Abstract
Overproduction of extracellular diphosphate due to hydrolysis of ATP by NPP1 leads to pathological calcium diphosphate (pyrophosphate) dihydrate deposition (CPPD) in cartilage, resulting in a degenerative joint disease that today lacks a cure. Here, we targeted the identification of novel NPP1 inhibitors as potential therapeutic agents for CPPD deposition disease. Specifically, we synthesized novel analogs of AMP (NPP1 reaction product) and ADP (NPP1 inhibitor). These derivatives incorporate several chemical modifications of the natural nucleotides including (1) a methylene group replacing the Pα,β-bridging oxygen atom to provide metabolic resistance, (2) sulfonate group(s) replacing phosphonate(s) to improve binding to NPP1’s catalytic zinc ions, (3) an acyclic nucleotide analog to allow flexible binding in the NPP1 catalytic site, and (4) a benzimidazole base replacing adenine. Among the investigated compounds, adenine-N9-(methoxy)ethyl-β-bisphosphonate, 10, was identified as an NPP1 inhibitor (Ki 16.3 μM vs. the artificial substrate p-nitrophenyl thymidine-5′-monophosphate (p-Nph-5′-TMP), and 9.60 μM vs. the natural substrate, ATP). Compound 10 was selective for NPP1 vs. human NPP3, human CD39, and tissue non-specific alkaline phosphatase (TNAP), but also inhibited human CD73 (Ki 12.6 μM). Thus, 10 is a dual NPP1/CD73 inhibitor, which could not only be of interest for treating CPPD deposition disease and calcific aortic valve disease but may also be considered for the immunotherapy of cancer. Compound 10 proved to be a promising inhibitor, which almost completely reduces NPPase activity in human osteoarthritic chondrocytes at a concentration of 100 μM.
Electronic supplementary material
The online version of this article (10.1007/s11302-019-09649-2) contains supplementary material, which is available to authorized users.
Keywords: Ecto-nucleotide pyrophosphatase/phosphodiesterase1 (NPP1), AMP analogs, ADP analogs, Acyclic nucleotide, Human chondrocytes, Calcium pyrophosphate dihydrate (CPPD)
Introduction
Calcium pyrophosphate dihydrate (CPPD) crystal deposition disease is a rheumatologic disorder with varied clinical manifestations due to precipitation of CPPD crystals in articular tissues. The knees, wrists, shoulder, ankles, and elbows are the commonly involved joints [1]. Recent studies have established a relationship between calcium diphosphate (previous nomenclature: pyrophosphate, PPi) crystal deposition at the joints and the pathogenesis of osteoarthritis [2]. Calcium diphosphate crystals can be detected in the synovial fluid causing stiffness and severe pain and eventually leading to cartilage damage [3, 4]. Since no treatment is available to prevent and/or dissolve CPP crystals, CPPD disease presents an unmet medical need [5]. Excess diphosphate (PPi) causes the deposition of calcium diphosphate crystals in the cartilage and synovial fluid [6–10]. Ecto-nucleotide pyrophosphatase/ phosphodiesterase1, NPP1, has been identified as the main PPi-generating enzyme of osteoblasts and chondrocytes [10–12]. The overexpression of NPP1 in CPPD diseased cartilage and, consequently, production of more PPi from extracellular ATP [13] shifts the delicate equilibrium between Pi and PPi, giving rise to overproduction of PPi, which later precipitates in the joints as insoluble calcium salt.
The extracellular PPi pool is derived both by NPP1 and intracellular export via the transmembrane ankylosis protein (ANK) in chondrocyte and osteoblast membranes [14]. AMP, the enzymatic product of NPP1, regulates the mineralization process [15].
Extracellular ATP is released from cells and acts on P2X receptors (ATP-gated ion channels) or G protein-coupled P2Y receptors (P2Y-Rs) [16–19]. ATP’s action on P2-Rs is regulated by extracellular nucleotidases hydrolysing ATP and thus terminating its signaling. The families of ectonucleotidases which degrade the released ATP are NTPDases (ecto-nucleoside triphosphate diphosphohydrolase), APs (alkaline phosphatases), NPPs (ecto-nucleotide pyrophosphatases/phosphodiesterases), and ecto-5′-nucleotidase (eN; CD73) [20, 21].
NPP1, the main subtype of NPP family, is found at the cell surface as transmembrane proteins, and extracellularly as secreted enzymes. NPP1 was reported to hydrolyze a broad spectrum of nucleotides [22]. Its main substrate is ATP, which is converted to AMP and PPi. p-Nitrophenyl 5′-thymidine monophosphate (p-Nph-5′-TMP) is frequently used as an alternative substrate for NPP1 due to simple colorimetrical detection using a microplate reader [23].
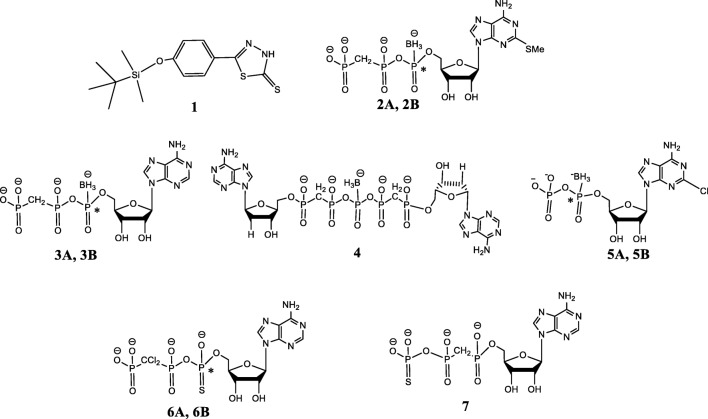
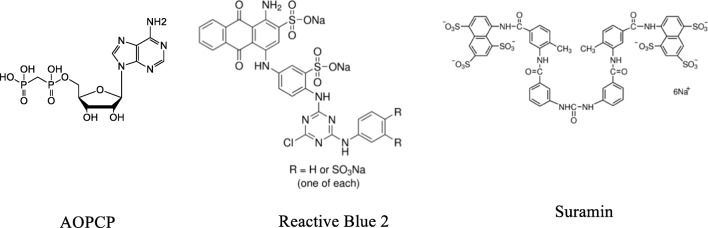
Several non-nucleotide-derived NPP1 inhibitors have been reported including polysulfonates, polysaccharides, polyoxometaletes, and diverse small heterocyclic compounds [22, 24]. Polysulfonates, e.g., Reactive Blue 2 and Suramin [25, 26], strongly inhibit human NPP1 (hNPP1) with Ki values in the nanomolar range, when p-Nph-5′-TMP or ATP were used as substrates. However, their selectivity for NPP1 is poor. The polyoxotungstate [TiW11CoO40]8− was found as the most potent inhibitor of human (h) NPP1 with a Ki 1.46 nM vs. ATP as a substrate [24, 27, 28], showing selectivity vs. human NTPDase1–3, NPP2–3, CD73, and tissue non-specific alkaline phosphatase (TNAP) [27, 28]. However, such polyanionic cluster compounds show limited stability and are not orally bioavailable. Oxadiazole and biscoumarin derivatives are weak non-competitive inhibitors of hNPP1 [29–32]. Quinazoline derivatives inhibited hNPP1, the best inhibitor displaying an IC50 36.2 nM vs. ATP as a substrate [28, 33, 34]. The quinazolines were NPP1-selective vs. NTPDase1–3, NPP3, CD73, and TNAP [34, 35], but showed high affinity binding to hERG potassium channels, which precluded their development as drugs due to expected cardiovascular side effects [34–36]. Recently, thiazolobenzimidazolone derivatives have been identified as potent uncompetitive NPP1 inhibitors, the best compound, 1 (Fig. 1), exhibiting a Ki 0.47 μM vs. ATP as a substrate. This scaffold, however, is hydrolytically unstable [37].
Fig. 1.
Selection of known NPP1 inhibitors
The most intensively investigated inhibitors of NPP1 are substrate analogs, namely, adenine nucleotide analogs, including Pα,β-methylene analogs, Pβ,γ-methylene analogs, 2-methylthio-adenine derivatives, nucleotides with oxidized ribose (dialdehyde derivatives), derivatives of diadenosine polyphosphates and nucleotide 2′(3′)-O-benzoylbenzoyl derivatives [22]. These nucleotide analogs generally exhibit a competitive mechanism of NPP1 inhibition [37–39]. Most of these analogs proved to be weak and non-selective NPP1 inhibitors.
Previously, we identified boranophosphate-modified ATP analogs, 2A/2B-diastereoisomers, and 3, as NPP1 inhibitors: 2A isomer, Ki 0.5 μM; 2B isomer, Ki 7 μM; and analog 3, Ki 56 μM vs. p-Nph-5′-TMP as a substrate [40, 41]. Pα-borano-substituted analogs showed complete resistance to hydrolysis in human blood serum over 24 h [42]. These nucleotides were also selective for NPP1 (85–90% inhibition at 100 μM) vs. NTPDase1–3 and −8 (0–10% inhibition at 100 μM) and NPP3 (10–25% inhibition at 100 μM) [43]. Selectivity is important since the blockade of other ecto-nucleotidases can cause side effects, e.g., immunogenic, antiproliferative, and antiangiogenetic effects by NTPDase1 or CD73 inhibition, or disturbed digestion of nucleotides from diets by NPP3 inhibitors.
We also found that the related di-2′-deoxyadenosine-Pα,β-Pδ,ε-dimethylene-penta-phosphonate, 4, is a selective inhibitor of NPP1 vs. NPP3 with an IC50 value of 13 μM and a Ki value of 9 μM vs. p-Nph-5′-TMP as a substrate [25]. In addition, at 100 μM 2-Cl-ADP-(α-borano), 5, A- and B-isomer, inhibited 90% and 85% of NPP1 activity, respectively [30].
We have also reported on nucleotides 6 and 7, bearing a thiophosphate moiety, as potent and selective NPP1 inhibitors [13]. ATP-α-CH2-γ-thio, 7, and ATP-α-thio-β,γ-CCl2 A-isomer 6 exhibited Ki values of 20 nM and 685 nM, respectively, vs. p-Nph-5′-TMP as a substrate [13]. Analog 7, although more potent than 6, showed less selectivity for NPP1. Analog 6 (at 100 μM) only slightly inhibited NPP3, NTPDase1, and NTPDase3 (38, 0, 22%) [13, 44].
NPP1 inhibitors have been suggested as potential therapeutic agents for CPPD deposition disease [29]. Indeed, recently, we have provided support for this hypothesis [42]. Specifically, we found that analog 6 inhibits NPPase activity in primary human chondrocytes cultured in monolayers. At 100 μM 6 inhibited the hydrolysis of 100 μM p-Nph-5′-TMP by 88%.
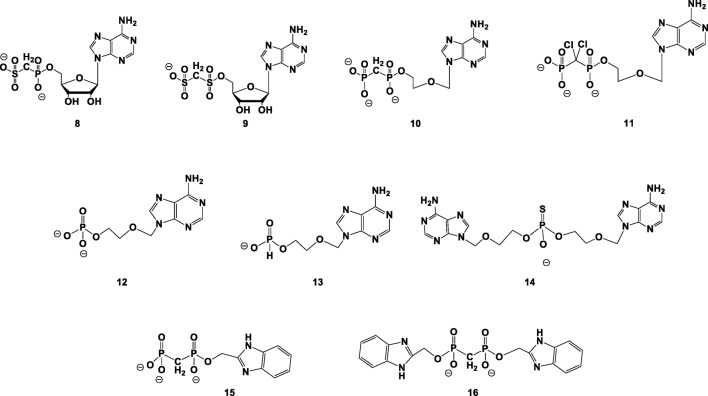
Our goal in the current study was to design and synthesize nucleotide analogs/mimics capable of inhibiting selectively NPP1 vs. various ectonucleotidases, as potential drug candidates for the treatment of OA/CPPD deposition disease. Specifically, we report on the synthesis of adenine nucleotide analogs/mimics 8–16 (Fig. 2); evaluation of their ability to inhibit hNPP1 and evaluation of their selectivity vs. other human ectonucleotidases including NPP3, CD39, and CD73; and finally, evaluation of their inhibition of NPPase activity vs. inhibition of TNAP in human osteoarthritic chondrocytes.
Fig. 2.
Adenine nucleotide analogs synthesized and evaluated here as NPP1 inhibitors
Results
Rational design of NPP1 inhibitors
Pre-requisites for nucleotide-based inhibitors of NPP1 are (1) resistance to hydrolysis by NPP1 and other ectonucleotidases and (2) potent, preferably selective inhibition of NPP1. Analogs 8–16 were designed here as potential NPP1 inhibitors based on the structure of the NPP1 catalytic site and the catalytic mechanism, as well as based on previous structure–activity relationships established in our group.
Specifically, we planned (1) stabilizing the Pα,β and Pβ,γ phosphodiester bonds by a CX2 (X = H, Cl) group; (2) improving the chelation of the NPP1 catalytic Zn2+ ion by phosphate bioisosters, such as sulfonate or thio-phosphonate moieties; (3) synthesizing an acyclic nucleotide analog for increasing the flexibility of the compound within the NPP1 catalytic site; and (4) maintaining/improving π-π interactions of the inhibitor with Tyr(340) within the NPP1 catalytic site [45] by replacing the adenine nucleobase by a benzimidazolyl moiety.
Specifically, we intended to achieve both inhibitory potency and enzymatic stability by replacing phosphate group(s) by a sulfonate- and a bis-sulfonate group, as in 7 and 9. We expected that the high affinity of the sulfonate moiety to Zn2+ ions will improve inhibition [46].
In addition, we considered ring-chain transformation of adenine nucleotides, namely, replacement of the ribose ring by a chain of comparable length, to help accommodate the molecule within the catalytic site due to increase in its flexibility. Derivatives 10–14 lack the ribose C2′ and C3′ atoms and hydroxyl groups (Fig. 2). It is noteworthy that the ribose 2′-OH binds to NPP1 via Tyr(340), while the ribose 3′-OH group does not participate in binding [43].
AMP, formed by the NPP1-catalyzed hydrolysis of ATP, inhibits the NPP1 reaction by a negative feedback [47]. Hence, we synthesized acyclic AMP analogs 12–14 as potential NPP1 inhibitors.
In analogs 15 and 16, we replaced the adenine nucleobase with a benzimidazolyl moiety for keeping π-π interactions with Tyr(340) within the active site of NPP1 [45], and possibly increasing the inhibitory potency of these analogs.
Synthesis of novel adenine nucleotide analogs 8–16
Adenosine-5′-(phosphoryl)methylene sulfonate 8 is a novel bioisoster of α,β-methylene-ADP (AOPCP). The latter has been reported as a weak and non-selective inhibitor of hNPP1 [24]. The 5′-Pα,β methylene group of 8 contributes to the chemical and metabolic stability of the compound, while replacement of the phosphonate group by a sulfonate group may improve binding to the NPP1 catalytic zinc ions [48].
Compound 8 was prepared from 2′,3′-methoxymethylidene-protected adenosine 17, treated with chloromethylene phosphorus dichloride at 0 °C, resulting in the formation of 18 which was treated with Na2SO3 [48], to add a sulfonate group as a Pβ-phosphate bioisoster (Scheme 1). Finally, the 2′,3′-methoxymethylidene protecting group was removed to yield the phosphonate-sulfonate isoster of ADP, 8. The product was separated on medium-pressure reverse phase column chromatography, followed by another separation on RP-HPLC. The final product was obtained in 11% yield, after replacing all Et3NH+ counter-ions with Na+ ions.
Scheme 1.
Synthesis of adenosine-5′-(phosphoryl)methylene-sulfonate, 8, and adenosine-5′-(sulfonyl)methylene-sulfonate, 9. Reagents and conditions: a (1) chloromethylene phosphorus dichloride, dry pyridine, Et3N, 0 °C, 5 h; (2) 0.2 M triethylammonium bicarbonate (TEAB), pH 8, 2 h, RT. b Na2SO3, microwave, 120 °C, 4.5 h. c (1) 10% HCl (pH 2.3), RT, 3 h; (2) 24% NH4OH, RT, 45 min; (3) Dowex 50WX8 hydrogen form, 10% NaOH. d (1) Methanedisulfonyl dichloride, dry pyridine, dry dichloromethane (DCM), 0 °C, 4 h; (2) 0.2 M TEAB, 2 h, RT
Adenosine-5′-(sulfonyl)methylenesulfonate, 9, was targeted as an analog of adenosine 5′-bisphosphonate, which bears two sulfonate groups capable of binding zinc ions. We have introduced a methylene-bis-sulfonate moiety at the 5′-position of protected adenosine, 17, by treating the latter with methane-disulfonyl dichloride at 0 °C. Next, the 2′,3′-protecting group was removed by 10% HCl (pH 2.3), and then by 24% NH4OH (pH 8.9) to give product 9 in 17% yield.
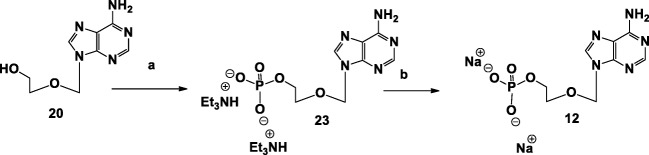
Adenine-N9-(methoxy)ethyl-β-bisphosphonate, 10, was targeted as a flexible analog of adenosine 5′-diphosphonate. We used methylenediphosphoryl-dichloride to introduce a methylene bis-phosphonate moiety at the primary alcohol of the acyclic adenine 20. Product 10 was obtained in 22% yield upon hydrolysis with triethylammonium bicarbonate (TEAB) solution (Scheme 2).
Scheme 2.
Synthesis of adenine-N9-(methoxy)ethyl-β-bisphosphonate, 10, and adenine-N9-(methoxy)ethyl-β-((dichloromethylene)bisphosphonate), 11. Reagents and conditions: a (1) CH2(PO2Cl)2, dry pyridine, dry DCM, − 30 °C, 4 h; (2) 0.2 M TEAB, 2 h. b Dowex 50WX8 hydrogen form, 10% NaOH. c 4-Toluene-sulfonyl chloride, dry pyridine, − 30 °C, 4 h. d (1) Cl2P2O6((Bu)3NH)2, acetonitrile, microwave, 120 °C, 45 min
Adenine-N9-(methoxy)ethyl-β-((dichloromethylene)bisphosphonate), 11, was synthesized as follows: adenine-(methoxy)ethanol, 20, was treated with 4-toluene-sulfonyl chloride to give 22, followed by displacement of the tosylate group with di-chloro-methylene bis-phosphonate to yield 11 in 25% yield (Scheme 2).
For the preparation of adenine-N9-(methoxy)ethyl-β-phosphate, 12, we treated 20 with phosphoryl chloride to introduce a monophosphate at the terminal alcohol, followed by hydrolysis with TEAB solution to give 12 in 15% yield (Scheme 3).
Scheme 3.
Synthesis of adenine-N9-(methoxy)ethyl-β-phosphate, 12. Reagents and conditions: a (1) POCl3, POMe3, − 15 °C, 1.5 h; (2) 0.2 M TEAB, 1 h, RT. b Dowex 50WX8 hydrogen form, 10% NaOH
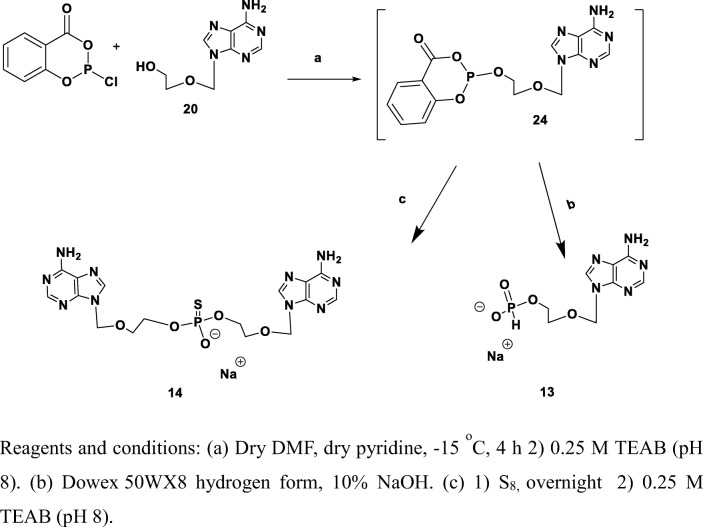
For the preparation of adenine-(methoxy)ethyl-β-(H-phosphonate), 13, we treated 20 with 2-chloro-4H-1,3,2-benzo-dioxaphosphorin-4-one followed by hydrolysis with TEAB solution (Scheme 4). Product 13 was obtained in 24% yield.
Scheme 4.
Synthesis of adenine-(methoxy)ethyl-β-(H-phosphonate), 13, and adenine-(methoxy)ethyl-β-(thiophosphate), 14. Reagents and conditions: a (1) dry DMF, dry pyridine, − 15 °C, 4 h; (2) 0.25 M TEAB (pH 8). b Dowex 50WX8 hydrogen form, 10% NaOH. c (1) S8, overnight; (2) 0.25 M TEAB (pH 8)
Adenine-(methoxy)ethyl-β-(thiophosphate), 14, was targeted as a flexible analog of adenosine-5′-thio-phosphate, which may in addition improve binding interactions with the NPP1 catalytic zinc ions. We used 2-chloro-4H-1,3,2-benzodioxaphosphorin-4-one to introduce a phosphite group at the terminal hydroxyl residue, followed by oxidation with elemental sulfur and finally hydrolysis with TEAB solution (Scheme 4). Product 14 was obtained in 9% yield.
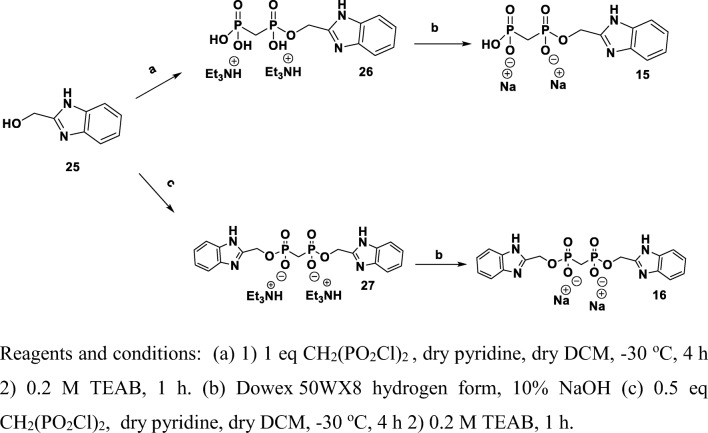
Benzimidazol-2-yl-methanol-bisphosphonate, 15, was prepared as an analog of compound 10. Compound 15 was obtained in 31% yield by treating benzimidazol-2-yl-methanol with methylenediphosphoryl-dichloride, followed by hydrolysis with TEAB solution (Scheme 5). When we treated benzimidazol-2-yl-methanol with 0.5 eq methylene bis(phosphonic-dichloride), we obtained benzimidazole dimer 16 in 8.2% yield.
Scheme 5.
Synthesis of benzimidazol-2-yl-methanol-bisphosphonate, 15, and benzimidazole-phosphonate, 16. Reagents and conditions: a (1) 1 eq CH2(PO2Cl)2, dry pyridine, dry DCM, − 30 °C, 4 h; (2) 0.2 M TEAB, 1 h. b Dowex 50WX8 hydrogen form, 10% NaOH. c (1) 0.5 eq CH2(PO2Cl)2, dry pyridine, dry DCM, − 30 °C, 4 h; (2) 0.2 M TEAB, 1 h
Evaluation of the ability of analogs 8–16 to inhibit human NPP1 and other ectonucleotidases
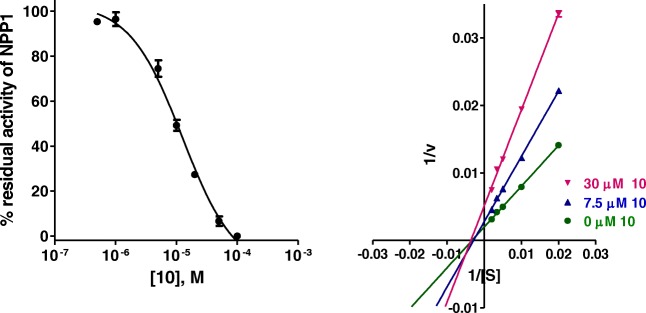
Analogs 8–16 were investigated for inhibition of human NPP1 (hNPP1) and, in addition, for their selectivity vs. other human ectonucleotidases including NPP3, NTPDase1 (CD39), and eN (CD73). The results are presented in Table 1. The data showed that only compound 10 significantly inhibited hNPP1 with a Ki value of 16.3 μM, when p-Nph-5′-TMP was applied as an artificial substrate. The Ki value of 10 was similar to that of the α,β-methylene-ADP (AOPCP) (Fig. 3), but much higher as compared to the standard NPP1 inhibitors—Reactive Blue 2 and Suramin (Table 1). Compound 10 was selective vs. human NPP3 and human CD39, but not vs. human CD73 (Ki value of 12.6 μM). A concentration-inhibition curve for 10 at hNPP1 is presented in Fig. 4. The subsequent investigation of the inhibition mechanism revealed a non-competitive inhibition, since all lines in the Lineweaver-Burk plot cross the X axis.
Table 1.
Evaluation of inhibitory activities of test compounds at various ectonucleotidases
| Compound | Ki ± SD (μM) (% inhibition ± SD at 10 μM) | |||
|---|---|---|---|---|
| Human NPP1a | Human NPP3a | Human CD39b | Human CD73c | |
| 8 | Inactived | Inactived | Inactived | 49.5 ± 0.7 |
| 9 | > 10 (12 ± 1) | Inactived | Inactived | > 10 (23 ± 3) |
| 10 | 16.3 ± 2.5 | Inactived | > 10 (22 ± 2) | 12.6 ± 1.4 |
| 11 | > 10 (4 ± 1) | Inactived | > 10 (24 ± 3) | > 10 (17 ± 4) |
| 12 | Inactived | Inactived | Inactived | > 10 (5 ± 3) |
| 13 | > 10 (6 ± 1) | Inactived | > 10 (33 ± 4) | Inactived |
| 14 | > 10 (13 ± 1) | Inactived | > 10 (15 ± 2) | Inactived |
| 15 | > 10 (21 ± 2) | Inactived | Inactived | > 10 (18 ± 6) |
| 16 | Inactived | > 10 (5 ± 1) | > 10 (9 ± 3) | > 10 (18 ± 5) |
| AOPCP | 1.28–16.5e | Inactived | > 10 (12 ± 5) | 0.197f |
| Reactive Blue 2 | 0.52g | 0.71g | 20.0h | 3.07i |
| Suramin | 0.26g | 0.04g | 300h | n.d.j |
aEvaluation of enzyme inhibition using 100 μM p-Nph-5′-TMP as a substrate
bEvaluation of enzyme inhibition using 100 μM ADP as a substrate
cEvaluation of enzyme inhibition using 5 μM [2,8-3H]AMP as a substrate
dNo inhibition at 10 μM
eLiterature value [22]
fLiterature value [49]
gLiterature value [50]
hLiterature value [26]
iLiterature value [49]
jNot determined
Fig. 3.
Previously reported NPP1 inhibitors
Fig. 4.
Concentration-inhibition curve of 10 at human NPP1 vs. 100 μM p-Nph-5′-TMP as a substrate (Ki = 16.3 ± 2.5 μM) and Lineweaver-Burk plot of NPP1 inhibition by 10. S, substrate concentration of p-Nph-5′-TMP (μM); v, velocity of enzyme (nmol/min/mg protein). Concentration of 10: green circle, 0 μM; blue triangle, 7.5 μM; violet triangle, 30 μM. Each experiment was performed in triplicates
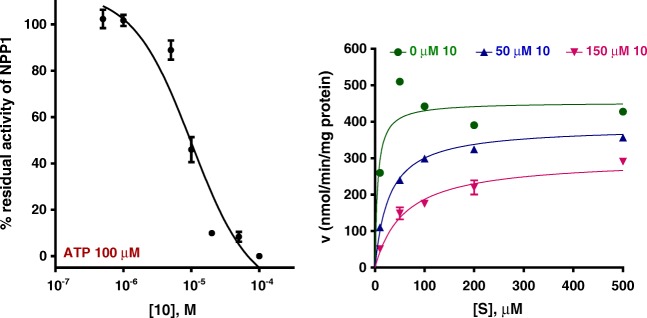
In previous studies, it have been found that inhibitors may display very different inhibitory potencies when tested vs. the artificial substrate p-Nph-5′-TMP, as compared to the natural substrate ATP [51]. Therefore, the inhibitory potency of compound 10 was subsequently investigated vs. the natural substrate ATP. The obtained Ki value with ATP was 9.60 ± 2.84 μM, which is lower than the Ki value obtained with p-Nph-5′-TMP as a substrate (16.3 μM) (Fig. 5). Interestingly, 10 showed a mixed inhibition of the enzyme (dominantly competitive inhibition) in the presence of ATP as a substrate (Fig. 5).
Fig. 5.
Concentration-inhibition curve of 10 at human NPP1 vs. 100 μM ATP as a substrate (Ki = 9.60 ± 2.84 μM) and Lineweaver-Burk plot of NPP1 inhibition by 10. S, substrate concentration of ATP (μM); v, velocity of enzyme (nmol/min/mg protein). Concentration of 10: green circle, 0 μM; blue triangle, 50 μM; violet triangle, 150 μM. Each experiment was performed in triplicates
Evaluation of the ability of analogs 8–16 to inhibit NPP1compared to as TNAP in human chondrocytes
Recently, we demonstrated that NPP1 is expressed in primary osteoarthritic human chondrocytes obtained from OA patients undergoing total knee replacement [42].
Here, we evaluated the ability of analogs 8–16 to inhibit NPPase activity in primary human chondrocytes cultured in monolayers at equimolar analog concentrations (100 μM) (Fig. 6a). Out of the studied series of analogs, compound 10 (at 100 μM) displayed the most promising inhibitory capabilities, showing 94% inhibition of NPPase activity in chondrocytes.
Fig. 6.
a Evaluation of the ability of analogs 8–16 to inhibit NPPase activity in human chondrocytes. NPPase activity was assayed by measuring the hydrolysis of the chromogenic substrate, p-Nph-5′-TMP. Analogs 8–16 and the natural substrate, ATP, were added at equimolar concentrations (100 μM). All values related to untreated human chondrocytes. b Human chondrocytes were incubated with or without analogs 8–16 and assayed for alkaline phosphatase activity by hydrolysis of p-nitrophenyl phosphate
To confirm that analogs 8–16 do not interfere with tissue non-specific alkaline phosphatase (TNAP) activity in adult human cartilage, we first tested their effects on primary human chondrocytes.
We found that analogs 8–16 did not inhibit TNAP activity (0% inhibition) as measured by p-nitrophenyl phosphate hydrolysis in human chondrocytes (Fig. 6b).
Evaluation of toxicity of analogs 8–16 in primary human chondrocytes
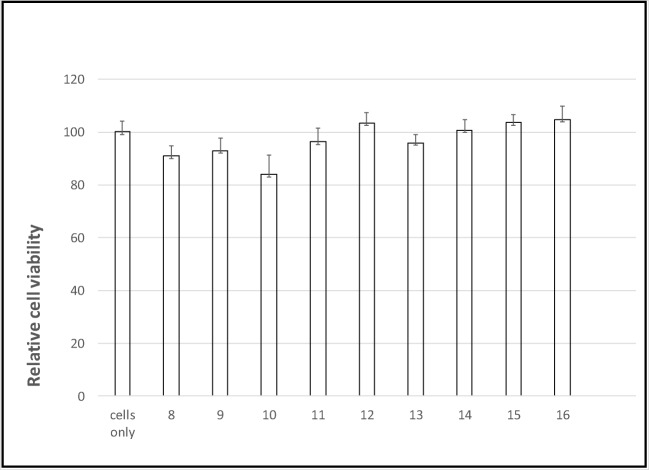
A prerequisite for the application of inhibitors 8–16 as therapeutic agents is the lack of toxicity. For this purpose, primary human chondrocytes were cultured with analogs 8–16 at a high concentration of 1 mM for 24 h. Thereafter, cell viability was measured relative to untreated controls by the XTT assay (Fig. 7). No significant decrease in cell’s viability was discernible at concentrations of up to 1 mM.
Fig. 7.
Analogs 8–16 are not toxic to primary human chondrocytes. Chondrocytes were incubated with analogs 8–16 at the indicated concentrations for 24 h, and then cell viability was assessed by the XTT assay
Discussion
Our quest for discovery of a drug candidate for OA/CPPD deposition disease relies on preventing PPi formation in human cartilage by inhibiting the major path of extracellular PPi formation, namely, hydrolysis of extracellular ATP by NPP1. For this purpose, we have designed and synthesized novel adenosine nucleotide analogs 8–16 as potential NPP1 inhibitors.
Evaluation of the inhibitory effect of analogs 8–16 at human NPP1 indicated that in this series, acyclic adenosine analog 10 showed the highest NPP1 inhibition, 44% at 10 μM. A Ki value of 16.3 μM was determined vs. p-Nph-5′-TMP as a substrate. The observed inhibitory potency of compound 10 was in the same range as the reported Ki value for AOPCP (Pα,β-methylene ADP) vs. p-Nph-5′-TMP (Ki 1.28 μM) [51]. Similar Ki values were also observed when the NPP1 inhibitor was tested vs. the natural substrate ATP (Ki 9.6 μM). This value was also similar to the reported values for AOPCP measured vs. ATP (Ki 16.5 μM) [51].
Importantly, NPP1 inhibitor 10 was selective vs. human NPP3 and NTPDase1 (CD39). Yet, it also inhibited human CD73 which further hydrolyzes AMP to adenosine (Ki 12.6 μM). Thus, 10 is a dual NPP1/CD73 inhibitor, which could not only be of interest for treating CPPD deposition disease but may also be considered for the immunotherapy of cancer. Furthermore, a dual NPP1/CD73 inhibitor is of importance as the treatment of calcific aortic valve disease (CAVD), the pathology of which is due to mineralization of the aortic valve promoted by adenosine [52]. Adenosine is generated in CAVD from ATP by the combined action of NPP1 and CD73.
Interestingly, the determined inhibition type of compound 10 was different for the artificial as compared to the natural substrate: when p-Nph-5′-TMP was employed as a substrate, a non-competitive inhibition type was found; while with ATP as the natural substrate, the inhibition was competitive. Compound 10 is an acyclic AOPCP analog, and other acyclic nucleotides have been reported to act as allosteric inhibitors of human NPP1 [38]. However, this is the first time that a nucleotide analog displays different inhibition modes vs. different NPP1 substrates. Our observations further confirm the hypothesis that the artificial substrate p-Nph-5′-TMP may additionally bind to a second binding site on NPP1 distinct from the orthosteric site [51].
Furthermore, inhibitor 10 reduced NPPase activity by 94% at 100 μM in human osteoarthritic chondrocytes, and was selective for NPP1 vs. TNAP, as observed by its inability to inhibit TNAP even at 100 μM. Inhibitor 10 is slightly more effective as an inhibitor of NPPase activity in primary chondrocytes cultured in monolayers than our previously reported analog 6. The latter compound (at 100 μM) inhibited the hydrolysis of p-Nph-5′-TMP (100 μM) by about 88% [42].
Surprisingly, replacement of the Pα,β-methylene group in 10 by Pα,β-dichloromethylene in 11 led to a loss of inhibitory potency. Likewise, replacement of an adenine ring by a benzimidazole ring and ribose ring-chain transformation in analogs 15 and 16 resulted in weak or inactive NPP1 inhibitors.
In summary, analog 10 was found to be the most promising NPP1 inhibitor in the tested series of analogs both at isolated hNPP1 enzyme, and in human osteoarthritic chondrocytes. The ancillary CD73 inhibitory activity of 10, which is in the same Ki range, could be beneficial for additional indications, e.g., to activate the immune system in cancer patients through enhancement of immunostimulatory ATP by NPP1 inhibition and prevention of the formation of immunosuppressive adenosine by CD73 blockade, as well as for the treatment of CAVD, where the combined action of NPP1 and CD73 generates adenosine which promotes the mineralization of the aortic valve.
Experiment
General
All commercial reagents were used without further purification, unless otherwise noted. All air- and moisture-sensitive reactions were conducted in flame-dried, nitrogen-flushed, two-neck flasks sealed with rubber septa, and the reagents were introduced with a syringe. All reactants for moisture-sensitive reactions were dried overnight in a vacuum oven. Progress of the reactions was monitored by TLC using precoated Merck silica gel plates (60F-253). Reactants and products were visualized using UV light. Compounds were characterized by NMR using a Bruker DPX-400 or DMX-600 spectrometer. 1H NMR spectra were recorded at 400 or 600 MHz. Nucleotides were also characterized by 31P NMR in D2O on Bruker AC-400 and DMX-600 spectrometers. High-resolution mass spectra were recorded on an AutoSpec-ESI mass spectrometer. Nucleotides were analyzed using electron spray ionization (ESI) on a Q-TOF microinstrument (Waters). Primary purification of the nucleotides was achieved on an LC (Isco UA-6) system using a column of Sephadex DEAE-A25, swollen in 1 M NaHCO3 or 1 M TEAB at 4 °C for 24 h. The resin was washed with deionized water before use. LC separation was monitored by UV detection at 260 nm. Final purification of the nucleotides was achieved on an HPLC (Merck-Hitachi) system using a semipreparative reversed-phase column [Gemini 5u C-18110A, 250 mm × 10 mm, 5 μm (Phenomenex, Torrance, CA)]. The details of the solvent system gradients used for the separation of each product are provided below. The purity of the nucleotides was evaluated on an analytical reversed-phase HPLC column system [Gemini 5u C-18110A, 150 mm × 3.60 mm, 5 μm (Phenomenex)] in two solvent systems, I and II. Solvent system I consisted of (A) 100 mM triethylammonium acetate (TEAA) (pH 7) and (B) CH3CN. Solvent system II consisted of (A) 46 mM PBS (pH 7.4) and (B) CH3CN. The products, obtained as Na+ salts, were generally ≥ 95% pure.
5′-(Chloromethylene)phosphonate-2′,3′-methoxymethylidene adenosine, 18
(Chloromethylene)phosphonic dichloride (200 mg, 1.2 mmol) was suspended in dry pyridine, followed by addition of dry triethylamine (0.9 mL, 0.15 mmol). The mixture was cooled in ice to 0 °C, and then 2′,3′-methoxymethylidene adenosine [53] (250 mg, 0.809 mmol) was added dropwise. The reaction mixture was stirred at 0 °C for 3 h, then warmed to 25 °C, and stirred for an additional 1 h. Finally, the reaction mixture was cooled in an ice bath to 0 °C and 0.2 M TEAB (15 mL) was added dropwise and the reaction mixture was stirred for 2 h. The reaction solution was evaporated and water (50 mL) was added and the solution was freeze-dried repeatedly, till a constant weight was attained. The residue was purified on a silica gel column, and the product was eluted using 50% methanol, 48.5% DCM, 1% hexane, and 0.5% triethylamine. The product was obtained as the triethylammonium salt, as a white solid (112 mg, 32% yield). 1H NMR (D2O, 400 MHz) δ: 8.23 (s, 1H), 7.64 (s, 1H), 4.20 (d, J 12 Hz, 1H), 6.25 (d, J 13 Hz,1H), 6.13 (d, J 13 Hz, 1H), 4.42 (m, J 15 Hz, 1H), 4.28 (m, 2H), 4.6 (t, J 18 Hz, 2H), 3.5 (d, J 11 Hz, 2H) 3.15 (s, 3H) ppm; 31P NMR (D2O, 161.96 MHz) δ: 13.45 (d, J 13 Hz) ppm. 13C NMR (D2O, 100.61 MHz) δ: 156, 152, 149, 140, 124, 118, 99, 85, 84, 64, 62, 60, 49 ppm. MS ESI (negative) m/z: 420.0, 421.2, 422.2 (M-H+).
Adenosine-5′-(phosphoryl)methylene-sulfonate, 8
5′-(Chloromethyl)phosphonate-2′,3′-methoxymethylidene adenosine, 8, (200 mg, 0.476 mmol) was dissolved in water (5 mL) in a microwave ampoule. The pH of the solution was adjusted to 10–11 with saturated NaOH. Next Na2SO4 (178 mg, 1.42 mmol) was added and the reaction mixture was stirred at 120 °C for 3 h, under microwave irradiation [48]. The ribose-protecting group was removed under these conditions. The crude mixture was separated on a reverse-phase column RP-18 using 90:10% TEAA/CH3CN as the eluent. The product was obtained without the protecting group upon further separation on HPLC 90:10% 1 M TEAA (pH 7.4)/CH3CN, isocratic elution, followed by replacing all Et3NH+ counter-ions with Na+ (22.4 mg, 11% yield). 1H NMR (D2O, 400 MHz) δ: 8.47 (s, 1H), 8.17 (s, 1H), 6.06 (d, J 12 Hz,1H), 4.72 (dd, J 6 Hz, J 2 Hz, 1H), 4.48 (t, J 13 Hz, 1H), 4.32 (s, 1H), 4.13 (dd, J 6 Hz, J 2 Hz, 2H), 3.37 (d, J 12 Hz, 2H), 31P NMR (D2O, 161.96 MHz) δ: 11.414 (s) ppm. 13C NMR (D2O, 100.61 MHz) δ: 156, 153, 149, 119, 140, 87, 84, 75, 71, 64, 49 ppm. HRMS ESI (negative) m/z calculated for C11H16N5O9PS2−: 423.0333, found 424.0334.
Adenosine-5′-(sulfonyl)methanesulfonate, 9
Methanedisulfonyldichloride (141 mg, 0.665 mmol) was suspended in dry DCM (5 mL). The mixture was cooled on ice to 0 °C and then dry pyridine (1 mL) was added drop wise followed by additional of 2′,3′-methoxymethylidene adenosine[53] (309 mg, 0.443 mmol) in pyridine (1 mL). The reaction mixture was stirred at 0 °C for 3 h and then warmed to RT and stirred for an additional 1 h. Next, the mixture was cooled on ice to 0 °C and 0.2 M TEAB (15 mL) was added dropwise to the mixture for 2 h. The solution was evaporated, water (50 mL) was added, and the solution was freeze-dried repeatedly, till a constant weight was attained. Methoxymethylidene-protecting group was removed in a mixture of 10% HCl (pH 2.3, 4 mL) and stirred for 3 h at RT. Then, 24% NH4OH (1.9 mL) was added until pH 9.8 was attained and the mixture was stirred for 1 h. The reaction solution was evaporated and water (50 mL) was added and the solution was freeze-dried repeatedly, till a constant weight was attained. Next the residue was subjected to ion-exchange chromatography (on DEAE-Sephadex A-25 chloride form, swollen overnight in 1 M NaHCO3 solution at 4 °C). The product was eluted with a gradient of 0–0.3 M (900 mL each) of TEAB (triethylammonium bicarbonate) solution, pH 7.6. The product was obtained at 0.05 M TEAB. Freeze-drying provided the product as a white solid. An additional separation was performed in high-pressure LC using reverse-phase column with isocratic elution using 90:10% 1 M TEAA(pH 7.4)/CH3CN as the eluent. The final product (48.3 mg, 17% yield) was produced by replacing all Et3NH+ counter-ions with Na+. 1H NMR (D2O, 400 MHz): δ, 8.54 (s, 1H), 7.96 (s, 1H), 6.07 (d, J 12 Hz, 1H), 4.64 (dd, J 7 Hz, J 2 Hz, 1H), 4.33 (t, J 15 Hz, 1H), 4.74 (m, 1H), 4.57(d, J 12 Hz, 2H), 4.84 (s, 2H) ppm. 13C NMR (D2O, 100.61 MHz) δ: 166, 136, 149, 119, 140, 93, 88, 77, 72, 68, 45 ppm. HRMS ESI (negative) m/z calculated for C11H15N5O9S2: 424.0238, found 424.0244.
Adenine-(methoxy)ethanol-(phosphoryl)methylene)phosphonate, 10
Methylenediphosphoryl-dichloride (165 mg, 0.665 mmol) was suspended in dry pyridine (5 mL). The mixture was cooled on ice to − 30 °C followed by the addition of adenine-(methoxy)ethanol (309 mg, 0.443 mmol) in pyridine (1 mL). The reaction mixture was stirred at − 30 °C for 3 h, and then warmed to RT and stirred for an additional 1 h. Next, the mixture was cooled on ice to 0 °C and 0.2 M TEAB (15 mL) was added dropwise to the mixture for 2 h. The solution was evaporated, water (50 mL) was added, and the solution was freeze-dried repeatedly, till constant weight was attained. Next, the residue was subjected to ion-exchange chromatography (on DEAE-Sephadex A-25 chloride form, swollen overnight in 1 M NaHCO3 solution at 4 °C). The product was eluted with a gradient of 0–0.5 M (900 mL each) of ammonium bicarbonate solution, pH 7.6. The product was obtained at 0.15 M NH4CO3. Freeze-drying provided the product as a white solid. An additional separation was performed on HPLC using reverse-phase column with isocratic elution using 90:10% 1 M TEAA (pH 7.4)/CH3CN as the eluent. The final product (48 mg, 22% yield) was produced by replacing all counter-ions with Na+. 1H NMR (D2O, 400 MHz) δ: 8.25 (s, 1H), 8.19 (s, 1H), 5.65 (s, 2H), 3.94 (t, J 7 Hz, 2H), 3.71 (t, J 7 Hz, 2H), 2.03 (t, 2H) ppm. 31P NMR (D2O, 161.96 MHz) δ: 20 (d, J 2 Hz, 1P), 14.9 (d, J 6 Hz, 1 P) ppm: 13C NMR (D2O, 100.61 MHz) δ: 156, 153, 149, 143, 119, 75, 69, 63, 28 ppm. HRMS ESI (negative) m/z calculated for C9H12N5O7P23−: 365.2112, found 365.2110.
Adenine-(methoxy)ethanol-(phosphoryl)methylene-dichloride)phosphonate, 11
p-Toluenesulfonyl chloride (270 mg, 0.015 mol) was dissolved in dry pyridine (5 mL). The solution was cooled to − 30 °C, and adenine-(methoxy)ethanol (200 mg, 0.03 mol) was added. The mixture was stirred for 4 h. Then 0.5 M TEAB was added and the solution was stirred at RT for 1 h. The solution was freeze-dried repeatedly, till constant weight was attained. The residue was separated on a silica column with a gradient of DCM:MeOH. The product was eluted with 10% MeOH.
Next, adenine-(methoxy)ethoxy 4-methylbenzenesulfonate (100 mg, 0.0027 mol) and dichloromethylene-diphosphate Bu3NH+ salt (1.64 g, 0.0027 mol) in acetonitrile (5 mL) has been introduced into a microwave ampoule. The reaction mixture was heated for 45 min at 130 °C. Finally, water (50 mL) was added and the solution was freeze-dried repeatedly, till constant weight was attained. Next, the residue was subjected to ion-exchange chromatography (on DEAE-Sephadex A-25 chloride form, swollen overnight in 1 M NaHCO3 solution at 4 °C). The product was eluted with a gradient of 0–0.5 M (900 mL each) of ammonium bicarbonate solution, pH 7.6, and was obtained at 0.15 M NH4CO3. Freeze-drying provided the product as a white solid. An additional separation was performed on HPLC using reverse-phase column with isocratic elution using 90:10% 1 M TEAA (pH 7.4)/CH3CN as the eluent. 1H NMR (D2O, 400 MHz) δ: 8.14 (s, 1H), 8.06 (s, 1H), 5.80 (s, 2H), 3.4 (t, J 7 Hz, 2H), 3.6 (t, J 7 Hz, 2H), 3.34 (s, 2H) ppm. 31P NMR (D2O, 161.96 MHz) δ: 10.57 (d, J 2 Hz, 1P), 8.45 (d, J 6 Hz, 1 P) ppm. 13C NMR (D2O, 100.61 MHz) δ: 156, 151, 149, 119, 140, 75, 66, 64, 49 ppm. HRMS ESI (negative) m/z calculated for C9H12N5O7P23−: 433.9629, found 433.9593.
Adenine-(methoxy)ethanol-phosphate, 12
Phosphoryl chloride (72 mg, 0.478 mmol) was suspended in dry trimethyl phosphate (5 mL). The mixture was cooled in dry ice bath and ethylene glycol to − 10 °C followed by additional of acyclic-adenosine (100 mg, 0.478 mmol) in trimethyl phosphate (1 mL). The reaction mixture was stirred at − 15 °C for 1.5 h. Next, the mixture was heated by ice to 0 °C and 0.2 M TEAB (15 mL) was added dropwise to the mixture and stirred for 1 h. The solution was evaporated, water (50 mL) was added, and the solution was freeze-dried repeatedly, till constant weight was attained. Next, the residue was subjected to ion-exchange chromatography (on DEAE-Sephadex A-25 chloride form, swollen overnight in 1 M NaHCO3 solution at 4 °C). The product was eluted with a gradient of 0–0.15 M (600 mL each) of ammonium bicarbonate solution, pH 7.6. The product was obtained at 0.08–0.1 M NH4HCO3. Freeze-drying provided the product as a white solid. The final product (22 mg, 15% yield) was produced by replacing all Et3NH+ counter-ions with Na+. 1H NMR (D2O, 400 MHz) δ: 8.07 (s, 1H), 7.92 (s, 1H), 5.51 (s, 2H), 3.76 (t, J 7 Hz, 2H), 3.61 (t, J 7 Hz, 2H). 31P NMR (D2O, 161.96 MHz) δ: 4.5 (s, 1P): 13C NMR (D2O, 100.61 MHz) δ: 155, 153, 149, 142, 118, 73, 69, 63 ppm. HRMS ESI (negative) m/z calculated for C8H12N5O5P1−: 288.0503, found 288.0499.
Adenine-(methoxy)ethoxy-phosphonate, 13
A solution of 2-chloro-4H-1,3,2-benzodioxaphosphorin-4-one (117 mg, 0.58 mmol) in anhydrous DMF (1 mL) was added via syringe to a solution of adenine-(methoxy)ethanol (100 mg, 0.478 mmol) and anhydrous pyridine (260 μL) in anhydrous DMF (1.5 mL) at 0 °C under nitrogen. The mixture was dripped into a cold 1 M TEAB solution (10 mL) until pH ≈ 8 was attained. The resulting mixture was stirred at RT for 30 min. During that time the color of the solution changed to yellow. The solution was extracted with ether (2 × 10 mL). The crude residue was separated on a DEAE-Sephadex A25 column with a linear gradient of ammonium bicarbonate (from 0.1 to 0.4 M ammonium bicarbonate, total gradient volume 600 mL). The relevant fraction was freeze-dried. The final product (32 mg, 24% yield) was produced by replacing all Et3NH+ counter-ions with Na+. 1H NMR (D2O, 400 MHz) δ: 7.94 (s, 2H), 7.81 (s, 2H), 7.07 (s, 1H), 5.50 (s, 1H) 5.30 (s, 4H), 3.5 (t, J 7 Hz, 4H), 3.4 (t, J 7 Hz, 4H) ppm. 31P NMR (D2O, 161.96 MHz) δ: 7.1 (d, J 630 Hz, 1P) ppm. 13C NMR (D2O, 100.61 MHz) δ: 155, 153, 149, 142, 118, 73, 69, 63 ppm. HRMS ESI (negative) m/z calculated for C9H12N5O7P23−: 272.0554, found 272.0554.
Adenine-(methoxy)ethoxy-thio-phosphate, 14
A solution of 2-chloro-4H-1,3,2-benzodioxaphosphorin-4-one (117 mg, 0.58 mmol) in anhydrous DMF (1 mL) was added via syringe to a solution of adenine-(methoxy)ethanol (100 mg, 0.478 mmol) and anhydrous pyridine (260 μL) in anhydrous DMF (1.5 mL) at 0 °C under nitrogen. The reaction mixture was stirred at RT for 2 h, and then elemental sulfur (25 mg, 0.77 mmol) was added at 0 °C. The color of the solution changed to orange and then to brown during stirring at RT for 1.5 h. The mixture was dripped into a cold 1 M TEAB solution (10 mL) until pH ≈ 7 was attained. The resulting mixture was stirred at RT for 30 min. During that time the color of the solution changed to yellow. The solution was extracted with ether (2 × 10 mL). The crude residue was separated on a DEAE-Sephadex A25 column with a linear gradient of ammonium bicarbonate (from 0.1 to 0.4 M ammonium bicarbonate, total gradient volume 600 mL). The relevant fraction was freeze-dried four times. The final product (13 mg, 9% yield) was produced by replacing all Et3NH+ counter-ions with Na+. 1H NMR (D2O, 400 MHz) δ: 8.08 (s, 1H), 7.99 (s, 1H), 5.45 (s, 2H), 3.5 (t, J 7 Hz, 2H), 3.4 (t, J 7 Hz, 2H) ppm. 31P NMR (D2O, 161.96 MHz) δ: 56.52 (s, 1P) ppm. 13C NMR (D2O, 100.61 MHz) δ: 155, 153, 149, 142, 118, 73, 69, 63 ppm. HRMS ESI (negative) m/z calculated for C9H12N5O7P23−: 495.1081, found 495.1084.
(Benzo-dimidazol-2-yl)methanol-((phosphoryl)methylene)phosphonate, 15
Methylenediphosphoryl-dichloride (165 mg, 0.665 mmol) was suspended in dry pyridine (5 mL). The mixture was cooled on ice to − 30 °C followed by the addition of (1H-benzo[d]imidazol-2-yl)methanol (309 mg, 0.443 mmol) in pyridine (1 mL). The reaction mixture was stirred at − 10 °C for 3 h, and then warmed to RT, and stirred for an additional 1 h. Next, the mixture was cooled on ice to 0 °C and 0.2 M TEAB (15 mL) was added dropwise to the mixture for 2 h. The solution was evaporated, water (50 mL) was added, and the solution was freeze-dried repeatedly till constant weight was attained. Next, the residue was subjected to ion-exchange chromatography (on DEAE-Sephadex A-25 chloride form, swollen overnight in 1 M NaHCO3 solution at 4 °C). The product was eluted with a gradient of 0–0.5 M (900 mL each) of ammonium bicarbonate solution, pH 7.6. The product was obtained at 0.15 M NH4CO3. Freeze-drying provided the product as a white solid. An additional separation was performed on HPLC using reverse-phase column with isocratic elution using 90:10% 1 M TEAA (pH 7.4)/CH3CN as the eluent. The final product (41 mg, 31% yield) was produced by replacing all counter-ions with Na+. 1H NMR (D2O, 400 MHz) δ: 7.58 (s, 1H), 7.25 (s, 1H), 5.15 (s, 1H), 2.14 (t, J 7 Hz, 2H). 31P NMR (D2O, 161.96 MHz) δ: 21.81 (d, 1P), 14.06 (d, 1P): 13C NMR (D2O, 100.61 MHz) δ: 153, 137, 123, 115, 59, 28 ppm. HRMS ESI (negative) m/z calculated for C9H12N2O6P23−: 307.0243, found 307.0235.
Di-(benzo-dimidazol-2-yl)methanol-((phosphoryl)methylene)phosphonate, 16
Methylenediphosphoryl-dichloride (55 mg, 0.225 mmol) was suspended in dry pyridine (5 mL). The mixture was cooled on ice to − 30 °C followed by the addition of (1H-benzo[d]imidazol-2-yl)methanol (309 mg, 0.443 mmol) in pyridine (1 mL). The reaction mixture was stirred at − 30 °C for 3 h, and then warmed to RT and stirred for an additional 1 h. Next, the mixture was cooled on ice to 0 °C and 0.2 M TEAB (15 mL) was added dropwise to the mixture for 2 h. The solution was evaporated, water (50 mL) was added, and the solution was freeze-dried repeatedly, till constant weight was attained. Next, the residue was subjected to ion-exchange chromatography (on DEAE-Sephadex A-25 chloride form, swollen overnight in 1 M NaHCO3 solution at 4 °C). The product was eluted with a gradient of 0–0.5 M (900 mL each) of ammonium bicarbonate solution, pH 7.6. The product was obtained at 0.1 M NH4CO3. Freeze-drying provided the product as a white solid. An additional separation was performed on HPLC using reverse-phase column with isocratic elution using 90:10% 1 M TEAA (pH 7.4)/CH3CN as the eluent. The final product (16 mg, 8.2% yield) was produced by replacing all counter-ions with Na+.1H NMR (D2O, 400 MHz) δ: 7.15 (s, 2H), 6.97 (s, 2H), 5.15 (s, 2H), 2.14 (t, J 7 Hz, 2H). 31P NMR (D2O, 161.96 MHz) δ: 19.53 (d, 1P) ppm. 13C NMR (D2O, 100.61 MHz) δ: 152, 136, 123, 114, 59, 26 ppm. HRMS ESI (negative) m/z calculated for C17H18N4O6P22−: 437.0774, found 437.0774.
Human NPP1 and NPP3 assay using p-Nph-5′-TMP as a substrate [28]
Compounds (8–16) were screened at 10 μM vs. 100 μM p-Nph-5′-TMP to study inhibition of NPP1 and NPP3. Purified enzyme, 0.975 μg (NPP1) and 5 μg (NPP3), which were prepared as previously described in Lee et al. [28], were diluted in the assay buffer (50 mM TRIS HCl, 2 mM CaCl2, and 0.2 mM ZnCl2 pH 9.0). The enzyme mixtures were incubated for 60 min at 37 °C, and the reactions were subsequently terminated by adding 20 μL of 1.0 N NaOH. The p-nitrophenolate formed was determined at 400 nm. Each analysis was repeated three times with triplicate measurements.
Human NPP1 assay using capillary electrophoresis [51]
Compound 10 identified as an inhibitor of NPP1 in the colorimetric assay was additionally tested at 10 μM vs. ATP (100 μM) as a substrate to confirm its potency vs. the natural substrate. NPP1 (130 μg) was dissolved in assay buffer (10 mM N-cyclohexyl-2-aminoethanesulfonic acid (CHES), 2 mM CaCl2, 1 mM MgCl2, pH 9.2). The mixture was incubated for 30 min at 37 °C, and the reaction was terminated by heating at 90 °C for 5 min. The capillary electrophoresis (CE) analysis was carried out using a P/ACE MDQ CE system (Beckman Instruments, Fullerton, CA, USA). Data collection and peak area analysis were performed by the P/ACE MDQ software 32 KARAT obtained from Beckman Coulter (Fullerton, CA, USA). A polyacrylamide-coated capillary was used [30 cm (20 cm effective length) × 50 μm (id) × 360 μm (od) purchased from Chromatographie Service GmbH (Langerwehe, Germany)]. Samples were injected electrokinetically by applying a voltage of − 6 kV for 60 s. Finally, analytes were separated by applying a separation voltage of − 15 kV, and detected by UV at 260 nm. Each analysis was repeated twice with triplicate measurements.
Determination of concentration-inhibition curves at human NPP1
Concentration-inhibition curves for the compound 10 at NPP1 were performed with p-Nph-5′-TMP and with ATP as a substrate. Seven different dilutions (final concentrations ranging from 0.5 to 100 μM in the colorimetric assay, 1 to 500 μM in the CE assay) were prepared in assay buffer. The assay conditions for the colorimetric p-Nph-5′-TMP assay and for the CE assay using ATP as a substrate were the same as described above.
Determination of inhibition type
The mechanism of inhibition at human NPP1 was determined employing different concentrations of 10, namely, 0-, 0.5-, and 2-fold of the IC50 value, measured vs. five different substrate concentrations ranging from 50 to 500 μM of p-Nph-5′-TMP, and from 10 to 500 μM of ATP, respectively. The assay procedure and operation conditions were same as described above. The experiment was conducted in triplicates. A Lineweaver-Burk plot was calculated using GraphPad Prism 5.0 for predicting the inhibition type of the compound.
Selectivity test vs. human NTPDase1 (CD39) [51]
The screening of the test compounds was performed at 10 μM vs. 100 μM ADP as a substrate as previously described [42]. The assay buffer was 10 mM HEPES, 1 mM MgCl2, and 2 mM CaCl2 (pH 7.4). The enzyme reaction was started by adding 130 μg of human CD39 and the reaction mixtures were incubated at 37 °C for 30 min. The released monophosphates were quantified at 623 nm after adding 0.96 mM malachite green reagent and 30 mM of ammonium molybdate reagent [54]. Three separate experiments were performed.
Selectivity test vs. rat CD73 [55]
The inhibition of rat CD73 [56] was investigated at 10 μM of test compounds or by preparing full concentration-inhibition curves in a concentration range of 0.03 to 1000 μM. Compounds were diluted in assay reaction buffer (25 mM Tris, 140 mM sodium chloride, 25 mM sodium dihydrogen phosphate solution, pH 7.4). After addition of 10 μL of the compound solution to 80 μL of assay reaction buffer and 10 μL of 1.23 μg/mL of rat CD73, the reaction was initiated by the addition of 10 μL of [2,8-3H]AMP (specific activity 3.7 × 109 Bq/mmol (100 mCi/mmol)), American Radio-labeled Chemicals, MO, USA, distributed by Hartman Analytic, Braunschweig, Germany), resulting in a final substrate concentration of 5 μM. After the reaction, which was performed for 20 min at 37 °C in a shaking water bath, 500 μL of cold precipitation buffer (100 mM lanthanum chloride, 100 mM sodium acetate, pH 4.0) was added to stop the reaction and to facilitate precipitation of free phosphate and unconverted [2,8-3H]AMP. After the precipitation was completed (after at least 30 min on ice), the mixture was separated by filtration through GF/B glass fiber filters using a cell harvester (M-48, Brandel, Gaitherburg, MD, USA). After washing each reaction vial three times with 500 μL of cold (4 °C) Milli-Q water, 5 mL of the scintillation cocktail ULTIMA Gold XR (PerkinElmer) was added, and radioactivity was measured by scintillation counting (TRICARB 2900 TR, Packard/PerkinElmer; counting efficacy: 49–52%). Three separate inhibition assays were performed each in triplicates.
Evaluation of the NPP1 and TNAP inhibitory activity of analogs 8–16 at human osteoarthritic chondrocytes
Preparation of human chondrocytes
Cartilage was obtained from the OA patients undergoing total knee replacement surgery. The study was approved by the institutional ethics committee according to the Declaration of Helsinki. All patients provided written informed consent.
Human articular chondrocytes were isolated from OA knee joints by sequential digestion and cultured as follows: cartilage slices were removed from the femoral condyles and tibial plateaus and washed in phosphate-buffered saline (PBS). Tissues were then minced with a scalpel, transferred into a digestion buffer containing Hank’s balanced salt solution (HBSS) with 4 WU of Liberase TM (Roche), and incubated on a gyratory shaker (65 rpm) at 37 °C overnight. Residual multicellular aggregates were removed by vortex and filtration through a nylon 80-μm mesh. The suspension was centrifuged (600 g, 10 min) to pellet cells. The cell pellet was treated with trypsin (37 °C for 10 min), washed three times PBS, and plated. In some cases the chondrocytes were sub-cultured once. The cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum and antibiotics until 80–90% confluence.
NPPase and TNAP colorimetric activity assays
For NPPase assays, cells (1.5 × 104 cells per well) were incubated in 200 μL HBSS and 0.1 mM p-nitrophenyl-thymidine 5′ monophosphate (Sigma-Aldrich. catalog #T4510) in 96-well plates at 37 °C with gentle agitation. Analogs 8–16 were added (0.1 mM, 0.01 mM, 0.001 mM) at the time points indicated, and absorbance at 405 nm was measured with an ELISA reader. TNAP assays were similarly performed except that the substrate was p-nitrophenyl phosphate (Sigma-Aldrich, catalog #P7998). A standard curve of 405 nm absorbance to p-nitrophenol (Fluka analytical, catalog #73560) concentration was used to calculate enzymatic activity.
XTT assay in human chondrocytes
Cell viability was determined by the tetrazolium salt XTT assay (Biological Industries Ltd., Beit Haemek, Israel) according to the manufacturer’s instructions.
Electronic supplementary material
(DOCX 6213 kb)
Abbreviations
- eNPP
Ecto-nucleotide pyrophosphatase/phosphodiesterase
- NTP
Nucleoside-5′-triphosphate
- p-Nph-5′-TMP
p-nitrophenyl ester thymidine 5′-monophosphate
- SD
Standard deviation
- CPPD
Calcium pyrophosphate dihydrate
- PPi
Pyrophosphate/diphosphate
- TEAB
Triethylammonium bicarbonate
- DCM
Dichloromethane
Compliance with ethical standards
Conflicts of interest
Molhm Nassir declares that he has no conflict of interest.
Uri Arad declares that he has no conflict of interest.
Sang-Yong Lee declares that he/she has no conflict of interest.
Shani Journo declares that he has no conflict of interest.
Salahuddin Mirza declares that he has no conflict of interest.
Christian Renn declares that he has no conflict of interest.
Christa E. Müller declares that she has no conflict of interest.
Bilha Fischer declares that she has no conflict of interest.
Ethical approval
The study was approved by the institutional ethics committee according to the Declaration of Helsinki. All patients provided written informed consent.
Footnotes
The first two authors (Molhm Nassir and Uri Arad) are of equal contribution.
All authors have approved the final article.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/20/2019
The original version of the article unfortunately contained an error.
References
- 1.Yamakawa K, Iwasaki H, Masuda I, Ohjimi Y, Honda I, Iyama K-I, Shono E, Naito M, Kikuchi M. Cartilage intermediate layer protein expression in calcium pyrophosphate dihydrate crystal deposition disease. J Rheumatol. 2002;29(8):1746–1753. [PubMed] [Google Scholar]
- 2.Gibilisco PA, Schumacher HR, Hollander JL, Soper KA. Synovial fluid crystals in osteoarthritis. Arthritis Rheum. 1985;28(5):511–515. doi: 10.1002/art.1780280507. [DOI] [PubMed] [Google Scholar]
- 3.Bjelle AO, Sundström BK. An ultrastructural study of the articular cartilage in calcium pyrophosphate dihydrate (CPPD) crystal deposition disease (chondrocalcinosis articularis) Calcif Tissue Int. 1975;19(1):63–71. doi: 10.1007/BF02563991. [DOI] [PubMed] [Google Scholar]
- 4.Nalbant S, Martinez J, Kitumnuaypong T, Clayburne G, Sieck M, Schumacher H., Jr Synovial fluid features and their relations to osteoarthritis severity: new findings from sequential studies. Osteoarthr Cartil. 2003;11(1):50–54. doi: 10.1053/joca.2002.0861. [DOI] [PubMed] [Google Scholar]
- 5.Abhishek A, Neogi T, Choi H, Doherty M, Rosenthal AK, Terkeltaub R. Unmet needs and the path forward in joint disease associated with calcium pyrophosphate crystal deposition. Arthritis Rheum. 2018;70:1182–1191. doi: 10.1002/art.40517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rachow JW, Ryan LM. Inorganic pyrophosphate metabolism in arthritis. Rheum Dis Clin N Am. 1988;14(2):289–302. [PubMed] [Google Scholar]
- 7.Terkeltaub RA. Inorganic pyrophosphate generation and disposition in pathophysiology. A J Phys. 2001;281(1):C1–C11. doi: 10.1152/ajpcell.2001.281.1.C1. [DOI] [PubMed] [Google Scholar]
- 8.Goding JW, Grobben B, Slegers H. Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2003;1638(1):1–19. doi: 10.1016/S0925-4439(03)00058-9. [DOI] [PubMed] [Google Scholar]
- 9.Johnson K, Vaingankar S, Chen Y, Moffa A, Goldring MB, Sano K, Jin-Hua P, Sali A, Goding J, Terkeltaub R. Differential mechanisms of inorganic pyrophosphate production by plasma cell membrane glycoprotein-1 and B10 in chondrocytes. Arthritis Rheum. 1999;42(9):1986–1997. doi: 10.1002/1529-0131(199909)42:9<1986::AID-ANR26>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 10.Johnson K, Hashimoto S, Lotz M, Pritzker K, Goding J, Terkeltaub R. Up-regulated expression of the phosphodiesterase nucleotide pyrophosphatase family member PC-1 is a marker and pathogenic factor for knee meniscal cartilage matrix calcification. Arthritis Rheum. 2001;44(5):1071–1081. doi: 10.1002/1529-0131(200105)44:5<1071::AID-ANR187>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Huang R, Rosenbach M, Vaughn R, Provvedini D, Rebbe N, Hickman S, Goding J, Terkeltaub R. Expression of the murine plasma cell nucleotide Pyrophosphohydrolase Pc-1 is shared by human liver, bone, and cartilage cells - regulation of Pc-1 expression in osteosarcoma cells by transforming growth factor-Beta. J Clin Invest. 1994;94(2):560–567. doi: 10.1172/JCI117370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson K, Moffa A, Chen Y, Pritzker K, Goding J, Terkeltaub R. Matrix vesicle plasma cell membrane glycoprotein-1 regulates mineralization by murine osteoblastic MC3T3 cells. J Bone Miner Res. 1999;14(6):883–892. doi: 10.1359/jbmr.1999.14.6.883. [DOI] [PubMed] [Google Scholar]
- 13.Nadel Y, Lecka J, Gilad Y, Ben-David G, Förster D, Reiser G, Kenigsberg S, Camden J, Weisman GA, Senderowitz H, Sévigny J, Fischer B. Highly potent and selective Ectonucleotide pyrophosphatase/phosphodiesterase I inhibitors based on an adenosine 5′-(α or γ)-Thio-(α,β- or β,γ)-methylenetriphosphate scaffold. J Med Chem. 2014;57(11):4677–4691. doi: 10.1021/jm500196c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villa-Bellosta R, Wang X, Millan JL, Dubyak GR, O’Neill WC. Extracellular pyrophosphate metabolism and calcification in vascular smooth muscle. Am J Physiol Heart Circ. 2011;301(1):H61–H68. doi: 10.1152/ajpheart.01020.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bollen M, Gijsbers R, Ceulemans H, Stalmans W, Stefan C. Nucleotide Pyrophosphatases/Phosphodiesterases on the move. Crit Rev Biochem Mol Biol. 2000;35(6):393–432. doi: 10.1080/10409230091169249. [DOI] [PubMed] [Google Scholar]
- 16.Agresti C, Meomartini M, Amadio S, Ambrosini E, Volonte C, Aloisi F, Visentin S. ATP regulates oligodendrocyte progenitor migration, proliferation, and differentiation: involvement of metabotropic P2 receptors. Brain Res Rev. 2005;48(2):157–165. doi: 10.1016/j.brainresrev.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22(3):364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- 18.Burnstock G. Potential therapeutic targets in the rapidly expanding field of purinergic signalling. J Clin Respir Med. 2002;2(1):45–53. doi: 10.7861/clinmedicine.2-1-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sak K, Boeynaems J-M, Everaus H. Involvement of P2Y receptors in the differentiation of haematopoietic cells. J Leukoc Biol Suppl. 2003;73(4):442–447. doi: 10.1189/jlb.1102561. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmied Arch Pharmacol. 2000;362(4–5):299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8(3):437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S-Y, Müller CE. Nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) and its inhibitors. MedChemComm. 2017;8(5):823–840. doi: 10.1039/C7MD00015D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henz SL, Fürstenau CR, Chiarelli RA, Sarkis JJF. Kinetic and biochemical characterization of an ecto-nucleotide pyrophosphatase/phosphodiesterase (EC 3.1. 4.1) in cells cultured from submandibular salivary glands of rats. Arch Oral Biol. 2007;52(10):916–923. doi: 10.1016/j.archoralbio.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Jj L, Choi HJ, Yun M, Kang Y, Jung JE, Ryu Y, Kim TY, Cha Y, Cho HS, Min JJ. Enzymatic prenylation and oxime ligation for the synthesis of stable and homogeneous protein–drug conjugates for targeted therapy. Angew Chem Int Ed Eng. 2015;54(41):12020–12024. doi: 10.1002/anie.201505964. [DOI] [PubMed] [Google Scholar]
- 25.Eliahu S, Lecka J, Reiser G, Haas M, Fo B, Lévesque SA, Pelletier J, Sévigny J, Fischer B. Diadenosine 5′, 5′′-(Boranated) polyphosphonate analogues as selective nucleotide pyrophosphatase/phosphodiesterase inhibitors⊥. J Med Chem. 2010;53(24):8485–8497. doi: 10.1021/jm100597c. [DOI] [PubMed] [Google Scholar]
- 26.Müller CE, Iqbal J, Baqi Y, Zimmermann H, Röllich A, Stephan H. Polyoxometalates—a new class of potent ecto-nucleoside triphosphate diphosphohydrolase (NTPDase) inhibitors. Bioorg Med Chem Lett. 2006;16(23):5943–5947. doi: 10.1016/j.bmcl.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Vollmayer P, Clair T, Goding JW, Sano K, Servos J, Zimmermann H. Hydrolysis of diadenosine polyphosphates by nucleotide pyrophosphatases/phosphodiesterases. FEBS J. 2003;270(14):2971–2978. doi: 10.1046/j.1432-1033.2003.03674.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee S-Y, Fiene A, Li W, Hanck T, Brylev KA, Fedorov VE, Lecka J, Haider A, Pietzsch H-J, Zimmermann H. Polyoxometalates—potent and selective ecto-nucleotidase inhibitors. Biochem Pharmacol. 2015;93(2):171–181. doi: 10.1016/j.bcp.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Khan KM, Fatima N, Rasheed M, Jalil S, Ambreen N, Perveen S, Choudhary MI. 1, 3, 4-Oxadiazole-2 (3H)-thione and its analogues: a new class of non-competitive nucleotide pyrophosphatases/phosphodiesterases 1 inhibitors. Bioorg Med Chem. 2009;17(22):7816–7822. doi: 10.1016/j.bmc.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Choudhary MI, Fatima N, Khan KM, Jalil S, Iqbal S. New biscoumarin derivatives-cytotoxicity and enzyme inhibitory activities. Bioorg Med Chem. 2006;14(23):8066–8072. doi: 10.1016/j.bmc.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 31.Grobben B, Claes P, Roymans D, Esmans EL, Van Onckelen H, Slegers H. Ecto-nucleotide pyrophosphatase modulates the purinoceptor-mediated signal transduction and is inhibited by purinoceptor antagonists. Br J Pharmacol. 2000;130(1):139–145. doi: 10.1038/sj.bjp.0703289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosoda N, Si H, Kanda Y, Katada T. Inhibition of phosphodiesterase/pyrophosphatase activity of PC-1 by its association with glycosaminoglycans. FEBS J. 1999;265(2):763–770. doi: 10.1046/j.1432-1327.1999.00779.x. [DOI] [PubMed] [Google Scholar]
- 33.Langer D, Hammer K, Koszalka P, Schrader J, Robson S, Zimmermann H. Distribution of ectonucleotidases in the rodent brain revisited. Cell Tissue Res. 2008;334(2):199–217. doi: 10.1007/s00441-008-0681-x. [DOI] [PubMed] [Google Scholar]
- 34.Pope MT, Kortz U. 2012. Polyoxometalates. Encyclo Inorg Bioinorg Chem
- 35.Shayhidin EE, Forcellini E, Boulanger MC, Mahmut A, Dautrey S, Barbeau X, Lagüe P, Sévigny J, Paquin JF, Mathieu P. Quinazoline-4-piperidine sulfamides are specific inhibitors of human NPP1 and prevent pathological mineralization of valve interstitial cells. Br J Pharmacol. 2015;172(16):4189–4199. doi: 10.1111/bph.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel SD, Habeski WM, Cheng AC, de la Cruz E, Loh C, Kablaoui NM. Quinazolin-4-piperidin-4-methyl sulfamide PC-1 inhibitors: alleviating hERG interactions through structure based design. Bioorg Med Chem Lett. 2009;19(12):3339–3343. doi: 10.1016/j.bmcl.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Lee S-Y, Perotti A, De Jonghe S, Herdewijn P, Hanck T, Müller CE. Thiazolo [3, 2-a] benzimidazol-3 (2H)-one derivatives: structure–activity relationships of selective nucleotide pyrophosphatase/phosphodiesterase1 (NPP1) inhibitors. Bioorg Med Chem. 2016;24(14):3157–3165. doi: 10.1016/j.bmc.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 38.Lee SY, Müller CE. Large-volume sample stacking with polarity switching for monitoring of nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) reactions by capillary electrophoresis. Electrophoresis. 2014;35(6):855–863. doi: 10.1002/elps.201300453. [DOI] [PubMed] [Google Scholar]
- 39.Fürstenau CR, Trentin DS, Barreto-Chaves MLM, Sarkis JJ. The effects of angiotensin II and genetic hypertension upon extracellular nucleotide hydrolysis by rat platelet ectoenzymes. Thromb Res. 2007;120(6):877–884. doi: 10.1016/j.thromres.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Stefan C, Jansen S, Bollen M. NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem Sci. 2005;30(10):542–550. doi: 10.1016/j.tibs.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Stefan C, Stalmans W, Bollen M. Threonine autophosphorylation and Nucleotidylation of the hepatic membrane protein PC-1. Eur J Biochem. 1996;241(2):338–342. doi: 10.1111/j.1432-1033.1996.00338.x. [DOI] [PubMed] [Google Scholar]
- 42.Danino O, Svetitsky S, Kenigsberg S, Levin A, Journo S, Gold A, Drexler M, Snir N, Elkayam O, Fischer B. Inhibition of nucleotide pyrophosphatase/phosphodiesterase 1: implications for developing a calcium pyrophosphate deposition disease modifying drug. Rheumatology. 2018;57(8):1472–1480. doi: 10.1093/rheumatology/key092. [DOI] [PubMed] [Google Scholar]
- 43.Lecka J, Ben-David G, Simhaev L, Eliahu S, Oscar J, Jr, Luyindula P, Pelletier J, Fischer B, Senderowitz H, Sévigny J. Nonhydrolyzable ATP analogues as selective inhibitors of human NPP1: a combined computational/experimental study. J Med Chem. 2013;56(21):8308–8320. doi: 10.1021/jm400918s. [DOI] [PubMed] [Google Scholar]
- 44.Sayer AH, Itzhakov Y, Stern N, Bilha F. characterization of complexes of Nucleoside-5′-phosphorothioate analogues with zinc ions. In Org Chem. 2013;52(19):10886–10896. doi: 10.1021/ic400878k. [DOI] [PubMed] [Google Scholar]
- 45.Chang L, Lee S-Y, Leonczak P, Rozenski J, De Jonghe S, Hanck T, Müller CE, Herdewijn P. Imidazopyridine-and purine-thioacetamide derivatives: potent inhibitors of nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) J Med Chem. 2014;57(23):10080–10100. doi: 10.1021/jm501434y. [DOI] [PubMed] [Google Scholar]
- 46.Chen A-H, Liu S-C, Chen C-Y, Chen C-Y. Comparative adsorption of cu (II), Zn (II), and Pb (II) ions in aqueous solution on the crosslinked chitosan with epichlorohydrin. J Hazard Mater. 2008;154(1):184–191. doi: 10.1016/j.jhazmat.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 47.van Meeteren LA, Ruurs P, Christodoulou E, Goding JW, Takakusa H, Kikuchi K, Perrakis A, Nagano T, Moolenaar WH. Inhibition of autotaxin by lysophosphatidic acid and sphingosine 1-phosphate. J Biol Chem. 2005;280(22):21155–21161. doi: 10.1074/jbc.M413183200. [DOI] [PubMed] [Google Scholar]
- 48.Meltzer D, Ethan O, Arguin G, Nadel Y, Danino O, Lecka J, Sevigny J, Gendron F-P, Fischer B. Synthesis and structure–activity relationship of uracil nucleotide derivatives towards the identification of human P2Y 6 receptor antagonists. Bioorg Med Chem. 2015;23(17):5764–5773. doi: 10.1016/j.bmc.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Bhattarai S, Freundlieb M, Pippel J, Meyer A, Abdelrahman A, Fiene A, Lee S-Y, Zimmermann H, Yegutkin GG, Sträter N. α, β-methylene-ADP (AOPCP) derivatives and analogues: development of potent and selective ecto-5′-nucleotidase (CD73) inhibitors. J Med Chem. 2015;58(15):6248–6263. doi: 10.1021/acs.jmedchem.5b00802. [DOI] [PubMed] [Google Scholar]
- 50.Iqbal J, Lévesque SA, Sévigny J, Müller CE. A highly sensitive CE-UV method with dynamic coating of silica-fused capillaries for monitoring of nucleotide pyrophosphatase/phosphodiesterase reactions. Electrophoresis. 2008;29(17):3685–3693. doi: 10.1002/elps.200800013. [DOI] [PubMed] [Google Scholar]
- 51.Lee S-Y, Sarkar S, Bhattarai S, Namasivayam V, De Jonghe S, Stephan H, Herdewijn P, El-Tayeb A, Müller CE. Substrate-dependence of competitive nucleotide pyrophosphatase/phosphodiesterase1 (NPP1) inhibitors. Front Pharmacol. 2017;8:54. doi: 10.3389/fphar.2017.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mahmut A, Boulanger M-C, Bouchareb R, Hadji F, Mathieu P. Adenosine derived from ecto-nucleotidases in calcific aortic valve disease promotes mineralization through A2a adenosine receptor. Cardiovasc Res. 2015;106(1):109–120. doi: 10.1093/cvr/cvv027. [DOI] [PubMed] [Google Scholar]
- 53.Gendron F-P, Halbfinger E, Fischer B, Duval M, D’Orléans-Juste P, Beaudoin AR. Novel inhibitors of nucleoside triphosphate diphosphohydrolases: chemical synthesis and biochemical and pharmacological characterizations. J Med Chem. 2000;43(11):2239–2247. doi: 10.1021/jm000020b. [DOI] [PubMed] [Google Scholar]
- 54.Baykov A, Evtushenko O, Avaeva S. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal Biochem. 1988;171(2):266–270. doi: 10.1016/0003-2697(88)90484-8. [DOI] [PubMed] [Google Scholar]
- 55.Freundlieb M, Zimmermann H, Müller CE. A new, sensitive ecto-5′-nucleotidase assay for compound screening. Anal Biochem. 2014;446:53–58. doi: 10.1016/j.ab.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 56.Baqi Y, Lee S-Y, Iqbal J, Ripphausen P, Lehr A, Scheiff AB, Zimmermann H, Bajorath JR, Müller CE. Development of potent and selective inhibitors of ecto-5′-nucleotidase based on an anthraquinone scaffold. J Med Chem. 2010;53(5):2076–2086. doi: 10.1021/jm901851t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 6213 kb)