Abstract
P2X3 receptors are involved with several pain conditions. Muscle pain induced by static contraction has an important socioeconomic impact. Here, we evaluated the involvement of P2X3 receptors on mechanical muscle hyperalgesia and neutrophil migration induced by static contraction in rats. Also, we evaluated whether static contraction would be able to increase muscle levels of TNF-α and IL-1β. Male Wistar rats were pretreated with the selective P2X3 receptor antagonist, A-317491, by intramuscular or intrathecal injection and the static contraction-induced mechanical muscle hyperalgesia was evaluated using the Randall–Selitto test. Neutrophil migration was evaluated by measurement of myeloperoxidase (MPO) kinetic–colorimetric assay and the cytokines TNF-α and IL-1β by enzyme-linked immunosorbent assay. Intramuscular or intrathecal pretreatment with A-317491 prevented static contraction-induced mechanical muscle hyperalgesia. In addition, A-317491 reduced static contraction-induced mechanical muscle hyperalgesia when administered 30 and 60 min of the beginning of static contraction, but not after 30 and 60 min of the end of static contraction. Intramuscular A-317491 also prevented static contraction-induced neutrophil migration. In a period of 24 h, static contraction did not increase muscle levels of TNF-α and IL-1β. These findings demonstrated that mechanical muscle hyperalgesia and neutrophil migration induced by static contraction are modulated by P2X3 receptors expressed on the gastrocnemius muscle and spinal cord dorsal horn. Also, we suggest that P2X3 receptors are important to the development but not to maintenance of muscle hyperalgesia. Therefore, P2X3 receptors can be pointed out as a target to musculoskeletal pain conditions induced by daily or work-related activities.
Keywords: P2X3 receptors, Muscle, Hyperalgesia, Static contraction, Cytokines, Neutrophils
Introduction
It is estimated that pain affects around 100 millions of Americans, and that annual cost, including treatment of pain and medical leave, is between $560 and $635 billions of dollars [1]. Musculoskeletal pain affects over 40% of the general population and has an important socioeconomic impact [2]. Low back pain, neck pain, and other musculoskeletal pain disorders are some of the main disability conditions in the world population [3]. Specifically, the static contraction is the type of muscle contraction most related to daily and work activities [4–7] and has been associated with development of muscle pain [8–10]. Recently, we developed a new model of muscle pain induced by static contraction and showed that this muscle pain is modulated by inflammatory mediators, such as bradykinin, prostaglandins, sympathetic amines, and neutrophils in rats [11].
It is widely known that ATP is an inflammatory mediator [12] important for several pain conditions [13, 14]. The involvement of ATP on pain is mediated by activation of purinergic P2X receptors (P2X1-P2X7) [15], especially the subtype P2X3, since it is expressed in key structures of the system related to pain signaling, such as superficial laminae of the spinal cord and C- and Aδ-primary afferent neurons [16, 17]. P2X3 are ionotropic receptors associated to Ca2+ and Na+ channels; therefore, its activation depolarizes or sensitizes nociceptive pathways [18]. P2X3 receptors have an important role on development of several pain conditions, including articular [19–21], cutaneous [22, 23], and muscle pain [24–26]. The intramuscular injection of ATP is able to induce muscle pain in mice [27, 28] and in humans [29]. Moreover, eccentric muscle contraction can increase P2X3 receptor mRNA in the masseter [30] and triceps sural muscle [31] and in afferent nociceptors [32]. The mechanism by which ATP, via activation of P2X3 receptors, contributes to muscle pain was not completely elucidated. We have previously demonstrated that the non-selective P2X3 receptor agonist, α,β-meATP, induces mechanical muscle hyperalgesia by a mechanism dependent on bradykinin, sympathetic amines, prostaglandins, neutrophil migration, TNF-α, and IL-1β [33].
Considering the clinical relevance of static contraction-induced muscle pain, the aim of this study was to evaluate whether P2X3 receptors are involved in the mechanical muscle hyperalgesia and neutrophil migration induced by static contraction. We also evaluated whether static contraction-induced muscle pain is modulated by the pro-inflammatory cytokines TNF-α and IL-1β.
Materials and methods
Animals
Male albino Wistar rats (200–250 g) from CEMIB (Multidisciplinary Center for Biological Research) UNICAMP were used and all the procedures followed the guidelines on using laboratory animals from IASP [34] and approved by the Committee on Animal Research of the State University of Campinas (license number 2448-1). The rats were housed in plastic cages with soft bedding (five per cage), in a 12-h light/dark cycle (lights on at 6:00 a.m.). Food and water were available ad libitum and the room was temperature controlled (23 °C). Before the test, rats were habituated to the test room for 1 h.
Muscle hyperalgesia induced by static contraction
The muscle hyperalgesia induced by static contraction was performed as described [11]. Initially, the rats were anesthetized with isoflurane and the static contraction was produced by applying electrical pulses through two electrodes (27 gauge) inserted into the belly of the gastrocnemius muscle. The electrical pulses were generated by a Grass S88X stimulator (Grass Technologies, West Warwick, RI, USA). The parameters used were monophasic current, repeated pulse, frequency of 50 Hz, and pulse duration of 19 ms for 1 h. In the sham group (control), the electrodes were placed but no stimulation was administered.
Drug
The following drug was used: the selective P2X3 receptor antagonist, 5-([(3-phenoxybenzyl) [(1S)-1,2,3,4-tetrahydro-1-naphthalenyl] [amino]carbonyl)-1,2,4-benzene-tricarboxylic acid (A-317491). The drug was dissolved in saline (0.9% NaCl) and was obtained from Sigma–Aldrich (St. Louis, MO, USA).
Intrathecal injections
A-317491 or vehicle (NaCl 0.9%) was administered intrathecally [22]. For injections, rats were anesthetized with 1/3 O2 to 2/3 N2O and isoflurane at 5% and 1.5% [32], respectively, and a 26-gauge needle was inserted in the subarachnoid space on the midline between the L4 and L5 vertebrae. The drug was injected at 1 μl/s with a total volume of 10 μl. The animals regained consciousness approximately 1 min after discontinuing the anesthesia.
Intramuscular injections
A-317491 or vehicle (NaCl 0.9%) was also administered into the gastrocnemius muscle [33]. For each injection, the needle was connected to a polyethylene catheter as well as to a Hamilton syringe (50 μl). The rats were briefly restrained, for a period no longer than 1 min. There was no evidence of stress and the total volume administered was 50 μl.
Mechanical muscle nociceptive threshold test
It was performed during light phase (9:00 a.m. to 5:00 p.m.) in a quiet room, with controlled temperature (23 °C) [35]. To measure the mechanical muscle hyperalgesia induced by static contraction and the role of the selective antagonist, the Randall–Selitto analgesimeter was used (Insight, Ribeirão Preto, SP, Brazil) [33, 36, 37]. A rounded tip with 2.0 mm of diameter was used to quantify the pain threshold of deep tissues [38]. Initially, the baseline muscle withdrawal threshold was quantified by three tests performed at 5-min intervals before static contraction. The mechanical muscle hyperalgesia was quantified as the change in mechanical nociceptive threshold calculated by subtracting the mean of measurements taken 1 h after the end of contraction [11]. Increases in hyperalgesia were represented by increase in the y-axis. All the experiments were performed by a blinded tester.
Enzyme-linked immunosorbent assay
An adaptation of enzyme-linked immunosorbent assay (ELISA) [39] was used to determine whether the static contraction was able to induce the release of TNF-α and IL-1β into the gastrocnemius muscle of rats. To this end, the gastrocnemius muscles were collected ½, 1, 3, 6, and 24 h after the end of static contraction or sham procedure. The tissues were weighed and homogenized in the same weight/volume proportion in a solution of phosphate-buffered saline (PBS) containing complete protease inhibitor cocktail, at 0.02% (Roche, Indianapolis, USA). The samples were centrifuged at 10,000 rpm for 15 min at 4 °C and the supernatants were stored at − 80 °C for posterior use to evaluate the protein levels. The cytokines were quantified by the following kits: TNF-α: Rat TNF-α DuoSet ELISA Kit (R&D Systems, catalog number DY510) and IL-1β: Rat IL-1β/IL-1F2 DuoSet ELISA Kit (R&D Systems, catalog number DY501). All procedures were performed following the instructions of the manufacturer (R&D Systems, Minneapolis, MN, USA) and were repeated twice to guarantee the authenticity of the results.
Myeloperoxidase activity measurement
To evaluate the role of P2X3 receptors on static contraction-induced neutrophil migration, the analysis of myeloperoxidase activity was used [40]. The gastrocnemius muscles were collected 1 h after the end of static contraction. The samples were weighed and stored at − 80 °C. The homogenizations were in buffer containing 0.1 M NaCl, 0.02 M NaPO4, and 1.015 M Na EDTA (pH 4.7, 500 μl). After that, the homogenized tissues were centrifuged at 2500g for 15 min. Pellets were resuspended in buffer (500 μl), followed by addition of 0.2% NaCl (500 μl) and, 30 s later, of 1.6% NaCl in glucose (500 μl) to induce hypotonic lyses. After another centrifugation, the pellet was resuspended in buffer containing 0.05 M NaPO4 and 0.5% hexadecyl-trimethylammonium bromide (HTAB) (pH 5.4). Samples were quickly frozen in liquid nitrogen, thawed three times to aid in lysis, and centrifuged for 15 min at 10,000g. The supernatant (50 μl) was added to 96-well microplate with 0.08 M NaPO4, followed by 3,3′5,5′-tetranethylbezidine (25 μl). The reaction was started by the addition of 0.5 mM H2O2 (100 μl) and stopped, 5 min later, by 4 M H2SO4 (50 μl). Optical density was read at 450 nm using an Epoch microplate spectrophotometer (BioTek, USA). Results were calculated by comparing a standard curve of neutrophil (> 95% purity) with the optical density of muscle tissue supernatant and were expressed by the number of neutrophils ×108/mg tissue. All the results were repeated twice to guarantee the authenticity of the results.
Statistical analysis
The results were represented by the decrease in mechanical muscle withdrawal threshold in grams (g), reported as mean ± SEM and obtained using GraphPad Prism 5.0 software. All data were analyzed using one-way ANOVA followed by post hoc testing using Tukey’s test or the Student’s t test. Significance was set at p < 0.05.
Results
Mechanical muscle hyperalgesia induced by static contraction is modulated by P2X3 receptors
To verify whether mechanical muscle hyperalgesia induced by static contraction was modulated by peripheral P2X3 receptors, the selective P2X3 receptor antagonist, A-317491, was administered into the gastrocnemius muscle previously to static contraction in the same muscle (right muscle) (Fig. 1a). Pretreatment (5 min) with A-317491 (6.0 and 60 μg/muscle, but not 0.6 μg/muscle) prevented static contraction-induced mechanical muscle hyperalgesia (p < 0.05, one-way ANOVA, Tukey’s test, Fig. 1b). A-317491 (60 μg/muscle) into the contralateral gastrocnemius muscle (left muscle) did not affect the static contraction-induced mechanical muscle hyperalgesia measured in the ipsilateral gastrocnemius muscle (right muscle) (p > 0.05, one-way ANOVA, Tukey’s test, Fig. 1b). A-317491 did not change behavioral responses in the sham group (p > 0.05, T test, Fig. 1b). The anti-hyperalgesic effect of A-317491 occurred in a dose-dependent manner with an ED50 of 5 μg (Fig. 1c).
Fig. 1.
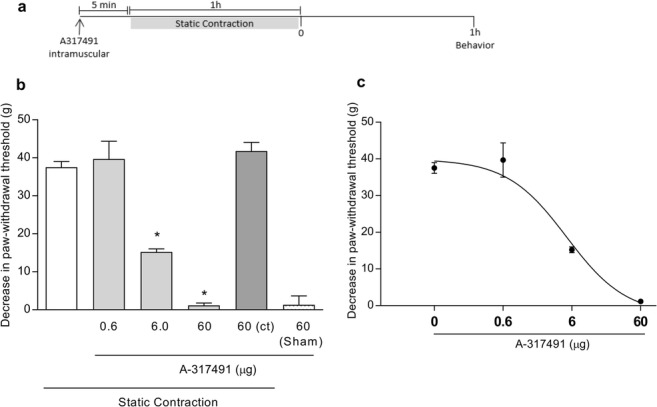
Involvement of peripheral P2X3 receptors in the mechanical muscle hyperalgesia induced by static contraction. a A-317491 was administered (intramuscular) 5 min before static contraction was performed. Behavior was quantified 1 h after static contraction. b Pretreatment with A-317491 (6.0 and 60 μg/muscle) prevented static contraction-induced mechanical muscle hyperalgesia when compared with the sham group, as indicated by the asterisk symbol (p < 0.05, Tukey’s test, n = 5). A-317491 (60 μg/muscle) into the contralateral (ct) gastrocnemius muscle (left muscle) did not affect the static contraction-induced mechanical muscle hyperalgesia measured in the ipsilateral gastrocnemius muscle (right muscle, p > 0.05, T test, n = 5). c Pretreatment with A-317491 reduced mechanical muscle hyperalgesia in a dose-dependent manner (non-linear regression analysis; ED50 = 5.0 μg; n = 5)
To verify whether the P2X3 receptors are involved in the development or maintenance of static contraction-induced mechanical muscle hyperalgesia, A-317491 was administered into the gastrocnemius muscle in different periods (Fig. 2a). A-317491 (60 μg/muscle) prevented the mechanical muscle hyperalgesia when administered 30 or 60 min after the beginning of static contraction (p < 0.05, one-way ANOVA, Tukey’s test, Fig. 2b), but not 30 or 60 min after the end of static contraction (p > 0.05, one-way ANOVA, Tukey’s test, Fig. 2b).
Fig. 2.
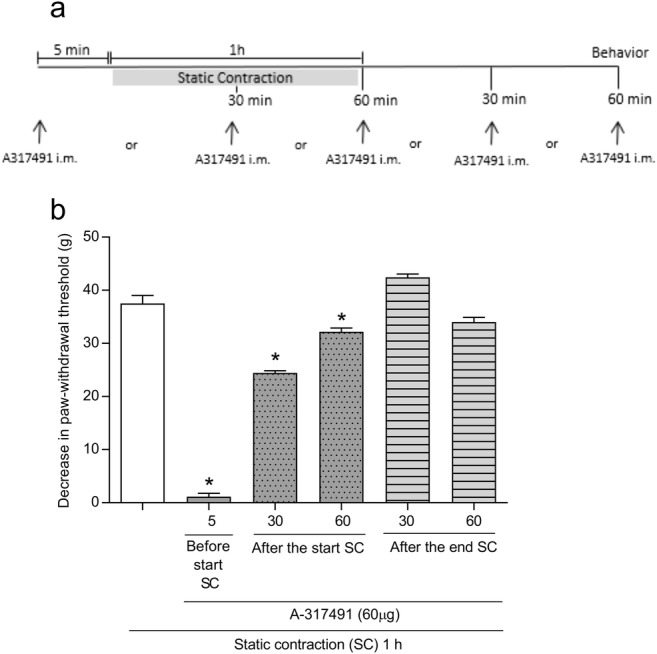
Involvement of peripheral P2X3 receptors in the maintenance mechanical muscle hyperalgesia induced by static contraction. a A-317491 was administered (intramuscular) 5 min before and 30 and 60 min after beginning of static contraction and 30 and 60 min after the end of static contraction. Behavior was quantified 1 h after static contraction. b Administration of A-317491 5 min before and 30 or 60 min after the beginning of static contraction prevented the mechanical muscle hyperalgesia, as indicated by the asterisk symbol (p < 0.05, Tukey’s test, n = 5). Administration of A-317491 30 or 60 min after the end of static contraction has no effect on mechanical muscle hyperalgesia (p > 0.05, ANOVA, n = 5)
To verify whether static contraction-induced mechanical muscle hyperalgesia was modulated by P2X3 receptors expressed on the spinal cord dorsal horn, A-317491 was administered intrathecally and previously to static contraction (Fig. 3a). Intrathecal administration of A-317491 (60 and 180 μg, but not 20 μg) prevented static contraction-induced mechanical muscle hyperalgesia (p < 0.05, one-way ANOVA, Tukey’s test, Fig. 3b). Intrathecal administration of NaCl 0.9% did not change the behavioral responses of the static contraction group (p > 0.05, T test, Fig. 3b). The anti-hyperalgesic effect of A-317491 occurred in a non-dose-dependent manner with an ED50 of 44.12 μg.
Fig. 3.
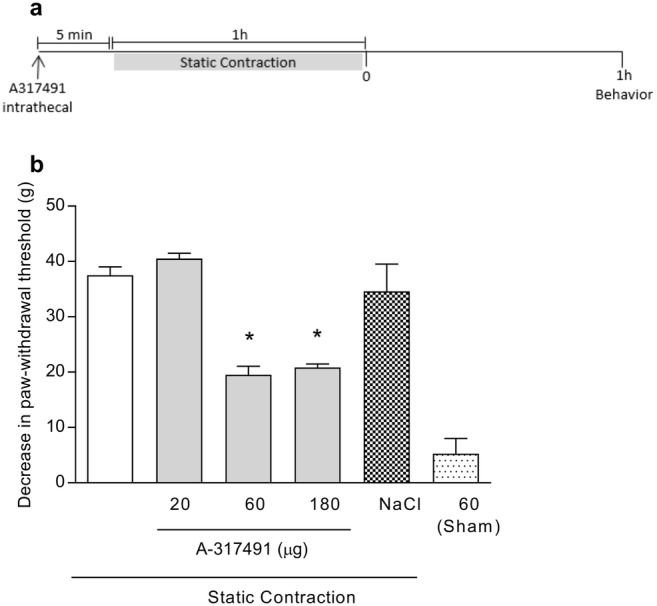
Involvement of dorsal horn P2X3 receptors in the mechanical muscle hyperalgesia induced by static contraction. a A-317491 was administered (intrathecal) 5 min before static contraction was performed. Behavior was quantified 1 h after static contraction. b Intrathecal pretreatment with A-317491 (60 and 180 μg) prevented the mechanical muscle hyperalgesia induced by static contraction (p < 0.05, Tukey’s test, n = 5), as indicated by the asterisk symbol, while having no effect in the sham group (p > 0.05, T test, n = 5)
Static contraction-induced increase in MPO activity is modulated by P2X3 receptors
To verify whether the static contraction-induced neutrophil migration [11] was modulated by P2X3 receptors, A-317491 was administered previously to static contraction (Fig. 4a). Pretreatment with A-317491 (60 μg/muscle, 5 min) prevented the increase in the myeloperoxidase (MPO) activity when compared with the control group (p < 0.05, one-way ANOVA, Tukey’s test, Fig. 4b).
Fig. 4.
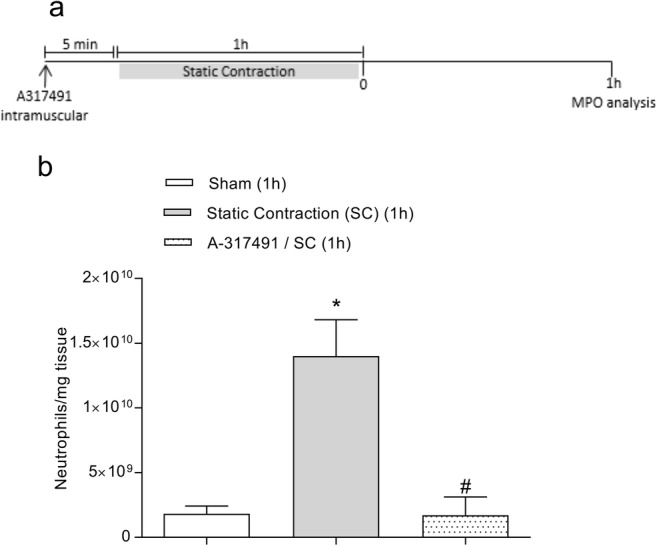
P2X3 receptors modulate neutrophil migration–induced by static contraction. a A-317491 was administered (intramuscular) 5 min before static contraction was performed. The muscle was collected 1 h after static contraction and used to MPO analysis. b Static contraction increased MPO activity (expressed by neutrophils/mg of tissue) when compared with the sham group, as indicated by the asterisk symbol (p < 0.05, Tukey’s test, n = 5). Pretreatment with A-317491 (60 μg/muscle) prevented neutrophil migration induced by static contraction when compared with the control group, as indicated by the number symbol (p < 0.05, Tukey’s test, n = 5)
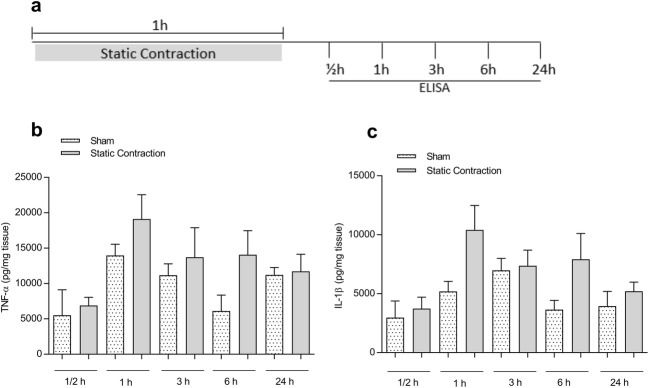
Static contraction did not increase muscle levels of TNF-α and IL-1β
To verify whether static contraction induces local release of the pro-inflammatory cytokines TNF-α and IL-1β, static contraction was performed in the gastrocnemius muscle of rats and the muscle concentrations of TNF-α and IL-1β were quantified ½, 1, 3, 6, and 24 h after the end of contraction (Fig. 5a). Static contraction did not increase the concentrations of TNF-α (Fig. 5b) and IL-1β (Fig. 5c) when compared with the sham group at any time point measured (p > 0.05, T test or one-way ANOVA, Tukey’s test).
Fig. 5.
Static contraction did not increase muscle levels of TNF-α and IL-1β. a A-317491 was administered (intramuscular) 5 min before static contraction was performed. The muscle was collected ½, 1, 3, 6, and 24 h after static contraction and used to ELISA analysis. b, c Static contraction did not increase muscle levels of TNF-α (b) and IL-1β (c), at any time point measured, when compared with the sham group (p > 0.05, T test or Tukey’s test, n = 5)
Discussion
In this study, we demonstrated that P2X3 receptors expressed on the gastrocnemius muscle and spinal cord dorsal horn seem to be essential to the mechanical muscle hyperalgesia induced by static contraction in the gastrocnemius muscle of rats. This is because both peripheral (muscle) and central (intrathecal) blockade of P2X3 receptors prevented the static contraction-induced mechanical muscle hyperalgesia. It is widely known that P2X3 receptors are involved in pain signaling of different etiologies and in different tissues, such as neuropathic (partial sciatic ligation and chronic constriction injury) and inflammatory (complete Freund’s adjuvant—CFA, carrageenan, formalin-persistent phase) pain in subcutaneous tissue [13, 22, 41], painful diabetic neuropathy [42], cancer-induced bone pain [43], endometriosis pain [44], articular hyperalgesia [19, 45], and visceral pain (colorectal distension, acetic acid–induced abdominal constriction, and trinitrobenzene sulphonic acid colitis) [46, 47]. Similar to subcutaneous tissue [22] and knee joint [19] of rats, in this present study, we also demonstrated that ATP, via activation of P2X3 receptors, is an important mediator on the development but not in maintenance of muscle hyperalgesia, since A-317491 prevented the mechanical muscle hyperalgesia when administered before and 30 or 60 min after the start of static contraction, but not 30 or 60 min after the end of it. Interestingly, the time course effect of A-317491 seems to be dependent on the inflammatory pain etiology, since after 48 h of an inflammation induced by the intraplantar administration of CFA, A-317491 fully blocked thermal hyperalgesia [41]. However, A-317491 before or after a surgery to induce postoperative pain did not decrease the mechanical allodynia observed 2 h and 1 or 2 days after surgery [41]. Taken together, our present results corroborate with previous studies that have demonstrated the involvement of P2X3 receptors on different musculoskeletal pain models, including CFA-induced masseter allodynia [26], eccentric contraction [25, 30, 48], and occlusal interference [24, 49]. Moreover, it was demonstrated that the activation of P2X3 receptors by its agonist (α,β-meATP) mediates musculoskeletal pain [33, 48].
In the present study, we used an animal model of musculoskeletal pain induced by static contraction, which is very similar to musculoskeletal pain frequently related to daily and work activities [11]. Specifically, work-related neck and shoulder pain is associated with static contraction of the trapezius muscle [9, 10, 50–52]. Low back disorders and pain in dental occupations are associated with prolonged static contraction of low back muscle in a sitting posture [53]; back pain during bedrest is primarily caused by low-intensity static contraction of low back muscles [54] and in patients with chronic myalgia or fibromyalgia, the static contraction of a muscle induces a marked increase in pain [55, 56]. We have recently demonstrated that this type of muscle contraction induces musculoskeletal pain by mechanisms that depend on the final inflammatory mediators released, e.g., prostaglandins and sympathetic amines, as well as neutrophil migration [11]. Therefore, our present results lead us to suggest that the static contraction induces ATP release, which in turn activates P2X3 receptors, increasing the susceptibility of muscle nociceptors to the final inflammatory mediators. Some mechanisms by which P2X3 receptors mediate nociceptive responses have already been reported [57, 58]. For example, it has been suggested that the P2X3 receptor is involved in endometriosis pain signal transduction via ERK signal pathway [44]. The peripheral mechanisms underlying the role of P2X3 receptors in inflammatory hyperalgesia are mediated by an indirect sensitization of the primary afferent nociceptors dependent on the previous pro-inflammatory cytokines released in different tissues [19, 22] and by a direct sensitization of the primary afferent nociceptors [22]. The vascular endothelial growth factor is involved in neuropathic pain transmission mediated by P2X3 receptors on primary sensory neurons [59]. In addition, the upregulation of P2X3 receptors in different cell types and tissues appears to be a potential peripheral mechanism involved in pain processes [19, 60–64].
The activation of P2X3 receptors produces pro-nociceptive effects in both primary afferent nociceptors and spinal cord. Although the spinal cord activation of P2X3 receptors have significant contributions to both inflammatory and neuropathic hyperalgesia [65], the peripheral activation of P2X3 receptors contributes to the development of inflammatory hyperalgesia but not of neuropathic hyperalgesia [65]. A dose comparison in the present study shows that the intramuscular blockade of P2X3 receptors appears to be more effective than the intrathecal blockade to reduce mechanical muscle hyperalgesia induced by static contraction. This difference may be related with the possible muscle but not central increase of ATP, which would be sufficient to bind to P2X3 receptors leading to membrane depolarization and cell excitation in the nociceptive primary afferent neurons. On the other hand, the activation of P2X3 receptors in supraspinal regions plays an inhibitory role in pain transmission [66].
It is important to clarify that although central and peripheral activation of P2X3 receptors are involved in nociceptive signaling of different etiologies and in different tissues [22, 45, 65, 67], the mechanisms by which these receptors contribute to pain process are not necessarily the same. For example, the present study demonstrated, for the first time, the involvement of P2X3 receptors in neutrophil migration induced by static contraction. It is well known that neutrophils have a role on different pain conditions [16, 17, 65, 67], including muscle pain [11, 30, 33, 68, 69]. Neutrophils invade skeletal muscles and are assumed to produce pro-inflammatory cytokines after exercise-induced muscle damage [70]. We have demonstrated that the non-selective P2X3 receptor agonist, α,β-meATP, induces muscle pain by a mechanism dependent on neutrophil migration [33]. Together, these results reinforce that endogenous ATP activates P2X3 receptors of muscle tissue and trigger the neutrophil migration to contribute to inflammatory muscle pain induced by different stimuli. Interestingly, the role of P2X3 receptors on neutrophil migration associated to inflammatory pain seems to be tissue dependent, since P2X3 receptors are involved with neutrophil migration induced by carrageenan on knee joint [45], but not with subcutaneous tissue [22] of rats. The mechanism by which P2X3 receptors modulate static contraction-induced neutrophil migration is unknown. However, considering that neutrophils modulate inflammatory pain by release of prostaglandins [71] and that static contraction-induced muscle pain is modulated by prostaglandins [11], we suggest that static contraction-induced release of ATP, which via activation of P2X3 receptors, contributed to release of prostaglandins triggered by neutrophils on muscle tissue.
Finally, we demonstrated that there was no increase in muscle levels of TNF-α and IL-1β in a period of 24 h in the end of static contraction. These data are supported by evidences that administration of carrageenan into the gastrocnemius muscle of rats does not increase muscle levels of TNF-α and the muscle levels of IL-1β only increase at 24 h after carrageenan [72]. It is important to point out that, similar to carrageenan, the static contraction induces muscle pain modulated by inflammatory mechanisms [11]. We cannot exclude a possible increase of these cytokines in periods after 24 h. In addition, static contraction is the type of contraction most related to fatigue in humans [73–75]. Therefore, it is possible to hypothesize that static contraction increased muscle blood flow [76, 77] by fatigue mechanisms [78–80] and promoted the clearance of muscle cytokines during contraction [81].
Conclusion
The findings of the present study demonstrated that P2X3 receptors are involved in the mechanical muscle hyperalgesia and neutrophil migration induced by static contraction. In addition, P2X3 receptors are essential to development, but not maintenance of this kind of muscle pain. Considering the static contraction-induced muscle pain has a functional characteristic and, therefore, is clinical and socioeconomic relevant, we point out the P2X3 receptors as important targets to control muscle pain induced by daily or work-related activities.
Funding information
This work was financially supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001 and by the Sao Paulo Research Foundation (FAPESP) (grant number 2011/11064-4; 2013/23448-7; 2012/10402-6).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Male albino Wistar rats (200–250 g) from CEMIB (Multidisciplinary Center for Biological Research) UNICAMP were used and all the procedures followed the guidelines on using laboratory animals from IASP [34] and approved by the Committee on Animal Research of the State University of Campinas (license number 2448-1).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Institute of Medicine Committee on Advancing Pain Research C, Education (2011) The National Academies Collection: Reports funded by National Institutes of Health. In: Relieving pain in America: a blueprint for transforming prevention, care, education, and research. National Academies Press (US) National Academy of Sciences., Washington (DC). doi:10.17226/13172
- 2.Cimmino MA, Ferrone C, Cutolo M. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2011;25(2):173–183. doi: 10.1016/j.berh.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 3.GBD Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (London, England) 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zennaro D, Laubli T, Krebs D, Klipstein A, Krueger H. Continuous, intermitted and sporadic motor unit activity in the trapezius muscle during prolonged computer work. J Electromyogr Kinesiol. 2003;13(2):113–124. doi: 10.1016/s1050-6411(02)00066-4. [DOI] [PubMed] [Google Scholar]
- 5.Forsman M, Birch L, Zhang Q, Kadefors R. Motor unit recruitment in the trapezius muscle with special reference to coarse arm movements. J Electromyogr Kinesiol. 2001;11(3):207–216. doi: 10.1016/s1050-6411(00)00054-7. [DOI] [PubMed] [Google Scholar]
- 6.Riley ZA, Terry ME, Mendez-Villanueva A, Litsey JC, Enoka RM. Motor unit recruitment and bursts of activity in the surface electromyogram during a sustained contraction. Muscle Nerve. 2008;37(6):745–753. doi: 10.1002/mus.20978. [DOI] [PubMed] [Google Scholar]
- 7.Garcia MG, Laubli T, Martin BJ. Long-term muscle fatigue after standing work. Hum Factors. 2015;57(7):1162–1173. doi: 10.1177/0018720815590293. [DOI] [PubMed] [Google Scholar]
- 8.Kadetoff D, Kosek E. The effects of static muscular contraction on blood pressure, heart rate, pain ratings and pressure pain thresholds in healthy individuals and patients with fibromyalgia. Eur J Pain. 2007;11(1):39–47. doi: 10.1016/j.ejpain.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Strom V, Roe C, Knardahl S. Work-induced pain, trapezius blood flux, and muscle activity in workers with chronic shoulder and neck pain. Pain. 2009;144(1–2):147–155. doi: 10.1016/j.pain.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Boix F, Roe C, Rosenborg L, Knardahl S. Kinin peptides in human trapezius muscle during sustained isometric contraction and their relation to pain. Journal of applied physiology (Bethesda, Md : 1985) 2005;98(2):534–540. doi: 10.1152/japplphysiol.01340.2003. [DOI] [PubMed] [Google Scholar]
- 11.Santos DFSS, Melo Aquino B, Jorge CO, Azambuja G, Schiavuzzo JG, Krimon S, Neves JDS, Parada CA, Oliveira-Fusaro MCG. Muscle pain induced by static contraction in rats is modulated by peripheral inflammatory mechanisms. Neuroscience. 2017;358:58–69. doi: 10.1016/j.neuroscience.2017.06.041. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton SG, McMahon SB. ATP as a peripheral mediator of pain. J Auton Nerv Syst. 2000;81(1–3):187–194. doi: 10.1016/s0165-1838(00)00137-5. [DOI] [PubMed] [Google Scholar]
- 13.Barclay J, Patel S, Dorn G, Wotherspoon G, Moffatt S, Eunson L, Abdel’al S, Natt F, Hall J, Winter J, Bevan S, Wishart W, Fox A, Ganju P. Functional downregulation of P2X3 receptor subunit in rat sensory neurons reveals a significant role in chronic neuropathic and inflammatory pain. J Neurosci. 2002;22(18):8139–8147. doi: 10.1523/JNEUROSCI.22-18-08139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goto T, Iwai H, Kuramoto E, Yamanaka A. Neuropeptides and ATP signaling in the trigeminal ganglion. Jpn Dent Sci Rev. 2017;53(4):117–124. doi: 10.1016/j.jdsr.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50(3):413–492. [PubMed] [Google Scholar]
- 16.Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Prog Neurobiol. 2001;65(2):107–134. doi: 10.1016/s0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 17.Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci. 1998;12(4–5):256–268. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- 18.Hattori M, Gouaux E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature. 2012;485(7397):207–212. doi: 10.1038/nature11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teixeira JM, Bobinski F, Parada CA, Sluka KA, Tambeli CH. P2X3 and P2X2/3 receptors play a crucial role in articular hyperalgesia development through inflammatory mechanisms in the knee joint experimental synovitis. Mol Neurobiol. 2017;54(8):6174–6186. doi: 10.1007/s12035-016-0146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliveira MC, Parada CA, Veiga MC, Rodrigues LR, Barros SP, Tambeli CH. Evidence for the involvement of endogenous ATP and P2X receptors in TMJ pain. Eur J Pain. 2005;9(1):87–93. doi: 10.1016/j.ejpain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Shinoda M, Ozaki N, Asai H, Nagamine K, Sugiura Y. Changes in P2X3 receptor expression in the trigeminal ganglion following monoarthritis of the temporomandibular joint in rats. Pain. 2005;116(1–2):42–51. doi: 10.1016/j.pain.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira MC, Pelegrini-da-Silva A, Tambeli CH, Parada CA. Peripheral mechanisms underlying the essential role of P2X3,2/3 receptors in the development of inflammatory hyperalgesia. Pain. 2009;141(1–2):127–134. doi: 10.1016/j.pain.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 23.de Oliveira Fusaro MC, Pelegrini-da-Silva A, Araldi D, Parada CA, Tambeli CH. P2X3 and P2X2/3 receptors mediate mechanical hyperalgesia induced by bradykinin, but not by pro-inflammatory cytokines, PGE(2) or dopamine. Eur J Pharmacol. 2010;649(1–3):177–182. doi: 10.1016/j.ejphar.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 24.Sun S, Qi D, Yang Y, Ji P, Kong J, Wu Q. Association of occlusal interference-induced masseter muscle hyperalgesia and P2X3 receptors in the trigeminal subnucleus caudalis and midbrain periaqueductal gray. Neuroreport. 2016;27(4):277–283. doi: 10.1097/WNR.0000000000000533. [DOI] [PubMed] [Google Scholar]
- 25.Noma N, Shinoda M, Honda K, Kiyomoto M, Dezawa K, Nakaya Y, Komiyama O, Imamura Y, Iwata K. Interaction of IL-1beta and P2X(3) receptor in pathologic masseter muscle pain. J Dent Res. 2013;92(5):456–460. doi: 10.1177/0022034513483770. [DOI] [PubMed] [Google Scholar]
- 26.Tariba Knežević P, Vukman R, Antonić R, Kovač Z, Uhač I, Simonić-Kocijan S. The role of P2X(3) receptors in bilateral masseter muscle allodynia in rats. Croat Med J. 2016;57(6):530–539. doi: 10.3325/cmj.2016.57.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makowska A, Panfil C, Ellrich J. ATP induces sustained facilitation of craniofacial nociception through P2X receptors on neck muscle nociceptors in mice. Cephalalgia : an international journal of headache. 2006;26(6):697–706. doi: 10.1111/j.1468-2982.2006.01095.x. [DOI] [PubMed] [Google Scholar]
- 28.Reitz M, Makowska A, Ellrich J. Excitatory and inhibitory purinergic control of neck muscle nociception in anaesthetized mice. Cephalalgia : an international journal of headache. 2009;29(1):58–67. doi: 10.1111/j.1468-2982.2008.01700.x. [DOI] [PubMed] [Google Scholar]
- 29.Mork H, Ashina M, Bendtsen L, Olesen J, Jensen R. Experimental muscle pain and tenderness following infusion of endogenous substances in humans. Eur J Pain. 2003;7(2):145–153. doi: 10.1016/S1090-3801(02)00096-4. [DOI] [PubMed] [Google Scholar]
- 30.Dessem D, Ambalavanar R, Evancho M, Moutanni A, Yallampalli C, Bai G. Eccentric muscle contraction and stretching evoke mechanical hyperalgesia and modulate CGRP and P2X(3) expression in a functionally relevant manner. Pain. 2010;149(2):284–295. doi: 10.1016/j.pain.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanna RL, Kaufman MP. Role played by purinergic receptors on muscle afferents in evoking the exercise pressor reflex. J Appl Physiol. 2003;94(4):1437–1445. doi: 10.1152/japplphysiol.01011.2002. [DOI] [PubMed] [Google Scholar]
- 32.Reinohl J, Hoheisel U, Unger T, Mense S. Adenosine triphosphate as a stimulant for nociceptive and non-nociceptive muscle group IV receptors in the rat. Neurosci Lett. 2003;338(1):25–28. doi: 10.1016/s0304-3940(02)01360-5. [DOI] [PubMed] [Google Scholar]
- 33.Schiavuzzo JG, Teixeira JM, Melo B, da Silva dos Santos DF, Jorge CO, Oliveira-Fusaro MC, Parada CA. Muscle hyperalgesia induced by peripheral P2X3 receptors is modulated by inflammatory mediators. Neuroscience. 2015;285:24–33. doi: 10.1016/j.neuroscience.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 34.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 35.Rosland JH. The formalin test in mice: the influence of ambient temperature. Pain. 1991;45(2):211–216. doi: 10.1016/0304-3959(91)90190-9. [DOI] [PubMed] [Google Scholar]
- 36.Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111(4):409–419. [PubMed] [Google Scholar]
- 37.Fujii Y, Ozaki N, Taguchi T, Mizumura K, Furukawa K, Sugiura Y. TRP channels and ASICs mediate mechanical hyperalgesia in models of inflammatory muscle pain and delayed onset muscle soreness. Pain. 2008;140(2):292–304. doi: 10.1016/j.pain.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi M, Ni Z, Yamashita T, Liang N, Sugawara K, Yahagi S, Kasai T. Excitability changes in human hand motor area induced by voluntary teeth clenching are dependent on muscle properties. Exp Brain Res. 2006;171(2):272–277. doi: 10.1007/s00221-006-0430-x. [DOI] [PubMed] [Google Scholar]
- 39.Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ. Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol. 1995;115(7):1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torres-Chavez KE, Sanfins JM, Clemente-Napimoga JT, Pelegrini-Da-Silva A, Parada CA, Fischer L, Tambeli CH. Effect of gonadal steroid hormones on formalin-induced temporomandibular joint inflammation. Eur J Pain. 2012;16(2):204–216. doi: 10.1016/j.ejpain.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Jarvis MF, Burgard EC, McGaraughty S, Honore P, Lynch K, Brennan TJ, Subieta A, Van Biesen T, Cartmell J, Bianchi B, Niforatos W, Kage K, Yu H, Mikusa J, Wismer CT, Zhu CZ, Chu K, Lee CH, Stewart AO, Polakowski J, Cox BF, Kowaluk E, Williams M, Sullivan J, Faltynek C. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci U S A. 2002;99(26):17179–17184. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou YF, Ying XM, He XF, Shou SY, Wei JJ, Tai ZX, Shao XM, Liang Y, Fang F, Fang JQ, Jiang YL. Suppressing PKC-dependent membrane P2X3 receptor upregulation in dorsal root ganglia mediated electroacupuncture analgesia in rat painful diabetic neuropathy. Purinergic Signal. 2018;14(4):359–369. doi: 10.1007/s11302-018-9617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansen RR, Nasser A, Falk S, Baldvinsson SB, Ohlsson PH, Bahl JM, Jarvis MF, Ding M, Heegaard AM. Chronic administration of the selective P2X3, P2X2/3 receptor antagonist, A-317491, transiently attenuates cancer-induced bone pain in mice. Eur J Pharmacol. 2012;688(1–3):27–34. doi: 10.1016/j.ejphar.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 44.Ding S, Zhu L, Tian Y, Zhu T, Huang X, Zhang X. P2X3 receptor involvement in endometriosis pain via ERK signaling pathway. PLoS One. 2017;12(9):e0184647. doi: 10.1371/journal.pone.0184647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teixeira JM, Oliveira MC, Nociti FH, Jr, Clemente-Napimoga JT, Pelegrini-da-Silva A, Parada CA, Tambeli CH. Involvement of temporomandibular joint P2X3 and P2X2/3 receptors in carrageenan-induced inflammatory hyperalgesia in rats. Eur J Pharmacol. 2010;645(1–3):79–85. doi: 10.1016/j.ejphar.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Zhang PA, Zhu HY, Xu QY, Du WJ, Hu S, Xu GY. Sensitization of P2X3 receptors in insular cortex contributes to visceral pain of adult rats with neonatal maternal deprivation. Mol Pain. 2018;14:1744806918764731. doi: 10.1177/1744806918764731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deiteren A, van der Linden L, de Wit A, Ceuleers H, Buckinx R, Timmermans JP, Moreels TG, Pelckmans PA, De Man JG, De Winter BY. P2X3 receptors mediate visceral hypersensitivity during acute chemically-induced colitis and in the post-inflammatory phase via different mechanisms of sensitization. PLoS One. 2015;10(4):e0123810. doi: 10.1371/journal.pone.0123810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shinoda M, Ozaki N, Sugiura Y. Involvement of ATP and its receptors on nociception in rat model of masseter muscle pain. Pain. 2008;134(1–2):148–157. doi: 10.1016/j.pain.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Qi D, Yang Y, Ji P, Kong J, Wu Q, Si H. Upregulation of the purinergic receptor subtype P2X3 in the trigeminal ganglion is involved in orofacial pain induced by occlusal interference in rats. J Oral Facial Pain Headache. 2016;30(1):51–60. doi: 10.11607/ofph.1459. [DOI] [PubMed] [Google Scholar]
- 50.Sjogaard G, Lundberg U, Kadefors R. The role of muscle activity and mental load in the development of pain and degenerative processes at the muscle cell level during computer work. Eur J Appl Physiol. 2000;83:99–105. doi: 10.1007/s004210000285. [DOI] [PubMed] [Google Scholar]
- 51.Eijckelhof BHW, Huysmans MA, Bruno Garza JL, Blatter BM, van Dieën JH, Dennerlein JT, van der Beek AJ. The effects of workplace stressors on muscle activity in the neck-shoulder and forearm muscles during computer work: a systematic review and meta-analysis. Eur J Appl Physiol. 2013;113(12):2897–2912. doi: 10.1007/s00421-013-2602-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hanvold TN, Wærsted M, Mengshoel AM, Bjertness E, Stigum H, Twisk J, Veiersted KB. The effect of work-related sustained trapezius muscle activity on the development of neck and shoulder pain among young adults. Scand J Work Environ Health. 2013;39(4):390–400. doi: 10.5271/sjweh.3357. [DOI] [PubMed] [Google Scholar]
- 53.Valachi B, Valachi K. Mechanisms leading to musculoskeletal disorders in dentistry. J Am Dent Assoc. 2003;134:1344–1350. doi: 10.14219/jada.archive.2003.0048. [DOI] [PubMed] [Google Scholar]
- 54.Baum K, Essfeld D. Origin of back pain during bedrest: a new hypothesis. Eur J Med Res. 1999;4:389–393. [PubMed] [Google Scholar]
- 55.Roe C, Knardahl S, Vollestad NK. Muscle activation during isometric contractions in workers with unilateral shoulder myalgia. J Musculoskelet Pain. 2000;8:57–73. [Google Scholar]
- 56.Umeda M, Corbin LW, Maluf KS. Examination of contraction-induced muscle pain as a behavioral correlate of physical activity in women with and without fibromyalgia. Disabil Rehabil. 2015;37:1864–1869. doi: 10.3109/09638288.2014.984878. [DOI] [PubMed] [Google Scholar]
- 57.Fabbretti E. ATP P2X3 receptors and neuronal sensitization. Front Cell Neurosci. 2013;7:236. doi: 10.3389/fncel.2013.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ford AP. In pursuit of P2X3 antagonists: novel therapeutics for chronic pain and afferent sensitization. Purinergic Signal. 2012;8:3–26. doi: 10.1007/s11302-011-9271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin J, Li G, Den X, Xu C, Liu S, Gao Y, Liu H, Zhang J, Li X, Liang S. VEGF and its receptor-2 involved in neuropathic pain transmission mediated by P2X2(/)3 receptor of primary sensory neurons. Brain Res Bull. 2010;83(5):284–291. doi: 10.1016/j.brainresbull.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 60.Xu J, Chu KL, Brederson JD, Jarvis MF, McGaraughty S. Spontaneous firing and evoked responses of spinal nociceptive neurons are attenuated by blockade of P2X3 and P2X2/3 receptors in inflamed rats. J Neurosci Res. 2012;90(8):1597–1606. doi: 10.1002/jnr.23042. [DOI] [PubMed] [Google Scholar]
- 61.Xiang Z, Xiong Y, Yan N, Li X, Mao Y, Ni X, He C, LaMotte RH, Burnstock G, Sun J. Functional up-regulation of P2X 3 receptors in the chronically compressed dorsal root ganglion. Pain. 2008;140(1):23–34. doi: 10.1016/j.pain.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leng C, Chen L, Gong X, Ma B, Gan W, Si Y, Xiao H, Li C. Upregulation of P2X2 and P2X3 receptors in rats with hyperalgesia induced by heroin withdrawal. Neuroreport. 2018;29(8):678–684. doi: 10.1097/WNR.0000000000001018. [DOI] [PubMed] [Google Scholar]
- 63.Liu M, Yang H, Fang D, Yang JJ, Cai J, Wan Y, Chui DH, Han JS, Xing GG. Upregulation of P2X3 receptors by neuronal calcium sensor protein VILIP-1 in dorsal root ganglions contributes to the bone cancer pain in rats. Pain. 2013;154(9):1551–1568. doi: 10.1016/j.pain.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 64.Tao J, Liu L, Fan Y, Wang M, Li L, Zou L, Yuan H, Shi L, Yang R, Liang S, Liu S. Role of hesperidin in P2X3 receptor-mediated neuropathic pain in the dorsal root ganglia. Int J Neurosci. 2019;9:1–10. doi: 10.1080/00207454.2019.1567512. [DOI] [PubMed] [Google Scholar]
- 65.McGaraughty S, Wismer CT, Zhu CZ, Mikusa J, Honore P, Chu KL, Lee CH, Faltynek CR, Jarvis MF. Effects of A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration. Br J Pharmacol. 2003;140(8):1381–1388. doi: 10.1038/sj.bjp.0705574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fukui M, Nakagawa T, Minami M, Satoh M, Kaneko S. Inhibitory role of supraspinal P2X3/P2X2/3 subtypes on nociception in rats. Mol Pain. 2006;5(2):19. doi: 10.1186/1744-8069-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jung YH, Kim YO, Lin H, Cho JH, Park JH, Lee SD, Bae J, Kang KM, Kim YG, Pae AN, Ko H, Park CS, Yoon MH, Kim YC. Discovery of potent antiallodynic agents for neuropathic pain targeting P2X3 receptors. ACS Chem Neurosci. 2017;8(7):1465–1478. doi: 10.1021/acschemneuro.6b00401. [DOI] [PubMed] [Google Scholar]
- 68.Pizza FX, Peterson JM, Baas JH, Koh TJ. Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J Physiol. 2005;562(Pt 3):899–913. doi: 10.1113/jphysiol.2004.073965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshida S, Hagiwara Y, Tsuchiya M, Shinoda M, Koide M, Hatakeyama H, Chaweewannakorn C, Yano T, Sogi Y, Itaya N, Sekiguchi T, Yabe Y, Sasaki K, Kanzaki M, Itoi E. Involvement of neutrophils and interleukin-18 in nociception in a mouse model of muscle pain. Mol Pain. 2018;14:1744806918757286. doi: 10.1177/1744806918757286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawanishi N, Mizokami T, Niihara H, Yada K, Suzuki K. Neutrophil depletion attenuates muscle injury after exhaustive exercise. Med Sci Sports Exerc. 2016;48(10):1917–1924. doi: 10.1249/MSS.0000000000000980. [DOI] [PubMed] [Google Scholar]
- 71.Cunha TM, Verri WA, Jr, Schivo IR, Napimoga MH, Parada CA, Poole S, Teixeira MM, Ferreira SH, Cunha FQ. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol. 2008;83(4):824–832. doi: 10.1189/jlb.0907654. [DOI] [PubMed] [Google Scholar]
- 72.Loram LC, Fuller A, Fick LG, Cartmell T, Poole S, Mitchell D. Cytokine profiles during carrageenan-induced inflammatory hyperalgesia in rat muscle and hind paw. J Pain. 2007;8(2):127–136. doi: 10.1016/j.jpain.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 73.Babault N, Desbrosses K, Fabre MS, Michaut A, Pousson M. Neuromuscular fatigue development during maximal concentric and isometric knee extensions. J Appl Physiol. 2006;100(3):780–785. doi: 10.1152/japplphysiol.00737.2005. [DOI] [PubMed] [Google Scholar]
- 74.Kahn JF, Monod H. Fatigue induced by static work. Ergonomics. 1989;32(7):839–846. doi: 10.1080/00140138908966846. [DOI] [PubMed] [Google Scholar]
- 75.Place N, Lepers R, Deley G, Millet GY. Time course of neuromuscular alterations during a prolonged running exercise. Med Sci Sports Exerc. 2004;36(8):1347–1356. doi: 10.1249/01.mss.0000135786.22996.77. [DOI] [PubMed] [Google Scholar]
- 76.Kilbom A, Persson J. Leg blood flow during static exercise. Eur J Appl Physiol Occup Physiol. 1982;48(3):367–377. doi: 10.1007/BF00430227. [DOI] [PubMed] [Google Scholar]
- 77.Osada T, Rädegran G (2016) Difference in muscle blood flow fluctuations between dynamic and static thigh muscle contractions: how to evaluate exercise blood flow by Doppler ultrasound. Phys Med Rehabil Res 1. doi:10.15761/PMRR.1000128
- 78.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol. 2010;109(4):966–976. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol. 2011;589(Pt 21):5299–5309. doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol. 2011;589:3855–3866. doi: 10.1113/jphysiol.2011.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Amann M, Eldridge MW, Lovering AT, Stickland MK, Pegelow DF, Dempsey JA. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J Physiol. 2006;575(Pt 3):937–952. doi: 10.1113/jphysiol.2006.113936. [DOI] [PMC free article] [PubMed] [Google Scholar]