Abstract
Urosepsis is a severe condition often caused by Escherichia coli that spontaneously have ascended the urinary tract to the kidneys causing pyelonephritis and potentially bacteraemia. The number of sepsis cases has been steadily increasing over the last decades, and there are still no specific, molecular supportive therapies for sepsis to supplement antibiotic treatment. P2X1 receptors are expressed by a number of immune cells including thrombocytes, which presently have been established as an important player in the acute immune response to bacterial infections. P2X1 receptor-deficient mice have been shown to be relatively protected against urosepsis, with markedly reduced levels of circulating proinflammatory cytokines and intravascular coagulation. However, here we show that continuous intravenous infusion with P2X1 receptor antagonist markedly accelerates development of a septic response to induced bacteraemia with uropathogenic E. coli. Mice exposed to the P2X1 receptor antagonists die very early with haematuria, substantially elevated plasma levels of proinflammatory cytokines, massive intravascular coagulation and a concomitant reduction in circulating thrombocytes. Interestingly, infusion of P2X1 receptor antagonists causes a marked acute reduction in circulating thrombocytes and a higher number of bacteria in the blood. These data support the notion that the number of functional thrombocytes is important for the acute defence against bacteria in the circulation and that the P2X1 receptor potentially could be essential for this response.
Electronic supplementary material
The online version of this article (10.1007/s11302-019-09658-1) contains supplementary material, which is available to authorized users.
Keywords: Sepsis, E. coli, P2X1, Antagonist, Thrombocytes
Introduction
Urinary tract infections (UTI) are exceedingly common and affect one out of three women under 24 years of age [1]. UTIs usually manifest as simple cystitis but can, in severe cases, particularly after instrumentation of the urinary tract, cause pyelonephritis [2] and potentially sepsis. Urinary tract infections are often caused by Escherichia coli (E. coli) [3] that are serotypically different from those found in the normal intestinal flora [4–6]. The E. coli that successfully invade the urinary tract produce several virulence factors [4–8], of which α-haemolysin (HlyA) is the most abundant. Notoriously, HlyA is more frequently isolated from patient samples with severe urinary tract infection [9]. In vitro biological effects of HlyA are intimately associated with ATP release and P2 receptor activation. ATP is released to the extracellular phase directly upon insertion of HlyA into biological membranes [10] and in the instances where HlyA cause fulminant cellular lysis. The HlyA induced cellular effects, including cellular damage, is to a very high extent, secondary to activation of P2 receptors in several cell types [11–14]. In vivo, P2 receptor activation is exceedingly important during urosepsis [15], and the P2X1 receptor abundance have been shown to be corelated to the degree of reduction in circulating erythrocytes during sepsis [16].
Interestingly, both P2X4 and P2X7 receptor-deficient mice exhibit an extreme sensitivity towards HlyA-producing E. coli and develop fulminant sepsis with majorly elevated proinflammatory cytokines and disseminated intravascular coagulation in a model of urosepsis [15]. Opposed to these phenotypes, P2X1 receptor-deficient mice are relatively protected against urosepsis. The survival is similar to the wild types, but the P2X1 receptor-deficient mice exhibit lower levels of proinflammatory cytokines in plasma and a marked reduction in the intravascular coagulation [15]. The notion that reduced P2X1 receptor activation is beneficial during a systemic response to bacterial infections is supported by an earlier study that demonstrated longer survival and reduced intravascular coagulation in P2X1 deficient mice after injection of lipopolysaccharide (LPS) from E. coli (O55:B5) as a model of endotoxemia [17]. In both instances, the tested P2X1 mice were on a C57bl/6j background and, thus, had a loss of function mutation in the P2X7 in the splice variant expressed in T lymphocytes [18] and known to lack nucleotide-binding oligomerisation domain, leucine-rich repeat and pyrin domain-containing family member 1b (Nlrp1b) susceptibility-allele, which is important for activation of caspase-1 in certain types of infection [19]. Mice on C57bl/6j background are less prone to develop sepsis in response to intravenous injection of uropathogenic E. coli [15] and are potentially a less favourable background to test whether the P2X1 receptor has any influence on the prognosis of sepsis. One could assume that a given effect of interference with the P2X1 during bacteraemia could be more pronounced in mice that do not have any of the mentioned genetic variants and thus, are more susceptible to uropathogenic E. coli as the Balb/c background. The viability among the outcome of P2X1 receptor knockout mice in various sepsis-like models could in theory result from using a relative immune resistant background. Thus, it is striking that another study that uses a different type of LPS from E. coli O111:B4 showed that P2X1 receptor knockout mice had reduced survival and increased intravascular coagulation after LPS exposure compared with wild type [20] also on a C57bl/6 J background.
Since the P2X1 receptor-deficient mouse is not available on Balb/c background, we instead tested the effect of continuous infusion of P2X1 receptor blockers on the outcome of induced bacteraemia with uropathogenic E. coli. Here, we show that mice, infused with the P2X1 receptor antagonists NF449 and NF279, as opposed to the P2X1 receptor-deficient mouse, die very early of urosepsis compared with vehicle controls. Surprisingly, infusion of both substances alone caused a marked and immediate reduction in circulating thrombocytes, which was paralleled by a reduced ability to limit the number of circulating bacteria.
Methods
Escherichia coli
The uropathogenic, HlyA-producing E. coli strain ARD6 (serotype: O6:K13:H1) was obtained from Statens Serum Institute (Copenhagen, Denmark). The bacteria were grown on agar plates containing lysogeny broth (LB) medium and kept for up to 1 month at 4 °C. For each experiment, a fresh liquid preparation of E. coli was cultured overnight at 37 °C at 250 rpm by transferring one colony to 4 ml LB medium. The following morning, the culture was centrifuged twice and resuspended in sterile saline. E. coli was counted by flow cytometry (Accuri C6, BD Biosciences). In all experiments, isolated bacteria were transferred to sterile saline (150 μl) and given as an injection into one of the lateral tail vein (iv) in a total volume of 150 μl.
Animals
Animal experiments with P2X antagonists (NF279 and NF449) were performed on Balb/cJ mice from Janvier Labs (Saint Berthevin Cedex, France). All mice were males, 8–10 weeks of age and with an average weight of 23.81 ± 0.16 g. P2X1 wild-type (P2X1+/+) and knockout mice (P2X1−/−) were bred at the Department of Biomedicine, Aarhus University by heterozygous breeding, and littermates were used. P2X1+/+ and P2X1−/− mice were on a mixed background (C57/BL6J/Balb/cJ). The P2X1+/+ and P2X1−/−mice used in this study were 7- to 10-week-old mice of mixed sex with a weight of 23.8 ± 0.7 g. P2X1−/− mice were originally developed by Richard J. Evans, University of Leicester, UK, who have kindly supplied us with breeder pairs.
Murine model of urosepsis
Sepsis was induced in mice on a Balb/cJ background according to previously described procedures [15]. For all experiments, mice were anaesthetised by ketamine (100 mg kg−1) and xylazine (10 mg kg−1) in sterile NaCl (0.9%) as subcutaneous injection and placed on a heating plate at 38 °C. The initial bolus injection was topped up every 30–45 min to sustain full anaesthesia for up to 6 h. E. coli were injected in one of the lateral tail veins in a total volume of 150 μl sterile saline. To monitor survival, the number of bacteria was adjusted to be fatal within 3 h for around 50% of the mice exposed to the uropathogenic bacteria alone (165 × 106 HlyA-producing E. coli iv). Our previous data demonstrated that this bacterial load caused a ceiling effect in terms of circulating proinflammatory cytokines [15]. Therefore, we harvested blood for further analysis in parallel experiments, where the bacterial load was reduced by a factor 0.25 corresponding to 41.3 × 106 HlyA-producing E. coli. The quality of blood samples from animals that have died spontaneously could not be compared with samples collected in live animals. Therefore, we collected the blood samples well ahead of fatal events in all of the compared groups. For both protocols, NF279, NF449 and saline were given as a bolus (40 mg kg−1) immediately before bacteria or saline were injected. Thereafter, the mice received a continuous infusion of P2X1 receptor antagonist (40 mg kg−1 h−1) or the corresponding sterile salt solution using a syringe pump from Harvard Apparatus (Holliston, Massachusetts, USA) with a flow of 100 μl h−1. The experiments performed in this study were approved by the Danish ethic committee for animal research “Dyreforsøgstilsynet” (2014-15-0201-00316).
Blood samples
Murine blood
Immediately before the mice were euthanised, blood was drawn from the abdominal inferior vena cava into a heparinised syringe and either used directly as a whole blood sample or after centrifugation at 1000g for 10 min at 4 °C to isolate plasma. Whole blood samples were used for thrombocyte count, and bacterial load is evaluated by counting the colony-forming units (CFU). Plasma was used for measurements of intravascular haemolysis, cytokine levels and thrombin-antithrombin (TAT) complexes.
Human blood
Blood was drawn from healthy volunteers in 5 ml EDTA-containing tubes on the day of the experiment. All subjects gave their written consent and the Danish Scientific Ethics Committee had approved the sampling procedure (Videnskabsetisk Komité-RegionMidt, M201100217). The blood was washed three times in 0.9% NaCl (twice at 1162g, 3 min, 4 °C), and once at 581g, 2 min, 4 °C), and the upper buffy coat containing the white blood cells was removed. The isolated erythrocytes were then washed once in HEPES buffered salt solution (HBS, 1162g, 3 min, 4 °C), and diluted in HBS for a 2.5% solution of washed erythrocytes, which was kept at 4 °C until use.
Haemolysis
Erythrocyte solution and HlyA were added to 96-well plates with or without agonists or antagonists for a final erythrocyte concentration of 1.25%. The plate was placed in an incubation chamber for 60 min at 37 °C under a constant swirl of 250 rpm. Thereafter, the plates were centrifuged (1162 g, 3 min, 4 °C), and the haemolytic activity was measured by transferring the supernatants to a clean 96-well plate and detecting the light absorbance (optical density, OD) at 410 nm on a spectrophotometer (Ultraspec III, LKB Biochrom). Haemolytic activity was calculated as a percentage of the maximal haemolysis induced by deionised water. In all experiments, a control without HlyA was subtracted from the samples. For measuring intravascular haemolysis, plasma was diluted (1:16) and measured as OD410. The remaining plasma was stored at − 20 °C for later evaluation of cytokines and levels of thrombin-antithrombin complexes.
Cytokines
TNF-α, IL-1β, KC (murine equivalent of human IL-8) and IL-6 were all measured on stored plasma samples (− 20 °C) on a flow cytometer (BD Accuri C6, BD Biosciences) according to the manufacturer’s instructions. Plasma was stored for a maximum of 30 days.
Thrombin-antithrombin (TAT) complexes
TAT complexes were measured in heparin-anticoagulated plasma samples with TAT Complexes Mouse ELISA Kit according to manufactures instructions (Abcam, Cambridge, UK). Plasma was stored for a maximum of 30 days.
Thrombocytes
The platelet-specific, hamster anti-mouse and anti-rat CD42d antibody and the FITC-conjugated secondary mouse anti-hamster antibody were from BD Biosciences. A whole blood sample (5 μl) was transferred to 60 μl PBS containing 2 μl antibody and incubated for 15 min. Afterwards, after 2 μl, secondary antibody was added and incubated for another 15 min. Then, a 20 μl sample was transferred to 1500 μl formaldehyde (0.02%), and the cells were investigated by flow cytometry (C6 Accuri) detecting 488 nm fluorescence, forward and side scatter. The cell count was back calculated to give the percentage of thrombocytes in the whole blood sample.
Bacterial load
Colony forming units (CFU) were determined in blood samples isolated from the animals before euthanasia. Whole blood was diluted 1/10, and 5 μl was plated on a blood agar plate and cultured overnight at 37 °C. The number of colonies was counted and expressed as CFU μl blood−1.
Materials
The P2X1 antagonists NF279, NF179, NF449 and Ro0437626 and the P2X1 agonist MRS2219 were purchased from Tocris Bioscience (Bristol, UK). Apyrase was from Sigma-Aldrich. All substances were dissolved in sterile isotonic saline (0.9% NaCl). CBA flex sets for measuring cytokines were from BD Biosciences. TAT Complexes Mouse ELISA Kit for measuring levels of thrombin-antithrombin was from Abcam. HBS is consisted of (in mM) the following: [Na+] 138, [K+] 5.3, [Cl−] 132.9, [Ca2+] 1.8, [Mg2+] 0.8, [SO42−] 0.8, [glucose] 5.6, [HEPES] 14, pH 7.4 at 37 °C. Phosphate-buffered saline (PBS): [Na+] 158, [K+] 4.5, [Cl−] 139.7, [HPO42−] 10, [H2PO4−] 1.8, pH 7.4 at 37 °C.
Statistics
Statistical analysis was performed using GraphPad Prism software. Survival studies were illustrated by Kaplan-Meier plot and analysed by a log-rank test. All other data were reported as mean ± standard error of the mean (SEM), and n indicated the number of individuals or mice unless otherwise stated in the figure legend. The data were tested for normal distribution by Kolmogorov-Smirnov test. Normally distributed data were tested by Student’s t test for single comparison and one-way ANOVA test for multiple comparisons to determine significant differences. In the few cases where the data did not meet the criteria of a normal distribution, the Mann-Whitney Wilcoxon matched pairs test for single comparison was used. A p value less than 0.05 was considered statistically significant.
Results
Our previous study on experimentally induced urosepsis revealed that the global lack of P2X7 or P2X4 receptor rendered mice much more susceptible to bacteraemia. They developed fulminant sepsis very rapidly after exposure to a bacterial load that caused 50% lethality in wild-type controls. The P2X7−/− and P2X4−/− mice died with massively elevated proinflammatory cytokine levels in plasma, acute tubular necrosis in the kidney and overstimulation of the coagulation system associated with a marked drop in circulating thrombocytes. In contrast, P2X1 receptor-deficient mice were relatively protected against the massive septic development. P2X1 receptor knockout mice did not have a statistically significant longer survival, but they did show markedly lower levels of circulating proinflammatory cytokines and substantially reduced activation of intravascular coagulation compared with wild-type controls [15]. The C57/bl6J background of P2X1−/− mice is known to have a T lymphocyte-related loss of function mutation in P2X7 [18], and to be deficient in NLRP-1b susceptibility allele [19]. Overall, this made the C57/bl6J mice less disposed to bacteraemia [15], and we speculated that acute inhibition of P2X1 receptors would be more pronounced in a Balb/cJ model.
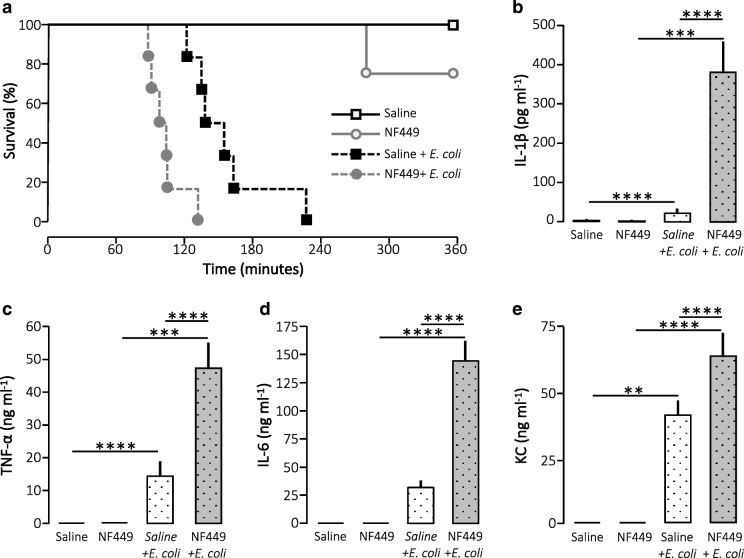
Thus, we tested the effect of the renowned P2X1 receptor antagonist NF449 in our murine model of urosepsis. Urosepsis is not easily modelled since mice do not readily develop sepsis after installation of bacteria in the urinary tract. Therefore, we used a previously established model that mimics a fast-developing urinary tract infection as seen after instrumentation of the urinary tract [15, 21]. We injected uropathogenic E. coli directly into the tail veins of the mice of anaesthetised mice and observed them in a period of 6 h. Since NF449 is known to have a relatively short half-life (T1/2), we chose to give the substance as continuous iv infusion in the mice during the observation period. We tested three infusion levels of NF449: 10 mg kg−1 h−1, 20 mg kg−1 h−1 and 40 mg kg−1 h−1. At 10 and 20 mg NF449 kg−1 h−1, the cytokine response to bacterial infection (2.5 h) was similar to mice infused with saline (Suppl. Figs. 1 and 2), and moreover, the survival during infection was similar to saline infusion at an NF449 infusion of 10 mg kg−1 h−1 (Suppl. Fig. 1). Thus, we used 40 mg kg−1 h−1 for the experiment, which corresponds to the dose of NF449 needed to increase tail bleeding in mice in a previous study [22]. Figure 1 clearly shows that NF449 did not protect the mice against developing sepsis during bacteraemia. Instead, mice that were continuously infused with NF449 died markedly faster upon exposure to HlyA-producing E. coli than the mice infused with saline after exposure to HlyA-producing E. coli (p = 0.003). Notably, infusion with NF449 alone did not affect the survival (Fig. 1a). In our experience, mice that die early in our model of urosepsis tend to have severely elevated proinflammatory plasma cytokines. Therefore, we measured the cytokine levels in the plasma 2.5 h after the onset of infection. Injection of HlyA-producing E. coli alone resulted in a statistically significant increase in plasma levels of IL-1β, TNF-α and the mouse equivalent of IL-8, keratinocyte chemoattractant (KC). Strikingly, this was substantially intensified in mice exposed to NF449 in addition to the HlyA-producing E. coli, which now also showed a statistically significant increase in IL-6 compared with control. Notably, NF449 alone did not have any influence on the baseline cytokine production.
Fig. 1.
Acute inhibition of P2X1 receptor (NF449; 40 mg kg−1 h−1) during E. coli-induced sepsis. a Kaplan-Meier plot shows survival over 6 h after subjection to HlyA-producing E. coli. Anesthetised mice were continuously infused iv with either saline or NF449. In addition, the mice were either injected iv with HlyA-producing E. coli (165 million) or saline, n = 2 for saline infusion, 4 for NF449 infusion, 8 for HlyA-producing E. coli during saline infusion and 8 for HlyA-producing E. coli during NF449 infusion. There was a significant difference (p < 0.05) between all four groups. The corresponding levels of b IL-1β, c TNF-α,d IL-6 and e KC 2.5 h after injection of HlyA-producing E. coli. The cytokine levels were measured in parallel in mice 2.5 h after injection 41.25 million HlyA-producing E. coli. The data are given as mean ± sem, n = 6 for saline infusion, 5 for NF449 infusion, 12 HlyA-producing E. coli during saline infusion and 10 HlyA-producing E. coli during NF449 infusion, *p < 0.05, **p < 0.01,***p < 0.001, ****p < 0.0001
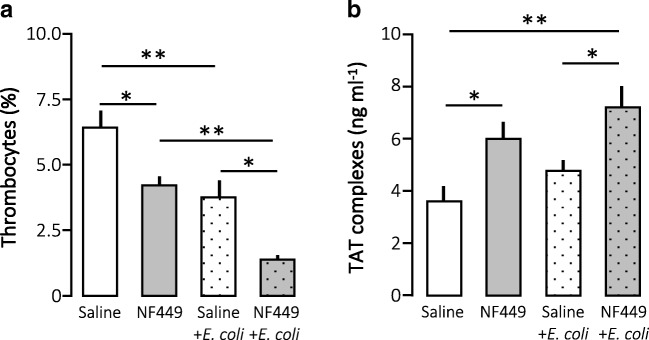
Normally, our model of urosepsis increases circulating levels of proinflammatory cytokines immensely. This elevation is closely associated with an increased activation of the coagulation system and a drop in circulating thrombocytes. We found a very similar pattern in the case of NF449 infusion. Figure 2a confirms our previously reported drop in thrombocyte number after exposure to HlyA-producing E. coli alone. This fall was intensified by NF449 infusion to a level where only approximately 1% of the circulating blood cells are platelets. Surprisingly, infusion of NF449 alone caused a statistically significant reduction of circulating thrombocytes, which is comparable to the fall observed in response to HlyA-producing E. coli alone. Figure 2b shows that in this experimental series the exposure to HlyA-producing E. coli alone did not cause a statistically significant increase in the plasma levels of TAT complexes. However, combined with NF449, HlyA-producing E. coli caused a statistically significant elevation of TAT. Parallel to the data on circulating thrombocytes, NF449 alone surprisingly increased the TAT complex level consistent with that the drug directly activating thrombocytes and the coagulation cascade in parallel.
Fig. 2.
The effect of P2X1 receptor inhibition (NF449; 40 mg kg−1 h−1) on thrombocyte and coagulation activation and erythrocyte damage in E. coli-induced sepsis. Anaesthetised mice were infused iv with sterile saline or NF449 (40 mg kg−1 h−1). In addition, the mice were either injected 41.3 millions of HlyA-producing E.coli or NF449 combined with HlyA-producing E.coli. A blood sample was collected after 2.5 h to determine a number of thrombocytes in blood, b intravascular coagulation measured as thrombin-antithrombin (TAT)-complexes. The bars indicate mean ± sem, n = 7–10, *p < 0.05, **p < 0.01,***p < 0.001, ****p < 0.0001
These data suggest that either the mice in the presence of NF449 have a much more substantial response to the bacterial load or the bacterial load becomes more pronounced in the presence of NF449. We tested this by plating out whole blood collected from the mice 2.5 h after injection of the HlyA-producing E. coli. Figure 3a shows that the presence of NF449 markedly affected the number of bacteria in the blood. Infusion of NF449 massively increases the number of circulating living bacteria compared with infusion of saline. Even though P2 receptors or equivalents have not been described in bacteria, NF449 may potentially show off-target effects that influence bacterial proliferation. Figure 3b illustrates that the presence of NF449 caused a marked increase in the number of bacteria when these were grown in LB medium in vitro. Even though that this effect was only seen at very high concentrations of NF449, they prevent us from concluding with certainty that the observed effects are indeed caused by inhibition of the murine P2X1 receptors.
Fig. 3.
Severity of infection after P2X1 receptor inhibition during E. coli-induced sepsis a shows number of colony forming units (CFU) in the blood collected 2.5 h after injection of 41.3 millions of HlyA-producing E. coli or saline to mice exposed to infusion with saline or NF449 (40 mg kg−1 h−1). The colonies were counted after 24 h of culture on blood plates. The bars indicate mean ± sem, n = 7–10, b shows the effect of NF449 on the growth of HlyA-producing E. coli in LB medium in vitro. The bars indicate mean ± sem, n = 5–7 *p < 0.05, **p < 0.01,***p < 0.001, ****p < 0.0001
Therefore, we set out to identify a P2X1 receptor antagonist that does not affect bacterial growth. Initially, we tested three other P2X1 receptor antagonists and one agonist in vitro. Figure 4 shows the previously demonstrated effect of P2X1 receptor inhibition of HlyA-induced haemolysis, which was observed for all antagonists tested, whereas the P2X1 receptor agonist MRS2211 slightly potentiated the HlyA-induced haemolysis in lower concentrations. The estimated the half maximal inhibitory concentration (IC50) was lowest for NF279, with approximately 7 μM, whereas Ro0437626 did not show full inhibition of the haemolytic process. Thus, we tested if any of the P2X1 receptor antagonists that fully inhibited the haemolytic process influenced the bacterial growth in vitro. Figure 4e shows the growth of HlyA-producing E. coli in LB medium alone or in the presence of either of the two P2X1 receptor blockers that caused complete inhibition in vitro (NF279 and NF157). Neither of the two substances had any influence on the growth of HlyA-producing E. coli (Fig. 4e). Moreover, apyrase up to 50 U ml−1 did not affect the bacterial growth, which led us to conclude that the effect of NF449 on the bacterial growth is unrelated to P2 receptors or P2-like receptors on the bacteria (Fig. 4f). Based on the concentration-response curve and the lack of effect on bacterial growth, we chose NF279 for the in vivo sepsis model.
Fig. 4.
The effects of P2X1 modulations on HlyA-induced haemolysis in vitro in human erythrocytes and P2X1 receptor antagonists and ATP scavenging and on E. coli growth in vitro. a NF279, n = 5; b NF157, n = 5; c Ro0437626, n = 5. All antagonists inhibit HlyA-induced haemolysis and the P2X1 receptor agonist d MRS2219, n = 5 slightly potentiates haemolysis in human erythrocytes. ARD6 bacteria suspended in LB-growth medium and counted over time from 0 to 4.5 h in the presence or absence of (e) the P2X1 antagonists NF279 or NF157, 250 μM or (f) the ATP scavenging enzyme apyrase, n = 6. N indicates number of repeats from 6 different cultures of ARD6. *p < 0.05, **p < 0.01
Figure 5a shows that infusion of NF279, similar to the infusion of NF449, markedly reduced the survival of Balb/cJ mice exposed to HlyA-producing E. coli. However, exposure to NF279 alone, in fact, did reduce the survival in itself (Fig. 5a). Consistent to the data obtained with NF449, infusion with NF279 also markedly amplified the plasma levels of all the tested proinflammatory cytokines (IL-1β, IL-6, TNF-α and KC; Fig. 5b–e) whereas NF279 alone did not affect the plasma cytokine levels. Moreover, NF279, similar to NF449, amplified the reduction in circulating thrombocytes upon exposure to HlyA-producing E. coli (Fig. 6a). Additionally, NF279 alone caused an even more substantial acute thrombocytopenia in the mice compared with NF449. This sudden drop in circulating thrombocytes is unlikely to reflect sudden intravascular activation of the thrombocytes because there was no parallel increase in thrombin-antithrombin complex formation (Fig. 6b).
Fig. 5.
The effect of the P2X1 receptor inhibition NF279 (40 mg kg−1 h−1) which do not affect bacterial growth in vitro in E. coli-induced sepsis. a Kaplan-Meier plot shows survival over 6 h after subjection to HlyA-producing E. coli. Anesthetised mice were continuously infused iv with either saline or NF279. In addition, the mice were either injected iv with HlyA-producing E. coli (165 million) or saline, n = 6 for all three groups. There was a statistically significant difference (p < 0.05) between mice exposed to E. coli + saline and E. coli + NF279 and between mice exposed to E. coli + NF279 and NF279 alone. The corresponding levels of b TNF-α, c IL-1β, d IL-6 and e KC, 2.5 h after injection of HlyA-producing E. coli. The cytokine levels were measured in parallel in mice 2.5 h after injection of 41.3 million HlyA-producing E. coli. The data are given as mean ± sem, 5 mice were infused with saline and injected iv with saline, 5 mice were infused with NF279 and injected iv with saline, 12 mice infused with saline and injected with HlyA-producing E. coli and 10 mice were infused with NF279 and injected with HlyA-producing E. coli, *p < 0.05, **p < 0.01,***p < 0.001, ****p < 0.0001
Fig. 6.
P2X1 receptor inhibition (NF279; 40 mg kg−1 h−1) in E. coli-induced sepsis. Anaesthetised mice were injected iv with 41.3 × 106 HlyA-producing E. coli for 2.5 h during constant infusion either saline or NF279. Blood sample was collected after 2.5 h to investigate whole blood and plasma. a Number of thrombocytes in blood, b intravascular coagulation measured as TAT-complexes, c colony forming units (CFU) in the blood collected 2.5 h after injection with HlyA-producing E. coli and cultured for 24 h, n = 5 for all four groups. *p < 0.05
Interestingly, P2X1 receptor inhibition with NF279, similarly to NF449, caused a marked increase in the number of circulating bacteria, as evident in whole blood samples taken from mice with experimentally induced bacteraemia. Since the whole blood sample is collected already after 2.5 h, the higher number of circulating bacteria is likely to reflect a reduced intravascular killing of the bacteria rather than an effect on proliferation. Thus, the data suggest that acute inhibition of P2X1 receptors may impair the immune response to acute infection, potentially via a thrombocyte-related effect.
Discussion
Sepsis is a severe condition defined as life-threatening organ dysfunction that results from a dysregulated host response to an infection. The prognosis of sepsis has improved over the last 40 years, and the mortality is currently around 20% of all cases of sepsis [23]. Unfortunately, the number of sepsis cases has been steadily increasing over the last decades, and no specific molecular therapies for sepsis have yet been approved (for review see [24]). One of the major causes of sepsis is infections arising from the urogenital area and is often caused by E. coli that has spontaneously ascended the urinary tract to the kidneys causing pyelonephritis and potentially bacteraemia. Alternatively, the bacteria can spread from the urinary tract to the bloodstream, secondary to instrumentation of the urinary tract. The E. coli that successfully ascend to the kidney often produce the virulence factor HlyA. The biological effect of this pore-forming virulence factor has been demonstrated to be intimately associated with purinergic signalling [11, 25]. ATP is released immediately after insertion of HlyA to the plasma membrane [10], and many of the following biological effects of HlyA are a consequence of activation of P2 receptors in an auto and paracrine fashion. HlyA has recently been demonstrated to be a critical factor in the development of sepsis in response to circulating E. coli in mice [21]. In that study, E. coli transfected with a plasmid containing the full HlyA operon were compared with the exact same strain transfected with a parallel plasmid, which had a substantial deletion in the HlyA gene. The data were exceedingly clear and showed that if a strain expresses HlyA, the injected mice develop fulminant sepsis whereas if HlyA secretion is absent the mice exhibit little if any systemic response to the bacteraemia [21]. This allowed us to conclude that HlyA is a virulence factor during bacteraemia that pushes an induced bacteraemia towards sepsis.
Since ATP is an important damage-associated molecular patterns (DAMP) molecule and essential in immune cell (for review see [26]) and thrombocyte activation [27–29], it is likely that the HlyA-induced ATP release is central in the systemic reaction to bacteraemia. Previous results confirm that meddling with P2 receptors has substantial impact on the prognosis of sepsis. Lately, our group made a systematic review of induced bacteraemia with uropathogenic E. coli in mice globally knocked out for P2X1, P2X4 or P2X7 receptors [15]. The P2X4 and P2X7 receptor-deficient mice showed a massive septic response to bacteraemia measured as extensive elevation in proinflammatory cytokines in plasma, drop in circulating thrombocytes, intravascular coagulation and haematuria [15]. Interestingly, we found that the complete opposite was the case in the P2X1 receptor-deficient mice, which exhibited a very modest increase in proinflammatory cytokines after injection with HlyA-producing E. coli compared with control [15]. As mentioned, the P2X1 receptor-deficient mouse was on a C57/bl6 background, which was remarkably immune resistant. Therefore, we tested whether we could observe a more pronounced phenotype by targeting the P2X1 receptor on Balb/cJ background.
Here, we tested the effect of infusion of established P2X1 receptor antagonists on the survival and immune response of Balb/cJ mice exposed to acute bacteraemia with uropathogenic E. coli. Infusion of NF449 and NF279 both caused a marked reduction in the survival of mice exposed to HlyA-producing E. coli. The reduced survival was associated with substantial activation of the immune system measured as a sizable elevation of all the measured pro-inflammatory cytokines, early haematuria, reduction in circulating thrombocytes and increase in intravascular coagulation. All these findings are consistent with animals exposed to the P2X1 receptor antagonists, which quickly develop a septic response to induced bacteraemia.
This reduced survival is in many ways a surprise and contradicts our previous data from P2X1 receptor-deficient mice, tested in the exact same model [15]. There are many obvious reasons as to why there may be a discrepancy between global knockout of a receptor and acute inhibition of the given receptor. In this instance, the substantial drop in circulating thrombocytes during infusion with NF449 comes to mind. This effect is in many ways peculiar because an inhibition of P2X1 receptors if anything should prevent activation of the thrombocytes [30–32]. Previous studies have reported a moderate reduction in thrombocyte activation after bolus injection with 10 mg kg−1 of NF449 [22]. This was substantiated by injection of a higher concentration of 50 mg kg−1 where NF449 supposedly also affect the P2Y1 and P2Y12 receptors on the thrombocytes [22]. Similarly, we find that a low infusion of 10–20 mg kg−1 h−1 did not influence the outcome of or the immune response to sepsis opposed to 40 mg kg−1 h−1. Thus, the observed effect on the thrombocytes may potentially be an effect of inhibition of all thrombocytic P2 receptors. Notably, we observed the same effect with infusion of NF279, where the result was even more pronounced. However, there are reports that other drugs that interfere with platelet function have been shown to cause thrombocytopenia. This is primarily true for acetylsalicylic acid, although the actions are not acute and rely mainly on stimulation of antibody formation and inhibition of megakaryocyte function [33]. Moreover, it has also been reported casuistically for P2Y12 receptor antagonists [34–36].
Whatever the cause is for the thrombocytopenia, early fall in platelet count is a negative clinical predictor for sepsis [37–40]. We found in our model of urosepsis complete consistency in the reduction of circulating thrombocytes, immune activation and early death of animals exposed to E. coli [15, 21]. A recent paper supports the notion that thrombocytes are implicated in the acute response to bacteria during sepsis regarding the role of thrombocytes in the septic response. The article demonstrates that mice lacking the damage-associated molecular pattern protein high-mobility group box 1 (HMGB1) specifically in thrombocytes die earlier of sepsis with higher bacterial load and higher levels of proinflammatory cytokines in plasma [41].
Thrombocytes have been established to be important in the first line of defence against intruding pathogens (for review see [42, 43]). Recently, thrombocytes have been shown to bind and trap fibrin coated E. coli in the circulation [44], and thus, severe thrombocytopenia may reduce the capacity for scavenging and inactivation of bacteria in general. Since both NF449 and NF279 cause acute thrombocytopenia in the mice, they may simultaneously reduce the mice defence against the injected E. coli. This could potentially explain why the mice infused with either NF449 or NF279 have a staggering higher number of bacteria in the bloodstream 2.5 h after injection of the same bacterial load, particularly because NF449 and NF279 have a minor and no effect, respectively, on the bacterial growth in vitro. The data support that the P2X1 antagonists increase the number of circulating bacteria and thereby have a more severe reaction to bacteraemia, possibly by compromising the number of functional circulating thrombocytes. It must be noted, however, that P2X1 receptors are expressed on neutrophil granulocytes, which in tissues are considered the first line of defence against intruding bacteria (for review see [45]). However, in the bloodstream, the slow process of phagocytosis has been challenged as the main route for neutralising invading bacteria [46]. Neutrophils have been shown to express P2X1 albeit much less prominently than thrombocytes [47]. There have been a few studies that imply a functional role of P2X1 receptors on neutrophils either promoting [48] or more recently, inhibiting neutrophil chemotaxis towards a pathogen target [49]. The notion that the P2X1 receptor promotes chemotaxia was supported by a study that showed that P2X1 receptor-deficient mice showed a lower degree of extravasation of neutrophils in response to LPS [17]. Since P2X1 receptors, to our knowledge, never has been linked to phagocytosis and that bacterial phagocytosis is likely to be less critical for bacterial clearance in the bloodstream, it is unlikely that neutrophil P2X1 receptors are responsible for the effects of NF449 and NF279 on the outcome of experimental urosepsis.
However, the theory that NF449 and NF279 reduced the first line defence against infectious agent via a marked reduction in the number of circulating thrombocytes could also potentially explain the discrepancy between the increased mortality of sepsis observed with P2X1 antagonists and the relative protection seen in P2X1−/− mice. We showed that the P2X1−/− mice had the same percentage of circulating thrombocytes as the wild-type littermate controls and thus, potentially be less susceptible to infection.
Thus, we speculate that the P2X1 receptor is essential for normal function of circulating thrombocytes and that an acute inhibition causes acute senescence or necrosis of the platelets. Since thrombocytes are implicated in binding bacteria in the bloodstream, it is reasonable to assume that a low circulating thrombocyte count will result in less sequestering of bacteria in the blood. This reduction of functional thrombocytes makes the animals more susceptible to the circulating bacteria that are now free in plasma because of the reduced thrombocyte binding. These findings have to be considered when considering interfering with P2 receptor signalling as a potential therapeutic target. Importantly, the data do certainly not support that P2X1 receptor as an appealing target during severe infection.
Electronic supplementary material
(PDF 45 kb)
(PDF 40 kb)
(PDF 45 kb)
Acknowledgements
We would like to cordially thank Richard J. Evans for supplying the P2X1 breeding pairs and Helle Jakobsen for skilled technical support and Julia Wehmöller for help with correction of the manuscript.
Funding information
The study was funded by the Independent Research Fund, Denmark (Danmarks Frie Forskningsfond): DFF-1331-00203A and the Lundbeck Foundation: R-192-2015-1362.
Compliance with ethical standards
Conflict of interest
Marianne Skals declares that she has no conflict of interest.
Anne-Sofie Greve declares that he has no conflict of interest.
Nanna Johnsen declares that she has no conflict of interest.
Mette G. Christensen declares that she has no conflict of interest.
Helle A. Praetorius declares that she has no conflict of interest.
Ethical approval
The experiments performed in this study were approved by the Danish ethic committee for animal research “Dyreforsøgstilsynet” (2014-15-0201-00316).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Marianne Skals and Anne-Sofie Greve contributed equally to this work.
References
- 1.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon. 2003;49(2):53–70. doi: 10.1067/mda.2003.7. [DOI] [PubMed] [Google Scholar]
- 2.Ragnarsdottir B, Svanborg C. Susceptibility to acute pyelonephritis or asymptomatic bacteriuria: host-pathogen interaction in urinary tract infections. Pediatr Nephrol. 2012;27(11):2017–2029. doi: 10.1007/s00467-011-2089-1. [DOI] [PubMed] [Google Scholar]
- 3.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113(Suppl 1A):5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 4.Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4(1):80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connell I, Agace W, Klemm P, Schembri M, Marild S, Svanborg C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci U S A. 1996;93(18):9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svanborg C. Urinary tract infections in children: microbial virulence versus host susceptibility. Adv Exp Med Biol. 2013;764:205–210. doi: 10.1007/978-1-4614-4726-9_17. [DOI] [PubMed] [Google Scholar]
- 7.Wullt B, Bergsten G, Connell H, Rollano P, Gebretsadik N, Hull R, Svanborg C. P fimbriae enhance the early establishment of Escherichia coli in the human urinary tract. Mol Microbiol. 2000;38(3):456–464. doi: 10.1046/j.1365-2958.2000.02165.x. [DOI] [PubMed] [Google Scholar]
- 8.Johnson JR, Stell AL. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J Infect Dis. 2000;181(1):261–272. doi: 10.1086/315217. [DOI] [PubMed] [Google Scholar]
- 9.Cavalieri SJ, Bohach GA, Snyder IS. Escherichia coli α-hemolysin: characteristics and probable role in pathogenicity. Microbiol Rev. 1984;48(4):326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skals M, Bjaelde RG, Reinholdt J, Poulsen K, Vad BS, Otzen DE, Leipziger J, Praetorius HA. Bacterial RTX toxins allow acute ATP release from human erythrocytes directly through the toxin pore. J Biol Chem. 2014;289:19098–19109. doi: 10.1074/jbc.M114.571414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skals MG, Jorgensen NR, Leipziger J, Praetorius HA. α -hemolysin from Escherichia coli uses endogenous amplification through P2X receptor activation to induce hemolysis. Proc Natl Acad Sci U S A. 2009;106:4030–4035. doi: 10.1073/pnas.0807044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsen CK, Skals M, Wang T, Cheema MU, Leipziger J, Praetorius HA. Python erythrocytes are resistant to α-Hemolysin from Escherichia coli. J Membr Biol. 2011;244(3):131–140. doi: 10.1007/s00232-011-9406-2. [DOI] [PubMed] [Google Scholar]
- 13.Fagerberg SK, Jakobsen MR, Skals M, Praetorius HA. Inhibition of P2X receptors protects human monocytes against damage by leukotoxin from Aggregatibacter actinomycetemcomitans and ⍺-hemolysin from Escherichia coli. Infect Immun. 2016;84(11):3114–3130. doi: 10.1128/IAI.00674-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen MG, Fagerberg SK, de Bruijn PI, Bjaelde RG, Jakobsen H, Leipziger J, Skals M, Praetorius HA. [Ca2+]i oscillations and IL-6 release induced by alpha-hemolysin from Escherichia coli require P2 receptor activation in renal epithelia. J Biol Chem. 2015;290(23):14776–14784. doi: 10.1074/jbc.M115.639526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greve AS, Skals M, Fagerberg SK, Tonnus W, Ellermann-Eriksen S, Evans RJ, Linkermann A, Praetorius HA. P2X1, P2X4, and P2X7 receptor knock out mice expose differential outcome of sepsis induced by ⍺-haemolysin producing Escherichia coli. Front Cell Infect Microbiol. 2017;7:113. doi: 10.3389/fcimb.2017.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagerberg SK, Patel P, Andersen LW, Lui X, Donnino MW, Praetorius HA. Erythrocyte P2X1 receptor expression is correlated with change in haematocrit in patients admitted to the ICU with blood pathogen-positive sepsis. Crit Care. 2018;22(1):181. doi: 10.1186/s13054-018-2100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maitre B, Magnenat S, Heim V, Ravanat C, Evans RJ, de la Salle H, Gachet C, Hechler B. The P2X1 receptor is required for neutrophil extravasation during lipopolysaccharide-induced lethal endotoxemia in mice. J Immunol. 2015;194(2):739–749. doi: 10.4049/jimmunol.1401786. [DOI] [PubMed] [Google Scholar]
- 18.Adriouch S, Dox C, Welge V, Seman M, Koch-Nolte F, Haag F. Cutting edge: a natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol. 2002;169(8):4108–4112. doi: 10.4049/jimmunol.169.8.4108. [DOI] [PubMed] [Google Scholar]
- 19.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38(2):240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 20.Lecut C, Faccinetto C, Delierneux C, van Oerle R, Spronk HM, Evans RJ, El Benna J, Bours V, Oury C. ATP-gated P2X1 ion channels protect against endotoxemia by dampening neutrophil activation. J Thromb Haemost. 2012;10(3):453–465. doi: 10.1111/j.1538-7836.2011.04606.x. [DOI] [PubMed] [Google Scholar]
- 21.Johnsen N, Hamilton ADM, Greve AS, Christensen MG, Therkildsen JR, Wehmoller J, Skals M, Praetorius HA (2019) Alpha-haemolysin production, as a single factor, causes fulminant sepsis in a model of Escherichia coli-induced bacteraemia. Cell Microbiol e13017. https://www.ncbi.nlm.nih.gov/pubmed/30761726 [DOI] [PubMed]
- 22.Hechler B, Magnenat S, Zighetti ML, Kassack MU, Ullmann H, Cazenave JP, Evans R, Cattaneo M, Gachet C. Inhibition of platelet functions and thrombosis through selective or nonselective inhibition of the platelet P2 receptors with increasing doses of NF449 [4,4′,4″,4″'-(carbonylbis(imino-5,1,3-benzenetriylbis-(carbonylimino)))tetrakis -benzene-1,3-disulfonic acid octasodium salt] J Pharmacol Exp Ther. 2005;314(1):232–243. doi: 10.1124/jpet.105.084673. [DOI] [PubMed] [Google Scholar]
- 23.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 24.Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585. doi: 10.1136/bmj.i1585. [DOI] [PubMed] [Google Scholar]
- 25.Skals M, Praetorius HA. Mechanisms of cytolysin-induced cell damage - a role for auto- and paracrine signalling. Acta Physiol. 2013;209:95–113. doi: 10.1111/apha.12156. [DOI] [PubMed] [Google Scholar]
- 26.Land WG, Agostinis P, Gasser S, Garg AD, Linkermann A. DAMP - induced allograft and tumor rejection: the circle is closing. Am J Transplant. 2016;16:3322–3337. doi: 10.1111/ajt.14012. [DOI] [PubMed] [Google Scholar]
- 27.Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem. 1998;273(4):2030–2034. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- 28.Daniel JL, Dangelmaier C, Jin J, Ashby B, Smith JB, Kunapuli SP. Molecular basis for ADP-induced platelet activation. I Evidence for three distinct ADP receptors on human platelets. J Biol Chem. 1998;273(4):2024–2029. doi: 10.1074/jbc.273.4.2024. [DOI] [PubMed] [Google Scholar]
- 29.Jarvis GE, Humphries RG, Robertson MJ, Leff P. ADP can induce aggregation of human platelets via both P2Y(1) and P(2T) receptors. Br J Pharmacol. 2000;129(2):275–282. doi: 10.1038/sj.bjp.0703046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darbousset R, Delierneux C, Mezouar S, Hego A, Lecut C, Guillaumat I, Riederer MA, Evans RJ, Dignat-George F, Panicot-Dubois L, Oury C, Dubois C. P2X1 expressed on polymorphonuclear neutrophils and platelets is required for thrombosis in mice. Blood. 2014;124(16):2575–2585. doi: 10.1182/blood-2014-04-571679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oury C, Kuijpers MJ, Toth-Zsamboki E, Bonnefoy A, Danloy S, Vreys I, Feijge MA, De VR, Vermylen J, Heemskerk JW, Hoylaerts MF. Overexpression of the platelet P2X1 ion channel in transgenic mice generates a novel prothrombotic phenotype. Blood. 2003;101(10):3969–3976. doi: 10.1182/blood-2002-10-3215. [DOI] [PubMed] [Google Scholar]
- 32.Oury C, Toth-Zsamboki E, Thys C, Tytgat J, Vermylen J, Hoylaerts MF. The ATP-gated P2X1 ion channel acts as a positive regulator of platelet responses to collagen. Thromb Haemost. 2001;86(5):1264–1271. [PubMed] [Google Scholar]
- 33.Kazama I, Baba A, Endo Y, Toyama H, Ejima Y, Matsubara M, Tachi M. Salicylate inhibits thrombopoiesis in rat megakaryocytes by changing the membrane micro-architecture. Cell Physiol Biochem. 2015;35(6):2371–2382. doi: 10.1159/000374039. [DOI] [PubMed] [Google Scholar]
- 34.Siao WZ, Chuang WY, Su CH, Huang SF, Tu WK, Chan KC. A rare case of ticagrelor-induced profound isolated thrombocytopenia. Acta Cardiol Sin. 2017;33(5):556–558. doi: 10.6515/ACS20161021C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubano JA, Chen K, Sullivan B, Vosswinkel JA, Jawa RS. Clopidogrel-associated thrombotic thrombocytopenic purpura following endovascular treatment of spontaneous carotid artery dissection. J Neurol Surg Rep. 2015;76(2):e287–e290. doi: 10.1055/s-0035-1566127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo YL, Li JJ, Yuan JQ, Qin XW, Zheng X, Mu CW, Hua YH. Profound thrombocytopenia induced by clopidogrel with a prior history of long-term safe administration. World J Cardiol. 2010;2(6):160–162. doi: 10.4330/wjc.v2.i6.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanderschueren S, De Weerdt A, Malbrain M, Vankersschaever D, Frans E, Wilmer A, Bobbaers H. Thrombocytopenia and prognosis in intensive care. Crit Care Med. 2000;28(6):1871–1876. doi: 10.1097/00003246-200006000-00031. [DOI] [PubMed] [Google Scholar]
- 38.Akca S, Haji-Michael P, de Mendonca A, Suter P, Levi M, Vincent JL. Time course of platelet counts in critically ill patients. Crit Care Med. 2002;30(4):753–756. doi: 10.1097/00003246-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Crowther MA, Cook DJ, Meade MO, Griffith LE, Guyatt GH, Arnold DM, Rabbat CG, Geerts WH, Warkentin TE. Thrombocytopenia in medical-surgical critically ill patients: prevalence, incidence, and risk factors. J Crit Care. 2005;20(4):348–353. doi: 10.1016/j.jcrc.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Venkata C, Kashyap R, Farmer JC, Afessa B. Thrombocytopenia in adult patients with sepsis: incidence, risk factors, and its association with clinical outcome. J Intensive Care. 2013;1(1):9. doi: 10.1186/2052-0492-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou H, Deng M, Liu Y, Yang C, Hoffman R, Zhou J, Loughran PA, Scott MJ, Neal MD, Billiar TR. Platelet HMGB1 is required for efficient bacterial clearance in intra-abdominal bacterial sepsis in mice. Blood Adv. 2018;2(6):638–648. doi: 10.1182/bloodadvances.2017011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeaman MR. Platelets: at the nexus of antimicrobial defence. Nat Rev Microbiol. 2014;12(6):426–437. doi: 10.1038/nrmicro3269. [DOI] [PubMed] [Google Scholar]
- 43.Hamzeh-Cognasse H, Damien P, Chabert A, Pozzetto B, Cognasse F, Garraud O. Platelets and infections - complex interactions with bacteria. Front Immunol. 2015;6:82. doi: 10.3389/fimmu.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaertner F, Ahmad Z, Rosenberger G, Fan S, Nicolai L, Busch B, Yavuz G, Luckner M, Ishikawa-Ankerhold H, Hennel R, Benechet A, Lorenz M, Chandraratne S, Schubert I, Helmer S, Striednig B, Stark K, Janko M, Bottcher RT, Verschoor A, Leon C, Gachet C, Gudermann T, Mederos YSM, Pincus Z, Iannacone M, Haas R, Wanner G, Lauber K, Sixt M, Massberg S. Migrating platelets are mechano-scavengers that collect and bundle bacteria. Cell. 2017;171(6):1368–1382. doi: 10.1016/j.cell.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol. 2014;15(7):602–611. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 46.Minasyan H. Phagocytosis and oxycytosis: two arms of human innate immunity. Immunol Res. 2018;66(2):271–280. doi: 10.1007/s12026-018-8988-5. [DOI] [PubMed] [Google Scholar]
- 47.Clifford EE, Parker K, Humphreys BD, Kertesy SB, Dubyak GR. The P2X1 receptor, an adenosine triphosphate-gated cation channel, is expressed in human platelets but not in human blood leukocytes. Blood. 1998;91(9):3172–3181. [PubMed] [Google Scholar]
- 48.Lecut C, Frederix K, Johnson DM, Deroanne C, Thiry M, Faccinetto C, Maree R, Evans RJ, Volders PG, Bours V, Oury C. P2X1 ion channels promote neutrophil chemotaxis through rho kinase activation. J Immunol. 2009;183(4):2801–2809. doi: 10.4049/jimmunol.0804007. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Qin W, Xu X, Xiong Y, Zhang Y, Zhang H, Sun B. Endotoxin-induced autocrine ATP signaling inhibits neutrophil chemotaxis through enhancing myosin light chain phosphorylation. Proc Natl Acad Sci U S A. 2017;114(17):4483–4488. doi: 10.1073/pnas.1616752114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 45 kb)
(PDF 40 kb)
(PDF 45 kb)