5%) (CG) |
Significantly higher for
USD 40 bonus programmes than programmes with no bonus P = 0.01 all other comparisons NS |
PPA at 3 and 6 months. Programme acceptance. | Cluster‐randomised so adjusted in main analyses; unadjusted data presented here |
| Windsor 1988 | 95 (A) 94 (B) 95 (C) 94 (D) | Continuous | 12 months | SCN ≤ 100 ng/mL | ≃ 6% (A) ≃ 18% (B) ≃ 5% (C) ≃ 10% (D) | Not reported | Social enhancement vs self‐help manual (± incentives) gave a continuous quit rate of 14.4% at 12 months, vs 5.8% | Incentives comparison was abandoned at 6 weeks |
1.1. Analysis.
Comparison 1 Incentives in mixed populations, Outcome 1 Smoking cessation (subgrouped by when incentives were provided).
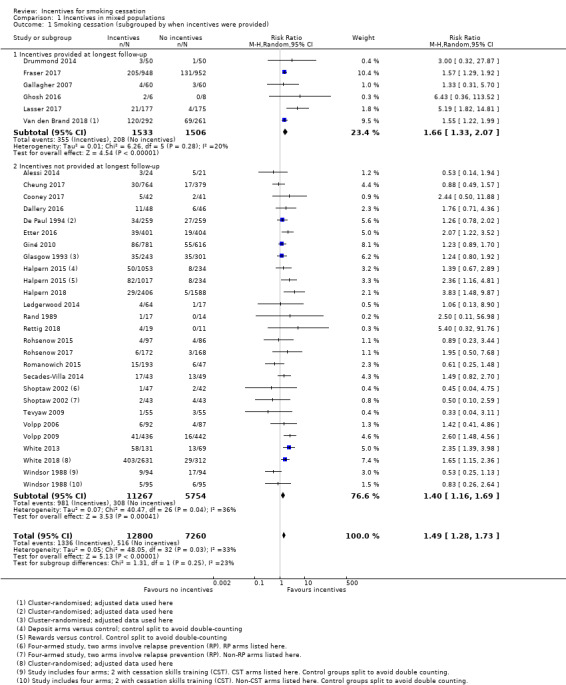
We conducted a meta‐analysis of 30 of the included studies for which there were sufficient data (33 comparisons) (Analysis 1.1). We excluded Ainscough 2017, Brunette 2017,and Hennrikus 2002 from formal analyses because no extractable data were available on programme participants at follow‐up.The primary result at longest follow‐up (six months or more) gave an RR for quitting with incentives compared with controls of 1.49 (95% CI 1.28 to 1.73; 30 RCTs (33 comparisons), adjusted N = 20,097, I2 = 33%). We also present this analysis in Table 1, with a grading of the certainty of the evidence.
To explore the effect of incentives offered up until the long‐term follow‐up point (six months or more) compared to those where longest follow‐up occurred after the incentive schedule had ended, we carried out a subgroup analysis. There was no significant difference in the results found between groups (P = 0.25, I2 = 24%, Analysis 1.1). Restricting results to only those studies which followed up beyond the provision of incentives yielded a statistically and clinically significant effect in favour of the intervention (RR 1.40, 95% CI 1.17 to 1.69; 28 RCTs; adjusted N = 17,058; I2 = 36%), suggesting that the impact of incentives continues for at least some time after incentives are no longer provided. In the group of studies where incentives were provided at longest follow‐up, the result was similar (RR 1.66, 95% CI 1.33 to 2.07; 6 RCTs; adjusted N = 3039; I2 = 20%).
In a subgroup analysis of trials recruiting participants in substance misuse treatment, results also suggested a favourable benefit of incentives for smoking cessation at longest follow‐up (no significant subgroup difference (P = 0.38; I2 = 0%; RR in substance abuse subgroup 1.24, 95% CI 0.81 to 1.89; 8 studies; N = 1055; I2 = 0%; Analysis 1.2.1). Although confidence intervals are wide, this reflects the smaller number of studies and participants in this group; the point estimate was consistent with the overall meta‐analysis which found a beneficial effect of the intervention.
1.2. Analysis.
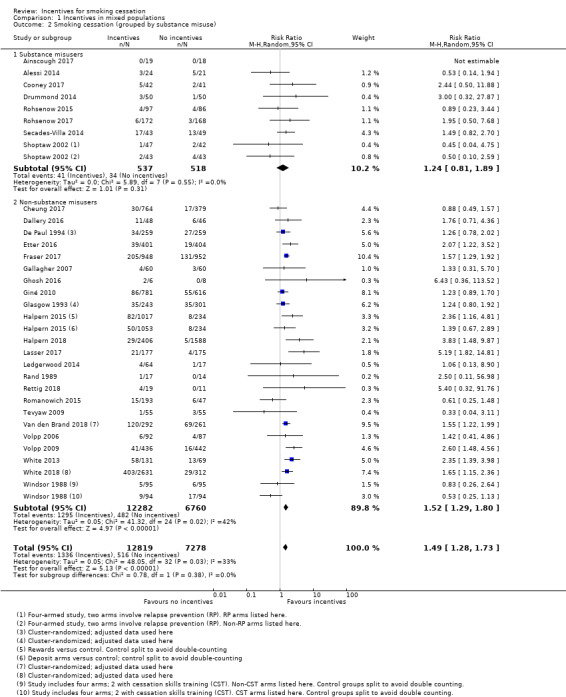
Comparison 1 Incentives in mixed populations, Outcome 2 Smoking cessation (grouped by substance misuse).
Although not always clearly reported, the financial amounts of incentives varied between trials, from zero (self‐deposits), to a range of between USD 45 up to USD 1185. There was no clear direction of effect between trials offering low or high total amounts of incentives, nor those encouraging redeemable self‐deposits. We ran an exploratory meta‐regression and found no significant association between the outcome and the total value of financial incentive (P = 0.180, Figure 3). Any such indirect comparison is particularly crude in this context, due to differences in the cultural significance of financial amounts (e.g. USD 50 might have different significance in different contexts).
3.
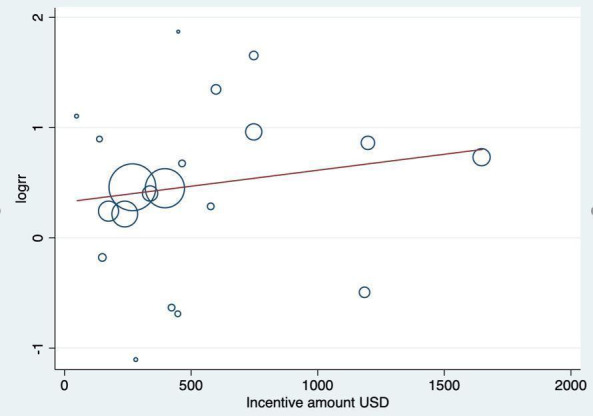
Exploratory meta‐regression testing association between incentive amount and effect estimate
Cheung 2017, a large trial recruiting from a community sample in Hong King as part of the 'Quit to win' contests, specifically examined the effect of small‐value financial incentives. They tested incentives where participants were 'early informed' against a group of participants who were 'late informed' about the incentive offer. There was no statistically significant difference in biochemically‐validated abstinence rates between the early‐informed and late‐informed groups. Overall there was a beneficial effect of the small financial incentive offer across both intervention groups when compared to a control group who were not offered an incentive.
The two largest trials included in this review update specifically evaluated financial incentives against deposit‐based incentives. Halpern 2018 found both deposits and incentives to be effective for long‐term smoking cessation, but no significant differences between the two forms of incentivisation (2% in the rewards group (95% CI 1.2 to 2.8) versus 2.9% in the redeemable deposit group (95% CI 2.0 to 3.8)) and a very high loss to follow‐up. Similarly, White 2018 found that both were effective and reported that "Deposit programs had a negligible effect on abstinence compared with no‐deposit programs" (reporting a 1.1 point increase, P = 0.53).
By far the largest trial among the included studies is White 2018, a nine‐arm cluster‐RCT recruiting 4190 participants drawn from employees at large workplaces in the Bangkok metropolitan area (101 worksites from 84 Bangkok area companies). The interventions were individual bonuses, team bonuses, self‐deposits and deposits plus bonuses (individual and team). The total incentive available varied by arm, and was equivalent to USD 20 (TBH 600 (Thai baht)) in arms that offered a smaller bonus (including the arm that combined a smaller bonus with deposits), and USD 40 (THB 1200) in the arms that offered a larger bonus (including the arm that combined a larger bonus with deposits and the arms with a team bonus). Bearing in mind that Thailand is a middle‐income country, the amounts given were relatively small compared to some of the other studies. Incentives were provided up until the end of the three‐month intervention period. All incentive arms did significantly better than the usual‐care control group at the study's 12‐month primary endpoint of validated sustained abstinence. As there were no significant differences between the individual‐ and group‐based arms, and no significant differences between deposit‐ or reward‐based arms, we combined the eight intervention arms into two groups (incentives versus control) for our analyses.
We conducted a sensitivity analysis removing studies at high risk of bias from the overall meta‐analysis for mixed populations. This resulted in an RR of 1.48 (95% CI 1.25 to 1.76; 25 RCTs; adjusted N = 13,986; I2 = 37%), which still clearly favours incentives. Removing both those studies at high and at unclear risk also yielded a statistically significant benefit in favour of the intervention (RR 1.97, 95% CI 1.57 to 2.57; 8 studies; adjusted N = 5037; I2 = 34%).
We constructed an exploratory funnel plot for the main meta‐analysis (Figure 4; Analysis 1.1, abstinence at longest follow‐up), but did not detect indications of publication bias, i.e. that small studies with negative findings might be under‐represented.
4.
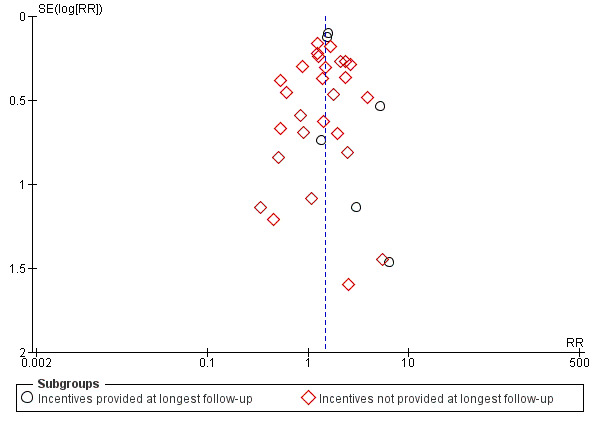
Funnel plot of comparison: 1 Incentives in mixed populations, outcome: 1.1 Smoking cessation (subgrouped by when incentives were provided).
Costs
Few studies reported on costs. Among all participants in Halpern 2015 achieving sustained abstinence at six months, the average cost per quitter ranged from USD 800 to USD 890. The trialists compared this outlay, even without any deposit contribution from the participant, to the estimated USD 5816 additional cost to employers of hiring a smoker rather than a non‐smoker in the USA (Berman 2014), and rated the intervention highly cost‐effective. Volpp 2009 offered no comment on potential cost benefits of incentive programmes, other than to report an estimate of savings per quitter to an employer (USD 3400 per year, MMWR 2002). White 2013 reported that the intervention, if rolled out to the smoking population in the study area, could translate to a decrease in smoking prevalence of 2% to 5%, and offered an incremental cost‐effectiveness analysis. The authors estimate that the cost per quitter from the intervention was USD 281 (95% CI USD 187 to USD 562), compared with quitting with nicotine gum (USD 1780, 95% CI USD 1414 to USD 2401) or with varenicline (USD 2073, 95% CI USD 1357 to USD 4388) in Thailand. The authors note that the intervention complies with the World Health Organization's ranking of "very cost‐effective" in Thailand, i.e. less than gross domestic product (USD 8600, purchasing power parity‐adjusted in 2011; World Bank 2012). Fraser 2017 reported that the overall cost per quitter for control group participants was on average USD 4268.26 while incentive group participants averaged USD 3601.37 per quit. Halpern 2018 reported the cost per successful quitter to be USD 7797.52 where free cessation aids were provided, compared to USD 3623.13 per quitter for the incentive group participants, and USD 3461.47 for the redeemable deposit group. Rettig 2018 did not report formal cost‐effectiveness data, but reported that “Over‐the‐counter nicotine replacement therapy was provided for free (estimated cost per participant USD240)” compared with low‐cost overall incentives (exact cost not reported).
Harms, disbenefits
Few studies formally evaluated harms or disbenefits of incentives specifically. Potential harms evaluated were attributable to smoking cessation itself or judged not attributable to the intervention. Gallagher 2007, reporting on an intervention in 180 people with schizophrenia or other serious mental illness, briefly considered whether smoking cessation may have worsened the participants' psychiatric symptoms, but found no evidence for this at end of intervention or at 36‐week follow‐up, using the Brief Symptom Inventory. However, the authors caution against placing too much weight on this finding, because of low power within the study to detect such differences. Alessi 2014 reported one participant was hospitalised for alcohol‐related heart, liver and lung problems, considered by the trialists not to be associated with the intervention programme. Brunette 2017, recruiting from a community mental health clinic, reported that 25 participants (4%) experienced a serious adverse event: 16 were hospitalised for psychiatric exacerbations, seven were hospitalised for medical reasons (pneumonia, lung cancer, and heart attack), and five study participants died. However it is not clear whether these events were related in any way to the intervention. Cheung 2017 speculated that incentive‐based interventions leading to 'cheating' or 'gaming' by participants may have occurred, in an attempt to ‘play the system’ to receive financial rewards. They suggest that 'loose' inclusion criteria for the study might have led to the inclusion of low‐rate/non‐daily/light smokers who might simply stop smoking for a day in order to win. Such 'cheating' was possible, but was not evaluated. None of the other included studies reported on any harms, unintended consequences or adverse events associated with the interventions; however, we consider in the Discussion section the implications of systematic deception in participants seeking to obtain unmerited rewards for abstinence, and other potential disbenefits of incentives interventions.
Pregnancy
Cessation
Details of the results at longest follow‐up (up to 24 weeks post‐partum) for nine of the 10 included studies in pregnant women in this review are tabulated in Table 4, and are displayed graphically where data were available in Analysis 2.1; Figure 4. One trial could not be included in the meta‐analysis: the MISS Project (Donatelle 2002) reported interim results only, i.e. for 298 women from a projected total of 600. We were unable to obtain further information on final numbers, or on quit rates achieved at any point.
2. Results of included studies: pregnancy.
| Study ID | Denominator | Abstinence | Time point | Biological criteria | Quit rate | Stat sig? | Other outcomes | Comment |
| Baker 2018 | 505 (IG) 509 (CG) |
7‐day PPA | 6 months | CO < 7 ppm | 74 (14.65%) (IG) 47 (9.23%) (CG) | P ≤ 0.01 | N of post‐birth home visits and phone calls taken; biochemically confirmed abstinence at the post‐birth week 1 visit; and self‐reported smoking status at the 2‐ and 4‐month visits | Engagement in treatment and cost effectiveness also cited on NCT record but NR |
| Donatelle 2000a | 112 (I) 108 (C) |
7‐day PPA | 8‐month gestation | Salivary cotinine < 30 ng/ml Thiocyanate < 100 ug/ml | 34/105; 32% (I) 9/102; 9% (C) |
Chi² = 18.4; P < 0.0001 | None stated | Differential losses to follow‐up; (I) 32% at 8m, vs (C) 51.5%. |
| 112 (I) 108 (C) |
7‐day PPA | 2m post‐partum | Salivary cotinine < 30 ng/ml Thiocyanate < 100 ug/ml | 22/103; 21% (I) 6/102; 5.9% (C) |
Chi2 = 11; P < 0.001 | None stated | Differential losses to follow‐up; (I) 36% at 2 months post‐partum, vs (C) 52% | |
| Donatelle 2000b | 67 (E1) 59 (E2) 60 (C) | "biochemically confirmed abstinence" | End of pregnancy | Salivary cotinine < 30 ng/ml Monthly CO < 5 ppm. | 19% (E1) 22% (E2) 12% (C) |
Not stated | None stated | Very little information available |
| Donatelle 2002 | 102 (E1) 96 (E2) 95 (C) |
Self‐report (telephone call) | 8 months gestation | Salivary cotinine < 30 ng/ml Monthly CO < 5 ppm | N.S. | Not stated. | High vs low incentives; cost per quitter |
Results are interim analysis only, based on 298 enrolled; target was 600. No further information available |
| Harris 2015 | 7 IG (CM) 10 CG (SCHB) |
PPA | Approximately 6 months | Urinary cotinine (cut‐off not defined) | 1 IG (CM) 3 CG (SCHB) |
Not stated but assume NS | Smoking reduction (time line follow‐back method), Stages of Change Ladder (SCL), Modified Fagerström Test ¨ of Nicotine Dependence (mFTND); Post‐treatment assessments measured birth outcomes (e.g. gestational age at birth, birth weight, and time spent in NICU) and smoking‐related variables | Follow‐up time point reported as 8.75 months pregnant (IG) and 8.19 months pregnant (CG). Randomised at (mean = 10.75 weeks pregnant), so follow‐up approximately 6 months |
| Heil 2008 | 37 (I) 40 (C) |
PPA | End of pregnancy | Urine cotinine < 80 ng/ml CO ≤ 6 ppm |
15/37; 41% (I) 4/40; 10% (C) |
P = 0.003 | Foetal growth | ‐ |
| antepartum CA; 24 weeks post‐partum |
Urine cotinine < 80 ng/ml CO ≤ 6 ppm |
3/37; 8% (I) 1/40; 3% (C) |
N.S. | Baby health Total voucher earnings |
‐ | |||
| Higgins 2014 | 44 (RCV; E1) 44 (CV; E2 42 (NCV; C) |
7‐day PPA | 28 wks gestation | Urinary cotinine ≤ 80 ng/ml CO < 4 ppm or 6 ppm |
18/40; 45% (E1) 14/39; 36% (E2) 7/39; 18% (C) |
N.S. | Foetal growth Birth outcomes | ‐ |
| 44 (RCV; E1) 44 (CV; E2 42 (NCV; C) |
7‐day PPA | 24 weeks post‐partum | Urinary cotinine ≤ 80 ng/ml CO < 4 ppm or 6 ppm |
7/40; 18% (E1) 6/39; 15% (E2) 3/39; 8% (C) |
‐ | Foetal growth Birth outcomes |
‐ | |
| Ondersma 2012 | 26 (E1) 28 (E2) 30 (E3) 26 (C) |
7‐day PPA |
30‐day CA 7‐day PPA |
Urinary cotinine ≤ 100 ng/ml CO < 4 ppm |
6/23: 26% (E1) 2/22: 10% (E2) 5/26: 19% (E3) 1/23: 4% (C) |
E1 P < 0.05 | ‐ | ‐ |
| 42 (E1) 28 (E2) 32 (C) |
PPA | 12 weeks | CO < 4 ppm Urine sample (for cocaine) |
13/42; 31% (E1) 0/28; 0% (E2) 0/32; 0% (C) |
‐ | ‐ | ‐ | |
| Tappin 2015a | 306 (I) 306 (C) |
"even a puff" in past 2 weeks "even a puff" in past 4 weeks < 5 cigs in past 8 weeks |
4 weeks 12 weeks (if quit at 4) 34 ‐ 38 weeks gest (all participants) |
CO < 10 ppm Cotinine: Urine 44.7 ng/ml; saliva 14.2 ng/ml |
69/306 (I) 26/303 (C) |
P < 0.001 | Adverse events engagement birth weight cost effectiveness |
3 controls dropped out after randomisation ‐ not included in denominators |
| 306 (I) 306 (C) |
still quit or < 5 cigs for since TQD | 6 months post‐natal (for 34/38‐week quitters) | Cotinine: Urine 44.7 ng/ml; saliva 14.2 ng/ml | 47/306 (I) 12/303 (C) |
P < 0.001 | ‐ | 3 controls dropped out after randomisation ‐ not included in denominators | |
| Tuten 2012 | 42 (E1) 28 (E2) 32 (C) |
Self‐reported 24‐hour PPA | 6 weeks PPA | None | 13/42; 31% (E1) 0/28; 0% (E2) 0/32; 0% (C) |
N.S. | Mean CPD | ‐ |
| 42 (E1) 28 (E2) 32 (C) |
Self‐reported 24‐hour PPA | 6 weeks PPA | None | 13/42; 31% (E1) 0/28; 0% (E2) 0/32; 0% (C) |
N.S. | Mean CPD | Abstinence not reported for this time point |
2.1. Analysis.
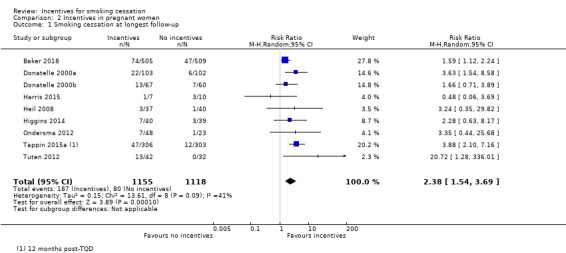
Comparison 2 Incentives in pregnant women, Outcome 1 Smoking cessation at longest follow‐up.
The trials demonstrated a clear benefit for the incentives groups over the controls. Taken together, nine trials in pregnant smokers (eight conducted in the USA and one in the UK) delivered an RR at longest follow‐up (up to 24 weeks post‐partum) of 2.38, 95% CI 1.54 to 3.69; 9 RCTs; N = 2273; I2 = 41%) in favour of incentives. This effect persisted in a sensitivity analysis removing the one study at high risk of bias (Donatelle 2000a) (RR 2.22, 95% CI 1.37 to 3.59; 8 RCTs; N = 2068; I2 = 41%).
We were unable to ascertain with any certainty whether the size of the rewards made a difference to outcomes, due to a paucity of relevant data. Three trials addressed the question of whether contingent rewards were more effective than non‐contingent fixed payments (Heil 2008; Higgins 2014; Tuten 2012). In these trials, scaled payments were given only as a reward for validated abstinence (contingent), while fixed payments were guaranteed provided that the participant attended and gave a biological sample, irrespective of her smoking status. All three trials favoured conditional over non‐conditional payments, with an RR of 3.33, 95% CI 0.97 to 11.38; 3 RCTs; N = 225; I2 = 18%; Analysis 2.3. None of the included trials compared an incremental with a fixed schedule, but with both payable only for validated abstinence, i.e. testing the role of variable rewards rather than contingency.
2.3. Analysis.
Comparison 2 Incentives in pregnant women, Outcome 3 Contingent rewards vs guaranteed payments.
Costs
While confirming that they had not conducted a cost‐benefit analysis, Heil 2008 reported that the average cost of the incentives per participant was USD 334. Tappin 2015a reports that the short‐term incremental cost per quitter was GBP 1127, with an incremental cost per quality‐adjusted life year of GBP 482. The NHS lower threshold is GBP 20,000, designated by the National Institute for Health and Care Excellence (NICE 2018) as an indicator of cost effectiveness (Tappin 2015b), suggesting that "financial incentives for smoking cessation in pregnancy are highly cost‐effective" (Boyd 2016). The remaining included studies in pregnancy did not report on costs.
Harms, disbenefits
None of the included pregnancy trials reported on harms or unintended consequences of the interventions, although Tappin 2015a offered some evidence on the likelihood of the participants 'gaming' to receive unmerited rewards. We consider this further in the Discussion section below.
Discussion
Summary of main results
Overall there is high‐certainty evidence that incentives improve smoking cessation rates at longest follow‐up in mixed‐population studies (Table 1). With moderate‐certainty evidence, the nine trials in 2273 pregnant women contributing to the meta‐analyses confirmed the efficacy of incentives at longest follow‐up, at or around the end of pregnancy (Table 2).
Previous reviews of incentive‐based interventions for smoking cessation have expressed concerns that the effect of incentives may be time‐limited. This would conform to a learning theory‐based explanation, that rewards are effective when consistently offered, but that the effect of the reward may be 'extinguished' when rewards cease. With regard to smoking cessation, where individuals may initially find quitting difficult but may adapt over time to this change, offering rewards that can initiate cessation seems to suggest that the long‐term effect overall may be maintained. This is plausible, because the incentives serve to support the initial, most difficult weeks (or months) of a quit attempt and the risk of relapse reduces over time. Findings from our meta‐analysis in mixed populations suggest that incentives continue to have a significant impact on sustained smoking cessation, even after they have finished. In our next update, when we anticipate further evidence will be available and therefore contribute further data points, we plan to conduct an analysis comparing quit rates at last incentive point to quit rates at subsequent follow‐ups where incentives are not provided; this would provide a more direct test of the lasting effect of incentives.
In our updated searches we identified four new trials that recruited people who misused substances (Ainscough 2017; Cooney 2017; Rohsenow 2015; Rohsenow 2017). This suggests that there is increasing focus and attention being paid to the importance of smoking cessation for this population, and incentives may offer a promising intervention strategy for this group in whom smoking rates remain high (Gentry 2017). Together with the four older trials recruiting from this population, our subgroup analysis suggested a positive benefit of incentives; confidence intervals were wide but results were consistent with the overall finding of a positive effect. However, we report these findings with caution, as the analysis is likely to be underpowered and more studies are needed.
We explored narratively the value of incentives offered across studies, and considered subgroup analysis. We concluded that this would not be possible, as the amounts offered varied considerably, and it was not possible to broadly group trials into 'low value' or 'high value' incentives, as the diverse cultural settings of studies and the 'meaning' of the total amount of incentives offered to participants can not be predicted. Even a small financial incentive offered to a factory worker in rural Thailand may be highly valuable and meaningful to individual participants. From the available evidence, we cannot conclude whether the value of the financial incentive has a discernible impact on the effectiveness of the intervention; this is therefore a question that future iterations of this review should seek to explore further. A possible approach would be to evaluate incentive size as a percentage of mean study participant income, but the most valuable data would come from studies directly comparing different incentive amounts, as then the population would not be a confounder.
The deposit‐refund trials merit particular attention, in the light of the discussions that have emerged from the Volpp 2009 study about whether the programme could be implemented in a real‐world setting. If implementation of an incentives programme is compromised by the costs incurred, then the model of participants depositing and forfeiting their own money is likely to be more attractive to employers and institutions seeking affordable behaviour change interventions. Although effect estimates appear to be consistent between deposit‐based and traditional reward schemes, low uptake rates compared with reward‐based interventions may limit the appeal and efficacy of such programmes (Volpp 2014). White 2013 reports a participation rate of 10.5% among eligible smokers, while Giné 2010 reports a rate of 10.6%. Halpern 2015 directly compares interventions funded entirely from trial resources to those funded partly by the participants themselves (USD 150). As discussed above, uptake rates proved to be a barrier, with 90% of those offered rewards accepting the intervention compared to only 13.7% of those required to put up a deposit, obliging the trialists to develop an adaptive model of randomisation in order to populate the deposit‐based arms. On an intention‐to‐treat basis, the rewards arms consistently delivered significantly more quitters than the deposit arms at all time points; however, in instrumental variable analysis which accounts for different rates of uptake (equivalent to a per protocol analysis), among participants prepared to accept either intervention the deposit arms outperformed the rewards arms, with six‐month quit rates of 53.4% and 17.1% respectively, and 12‐month quit rates of 18.5% and 8.8% respectively.
White 2013 used community‐based health workers to support smokers attempting to quit in a region of Thai villages, using a deposit‐refund intervention. The six‐month success rates were impressive, at 44.3% for the intervention group compared with 18.8% among the controls; however, the unusually high quit rate for the control group suggests that this population may have represented 'low‐hanging fruit' (easy quitters who may never have received support to stop smoking before), and that these findings are not readily generalisable to areas with longstanding and established tobacco control programmes. The two largest trials included in this review update specifically evaluated financial incentives against deposit‐based incentives. White 2018 and Halpern 2018 found both deposits and incentives to be effective for long‐term smoking cessation, but no significant differences between the two forms of incentivisation. This suggests that although it may be more difficult to recruit smokers into deposit‐based programmes, once they are in they appear to be strongly committed to the process and can achieve high quit rates.
While the findings of this review are encouraging, it is important to note that there may be substantial barriers to implementing incentives in routine care or as part of mainstream services. Public opinion regarding incentives is often negative (Berlin 2018; Giles 2015; Hoddinott 2014) with incentives seen as ‘rewarding’ behaviour change for a ‘habit’ that is perceived as self‐inflicted (smoking). This may limit the extent to which trial results can inform changes in policy and practice. An additional challenge is resource constraints. Real‐world implementation requires funders (such as the NHS or local government in the UK who fund smoking cessation services) to prioritise these schemes over other approaches to smoking cessation. For further consideration are the possible harms and disbenefits of incentives schemes. Those who relapse to smoking and do not receive a financial incentive may conceivably disengage from subsequent cessation attempts. This potential harm warrants monitoring in future trials.
Deception
Only six of the included trials tested for smoking status at baseline, with cotinine or exhaled carbon monoxide (CO) (Brunette 2017; Cheung 2017; Etter 2016; Halpern 2015; Harris 2015; Romanowich 2015). Halpern 2015 only tested eligibility in a 5% sample of enrolled participants, who were paid USD 100 for supplying a cotinine assay. Of those asked to submit a baseline sample, nine (6%) returned a negative assay and 21 (14%) did not return a sample, suggesting that up to 20% of participants could have been non‐smokers. However, as the rates were comparable across all arms of the trial, and sensitivity analyses adjusting for this possible level of deception made no difference to effect estimates, we conclude that biochemical validation of smoking status at baseline may not impact on overall trial outcomes, since deception about baseline smoking status is likely to be equally distributed through randomisation.
It was a condition for inclusion in this review that studies used some form of biochemical verification to confirm the smoking status of those claiming abstinence when rewards were due. This procedure is the recommended gold standard for good trial design in smoking cessation studies (SRNT 2002). It may also be particularly important that quitters in an incentives‐based trial are shown to be truly abstinent at the evaluation points, since deception may be a justifiable critique to be directed at incentive‐based interventions. Volpp 2006 addressed the likelihood of participants modifying their smoking behaviour in anticipation of being contacted for follow‐up assessment and cotinine testing, and concluded that this was unlikely, since although participants knew that they would be biologically tested, they were unaware of how long nicotine metabolites would be detectable or the exact date on which they would be checked. Where reported, most included studies demonstrated good correspondence between self‐reported claims of abstinence and biochemical verification. As incentives were contingent upon biochemically‐confirmed abstinence, and our review outcome of longest follow‐up was robust to the removal of studies without biochemical verification at longest follow‐up (e.g. studies at high risk of detection bias), it is unlikely that deception impacted our findings.
Work on incentives for pregnant smokers trying to quit has been concerned with directing attention to the risks of deception or 'gaming', particularly the likelihood of delaying a quit attempt to coincide with a rewards programme, and the likelihood of misrepresenting smoking status, either to gain admission to an incentive programme or to receive unmerited rewards for abstinence (Marteau 2013). Ierfino 2015, in a longitudinal cohort study of 239 pregnant smokers, found no evidence of gaming to enter an incentives programme, but detected a 4% level of deception to win vouchers for abstinence (e.g. falsely reporting abstinence). Tappin 2015a used residual routine blood samples (i.e. taken for non‐study‐related purposes) collected from the final 200 women enrolling in their study, to cross‐check the smoking status of self‐reporting quitters. Residual bloods were available for 18 of the 69 intervention women who self‐reported abstinence at 34/38 weeks, and had this confirmed by saliva or urinary cotinine; 78% of these samples (14/18) confirmed their non‐smoking status. Similarly, five residual samples for the 26 control participants with confirmed abstinence at 34/38 weeks corroborated 80% (4/5) of the results. While this suggests some overestimation of the true quit rates, the level of deception appeared to be similar across both groups, and confirmed the veracity of 80% of the self‐reported quitters. A large pregnancy trial included in this new update of the review (Baker 2018) reported that "It is possible that some participants quit or reduced their smoking just prior to the 6‐month visit. Breath CO, which was used in this trial, has a relatively brief half‐life; serum cotinine might have been more sensitive to detecting temporally remote smoking'" A much smaller pregnancy trial (Harris 2015) more robustly combined both CO and urinary cotinine testing to confirm abstinence. Taken together, these results suggest that the likelihood of deception in the pregnancy incentives trials is also low.
Overall completeness and applicability of evidence
This review includes a number of studies from diverse cultural settings (White 2018 recruited from worksites in Bangkok, Giné 2010 approached people smoking in the street in Philippines cities, Van den Brand 2018 recruited from workplaces in the Netherlands, Etter 2016 recruited a community sample in Switzerland, and Fraser 2017 contributes new evidence from 1900 US community‐based smokers), suggesting that the impact of incentives can be considered to be generalisable across these populations, although more evidence is needed from low‐ and middle‐income countries. Future reviews might consider analysis of outcomes by population setting. Comparison of clinical versus community‐based populations may be meaningful, as different populations may be more or less motivated to quit, and have differential access to adjunctive support. Population setting may also have relevance for scalability of incentive‐based interventions.
We have followed standard Cochrane methods to identify and evaluate the studies contributing to this review, and are confident that we have not missed any significant published trials. We have sought missing or incomplete data, and have contacted authors where possible to clarify our interpretation of their work. The increased diversity of populations included within this review, from diverse cultural settings, across different healthcare systems, and including unique populations such as pregnant women and those in treatment for substance misuse and distinct clinical populations has extended the applicability of this review, in line with recent trends in public health approaches to incentivising behaviour change.
Certainty of the evidence
Mixed‐population studies:
We rated the overall certainty of the evidence in this group of trials as high (see Table 1). Although there were concerns about risk of bias, particularly in older trials, our sensitivity analyses excluding trials at high risk of bias and excluding studies at both high and unclear risk of bias did not change the positive effect of incentives on abstinence rates. We upgraded the quality of evidence from the previous version of this review, suggesting that we can have increased confidence in our reported findings and the estimate of effect. Due to the inclusion of several high‐quality large RCTs which detected significant effects in favour of the intervention, the estimate of effect has increased overall.
Pregnancy studies:
The included trials covering pregnant smokers are rated as being of moderate certainty (Table 2), suggesting that the true effect is likely to be similar to what we have found, although further research may have an impact on the estimate of effect. Certainty was limited by concerns about risk of bias, with most studies judged to be at unclear risk. All the trials were conducted within the last 19 years, and have benefited from advances in trial methodology and reporting expectations. Only four trials (Baker 2018; Harris 2015; Ondersma 2012; Tappin 2015a) were deemed to have reported adequate randomisation procedures.
Potential biases in the review process
We followed standard Cochrane methods which are designed to reduce risks of bias. Our funnel plot for the main analysis in mixed populations did not suggest evidence of asymmetry. We searched trial registries, but we cannot rule out the possibility that we may have missed unpublished, unregistered studies.
Agreements and disagreements with other studies or reviews
A number of systematic reviews addressing incentives for smoking cessation have been published in recent years. Two reviews address smoking among other public health interventions based on incentives: Giles 2014 evaluated 16 studies of incentivised health behaviour change, 10 of which focused on smoking cessation. Using most of the same studies as in previous versions of our review, they demonstrated a benefit for smoking cessation up to six months follow‐up (RR 2.48, 95% CI 1.77 to 3.46) and at later follow‐up (more than six months) (RR 1.50, 95% CI 1.05 to 2.14), but with high heterogeneity (I2 = 76%). Two further reviews were confined to smoking cessation only: Leeks 2010 demonstrated a 4.4% benefit at a median of 12 months follow‐up for worksite‐based cessation programmes which included incentives or competitions. Sigmon 2012b explored incentive programmes within particular high‐risk population subgroups, including substance abusers, adolescents and young adults, and people diagnosed with pulmonary disease, and also highlighted the use of shaping procedures for hard‐to‐treat smokers, the promise of developing technologies for delivery of the intervention, and varying the scale of the incentive.
We are aware of two reviews which synthesise evidence on incentive schemes in pregnant women. Higgins 2012 summarised a series of six trials of incentives in pregnant smokers, conducted by two US‐based research groups and particularly addressing birth outcomes. Findings are similar, stating that such interventions "hold promise" as a mechanism for increasing cessation rates in this population of smokers. As highlighted above, however, studies suggest that implementing incentives may be difficult, regardless of the evidence. Our results in pregnant women are similar to those reported in the Pregnancy and Childbirth Cochrane Review (Chamberlain 2017), but there are some key differences. The Chamberlain 2017 review assessed effectiveness of psychosocial interventions for smoking cessation in pregnancy, and included a subset of studies using incentives. Their primary outcome was abstinence around the end of pregnancy, while ours is at longest follow‐up, i.e. post‐partum where available. Unlike in our review, they did not exclude prespecified withdrawals (termination, foetal demise) from the denominator. The Chamberlain 2017 meta‐analysis included only data comparing contingent incentives with alternative interventions. They identified insufficient data for comparisons with usual care and substantial heterogeneity (I2 = 93%) when assessing incentives compared with less intensive interventions, precluding pooling of data. We include six trials which do not appear in the Chamberlain 2017 meta‐analysis. Their main result (incentives versus alternative interventions for smoking abstinence around the end of pregnancy) covers 212 women, giving a risk ratio of 2.26 (95% CI 1.36 to 4.09) (Chamberlain 2017). Our own analysis of the same (secondary) outcome in 1244 women delivers an RR of 2.79 (95% CI 2.10 to 3.72) (Analysis 2.2).
2.2. Analysis.
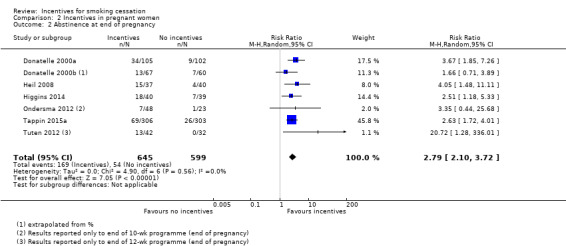
Comparison 2 Incentives in pregnant women, Outcome 2 Abstinence at end of pregnancy.
Authors' conclusions
Implications for practice.
There is high‐certainty evidence that incentives boost long‐term cessation rates (six months or more) in mixed‐population studies. This effect appears to persist following their discontinuation, suggesting that even a short incentivised intervention may have long‐term benefits.
There is moderate‐certainty evidence that incentives also boost the long‐term cessation rates of pregnant women who smoke, which continues post‐partum.
Low‐ to moderate‐value incentives appear to achieve sustained success rates beyond the end of the reward schedule, suggesting that even modest incentive schemes may be effective at encouraging long‐term smoking abstinence.
Deposit‐refund trials may be prone to low rates of uptake compared to reward‐based programmes; however, people who do sign up and contribute their own money achieve comparable or higher quit rates than reward‐only participants.
Although concern has been expressed about incentive‐based interventions attracting smokers motivated more by the material rewards than by the desire to quit, there was little evidence that levels of deception varied between experimental and control participants, or that rates of disconfirmation were unacceptably high. The motivation for entering an incentive‐based cessation scheme may be less important than eventual engagement in promoting smoking cessation.
Implications for research.
Evaluation of different incentive reward schedules for smoking cessation is needed.
Further large, well‐conducted trials are needed on the effectiveness of using incentives for smoking cessation in low‐ and middle‐income countries and in pregnant women.
Trials are needed that directly compare high‐ and low‐value incentives to assess whether there is a difference in effect. A possible approach would be to evaluate incentive value as a percentage of mean study participant income.
The affordability and cost effectiveness of incentive programmes should be tested in real‐world settings, as part of the evaluation process.
Implementation and acceptability of incentives in real‐world settings should be formally evaluated, including directly comparing or assessing the value of incentives alongside other smoking cessation interventions.
Trials in pregnant women should explore the effect of financial versus deposit‐based incentives.
Potential disbenefits and harms of incentives interventions require monitoring in future trials.
What's new
| Date | Event | Description |
|---|---|---|
| 21 January 2019 | New citation required and conclusions have changed | Certainty of evidence for studies in mixed populations upgraded from low to high. Previously included non‐randomised studies now excluded. |
| 21 January 2019 | New search has been performed | Search run 30th July 2018. Review updated with 16 new included studies in mixed populations and 2 new included studies in pregnant women. |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 6 January 2015 | New citation required and conclusions have changed | Review split into 'Incentives and contingency management' (this update) and 'Competitions' (companion update). 5 trials transferred to the Competitions update, and 7 new trials added, plus a complete new section (9 trials) on pregnant women. |
| 16 December 2014 | New search has been performed | New searches conducted, and entire review reformatted and expanded. 16 new trials added. Non‐randomised trials excluded. Analysis changed from OR to RR. |
| 14 April 2011 | Amended | Minor typographical errors corrected |
| 24 November 2010 | New search has been performed | 15 new trials added: 2 included, 13 excluded. |
| 24 November 2010 | New citation required and conclusions have changed | New included study (Volpp 2009) found long‐term positive effects of their incentive‐based trial. Risk of bias tables added for all studies. |
| 6 August 2008 | Amended | Source of support added |
| 29 April 2008 | New citation required but conclusions have not changed | Name change for first author |
| 2 April 2008 | New search has been performed | Two new included studies, nine new excluded studies, conclusions unchanged. |
| 2 April 2008 | Amended | Converted to new review format. |
Acknowledgements
Kate Cahill was lead author on previous versions of this review and her contributions are gratefully acknowledged. For the previous version of this review, we are profoundly grateful to Scott Halpern, Theresa Marteau and Justin White for exceptional support and co‐operation in supplying data and information for use in this review. We would also like to thank Sheila Alessi, Carla Berg, Suzanne Colby, Sandra Gallagher, Xavier Giné, Suzy Bird Gulliver, Tomoko Hiragaya, Leonard Jason, Susan McMahon, Steven Ondersma, Erin Rotherham‐Fuller, Damaris Rohsenow, Steven Shoptaw, David Tappin, Tracy Tevyaw, Andrea Troxel, and Kevin Volpp for additional data or clarification. Thanks also to Frances Kellie from the Cochrane Pregnancy and Childbirth Group for collaborative support with the inclusion of the pregnancy trials.
For this version of the review, we are grateful to Thomas Ainscough, Ivan Berlin, Patrick Calhoun, Severin Haug, Richard Lamb, Marita Lynagh, Payam Sheikhattari, Floor van den Brand, Justin White, and Sarah Wilson for answering queries regarding included, ongoing, and excluded studies, and to Clare Miles for her assistance with data extraction.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure and Cochrane Programme Grant funding to the Cochrane Tobacco Addiction Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health and Social Care. JHB is also part‐funded by the NIHR Oxford Biomedical Research Centre (BRC).
We would also like to thank Justine White and Floor van den Brand for peer reviewing this review, and Sandra Wilcox and Lee Bromhead for providing consumer review.
Appendices
Appendix 1. CRS search strategy
incentive*:ti,ab,MH,EMT,KW,KY,XKY
2 competition*:ti,ab,MH,EMT,KW,KY,XKY
3 contest*:ti,ab,MH,EMT,KW,KY,XKY
4 lotter*:ti,ab,MH,EMT,KW,KY,XKY
5 reward*:ti,ab,MH,EMT,KW,KY,XKY
6 prize*:ti,ab,MH,EMT,KW,KY,XKY
7 contingent payment*:ti,ab,MH,EMT,KW,KY,XKY
8 deposit contract*:ti,ab,MH,EMT,KW,KY,XKY
9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
Data and analyses
Comparison 1. Incentives in mixed populations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation (subgrouped by when incentives were provided) | 30 | 20060 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.28, 1.73] |
| 1.1 Incentives provided at longest follow‐up | 6 | 3039 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.33, 2.07] |
| 1.2 Incentives not provided at longest follow‐up | 24 | 17021 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.16, 1.69] |
| 2 Smoking cessation (grouped by substance misuse) | 31 | 20097 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [1.28, 1.73] |
| 2.1 Substance misusers | 8 | 1055 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.81, 1.89] |
| 2.2 Non‐substance misusers | 23 | 19042 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.29, 1.80] |
Comparison 2. Incentives in pregnant women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at longest follow‐up | 9 | 2273 | Risk Ratio (M‐H, Random, 95% CI) | 2.38 [1.54, 3.69] |
| 2 Abstinence at end of pregnancy | 7 | 1244 | Risk Ratio (M‐H, Random, 95% CI) | 2.79 [2.10, 3.72] |
| 3 Contingent rewards vs guaranteed payments | 3 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 3.33 [0.97, 11.38] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ainscough 2017.
| Methods | Randomised pilot study Country: UK Setting: Outpatient drug addiction treatment centre |
|
| Participants | 37 smokers aged 18+ undergoing pharmacological treatment for opioid addiction Intervention n = 19, control n = 18 Mean CPD = 10. women n = 12 (32%). Mean age, ethnicity, not reported |
|
| Interventions | Both groups received standard smoking cessation treatment (manualised behavioural support and NRT according to NCSCT and NICE guidance over 6 weeks) Experimental Group(s): Contingency management for abstinence. Following an escalating with reset schedule, where rewards increase in a set increment value for each successive verified display of the desired behaviour. When the desired behaviour is not observed, no reward is given, and the reward value for the next verified display of the desired behaviour is reset to that of the initial reward. Reward values then begin to rise again in the same way as before. The desired behaviour in the experimental group is smoking abstinence, defined as breath CO reading of < 10 ppm Control Group: Contingency management for attendance. The intervention followed an escalating with reset schedule as described above, but the incentives were for attendance at smoking cessation treatment at the clinic that week, not abstinence Theoretical basis for intervention: not reported Duration of intervention: 5 weeks in total, starting in week 2 of the standard stop‐smoking services treatment and ending in week 6 Length of follow‐up: 6 months |
|
| Outcomes | Point prevalence abstinence at 6 months. Aimed to CO‐verify with cut off at < 10 ppm | |
| Notes | New for 2019 update Trial encountered many problems and only 1 person was followed up at 6 months. Unpublished study, author provided outcome data by personal correspondence. Funding: "This work was funded as part of TSA’s PhD studentship by the Medical Research Council and the Institute of Psychiatry, Psychology and Neuroscience (MRC/IoP Excellence Studentship). LSB is funded by a Cancer Research UK (CRUK)/BUPA Foundation Cancer Prevention Fellowship (C52999/ A19748). LSB and AM are members of the UK Centre for Tobacco and Alcohol Studies, a UK Clinical Research Collaboration Public Health Research: Centre of Excellence. Funding from the Medical Research Council, British Heart Foundation, Cancer Research UK, Economic and Social Research Council and the National Institute for Health Research under the auspices of the UK Clinical Research Collaboration is gratefully acknowledged 35 (MR/K/K023195/1). Neither the funding bodies nor study sponsors had any role in study design; collection, management, analysis and interpretation of data; writing of the report and the decision to submit the report for publication." Decarations of interest: "JS has contributed to UK guidelines which include consideration of the potential role of contingency management in the management of addiction problems (NICE, 2007; chaired by JS), and JS also chaired the broader scope pan‐UK working group preparing the 2007 and 2017 editions of the ‘Orange Book’ (‘Guidelines on the Management of Drug Misuse & Dependence’) for the UK Departments of Health, providing guidance on management and treatment of drug dependence and misuse, which include guidance on possible inclusion of contingency management. JS’s institution has received support and funding from the Department of Health (England) and National Treatment Agency (England), and JS and JS’s institution have provided funded consultancy advice on possible novel addiction treatments, products and formulations to a range of pharmaceutical companies but these do not have any connection to the intervention being investigated in this paper. JS’s employer (King’s College London) has registered intellectual property on a novel buccal naloxone with which JS is involved, and JS has been named in a patent registration by a pharmaceutical company as inventor of a potential novel concentrated nasal spray, but these do not have any connection to the work being reported in this paper. A fuller account of JS’s interests is at http://www.kcl.ac.uk/ioppn/depts/addictions/people/hod.aspx. JS is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust and King's College London and is an NIHR Senior Investigator" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomisation will be performed by the principal investigator (PI), using the service provided by the company ‘sealed envelope’, 25 and will be performed using random permuted blocks within strata. Randomisation will be stratified based on participants’ current smoking frequency (between 10 and 20 per day, and more than 20 per day).” |
| Allocation concealment (selection bias) | Unclear risk | Not discussed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome not biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only one participant was successfully followed up |
Alessi 2014.
| Methods | Randomised controlled trial Country: Connecticut, USA Setting: Residential substance use disorder clinic |
|
| Participants | 45 smokers, aged 18+, smoking 10+ CPD. All participants were men Intervention n = 24, control n = 21. Mean age 37, ethnicity 84% non‐Hispanic. Mean FTND 3.8. Mean CPD 18.6 |
|
| Interventions | All participants: 2 quit‐smoking preparation sessions, i.e. in session 1: 2 x CO samples, 30‐minute counselling session and a self‐help quit guide; then in session 2 (4 days later) review of progress and obstacles, quit plan updated and TQD set Participation rewards: Everyone got USD 15 for intake, USD 25 per follow‐up, and a USD 1 gift certificate or item (snacks and gum) for each CO and cotinine sample, irrespective of smoking status Control Group: Monitoring only; 2 x CO samples a day Monday to Friday for 4 weeks, plus brief individualised support/feedback (5 mins) from research staff. CPD tracked at every session; cotinine tested on Mondays Experimental Group: As Controls, + incentives: In week 1 a “guaranteed prize” bowl with 70 cards, of which 64 had a USD 1 prize, e.g. toiletries, sports drink, gum, 5 worth a USD 20 prize, e.g. exercise weights, portable games, Barnes and Noble gift cards, and 1 for USD 100 (linens, TV, and DVD player). Week 1 started with 1 draw for an abstinent CO test, rising by 1 for each consecutive abstinent test, capping at 5. A positive test or unexpected missed sample reset back to 1 In weeks 2 to 4, standard prize bowl contained 500 cards, 50% worth a prize; 219 were USD 1 prizes, 30 were USD 20 prizes, and 1 USD 100 prize. A cotinine‐negative test gave 5 bonus draws. Participants could earn 150 draws from this bowl for negative CO samples and 15 draws for negative cotinine samples. Increased draw entitlements from week 1 carried over to weeks 2 to 4 CM participants could earn up to 190 draws for negative CO tests, with average expected maximum earnings of USD 426.56, and 15 draws for negative cotinine tests, averaging USD 46.43 |
|
| Outcomes | % reduction in CPD; 7‐day PPA at 4, 8, 12 and 24 weeks Biochemical verification: twice daily (Monday to Friday) CO < 6 ppm; weekly (Monday) cotinine < 30 ng/ml |
|
| Notes | Additional information supplied by the author Funding: "This study and the preparation of this report were funded by National Institutes of Health grants R21‐DA021836; R21‐DA029215; R01‐DA013444; R01‐DA027615; R01‐DA024667; P30‐DA023918 and P50‐DA092410. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomization to one of two conditions occurred using an urn procedure” and “stratifying on at least one CO ≤ 6 ppm during baseline”. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very few losses; 2 participants left the treatment centre before completion |
Baker 2018.
| Methods | 2‐group randomised clinical trial. 2012 to 2016 Country: USA Setting: A perinatal support programme (First breath (FB)). Private and community health clinics providing perinatal healthcare services across Wisconsin as part of the FB programme |
|
| Participants | 1014 pregnant women, aged 18+, smoking daily (at least 1 CPD each day for at least 1 week) at some time within the last 6 months, enrolled in Wisconsin Medicaid (BadgerCare Plus or Medicaid SSI). Intervention n = 505, control n = 509. Mean age 26, ethnicity: % white CG: 47.2% IG: 45.4%, black or African American CG: 36.9% IG: 39.8%, Asian CG: 0.8% IG: 0.2%, American Indian/Alaska Native CG: 2.0% IG: 1.0%, 'Other' CG: 2.8% IG: 1.0%, Refused/do not know/missing CG: 7.5% IG: 8.5%, Hispanic CG: 5.3% IG: 4.8%, Non‐Hispanic CG: 81.7% IG: 81.8%, Refused to answer/missing CG: 13.0% IG: 13.5% Education % Less than high school CG: 3.7% IG: 4.2%, Some high school CG: 20.6% IG: 20.6%, High school or GED CG: 34.2% IG: 34.3%, Some college or 2‐year degree CG: 25.55 IG: 22.0%, College degree CG: 3.0% IG: 5.4%, Refused to answer/missing CG: 13.0% IG: 13.7%. CPD: 1 to 10 CG: 39.3% IG: 38.4%; 11 to 20 CG: 39.1% IG: IG: 39.4%; 20+ CG: 17.5% IG: 19.4%; Refused to answer/missing CG: 4.1% IG: 2.8% |
|
| Interventions |
Control Group: The study compensated all participants USD 40 for study registration/enrolment and USD 40/visit for attendance at post‐birth Visit 1 (1 to 3 weeks post‐birth) and post‐birth Visit 4 (at month 6). Participants attending visits 1 and 4 completed CO testing to biochemically verify self‐reports of abstinence from smoking; participants with CO test values of 7 ppm were considered to be abstinent. Thus, control condition participants could receive up to USD 120 Experimental Group(s): Incentive condition participants received a further USD 25/visit for any of the 6 pre‐birth visits they completed, USD 25/visit for attendance at post‐birth visits 2 and 3, USD 20/call for completion of 5 post‐birth calls, and USD 40/visit for biochemically‐confirmed abstinence at post‐birth visits 1 and 4. Thus, incentive condition participants could receive up to USD 500 for meeting all payment criteria Theoretical basis for intervention: not reported Duration of intervention: 6 months post‐birth Length of follow‐up: 6 months post‐birth |
|
| Outcomes | PPA at 6 months with cut‐off CO < 7 ppm. Number of post‐birth home visits and phone calls taken; biochemically‐confirmed abstinence at the post‐birth week 1 visit; and self‐reported smoking status at the 2‐ and 4‐month visits Engagement in treatment and cost effectiveness also cited on NCT record but not reported |
|
| Notes | New for 2019 update’ Previously listed as ongoing Funding: not reported Decarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | FB staff used randomisation tables prepared by the UW‐CTRI to randomise women upon consent. Separate computer determined randomisation tables were created based on race (white/non‐white) and county with proportional randomisation (1:1) into the incentive and control conditions |
| Allocation concealment (selection bias) | Unclear risk | Allocation not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Results were CO‐verified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For the primary outcome, 316 of 509 (37.9%) control condition participants had missing data; 145 of 505 (28.7%) incentive condition participants had missing data. Participants with missing data for the primary outcome were counted as smoking (ITT) |
| Other bias | Unclear risk | 6‐month follow‐up stated for primary outcome, but Table 2 results reports "4‐6 months" follow‐up |
Brunette 2017.
| Methods | 3‐arm RCT, 2012 to 2018 Country: USA Setting: 10 New Hampshire community mental health clinics |
|
| Participants | 661 community‐dwelling adult Medicaid beneficiaries (low income) with a mental illness diagnosis who were receiving services at a community mental health clinics (CMHC). 22% schizophrenia, 23% bipolar, 24% major depression, 31% anxiety and other disorders. Average 17 CPD at baseline. Intervention: Prescriber visit (PV) plus quitline (PV+Q) n = 303, PV+ CBT n = 212; Control (PV) only n = 146. Mean age 46. 426 women (64%). Ethnicity: n = 610, 93% white. Employment: n = 545, 82% not employed. Education: n = 549 high school graduate (83%) | |
| Interventions |
Control Group: Usual care prescriber visit for smoking cessation. All conditions included a visit with participants’ existing CMHC psychiatrist or nurse practitioner to discuss cessation medications and NRT and to obtain a prescription if they decided to use pharmacotherapy (PV). CMHC prescribers were trained with a yearly 45‐minute session of group training in safety, efficacy, and techniques for providing brief tobacco cessation counselling with evidence‐based pharmacotherapy tailored to smokers with mental illnesses. NRT (single product) and cessation medications (varenicline and bupropion) were covered by Medicaid. All participants received a participation reward of USD 30 Experimental Groups: PV+Q: PV plus facilitated quitline counselling (PV+Q). Participants met with their prescriber as described above, for which USD 15 was provided, and received a supported referral to the New Hampshire Tobacco Helpline, which provides an average of 3 manualised telephone counselling sessions to help smokers quit and to support abstinence. Participants’ cellphone records or helpline staff verified participation, enabling rewards for up to 3 calls (USD 20 each) PV+CBT: Participants met with their prescriber as described above for which a USD 15 participation reward was provided. Programme co‐ordinators explained how to use telephone counselling and forwarded a referral to the telephone CBT therapist, who initiated the first call. The CBT used was a manualised adaptation of the 12‐session Freedom From Smoking programme for people with severe mental illnesses provided by experienced tobacco treatment specialists. Participants received a USD 5 participation reward for each completed session, confirmed by counsellors’ records. Incentives for smoking abstinence. Within each intervention, half of participants were randomly assigned to receive monetary incentives contingent upon abstinence during 1 x 4‐week cessation attempt. Programme co‐ordinators explained how to use the abstinence incentive intervention. Participants agreed to come in to the clinic for abstinence confirmation after they initiated a quit attempt. Participants in the abstinence rewards conditions received USD 50 in cash for verified abstinence on Mondays, Wednesdays, and Fridays in the first 2 weeks of the quit attempt. The incentives were contingent on breath CO of 6 ppm on the first day and 4 ppm on subsequent days and urine cotinine sample, 100 ng/mL in the second week for those not using NRT. Participants could return in the third and fourth weeks for additional incentives (i.e. USD 75 for verified abstinence with the same criteria). Participants could earn up to USD 450 during the 4 weeks after quitting Theoretical basis for intervention: Behavioural reinforcement theory. CBT Duration of intervention: 1 month Length of follow‐up: 12 months |
|
| Outcomes | 12‐month PPA confirmed by CO breath test and urinary cotinine. Expired breath CO ≤ 4 ppm and urine cotinine < 100 ng/mL (or solely breath CO if participant was using NRT). Treatment programme participation, medications | |
| Notes | New for 2019 update Half of participants in each intervention group randomised to receive incentives. N randomised to incentives not reported. 25 participants (4%) experienced a serious adverse event: 16 were hospitalised for psychiatric exacerbations, 7 were hospitalised for medical reasons (pneumonia, lung cancer, and heart attack), and 5 study participants died Funding: "This research received financial support from the Centers for Medicare and Medicaid Services (Medicaid Incentives for the Prevention of Chronic Diseases grant 1B1CMS330880) and from the New Hampshire Department of Health and Human Services (NHDHHS)." Decarations of interest: "Dr. Brunette reports receipt of research funding from Alkermes. The other authors report no financial relationships with commercial interests." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "equipoise randomization that allowed participants to opt out of one of the cessation treatment conditions or allowed randomization to any of the three options. This strategy is recommended for comparative effectiveness trials that include more than two treatments. Randomization strata were defined by conditions to which the participant was willing to be randomly assigned. Within the stratum, a participant was then randomly assigned with equal probability to the selected treatment condition options. Computer‐generated tables for each strata within each site were used for random assignment. In addition, participants were randomly assigned to receive incentives for biologically verified abstinence or no incentives. After randomization, participants were encouraged to initiate their assigned interventions, but interventions could be accessed for the one year study period." Quote: “Within each intervention, half of participants were randomly assigned to receive monetary incentives contingent upon abstinence during one four‐week cessation attempt” |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Full results not reported – unclear how many participants randomised to incentives/no incentives, values for the primary outcome only presented as % in a figure |
Cheung 2017.
| Methods | 3‐arm RCT. 2013 to 2016. Country: Hong Kong Setting: Shopping malls and public areas in all 18 districts in Hong Kong |
|
| Participants | 1143 participants in the Hong Kong 4th QTW Contest in 2013. Adult daily smokers who smoked at least 1 CPD in the past 3 months with exhaled CO ≥ 4 ppm. Intervention groups: early‐informed (EI) n = 379, late‐informed (LI) n = 385; Control group: 379 Mean age 45. 208 (16.4%) women. Mean 15.2 CPD at baseline. Had secondary education (64.1%). Ethnicity not reported. Monthly household income (HKD) (USD 1 = HKD 7.8) Below 10,000 EI: 94 (24.8) LI: 108 (28.0) CG: 115 (30.3), 10,000 – 19,999 EI: 128 (33.8) LI: 129 (33.5) CG: 116 (30.6) 20,000 or more EI: 140 (36.9) LI: 131 (38.4) CG: 124 (32.8) |
|
| Interventions | All groups offered cash incentive if biochemically validated quit rate at 3 months (HKD 500/USD 64) Control Group: The control group was not informed about the incentive at any telephone follow‐up, but the validated quitters at either 3‐ and 6‐month follow‐up received the incentive at 6‐month follow‐up At 3‐month follow‐up, validated quitters participated in a lucky draw organised by the Council on Smoking and Health (COSH), in which each of the 5 winners obtained a gift voucher of HKD 10,000 (USD 1282). All participants were informed about this grand prize at the enrolment To ensure fairness, all quitters received the incentive, and once only All participants received brief smoking cessation advice based on the AWARD protocol at enrolment, 1‐week and 1‐month follow‐up, a pocket‐sized self‐help education card and a 12‐page self‐help booklet Experimental Groups: Early informed group ‐ At 1‐week and 1‐month telephone follow‐up, the early‐informed group was informed about the incentive, which was offered to the validated quitters at 3‐month follow‐up Late informed group ‐ At 3‐month follow‐up, the late‐informed group was informed that they would receive the incentive if they quit and passed the biochemical validation at 6‐month follow‐up. Theoretical basis for intervention: Health Action Process Approach (HAPA) model Duration of intervention: 6 months Length of follow‐up: 6 months |
|
| Outcomes | Point prevalence abstinence at 6 months follow up, verified by exhaled CO ≤ 4 ppm and saliva cotinine level ≤ 10 ng/ml. Quit attempts (longest duration and number of quit attempts (no smoking for at least 24 hours)); cessation aids. | |
| Notes | New for 2019 update ‘loose inclusion criteria for the study might have led to the inclusion of low‐rate/nondaily/light smokers who might simply stop smoking for a day in order to win. Such “cheating” was possible’ Funding: "This work was supported by the Hong Kong Council on Smoking and Health (COSH)." Decarations of interest: "Prof. Tai‐hing Lam is the principal investigator of the FAMILY project, which was funded by the Hong Kong Jockey Club Charities Trust. All other authors do not have connection with the tobacco, alcohol, pharmaceutical or gaming industries, and nobody was substantially funded by these organizations." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was used to individually and randomly allocate the participants recruited on each recruitment day into 3 RCT groups. One investigator (YTCD) used random.org to generate random permutations of the 3 RCT groups and allocated these permutations to the blocks with a size equal to 3, 6 or 9. These blocks of permutations were assigned to the participant lists on each recruitment day. Other research staff conducted the telephone call and the intervention based on the allocation list at 1‐week and 1‐month telephone follow‐up |
| Allocation concealment (selection bias) | Low risk | All participants and recruitment staff were prevented from knowing the allocation procedures at enrolment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data completion at 6 months above 50% in all arms and no substantial between‐group differences: Early informed: n = 228, 60.2%; Late informed: n = 221, 57.4%; Control group: n = 228, 60.2% |
| Other bias | Unclear risk | Participants took part in a Quit and win contest (and may have already been randomised as part of that study) before being randomised to this trial. Potentially already motivated sample (incentivised by competition) |
Cooney 2017.
| Methods | RCT. Dates not reported Country: USA Setting: US. Department of Veterans Affairs (VA) intensive outpatient substance use treatment programme |
|
| Participants | 83 adults age 18+. Met DSM‐IV criteria for alcohol abuse or dependence with last drink occurring within 90 days, reported current cigarette smoking of 10 or more CPD with at least a 3‐year smoking history, substance detoxification when necessary was completed prior to randomisation. If history of recent cannabis use, required to have negative urine toxicology screen for cannabis at time of enrolment. Intervention group: n = 42; Control group: n = 41. Mean age 49.9. Mean 20 CPD at baseline. Women n = 3 (2.4%). Ethnicity: 67% white: CBT + NRT: Hispanic n = 3 (7.3%), white n = 26 (63.4%), African American n = 11 (26.8%), Other n = 1 (2.4%) CBT plus NRT plus CM: Hispanic n = 0 (0%), white n = 30 (71.4%), African American n = 12 (28.6%), Other n = 0 (0%). Socioeconmic status: Homeless 38.6%, employed: CBT + NRT n = 11 (26.8%) CBT + NRT + CM n = 16 (30.1%). Other drug use diagnosis (CG) 15 (36.6%) (CM) 18 (42.9%) |
|
| Interventions |
Control Group: CBT + NRT: 3 x 40‐minute sessions (120 minutes total) at weekly intervals, focused on preparation to quit smoking, coping with nicotine withdrawal, and relapse prevention. All prescribed an 8‐week course of nicotine patch therapy beginning on TQD with 21 mg dose for 4 weeks, then 14 mg for 2 weeks, then 7 mg for 2 weeks Experimental Group(s): CBT + NRT + CM: same behavioural content and strategies as CG, but delivered across 12 shorter daily10‐minute sessions (120 min total) to correspond with daily CM sessions. Escalating financial incentives for biochemically‐confirmed smoking abstinence following TQD. Readings of 5 ppm or less were reinforced with a progressive monetary incentive, along with a reset condition for tobacco lapse. Participants were eligible to receive financial reinforcements twice daily from session 5 through 12. Reinforcement began with USD 5 at the beginning of day 1, USD 5.50 at the end of day 1, USD 6 and USD 6.50 on day 2, USD 7 and USD 7.50 on day 3 and so on, up through USD 12 and USD 12.50 on day 8, totaling a potential maximum of USD 140 in financial reinforcement for 8 days of tobacco abstinence. Reinforcement was withheld for CO ≤ 5 ppm and was reset to USD 5 for the first CO ≤ 5 ppm after a smoking lapse. Vouchers for financial reinforcements were given to participants by the study therapist Theoretical basis for intervention: CBT Duration of intervention: 8 weeks (12 days CM but 8 weeks NRT) Length of follow‐up: 6 months |
|
| Outcomes | PPA at 6 months. CO "Readings of ≤ 5 parts per million (ppm) were considered corroboration of smoking abstinence". Smoking at 1½ weeks after quit date, 1 month. Alcohol use, drug use | |
| Notes | New for 2019 update Funding: not reported Decarations of interest: "Judith Cooney has worked as a promotional speaker for Pfizer, Inc" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomisation computer programme |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High retention rates |
| Other bias | Unclear risk | Quote: "Design with an experimental condition that integrated CM within 12 brief CBT sessions compared with a more conventional, three‐session CBT without CM treatment schedule. This design does not allow us to dismantle the effects of frequently scheduled CBT from the effects of CM procedures" |
Dallery 2016.
| Methods | RCT. 2010 to 2016 Country: USA Setting: community smokers, recruited online |
|
| Participants | 94 adult community smokers, motivated to quit, with at least a 2‐year history of daily smoking. Mean age 36, mean CPD at baseline = 17. Submission‐contingent group (SC) n = 46; abstinence‐contingent group (AC) n = 48. 56% women. Ethnicity: AC: European American 77%; Hispanic American 4%; African American 15%; native American 0%; Asian 4%; More than 1 race 0%. SC: European American 71%; Hispanic American 7%; African American 7%; Native American 4%; Asian 9%; More than one race 2%. SES: AC: Less than high school 2%; High school degree, no college 8%; Some college 44%; Associate professional degree 13%; Bachelor’s degree or higher 37%. SC: Less than high school 2%; High school degree, no college 0%; Some college 48%; Associate professional degree 13%; Bachelor’s degree or higher 37% | |
| Interventions |
Control Group: submission‐contingent (SC) group: financial incentives based on submitting CO samples (regardless of abstinence status). Participants could earn a maximum of USD 480 in incentives based on abstinence or CO submissions. Participants in both groups were provided with the same CO‐based goals. The only difference between groups was the target behaviour to receive incentives: the AC group had to meet video‐verified CO cut‐points and the SC group had to submit videos. A USD 50 deposit was required from all participants. Deposits were made to PayPal via debit, credit card or direct bank transfer. The first USD 50 earned went toward reimbursement for the initial deposit incentive. Participants were loaned a CO monitor (Bedfont piCO+ Smokerlyzer; Bedfont Scientific Ltd, Maidstone, UK) and a web‐camera. Each participant’s homepage consisted of a graph of CO sample results, voucher earnings history, a ‘post video’ button and a display showing their previous sample’s date/time, and the earliest date/time at which they could provide their next sample Experimental Group(s): abstinence‐contingent (AC) group: financial incentives based on abstinence Tapering: For participants in the AC group, the average CO during baseline was calculated and every predetermined reduction from this value resulted in USD 3.00. The reductions were calculated for each participant such that the last day of this phase would serve as their quit day, with a target CO ≤ 4 ppm Induction: For participants in the AC group, CO samples were judged as either positive or negative (≤ 4 ppm.). A USD 3.00 incentive was awarded for the first negative sample, and increased by USD 0.25 for each consecutive negative sample. In addition, every third consecutive negative CO resulted in a USD 5.00 bonus Theoretical basis for intervention: not reported Duration of intervention: total length of intervention appears to be 49 days. 7 weeks stated in abstract Length of follow‐up: 6 months |
|
| Outcomes | PPA CO‐verified by video at 6 months. CO ≤ 4 ppm. PPA at week 4 and 3‐month follow‐up. Treatment acceptability, behavioural change | |
| Notes | New for 2019 update Funding: "Research and preparation of this paper was supported by Grants R01DA023469 (Principal Investigator: J.D.) and P30DA029926 (Principal Investigator: L.M.) from the National Institute on Drug Abuse." Decarations of interest: none reported by authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was automated by an Excel macro that coded each participant based on smoking severity and gender, and then assigned the participant to the group with a lower number of participants with that combined smoking severity and sex code. If smoking severity and gender distributions were equal, then the participant was assigned randomly to a group" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated by online video (participant takes CO test and videos results to research team) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low and similar rates of attrition in each arm |
De Paul 1994.
| Methods | Cluster‐randomised 3‐arm controlled trial Country: USA Setting: 63 Chicago worksites, stratified on size and type of business. |
|
| Participants | 844 smokers (280 self‐help (SH), 281 incentives (I), 283 Group (G)), Mean age 37.8, 37% women, mean % black 20.5, mean years education 13.8, mean years smoking 19.9, mean CPD 21.5. Sex and race differed across conditions and were controlled for in all analyses |
|
| Interventions | 1. SH (also = M) 5‐day cessation TV programme 'Smoke‐free in the 90s' + 8‐page newspaper supplement, self‐help ALA manual Freedom from smoking in 20 days 2. I (also = IM) as SH, plus USD 1 per day for each day abstinent up to 6 months (maximum USD 175) 3. G (also = GIM) as I, plus group meetings twice a week for first 3 weeks, + 14 'booster' meetings over 6 months; programme included a 'buddy' system, and tips in booster sessions on living with a smoker, weight control, exercise and stress management | |
| Outcomes | Baseline, post‐test (3 weeks), and at 6, 12, 18 and 24 months. Lottery system used to boost follow‐up return rates. CO samples at all assessments, + cotinine at 6 months. ICC calculated (no significant between‐firm effects detected) | |
| Notes | Only groups SH and I are used for the comparison, to isolate the effects of the incentives. Results extrapolated from percentages Additional information supplied by the authors Study was funded by the National Institute of Heart, Lung, and Blood | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified by size and type and then randomised. Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1/63 companies dropped out before intervention, 1/62 during intervention, 3/61 during first 6 months Only Grps I (SH only) and II (SH + incentive) used for this review; attrition by post‐test was SH: 32.3%, Incentives: 23.3%; 6 months SH: 43.3%, Incentives: 46.5%; 12 months SH: 52.5%, Incentives: 47.2%; Although losses by 12 months are high, there was no significant difference in levels of missing data between the 2 groups. Across groups, those who were younger, heavier smokers, with lower health ratings, less effort to quit, more confident of quitting and with less helpful coworkers were more likely to drop out |
| Other bias | Unclear risk | Allocation was by worksite, but analysis by individual participant. The last scheduled rewards were paid out to coincide with the final assessment, and may therefore have confounded that result |
Donatelle 2000a.
| Methods | Study design: RCT, conducted June 1996 to June 1997; data collection completed January 1998
Country: USA
Setting: 4 Oregon Women, Infants, and Children (WIC) programme sites SOS (Significant Other Supporter) Programme I |
|
| Participants | 220 women smokers (112 intervention, 108 control) Aged 15+, ≤ 28 weeks gestation, literate in English; withdrawal criteria included termination and foetal death | |
| Interventions | All participants received a USD 5 participation voucher at each of 3 assessments. Everyone at baseline was given verbal and written advice on importance of smoking cessation by WIC‐ or SOS‐trained staff, + self‐help kit A pregnant woman’s guide to quit smoking Experimental Group(s): Each participant was asked to designate a social supporter, preferable female non‐smoker. Both were eligible for vouchers if participant quit. Participant was phoned monthly (up to 10 months) to report smoking status. If she reported quit and supplied confirmatory saliva sample, she got a USD 50 voucher, and “their social supporter received a voucher as well” (i.e. USD 50 for 1st quit month, USD 25 for other quit months, and USD 50 for final quit month). Vouchers were funded by contributions from 10 local 'community partners' (businesses, foundations and healthcare organisations) Control Group: Baseline advice and quit kit, + monthly phone calls to determine smoking status |
|
| Outcomes | 7‐day PPA at 8 months gestation, and at 2 months post‐partum Biochemical validation by salivary cotinine < 30 ng/ml, and salivary thiocyanate < 100 mg/ml |
|
| Notes | New for 2015 update Funding: grant from Robert Wood Johnson Foundation Smoke‐Free Families Program, + support from local businesses and healthcare facilities |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Relatively high, but comparable with non‐participant attenders at the WIC clinic Losses: Intervention: 32% at 8 months gestation, 36% at 2 months post‐partum; Control: 51.5% at 8 months gestation, 52% at 2 months post‐partum |
Donatelle 2000b.
| Methods | Study design: RCT, dates not stated
Country: USA
Setting: 8 Oregon Women, Infants, and Children (WIC) programme counties SOS (Significant Other Supporter) Programme III |
|
| Participants | Probably similar to Donatelle 2000a; 186 pregnant smokers, randomised to E1 (N = 67); E2 (N = 59); Controls (N = 60) | |
| Interventions | All participants received a 5As intervention (Ask, Advise, Assess, Assist, Arrange) E1 group: USD 25 voucher (local department store) for each month achieving validated abstinence E2 group: USD 25 voucher (local department store) for achieved abstinence + immediate feedback on risks to the foetus associated with CO results. CO ≤ 5 ppm confirmed monthly abstinence |
|
| Outcomes | Abstinence at end of pregnancy Biochemical validation by salivary cotinine < 30 ng/ml. CO < 5 ppm monthly |
|
| Notes | New for 2015 update Minimal information; emails to the author elicited no responses Funding not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
Donatelle 2002.
| Methods | Study design: RCT, 3‐group design; interim report on data from August 2001 to September 2002 Country: USA Setting: 9 Oregon private practice prenatal clinics The MISS project | |
| Participants | 298 "predominantly low‐income, high risk pregnant women", smoking "even a puff" in the last 7 days, aged 15+, < 29 weeks gestation, literate in English 79% had Medicaid or Oregon Health Plan insurance; 24% had private insurance; mean age 24.1 years, mean education years 11.9 Target enrolment was 600 |
|
| Interventions | All participants received a 5As intervention (Ask, Advise, Assess, Assist, Arrange), + a copy of A Pregnant Woman's Guide to Quit Smoking, + local cessation resource guide E1 group (102 women): USD 25 voucher (local department store) for each month achieving validated abstinence E2 group (96 women): USD 75 voucher (local department store) for achieved abstinence Control group (95 women): standard care as above |
|
| Outcomes | Abstinence at 8 months gestation, + phone call post‐partum and a salivary cotinine test for self‐reported non‐smokers Biochemical confirmation: salivary cotinine < 30 ng/ml; CO < 5 ppm at monthly tests |
|
| Notes | New for 2015 update Funding: grant from Robert Wood Johnson Foundation Smoke‐Free Families Program Too little information to adequately assess risk of bias; emails to the author elicited no responses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information. 298 reported enrolled, but results given for only 293 |
Drummond 2014.
| Methods | Randomised factorial 4‐arm controlled trial, subset of a prospective, longitudinal cohort study (ALIVE: AIDS linked to the Intravenous Experience study) Country: USA Setting: Baltimore, MD Study conducted March 2011 to February 2012 |
|
| Participants | 100 injecting drug‐using patients randomised to 4 groups: Usual care (26); Lung age (24); Contingency management (26); Lung age + contingency management arm (24). 47% women, median age 50 (IQR 45 to 56); median FTND 4 (IQR 2 to 5); median pack years 19 (IQR 12.5 to 31); Ethnicity % African‐American: UC 92; LA 75; CM 100; LA+CM 88 | |
| Interventions | 6‐month programme. Cotinine blood test at 6 months for everyone 1. Usual care (UC; control) group: Baseline visit, + visits at 1, 2, 4 weeks, and then 2, 3 and 6 months. Participants completed questionnaires, self‐efficacy, motivation to quit, level of addiction, eCO testing, spirometry At all visits participants reviewed their spirometry results, and got guidance on quitting based on this 2. Lung age (LA) group: As UC, + written report giving their chronological age and their lung age. ‘Abnormal’ = lung age exceeding chronological age 3. Contingency management (CM) Group: As UC, + monetary compensation for verified cessation. On each visit, CO < 7 ppm = USD 25 at 1st visit, increasing by USD 5 for each consecutive negative sample, to a maximum of USD 50. If negative sample, no payment and schedule reverted to starting point 4. Combined (LA + CM) group: Combined the CM and lung age elements |
|
| Outcomes | Primary: 7‐day PPA at 6 months, cotinine‐confirmed Secondary: N of visits attended, smoking rates, N of quit attempts, change in FTND score, change in self‐efficacy score |
|
| Notes | New for 2015 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “120 sequentially numbered opaque sealed envelopes were externally prepared that included random assignment to one of four interventions. Randomization sequence was computer‐generated using a block randomization approach with randomly ordered four and eight sample blocks” |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | > 80% followed up at 6m across all groups |
| Other bias | Unclear risk | Ethnicity significantly different for LA group from others (P = 0.03), but no report of interaction with key outcomes. Authors stated they had predetermined to combine CM and non‐CM arms if they found no interaction between lung age and CM |
Etter 2016.
| Methods | 2‐arm, parallel‐group, individually randomised controlled trial. 2011 to 2016 Country: Switzerland |
|
| Participants | 805 regular smokers, smoking at least 5 CPD for at least 1 year. Baseline CO reading of at least 10 ppm. Baseline saliva cotinine reading of NicAlert level 1 or higher (≥ 10 ng/mL). Age > 18 years old CG n = 404, IG n = 401 Mean age 42, Mean CPD = 16. Median annual income, CG: USD 20,700 IG: USD 19,200. Occupation, % Unemployed CG: 18 IG: 20 Student CG: 45 IG: 42 Mother at home CG: 3 IG: 1 Manual CG: 12 IG: 15 Clerical, administrative CG: 13 IG: 12 Professional CG: 9 IG: 10. Mean years of education: 15 | |
| Interventions |
Control Group: Internet‐based support Experimental Group(s): financial rewards of up to CHF 1,500 (USD 1650 in 2013) were paid to those participants biochemically verified as abstinent. Incentives given 6 times during 6 months: CHF 100, 150, 200, 300, 350, and 400 at 1, 2, and 3 weeks, and 1, 3, and 6 months, respectively (USD 110, USD 165, USD 220, USD 330, USD 385, and USD 440, respectively). If participants smoked or missed an assessment, the value of the next reward was reset to the value of the previous reward they had received Theoretical basis for intervention: not reported Duration of intervention: 6 months Length of follow‐up: 18 months |
|
| Outcomes | Continuous abstinence between 6 and 18 months, CO‐verified (0 to 3 ppm) and cotinine,10 ng/ml Quit attempts during the intervention phase (number, duration and dates), cigarette consumption, motivation to quit, confidence in ability to quit, Use of the online smoking cessation programme |
|
| Notes | New for 2019 update Previously listed as ongoing. Relatively affluent population compared to other countries Funding: "From the Institute of Global Health, Faculty of Medicine, University of Geneva, Geneva, Switzerland. The study was funded by the Swiss Tobacco Prevention Fund (Swiss Federal Office of Public Health), grant 11.001733. Dr. Etter’s salary was paid by the University of Geneva. The authors have reported that they have no relationships relevant to the contents of this paper to disclose." Decarations of interest: "The Swiss Tobacco Prevention Fund, which funded the study, suggested that the follow‐up should be extended to 12 instead of 6 months after the final incentives were received, but had no other role in the conduct of the study." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "sealed opaque envelopes drawn by participants. Neither the researchers nor the participants could know in advance the content of the envelopes. We did not use blocks for randomization. Participants could not be blinded to their assignment group. Researchers were not blinded, but online data collection at follow‐up was computerized" |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "very high follow‐up rates." "All randomized participants were included in the denominator and participants with missing data at follow‐up were counted as smokers." |
Fraser 2017.
| Methods | 2‐group randomised clinical trial. 2013 to 2017 Country: USA Setting: recruited from Wisconsin Quit Line, primary care clinics, and community advertisements |
|
| Participants | 1900 community‐dwelling smokers accessing a quit line or recruited from primary care or advertising CG: n = 952; IG: n = 948 60% women. Mean age 45. Mean CPD = 16. 51% and 41% black and white respectively. Education: 61% high school or less |
|
| Interventions |
Control Group: All participants were incentivised for participating in baseline and 6‐month follow‐up biochemical assessment visits. Quit line coaching included a pre‐quit call that typically occurred at study enrolment and 4 additional proactive calls. Participants could also initiate calls to the WTQL for additional assistance. WTQL quit coaches made 3 attempts (per protocol) on different days to reach a participant for each proactive call, leaving messages at least twice if possible. Those callers not reached on the first 2 proactive calls were sent a letter urging them to call. Study participants also received a mailed quit guide, access to recorded medication information (by phone), and access to Web Coach®, an online cessation programme maintained by the quit line. WTQL quit coaches routinely recommended that participants obtain a prescription for a Medicaid‐approved smoking cessation medication from their primary care provider (at minimal or no co‐pay). Participants in the control condition could receive a total incentive of USD 80: USD 40 each for attendance at the baseline and 6‐month follow‐up biochemical assessment visits. Compensation was in the form of prepaid Visa gift cards and took 2 to 4 weeks from the point of contact Experimental Group(s): All participants were incentivised for participating in baseline and 6‐month follow‐up biochemical assessment visits. Incentive condition participants were additionally incentivised for participating in WTQL calls and for biochemically determined abstinence at the 6‐month follow‐up visit. Participants in the Incentive condition could receive a total payment of USD 270: USD 30/call for up to 5 WTQL calls, USD 40/visit for attending the baseline and 6‐month follow‐up assessment visit, and USD 40 for producing biochemical evidence of abstinence at the 6‐month follow‐up visit Theoretical basis for intervention: None specified Duration of intervention: 6 months Length of follow‐up: 6 months |
|
| Outcomes | PPA at 6 months. CO ≥ 7 ppm. Clinics chose different cut‐scores for the urine cotinine test; Almost all the clinics chose to define smoking as a value that exceeded either 50 ng/mL, 100 ng/mL, or 200 ng/mL, depending on the clinic. 4 clinics used 300 ng/mL as the smoking cut‐score. Treatment engagement, medication use | |
| Notes | New for 2019 update Funding: "This research was supported by Funding Opportunity Number 1B1CMS330876 from the Centers for Medicare & Medicaid Services." Decarations of interest: "No financial disclosures were reported by the authors of this paper." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation occurred by computer‐generated lists (Appendix, available online), with order stratified by county and race |
| Allocation concealment (selection bias) | Unclear risk | Quote: “These randomization lists were supplied by the research team to the Wisconsin Tobacco QL and then programmed to automatically determine randomization at the time required in the consent protocol” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 552 intervention (58%) and 562 control followed up at 6 months (59%) |
Gallagher 2007.
| Methods | Randomised 3‐arm controlled trial Country: USA Setting: 3 psychiatric case management sites in La Frontera, Arizona |
|
| Participants | 180 smokers, aged 18+, English‐speaking, smoked at least 10 CPD for at least 3 years, CO > 10 ppm. Diagnosed with DSM‐IV Axis 1 psychotic‐spectrum or affective disorders resulting in long‐term mental illness and experiencing significant symptoms and functional impairment. 48% women, 76% Anglo, av age 43, av FTQ 6.1, av CPD 24.8 Not required to commit to cessation, but 98% expressed interest either in quitting or in reducing Randomised to contingent reinforcement (CR, n = 60), contingent reinforcement + NRT patches (CR+NRT, n = 60), or self‐help (Control, n = 60) | |
| Interventions | 1. CR: Weekly visits weeks 1 to 4 (Phase 1), fortnightly weeks 6 to 12 (Phase II), monthly weeks 16 to 24 (Phase III). Payments USD 25 for baseline assessment and USD 5 per visit, plus USD 20 per abstinent visit in Phase I, USD 40 in Phase II, USD 60 in Phase III, and USD 80 if abstinent at 36‐week follow‐up. Maximum payable USD 580 for attendance + abstinence. At each visit weight, pulse rate, smoking status, intention to quit, withdrawal symptoms, CO, BP measured 2. CR+NRT: As CR Group, plus 16‐week course of 21 mg NRT patches, plus supporting instructions 3. Control: Visits at baseline and weeks 20 and 36, plus encouraged to use the community smoker helpline, ALA and ACS self‐help information. In all groups, salivary cotinine measured at baseline and at weeks 20 and 36; brief symptom inventory (psychiatric symptoms) at baseline and weeks 6, 16 and 36; FTND at baseline and at weeks 10, 24, 36 | |
| Outcomes | Abstinence at weeks 20, 36 Verified by expired CO < 10 ppm and by salivary cotinine < 15 ng/mL Other outcomes: smoking reduction, change in psychiatric symptoms | |
| Notes | Additional information supplied by the author New for 2008 update Study funded by Arizona Biomedical Research Commission | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Unconcealed computer‐generated random‐number lists [personal communication] |
| Allocation concealment (selection bias) | High risk | Study staff oversaw allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No significant differences between groups: Attrition for CR at weeks 20 and 36 was 37% and 43%; CR+NRT at weeks 20 and 36 was 35% and 36%; and Controls 52% at both time points |
Ghosh 2016.
| Methods | 2‐arm RCT (dates not reported) Country: USA Setting: Head and Neck Surgery clinic at the Philadelphia Veterans Affairs Medical Center |
|
| Participants | 14 patients with a previous diagnosis of head and neck cancer who had completed treatment or were undergoing treatment. Age 18+. Actively smoking at least, on average, five cpd. CG: n = 8 IG: n = 6. 'the majority' male gender. Mean age 60. all black/African American. Education: 1 to 3 years at college. Income: $30,000 to 39,999. CPD reported as packs per day CG; 1, IG: 1.5 to 2. |
|
| Interventions |
Control Group: Participants in each study arm were offered free enrolment in a Veterans Administration‐sponsored smoking cessation course. Attendance was recorded at each of the 3 classroom sessions. For all participants, a payment of USD 50 was made for each class attended. Payments for attendance at each class took place at the conclusion of the class on that day Experimental Group(s): information about smoking cessation and financial incentives in the form of cash payments at specific time intervals if class attendance or smoking abstinence was confirmed At 30 days: USD 150; At 3 months: USD 150; At 6 months USD 150 Theoretical basis for intervention: not reported Duration of intervention: 6 months Length of follow‐up: 6 months reported but methods state 12 months |
|
| Outcomes | PPA CO‐confirmed at 6 months, but cut‐off not defined | |
| Notes | New for 2019 update Contacted author to request clarification on some issues but did not hear back Funding: not reported Decarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation was performed, at the time of study enrolment, by the physician or clinical staff (physician assistant), according to a specific schema. Slips of paper were sequentially numbered with integers from 100 to 299, and for each person enrolling in the study 1 slip was selected at random. The number on the slip of paper in the envelope became the participant’s study identification number. Group assignment was as follows: even numbers were assigned to the control group (information only), while odd numbers were assigned to the experimental group (financial incentives plus information) |
| Allocation concealment (selection bias) | High risk | See above |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Biochemically verified, but cut‐off level was not defined |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 24 randomised, only 14 included in analysis (others were post‐randomisation exclusions but numbers not reported by group). All lost to follow‐up at 6 months apart from 2 in intervention group |
| Other bias | High risk | Inconsistent reporting of length of follow‐up. Methods state 12 month follow‐up but only 6‐month follow‐up reported |
Giné 2010.
| Methods | Randomised 3‐arm controlled trial. Study conducted between August 2006 and May 2007 Country: Philippines (Mindanao); the CARES study (Committed Action to Reduce and End Smoking), described as randomised |
|
| Participants | 2000 smokers aged ≥ 18, identified as "obvious smokers", and approached in the street by Green Bank marketing employees 3 waves of recruitment: 1) and 2) Butuan city; August 2006 to December 2006; 3) Ampayon; February 2007 to May 2007. Totals were 418 smokers enrolled from waves 1 and 2, and 515 from wave 3. Allocation distributed as: Wave 1: 1a: 45%; 1b: 45%; cue cards: 5%; controls: 5% (to verify acceptability of CARES offer) Wave 2: 1a: 15%; 1b: 15%; cue cards: 30%; controls: 40% (to balance up numbers of cue card and control participants) Wave 3: presumably roughly 1:1:1 (no without‐deposit CARES assignments) |
|
| Interventions | All individuals approached were given a pamphlet on dangers of smoking, and a quitting tip sheet People agreeing to participate were given a brief baseline survey (age, smoking status) 1a: CARES + deposit collection (most were visited weekly by a bank employee to collect the money) 1b: CARES without deposit collection (pt had to go to a bank to deposit money) Both the CARES groups were encouraged to deposit the money they would normally spend on cigarettes (minimum 50 pesos (˜USD 1)) in a non‐interest‐bearing account 2: Cue cards: pocket‐sized graphic depictions of negative health consequences of smoking; choice of 1 from 4 different images 3. Control (no additional intervention) |
|
| Outcomes | All participants contacted and tested (NicCheck urine test for nicotine and cotinine) for smoking status; those proved abstinent received their deposit money back at 6‐months test. Those who could not be reached, did not attend, or who failed the test, forfeited their money to charity. Pre‐stated assessment at 6 months (PPA), and 'surprise' test at 12 months; continuous abstinence defined as abstinent at both time points. All non‐CARES participants received 30 pesos for attending for testing at 6 months; all participants, including CARES, received 30 pesos for attending 12 months test |
|
| Notes | New for 2015 update. Study was funded by the World Bank, and implemented by marketers of the Green Bank of Caraga Additional information supplied by the authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomised with stickers on backs of baseline questionnaires for waves 1 and 2; for wave 3, researchers used a calculation (residual of (birth date (dd+mm+yy)/3)) to allocate participants, 0s to CARES, 1s to cue cards, and 2s to control |
| Allocation concealment (selection bias) | Unclear risk | Researchers revealed allocation concealed under a sticker on the back of the baseline face‐to‐face questionnaire for waves 1 and 2. Algorithm effectively concealed allocation for wave 3. Change in methods may have compromised concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No differences between the groups; 36% attrition across the board in Wave 3 at 12 months. Nothing reported for waves 1 and 2 |
Glasgow 1993.
| Methods | Cluster‐randomised controlled trial Country: USA Setting: 18 Oregon worksites (8 experimental, 10 control), stratified on number of employees and estimated smoking prevalence |
|
| Participants | Smokers defined as ≥ 7 cigs/week. Smoking prevalence av 21.5%; av age 40.5, 37% M, av 18.5 CPD. Smokers in intervention sites had higher education levels, and rated themselves more likely to try and quit smoking within next 6 months. 23% of baseline smokers in intervention sites joined the programme. 474 participants in intervention sites, 623 in control sites | |
| Interventions | 1. Experimental: USD 10 for each monthly PPA over 1 year of programme + monthly worksite lottery (USD 5 to USD 20 first 6 months, then minimum USD 50 for 2nd 6 months). 12‐month sweepstake for USD 200, USD 100 and USD 50 at each worksite. Also 'good buddy' nonsmokers' lottery prize. No formal quitting support 2. Control: Baseline and follow‐up surveys at 1 year, 2 years | |
| Outcomes | PPA at 1 year, 2 years Validation: CO < 9 ppm and salivary cotinine | |
| Notes | This is the HIP study (Health Incentives Program). Great variability in outcome across sites (2‐year follow‐up cessation rates Int: 14 to 33%, Control: 9 to 27%), with 30% lost to follow‐up at 2 years Study was funded by National Cancer Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the worksites were then randomized". Method not stated |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 12 months, 19% of incentives group had left, vs 24% of no‐incentives group At 24 months, 27% of incentives group had left, vs 32% of no‐incentives group; No statistically significant differences |
| Other bias | Unclear risk | Data extrapolated from percentages |
Halpern 2015.
| Methods | 5‐arm randomised controlled trial. Study conducted between February and October 2012 Country: USA Setting: Web‐based and worksite‐based across the USA |
|
| Participants | Employees of CVS/Caremark (retail pharmacy outlets) and their families and friends. Aged 18+, smoking at least 5 cpd, with internet access, and interested in learning about ways to quit. Mean age 33, 63% women, 74% income < USD 60,000, 79% white, mean CPD 15, 64% in preparation stage of change (ready to quit within 30 days) |
|
| Interventions | All participants were paid for completing questionnaires and submitting samples, and all used the Way to Health web‐based portal for communicating, and accounting A random sample of 5% of enrolled participants were invited for cotinine screening and offered USD 100 for completing the cotinine assay, to discourage non‐smokers from signing up Control Group (N = 468): Usual care, i.e. information about local SC services, ACS cessation guides, and for the 41% on health benefits free access to behavioural support and NRT 1. Individual rewards (N = 498): usual care, plus participants received USD 200 for sustained abstinence at each of 14 days, 30 days and 6 months, + a 6‐month USD 200 bonus for sustained abstinence at that point 2. Collaborative rewards (N = 519): usual care, plus participants grouped into teams of 6, linked by proximal TQDs. Rewards for sustained abstinence were given at 14 days, 30 days and 6 months, calculated at USD 100 per successful quitter in the group, i.e. up to USD 600 per person at each time point if all 6 remained quit, + USD 200 sustained abstinence bonus at 6 months. Linked by a web‐based chat room throughout study, for mutual support 3. Individual deposits (N = 582): usual care, plus participants put up USD 150 of their own money (by debit or credit card) within 60 days of enrolment or prior to the TQD, whichever came first. They received USD 200 for confirmed abstinence at each of 14 days, 30 days and 6 months, + a 6‐months USD 200 sustained abstinence bonus. 4. Competitive deposits (pari‐mutuel principle) (N = 471): usual care, plus participants put up USD 150 of their own money (by debit or credit card) within 60 days of enrolment or prior to the TQD, whichever came first. Participants were grouped into teams of 6, linked by proximal TQDs. USD 600 per person was available, distributed at 14 days, 30 days and 6 months to successful quitters only, + USD 200 sustained abstinence bonus at 6 months. So USD 1200 potentially available at each time point for sustained abstinence, e.g. if 2 people quit at 14 days but relapsed by 30, the 2 quitters would get USD 600 each at 14 days and then no more rewards for anyone in the group. Participants got anonymised descriptions of their team‐mates to encourage competitive interaction All intervention arms offered potentially the same financial returns, i.e. USD 800 per quitter, but for the 2 deposit arms this includes USD 150 of the participants' own money |
|
| Outcomes | Primary: Sustained abstinence (14 days, 30 days, 6 months) at 6 months, cotinine‐verified (anabasine/anabitine for NRT users) Biochemical verification: salivary cotinine < 10 ng/ml (urinary anabasine/anabitine < 3 ng/ml) Secondary outcomes: initial quit rate at 14 days, sustained abstinence at 30 days and at 12 months; self‐reported quit rates at 12 months; rates of acceptance of the assigned intervention |
|
| Notes | New for 2015 update Funding was from National Cancer Insitute grant R01 CA159932 (SDH) and National Institute of Aging grant RC2 AG036592 (DAA and KGV), and through in‐kind support from the host company |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomized individually”, stratified on 2 variables: access to full company‐sponsored healthcare benefits; household income (USD 60,000 [median company income]) “We developed an adaptive randomization algorithm that updated the assignment probabilities to the five arms after every third enrolled patient. Updated probabilities reflected the inverse of the proportion of participants assigned to that arm who accepted the intervention, relative to total acceptance across arms”. |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses not reported; ITT analyses included all randomised. Compliance rates were rewards 89.9%, deposits 13.9% |
Halpern 2018.
| Methods | 5‐arm RCT. 2015 to 2017 Country: USA Setting: Employees of companies using wellness programme |
|
| Participants | N = 6006 employees and spouses of company wellness programmes. CG; n overall = 3600, consisting of n = 813 usual care; n = 1588 free cessation aids; n = 1199 free e‐cigarettes. IG: Reward group n = 1198, redeemable deposit n = 1208 | |
| Interventions |
Control Group: access to information about benefits of smoking cessation and motivational text message service plus free cessation aids (NRT, bupropion or varenicline with NJOY EC if standard therapies tried and did not work) Experimental Groups: REWARD Group: as control, plus USD 600 in rewards for sustained abstinence. eligible to earn USD 100, USD 200, and USD 300 if at 1, 3, and 6 months after the quit date, respectively, they submitted blood or urine samples for testing and the samples were negative for nicotine metabolites REDEEMABLE DEPOSITS group: as control, plus USD 600 in redeemable funds deposited in separate account for each participant with money removed from account if cessation milestones not met (same schedule as rewards group) Theoretical basis for intervention: not reported Duration of intervention: 6 months Length of follow‐up: 12 months |
|
| Outcomes | PPA at 12 months. "Co verified cut off 20 ng per milliliter was the primary method for confirming abstinence". Urine sample with an anabasine level of < 3 ng per milliliter or a blood carboxyhaemoglobin level of less than 4%. For users of e‐cigarettes who had a positive cotinine sample (cotinine level ≥ 20 ng per milliliter), also accepted a blood carboxyhaemoglobin level of < 4%. PPA for quitting at 1 month and sustained abstinence rates at 3 months and 6 months | |
| Notes | New for 2019 update Funding: "Supported by a grant from the Vitality Institute to the University of Pennsylvania Center for Health Incentives and Behavioral Economics." Decarations of interest: none reported by authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated, but low levels of completion. However, "Because the definition of the primary outcome was biochemically confirmed sustained abstinence, participants who did not submit samples were coded as not having met the primary outcome" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Poor post‐randomisation intervention engagement. 6006 randomised and included in ITT analysis. Only 1191 followed up as 'engaged' cohort, included in secondary analysis. At 12 months, very few participants completed self‐report assessment: 6/1588 control; 21/1198 rewards; 33/1208 redeemable |
Harris 2015.
| Methods | Pilot RCT. Dates not stated Country: USA Setting: rural Appalachia Ohio and Kentucky prenatal clinics |
|
| Participants | 17 pregnant women (mean = 10¾ weeks pregnant) aged 18+, daily smokers (reporting smoking at least 2 CPD verified by breath CO readings and urinary cotinine levels). IG: CM (n = 7) CG: SCHB (phone‐delivered counselling) (n = 10). Mean age 24. 88% identified as white. Mean CPD = 12. SES and education not reported | |
| Interventions |
Control Group: SCHB participants received 5 telephone calls from a registered nurse, and as many as 5 check‐in calls Experimental Group(s): a 6‐week web‐based CM programme, with 2 follow‐up sessions that occurred after the 6‐week programme ended but before birth. The web‐based CM programme was used to verify breath CO measurements. Each participant was loaned a piCO Smokerlyzer, web camera, and if necessary a laptop computer with Internet access. The Motiv‐8 portion of the CM programme lasted 6 weeks and consisted of 5 phases: Baseline (7 days), Shaping (4 days), Abstinence (21 days), Thinning (5 days), and Return to Baseline (5 days). During each phase, participants submitted video recordings of themselves twice a day (at least 8 hours apart) giving breath samples using the Smokerlyzer. They could earn vouchers exchangeable for online purchases with major retailers (e.g. Best Buy, Wal‐Mart) for criterion breath samples based on programme phase. For the Abstinence phase, participants were required to have breath CO levels 4 ppm to indicate abstinence and to earn vouchers. Escalating pay schedule: 1st sample that met criteria earned voucher of USD 1; vouchers then increased in value by USD 0.25 for each consecutive breath sample that met the abstinence criterion. USD 5.00 bonus for every 6 consecutive breath samples that met the abstinence criterion. Participants could earn a maximum of approximately USD 800 during study participation. If sample did not meet criterion, then the participant did not receive reinforcement and the value of the next voucher was reset to USD 1.00. However, if, after a reset, the participant provided 3 consecutive samples that met the criterion for recent abstinence, then the voucher returned to the value at which the reset occurred In addition, 2 spot checks (at random times, participants aware in advance) during remaining months of pregnancy following programme end – if abstinent, participant received USD 100 in cash Participants were aware these follow‐up sessions would occur. These appointments were intended to extend incentives for abstinence later into pregnancy Theoretical basis for intervention: not reported Duration of intervention: 6 weeks but with pre‐birth follow up (intervention) appointments Length of follow‐up: Until end of pregnancy. Mean 8.47 months |
|
| Outcomes | PPA at end of pregnancy verified by urinary cotinine (cut‐off not defined). Smoking reduction (time line follow‐back method), Stages of Change Ladder (SCL), Modified Fagerström Test of Nicotine Dependence (mFTND); Post‐treatment assessments measured birth outcomes (e.g. gestational age at birth, birth weight, and time spent in NICU) and smoking‐related variables |
|
| Notes | New for 2019 update Funding: Not reported Decarations of interest: "The authors report no conflict of interest or relevant financial relationships" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number generator available via the Internet (Research Randomizer, n.d.) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants reported as followed up |
Heil 2008.
| Methods | Study design: RCT; conducted between 2001 and 2003 Country: USA Setting: 4 group obstetric practices and the Women, Infants, and Children (WIC) programme in Burlington, VT | |
| Participants | 82 women, gestational age ≤ 20 weeks, smoked at all in the previous 7 days, locally resident, English‐speaking Mean age 24, Education 11.8 years, mean CPD 18.6; mean gestation 9 weeks |
|
| Interventions | Abstinence monitoring for first 5 days (Monday to Friday) for all participants, then twice weekly (Mondays, Thursdays) for next 7 weeks, then once a week (Wednesday) for 4 weeks, then every other Wednesday until delivery. In post‐partum period, monitoring increased to every Wednesday for first 4 weeks, then bi‐weekly (every other Wednesday) for next 8 weeks to week 12. Final 24‐week post‐partum testing
All participants received standard care from their clinic, over and above trial conditions. Also a pamphlet from study staff at baseline, plus another for those not smoking at end of pregnancy. NRT was discouraged for all participants, as it might contaminate testing Experimental Group (40 women): Contingent vouchers: awarded for proven abstinence during first 5 days. From 2nd week vouchers given for urine cotinine ≤ 80 ng/ml. Vouchers entirely contingent on biochemical specimens, not on self‐report. Values started at USD 6.25, increasing by USD 1.25 per consecutive negative sample, to a maximum of USD 45, where they stayed until a missed visit or a positive test. If reset required, value went back to start point, but 2 valid tests restored to previous level Control Group (37 women): Vouchers delivered independent of smoking status, at USD 15 per visit antepartum and USD 20 per visit post‐partum. This would average the mean payments earned in the other group Voucher‐based reinforcement therapy (VBRT) applied until 12 weeks post‐partum |
|
| Outcomes | Abstinence at end of pregnancy, 12 and 24 weeks post‐partum Biochemical confirmation by urine cotinine < 80 ng/ml, apart from CO ≤ 6 ppm for first week |
|
| Notes | New for 2015 update Funding was from research grants R01DA14028 (STH) and GCRC M01RR109 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was stratified based on the clinic where participants received their pre‐natal care". Participants "were assigned randomly". |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Relatively high compliance (83% to 95%) with assessment schedules, and no differences between groups. Withdrawals only for termination or foetal death. 3 intervention and 2 control participants removed from the denominators |
Hennrikus 2002.
| Methods | Cluster‐randomised controlled trial Country: USA Setting: 24 Minneapolis‐St Paul worksites; 2 x 3 factorial design, stratified by gender and education |
|
| Participants | 2402 current smokers (smoked at least 100 cigs in lifetime). Mean age 39, 56.2% women, 62% married/partner | |
| Interventions | 1. Group: 13 group sessions over 2 months. 2. Phone: sent printed materials, inc ALA Freedom from Smoking + 3 to 6 telephone counselling sessions 3. Choice: free choice between group or phone programmes. All programmes offered 3 times over 18 months; smokers could join more than once. Half of the sites in each intervention were offered direct incentives for participation and for quitting: Quitters at 1 month won USD 20 and entered lottery for grand prize (USD 500 as 1 prize (5 sites), 2 x USD 250 (6 sites) or 4 x USD 125 (1 site)). Drawn about every 6 months | |
| Outcomes | Baseline, 7‐day PPA at 12 months, 24 months Validation: self‐report, countersigned by friend or family member for monthly abstinence. Grand draw prize winners + 24 months random sample of quitters (paid USD 25 for compliance) tested for salivary cotinine | |
| Notes | Study was funded by the National Heart, Lung, and Blood Institute. Data extrapolated from percentages | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Four worksites were randomly assigned to each of the 6 experimental conditions". Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not all participants had abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 12 months 14.5% lost, and at 24 months 18.3% lost |
| Other bias | High risk | Group dropouts were not followed up; phone dropouts were rung for up to 10 times for each counselling session, and were then left messages or sent letters |
Higgins 2014.
| Methods | Study design: 3‐arm RCT; December 2006 to June 2012 Country: USA Setting: Group obstetric practices and the WIC office in Burlington, VT |
|
| Participants | 130 women; mean age 24; mean gestational age 10 weeks; mean CPD pre‐pregnancy 18; while pregnant 8 12 women (RCV: 4; UCV: 5; NCV: 3) withdrawn from denominators because of termination or foetal death |
|
| Interventions | All participants received standard antenatal care for smoking; study staff delivered additional counselling as 4 sessions over first 2 weeks, at final antepartum visit and during 3 post‐partum study visits. Those who quit during pregnancy got brief counselling at routine visits whenever they reported temptations to smoke. Study staff used a printed booklet tailored for pregnant smokers, Need help putting out that cigarette? (ACOG 2001, URL in study report) Women chose one of next 2 Mondays as a quit date; were then monitored from Monday to Friday that week, Monday and Thursday next 7 weeks, then every Wednesday for 4 weeks, then every other Wednesday until delivery for the UCV and NCV groups; in the RCV group it was every other week to week 12 and then every 3rd week through to delivery. After delivery all 3 groups were on weekly monitoring for 4 weeks, then every other week through to 12 weeks post‐partum. 1 final assessment at 24 weeks. Relapsers could continue the schedule or recycle back round the entire process (only offered once per woman). This was used fairly equally by all 3 groups (RCV 40%, UCV 46% and NCV 41%) Experimental Groups: 1. Usual contingent voucher (39 women): First 5‐day week validated by CO, thereafter by urine cotinine. Vouchers based exclusively on valid biotesting, not self‐report. Vouchers began at USD 6.25 and rose by USD 1.25 each time to a max of USD 45. Missed or positive results meant schedule was reset, but 2 passes reset the schedule to former point 2. Revised contingent voucher (40 women): Same pattern as above, but with potentially USD 296.25 available early in weeks 1 to 6 by maintaining a ≤ 4 ppm breath CO in week 1 (i.e. USD 18.75 day 1 to USD 33.75 day 5 (going up by USD 3.75 per day)), testing cotinine‐negative on 2nd Monday for an additional USD 87.50 and thereafter testing negative twice a week to week 6. The 2nd test each week increased by USD 15.50 if it was negative and the first had also been negative. This was meant to reinforce early continuous abstinence Non‐contingent voucher [Control Group] (39 women): Voucher value was USD 15 per antepartum and USD 20 per post‐partum visit, irrespective of smoking status. Total available earnings were comparable across all 3 groups Duration: to 24 weeks post‐partum |
|
| Outcomes | 7‐day PPA at baseline, 1 month, end of pregnancy, 2, 4, 8, 12 and 24 weeks post‐partum Biochemical validation: CO ≤ 4 ppm or 6 ppm, + urine cotinine ≤ 80 ng/ml |
|
| Notes | New for 2015 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated; they were "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses not reported, apart from withdrawals and foetal demise, but ITT analyses conducted |
Lasser 2017.
| Methods | Unblinded, randomised clinical trial. 1 May 2015 to 4 September 2017 Country: USA Setting: Boston Medical Center, a large urban hospital |
|
| Participants | 352 low‐SES and minority daily smokers. Age of 18+, smoking 10 or more CPD in the past week; in contemplation or preparation stage of readiness to quit smoking; having a primary care clinician in the Section of General Internal Medicine or Department of Family Medicine. CG n = 175; IG n = 177. 54% women. Mean age 50. Mean cpd = 15. 'majority reported belonging to a racial/ethnic minority group'. SES: ≤ $20 000 193 (55%); > $20 000 90 (26%); Refused/do not know 69 (20%). Education: ≤ High school 80 (23%); High school or GED 137 (39%); > High school 133 (38%) |
|
| Interventions |
Control Group: Enhanced traditional care control participants received a low‐literacy smoking cessation brochure and a list of hospital and community resources for smoking cessation Experimental Group(s): up to 4 hours of participant navigation delivered over 6 months, and financial incentives for biochemically‐confirmed smoking cessation at 6 and 12 months following enrolment. USD 250 for smoking cessation 6 months after study enrolment, as confirmed by a salivary cotinine, and an additional $500 for an additional 6 months after the initial cessation (12‐month time point), confirmed by a salivary cotinine. Participants who did not quit smoking at 6 months and who had been unaware of the exact dollar amount of the incentive were given a 'second chance' to quit smoking and earn USD 250 at 12 months, having been notified of the exact amount of the incentive Theoretical basis for intervention: The Social Contextual Model; operant conditioning for incentives Duration of intervention: 12 months Length of follow‐up: 12 months |
|
| Outcomes | Continued abstinence biochemically verified by saliva or urine cotinine (≥ 10 ng/ml) or anabasine test (for those on NRT, < 3 ng/mL). Receipt of counselling, medications | |
| Notes | New for 2019 update In terms of misreport or no measurement 21 out of 41 self‐reported at 12 months confirmed quit from Int group versus 4/19 control group Funding: "This study was supported by American Cancer Society (grant No. 125785‐RSG‐14‐034‐01CPPB). The funder/sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication." Decarations of interest: "Dr Quintiliani was a consultant on a research grant to Partners HealthCare Inc unrelated to the work presented in this article. No other conflicts are reported." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomized participants using a random number generator with allocation concealment to a research assistant using sealed envelopes. Randomization was stratified by stage of change (contemplation vs preparation) with regard to smoking cessation" |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes given to research assistant |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically verified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention: 48 lost to follow‐up, Control: 53 lost to follow‐up (at 12 months). ITT analyses used |
Ledgerwood 2014.
| Methods | Randomised controlled trial; conducted December 2007 to January 2011 Country: Michigan, USA Setting: University clinic |
|
| Participants | 81 smokers, aged ≥ 18, FTND ≥ 4, literate in English; recruited through newspaper ads, bulletin boards, health fairs, broadcast messages in a health centre and a university All participants had to submit 5+ CO samples during 1st week, to qualify to enter the study. Received USD 1 per sample, + a bonus of USD 20 if all 10 samples submitted in week 1 N = Standard care (SC): 17; Traditional contingency management (TCM): 28; Enhanced contingency management (ECM): 36; 61% women, mean age 44.8, mean FTND 6.3 |
|
| Interventions | 1. SC: Weeks 2 to 5: Monitoring of CO and cotinine + brief counselling (≃ 5 mins) twice a day, 5 days a week for 4 weeks; participants received USD 1 per sample, regardless of result, + weekly bonus of USD 20 for submitting all 10 samples 2. TCM: as SC, + chances to win prizes for every negative CO or cotinine or both. On Day 1, participant drew for a prize if CO down by at least 3 ppm; thereafter draws only if CO ≤ 6ppm. Weekly N of draws increased by 1 a day for every day (2 tests) abstinent, up to 5 daily draws by end of week. TCM urn contained 250 slips of paper: 50% had some kind of reward, i.e. 44.8% small (worth around USD 1, e.g. snacks, toiletries); 4.8% large (worth around USD 20, e.g. gift certificates, electronics); and 0.4% jumbo (worth USD 100, e.g. DVD player, gift certificate). In weeks 3 to 5, if Monday cotinine ≤ 100 ng/ml (i.e. weekend abstinence), participant had 5 draw chances. In whole 5‐week study course 180 draws and 15 bonus draws were possible 3. ECM: as TCM, but in 1st week prizes were guaranteed for negative tests. In week 1, ECM urn had 91.2% small, 8% large, and 0.8% jumbo rewards. For remaining 3 weeks, urn contained 65.8% no prize, 30% small, 4% large, and 0.2% jumbo |
|
| Outcomes | PPA, cotinine‐verified, at 2 months and 6 months Other outcomes: Prize money won; differences between TCM and ECM schedules |
|
| Notes | New for 2015 update Funding by NIH grant, and Helene Lycaki/Joe Young Sr funding through the State of Michigan |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All participants had to have submitted at least 5 samples to qualify for entry. Quote: "Statistician‐prepared sequentially numbered randomization envelopes concealed group assignment until assigned". Stratified by gender and by any CO ≤ 6ppm (none vs 1 or more) on treatment day 1 (quit date). Randomised in a ratio of 2:1 CM:control |
| Allocation concealment (selection bias) | Low risk | Envelopes concealed allocation until assigned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 months: SC 1/17; TCM 6/28; ECM 5/36 lost 6 months: SC 2/17; TCM 7/28; ECM 10/36 lost All analyses conducted as ITT, and differences non‐significant |
Ondersma 2012.
| Methods | Study design: 4‐arm factorial RCT; July 2008 to November 2009 Country: USA Setting: 4 prenatal clinics in Detroit, MI | |
| Participants | 110 pregnant smokers, aged 18+, gestation < 27 weeks. Allocated to CD‐5As (N = 26); CM‐Lite (N = 28); CD‐5As+CM‐Lite (N = 30): TAU (N = 26). Mean age 27.9 years, 81.8% B; mean CPD 8. |
|
| Interventions |
Control (TAU): Standard care from prenatal clinic staff, without any input from research team. Participants used PC tablets to complete a brief series of questions about their musical preferences, watched a series of tailored music videos, and answered questions about the videos, i.e. computer time was blinded, and comparable with intervention groups All participants completed a baseline 11‐item assessment of ease of use, enjoyment, helpfulness, satisfaction Experimental Groups: 1. CD‐5As: PC tablet, with interactive software; participants accessed with headphones, for privacy. Content was 5As programme (Ask, Advise, Assess, Assist, Arrange), or 5Rs (Relevance, Risks, Rewards, Roadblocks, Repetition) for those unwilling to set a quit date. Included a 4‐ to 6‐minute professional video of black male obstetrician + 3 testimonials , tailored to participant’s reactivity, defensiveness, quit status. All gave positive advice to quit. Programme led participant through advice, feedback, plan development, support options Participants at baseline completed 5‐item additional assessment of likelihood of quitting, intention to quit, confidence in ability to quit, readiness to quit, desire to quit 2. CM‐Lite: designed for non‐treatment‐seeking participants in a healthcare setting. No proactive tracking, but relying on participant to request verification of smoking status. Testing offered only at antenatal visits, rather than multiple times a day. Participants eligible for unlimited incentivisation attempts, but only 5 reinforcement vouchers available (retail gift cards worth USD 50) Programme was delivered by a website, which took participants through verification process and recorded result (urinary cotinine test) 3. Combination of CD‐5As and CM‐Lite |
|
| Outcomes | Follow‐up at 10 weeks 7‐day PPA, 30‐day CA, validated by CO < 4 ppm, urinary cotinine (Nicalert strips) < 100 ug/ml Mean N of samples submitted, and modal N of negative samples; mean amount of gift vouchers earned; mean amount earned among those submitting a sample; help‐seeking behaviour |
|
| Notes | New for 2015 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer for brief intervention (1:1), then by random‐number generator (www.randomization.com) for CM component |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | CD‐5As: 3/26 lost; CM‐Lite 6/28 lost; CD‐5As+CM‐Lite 4/30 lost; TAU 3/26 lost. All participants included in ITT analyses |
Rand 1989.
| Methods | Quasi‐randomised 3‐arm controlled trial. Country: USA Setting: Employees at a Baltimore hospital |
|
| Participants | 51 smokers, av age 38.1, 74.5% women, CPD 26 | |
| Interventions | Introductory lecture, ACS brochure Clearing the Air, baseline measures taken. After confirmed 5‐day abstinence (USD 25 reward), participants assigned either to: 1. Contingent Group: Contingent payment/frequent monitoring: checked twice a week at random times, paid USD 4 per CO < 11 ppm 2. Non‐contingent Group: Non‐contingent payment/frequent monitoring: checked twice a week at random times, paid USD 4 per CO sample, regardless of reading 3. Control Group: Non‐contingent payment/infrequent monitoring: checked monthly at random times, paid USD 40 per CO sample, regardless of reading. Programme lasted 26 weeks | |
| Outcomes | 3 x daily CO samples < 11 ppm to confirm 5‐day qualifying abstinence. Monthly survival analysis (continuous cessation) to 6 months. Dropouts and relapsers treated as continuing smokers | |
| Notes | Study funded by National Institute on Drug Abuse | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not stated; "subjects were randomly assigned to one of three follow‐up groups" after 5 days confirmed abstinence. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4/51 participants dropped out in abstinence week; by 6 months, 6 contingents, 1 non‐contingent and 4 controls had dropped out. Although dropout reasons were generally unrelated to participation (e.g. heart attack, pregnancy, work move), significantly more contingents dropped out (Chi2 = 13.63, P = 0.005) |
Rettig 2018.
| Methods | Pilot RCT. 2014 to 2018 Country: USA Setting: Johns Hopkins Cancer Treatment Centers |
|
| Participants | 30 smokers with head and neck or thoracic malignancies undergoing radiation therapy. Age 18+. Reported smoking cigarettes within the previous 14 days. CG: n = 11; IG: n = 19.
Mean age = 55. Mean CPD = 8. N = 11 women (38%). Ethnicity: white: 18 (62%); black or other non‐white 11 (38%). Education: Did not finish high school n = 7 (24%); High school graduate or GED n = 11 (38%); Post‐high school n = 11 (38%). Income: < USD 15,100: 11 (38%); USD 15,000 to USD 49,999: 6 (21%); USD 50,000 to USD 99,999: 4 (14); > USD 100,000: 7 (24%) Mental health history: anxiety: 3 (10%) depression: 6 (21%) bipolar disorder: 2 (7%) HADS‐D score < 8 (not depressed): 20 (69%); 8+ (depressed): 9 (31%). Ever used injection drugs No: 23 (79%) Yes: 6 (21%) |
|
| Interventions |
Control Group: ‘Enhanced usual care’: single counselling session at the baseline visit. The baseline visit for the control group comprised 4 intervention components to constitute enhanced usual care: (1) brief counselling by a trained tobacco treatment specialist consistent with the '5 As' recommended by the United States Department of Health and Human Services37; (2) a smoking cessation workbook tailored to people with cancer; (3) contact information for local and national smoking cessation resources, including some offering free nicotine replacement therapy; and (4) mental health screening to evaluate depressive symptoms Experimental Group: Usual care plus up to 4 additional daily visits during the first week. At baseline, the intervention group received the smoking cessation workbook and underwent intensive tobacco treatment specialist motivational interviewing, with brief follow‐up motivational interviewing sessions at subsequent study visits, daily for the first week, then weekly for 8 weeks. Other additional interventions received included: enrolment in the National Cancer Institute’s free smokefreetxt text‐messaging programme (smokefree.gov); contingency management at each visit, by which participants received USD 5 gift cards for biochemically‐confirmed smoking abstinence; and guided pharmacotherapy. Pharmacotherapeutic options offered were combination nicotine replacement therapy (patch/gum, patch/lozenge, or patch/nasal spray), bupropion, and varenicline. Participants were permitted to opt out of intervention components Theoretical basis for intervention: not reported Duration of intervention: 8 weeks Length of follow‐up: 12 months |
|
| Outcomes | PPA at 12 months, CO‐verified, confirmed as exhaled CO 8 ppm. Smoking abstinence at 1, 2, 3, 4, 5, 6, 7 and 8 weeks, and at 3 and 6 months. Smoking intensity (total cigarettes per previous 7 days), reduction from baseline, and total cigarettes smoked | |
| Notes | New for 2019 update Funding: "This work was supported by the National Institute of Dental and Craniofacial Research and National Institutes of Health Research Training in Otolaryngology grant (grant number 2T32DC000027026) and the Maryland Department of Health and Mental Hygiene Cigarette Restitution Fund (grant number PHPA‐G2034). The study sponsors had no role in study design or in the collection, analysis, or interpretation of data" Decarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to enhanced usual care (“control”) or intervention groups using 1:1 block randomisation with stratification by cancer site (head and neck versus thoracic) and sex. Randomisation was generated using SAS software (Cary, NC) |
| Allocation concealment (selection bias) | Low risk | Allocation concealed in sequentially‐numbered opaque envelopes until study group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One participant in the control group was lost to follow‐up after the baseline visit; therefore, the analytic cohort comprised 29 participants (19 intervention and 10 control)" |
Rohsenow 2015.
| Methods | 4‐arm RCT. Recruitment: June 2002 to June 2006. End date not reported Country: USA Setting: a state‐funded inner‐city 28‐day residential substance abuse treatment programme |
|
| Participants | 184 people meeting current DSM‐IV substance‐use disorder (SUD) criteria, smoking at least 10 CPD for the past 6 months, not engaged in smoking treatment. CG: n = 86; IG (CV): n = 98. Gender N = 102, 55.4% women. Mean age = 34.5. Mean CPD = 22.3. Ethnicity: 83.2% (n = 153) were white, 9.2% (n = 17) were black, 7.5% (n = 14) were of other races; 6.6% (n = 12) were Hispanic. SES: mean legal income was USD 9487 in the past year, mean education level was 12.2 years. 71.2% alcohol abuse or dependence, 73.9% cocaine abuse or dependence, 52.8% opiate abuse or dependence, and 37% marijuana abuse or dependence | |
| Interventions |
Control Group: BA (Brief advice): 1 x 15‐minute session to promote motivation to quit, adapted for SUD recovery issues. Advice given re: quit date, NRT, support groups, resources, smoking cessation pamphlets and corrective information, if needed. Followed up after first session at 7, 14 and 19 days (10 to 15 minutes each). Progress asked and revision of goals, if necessary Experimental Group(s): MI (motivational interviewing): 1 x 45‐minute session providing education re: smoking cessation, discussion of pros and cons, health risks and costs, corrective information, goal‐setting + written smoking cessation pamphlets. Followed up after first session at 7, 14 and 19 days (15 to 30 minutes each). Progress asked and revision of goals, if necessary Intervention and control groups then randomised into receiving either Contingency Vouchers (CV) or Non‐Contingency vouchers (NCV) Contingent voucher procedures were provided during a 5‐day reduction phase plus a 14‐day abstinent phase. CO monitoring used an EC50 Micro III Smokerlyzer. In NCV participants could earn the same payments a day for 19 days as those randomised to CV, simply for providing breath samples as scheduled Theoretical basis for intervention: MI Duration of intervention: 4 sessions for MI; 4 sessions for BA. 19 days Length of follow‐up: MI: 12 months |
|
| Outcomes | PPA at 12 months. CO level ≤ 4 ppm and salivary cotinine level ≤ 15 ng/ml. Timeline follow‐back method to assess smoking reduction (number of CPD), number of heavy‐drinking days, number of drug‐use days, and relapse to any heavy drinking or drug use over the 12 months | |
| Notes | New for 2019 update Funding: "Supported by 1 RO1 DA13616 from the National Institute on Drug Abuse; two Senior Career Research Scientist Awards from the Department of Veterans Affairs (DJR and PMM); and K05AA019681 from the National Institute on Alcohol Abuse and Alcoholism. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs." Decarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified random assignment, using urn randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated. CO verification not required for those in prison at follow‐up Quote: "At follow‐up, people with a CO 4 ppm, cotinine 15 ng/mL (if not using NRT), or missing CO or cotinine data, or with self‐reported smoking were coded as having smoked with the following exception: if the participant was in prison, self‐report was accepted since biological verification equipment was not allowed so lack of verification was unrelated to participant decision (Number of prisoners claiming abstinence: n = 2 at 3 months, n = 1 at 6 months, n = 3 at 12 months.)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12‐month follow‐up was completed by 139 (75.5%) |
Rohsenow 2017.
| Methods | RCT. Initial start date not reported Country: USA Setting: 2 inner‐city state‐funded residential SUD treatment programmes |
|
| Participants | 340 smokers who had not sought smoking treatment in a residential SUD treatment programme, meeting current DSM‐IV SUD criteria and smoking at least 10 CPD for the past 6 months. CG (NV) n = 168. IG (CV) n = 172. Gender: 33% women. Mean age: 37.58. Mean CPD = 19.48. Ethnicity: white: 86%; black/ African American: 10%; Asian: < 1%; multi‐racial: 2%. Annual Income: USD 0 to USD 9999: 59%; USD 10,000 to USD 29,999: 26%; USD 30,000 to USD 49,999: 9%; USD 50,000+ 6%. Education years, mean = 12.09. Alcohol use disorder: 76%; opiate use disorder: 49%; cocaine use disorder: 60%; marijuana use disorder: 36% |
|
| Interventions |
Control Group: NV: vouchers not contingent on smoking status. (USD 8 per sample), plus a USD 40 bonus for providing all 33 samples (total possible = USD 304). All received BA, a standard of care for smokers not seeking smoking treatment, then 7, 14, and 19 days later (subsequent sessions, 10 to 15 minutes). 4 sessions in total. and up to 8 weeks of NRT. To encourage participants to provide a breath CO sample regardless of whether they had been smoking, USD 1 was provided simply for providing the sample (non‐contingent) regardless of results (total possible = USD 33). Experimental Group(s): CV: 14 days of vouchers for smoking abstinence (based on CO readings twice a day) after a 5‐day smoking reduction period. Reduction phase. USD 2 per test for a 25% reduction from baseline CO, USD 4 for 50% reduction, and USD 6 for a 75% or greater reduction. Abstinence phase. Escalating schedule of payments provided increasing levels of payments in vouchers for each successive CO reading ≤ 6 ppm. USD 3 for the first sample, increasing by USD 0.50 for each consecutive negative test to USD 16.50 for the 28th consecutive abstinent breath sample, plus USD 10 bonuses provided every time 3 consecutive readings showed abstinence. Whenever a breath sample did not meet the criterion for abstinence, the participant earned no voucher and the payment schedule reverted to the initial USD 3 level, then after 3 consecutive abstinent samples the schedule returned to the payment level at which the reset occurred Total possible payment. Participants who completed all 19 days of samples and missed no more than 3 of the scheduled breath tests earned a USD 40 bonus voucher (total possible = USD 433 plus USD 33 for showing up = USD 466) Theoretical basis for intervention: not reported Duration of intervention: 19 days vouchers plus 8 weeks NRT Length of follow‐up: 12 months |
|
| Outcomes | PPA at 12 months, CO level ≤ 4 ppm and salivary cotinine ≤ 15 ng/ml At 1, 3, 6 months, the Timeline Followback interview for number of cigarettes each day, number of days of drug use, and number of heavy drinking days. At pretreatment and at 1 month, participants completed a Smoking Self‐Efficacy Questionnaire |
|
| Notes | New for 2019 update Funding: "Supported by 1 R01 DA023995 from the National Institute on Drug Abuse; a Senior Career Research Scientist Award from the Department of Veterans Affairs to the first author; and K05AA019681 from the National Institute on Alcohol Abuse and Alcoholism. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the official views of the National Institutes of Health." Decarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data for Intervention: 27% (CV) and 32% (NCV). Judged low as below 50% and ITT used |
Romanowich 2015.
| Methods | RCT June 2005 to November 2010 Country: USA Setting: San Antonio |
|
| Participants | 146 volunteers who worked at the University of Texas Health Science Center at San Antonio, lived near that centre, or both Smoking > 15 CPD, regularly for at least 1 year, and planning to quit smoking within the next month. Age 18+ with CO > 15 ppm All participants were classified as early success (ES) from the results of a 5‐visit baseline fixed reinforcement period before randomisation Participants were all classified as ES based on their performance during a 5‐visit abstinence trial. During this trial, participants received USD 5.00 for each breath sample. CG: n = 47, IG HTT percentile criterion: n = 37; IG HTT fixed n = 40; IG‐ES Escalating: n = 59, IG‐ES Fixed: n = 58. Mean age 41. Mean CPD = Control: 21.9 (6.3), Escalating: 21.7 (5.3), Fixed: 24.3 (6.9). Gender (% female) = Control:16 (55), Escalating: 25 (42), Fixed: 26 (45). Ethnicity: white: Control: 16 (55%), Escalating: 43 (73%), Fixed 39 (67%). Income: < USD 15,000 Control: 8 (28%), Escalating: 26 (44%), Fixed: 29 (50%); USD 15,000 to USD 24,999 Control: 7 (24%), Escalating: 12 (20%), Fixed: 13 (22%); USD 25,000 to USD 34,999 Control: 7 (24%), Escalating: 6 (10%), Fixed: 6 (10%); > USD 35,000 Control: 7 (24%), Escalating: 15 (25%), Fixed: 10 (17%). Education: GED or high school: Control: 11 (38%), Escalating: 21 (36%), Fixed: 23 (40%); Vo tech or associated: Control: 12 (41%); Escalating: 21 (36%); Fixed: 22 (38%); Bachelors+: Control: 6 (21%), Escalating: 17 (29%), Fixed: 13 (22%) |
|
| Interventions |
Control Group: Payments for CO tests, not contingent on abstinence. a two‐in‐three chance of receiving a payment on any visit (the probability for each visit was independent of other visits), regardless of their breath CO sample Experimental Group(s): Escalating reinforcement group: Specifically, the value of the payment available started at USD 5.00 and increased by USD 0.50 with the delivery of each breath CO sample Fixed reinforcement group: the value of the potential payment for these participants was always USD 19.75, regardless of how many consecutive criterion breath CO samples they had previously submitted For both escalating and fixed reinforcement groups, the total payment amount possible was USD 1185.00 over the 60‐visit intervention period Theoretical basis for intervention: not reported Duration of intervention: 60 visits, approximately 12 weeks Length of follow‐up: 6 months |
|
| Outcomes | PPA at 6 months. CO criterion of < 4 ppm . saliva cotinine level < 20 ng/ml. Use of smoking cessation medication. CPD in past 6 week at 6 months | |
| Notes | New for 2019 update CO cut‐off of < 3 ppm stated in NCT entry but < 4 ppm stated in e‐mail correspondence with author Funding: "The research reported in this paper was supported by Grant DA013304 to R. J. Lamb." Decarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not computer‐generated Quote: "Random assignment to one of the three groups was accomplished by assigning two participants to the escalating reinforcement group, two to the fixed reinforcement group, and one to the control group from each group of five participants who completed the abstinence trial" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | > 80% follow‐up across all conditions (data supplied by authors). Quote: "All participants randomly assigned to one of the study conditions were included in the analysis. All missing data points were counted as not meeting the breath CO criterion (i.e. positive) for that visit." |
Secades‐Villa 2014.
| Methods | Randomised controlled trial Country: Spain Setting:Community‐based, conducted in Addictive Behaviors Clinic at Oviedo University |
|
| Participants | 92 smokers of > 10 CPD, aged > 18, motivated to quit, recruited by flyers, local media ads and word of mouth Mean 64.1% women; mean age 45.8 (SD 12.1); mean CPD 21.7 (SD 8.7); mean FTND 5.7 (SD 1.8); CBT 35.4% in full‐time work, CBT+CM 55.8% |
|
| Interventions | 1. CBT (control) group: Group‐based counselling, 5 to 6 participants. 1‐hour sessions, weekly over 6 weeks. Main technique nicotine fading, based on weekly 30% reduction, with abstinence required from week 5 onwards. Also info about tobacco, a behavioural contract, self‐monitoring, withdrawal strategies, physiological feedback, social reinforcement, relapse prevention Cotinine and CO collected twice a week, i.e. 11 samples over the 6 weeks 2. CBT + CM: As CBT, plus voucher system, beginning in week 5 CBT session; negative ≤ 80 ng/ml. First negative specimen earned 80 points (1 point = EUR 1), with a 20‐point increase for each subsequent and consecutive negative sample. Missing samples counted as negative, and missing or failed set the reward back to 80 points. Max value EUR 300 (3 consecutive negative specimens) Points could be exchanged for vouchers for “leisure activities, cinema, theatre, museums, sports events, gyms, adventure sports, meals in restaurants, training, purchases in department stores, bookshops, clothes shops and art shops, and spa and beauty services”. |
|
| Outcomes | Primary: 7‐day PPA at EoT, at 1 month and at 6 months; CA at 6 months (all 3 time point tests to be negative) Biochemical validation by CO < 4 ppm, cotinine < 80 ng/ml Secondary: Treatment retention; % attending throughout the 6‐week course Testing was twice a week, rather than daily+ |
|
| Notes | New for 2015 update Funding was from Spanish Ministry of Science and Innovation grant PS12011‐22804, and predoctoral grants BP12‐037; FOundation for the Promotion of Applied Science Research and Technology in Asturias; and Spanish Ministry of Economy and Competitiveness (BES‐2012‐053988). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Eligible participants were randomly assigned …, in accordance with a computer‐generated randomization list” (p. 64) |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition at 6 months not reported (at 1 month, 10 control and 1 intervention lost to follow‐up) |
Shoptaw 2002.
| Methods | Randomised 4‐arm controlled trial Country: USA Setting: 3 narcotic treatment centres in LA |
|
| Participants | 175 smokers (≥ 10 CPD, expired CO > 8 ppm, cotinine > 30 ng/mL), av age 44, 39.5% women, av 22.1 CPD. No significant differences between groups, except group 3 reported higher cocaine use than other groups | |
| Interventions | 2‐week baseline and randomisation period, then 12 weeks treatment with NRT patches, tapered from 21 mg for 8 weeks, to 14 mg for 2 weeks and 7 mg for 2 weeks. CO and urine samples taken x 3/week. Randomised to: Group 1. NRT patch only Group 2 NRT patch + RP Group 3. NRT patch + CM: USD 2 for 1st CO sample < 8 ppm; each consecutive sample rewarded with voucher increased by USD 0.50, + bonus USD 5 for every 3 consecutive samples. If a sample > 8 ppm, reward process reverted to USD 2 level again, but was restored to previous scale after 1 round of 3 consecutive samples < 8 ppm. Participants could earn up to USD 447.50 Group 4. NRT patch + RP + CM (see group 3 procedure) | |
| Outcomes | Baseline measures, + thrice‐weekly breath and urine samples throughout 12 weeks treatment, + weekly self‐report, and same measures at 6 months and 12 months. Participants with missing data were counted as continuing smokers | |
| Notes | Additional outcome data supplied by the authors Study funded by National Institute on Drug Abuse, and National Cancer Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "an urn randomization procedure". A randomised 2 x 2 repeated measures design |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Group A (patch only): 6 months 5/43, 12 months 7/43 dropped out; Group B (patch + CM): 6 months 6/43, 12 months 8/43 dropped out |
Tappin 2015a.
| Methods | Phase II single‐blind randomised controlled trial, conducted July 2012 to September 2013 Country: UK Setting: Large health board area, inner city, Greater Glasgow and Clyde (Scotland) |
|
| Participants | 612 pregnant smokers, aged 16+, English‐speaking, gestation 24+ weeks, exhaled CO > 7 ppm. Intervention n = 306, control n = 306. | |
| Interventions |
Control: Standard care: All smokers identified at maternity booking referred to the stop‐smoking services (SSS), who attempted to contact them. SSS set up a 1‐hour session to discuss cessation, + 4 weekly phone calls to support, and 10 weeks free NRT if wished. SSS contacts at 4 weeks, 12 weeks (if quit at 4), 34 to 38 weeks gestation, and 6 months post‐natal if quit at 34 to 38 weeks Experimental Group: As control, plus: up to GBP 400 of shopping vouchers (Love2shop), for engagement or for quitting, or both: GBP 50 for attending the 1‐hour face‐to‐face and setting a TQD (engagement). At 4‐week phone check‐up, if self‐reported no smoking for past 2 weeks had a researcher visit and CO breath test < 10 ppm; if OK, another GBP 50 voucher Routine phone call at 12 weeks (for those quit at 4) + CO test, GBP 100 voucher if validated Some time between 34 and 38 weeks gestation, all participants contacted by helpline staff. Researchers visited self‐reported quitters for CO and cotinine, and gave GBP 200 for confirmed intervention quitters To minimise losses to follow‐up, all participants (intervention and control) reporting smoking status and with saliva or urine sample at final follow‐up given a GBP 25 shopping voucher (engagement) |
|
| Outcomes | Abstinence at 4 weeks for all participants (2‐week PPA, CO < 10 ppm); 12 weeks, if quit at 4, intervention only (4‐week PPA, CO < 10 ppm); 34 to 38 weeks gestation, all participants (< 5 cigs in past 8 weeks, CO < 10 ppm, cotinine (urine < 44.7 ng/ml; saliva < 14.2 ng/ml) if self‐reported quit); 6 months post‐natal for confirmed quitters at 34 to 38 weeks: still quit or < 5 cigs since quit date, cotinine‐confirmed. | |
| Notes | Published after last search date Change to protocol meant that research team were allowed to collect routine blood samples (residual) in late pregnancy (32 to 42 weeks) from the last 200 women enrolled |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The Glasgow clinical trials unit embedded the randomisation in the trial database using randomised permuted blocks, with a block length of four”. |
| Allocation concealment (selection bias) | Low risk | Quote: “Allocation was concealed from staff and clients until after consent and recruitment.” “The helpline … contacted women, confirmed that all selection criteria had been met, enrolled participants using telephone consent, and conducted concealed random allocation”. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition equal across groups: 43/303 (14%) control, 46/306 (15%) incentives. ITT analysis assumed all lost to follow‐up were continuing smokers, and cross‐checked this where possible by residual blood samples |
Tevyaw 2009.
| Methods | Randomised controlled trial Country: USA Setting: Public and private colleges and universities |
|
| Participants | 110 young adult smokers, with a baseline CO ≥ 10 ppm. Mean age 19.7, 38.2% women, av 12 CPD, 77% white. No significant differences between groups on any demographic variables. Motivation to quit not required (51% reported no plans to quit). | |
| Interventions | 2 x 2 psychosocial condition x reinforcement condition 1. 3 sessions motivational enhancement therapy (MET) counselling over 2 weeks, with either contingent or non‐contingent rewards 2. 3 sessions of progressive muscle relaxation control (REL), with either contingent or non‐contingent rewards 3 weeks of reinforcement, with CO samples collected in person twice daily from each participant All participants received USD 75 for completion of baseline interview, and cash payments for follow‐ups, i.e. USD 25 at 1 month, USD 35 at 3 months, USD 75 at 6 months, + USD 40 for timely completion of all 3 follow‐ups (i) Non‐contingent rewards: USD 5 for each sample, + USD 10 per week for attending ≥ 80% of sample collections. Total available USD 240 (ii) Contingency management rewards: Week 1: USD 1 for reduction of 25% to 49%, USD 2 for 50% to 74%, USD 3 for > 75%. Weeks 2 to 3: Payments for abstinence (< 5 ppm): USD 3 for 1st abstinent sample, increasing by 50c for each subsequent abstinent sample. Additional USD 1 for 2 consecutive abstinent samples. Non‐abstinent sample meant no bonus for that reading and the clock set back to USD 3 for next abstinent reading. After a reset, 4 consecutive abstinent samples reset the bonus to the pre‐reset level. Total available USD 285.50 | |
| Outcomes | 7‐day PPA at 6 months. Validation: CO < 5 ppm for daily samples; cotinine < 15 ng/mL for non‐attenders sending in samples at follow‐up | |
| Notes | New for 2011 update. Additional information supplied by the authors Study funded by National Institute on Drug Abuse, and Dept of Veterans Affairs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "participants were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 months attrition: CM+MET: 2/28; CM+REL: 2/27; NR+MET: 1/27; NR+REL 1/27 |
Tuten 2012.
| Methods | Study design: 3‐armed RCT; May 2005 to January 2009 Country: USA Setting: Center for Addiction and Pregnancy, Baltimore, MD |
|
| Participants | 102 pregnant, methadone‐maintained smokers, aged 18+, ≤ 30 weeks gestation, nicotine‐dependent or smoking 10+ CPD Contingent behavioural incentives (CBI): 42; non‐contingent behavioural incentives (NCBI): 28; treatment as usual (TAU): 32 Mean age 30.7; mean CPD 18; % unemployed 94.8; mean gestational age 16.5 weeks |
|
| Interventions | All participants completed an initial 8‐day residential course, then went to outpatient status. In 1st week, all completed an Addiction Severity Test (ASI), a structured clinical interview for DSM‐IV disorders (SCID), revised FTND. CO testing 3 times a week, urine samples 3 times a week (cotinine) + random cocaine testing once a week ASI repeated at 1 month and 3 months and at 6 weeks post‐partum, + CO and urine tests. At each testing all participants received brief (10 mins) MI feedback. Standard info on adverse effects of smoking for mother and baby All this was classified as ‘Treatment as usual (TAU)’. Experimental Group: CBI: 12 weeks of eligibility for CBI rewards contingent on reduction or abstinence. Incentives for each negative breath test on Mondays, Wednesdays and Fridays, as follows: week 1: any reduction; weeks 2 to 4 10% reduction; weeks 5 to 7: 25% reduction; weeks 8 to 9: 50% reduction; weeks 10 to 11: 75% reduction; week 12 – delivery: abstinence (CO < 4 ppm) Voucher started at USD 7.50 and increased by USD 1 a day up to USD 41.50. If negative sample missed through the 12 weeks, schedule was reset to USD 7.50. If she achieved 5 consecutive negative tests, the voucher value was restored to former level NCBI: “pseudo‐yoked” schedules. NCBI participants were each yoked to a random participant in the pilot study (i.e. had submitted CO samples for at least 2 weeks). Participants were told that their behaviour did not determine rewards received, but that they would receive incentives in line with a previously established schedule. NCBI participants had to give breath and urine samples to receive their scheduled incentive. They were eligible for 12 weeks or until delivery |
|
| Outcomes | Primary target outcome was reduction. Abstinence measured at end of 12‐week programme, and 6 weeks post‐partum Cessation was PPA, biochemically verified (CO < 4 ppm; urinary cotinine < 300 ng/ml) |
|
| Notes | New for 2015 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were assigned randomly" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | CBI: 8/42 lost; NCBI 4/28 lost; TAU 7/32 lost, but all included in ITT analyses. |
Van den Brand 2018.
| Methods | Cluster‐RCT; 2016 to 2018 Country: The Netherlands Setting: Companies of varying size and from different industry types in the Netherlands. Companies were required to facilitate a smoking cessation training programme at the workplace during or directly after working hours |
|
| Participants | 604 employed smokers, aged 18+, had smoked tobacco for at least 1 pack year. Mean age 45. IG n = 319; CG n = 285. CPD at baseline ≤ 10 IG n = 58 (18%) CG n = 55 (19%); 11 to 20 IG n = 179 (56%) CG n = 159 (56%); 21 to 30 IG n = 59 (18%) CG n = 58 (20%); ≥ 31 IG n = 9 (3%) CG n = 3 (1%); missing IG n = 14 (4%) CG n = 10 (4%). Ethnicity not reported. Income level: low IG n = 111 (35%), CG n = 68 (24%); middle IG n = 91 (29%), CG n = 84 (29%); high IG n=76 (24%) CG n = 105 (37%); missing IG n = 41 (13%) CG n = 28 (10%). Education level low: IG n = 97 (30%); CG n = 62 (22%); middle IG n = 136 (43%); CG n = 119 (42%); high IG n = 75 (24%); CG n = 90 (32%); missing IG n = 11 (3%); CG n = 14 (5%) | |
| Interventions |
Experimental Group(s): Participants could earn 4 vouchers with a total worth of EUR 350. The first EUR 50 voucher was received on the condition of biochemically validated smoking abstinence at the end of the smoking cessation training programme. The second and third EUR 50 vouchers could be earned when participants were abstinent 3 and 6 months after finishing the cessation programme. At the end of the study (12 months after completion of the cessation programme), participants could earn an additional EUR 200 voucher The vouchers were sent by email in the form of a digital code that could be exchanged in a web shop for a large range of products or activities. Control Group: A smoking cessation group training programme consisting of a 90‐minute session each week for 7 weeks. The pre‐existing training programme was designed to help participants to initiate a quit attempt and guide them through the first few difficult weeks of quitting smoking, with an important role for group dynamics and peer support. Participants quit together at the start of the third session, and had quit smoking for about 1 month at the last session |
|
| Outcomes | Primary: continuous abstinence at 12 months. Cut‐off point 9 ppm Secondary outcomes: 3 and 6 months biochemically validated abstinence, and self‐reported abstinence |
|
| Notes | New for 2019 update Funding: "This study is funded by the Dutch Cancer Society (grant number: UM 2015–7943)" Decarations of interest: "DK received an unrestricted grant from Pfizer for an investigator‐initiated trial on the effectiveness of practice nurse counseling and varenicline for smoking cessation in primary care (Dutch Trial Register NTR3067). OS received institutional research grants from Pfizer for investigator‐initiated trials." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was generated by a digital programme using the biased urn method, in order to maintain allocation to intervention groups as balanced as possible. The randomisation programme was written by a statistician (BW), but companies were randomly allocated by an independent research assistant not involved in the study |
| Allocation concealment (selection bias) | Low risk | Group allocation was not revealed to participants or employers until the start of the first training session |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 post‐randomisation exclusion (reported) |
Volpp 2006.
| Methods | Randomised controlled trial Country: USA Setting: Philadelphia VA Medical Center |
|
| Participants | All outpatient self‐identified smokers invited to complete baseline survey. 404 surveyed, 179 eligible: 92 invited to join Incentives group, 87 to join Control group Mean age 52, 6% women, 25% white, 41.7% completed high school or GED, av 22 CPD, mean years smoking 30, 35% Fagerström score > 7, 17% smoking > 2 packs a day | |
| Interventions | 1. 5 free fortnightly sessions of SC programme, standardised group counselling, plus NRT patches every 2 weeks (4 weeks x 21 mg, 2 weeks x 14 mg, 2 weeks 7 mg) 2. As 1, plus USD 20 per session attended, + USD 100 if quit ˜ 30 days after programme completion (75 days post‐quit date) Incentives and control groups conducted separately, to avoid contamination, but same instructor, blinded to assignment and not involved in rewards distribution | |
| Outcomes | Primary: Initial enrolment within the programme (= attended 1st session) Secondary: Cumulative attendance, programme completion 7‐day PPA at ˜ 1 month post‐completion (75 days post‐quit date), and at 6 months post‐completion (˜ 7.5 months post‐quit date) among those who had quit at earlier time point. ITT analysis, included all 179 eligible smokers, whether or not they had joined the cessation programme Validation: urinary cotinine (< 500 ng/mL). USD 20 reimbursement for attending for validation procedure | |
| Notes | Sample size estimate of 100 per group would give > 80% power to test enrolment at 5% level of significance, with a 1‐sided test of equality of proportions New for 2008 update. Study funded by VA Health Services Research and Development; Center for Health Equity Research and Promotion; Leonard Davis Institute of Health Economics of the University of Pennsylvania School of Medicine; National Institute on Drug Abuse; National Cancer Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by permuted blocks of 4, stratified by level of smoking (± 2 packs per day) |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered surveys by computer‐generated lists of random numbers |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses at 1 month: Intervention 29/92, Control 25/87; Losses at 6 months: Intervention 31/92, Control 26/87 Enrolled in SC programme: Intervention 38, Control 17; Completion rates Intervention 23/38, Control 10/17 |
Volpp 2009.
| Methods | Randomised controlled trial Country: USA Setting: Multiple worksites of General Electric Energy Company Results were adjusted for stratification variables, i.e. worksite, income and amount smoked |
|
| Participants | 878 smokers, randomised to Int (436) or control (442). Av age 45, 35% women, av 20 CPD, 25% high school or lower, 65% income > 500% of poverty level. Motivation to quit not required. No significant baseline differences between groups on any demographic variables | |
| Interventions | All participants given information on local community‐based SC services, + received standard employee benefits, e.g. physician visits, SC pharmacotherapies. All received USD 20 per telephone interview at baseline and at 3 follow‐ups, plus USD 25 per confirmatory sample returned Intervention: Told they would receive USD 100 for completing an SC course, USD 250 for confirmed abstinence at 6 months, and USD 400 for confirmed sustained additional 6‐month abstinence |
|
| Outcomes | Prolonged abstinence at 9 months or 12 months. Those not abstinent at 3 months were retested at 6 months , and followed from then if abstinent All abstinent at both follow‐ups were assessed again 6 months later, i.e. at 15 months or 18 months 9 to 12 months endpoint used in 6‐month MA, and 15 to 18 months endpoint in 12‐month MA Validation: Cotinine by saliva or urine | |
| Notes | New for 2011 update. Additional information supplied by the author Study was funded by Centers for Disease Control and Prevention and by Pennsylvania Dept of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "performed in permuted blocks of four", stratified by level of smoking (± 2 packs per day), income and worksite. |
| Allocation concealment (selection bias) | Low risk | Quote: "assignments were concealed until all eligible criteria had been entered" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 6 months 50/436 (Intervention) and 47/442 (Control) lost to follow‐up, and 16 and 12 withdrew; At 6 to 12 months 43/436 (Intervention) and 35/442 (Control) lost to follow‐up, and 13 and 12 withdrew. At 12 to 18 months 4/436 (Intervention) and 2/442 (Control) lost to follow‐up, and 1 and 0 withdrew |
White 2013.
| Methods | Randomised controlled trial; conducted from December 2010 to March 2011 Setting: Thailand rural villages |
|
| Participants | 215 smokers (10.5% of eligible smokers in 30/42 villages) Participants grouped in 2‐person teams, either choosing their own partner or being randomly assigned based on village and gender. Controls also paired up N = 128 experimental; 68 control; 13% women, mean age 51, mean CPD 13.5 |
|
| Interventions | All participants received an initial group counselling session, and a further session at 3‐month follow‐up
Intervention Grp: signed a 'team commitment' contract: a) Opened a savings account, with a minimum deposit of THB 50 (USD 1.67), and a starter bonus of THB 150 (USD 5), with an extra bonus of THB 150 if the account balance reached THB 150 over the 10‐week deposit period. Community Health Workers visited weekly for the 10‐week duration, to try to elicit additional voluntary contributions b) Cash bonus of THB 1200 (USD 40) to each partner if both were abstinent at 3 months c) Weekly supportive text messages Intervention group received deposits back if verified quit at 3 months |
|
| Outcomes | 7‐day PPA at 3 months, 6 months, 13 to 16 months ("14 months"); urine cotinine verified at 3 months and 6 months , but 14 months self‐report only Participants not attending at 3 months and 6 months were contacted by CHW or by phone, and tested at home if claimed abstinent Other outcomes: % receiving 3‐month bonus; %s quit as teams at 3 months, 6 months and 14 months; partner choice vs random assignment; team vs individual enhancing likelihood of quitting; impact of text messages; cost effectiveness |
|
| Notes | New for 2015 update; Funded by grants from the US National Institute on Aging and the US National Institute for Child Health and Development Additional information supplied by the author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated random numbers" by an independent programmer |
| Allocation concealment (selection bias) | Low risk | Quote: "...concealing the sequence from other field staff and participants" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention: 1/128 death, 3/128 missing baseline data; Control: 1/68 missing baseline data |
White 2018.
| Methods | Cluster‐RCT; 2015 to 2017 Country: Thailand Setting: Large workplaces in the Bangkok metropolitan area |
|
| Participants | 4190 employees at large workplaces in the Bangkok metropolitan area (101 worksites from 84 Bangkok area companies) Age 18+; Healthy volunteers. Motivated to quit smoking. Smoker of 100+ cigarettes during lifetime and at least 10+ cigarettes a week. CG: n = 444. IG(s): 2) USD 20 individual bonus n = 508; 3) USD 40 individual bonus n = 482; 4) team bonus n = 495; 5) deposits n = 397; 6) deposits plus teammate (no bonus) n = 364; 7) deposits plus USD 20 individual bonus n = 515; 8) deposits plus USD 40 individual bonus n = 489; 9) deposits plus team bonus n = 496. Gender: the per cent male varied from 94.9% to 99.3%. Age: The per cent over age 45 varied from 6.5% to 18.5%. Mean CPD = 8. Ethnicity: not reported |
|
| Interventions |
Control Group: Participants in the control group (1) received usual care only, consisting of 2 elements: in‐person group counselling on smoking cessation and text messaging support with quitting. The group counselling consisted of 90 minutes of counselling delivered at each worksite by a trained smoking cessation counsellor. The text messaging programme, developed by the Thai Health Professional Alliance against Tobacco, provided 1 to 3 messages a day for 28 days, with advice, support, and encouragement for quitting smoking Experimental Group(s): 9 randomisation groups (8 experimental) consisting of a combination of 4 intervention components: usual care, refundable deposits, a teammate, and a cash bonus: 2) USD 20 individual bonus, 3) USD 40 individual bonus, 4) team bonus, 5) deposits, 6) deposits plus teammate (no bonus), 7) deposits plus USD 20 individual bonus, 8) deposits plus USD 40 individual bonus, 9) deposits plus team bonus. "Deposits. Participants in deposit programmes (groups 5 to 9) were asked to provide refundable deposits contingent on smoking abstinence. These participants made an minimum initial contribution of USD 3 (THB (Thai baht)100) at the enrolment meeting, which was kept under the care of an appointed company representative. Participants then received a personal deposit box, made out of metal and designed to be tamper‐proof (pictured in Supplemental Figure S1). Participants were free to make additional voluntary contributions in the box until the 3‐month follow‐up assessment. Study personnel encouraged participants to contribute at least as much as they had typically spent on tobacco. Participants gave the project an additional USD 5 as collateral for the safe return of the box, in order to prevent tampering or theft. At the 3‐month follow‐up assessment, study personnel opened each box using a can opener and recorded the total balance. All deposits were returned to the participant if the person was confirmed to be abstinent during the 3‐month assessment. Deposits were forfeited to the project if the person was found to have smoked Teammate. Participants in team‐based programmes (groups 4, 6, and 9) were randomly assigned to another participant from the same worksite as a teammate. Team assignment was stratified by work shift and native language in order to facilitate opportunities for communication. Pairings were announced at the enrollment meeting at each worksite Cash bonus. Participants in groups 2 and 7 were eligible for a cash bonus of USD 20 (THB 600) for abstaining from smoking at 3 months. Participants in groups 3 and 8 were eligible for a bonus of USD 40 for abstinence at 3 months. On average, this amount was roughly equivalent to 1 or 2 days’ wages, respectively. Participants in groups 4 and 9 were eligible for a team bonus of USD 40 only if both team members abstained from smoking at 3 months" Theoretical basis for intervention: not reported Duration of intervention: 3 months Length of follow‐up: 12 months |
|
| Outcomes | 7‐day point prevalence. Biochemically verified by urine cotinine test. cut‐off level of 200 ng/mL. PPA at 3 and 6 months. Programme acceptance | |
| Notes | New for 2019 update Results data are from manuscript in preparation provided by the author. Home‐based depositing strategy did not appear to lead to consistent use (Supp. Table S7), especially in the absence of regular reminders.The size of the worksites did not lend itself to the strategy we employed for pairing teammates. Many teammates did not know each other, and did not interact during the study period Funding/declaration of interest: sponsors and collaborators: University of California, Berkeley, National Institutes of Health (NIH), National Institute on Drug Abuse (NIDA), Mahidol University |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Worksites were digitally cluster‐randomised to usual care or one of 8 incentive designs |
| Allocation concealment (selection bias) | Low risk | A co‐author implemented the random allocation sequences using computer‐generated random numbers, concealing the sequence from field staff, company employees, and participants until after the baseline survey was completed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis.
Quote: "Eight smokers (< 0.02%) were omitted from the analyses due to missing baseline covariate data, providing a denominator of 4182 for all intent‐to‐treat analyses". Per protocol analyses presented and yield consistent results. Follow‐up rates ranged between 57 to 70% across all arms at 12m" (data supplied by author). |
Windsor 1988.
| Methods | Study design: 2 x 2 factorial pretest/post‐test control group design Country: USA Setting: University of Alabama, Birmingham |
|
| Participants | 378 smokers over 21 months recruitment, mean age 37, CPD 25; sex ratio not stated | |
| Interventions | Baseline survey, ALA Freedom from smoking in 20 days self‐help manual and A lifetime of freedom from smoking maintenance manual at quit date. Method 1: Controls: manuals only, brief chat Method 2: Cessation skills training (diary, deep breathing), contract to quit, and quit smoking 'buddy' (with buddy education) Method 3: Monetary incentives: USD 25 after 6 weeks confirmed cessation, and after 6 months confirmed cessation Group A: Method 1 only Group B: Methods 1 and 2 Group C: Methods 1 and 3 Group D: Methods 1, 2 and 3 | |
| Outcomes | 6 weeks, 6 months, 1 year. Baseline measure and saliva were obtained for thiocyanate (SCN) analysis of smoking status (≤ 100 ng/mL) Participants smoking > 2 cigs more than once in a follow‐up period counted as a smokers. Lost to follow‐up counted as continuing smokers | |
| Notes | As no significant effect of incentives was detected after 6 weeks, the authors collapsed Groups A and C for comparison with Groups B and D collapsed, to test programme efficacy Our MAs were conducted using Group C vs Group A (Windsor 1988), and Group D vs Group B (Windsor (B) 1988) Study funded by National Heart, Lung, and Blood Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated assignment method". |
| Allocation concealment (selection bias) | Low risk | Quote: "labels were placed in separately sealed envelopes". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Employees lost to follow‐up were counted as smokers. The data indicate that these individuals were equally distributed among groups". N lost to follow‐up in each group not provided |
| Other bias | Unclear risk | The last scheduled rewards were paid out to coincide with the final assessment, and may therefore have confounded that result. Data extrapolated from percentages |
ACS: American Cancer Society; ALA: American Lung Association; av: average (mean); B: black; CA: continuous abstinence; CBT: cognitive behavioural therapy; CHW: community health worker; CM: contingency management; CO: carbon monoxide; COPD: chronic obstructive pulmonary disease; CPD: cigarettes per day; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Edition 4; EoT: end of treatment; ICC: Intra‐class correlation coefficient; FEV: forced expiratory volume; FTND: Fagerström test for nicotine dependence; FVC: forced vital capacity; NICU: neonatal intensive care unit; NRT: nicotine replacement therapy; pp: post‐partum; PPA: point prevalence abstinence; ppm: parts per million; QTW: quit to win; RP: relapse prevention; SC: smoking cessation; SCN: saliva thiocyanate; TAU: treatment as usual
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alessi 2008 | Outcome was reduction rather than smoking cessation, and participants were followed for 12 weeks only New for 2011 update |
| Berg 2014 | Too short; follow‐up to 12 weeks New for 2015 update |
| Bowers 1987 | Outcomes were reduced CO and changes in blood pressure, not smoking cessation |
| Businelle 2014 | Too short, 5 weeks duration New for 2015 update |
| Cavallo 2007 | Randomised trial of school children, lasting 1 month New for 2008 update |
| Chivers 2008 | RCT of variable contingency management schedules; programme lasted for 2 weeks only. New for 2011 update. |
| Correia 2006 | 3‐week trial of college students, randomised to high or low payments for abstinence New for 2008 update |
| Crowley 1991 | Study 1 was not a controlled trial. Studies 2 and 3 did not give detailed 6‐month follow‐up |
| Crowley 1995 | Intervention is a competition (lottery ticket) rather than an incentive |
| Cummings 1988 | Follow‐up was only 3 months |
| Curry 1991 | Incentives were for use of the materials, not for smoking cessation |
| Dallery 2008 | RCT of deposit vs vouchers for abstinence; programme lasted only 20 days New for 2011 update |
| De Paul 1989 | Incentives component cannot be evaluated separately from the other components of the intervention |
| Donatelle 2000c | SOS II Programme: Non‐randomised; used the control group from Donatelle 2000a as historical controls New for 2015 update; pregnancy trial |
| Dunn 2008 | RCT of contingency management; study only lasted 2 weeks New for 2011 update |
| Dunn 2010 | Same intervention as Dunn 2008, but with bupropion an added option |
| Elliott 1968 | No control group, and followed up for 3 months only |
| Emont 1992 | Aim of the study was to enhance recruitment, not smoking cessation |
| Fortmann 1995 | Intervention being tested was self‐help versus nicotine gum. All participants could receive quitting incentive payment |
| Gadomski 2010 | Cohort study, not an RCT New for 2011 update |
| Gilbert 1999 | Long‐term incentives were for attending follow‐up, not for smoking cessation |
| Gilbert 2002 | No long‐term follow‐up outcomes beyond 31 days |
| Glover 2015 | Trial only 8 weeks duration, not necessarily to end of pregnancy or beyond New for 2015 update; pregnancy trial |
| Gottlieb 1990 | Competition was used as a recruitment tool, not for smoking cessation |
| Graham 2007 | No control group; incentives were paid for recruitment rather than cessation New for 2011 update |
| Gulliver 2004 | Participants randomised to partner support or no partner support; everyone attending follow‐up got a raffle ticket, regardless of smoking status New for 2015 update; pregnancy trial |
| Hanewinkel 2007 | Smoking prevention trial among school children in Germany, Finland and Netherlands. See Schools prevention review New for 2008 update |
| Haug 2017 | Intervention targets both smoking and alcohol use |
| Hertzberg 2013 | CM RCT in PTSD patients, only followed up for 3 months New for 2015 update |
| Higgins 2004 | Study previously included, but excluded from the 2019 update because only 16 out of 53 participants were randomly allocated |
| Hunt 2010 | Not randomised; only followed up for 3 months New for 2015 update |
| Jason 1990 | Some baseline differences between (non‐randomised) experimental and control companies, and intervention included several programme options as well as the incentive component |
| Jeffery 1988 | Intervention being tested was reduction versus cessation. Incentives were available to both groups |
| Jeffery 1989 | Not a controlled trial. All participants were eligible for incentives |
| Jeffery 1993 | Incentives were for attendance, not for cessation |
| Kassaye 1984 | Objectives were cessation and reduction, and long‐term follow‐up outcomes were not fully reported |
| Kendzor 2015 | Follow‐up only 3 months New for 2015 update |
| Kollins 2010 | Not a randomised trial, and participants were followed for 24 days New for 2011 update |
| Lamb 2004 | Trial of 102 adult smokers, rewarded for reduced CO in daily breath samples. Lasted 3 months, and outcome was lowered CO rather than cessation New for 2008 update |
| Lamb 2007 | Aim was reduction, not cessation; only followed up for 3 months New for 2015 update |
| Lamb 2010 | RCT of contingency management; followed for 3 months New for 2010 update |
| Lussier 2005 | Trial of 63 adult smokers randomised to 14‐, 7‐ or 1‐day contingency payments for abstinence. Lasted 2 weeks |
| MacKillop 2009 | Only followed up for 8 weeks New for 2015 update |
| Mantzari 2012 | Qualitative report; not an RCT New for 2015 update; pregnancy article |
| McDonell 2013 | Intervention targets psycho‐stimulant use |
| Meredith 2011 | Followed up only for 2 weeks New for 2015 update |
| Monti 2006 | 50 adult smokers randomised to MET+contingency payment, relaxation+contingency payment, MET+ noncontingency reinforcement or relaxation+noncontingency reinforcement. Followed for 3‐week treatment period New for 2008 update |
| Mooney 2004 | 97 adult smokers randomised to standard care, information or information+contingent payment. Lasted 15 days, and outcome was increased use of nicotine gum, not cessation New for 2008 update |
| NCT00508560 | Study terminated before completion |
| NCT00718835 | Follow‐up less than 6 months |
| NCT00807742 | Follow‐up less than 6 months |
| NCT00960375 | Follow‐up less than 6 months |
| NCT01040260 | Competition rather than incentives as intervention |
| NCT01145001 | Follow‐up less than 6 months |
| NCT01303081 | Follow‐up less than 6 months |
| NCT02195570 | Study terminated before completion |
| Nowicki 1984 | Complex intervention, including monthly lottery; cannot separate out the effect of the components New for 2015 update; pregnancy trial |
| Olsen 1990 | Incentives could not be evaluated separately from other components |
| Ormston 2015 | Not an RCT; report on the "quit4u" stop smoking service New for 2015 update; pregnancy study |
| Pardell 2003 | No baseline measurements reported, and incentives could not be separated from other programme components |
| Parker 2007 | Complex intervention, including USD 100 lottery for 30‐day abstinence. Cannot separate out the components New for 2015 update; pregnancy trial |
| Paxton 1980 | Study previously included, but excluded from the 2019 update because participants were not randomised |
| Paxton 1981 | Study previously included, but excluded from the 2019 update because participants were not randomised |
| Paxton 1983 | Study previously included, but excluded from the 2019 update because participants were not randomised |
| Perkins 2010 | Cross‐over RCT, lasted for 6 weeks New for 2011 update |
| Poole 2001 | Prospective cohort study, not a controlled trial |
| Radley 2013 | Not an RCT; report on the 'Give it up for baby' programme New for 2015 update; pregnancy study |
| Rohsenow 2005 | 187 substance abusers randomised to contingency reinforcement or non‐contingent reinforcement for 19 days. No results reported for CR group New for 2008 update |
| Roll 2008 | RCT of deduction CM vs incremental CM; Trial only lasted 5 days, and abstinence was for 48 hours New for 2011 update |
| Romanowich 2010 | No non‐incentive control group, and only followed up for 3 months New for 2015 update |
| Romanowich 2013 | No non‐incentive group, and only followed up for 3 months New for 2015 update |
| Romanowich 2014 | No non‐incentive group, and only followed up for 3 months New for 2015 update |
| Sheikhattari 2016 | Less than 6 months follow‐up |
| Sigmon 2012a | No non‐incentive group, and only 3 months follow‐up New for 2015 update |
| Sloan 1990 | Non‐experimental design, with no control group |
| Spring 1978 | Long‐term follow‐up outcomes not fully reported |
| Stitzer 1985 | Outcome was reduced CO, and only 6 weeks follow‐up |
| Stoops 2009 | RCT of contingency management; programme lasted 6 weeks New for 2010 update |
| Strecher 1983 | No long‐term outcomes, and no control group |
| Tanaka 2006 | Complex intervention, in which impact of incentive cannot be isolated New for 2011 update |
| Tidey 2011 | Only followed up for 2 weeks New for 2015 update |
| Wagner 2013 | Ineligible interventions in comparison study of two RCTs (no incentives) New for 2015 update |
| Walsh 1997 | Complex intervention, including a lottery for quitters. Cannot separate out the lottery component New for 2015 update; pregnancy study |
| Winett 1973 | Incentives were paid for attendance, reduction and cessation; no true control group (no incentives) |
| Winhusen 2014 | Unable to establish length of follow‐up (no response from author) New for 2015 update |
| Wiseman 2005 | 20 cocaine users randomised to contingent or non‐contingent payments. Treatment period and follow‐up lasted 2 weeks New for 2008 update |
| Yi 2008 | Outcome was reduction rather than smoking cessation, and duration of study only 5 days New for 2011 update |
| Yoon 2009 | RCT of variable contingency payments for abstinence; followed up for 2 weeks New for 2011 update |
CO: carbon monoxide
Characteristics of studies awaiting assessment [ordered by study ID]
NCT02713594.
| Methods | Open‐label, parallel assignment randomised controlled trial |
| Participants | 1900 Medicaid smokers |
| Interventions | Control: "Counseling from the Wisconsin Tobacco Quit Line (WTQL) consisted of 5 proactive calls to the participant to help them successfully quit tobacco use, plus ad hoc calls at the participant's initiation; also, WTQL coaches encouraged participants to see their health care provider to obtain Medicaid‐approved smoking cessation medications to help them quit smoking." Experimental: Control intervention plus financial incentives. "Participants in the Incentive condition received $30 per call for up to five WTQL calls taken; in addition, Incentive condition participants received $40 for producing biochemical evidence of abstinence at the 6‐month follow‐up visit." |
| Outcomes | Primary outcome: biochemically verified smoking abstinence (measured by urine cotinine or expired CO) assessed at 6 months follow‐up Secondary outcomes: engagement in treatment (number of calls completed) and cost‐effectiveness |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Berlin 2016.
| Trial name or title | Financial incentives for smoking cessation in pregnancy (FISCP): randomised, multicentre study |
| Methods | Single‐blind, randomised, 2 parallel groups, national superiority trial run in 16 maternity wards all over France |
| Participants | 398 pregnant smokers aged ≥18 years, smoking at least 5 manufactured or 3 roll‐your‐own cigarettes a day, and pregnant for < 18 weeks of amenorrhoea |
| Interventions | Control group: After a predefined quit date, participants in the control group will receive EUR 20 vouchers at the completion of each visit but no financial incentive for smoking abstinence Intervention group: participants will be rewarded for their abstinence by vouchers on top of the EUR 20 show‐up fee. The amount of reward for abstinence will increase as a function of duration of abstinence to stimulate longer periods of abstinence |
| Outcomes | Complete abstinence from quit date to the last predelivery visit |
| Starting date | April 2016 |
| Contact information | Dr Ivan Berlin ivan.berlin@aphp.fr Phone: 33(0)142161678 |
| Notes | Pregnancy trial Clinicaltrials.gov identifier: NCT02606227 |
Lynagh 2012.
| Trial name or title | ENtiCe Project ‐ Encouragement for Nicotine Cessation in pregnant smokers |
| Methods | 3‐armed randomised trial (smaller incentives, larger incentives, control (no incentive)); non‐concealed allocation, randomising by day and antenatal session. No blinding |
| Participants | 90 pregnant women who smoke, aged 16+, < 31 weeks gestation |
| Interventions | 1; Small (AUD 20) incentives; 2. Large (AUD 40) incentives; 3. (Control) usual care. Incentive starts at AUD 20/AUD 40, and increases by AUD 20/AUD 40 for every consecutive abstinent check. Up to AUD 720/AUD 1440 available if quit throughout programme |
| Outcomes | Primary: Consenting to participate; acceptance of cash incentives; 7‐day PPA, self‐reported and cotinine‐verified, over 8 routine a/n sessions (10 weeks) Secondary: abstinence confirmed by cotinine content of hair. |
| Starting date | Registered April 2012 |
| Contact information | Dr Marita Lynagh (marita.lynagh@newcastle.edu.au) |
| Notes | Pregnancy trial ACTRN12612000399897 |
Meyer 2015.
| Trial name or title | Financial incentives promote smoking abstinence among patients with pulmonary disease |
| Methods | Randomised controlled trial |
| Participants | 30 daily smokers diagnosed with chronic obstructive pulmonary disease (COPD) (FEV1/FVC < 70%) |
| Interventions | "Participants visit the clinic daily for 14 days and provide breath and urine samples for biochemical verification of smoking status. Incentive participants earn financial incentives delivered contingent upon smoking abstinence. Controls receive vouchers of the same value but independent of smoking status." |
| Outcomes | "Abstinence is defined as a breath CO level 6 ppm during Study Days 1–5 and a urinary cotinine level 80 ng/ml on Days 6–14." |
| Starting date | Not stated |
| Contact information | Stacey Sigmon: UHC‐SATC Room 1415, 1 South Prospect Street, Burlington, VT 05401, United States stacey.sigmon@uvm.edu |
| Notes |
NCT00064922.
| Trial name or title | Incentive program for female substance abusers who smoke |
| Methods | 3‐arm intervention trial (not clear if randomised or not) |
| Participants | 90 substance‐abusing women, aged > 15 |
| Interventions | 2 voucher incentive programmes; 1 targeting abstinence alone, and the other additional incentives for negative BAL and urinalysis |
| Outcomes | Not stated; would include abstinence |
| Starting date | January 2002 |
| Contact information | Leslie Amass (leslie.amas@uchsc.edu) |
| Notes |
NCT00079469.
| Trial name or title | Contingency management to enhance smoking cessation for cancer survivors: a proof of concept trial |
| Methods | Multicentre RCT |
| Participants | Smokers of at least 2 years who had been diagnosed and completed treatment for cancer at least 6 months, but not more than 5 years, before study entry |
| Interventions | 12 weeks bupropion + 6 weeks counselling for all participants; intervention arm get CM payments for abstinence in weeks 1 to 6. |
| Outcomes | Primary: Feasibility of study; 7‐day PPA at weeks 12, 24 Secondary: Characteristics of participants determining success |
| Starting date | February 2004 to August 2004 |
| Contact information | Glen D Morgan |
| Notes |
NCT00273793.
| Trial name or title | Increasing contingency management success in smoking cessation |
| Methods | Open‐label RCT |
| Participants | 240 adult smokers |
| Interventions | Contingent and non‐contingent incentives, with fixed and variable schedules, for hard‐to‐treat and easy‐to‐treat smokers |
| Outcomes | CO‐verified abstinence at 6‐month follow‐up |
| Starting date | June 2005 |
| Contact information | Richard J Lamb, University of Texas |
| Notes |
NCT00408265.
| Trial name or title | Smoking cessation in substance abuse treatment patients: a feasibility study |
| Methods | Open‐label RCT |
| Participants | Substance‐abusing men (N not given) |
| Interventions | Self‐help materials vs self‐help materials + CM component, i.e. rewards equivalent to USD 1, USD 20, USD 100 for validated abstinence Participants checked 4 times a week in weeks 1 to 4, twice a week in weeks 5 to 8, weekly in weeks 9 to 12. Follow‐ups at 1, 3 and 6 months following TQD |
| Outcomes | Primary: % negative CO readings; % negative cotinine readings; longest period of continuous abstinence Secondary: Self‐reported smoking; objective substance use; self‐reported substance use; treatment retention |
| Starting date | January 2004 to March 2007 |
| Contact information | Sheila M Alessi (salessi@uchc.edu) |
| Notes |
NCT00683280.
| Trial name or title | Contingency management and pharmacotherapy for smoking cessation |
| Methods | Open‐label Phase II RCT |
| Participants | 70 adult smokers, motivated to quit (59 recruited) |
| Interventions | 12‐week course of varenicline alone vs 12‐week course of varenicline + CM rewards for validated abstinence at weeks 5, 12 and 24 |
| Outcomes | Primary: Abstinence validated by CO, cotinine Secondary: Changes from baseline in ambulatory 24‐hour BP |
| Starting date | May 2008 to August 2010 |
| Contact information | Prof Sheila M Alessi (salessi@uchc.edu) |
| Notes |
NCT00690131.
| Trial name or title | An integrated approach to smoking cessation in severe mental illness (SMI) |
| Methods | Open‐label RCT |
| Participants | 50 adult smokers with severe and persistent mental illness |
| Interventions | Group counselling, pharmacotherapies and CM with financial incentives for reductions in smoking |
| Outcomes | Smoking abstinence and number of quit attempts at 3‐month follow‐up |
| Starting date | June 2008 |
| Contact information | Melanie E Bennett, University of Maryland |
| Notes |
NCT01484717.
| Trial name or title | Interactive voice response technology to mobilise contingency management for smoking cessation |
| Methods | Open‐label RCT |
| Participants | 90 smokers motivated to quit |
| Interventions | NRT (8 weeks of patches) + brief telephone counselling for all participants. Intervention arm also receives chance to win prizes for negative breath tests |
| Outcomes | Primary: Longest duration of abstinence (up to 24 weeks) Secondary: not stated |
| Starting date | January 2012 |
| Contact information | Shelia M Alessi (salessi@uchc.edu) |
| Notes |
NCT01736982.
| Trial name or title | Contingency management for smoking cessation in the homeless |
| Methods | Open‐label RCT |
| Participants | 70 homeless smokers |
| Interventions | Standard care: 8 weeks NRT, breath sample monitoring, standard SC counselling; Intervention: as standard care + prizes for negative breath samples |
| Outcomes | Longest duration of abstinence (Week 4) |
| Starting date | October 2012 |
| Contact information | Eileen M Ciesielski (echiesielski@uhc.edu) |
| Notes |
NCT01789710.
| Trial name or title | Contingency management for smoking cessation in homeless smokers |
| Methods | Single‐arm trial |
| Participants | 30 homeless smokers |
| Interventions | Internet‐based smoking cessation programme, plus NRT and bupropion, plus 4 counselling sessions. Participants will use smartphone to relay images of verification. Payment contingent on CO readings |
| Outcomes | Breath CO, throughout study to 12 months |
| Starting date | January 2013 |
| Contact information | Jean C Beckham |
| Notes |
NCT01826331.
| Trial name or title | Incentives for participation versus outcomes |
| Methods | Single‐blind randomised controlled trial |
| Participants | 880 smokers |
| Interventions | 1. Those incentivised for participation in an evidence‐based treatment designed for smokers at each stage of change 2. Those incentivised for biologically validated prolonged abstinence at 6 and 12 months who could also choose to participate in the TTM (Transtheoretical Model)‐tailored intervention 3. An assessment‐only control condition |
| Outcomes | Smoking abstinence at 24 months |
| Starting date | March 2014 |
| Contact information | James O Prochaska, Ph.D, University of Rhode Island jop@uri.edu |
| Notes |
NCT01965405.
| Trial name or title | Smoking cessation for people living with HIV/AIDS |
| Methods | Open‐label RCT |
| Participants | 400 smokers with HIV/AIDS |
| Interventions | Standard care (controls): bupropion + brief counselling; Phase 1: as standard care + high‐value prize contingency management for validated abstinence; Phase 2a: Non‐responders A: bupropion, brief counselling + monitored support to quit; Phase 2a: Non‐responders B: as A, + chance to win prizes for validated abstinence. 2b: Responders A: Bupropion, no additional treatment; 2b: Responders B: bupropion, continued monitoring + low‐intensity prize contingency management All participants received USD 35 for intake, and USD 25 for each follow‐up interview at post‐phase 1, post‐phase 2, 6 and 12 months |
| Outcomes | Primary: Urinary cotinine at all test points, up to 12 months; Longest duration of continuous abstinence; 7‐day PPA at all time points; CO result at all time points |
| Starting date | August 2013 |
| Contact information | Lisa Sulkowski (lulkows@med.wayne.edu) |
| Notes |
NCT02210832.
| Trial name or title | Financial incentives for smoking cessation among disadvantaged pregnant women |
| Methods | Open‐label RCT |
| Participants | 345 pregnant smokers |
| Interventions | Controls: Best practice (5As) + referral to pregnancy‐specific quit line; Intervention: as controls, + financial incentives for validated abstinence (vouchers, available through to 12 weeks post‐partum) Also a group of never‐smoker pregnant women matched to smokers on key demographic variables, for comparison through to 12 months |
| Outcomes | Primary: validated 7‐day PPA at final ante‐partum appointment (around 28 weeks gestation) Secondary: validated 7‐day PPA post‐partum, i.e. weeks 4, 8, 12, 24 and 48; birth outcomes; maternal and baby heath utilisation measures; cost effectiveness |
| Starting date | January 2014 |
| Contact information | Mary Ellen Lynch (mlynch1@uvm.edu) and Kylie N Johnson (kjohns@uvm.edu) |
| Notes | Pregnancy trial |
NCT02237898.
| Trial name or title | Harnessing the power of technology: MOMBA for post‐partum smoking |
| Methods | Phase 1 RCT |
| Participants | 40 pregnant smokers |
| Interventions | Controls; traditional office‐based CM programme, delivering financial incentives to abstinent post‐partum women; Intervention: MOMBA smart‐phone Sensodrone app to relay CO monitoring of abstinence N.B. All participants receive financial incentives; mode of delivery was the variable being tested |
| Outcomes | Primary: Acceptability; feasibility Secondary: short‐term abstinence; long‐term (7‐day PPA) |
| Starting date | September 2014 |
| Contact information | Ruth M Arnold (ruth.arnold@yale.edu) and Heather Howell (heather.howell@yale.edu) |
| Notes | Pregnancy trial (pilot) |
NCT02245308.
| Trial name or title | Abstinence Reinforcement Therapy (ART) for homeless veteran smokers |
| Methods | Open‐label RCT |
| Participants | 165 veteran homeless smokers |
| Interventions | Controls: VA smoking cessation clinic for standard care treatment, including group counselling, individual counselling, self‐help materials. Intervention: tele‐health programme, combining CBT‐based support, tele‐medicine clinic for pharmacological aids (nicotine patches, bupropion), and mobile contingency management |
| Outcomes | Primary: validated 6‐month abstinence Secondary: QALY, resource utilisation, intervention delivery costs and participant time costs |
| Starting date | September 2014 |
| Contact information | Jean C Beckham |
| Notes |
NCT02266784.
| Trial name or title | Contingency management, quitting smoking, and ADHD (ADQUIT) |
| Methods | Phase 1 open‐label RCT |
| Participants | 40 smokers with ADHD |
| Interventions | Controls: Supportive counselling + NRT patches, 8‐week programme; Intervention: as for controls, + escalating financial rewards for continuous abstinence, reset in case of failure or missed visits; all participants checked at 3 and 6 months |
| Outcomes | Primary: Change in motivation to quit; change in readiness to change behaviour Secondary: Chamge in smoking behaviour in ADHD population at 3 months, 6 months; decreasing effects of quitting smoking |
| Starting date | October 2014 |
| Contact information | Joseph S English (engli009@mc.duke.edu) and Denny A Hood (denny.hood@dm.duke.edu) |
| Notes |
NCT02506829.
| Trial name or title | Financial Incentives for Smoking Treatment (FIESTA) |
| Methods | Parallel‐assignment randomised controlled trial |
| Participants | 182 urban veteran smokers |
| Interventions | Financial incentives intervention: "Usual care in hospital, referral to a smoking cessation Quitline on discharge from hospital. Financial incentives for: a) speaking with a coach from the Smoker's Quitline ($50), b) completion of another community‐based smoking‐cessation program ($50), and/or c) use of pharmacotherapies for smoking cessation at 2 weeks ($50); and d) for smoking cessation, confirmed with the use of a cotinine test at 2 months ($150); and e) for smoking cessation, confirmed with the use of a cotinine test at 6 months after study enrollment ($250)." Control: "Usual care in hospital, referral to a smoking cessation Quitline on discharge from hospital." |
| Outcomes | Primary: Self‐report and biochemically verified (by salivary cotinine) smoking abstinence at 6 months Secondary: Self‐report smoking abstinence at 6 months; use of evidence‐based treatments; quality of life (measured with EQ5‐D); short‐ and long‐term return on investment (cost analysis) |
| Starting date | July 2015 |
| Contact information | Dr Joseph Ladapo JLadapo@mednet.ucla.edu Dr Scott Sherman Scott.Sherman@nyumc.org |
| Notes |
NCT02596061.
| Trial name or title | In It To Quit: commitment contracts for smoking cessation (I2Q) |
| Methods | Open‐label parallel‐assignment 3‐group randomised controlled trial |
| Participants | 311 low‐moderate income smokers |
| Interventions | Intervention 1 ‐ Rewards Only :Participants have access to a website where they can self‐report smoking status, add virtual supporters, and submit journal entries. Participants receive incentives for completing these activities and for using counselling services at the clinic. At the 2‐month mark, these participants come to the clinic for a biochemical verification of their smoking status. Their rewards are contingent on passing this verification test. They are also asked to return to the clinic 6 and 12 months after enrolment to complete the smoking tests and are compensated for these 2 visits. Intervention 2 ‐ Pre‐Committment: In addition to activities in intervention group 1, at enrollment, participants are offered the chance to set aside some of their future rewards for a deposit contract that lasts for 4 months and starts 2 months after the rewards contract. If at the end of 6 months the participants pass the second verification test, their rewards are returned to them Control: No intervention |
| Outcomes | Primary outcome: continuous abstinence from smoking between months 2 and 12, i.e. biochemically verified abstinence at all 3 measurements |
| Starting date | November 2015 |
| Contact information | Dr Daren Anderson: andersd@chc1.com |
| Notes |
NCT02737566.
| Trial name or title | Small financial incentives to promote smoking cessation (Prevail II) |
| Methods | Open‐label parellel‐assignment randomised controlled trial |
| Participants | 320 English‐speaking adult smokers of at least 5 cigarettes a day, in receipt of Medicaid or uninsured |
| Interventions | Experimental: standard care plus financial incentives ("the opportunity to earn small gift cards for biochemically‐verified abstinence through 12 weeks post‐quit. The amount of the gift cards will escalate each week from the quit date through 4 weeks post‐quit with continuous abstinence. Participants who are non‐abstinent at any visit may earn incentives for abstinence at the next visit, but the amount will reset to the starting level. Participants may additionally earn an additional gift card for abstinence at the 8 and 12 weeks post‐quit visits." Control: standard care (weekly smoking cessation counselling and pharmacotherapy) |
| Outcomes | Primary outcome: biochemically verified 7‐day point prevalence smoking cessation at 26 weeks Secondary outcome: biochemically verified 7‐day point prevalence smoking cessation at 12 weeks |
| Starting date | January 30th 2017 |
| Contact information | Principal investigator: Darla Kendzor Email: Darla‐Kendzor@ouhsc.edu |
| Notes |
NCT02952703.
| Trial name or title | Disseminating and implementing a smoking cessation program for pregnant and postpartum women |
| Methods | Single‐blind parallel‐assignment randomised controlled trial |
| Participants | 185 |
| Interventions | Intervention: "Striving to quit". Includines "additional pre‐natal counseling (in‐person and telephonic); post delivery counseling (in‐person and telephonic) and incentives" Control: Brief pre‐natal smoking cessation counselling |
| Outcomes | Primary outcome: biochemically verified smoking abstinence (breath CO < 9ppm, 6 months post‐intervention) Secondary outcome: motivation to quit/remain quit meausred on a 5‐point Likert scale at 6 months |
| Starting date | May 2018 |
| Contact information | Principal investigator: Michael Fiore Email: mcf@ctri.wisc.edu |
| Notes | Pregnancy trial |
NCT03163056.
| Trial name or title | Smoking cessation treatment for depressed smokers |
| Methods | Open‐label parallel‐assignment randomised controlled trial |
| Participants | 150 adult smokers of 10+ cigarettes a day for at least 1 year, meeting diagnostic criteria for current unipolar major depression and nicotine dependence |
| Interventions | Control group 1: group‐based cognitive behaviour treatment for smoking cessation Control group 2: as per control group 1 plus behavioural activation strategies Intervention group: as per groups 1 and 2 plus contingency management. From the 5th session onwards "Participants providing negative specimens of both CO (≤4 ppm) and cotinine (80 ng/ml) earned points exchangeable for rewards (e.g. cinema tickets) on a schedule of escalating magnitude of reinforcement (from 10€ voucher value with maximum possible earnings of 175€ in vouchers)." |
| Outcomes | Primary outcomes: 7‐day point prevalence smoking abstinence biochemically verified with CO and cotinine (longest follow‐up 6 months) Secondary outcomes: depression; behavioural activation; environmental reward; cigarette craving; anxiety; impulsivity |
| Starting date | January 26th 2015 |
| Contact information | Dr Roberto Secades‐Villa: secades@uniovi.es |
| Notes |
NCT03528304.
| Trial name or title | Native Women's Wellness: contingency management for tobacco cessation and weight loss (NWW) |
| Methods | Open‐label factorial assignment randomised controlled trial |
| Participants | 125 women aged 18 ‐ 44 of American Indian or Alaska Native heritage, currently smoking and overweight and not interested in using NRT |
| Interventions | No‐intervention control Intervention 1: contingency management for smoking cessation. As part of the CM intervention, women attend visits for smoking are rewarded with prizes for abstaining from smoking Intervention 2: contingency management for smoking cessation and weight loss. As part of the CM intervention, women attend visits for smoking and weight loss assessment and are rewarded with prizes for abstaining from smoking and for losing some weight The study also included a third arm which does not meet the inclusion criteria for this review, involving contingency management alone |
| Outcomes | Primary outcome: smoking abstinence at 16 weeks |
| Starting date | September 2010 |
| Contact information | Dr Dedra Buchwald Email: dedra.buchwald@wsu.edu |
| Notes | Pregnant women and those planning to become pregnant in the next 4 months were excluded |
NCT03551704.
| Trial name or title | Smoking cessation treatment for substance use dependents |
| Methods | Open‐label parallel‐assignment randomised controlled trial |
| Participants | 120 adults who have smoked at least 10 cigarettes a day for the past year undergoing outpatient cocaine or alcohol treatment and meeting diagnostic criteria for nicotine dependence |
| Interventions | Control group: cognitive behavioural therapy plus episodic future thinking Intervention group: as per control group, plus contingency management. Participants will be provided with "incentives to promote and reinforce abstinence contingent on biochemical verification. The schedule will incorporate an increasing magnitude of reinforcement." |
| Outcomes | Primary outcomes: 1. Changes in smoking abstinence (period of at least 24 hours without smoking or not smoking in the last 7 days, assessed with CO and cotinine samples) 2. Changes in continuous abstinence (not smoking even a puff since the quit date, verified with CO and cotinine samples) Secondary outcomes: Changes in other substance abstinence; changes in drug demand; changes in health‐related quality of life; changes in depression; changes in impulsivity; cost‐analysis |
| Starting date | January 15th 2018 |
| Contact information | Dr Roberto Secades‐Villa: secades@uniovi.es |
| Notes |
Secades‐Villa 2015.
| Trial name or title | CM of smoking abstinence vs CM with shaping for smoking cessation among treatment‐seeking patients |
| Methods | Randomised controlled trial |
| Participants | 47 smokers in a community setting |
| Interventions | Contingency management (CM) intervention where participants earned voucher‐based incentives contingent on providing biochemical evidence (a negative urine cotinine test) of smoking abstinence Contingency management with shaping (CMS): participants were set intermediate criteria for incentive delivery between the present behaviour and total abstinence. "CMS reinforce progressive reductions in smoking according to a percentile schedule." |
| Outcomes | Smoking abstinence at post‐treatment assessment; treatment completion; total days abstinent |
| Starting date | Not reported |
| Contact information | Dr Roberto Secades‐Villa: secades@uniovi.es |
| Notes |
Wilson 2016.
| Trial name or title | Abstinence Reinforcement Therapy (ART) for rural veterans |
| Methods | Randomised control trial |
| Participants | 310 rural Veteran smokers |
| Interventions | Intervention: Abstinence Reinforcement Therapy (ART) which combines evidence‐based cognitive‐behavioural telephone counselling (TC), a tele‐medicine clinic for access to NRT and mobile contingency management (mCM), in which compensation is given based on smoking abstinence. Control condition: TC and NRT alone |
| Outcomes | Primary outcome: self‐report and biochemically validated prolonged smoking abstinence at 6 months and 12 months |
| Starting date | Not reported |
| Contact information | Sarah Wilson: sarah.wilson@duke.edu |
| Notes |
Differences between protocol and review
For this 2019 update, we removed the separate six‐month follow‐up time point analysis for studies of mixed populations, as long‐term follow‐up was our focus for this review.
For this 2019 update we excluded non‐randomised studies and changed the analysis from odds ratios to risk ratios, in accordance with standard methods of the Cochrane Tobacco Addiction Group. We also introduced a new subgroup analysis within mixed‐population studies of trials recruiting from substance misuse populations (community or inpatient clinics), since a number of new studies included in this update recruited from this specific population and there is reason to believe the effect of incentives could be different in this population.
For the 2019 update we changed the 'Risk of bias' assessments from evaluating performance and detection bias in one domain, to assessing detection bias alone, based on whether studies biochemically validated abstinence. We did this because the interventions being studied preclude effective blinding of participants and study personnel, and in order to simplify and clarify the assessment of detection bias.
We included sensitivity analysis to explore the relative size of incentives offered, and sub‐group analysis to explore potential impact of studies where incentives were continually offered up until the long term follow up point.
Contributions of authors
CN (guarantor of the review) extracted data, conducted the analyses and wrote the review. SG double‐data extracted and contributed to the analysis and writing of the review. JHB contributed to the design of the update, double‐data extracted, checked data extraction and contributed to the writing of the review. RP checked the statistical analysis and commented on the review. JLB ran the searches, checked the inclusion/exclusion and critical appraisal and contributed to the writing of the review. LB contributed to the writing and editing of the review.
Sources of support
Internal sources
Nuffield Department of Primary Care Health Sciences, University of Oxford, UK.
JHB is funded by the National Institute of Health Research School for Primary Care Research (NIHR SPCR), UK.
External sources
NHS Research and Development Fund, UK.
Declarations of interest
CN: none known. SG: none known. JLB: none known. LB: is co‐author of one of the trials included in the review (Tappin 2015a) and some of the studies cited as supporting evidence in the Background and Discussion sections (Berlin 2018; Hoddinott 2014). RP: none known. JHB: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Ainscough 2017 {published data only}
- Ainscough TS, Brose LS, Strang J, McNeill A. Contingency management for tobacco smoking during opioid addiction treatment: a randomised pilot study. BMJ Open 2017;7(9):e017467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alessi 2014 {published data only}
- Alessi SM, Petry NM. Smoking reductions and increased self‐efficacy in a randomized controlled trial of smoking abstinence ‐ contingent incentives in residential substance abuse treatment patients. Nicotine & Tobacco Research 2014;16(11):1436‐45. [NCT00683033] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2018 {published data only}
- Baker TB, Fraser DL, Kobinsky K, Adsit R, Smith SS, Khalil L, et al. A randomized controlled trial of financial incentives to low income pregnant women to engage in smoking cessation treatment: effects on post‐birth abstinence. Journal of Consulting and Clinical Psychology 2018;86(5):464‐73. [DOI] [PubMed] [Google Scholar]
- NCT01469490. Striving to quit: First Breath (STQ FB). www.clinicaltrials.gov/ct2/show/NCT01469490 (first received 3rd April 2012).
Brunette 2017 {published data only}
- Brunette MF, Pratt SI, Bartels SJ, Scherer EA, Sigmon SC, Ferron JC, et al. Randomized trial of interventions for smoking cessation among medicaid beneficiaries with mental illness. Psychiatric Services 2017;69(3):274‐80. [DOI] [PubMed] [Google Scholar]
- NCT02515981. New Hampshire medicaid wellness incentive program. clinicaltrials.gov/show/NCT02515981 (first received 5th August 2015).
Cheung 2017 {published data only}
- Cheung YT, Wang MP, Li HC, Kwong A, Lai V, Chan SS, et al. Effectiveness of a small cash incentive on abstinence and use of cessation aids for adult smokers: a randomized controlled trial. Addictive Behaviours 2017;66:17‐25. [DOI] [PubMed] [Google Scholar]
- NCT01928251. Promoting smoking cessation in the community via quit to win contest 2013. clinicaltrials.gov/show/NCT01928251 (first received 23 August 2013).
Cooney 2017 {published data only}
- Cooney JL, Cooper S, Grant C, Sevarino K, Krishnan‐Sarin S, Gutierrez IA, et al. A randomized trial of contingency management for smoking cessation during intensive outpatient alcohol treatment. Journal of Substance Abuse Treatment 2017;72:89‐96. [DOI] [PubMed] [Google Scholar]
Dallery 2016 {published data only}
- Dallery J, Raiff BR, Kim SJ, Marsch LA, Stitzer M, Grabinski MJ. Nationwide access to an internet‐based contingency management intervention to promote smoking cessation: a randomized controlled trial. Addiction 2016;112(5):875‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Paul 1994 {published data only}
- Jason LA, McMahon SD, Salina D, Hedeker D, Stockton M, Dunson K, et al. Assessing a smoking cessation intervention involving groups, incentives, and self‐help manuals. Behavior Therapy 1995;26:393‐408. [Google Scholar]
- Jason LA, Salina D, McMahon SD, Hedeker D, Stockton M. A worksite smoking intervention: a 2 year assessment of groups, incentives, and self‐help. Health Education Research 1997;12(1):129‐38. [DOI] [PubMed] [Google Scholar]
- McMahon SD, Jason LA. Social support in a worksite smoking intervention. Behavior Modification 2000;24(2):184‐201. [DOI] [PubMed] [Google Scholar]
- McMahon SD, Jason LA. Stress and coping in smoking cessation: a longitudinal examination. Anxiety, Stress and Coping 1998;11:327‐43. [Google Scholar]
- McMahon SD, Jason LA, Salina D. Stress, coping, and appraisal in a smoking cessation intervention. Anxiety, Stress and Coping 1994;7(2):161‐71. [Google Scholar]
Donatelle 2000a {published data only}
- Donatelle RJ, Hudson D, Dobie S, Goodall A, Hunsberger M, Oswald K. Incentives in smoking cessation: status of the field and implications for research and practice with pregnant smokers. Nicotine & Tobacco Research 2004;6(Suppl 2):S163‐79. [DOI] [PubMed] [Google Scholar]
- Donatelle RJ, Prows SL, Champeau D, Hudson D. Randomised controlled trial using social support and financial incentives for high risk pregnant smokers: Significant Other Support (SOS) program. Tobacco Control 2000;9(Supplement III):iii67‐iii69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donatelle 2000b {published data only}
- Donatelle RJ, Hudson D, Dobie S, Goodall A, Hunsberger M, Oswald K. Incentives in smoking cessation: status of the field and implications for research and practice with pregnant smokers. Nicotine & Tobacco Research 2004;6(Suppl 2):S163‐79. [DOI] [PubMed] [Google Scholar]
- Donatelle RJ, Prows SL, Champeau D, Hudson D. Using social support, biochemical feedback, and incentives to motivate smoking cessation during pregnancy: comparison of three intervention trials. Poster at American Public Health Association meeting, Boston MA 2000.
Donatelle 2002 {published data only}
- Donatelle RJ, Hudson D, Dobie S, Goodall A, Hunsberger M, Oswald K. Incentives in smoking cessation: status of the field and implications for research and practice with pregnant smokers. Nicotine & Tobacco Research 2004;6(Suppl 2):S163‐79. [DOI] [PubMed] [Google Scholar]
- Donatelle RJ, Hudson D, Lichtenstein E. Using 5 A's and incentives to promote prenatal smoking cessation [presentation to National Conference of Tobacco or Health, 2002]. ncth.confex.com/ncth/responses/2002/257.ppt 2002 (retrieved 10 August 2004).
Drummond 2014 {published data only}
- Drummond MB, Astemborski J, Lambert AA, Goldberg S, Stitzer ML, Merlo CA, et al. A randomized study of contingency management and spirometric lung age for motivating smoking cessation among injection drug users. BMC Public Health 2014;14:761. [NCT01334736] [DOI] [PMC free article] [PubMed] [Google Scholar]
Etter 2016 {published data only}
- Etter J‐F. Financial incentives for smoking cessation in low‐income smokers: study protocol for a randomized controlled trial. Trials 2012;13:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter J‐F, Schmid F. Effects of large financial incentives for long‐term smoking cessation: a randomized trial. Journal of the American College of Cardiology 2016;68(8):777‐85. [DOI] [PubMed] [Google Scholar]
Fraser 2017 {published data only}
- Fraser DL, Fiore MC, Kobinsky K, Adsit R, Smith SS, Johnson ML, et al. A randomized trial of incentives for smoking treatment in medicaid members. American Journal of Preventive Medicine 2017;53(6):754‐63 MISC3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02713594. Wisconsin tobacco quit line medicaid incentive evaluation. clinicaltrials.gov/show/nct02713594 (first received 21 April 2017).
Gallagher 2007 {published data only}
- Gallagher SM, Penn PE, Schindler E, Layne W. A comparison of smoking cessation treatments for persons with schizophrenia and other serious mental illnesses. Journal of Psychoactive Drugs 2007;39(4):487‐97. [DOI] [PubMed] [Google Scholar]
Ghosh 2016 {published data only}
- Ghosh A, Philiponis G, Bewley A, Ransom ER, Mirza N. You can't pay me to quit: The failure of financial incentives for smoking cessation in head and neck cancer patients. Journal of Laryngology and Otology 2016;130(3):278‐83. [DOI] [PubMed] [Google Scholar]
Giné 2010 {published and unpublished data}
- Giné X, Karlan D, Zinman J. Put your money where your butt is: a commitment contract for smoking cessation. American Economic Journal: Applied Economics 2010;2:213‐35. [Google Scholar]
Glasgow 1993 {published data only}
- Glasgow RE, Hollis JF, Ary DV, Boles SM. Results of a year‐long incentives‐based worksite smoking‐cessation program. Addictive Behaviors 1993;18(4):455‐64. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Hollis JF, Ary DV, Lando HA. Employee and organizational factors associated with participation in an incentive‐based worksite smoking cessation program. Journal of Behavioral Medicine 1990;13(4):403‐18. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Hollis JF, Pettigrew L, Foster L, Givi MJ, Morrisette G. Implementing a year‐long worksite‐based incentive program for smoking cessation. American Journal of Health Promotion 1991;5(3):192‐9. [DOI] [PubMed] [Google Scholar]
Halpern 2015 {published data only}
- Halpern SD, French B, Small DS, Saulsgiver K, Harhay MO, Audrain‐McGovern J, et al. A randomized trial of four financial incentive programs for smoking cessation. New England Journal of Medicine 2015;372(22):2108‐17. [DOI: 10.1056/NEJMoa1414293] [DOI] [PMC free article] [PubMed] [Google Scholar]
Halpern 2018 {published data only}
- Halpern SD, Harhay MO, Saulsgiver K, Brophy C, Troxel AB, Volpp KG. A pragmatic trial of e‐cigarettes, incentives, and drugs for smoking cessation. New England Journal of Medicine 2018;378(24):2302‐10. [DOI] [PubMed] [Google Scholar]
- NCT02328794. Randomized clinical trial to reduce harm from tobacco. clinicaltrials.gov/show/nct02328794 (first received 31 December 2014).
Harris 2015 {published data only}
- Harris M, Reynolds B. A pilot study of home‐based smoking cessation programs for rural, Appalachian, pregnant smokers. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2015;44(2):236‐45. [DOI] [PubMed] [Google Scholar]
Heil 2008 {published data only}
- Heil SH, Higgins ST. Characterizing nicotine withdrawal and craving in pregnant cigarette smokers. 66th Annual Meeting of the College on Problems of Drug Dependence; June 12 ‐ 17; San Juan Puerto Rico. 2004.
- Heil SH, Higgins ST, Bernstein IM, Solomon LJ, Rogers RE, Thomas CS, et al. Effects of voucher‐based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction 2008;103(6):1009‐18. [ClinicalTrialsID: NCT00497068] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Higgins ST, Solomon LJ, Lynch ME, McHale L, Dumeer A, et al. Voucher‐based incentives for abstinence from cigarette smoking in pregnant and postpartum women. Society for Research on Nicotine and Tobacco 13th Annual Meeting; Feb 21 ‐ 24; Austin, TX, USA. 2007.
- Heil SH, Tidey JW, Holmes HW, Badger GJ, Higgins ST. A contingent payment model of smoking cessation: effects on abstinence and withdrawal. Nicotine & Tobacco Research 2003;5(2):205‐13. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Bernstein IM, Washio Y, Heil SH, Badger GJ, Skelly JM, et al. Effects of smoking cessation with voucher‐based contingency management on birth outcomes. Addiction 2010;105(11):2023‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Badger GJ, Mongeon JA, Solomon LJ, McHale L, et al. Biochemical verification of smoking status in pregnant and recently postpartum women. Experimental and Clinical Psychopharmacology 2007;15(1):58‐66. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dumeer AM, Thomas CS, Solomon LJ, Bernstein IM. Smoking status in the initial weeks of quitting as a predictor of smoking‐cessation outcomes in pregnant women. Drug and Alcohol Dependence 2006;85(2):138‐41. [DOI] [PubMed] [Google Scholar]
- Jones C, Gaalema D, Shephard DS, Erten M, Stoeckel M, Day S, et al. Incentive‐based treatments to promote smoking abstinence during pregnancy: findings from the Vermont Center on Behavior and Health. Value in Health 2014;17(3):A217. [Google Scholar]
- Linares Scott TJ, Heil SH, Higgins ST, Badger GJ, Bernstein IM. Depressive symptoms predict smoking status among pregnant women. Addictive Behaviors 2009;34(8):705‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon LJ, Higgins ST, Heil SH, Badger GJ, Thomas CS, Bernstein IM. Predictors of postpartum relapse to smoking. Drug and Alcohol Dependence 2007;90(2‐3):224‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Experimental and Clinical Psychopharmacology 2007;15(2):176‐86. [DOI] [PubMed] [Google Scholar]
Hennrikus 2002 {published data only}
- Hennrikus DJ, Jeffery RW, Lando HA, Murray DM, Brelje K, Davidann B, et al. The SUCCESS project: the effect of program format and incentives on participation and cessation in worksite smoking cessation programs. American Journal of Public Health 2002;92(2):274‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando HA, Thai DT, Murray DM, Robinson LA, Jeffery RW, Sherwood NE, et al. Age of initiation, smoking patterns, and risk in a population of working adults. Preventive Medicine 1999;29(6 Pt 1):590‐8. [DOI] [PubMed] [Google Scholar]
- Martinson BC, Murray DM, Jeffery RW, Hennrikus DJ. Intraclass correlation for measures from a worksite health promotion study: estimates, correlates and applications. American Journal of Health Promotion 1999;13(6):347‐57. [DOI] [PubMed] [Google Scholar]
- Sherwood NE, Hennrikus DJ, Jeffery RW, Lando HA, Murray DM. Smokers with multiple behavioral risk factors: how are they different?. Preventive Medicine 2000;31(4):299‐307. [DOI] [PubMed] [Google Scholar]
Higgins 2014 {published data only}
- Higgins ST, Washio Y, Lopez AA, Heil SH, Solomon LJ, Lynch ME, et al. Examining two different schedules of financial incentives for smoking cessation among pregnant women. Preventive Medicine 2014;68:51‐7. [DOI: 10.1016/j.ypmed.2014.03.024] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lasser 2017 {published data only}
- Lasser KE, Quintiliani LM, Truong V, Xuan Z, Murillo J, Jean C, et al. Effect of patient navigation and financial incentives on smoking cessation among primary care patients at an urban safety‐net hospital: a randomized clinical trial. JAMA Internal Medicine 2017;177(12):1798‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser KE, Quintiliani LM, Truong V, Xuan Z, Pbert L. Patient navigation to promote smoking cessation in primary care: preliminary findings from an ongoing randomized controlled trial. Conference: 40th Annual Meeting of the Society of General Internal Medicine, SGIM 2017 2017, issue 2 Supplement 1:S266.
- Quintiliani LM, Russinova ZL, Bloch PP, Truong V, Xuan Z, Pbert L, et al. Patient navigation and financial incentives to promote smoking cessation in an underserved primary care population: a randomized controlled trial protocol. Contemporary Clinical Trials 2015;45(Pt B):449‐57. [DOI] [PubMed] [Google Scholar]
Ledgerwood 2014 {published data only}
- Ledgerwood DM, Arfken CL, Petry NM, Alessi SM. Prize contingency management for smoking cessation: a randomized trial. Drug and Alcohol Dependence 2014;140:208‐12. [ClinicalTrials.gov: NCT00865254] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ondersma 2012 {published data only}
- Ondersma SJ, Chase SK, Svikis DS, Schuster CR. Computer‐based motivational intervention for perinatal drug use. Journal of Substance Abuse Treatment 2005;28(4):305‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Svikis DS, Lam PK, Connors‐Burge VS, Ledgerwood DM, Hopper JA. A randomized trial of computer‐delivered brief intervention and low‐intensity contingency management for smoking during pregnancy. Nicotine & Tobacco Research 2012;14(3):351‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rand 1989 {published data only}
- Rand CS, Stitzer ML, Bigelow GE, Mead AM. The effects of contingent payment and frequent workplace monitoring on smoking abstinence. Addictive Behaviors 1989;14(2):121‐8. [DOI] [PubMed] [Google Scholar]
Rettig 2018 {published data only}
- Rettig EM, Fakhry C, Hales RK, Kisuule F, Quon H, Kiess AP, et al. Pilot randomized controlled trial of a comprehensive smoking cessation intervention for patients with upper aerodigestive cancer undergoing radiotherapy. Head & Neck 2018;40(7):1534‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rohsenow 2015 {published data only}
- Rohsenow DJ, Tidey JW, Martin RA, Colby SM, Sirota AD, Swift RM, et al. Contingent vouchers and motivational interviewing for cigarette smokers in residential substance abuse treatment. Journal of Substance Abuse Treatment 2015;55:29‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rohsenow 2017 {published data only}
- Rohsenow DJ, Martin RA, Tidey JW, Colby SM, Monti PM. Treating smokers in substance treatment with contingent vouchers, nicotine replacement and brief advice adapted for sobriety settings. Journal of Substance Abuse Treatment 2017;72:72‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Romanowich 2015 {published data only}
- NCT00273793. Increasing contingency management success in smoking cessation. clinicaltrials.gov/show/NCT00273793 (first received 7 June 2012).
- Romanowich P, Lamb RJ. The effects of fixed versus escalating reinforcement schedules on smoking abstinence. Journal of Applied Behavior Analysis 2015;48(1):25‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Secades‐Villa 2014 {published data only}
- López‐Núñez C, Alonso‐Pérez F, Pedrosa I, Secades‐Villa R. Cost‐effectiveness of a voucher‐based intervention for smoking cessation. American Journal of Drug and Alcohol Abuse 2016;42(3):296‐305. [DOI] [PubMed] [Google Scholar]
- López‐Núñez C, Pérez FA, Weidberg SE, Pericot‐Valverde I, Secades‐Villa R. Incremental cost‐effectiveness of a voucher‐based CM protocol added to a CBT for smoking cessation. Drug and Alcohol Dependence 2015;156:e134. [Google Scholar]
- Secades‐Villa R, García‐Rodríguez O, López‐Núñez C, Alonso‐Pérez F, Fernández‐Hermida JR. Contingency management for smoking cessation among treatment‐seeking patients in a community setting. Drug and Alcohol Dependence 2014;140:63‐8. [DOI] [PubMed] [Google Scholar]
Shoptaw 2002 {published data only}
- Shoptaw S, Rotheram‐Fuller E, Yang X, Frosch D, Nahom D, Jarvik ME, et al. Smoking cessation in methadone maintenance. Addiction 2002;97(10):1317‐28. [DOI] [PubMed] [Google Scholar]
Tappin 2015a {published data only}
- Boyd KA, Briggs AH, Bauld L, Sinclair L, Tappin D. Are financial incentives cost‐effective to support smoking cessation during pregnancy?. Addiction 0126;111(2):360‐70. [DOI] [PubMed] [Google Scholar]
- Tappin D, Bauld L, Purves D, Boyd K, Sinclair L, MacAskill S, et al. Financial incentives for smoking cessation during pregnancy: randomised controlled trial. Lancet in press (www.thelancet.com/pdfs/journals/lancet/PIIS0140‐6736%2814%2962130‐9.pdf). [DOI] [PubMed]
- Tappin D, Bauld L, Purves D, Boyd K, Sinclair L, MacAskin S, et al. Cessation in Pregnancy Incentives Trial (CPIT) team. Financial incentives for smoking cessation in pregnancy: randomised controlled trial. BMJ 2015;350(27 January 2015):h134. [DOI] [PubMed] [Google Scholar]
- Tappin DM, Bauld L, Tannahill C, Caestecker L, Radley A, McConnachie A, et al. The Cessation in Pregnancy Incentives Trial (CPIT): study protocol for a randomized controlled trial. Trials 2012;13:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tevyaw 2009 {published data only}
- Tevyaw TO, Colby SM, Tidey JW, Kahler CW, Rohsenow DJ, Barnett NP, et al. Contingency management and motivational enhancement: a randomized clinical trial for college students. Nicotine & Tobacco Research 2009;11(6):739‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tuten 2012 {published data only}
- Tuten M, Fitzsimona H, Chisolm MS, Nuzzo PA, Jones HE. Contingent incentives reduce cigarette smoking among pregnant, methadone‐maintained women: results of an initial feasibility and efficacy randomized clinical trial. Addiction 2012;107(10):1868‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van den Brand 2018 {published data only}
- NTR5657. Incentives for workplace smoking cessation. www.trialregister.nl/trial/5537 (first received 27 January 2016).
- Brand FA, Nagelhout GE, Winkens B, Evers SM, Kotz D, Chavannes NH, et al. The effect of financial incentives on top of behavioral support on quit rates in tobacco smoking employees: study protocol of a cluster‐randomized trial. BMC Public Health 2016;16(1):1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand FA, Nagelhout, GE, Winkens B, Chavannes NH, Schayck OC. Effect of a workplace‐based group training programme combined with financial incentives on smoking cessation: a cluster‐randomised controlled trial. Lancet Public Health 2018;3(11):e536‐44. [DOI] [PubMed] [Google Scholar]
Volpp 2006 {published data only}
- Volpp KG, Levy AG, Asch DA, Berline JA, Murphy JJ, Gomez A, et al. A randomized controlled trial of financial incentives for smoking cessation. Cancer Epidemiology, Biomarkers and Prevention 2006;15(1):12‐8. [DOI] [PubMed] [Google Scholar]
Volpp 2009 {published data only}
- Kim A, Kamyab K, Zhu J, Volpp K. Why are financial incentives not effective at influencing some smokers to quit? Results of a process evaluation of a worksite trial assessing the efficacy of financial incentives for smoking cessation. Journal of Occupational and Environmental Medicine 2011;53(1):62‐7. [DOI] [PubMed] [Google Scholar]
- Kim AE, Towers A, Renaud J, Zhu J, Shea JA, Galvin R, et al. Application of the RE‐AIM framework to evaluate the impact of a worksite‐based financial incentive intervention for smoking cessation. Journal of Occupational and Environmental Medicine 2012;54(5):610‐4. [DOI] [PubMed] [Google Scholar]
- Troxel AB, Volpp KG. Effectiveness of financial incentives for longer‐term smoking cessation: evidence of absence or absence of evidence?. American Journal of Health Promotion 2012;26(4):204‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpp KG, Das A. Comparative effectiveness ‐ thinking beyond medication A versus medication B. New England Journal of Medicine 2009;361(4):331‐3. [DOI] [PubMed] [Google Scholar]
- Volpp KG, Galvin R. Reward‐based incentives for smoking cessation: how a carrot became a stick. JAMA 2014;311(9):909‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpp KG, Troxel AB, Pauly MV, Glick HA, Puig A, Asch DA, et al. A randomized, controlled trial of financial incentives for smoking cessation. New England Journal of Medicine 2009;360(7):699‐709. [clinicaltrials.gov ID NCT00128375] [DOI] [PubMed] [Google Scholar]
White 2013 {published data only}
- White JS, Dow WH, Rungruanghiranya S. Commitment contracts and team incentives: a randomized controlled trial for smoking cessation in Thailand. American Journal of Preventive Medicine 2013;45(5):533‐42. [ClinicalTrials.gov: NCT01311115] [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2018 {published data only}
- NCT02421224. Social and monetary incentives for smoking cessation at large employers (SMILE). clinicaltrials.gov/show/NCT02421224 (first received 20 April 2015).
Windsor 1988 {published data only}
- Windsor RA, Lowe JB. Behavioral impact and cost analysis of a worksite self‐help smoking cessation program. Progress in Clinical and Biological Research 1989;392:231‐42. [PubMed] [Google Scholar]
- Windsor RA, Lowe JB, Bartlett EE. The effectiveness of a worksite self‐help smoking cessation program: a randomized trial. Journal of Behavioral Medicine 1988;11(4):407‐21. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alessi 2008 {published data only}
- Alessi SM, Petry NM, Urso J. Contingency management promotes smoking reduction in residential substance abuse patients. Journal of Applied Behavior Analysis 2008;41:617‐22. [Clinicaltrials.gov ID NCT00408265] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berg 2014 {published data only}
- Berg CJ, Stratton E, Sokol M, Santamaria A, Bryant L, Rodriguez R. Novel incentives and messaging in an online college smoking intervention. American Journal of Health Behavior 2014;38(5):668‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowers 1987 {published data only}
- Bowers TG, Winett RA, Frederiksen LW. Nicotine fading, behavioral contracting, and extended treatment: effects on smoking cessation. Addictive Behaviors 1987;12(2):181‐4. [DOI] [PubMed] [Google Scholar]
Businelle 2014 {published data only}
- Businelle MS, Kendzor DE, Kesh A, Cuate EL, Poonawalla IB, Reitzel LR, et al. Small financial incentives increase smoking cessation in homeless smokers: a pilot study. Addictive Behaviors 2014;39(3):717‐20. [DOI] [PubMed] [Google Scholar]
Cavallo 2007 {published data only}
- Cavallo DA, Cooney JL, Duhig AM, Smith AE, Liss TB, McFetridge AK, et al. Combining cognitive behavioral therapy with contingency management for smoking cessation in adolescent smokers: a preliminary comparison of two different CBT formats. American Journal on the Addictions 2007;16(6):468‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhig A, Cavallo D, McFetridge A, Liss T, Dahl T, Krishnan‐Sarin S. Contingency management for smoking cessation in adolescent smokers (POS2‐51). Society for Research in Nicotine and Tobacco 12th Annual Meeting February 15‐18, Orlando FLA. 2006:87.
- Krishnan‐Sarin S, Duhig AM, McKee SA, McMahon TJ, Liss T, McFetridge A. Contingency management for smoking cessation in adolescents. Experimental and Clinical Psychopharmacology 2006;14(3):306‐10. [DOI] [PubMed] [Google Scholar]
- Krishnan‐Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, et al. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug and Alcohol Dependence 2007;88(1):79‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chivers 2008 {published data only}
- Chivers LL, Higgins ST, Heil SH, Proskin RW, Thomas CS. Effects of initial abstinence and programmed lapses on the relative reinforcing effects of cigarette smoking. Journal of Applied Behavior Analysis 2008;41(4):481‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Correia 2006 {published data only}
- Correia CJ, Benson TA. The use of contingency management to reduce cigarette smoking among college students. Experimental and Clinical Psychopharmacology 2006;14(2):171‐9. [DOI] [PubMed] [Google Scholar]
Crowley 1991 {published data only}
- Crowley TJ, MacDonald MJ, Zerbe GO, Petty TL. Reinforcing breath carbon monoxide reductions in chronic obstructive pulmonary disease. Drug and Alcohol Dependence 1991;29(1):47‐62. [DOI] [PubMed] [Google Scholar]
Crowley 1995 {published data only}
- Crowley TJ, Macdonald MJ, Walter MI. Behavioral anti‐smoking trial in chronic obstructive pulmonary disease patients. Psychopharmacology 1995;119(2):193‐204. [DOI] [PubMed] [Google Scholar]
Cummings 1988 {published data only}
- Cummings KM, Hellmann R, Emont SL. Correlates of participation in a worksite stop‐smoking contest. Journal of Behavioral Medicine 1988;11(3):267‐77. [DOI] [PubMed] [Google Scholar]
Curry 1991 {published data only}
- Curry SJ, Wagner EH, Grothaus LC. Evaluation of intrinsic and extrinsic motivation interventions with a self‐help smoking cessation program. Journal of Consulting and Clinical Psychology 1991;59(2):318‐24. [DOI] [PubMed] [Google Scholar]
Dallery 2008 {published data only}
- Dallery J, Meredith S, Glenn IM. A deposit contract method to deliver abstinence reinforcement for cigarette smoking. Journal of Applied Behavior Analysis 2008;41(4):609‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Paul 1989 {published data only}
- Jason LA, Lesowitz T, Michaels M, Blitz C, Victors L, Dean L, et al. A worksite smoking cessation intervention involving the media and incentives. American Journal of Community Psychology 1989;17(6):785‐99. [DOI] [PubMed] [Google Scholar]
- Salina D, Jason LA, Hedeker D, Kaufman J, Lesondak L, McMahon SD, et al. A follow‐up of a media‐based, worksite smoking cessation program. American Journal of Community Psychology 1994;22(2):257‐71. [DOI] [PubMed] [Google Scholar]
Donatelle 2000c {published data only}
- Donatelle RJ, Prows SL, Champeau D, Hudson D. Using social support, biochemical feedback, and incentives to motivate smoking cessation during pregnancy: comparison of three intervention trials. Poster at American Public Health Association meeting, Boston MA, USA 2000.
Dunn 2008 {published data only}
- Dunn KE, Saulsgiver KA, Sigmon SC. Contingency management for behavior change: applications to promote brief smoking cessation among opioid‐maintained patients. Experimental and Clinical Psychopharmacology 2011;19(1):20‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Thomas CS, Heil SH, Higgins ST. Voucher‐based contingent reinforcement of smoking abstinence among methadone‐maintained patients: a pilot study. Journal of Applied Behavior Analysis 2008;41(4):527‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunn 2010 {published data only}
- Dunn KE, Saulsgiver KA, Sigmon SC. Contingency management for behavior change: applications to promote brief smoking cessation among opioid‐maintained patients. Experimental and Clinical Psychopharmacology 2011;19(1):20‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Sigmon SC, Reimann EF, Badger GJ, Heil SH, Higgins ST. A contingency management intervention to promote initial smoking cessation among opioid‐maintained patients. Experimental and Clinical Psychopharmacology 2010;18(1):37‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elliott 1968 {published data only}
- Elliott R, Tighe T. Breaking the cigarette habit: effects of a technique involving threatened loss of money. Psychological Record 1968;18(4):503‐13. [Google Scholar]
Emont 1992 {published data only}
- Emont SL, Cummings KM. Using a low‐cost, prize‐drawing incentive to improve recruitment rate at a work‐site smoking cessation clinic. Journal of Occupational Medicine 1992;34(8):771‐4. [DOI] [PubMed] [Google Scholar]
Fortmann 1995 {published data only}
- Fortmann SP, Killen JD. Nicotine gum and self‐help behavioral treatment for smoking relapse prevention: results from a trial using population‐based recruitment. Journal of Consulting and Clinical Psychology 1995;63(3):460‐8. [DOI] [PubMed] [Google Scholar]
Gadomski 2010 {published data only}
- Gadomski A, Adams L, Tallman N, Krupa N, Jenkins P. Effectiveness of a combined prenatal and postpartum smoking cessation program. Maternal and Child Health Journal 2010;15(2):188‐97. [DOI] [PubMed] [Google Scholar]
Gilbert 1999 {published data only}
- Gilbert DG, Crauthers DM, Mooney DK, McClernon FJ, Jensen RA. Effect of monetary contingencies on smoking relapse: influences of trait depression, personality, and habitual nicotine intake. Experimental and Clinical Psychopharmacology 1999;7(2):174‐81. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Plath LC, Jensen RA, Meliska CJ. Effects of smoking abstinence on mood and craving in men: influences of negative‐affect‐related personality traits, habitual nicotine intake and repeated measurements. Personality and Individual Differences 1998;25(3):399‐423. [Google Scholar]
Gilbert 2002 {published data only}
- Gilbert DG, McClernon FJ, Rabinovich NE, Plath LC, Masson CL, Anderson AE, et al. Mood disturbance fails to resolve across 31 days of cigarette abstinence in women. Journal of Consulting and Clinical Psychology 2002;70(1):142‐52. [DOI] [PubMed] [Google Scholar]
Glover 2015 {published data only}
Gottlieb 1990 {published data only}
- Gottlieb NH, Nelson A. A systematic effort to reduce smoking at the workplace. Health Education Quarterly 1990;17(1):99‐118. [DOI] [PubMed] [Google Scholar]
Graham 2007 {published data only}
- Graham AL, Cobb NK, Raymond L, Sill S, Young J. Effectiveness of an internet‐based worksite smoking cessation intervention at 12 months. Journal of Occupational and Environmental Medicine 2007;49(8):821‐8. [DOI] [PubMed] [Google Scholar]
Gulliver 2004 {published data only}
- Gulliver SB, Colby SM, Hayes K, Raffa SD. Tobacco cessation treatment for pregnant smokers: incorporating partners and incentives. Medicine and Health Rhode Island 2004;87(1):9‐12. [PubMed] [Google Scholar]
Hanewinkel 2007 {published data only}
- Etter JF, Bouvier P. Some doubts about one of the largest smoking prevention programmes in Europe, the smokefree class competition. Journal of Epidemiology and Community Health 2006;60(9):757‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanewinkel R. "Be smart ‐ don't start". Results of a non‐smoking competition in Germany 1997‐2007. Gesundheitswesen 2007;69(1):38‐44. [DOI] [PubMed] [Google Scholar]
- Hanewinkel R, Wiborg G. Diffusion of the non‐smoking campaign "Be smart ‐ don't start" between 1997 and 2003 in Germany. Gesundheitswesen 2003;65(4):250‐4. [DOI] [PubMed] [Google Scholar]
- Hanewinkel R, Wiborg G. Primary and secondary prevention of smoking in adolescents: results of the campaign "Be smart ‐ don't start". Gesundheitswesen 2002;64(8‐9):492‐8. [DOI] [PubMed] [Google Scholar]
- Hanewinkel R, Wiborg G, Abdennbi K, Ariza C, Bollars C, Bowker S, et al. European smokefree class competition: a measure to decrease smoking in youth. Journal of Epidemiology and Community Health 2007;61(8):750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanewinkel R, Wiborg G, Isensee B, Nebot M, Vartiainen E. "Smoke‐free Class Competition": Far‐reaching conclusions based on weak data. Preventive Medicine 2006;43(2):150‐1. [DOI] [PubMed] [Google Scholar]
- Pick O, Nolte B, Koller M, Vogelmeier M, Engenhart‐Cabilic R. Significance of a health campaign in 11‐ to 14‐year‐old school children for smoking prevention ‐ Effect evaluation on the school competition "Be smart ‐ don't start". Gesundheitswesen 2005;67(7):542. [Google Scholar]
- Schneider S, Mohnen SM, Tonges S, Potschke‐Langer M, Schulze A. Are competitions an appropriate instrument for youth smoking cessation? A 1‐year follow‐up of the Germany‐wide 'Smoke‐free 2004' campaign. Medizinische Klinik 2006;101(9):711‐7. [DOI] [PubMed] [Google Scholar]
- Schulze A, Mons U, Edler L, Potshcke‐Langer M. Lack of sustainable prevention effect of the "Smoke‐free Class Competition" on German pupils. Preventive Medicine 2006;42(1):33‐9. [DOI] [PubMed] [Google Scholar]
- Wiborg G, Hanewinkel R, Kliche KO. "Be smart ‐ don't start" campaign to prevent children from starting to smoke: an analysis according to type of school they attend. Deutsche Medizinische Wochenschrift 2002;127(9):430‐6. [DOI] [PubMed] [Google Scholar]
Haug 2017 {published data only}
- Haug S, Paz Castro R, Kowatsch T, Filler A, Schaub MP. Efficacy of a technology‐based, integrated smoking cessation and alcohol intervention for smoking cessation in adolescents: Results of a cluster‐randomised controlled trial. Journal of Substance Abuse Treatment 2017;82:55‐66. [DOI] [PubMed] [Google Scholar]
Hertzberg 2013 {published data only}
- Hertzberg JS, Carpenter VL, Kirby AC, Calhoun PS, Moore SD, Dennis MF, et al. Mobile contingency management as an adjunctive smoking cessation treatment for smokers with Posttraumatic Stress Disorder. Nicotine & Tobacco Research 2013;15(11):1934‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2004 {published data only}
- Higgins ST, Bernstein IM, Washio Y, Heil SH, Badger GJ, Skelly JM, et al. Effects of smoking cessation with voucher‐based contingency management on birth outcomes. Addiction 2010;105(11):2023‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Badger GJ, Skelly JM, Solomon LJ, Bernstein IM. Educational disadvantage and cigarette smoking during pregnancy. Drug & Alcohol Dependence 2009;104(Suppl 1):s100‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dumeer AM, Thomas CS, Solomon LJ, Bernstein IM. Smoking status in the initial weeks of quitting as a predictor of smoking‐cessation outcomes in pregnant women. Drug and Alcohol Dependence 2006;85(2):138‐41. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Solomon LJ, Bernstein IM, Lussier JP, Abel RL, et al. A pilot study on voucher‐based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine & Tobacco Research 2004;6(6):1015‐20. [DOI] [PubMed] [Google Scholar]
- Higgins TM, Higgins ST, Heil SH, Badger GJ, Skelly JM, Bernstein IM, et al. Effects of cigarette smoking cessation on breastfeeding duration. Nicotine & Tobacco Research 2010;12(5):483‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Experimental and Clinical Psychopharmacology 2007;15(2):176‐86. [DOI] [PubMed] [Google Scholar]
Hunt 2010 {published data only}
- Hunt YM, Rash CJ, Burke RS, Parker JD. Smoking cessation in recovery: comparing 2 different cognitive behavioral treatments. Addictive Disorders & Their Treatment 2010;9(2):64‐74. [Google Scholar]
Jason 1990 {published data only}
- Jason LA, Jayaraj S, Blitz CL, Michaels MH, Klett LE. Incentives and competition in a worksite smoking cessation intervention. American Journal of Public Health 1990;80(2):205‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeffery 1988 {published data only}
- Jeffery RW, Pheley AM, Forster JL, Kramer FM, Snell MK. Payroll contracting for smoking cessation: a worksite pilot study. American Journal of Preventive Medicine 1988;4(2):83‐6. [PubMed] [Google Scholar]
Jeffery 1989 {published data only}
- Jeffery RW, Forster JL, Schmid TL. Worksite health promotion: feasibility testing of repeated weight control and smoking cessation classes. American Journal of Health Promotion 1989;3(4):11‐16. [DOI] [PubMed] [Google Scholar]
Jeffery 1993 {published data only}
- Jeffery RW, Forster JL, Baxter JE, French SA, Kelder SH. An empirical evaluation of the effectiveness of tangible incentives in increasing participation and behavior change in a worksite health promotion program. American Journal of Health Promotion 1993;8(2):98‐100. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, Forster JL, French SA, Kelder SH, Lando HA, McGovern PG, et al. The Healthy Worker Project: a work‐site intervention for weight control and smoking cessation. American Journal of Public Health 1993;83(3):395‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kassaye 1984 {published data only}
- Kassaye WW. Assessment of the impact of monetary incentives upon smoking behavior. Journal of Drug Education 1984;14(2):109‐18. [DOI] [PubMed] [Google Scholar]
Kendzor 2015 {published data only}
- Kendzor DE, Businelle MS, Poonawalla IB, Cuate EL, Kesh A, Rios DM, et al. Financial incentives for abstinence among socioeconomically disadvantaged individuals in smoking cessation treatment. American Journal of Public Health 2015; Vol. 105, issue 6:1198‐205. [DOI] [PMC free article] [PubMed]
Kollins 2010 {published data only}
- Kollins SH, McClernon FJ, Voorhees EE. Monetary incentives promote smoking abstinence in adults with attention deficit hyperactivity disorder (ADHD). Experimental and Clinical Psychopharmacology 2010;18(3):221‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamb 2004 {published data only}
- Lamb RJ, Morral AR, Kirby KC, Iguchi MY, Galbicka G. Shaping smoking cessation using percentile schedules. Drug and Alcohol Dependence 2004;76(3):247‐59. [DOI] [PubMed] [Google Scholar]
- Romanowich P, Lamb RJ. The relationship between in‐treatment abstinence and post‐treatment abstinence in a smoking cessation treatment. Experimental and Clinical Psychopharmacology 2010;18(1):32‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamb 2007 {published data only}
- Lamb RJ, Morral AR, Kirby KC, Javors MA, Galbicka G, Iguchi M. Contingencies for change in complacent smokers. Experimental and Clinical Psychopharmacology 2007;15(3):245‐55. [DOI] [PubMed] [Google Scholar]
Lamb 2010 {published data only}
- Lamb RJ, Kirby KC, Morral AR, Galbicka G, Iguchi MY. Shaping smoking cessation in hard‐to‐treat smokers. Journal of Consulting and Clinical Psychology 2010;78(1):62‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lussier 2005 {published data only}
- Lussier JP, Higgins ST, Badger GJ. Influence of the duration of abstinence on the relative reinforcing effects of cigarette smoking. Psychopharmacology Berl 2005;181(3):486‐95. [DOI] [PubMed] [Google Scholar]
MacKillop 2009 {published data only}
- McKillop J, Kahler CW. Delayed reward discounting predicts treatment response for heavy drinkers receiving smoking cessation treatment. Drug and Alcohol Dependence 2009;104(3):197‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantzari 2012 {published data only}
- Mantzari E, Vogt F, Marteau TM. The effectiveness of financial incentives for smoking cessation during pregnancy: is it from being paid or from the extra aid?. BMC Pregnancy and Childbirth 2012;12(24):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonell 2013 {published data only}
- McDonell MG, Srebnik D, Angelo F, McPherson S, Lowe JM, Sugar A, et al. A randomized controlled trial of contingency management for psycho‐stimulant use in community mental health outpatients with co‐occuring serious mental illness. American Journal of Psychiatry 2013;170(1):94‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meredith 2011 {published data only}
- Meredith SE, Grabinski MJ, Dallery J. Internet‐based group contingency management to promote abstinence from cigarette smoking: a feasibility study. Drug and Alcohol Dependence 2011;118(1):23‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Monti 2006 {published data only}
- Monti P, Tevyaw TO, Tidey J, Colby S, Kahler C, Barnett N, et al. Combining motivational enhancement and contingency management for young adult smokers (SYM3D). Society for Research on Nicotine and Tobacco, 12th Annual Meeting February 15‐18, Orlando FLA. 2006.
- Ohmura Y, Takahashi T, Kitamura N. Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacology Berl 2005;182(4):508‐15. [DOI] [PubMed] [Google Scholar]
- Tevyaw TO, Tidey J, Colby S, Kahler C, Barnett N, Luboyeski E, et al. Contingency management and MET for young adult smokers: preliminary results (RP‐065). Society for Research on Nicotine and Tobacco, 11th Annual Meeting March, Prague, Czech Republic. 2005.
Mooney 2004 {unpublished data only}
- Mooney ME. Interventions to increase use of nicotine gum: a randomized, controlled single‐blind trial. Dissertation Abstracts International: Section B: The Sciences and Engineering 2004; Vol. 64:4052.
NCT00508560 {unpublished data only}
- NCT00508560. Contingency management for smoking cessation among veterans with schizophrenia or other psychoses. clinicaltrials.gov/ct2/show/NCT00508560 (first received 30th June 2017).
NCT00718835 {unpublished data only}
- NCT00718835. Incentive‐based smoking cessation for methadone patients. clinicaltrials.gov/ct2/show/NCT00718835 (first received 21 July 2008).
NCT00807742 {unpublished data only}
- NCT00807742. Contingency management for smoking in substance abusers. www.clinicaltrials.gov/ct2/show/NCT00807742 (first received 12th December 2008).
- Rohsenow DJ, Monti PM, Colby SM, Martin RA. Brief interventions for smoking cessation in alcoholic smokers. Alcoholism: Clinical and Experimental Research 2002;26(12):1950‐1. [DOI] [PubMed] [Google Scholar]
NCT00960375 {unpublished data only}
- NCT00960375. Smoking cessation for veterans with severe and persistent mental illness. www.clinicaltrials.gov/ct2/show/NCT00960375 (first received August 17th 2009).
NCT01040260 {unpublished data only}
- NCT01040260. Low‐cost contingency management for smoking cessation. www.clinicaltrials.gov/ct2/show/study/NCT01040260 (first received 29th December 2009).
NCT01145001 {unpublished data only}
- Krishnan‐Sarin S. Enhancing a high school‐based smoking cessation program. https://clinicaltrials.gov/ct2/show/NCT01145001 (first received 16th June 2010).
NCT01303081 {unpublished data only}
- NCT01303081. Pilot randomized controlled trial of financial incentives for smoking cessation. www.clinicaltrials.gov/ct2/show/NCT01303081 (first received 24th February 2011).
NCT02195570 {unpublished data only}
- NCT02195570. Contingency management for smoking cessation in pregnant women. www.clinicaltrials.gov/ct2/show/NCT02195570 (first received 21st July 2014).
Nowicki 1984 {published data only}
- Nowicki P, Gintzig L, Hebel JR, Latham R, Miller V, Sexton M. Effective smoking intervention during pregnancy. Birth 1984;11(4):217‐24. [DOI] [PubMed] [Google Scholar]
- Sexton M, Hebel JR. A clinical trial of change in maternal smoking and its effects on birth weight. JAMA 1984;251(7):911‐5. [PubMed] [Google Scholar]
Olsen 1990 {published data only}
- Olsen GW, Lacy SE, Sprafka JM, Arceneaux TG, Potts TA, Kravat BA, et al. A 5‐year evaluation of a smoking cessation incentive program for chemical employees. Preventive Medicine 1991;20(6):774‐84. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Shellenberger RJ, Lacy SE, Fishbeck WA, Bond GG. A smoking cessation incentive program for chemical employees: design and evaluation. American Journal of Preventive Medicine 1990;6(4):200‐7. [PubMed] [Google Scholar]
Ormston 2015 {published data only}
Pardell 2003 {published data only}
- Pardell H, Faixedas MT, Salto E, Valverde A, Tresserras R, Taberner JL, et al. Influence of an economical incentive on smoking cessation at community level. Society for Research on Nicotine and Tobacco 5th European Meeting 2003 November 20‐22 Padua: Abstract book. 2003; Vol. Poster 41.
Parker 2007 {published data only}
- Parker DR, Windsor RA, Roberts MB, Hecht J, Hardy NV, Strolla LO, et al. Feasibility, cost and cost‐effectiveness of a telephone‐based motivational intervention for underserved pregnant smokers. Nicotine & Tobacco Research 2007;9(10):1043‐51. [DOI] [PubMed] [Google Scholar]
Paxton 1980 {published data only}
- Paxton R. The effects of a deposit contract as a component in a behavioural programme for stopping smoking. Behaviour Research and Therapy 1980;18(1):45‐50. [DOI] [PubMed] [Google Scholar]
Paxton 1981 {published data only}
- Paxton R. Deposit contracts with smokers: varying frequency and amount of repayments. Behaviour Research and Therapy 1981;19(2):117‐23. [DOI] [PubMed] [Google Scholar]
Paxton 1983 {published data only}
- Paxton R. Prolonging the effects of deposit contracts with smokers. Behaviour Research and Therapy 1983;21(4):425‐33. [DOI] [PubMed] [Google Scholar]
Perkins 2010 {published data only}
- Perkins KA, Lerman C, Fonte CA, Mercincavage M, Stitzer ML, Chengappa KN, et al. Cross‐validation of a new procedure for early screening of smoking cessation medications in humans. Clinical Pharmacology and Therapeutics 2010;88(1):109‐14. [DOI] [PubMed] [Google Scholar]
Poole 2001 {published data only}
- Poole K, Kumpfer K, Pett M. The impact of an incentive‐based worksite health promotion program on modifiable health risk factors. American Journal of Health Promotion 2001;16(1):21‐6. [DOI] [PubMed] [Google Scholar]
Radley 2013 {published data only}
- Radley A, Ballard P, Eadie D, MacAskill S, Donnelly L, Tappin D. Give It Up For Baby: outcomes and factors influencing uptake of a pilot smoking cessation incentive scheme for pregnant women. BMC Public Health 2013;13:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rohsenow 2005 {unpublished data only}
- Rohsenow D, Martin R, Tidey J, Monti P, Swift R. Motivational and contingency interventions for unmotivated smokers in substance abuse treatment (PA‐7). Society for Research on Nicotine and Tobacco, 11th Annual Meeting 2005 March, Prague, Czech Republic. 2005.
Roll 2008 {published data only}
- Roll JM, Howard JT. The relative contribution of economic valence to contingency management efficacy: a pilot study. Journal of Applied Behavior Analysis 2008;41(4):629‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Romanowich 2010 {published data only}
- Romanowich P, Lamb RJ. Effects of escalating and descending schedules of incentives on cigarette smoking in smokers without plans to quit. Journal of Applied Behavior Analysis 2010;43(3):357‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Romanowich 2013 {published data only}
- Romanowich P, Lamb RJ. The effect of framing incentives as either losses or gains with contingency management for smoking cessation. Addictive Behaviors 2013;38(4):2084‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Romanowich 2014 {published data only}
- Romanowich P, Lamb RJ. The effects of percentile versus fixed criterion schedules on smoking with equal incentive magnitude for initial abstinence. Experimental and Clinical Psychopharmacology 2014; Vol. 22, issue 4:348‐55. [DOI] [PMC free article] [PubMed]
Sheikhattari 2016 {published data only}
- Sheikhattari P, Apata J, Kamangar F, Schutzman C, O'Keefe A, Buccheri J, et al. Examining smoking cessation in a community‐based vs. clinic‐based intervention using community‐based participatory research. Journal of Community Health 2016;41(6):1146‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sigmon 2012a {published data only}
- Sigmon SC, Patrick M, Saulsgiver K, Higgins S. Using financial incentives to sustain smoking abstinence among opioid‐maintained patients. 74th Annual Scientific Meeting of the College on Problems of Drug Dependence; June 9‐14, Palm Springs (CA), USA. 2012:Abstract 624.
Sloan 1990 {published data only}
- Sloan RP, Dimberg L, Welkowitz LA, Kristiansen MA. Cessation and relapse in a year‐long workplace quit‐smoking contest. Preventive Medicine 1990;19(4):414‐23. [DOI] [PubMed] [Google Scholar]
Spring 1978 {published data only}
- Spring FL, Sipich JF, Trimble RW, Goeckner DJ. Effects of contingency and noncontingency contracts in the context of a self‐control‐oriented smoking modification program. Behavior Therapy 1978;9(5):967‐8. [Google Scholar]
Stitzer 1985 {published data only}
- Stitzer ML, Bigelow GE. Contingent payment for carbon monoxide reduction: effects of pay amount. Behavior Therapy 1983;14(5):647‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent reinforcement for carbon monoxide reduction: within‐subject effects of pay amount. Journal of Applied Behavior Analysis 1984;17(4):477‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer ML, Bigelow GE. Contingent reinforcement for reduced breath carbon monoxide levels: target‐specific effects on cigarette smoking. Addictive Behaviors 1985;10(4):345‐9. [DOI] [PubMed] [Google Scholar]
Stoops 2009 {published data only}
- Stoops WW, Dallery J, Fields NM, Nuzzo PA, Schoenberg NE, Martin CA, et al. An internet‐based abstinence reinforcement smoking cessation intervention in rural smokers. Drug & Alcohol Dependence 2009;105(1‐2):56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strecher 1983 {published data only}
- Strecher VJ. A minimal‐contact smoking cessation program in a health care setting. Public Health Reports 1983;98(5):497‐502. [PMC free article] [PubMed] [Google Scholar]
Tanaka 2006 {published data only}
- Tanaka H, Yamato H, Tanaka T, Kadowaki T, Okamura T, Nakamura, M, et al. Effectiveness of a low‐intensity intra‐worksite intervention on smoking cessation in Japanese employees: a three‐year intervention trial. Journal of Occupational Health 2006;48(3):175‐82. [DOI] [PubMed] [Google Scholar]
Tidey 2011 {published data only}
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Reid N. Effects of contingency management and bupropion on cigarette smoking in smokers with schizophrenia. Psychopharmacology 2011;217(2):279‐87. [ClinicalTrials.gov: NCT00136760] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagner 2013 {published data only}
- Wagner F, Sheikhattan P. Improving the retention rates of two evidence‐based smoking cessation trials designed through a participatory approach. European Psychiatry 2013;28(S1):1968. [Google Scholar]
Walsh 1997 {published data only}
- Walsh RA, Redman S, Brinsmead MW, Byrne JM, Melmeth A. A smoking cessation program at a public antenatal clinic. American Journal of Public Health 1997;87(7):1201‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winett 1973 {published data only}
- Winett RA. Parameters of deposit contracts in the modification of smoking. Psychological Record 1973;23:49‐60. [Google Scholar]
Winhusen 2014 {published data only}
- Winhusen T, Stitzer M, Woody G, Brigham G, Kropp F, Ghitza U, et al. Design considerations for a study to evaluate the impact of smoking cessation treatment on stimulant use outcomes in stimulant‐dependent individuals. Contemporary Clinical Trials 2012;33(1):197‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen TM, Brigham GS, Kropp F, Lindblad R, Gardin JG, Penn P, et al. A randomized trial of concurrent smoking cessation and substance use disorder treatment in stimulant‐dependent smokers. Journal of Clinical Psychiatry 2014;75(4):336‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen TM, Kropp F, Theobald J, Lewis DF. Achieving smoking abstinence is associated with decreased cocaine use in cocaine‐dependent patients receiving smoking‐cessation. Drug and Alcohol Dependence 2014;134:391‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiseman 2005 {published data only}
- Wiseman EJ, Williams DK. Effectiveness of payments for reducing carbon monoxide levels and noncontingent payments on smoking behaviors in cocaine‐abusing outpatients wearing nicotine or placebo patches. Experimental and Clinical Psychopharmacology 2005;13(2):102‐10. [DOI] [PubMed] [Google Scholar]
Yi 2008 {published data only}
- Yi R, Johnson MW, Giordano LA, Landes RD, Badger GJ, Bickel WK. The effects of reduced cigarette smoking on discounting future rewards: an initial evaluation. Psychological Record 2008;58(2):163‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoon 2009 {published data only}
- Yoon JH, Higgins ST, Bradstreet MP, Badger GJ, Thomas CS. Changes in the relative reinforcing effects of cigarette smoking as a function of initial abstinence. Psychopharmacology Berl 2009;205(2):305‐18. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT02713594 {published data only}
- NCT02713594. Wisconsin tobacco quit line medicaid incentive evaluation. clinicaltrials.gov/show/nct02713594 first received 18 March 2016.
References to ongoing studies
Berlin 2016 {published data only}
- Berlin N, Goldzahl L, Jusot F, Berlin I. Protocol for study of financial incentives for smoking cessation in pregnancy (FISCP): randomised, multicentre study. BMJ Open 2016;6(7):e011669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02606227. Financial incentive for smoking cessation in pregnancy. clinicaltrials.gov/show/nct02606227 (first received 17 November 2015).
Lynagh 2012 {published and unpublished data}
- ACTRN12612000399897. ENti‐Ce Project ‐ Encouragement for Nicotine Cessation in pregnant smokers. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=347842 (first received 16 March 2012).
- Lynagh M, Bonevski B, Sanson‐Fisher R, Symonds I, Scott A, Hall A, et al. An RCT protocol of varying financial incentive amounts for smoking cessation among pregnant women. BMC Public Health 2012;12:1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meyer 2015 {published data only}
- Meyer AC, Streck JM, Ochalek TA, Hruska B, Teneback CC, Dixon AE, et al. Financial incentives promote smoking abstinence among patients with pulmonary disease. Drug and Alcohol Dependence 2015;156:e150‐1. [Google Scholar]
NCT00064922 {unpublished data only}
- NCT00064922. Incentive programs for female substance abusers who smoke. clinicaltrials.gov/ct2/show/NCT00064922 (first received 16 July 2003).
NCT00079469 {unpublished data only}
- NCT00079469. Bupropion and counseling with or without contingency management to enhance smoking cessation in cancer survivors who continue to smoke. clinicaltrials.gov/ct2/show/NCT00079469 (first received 10 March 2004).
NCT00273793 {unpublished data only}
- NCT00273793. Increasing contingency management success using shaping. clinicaltrials.gov/ct2/show/NCT00273793 (first received 9 January 2006).
NCT00408265 {unpublished data only}
- NCT00408265. Smoking cessation in substance abuse treatment patients: a feasibility study. clinicaltrials.gov/ct2/show/NCT00408265 (first received 6 December 2006).
NCT00683280 {unpublished data only}
- NCT00683280. Contingency management and pharmacotherapy for smoking cessation. www.clinicaltrials.gov/ct2/show/NCT00683280 (first received 23rd May 2008).
NCT00690131 {unpublished data only}
- NCT00690131. An integrated approach to smoking cessation in Severe Mental Illness (SMI). clinicaltrials.gov/ct2/show/NCT00690131 (first received 4 June 2008).
NCT01484717 {unpublished data only}
- NCT01484717. Interactive Voice Response technology to mobilize contingency management for smoking cessation. www.clinicaltrials.gov/ct2/show/NCT01484717 (first received 2nd December 2011).
NCT01736982 {unpublished data only}
- NCT01736982. Contingency management for smoking cessation in the homeless. www.clinicaltrials.gov/ct2/show/NCT01736982 (first received 29th November 2012).
NCT01789710 {unpublished data only}
- NCT01789710. Contingency management for smoking cessation in homeless smokers. www.clinicaltrials.gov/ct2/show/NCT01789710 (first received 12th February 2013).
NCT01826331 {published data only}
- NCT01826331. Incentives for participation versus outcomes. clinicaltrials.gov/show/nct01826331 (first received 8th April 2013).
NCT01965405 {unpublished data only}
- NCT01965405. Smoking cessation for people living with HIV/AIDS. www.clinicaltrials.gov/ct2/show/NCT01965405 (first received 18th October 2013).
NCT02210832 {unpublished data only}
- NCT02210832. Financial incentives for smoking cessation among disadvantaged pregnant women. www.clinicaltrials.gov/ct2/show/NCT01526265 (first received 3rd February 2012).
NCT02237898 {unpublished data only}
- NCT02237898. Harnessing the power of technology: MOMBA for postpartum smoking. www.clinicaltrials.gov/ct2/show/NCT02237898 (first received September 11th 2014).
NCT02245308 {unpublished data only}
- NCT02245308. Abstinence reinforcement therapy (ART) for homeless veteran smokers. www.clinicaltrials.gov/ct2/show/NCT02245308 (first received 19th September 2014).
NCT02266784 {unpublished data only}
- NCT02266784. Contingency management, quitting smoking, and ADHD (ADQUIT). www.clinicaltrials.gov/ct2/show/NCT02266784 (first received 17th October 2014).
NCT02506829 {published data only}
- NCT02506829. Financial incentives for smoking treatment. clinicaltrials.gov/show/NCT02506829 (first received 23rd July 2015).
NCT02596061 {published data only}
- NCT02596061. In it to quit: commitment contracts for smoking cessation. clinicaltrials.gov/show/nct02596061 (first received 4th November 2015).
NCT02737566 {published data only}
- NCT02737566. Small financial incentives to promote smoking cessation. clinicaltrials.gov/show/nct02737566 (first received 14th April 2016).
NCT02952703 {published data only}
- NCT02952703. Disseminating and implementing a smoking cessation program for pregnant and postpartum women. clinicaltrials.gov/show/nct02952703 (first received 2nd November 2016).
NCT03163056 {published data only}
- NCT03163056. Smoking cessation treatment for depressed smokers. clinicaltrials.gov/ct2/show/NCT03163056 (first received 22nd May 2017).
NCT03528304 {published data only}
- NCT03528304. Native women's wellness: contingency management for tobacco cessation and weight loss. clinicaltrials.gov/ct2/show/NCT03528304 (first received May 17th 2018).
NCT03551704 {published data only}
- NCT03551704. Smoking cessation treatment for substance use dependents. clinicaltrials.gov/ct2/show/NCT03551704 (first received 11th June 2018).
Secades‐Villa 2015 {published data only}
- Secades‐Villa R, López‐Núñez C, Pericot‐Valverde I, Alonso‐Pérez F, Garcia‐Rodriguez O. CM of smoking abstinence vs. CM with shaping for smoking cessation among treatment‐seeking patients. Drug and Alcohol Dependence 2015;146:e93. [DOI] [PubMed] [Google Scholar]
Wilson 2016 {published data only}
- NCT01723163. Abstinence Reinforcement Therapy (ART) for rural veteran smokers. www.clinicaltrials.gov/ct2/show/NCT01723163 (first received 7th November 2012).
- Wilson SM, Hair LP, Hertzberg JS, Kirby AC, Olsen MK, Lindquist JH, et al. Abstinence reinforcement therapy (ART) for rural veterans: methodology for an mHealth smoking cessation intervention. Contemporary Clinical Trials 2016;50:157‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Adams 2014
- Adams J, Giles EL, McColl E, Sniehotta FF. Carrots, sticks and health behaviours: a framework for documenting the complexity of financial incentive interventions to change health behaviours. Health Psychology Review 2014;8(3):286‐95. [DOI] [PubMed] [Google Scholar]
Ballard 2009
- Ballard P, Radley A. Give it up for Baby: a smoking cessation intervention for pregnant women in Scotland. Cases in Public Health Communication & Marketing 2009; Vol. 3:147‐60.
Berlin 2018
- Berlin N, Goldzahl L, Bauld L, Hoddinott P, Berlin I. Public acceptability of financial incentives to reward pregnant smokers who quit smoking: a United Kingdom‐France comparison. European Journal of Health Economics 2018;19(5):697‐708. [DOI: 10.1007/s10198-017-0914-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berman 2014
- Berman M, Crane R, Seiber E, Munur M. Estimating the cost of a smoking employee. Tobacco Control 2014;23:428‐33. [DOI] [PubMed] [Google Scholar]
Boyd 2016
- Boyd KA, Briggs AH, Bauld L, Sinclair L, Tappin D. Are financial incentives cost‐effective to support smoking cessation during pregnancy?. Addiction 2016;111(2):360‐70. [DOI: 10.1111/add.13160] [DOI] [PubMed] [Google Scholar]
Chamberlain 2017
- Chamberlain C, O'Mara‐Eves A, Porter J, Coleman T, Perlen SM, Thomas J, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.CD001055.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crossland 2015
- Crossland N, Thomson G, Morgan H, Dombrowski SU, Hoddinott P, the 'BIBS' study team. Incentives for breastfeeding and for smoking cessation in pregnancy: an exploration of types and meanings. Social Science and Medicine 2015;128(1):10‐7. [DOI] [PubMed] [Google Scholar]
Fanshawe 2017
- Fanshawe TR, Halliwell W, Lindson N, Aveyard P, Livingstone‐Banks J, Hartmann‐Boyce J. Tobacco cessation interventions for young people. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD003289.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fanshawe 2019
- Fanshawe TR, Hartmann‐Boyce J, Perera R, Lindson N. Competitions for smoking cessation. Cochrane Database of Systematic Reviews 2019, Issue 2. [DOI: 10.1002/14651858.CD013272] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gentry 2017
- Gentry S, Craig J, Holland R, Notley C. Smoking cessation for substance misusers: A systematic review of qualitative studies on participant and provider beliefs and perceptions. Drug and Alcohol Dependence 2017;180:178‐92. [DOI] [PubMed] [Google Scholar]
Giles 2014
- Giles EL, Robalino S, McColl E, Sniehotta FF, Adams J. The effectiveness of financial incentives for health behaviour change: systematic review and meta‐analysis. PLOS One 2014;9(3):e90347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giles 2015
- Giles E, Sniehotta FF, McColl E, Adams J. Acceptability of financial incentives and penalties for encouraging uptake of healthy behaviours: focus groups. BMC Public Health 2015;15:58. [DOI: 10.1186/s12889-015-1409-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gneezy 2011
- Gneezy U, Meier S, Ray‐Biel P. When and why incentives (don't) work to modify behaviour. Journal of Economic Perspectives 2011;25(4):191‐210. [Google Scholar]
Higgins 2002
- Higgins ST, Alessi SM, Dantona RL. Voucher‐based incentive. A substance abuse treatment innovation. Addictive Behaviors 2002;27(6):887‐910. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2012
- Higgins ST, Washio Y, Heil SH, Solomon LJ, Gaalema DE, Higgins TM, et al. Financial incentives for smoking cessation among pregnant and newly postpartum women. Preventive Medicine 2012 Nov [Epub ahead of print];55(Suppl):S33‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Hoddinott 2014
- Hoddinott P, Morgan H, MacLennan G, Sewel K, Thomson G, Bauld L, et al. Public acceptability of financial incentives for smoking cessation in pregnancy and breastfeeding: a survey of the British public. BMJ Open 2014;4(7):e005524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ierfino 2015
- Ierfino D, Mantzari E, Hirst J, Jones T, Aveyard P, Marteau T. Financial incentives for smoking cessation in pregnancy: a single arm intervention study assessing cessation and gaming. Addiction 2015;110(4):680‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jochelson 2007
- Jochelon K. Paying the patient: improving health using financial incentives. www.kingsfund.org.uk/sites/default/files/Paying_the_Patient.pdf 2007.
Lagarde 2009
- Lagarde M, Haines A, Palmer N. The impact of conditional cash transfers on health outcomes and use of health services in low and middle income countries. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008137] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leeks 2010
- Leeks KD, Hopkins DP, Soler RE, Aten A, Chattopadhyah SK: Task Force on Community Preventive Services. Worksite‐based incentives and competitions to reduce tobacco use: a systematic review. American Journal of Preventive Medicine 2010;38(2S):S263‐74. [DOI] [PubMed] [Google Scholar]
Lynagh 2013
- Lynagh MC, Sanson‐Fisher RW, Bonevski B. What's good for the goose is good for the gander. Principles for the use of financial incentives in health behaviour change. International Journal of Behavioral Medicine 2013;20(1):114‐20. [DOI] [PubMed] [Google Scholar]
Marteau 2009
- Marteau TM, Ashcroft RE, Oliver A. Using financial incentives to achieve healthy behaviour. BMJ 2009;338:b1415. [DOI] [PubMed] [Google Scholar]
Marteau 2013
- Marteau TM, Thorne J, Aveyard P, Hirst J, Sokal R. Financial incentives for smoking cessation in pregnancy: protocol for a single arm intervention study. BMC Pregnancy and Childbirth 2013;13:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinson 1999
- Martinson BC, Murray DM, Jeffery RW, Hennrikus DJ. Intraclass correlation for measures from a worksite health promotion study: estimates, correlates, and applications. American Journal of Health Promotion 1999;13(6):347‐57. [DOI] [PubMed] [Google Scholar]
Miglin 2017
- Miglin R, Kable JW, Bowers ME, Ashare RL. Withdrawal‐related changes in delay discounting predict short‐term smoking abstinence. Nicotine and Tobacco Research 2017;19(6):694‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
MMWR 2002
- Centers for Disease Control and Prevention. Annual smoking‐attributable mortality, years of potential life lost, and economic costs‐‐United States, 1995‐1999. MMWR; Morbidity and Mortality weekly report 2002;51(14):300‐3. [PubMed] [Google Scholar]
NICE 2010
- National Institute of Health and Clinical Excellence (NICE). Should incentives be used to help people quit unhealthy habits? NICE's Citizens Council reveals its view. www.nice.org.uk/newsroom/pressreleases/CitizensCouncilIncentives.jsp (accessed 30/11/2010).
NICE 2018
- National Institute for Health and Care Excellence. Guidelines to the Methods of Technology Appraisal. https://www.nice.org.uk/Media/Default/About/what‐we‐do/NICE‐guidance/NICE‐technology‐appraisals/technology‐appraisal‐processes‐guide‐apr‐2018.pdf 2018 (accessed 25th June 2019).
Paes‐Sousa 2011
- Paes‐Sousa R, Santos LM, Miazaki ES. Effects of a conditional cash transfer programme on child nutrition in Brazil. Bulletin of the World Health Organization 2011;89(7):496‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Powell‐Jackson 2011
Promberger 2012
- Promberger M, Dolan P, Marteau TM. "Pay them if it works"; discrete choice experiments on the acceptability of financial incentives to change health related behaviour. Social Science and Medicine 2012;75(12):2509‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robertson 2018
- Robertson L, Gendall P, Hoek J, Marsh L, McGee R. Perceptions of financial incentives for smoking cessation: a survey of smokers in a country with an endgame goal. Nicotine & Tobacco Research 2018;15(20):1481‐8. [DOI: 10.1093/ntr/ntx268] [DOI] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Sigmon 2012b
- Sigmon SC, Patrick ME. The use of financial incentives in promoting smoking cessation. Preventive Medicine 2012;55:S24‐S32. [DOI] [PMC free article] [PubMed] [Google Scholar]
SRNT 2002
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research 2002;4(2):149‐59. [DOI] [PubMed] [Google Scholar]
Strickland 2014
- Strickland S. Does it work to pay people to live healthier lives?. BMJ 2014;348:g2458. [DOI] [PubMed] [Google Scholar]
Tappin 2015b
Thomson 2014
- Thomson G, Morgan H, Crossland N, Bauld L, Dykes F, Hoddinottt P, the BIBS team. Unintended consequences for behaviour change and maintenance around childbirth of incentive provision. PLOS One 2014;9(10):e111322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van den Brand 2017
- Brand FA, Nagelhout GE, Reda AA, Winkens B, Evers SM, Kotz D, et al. Healthcare financing systems for increasing the use of tobacco dependence treatment. Cochrane Database of Systematic Reviews 2017, Issue 9. [DOI: 10.1002/14651858.CD004305.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Volpp 2014
- Volpp KG, Galvin R. Reward‐based incentives for smoking cessation: how a carrot became a stick. JAMA 2014;311(9):909‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2018
- World Health Organization. Tobacco: Key Facts. World Health Organization Fact Sheets 9 March 2019:www.who.int/news‐room/fact‐sheets/detail/tobacco.
World Bank 2012
- The World Bank. World Databank. www.databank.worldbank.org 2012 (accessed 18th August 2014).
References to other published versions of this review
Cahill 2008
- Cahill K, Perera R. Competitions and incentives for smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD004307.pub3] [DOI] [PubMed] [Google Scholar]
Cahill 2011
- Cahill K, Perera R. Competitions and incentives for smoking cessation. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD004307.pub4] [DOI] [PubMed] [Google Scholar]
Cahill 2015
- Cahill K, Hartmann‐Boyce J, Perera R. Incentives for smoking cessation. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD004307.pub5] [DOI] [PubMed] [Google Scholar]
Hey 2003
- Hey K, Perera R. Competitions and incentives for smoking cessation. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD004307] [DOI] [PubMed] [Google Scholar]
Hey 2005
- Hey K, Perera R. Competitions and incentives for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004307.pub2] [DOI] [PubMed] [Google Scholar]


