Abstract
Previous studies have shown that T cell receptor (TCR) and CD28 coreceptor stimulation involves rapid ATP release, autocrine purinergic feedback via P2X receptors, and mitochondrial ATP synthesis that promote T cell activation. Here, we show that ADP formation and autocrine stimulation of P2Y1 receptors are also involved in these purinergic signaling mechanisms. Primary human CD4 T cells and the human Jurkat CD4 T cell line express P2Y1 receptors. The expression of this receptor increases following T cell stimulation. Inhibition of P2Y1 receptors impairs the activation of mitochondria, as assessed by mitochondrial Ca2+ uptake, and reduces cytosolic Ca2+ signaling in response to TCR/CD28 stimulation. We found that the addition of exogenous ADP or overexpression of P2Y1 receptors significantly increased IL-2 mRNA transcription in response to TCR/CD28 stimulation. Conversely, antagonists or silencing of P2Y1 receptors reduced IL-2 mRNA transcription and attenuated T cell functions. We conclude that P2Y1 and P2X receptors have non-redundant, synergistic functions in the regulation of T cell activation. P2Y1 receptors may represent potential therapeutic targets to modulate T cell function in inflammation and host defense.
Electronic supplementary material
The online version of this article (10.1007/s11302-019-09653-6) contains supplementary material, which is available to authorized users.
Keywords: T lymphocytes, Immune cells, Cell activation, Purinergic signaling
Introduction
Extracellular ATP is an important regulator of immune cells [1–3]. T cells respond to the ligation of their T cell receptors (TCR) and CD28 coreceptors with rapid release of ATP into the extracellular space [4]. The released ATP stimulates purinergic receptors that contribute to T cell activation by amplifying TCR/CD28 signaling at the immune synapse [4–6]. The purinergic receptor family comprises three major subgroups: (1) four P1 receptors that are G protein-coupled receptors and respond to the ATP breakdown product adenosine; (2) eight P2Y receptors that are also G protein-coupled receptors and bind to ATP, ADP, and other nucleotides; and (3) seven P2X receptors that respond to ATP and act as ATP-gated Ca2+ channels [7–9].
Stimulated T cells release ATP via pannexin-1 (panx1) channels, resulting in the activation of P2X1, P2X4, and P2X7 receptors that facilitate cellular Ca2+ influx, which promotes downstream signal transduction pathways that lead to IL-2 expression and T cell proliferation [4, 5]. During T cell activation panx1 channels, P2X1 and P2X4 receptors, and mitochondria accumulate at the immune synapse that forms between T cells and antigen-presenting cells (APCs) [6, 10]. Mitochondria are responsible for the production of the ATP that is released from T cells in response to TCR/CD28 stimulation [11]. The accumulation of mitochondria, panx1, and P2X receptors at the immune synapse triggers localized Ca2+ influx that further stimulates mitochondria and propagates cell signaling pathways in response to TCR/CD28 activation [6, 11].
However, ATP that is released into the extracellular space can also be hydrolyzed to ADP, AMP, and adenosine by a number of ectonucleotidases that are expressed on the cell surface of virtually all mammalian cell types, including T cells [12]. In T cells, ADP formed by these ectonucleotidases may activate specific P2Y receptors that preferably bind to ADP [13]. While the contributions of ATP and P2X receptors to T cell activation have been extensively studied, little is known about whether and how ADP-selective P2Y receptors are involved in the regulation of T cells. Here, we show that P2Y1 receptors are abundantly expressed ADP receptors in CD4 T cells and that T cell stimulation leads to an increase in extracellular ADP that stimulates P2Y1 receptors. We show that this ADP-mediated autocrine feedback mechanism contributes to T cell activation and the functional T cell responses induced by TCR/CD28 stimulation. We conclude that P2Y1 receptors work in synergy with P2X receptors in the regulation of T cell activation.
Materials and methods
Reagents
Rabbit p44/42 MAPK (ERK1/2) and phospho-p44/42 MAPK (pERK1/2; Thr202/Tyr204) antibodies were from Cell Signaling Technology (Beverly, MA, USA). Rabbit P2Y1 (Cat. # APR-021) and P2Y12 (Cat. # APR-020) antibodies recognizing extracellular epitopes of human P2Y1 and P2Y12 receptors, respectively, were purchased from Alomone Labs (Jerusalem, Israel). Secondary phycoerythrin (PE)- or DyLight 488-labeled donkey anti-rabbit antibodies (clone: Poly4064) were from Biolegend (San Diego, CA). Mouse anti-human CD3 (clone: HIT3a) and anti-CD28 antibodies (clone: CD28.2) were from BD Biosciences (San Jose, CA), and goat anti-mouse IgG Fc antibodies were from Pierce (Thermo Fisher Scientific). Fluo-4 AM was purchased from Molecular Probes (Thermo Fisher Scientific, Waltham, MA). 5-BDBD, MRS2279, and 2-methylthioadenosine diphosphate trisodium salt (Me-S-ADP) were purchased from Tocris Bioscience (R&D Systems, Minneapolis, MI). All other reagents were from Sigma-Aldrich (St. Louis, MO) unless otherwise stated.
Cell isolation and cell culture
All studies involving human subjects were approved by the Institutional Review Board of Beth Israel Deaconess Medical Center, and written informed consent was obtained prior to blood draws. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood of healthy volunteers by density gradient centrifugation (Ficoll-Paque Plus, GE Healthcare, Pittsburgh, PA), and CD4 T cells were purified from PBMCs using CD4 T cell isolation kits according to the manufacturer’s instructions (Miltenyi Biotec, San Diego, CA). Purified CD4 T cells were suspended in RPMI-1640 medium (ATCC, Mannassas, VA) supplemented with 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco, Thermo Fisher Scientific, Waltham, MA) and maintained at 37 °C in 5% CO2 in a humidified atmosphere. For some experiments, CD4 T cells were expanded by incubation for 6 days in the presence of 30 U/ml interleukin-2 (Roche, Mannheim, Germany) and human T-activator CD3/CD28 Dynabeads (Thermo Fisher Scientific) at a cell to bead ratio of 1:1. Jurkat T cells (clone E6-1; ATCC, Manassas, VA) were maintained in fully supplemented cell culture medium at 37 °C and 5% CO2.
Jurkat cell transfection
Transfections were carried out by electroporation using a Neon transfection system (Invitrogen, Thermo Fisher Scientific). Jurkat cells were transfected with 10 μg plasmid DNA or 100 nM siRNA, transferred to antibiotic-free cell culture medium, and cultured for 48 h if not stated otherwise.
Extracellular nucleotides
To assess the release of nucleotides in response to T cell stimulation, purified CD4 T cells (5 × 105 in 500 μl of cell culture medium) were stimulated with anti-CD3/CD28 antibody-coated Dynabeads at a bead-to-cell ratio of 1:1. The capability of CD4 T cells to hydrolyze ATP or ADP was tested by incubating cells (5 × 105) with 6 μM ATP or ADP. Reactions were stopped at the indicated times by placing samples in an ice water bath. Cell-free supernatants were harvested by centrifugation using a cooled microcentrifuge and stabilized with 0.4 M perchloric acid. Concentrations of ATP and its breakdown products in cell culture supernatants or plasma samples were determined by high-performance liquid chromatography (HPLC) as previously described [14].
Real-time quantitative PCR (qPCR)
IL-2 mRNA levels were measured by qPCR in Jurkat or primary T cells (5 × 105) stimulated for 4 h with anti-CD3/CD28 antibody-coated microbeads in the presence or absence of receptor agonists or antagonists as indicated. Total RNA was extracted using the RNAqueous RNA extraction kit (Ambion, Austin, TX) according to the manufacturer’s instructions. cDNA was synthesized from equal amounts of total RNA using Superscript III reverse transcriptase, oligo(dT), and random hexamer primers (Invitrogen, Thermo Fisher Scientific). qPCR was performed on a LightCycler 480 instrument (Roche Diagnostics, Mannheim, Germany) using LightCycler 480 Probes Master and RealTime ready single assays (Roche Diagnostics) for IL-2 (Assay 100958), succinate dehydrogenase complex, subunit A (SDHA; assay ID 102136), and TATA box binding protein (TBP; assay ID 101145). IL-2 expression was normalized against SDHA and TBP expression. P2X1, P2X4, P2X7, P2Y1, P2Y12, and P2Y13 receptor mRNA levels were determined in primary CD4 T cells or Jurkat cells using prevalidated QuantiTect primer assays (Qiagen, Valencia, CA), iQTM SYBR® Green supermix (Bio-Rad, Hercules, CA) and a Mastercycler® ep realplex instrument (Eppendorf, Hamburg, Germany). Receptor expression was normalized to β-actin, and the comparative Ct method was used for relative quantification of gene expression.
Flow cytometry
Flow cytometry was used to assess the surface expression of P2Y1 and P2Y12 receptors in freshly isolated CD4 T cells or in CD4 T cells stimulated for 72 h with anti-CD3/CD28 antibody-coated microbeads. Briefly, 5 × 105 T cells were stained in a total volume of 100 μl containing 1.6 μg of primary antibodies or appropriate isotype controls for 1 h on ice. Cells were washed and incubated with PE-labeled secondary antibodies (1:500) for 15 min. Samples were analyzed with an Attune® Acoustic Focusing Cytometer and Attune® Cytometric Software (Applied Biosystems, Beverly, MA).
P2Y1 receptor silencing
Pre-designed validated siRNA targeting the P2Y1 receptor and a nonsense control siRNA were purchased from Ambion (Thermo Fisher Scientific). Successful knockdown was confirmed by qPCR. Transfection efficiency was tested using a Cy3-conjugated nonsense siRNA (Ambion) and found to be > 90%.
P2Y1 receptor overexpression
P2Y1 receptor cDNA (NM_002563.2) was amplified from cDNA of a healthy donor by PCR using the following primers: P2Y1 sense: 5′-GAA TTC GCC ACC ATG ACC GAG GTC TGT G-3′ and P2Y1 antisense: 5′-TCT AGA TCA CAG GCT TGT ATC TCC-3′. The primers introduce an EcoRI restriction site upstream of the start codon and a XbaI restriction site at the 3′-end. PCR products and the pCMV-XL5 vector (OriGene Technologies, Rockville, MD) were digested, purified, and ligated together. As a negative control, the multiple cloning site was removed from the pCMV-XL-5 vector by digesting the plasmid with EcoRI. Plasmids were transformed into SoloPack competent cells (StrataClone, Thermo Fisher Scientific) and screened, and correct orientation and sequence of the insert were confirmed by DNA sequencing (Eurofins Genomics, Germany). P2Y1 receptor overexpression was confirmed by qPCR.
Immunocytochemistry
Jurkat cells were placed into fibronectin-coated glass bottom chamber slides (Lab-Tek, Rochester, NY), and non-adherent cells were removed by carefully washing the wells with cell culture medium. Cells were reconstituted in fully supplemented culture medium and incubated in the presence or absence of anti-CD3/CD28 antibody-coated microbeads for up to 30 min at 37 °C and 5% CO2. Cells were fixed with 4% paraformaldehyde in PBS for 10 min, incubated with blocking buffer (1% BSA and 0.3 M glycine in PBS) for 1 h, and stained with antibodies (1:100) directed against extracellular epitopes of human P2Y1 or P2Y12 receptors (Alomone Labs) overnight. Cells were washed and incubated with DyLight488-labeled secondary antibodies (1:2000; Biolegend) for 1 h, and receptor expression was assessed with a Leica DMI6000B inverted fluorescence microscope (Leica Microsystems, Wetzlar, Germany) using a fluorescein isothiocyanate (FITC) filter set, a ×100 oil objective (numerical aperture [NA] 1.3), and a Leica DFC365 FX camera. Cells that were stained with secondary antibodies only were included as negative controls to verify specific binding.
Imaging of cytosolic and mitochondrial Ca2+
To assess cytosolic Ca2+ levels, CD4 T cells were loaded with Fluo-4 AM (4 μM) for 20 min at 37 °C. Changes in mitochondrial Ca2+ were studied in Jurkat cells expressing the mitochondrial Ca2+ biosensor mito-CAR-GECO1 (#46022, Addgene, Cambridge, MA) [15]. Cells were placed into fibronectin-coated 8-well glass bottom dishes. Fluorescence live-cell imaging was performed with the Leica DMI6000B microscope described above equipped with a temperature-controlled (37 °C) stage incubator (Ibidi, Fitchburg, WI). Cells were pretreated for 10 min with P2 receptor inhibitors as indicated and stimulated with anti-CD3, anti-CD28, and anti-mouse IgG antibodies (0.5 μg/ml each) to induce TCR/CD28 cross-linking, and changes in cytosolic and mitochondrial Ca2+ levels were recorded. Fluorescence images were captured at a frame rate of 60 frames per minute through a ×63 oil objective (NA 1.4) using FITC and tetramethylrodamine (TRITC) filter sets (Leica Microsystems) and LeicaLAS microscope imaging software. Images were analyzed with ImageJ software (National Institutes of Health).
Immunoblotting
Immunoblotting was performed as previously described [6]. P2Y1 and P2Y12 receptor expression was analyzed in CD4 T cells before and after stimulating cells for 72 h with anti-CD3/CD28 antibody-coated beads. Mouse anti-β-actin antibodies (Sigma-Aldrich) were used as a loading control. ERK 1/2 MAPK activation was assessed using antibodies that recognize the phosphorylated forms of ERK1/2 (Thr202/Tyr204). Total ERK 1/2 MAPKs served as loading controls (Cell Signaling Technology, Beverly, MA, USA).
Statistical analysis
Unless otherwise indicated, data are presented as mean ± standard deviation (SD). Differences between groups were tested for statistical significance using the two-tailed unpaired Student’s t test or the Mann-Whitney test depending on whether data were normally distributed or not. For multiple comparisons, one-way analysis of variance (ANOVA) followed by post hoc Holm-Sidak’s test were used. Differences were considered statistically significant at p < 0.05.
Results
T cell stimulation triggers rapid ATP release and the accumulation of extracellular ATP, ADP, and AMP
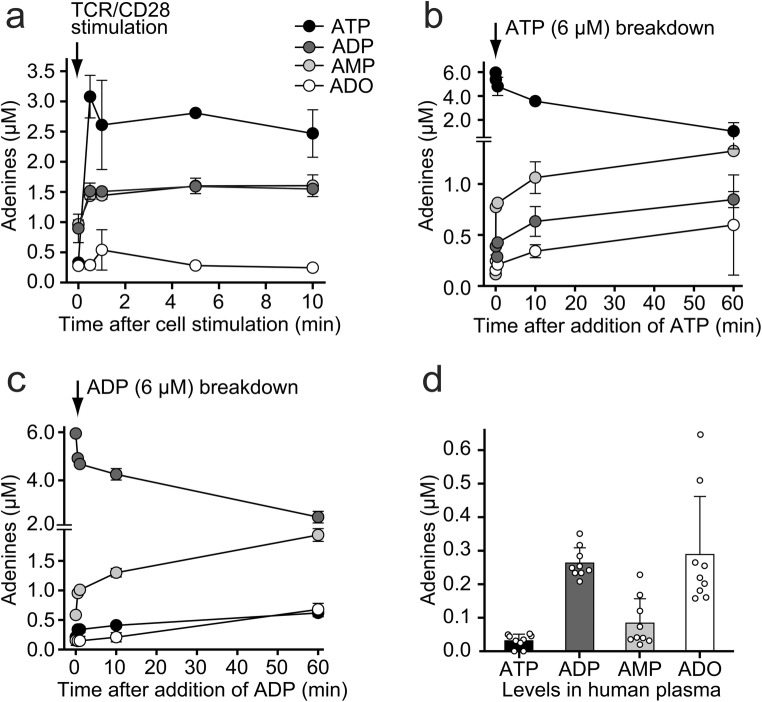
Previous work has shown that TCR/CD28 stimulation of CD4 T cells causes rapid cellular ATP release [4, 5, 11]. We showed that the released ATP binds primarily to P2X1 and P2X4 receptors that accumulate at the immune synapse where ATP facilitates localized Ca2+ influx that promotes T cell activation [6]. However, CD4 T cells also express ectonucleotidases including ectonucleoside triphosphate diphosphohydrolases (ENTPD) 1 and ENTPD2, also known as CD39 and CD39L1, respectively, which convert extracellular ATP to ADP and AMP [12, 16, 17]. In the current study, we used HPLC analysis to study the kinetics of the release of ATP from stimulated T cells and of the conversion of released ATP to ADP, AMP, and adenosine. In agreement with previous reports, we found that TCR/CD28 stimulation caused rapid ATP release from purified human CD4 T cells (Fig. 1a). We found that extracellular ATP concentrations rose more than tenfold above baseline levels and that this increase in extracellular ATP was accompanied by a concomitant increase in extracellular ADP and AMP concentrations. These findings suggest that a portion of the released ATP is rapidly hydrolyzed to ADP and AMP by ectonucleotidases (Fig. 1a). This possibility is supported by our finding that unstimulated primary CD4 T cells and the human CD4 Jurkat T cell line (data not shown) were able to rapidly convert exogenously added ATP to ADP, AMP, as well as adenosine (Fig. 1b). CD4 T cells were also able to hydrolyze exogenously added ADP, converting nearly 50% of extracellular ADP to AMP and adenosine in less than 1 h (Fig. 1c). We found that peripheral plasma of healthy human subjects contains high levels of ADP that average 260 nM. ADP levels were on average eight times higher than the corresponding ATP levels in those plasma samples (Fig. 1d). Average adenosine levels were similar to those of ADP, but the individual adenosine concentrations differed considerably from subject to subject. Taken together, these findings indicate that stimulation of T cells via TCR/CD28 causes rapid ATP release and that extracellular ATP is converted to ADP that accumulates together with adenosine in the plasma of healthy human subjects.
Fig. 1.
T cell stimulation triggers rapid ATP release and accumulation of extracellular ATP, ADP, and AMP. a ATP, ADP, and AMP accumulate in the extracellular space of stimulated T cells. Purified human CD4 T cells were stimulated with anti-CD3/CD28 antibody-coated beads, and ATP, ADP, AMP, and adenosine (ADO) concentrations in the cell supernatants were measured with HPLC at the indicated times. b–c T cells are able to hydrolyze extracellular ATP and ADP. Exogenous ATP (b) or ADP (c) each at a final concentration of 6 μM was added to primary human CD4 T cells, and hydrolytic breakdown was monitored over time. Data shown (a–c) are means ± SD of n = 3 experiments with different cell preparations. d Human plasma from healthy subjects contains high levels of ADP. Plasma samples were collected from healthy human subjects, and the concentrations of ATP, ADP, AMP, and adenosine were determined with HPLC. Data shown are means ± SEM (n = 9)
CD4 T cells express ATP and ADP receptors
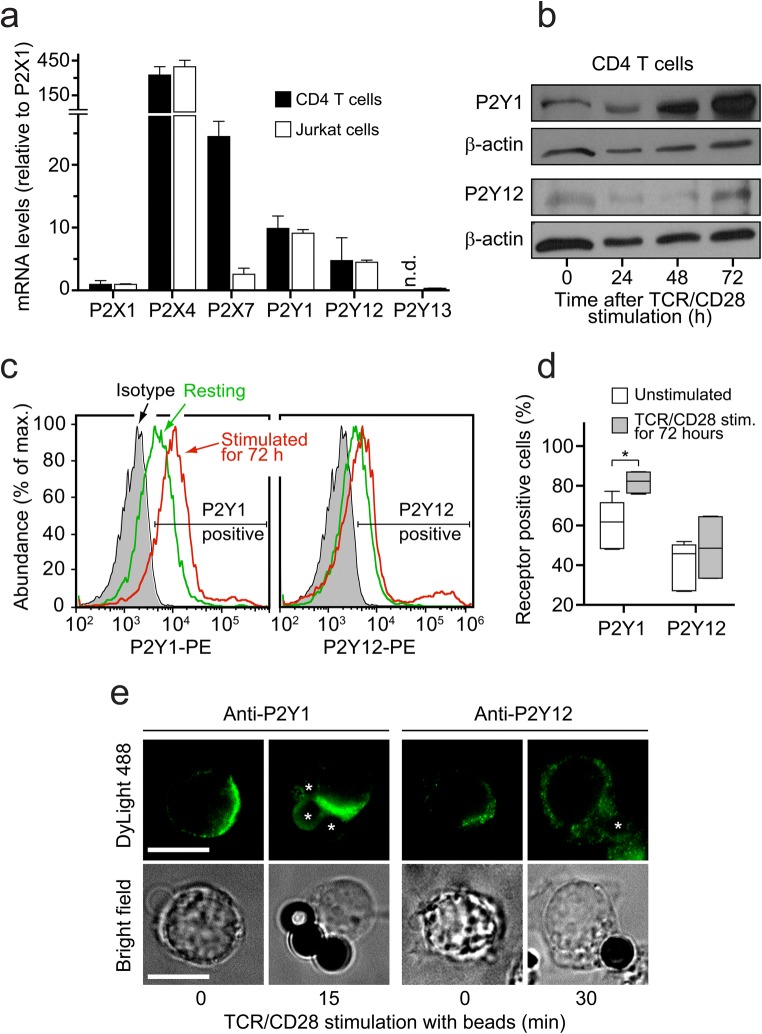
We have previously shown that ATP regulates the functions of CD4 T cells by stimulating P2X1, P2X4, and P2X7 receptors [4, 6]. Among these ATP selective P2 receptors, P2X4 receptors are abundantly expressed in CD4 T cells and have particularly important roles in the regulation of T cell migration, immune synapse formation, and T cell activation [6, 18]. Of the nineteen known P2 receptors, several members are known to respond to ADP, including P2Y1, P2Y12, and P2Y13 receptors [8, 19]. Therefore, we examined the expression of these P2Y receptors in CD4 T cells. Using qPCR analysis, we found that primary human CD4 T cells and Jurkat T cells express mRNA encoding P2Y1 and P2Y12 receptors in addition to P2X1, P2X4, and P2X7 receptors (Fig. 2a). P2Y12 mRNA expression levels were lower than those of P2Y1. Because P2Y13 receptor expression was barely detectable in primary CD4 T cells and Jurkat cells, we focused on P2Y1 and P2Y12 receptors. Immunoblot analysis of P2Y1 and P2Y12 receptor expression in primary CD4 T cells revealed strong expression of P2Y1 but weak expression of P2Y12 receptors (Fig. 2b). Analyzing CD4 T cells before and after stimulation via TCR/CD28 showed that P2Y1 receptor expression levels increased markedly within 48 h after cell stimulation (Fig. 2b). By contrast, P2Y12 receptor expression levels were barely detectable in unstimulated CD4 T cells and there was no detectable increase in expression levels in response to TCR/CD28 stimulation (Fig. 2b).
Fig. 2.
CD4 T cells express ATP-selective P2X and ADP-selective P2Y receptors. a P2 receptor mRNA levels in purified CD4 T cells were determined with qPCR using CD4 T cells isolated from healthy human subjects or Jurkat T cells (mean ± SD, n = 3; n.d., not detected). b Total protein expression of P2Y1 and P2Y12 receptors in human CD4 T cells before and after TCR/CD28 stimulation. The results shown are representative of n = 3 experiments with cells from different subjects. c–d Cell surface expression of P2Y1 and P2Y12 receptors on human CD4 T cells was assessed with flow cytometry before or 72 h after TCR/CD28 stimulation. Representative histograms (c) and summarized data (d) of unstimulated (n = 5) or stimulated (n = 4) cell preparations with cells from different donors are shown (*p < 0.05, Mann-Whitney test). e Polarized P2Y1 receptor expression and translocation to the immune synapse during T cell stimulation. The cell surface expression of P2Y1 and P2Y12 receptors on Jurkat T cells was analyzed with immunofluorescence imaging before and after stimulation of cells with anti-CD3/CD28 antibody-coated beads (indicated with asterisks) to mimic the formation of an immune synapse. Images are representative of separate experiments (n = 3) comprising at least 50 cells. Scale bar 10 μm; ×100 oil objective
These findings were paralleled by results obtained with flow cytometric analyses. P2Y1 and P2Y12 receptor expression on the cell surface of primary human CD4 T cells was analyzed before or 72 h after stimulation via TCR/CD28. We found that about 60% of all unstimulated CD4 T cells expressed P2Y1 receptors, while only 40% of cells were modestly positive for P2Y12 receptors (Fig. 2c, d). The expression of P2Y1 receptors increased significantly 72 h after TCR/CD28 stimulation, while little change in P2Y12 receptor expression was noted (Fig. 2d).
Finally, we used fluorescence microscopy to examine the expression and distribution of P2Y1 and P2Y12 receptors on the cell surface of Jurkat cells. We found that both receptors are present on the cell surface, but that the expression of P2Y1 receptors was more pronounced than that of P2Y12 receptors (Fig. 2e). Moreover, P2Y1 but not P2Y12 receptors translocated to the immune synapse that was formed by stimulation of cells with anti-CD3/CD28 antibody-coated beads. Taken together, these findings demonstrate that CD4 T cells express ADP-sensitive P2Y1 receptors, suggesting that P2Y1 receptors work hand-in-hand with P2X receptors as components of the purinergic signaling mechanism that regulates T cell activation.
P2Y1 and P2X4 receptors contribute to Ca2+ signaling in response to T cell stimulation
P2X4 receptors act as ATP-gated Ca2+ channels that facilitate Ca2+ influx following T cell stimulation [6, 9, 18]. By contrast, P2Y1 receptors are G protein-coupled receptors that signal through Gq/G11 [20]. Thus, P2X4 and P2Y1 receptors are expected to regulate T cell activation through modulation of intracellular Ca2+ levels and associated signaling pathways. We used live-cell imaging and the fluorescent Ca2+ probe Fluo-4-AM to study the roles of P2X4 and P2Y1 receptors in the Ca2+ signaling response of primary human CD4 T cells following TCR/CD28 stimulation. We found that inhibition of P2X4 receptors with the specific antagonist 5-BDBD virtually completely abolished Ca2+ signaling in response to TCR/CD28 stimulation (Fig. 3a). Pretreatment with the non-specific P2 receptor antagonist suramin also markedly reduced Ca2+ signaling (Fig. 3b–c). Inhibition of P2Y1 receptors with the selective antagonist MRS2279 decreased Ca2+ signaling and delayed the peak of cytosolic Ca2+ levels by about 2 min when compared with control cells. These data demonstrate that ATP release and autocrine stimulation of P2X4 and P2Y1 receptors contributes to the Ca2+ signaling response that regulates T cell activation following TCR/CD28 stimulation.
Fig. 3.

P2Y1 and P2X4 receptors regulate cytosolic Ca2+ signaling in response to TCR/CD28 stimulation. a Primary human CD4 T cells were loaded with the cytosolic Ca2+ probe Fluo-4 AM, pretreated for 10 min with specific antagonists of P2X4 (5-BDBD, 10 μM) or P2Y1 (MRS2279, 10 μM) receptors or with the general P2 receptor antagonist suramin (100 μM). Then, cells were stimulated by TCR/CD28 cross-linking, and changes in cytosolic Ca2+ levels were recorded over time using live-cell fluorescence microscopy. Traces of individual cells are shown in gray, and averages of all cells studied (n = 120–350) are shown in red. Data shown are representative of different experiments (n ≥ 3) with cells from different donors. b Averaged mean fluorescence values (+ SEM) of cells from at least three different donors (n = 120-350 per experiment). c Peak Ca2+ levels following TCR/CD28 stimulation; mean ± SEM; *p < 0.05 vs. control, one-way ANOVA
P2Y1 and P2X4 receptors regulate mitochondrial Ca2+ uptake in stimulated T cells
Mitochondria generate the ATP that T cells release in response to TCR/CD28 or chemokine receptor stimulation [11, 18]. The production of ATP occurs through oxidative phosphorylation in mitochondria, which requires the uptake of Ca2+ by mitochondria [21, 22]. Because P2X receptors function as ATP-gated Ca2+ channels, their stimulation by ATP facilitates cellular Ca2+ influx that delivers the Ca2+ that is needed by mitochondria to generate ATP [11, 23, 24]. Recently, we have shown that P2X4 receptors of T cells have a prominent role in facilitating mitochondrial Ca2+ uptake in response to stimulation of the chemokine receptor CXCR4 by SDF-1α [18]. The findings shown above indicate that P2X4 receptors are essential for the increase in intracellular Ca2+ following TCR/CD28 stimulation and that P2Y1 receptors also contribute to T cell activation. Therefore, we studied how P2X4 and P2Y1 receptors contribute to mitochondrial Ca2+ uptake in response to TCR/CD28 stimulation using Jurkat T cells that were transfected to express the mitochondrial Ca2+ indicator mito-CAR-GECO1 [15]. TCR/CD28 stimulation triggered a sharp increase in mitochondrial Ca2+ uptake that was nearly completely abolished by pretreatment of Jurkat cells with the P2X4 receptor antagonists 5-BDBD (Fig. 4). Inhibition of P2 receptors with suramin and of P2Y1 receptors with MRS2279 markedly blunted mitochondrial Ca2+ uptake in response to TCR/CD28 stimulation (Fig. 4b, c). Taken together, these results indicate that P2X4 and P2Y1 receptors work in synergy to regulate mitochondrial Ca2+ uptake following TCR/CD28 stimulation.
Fig. 4.
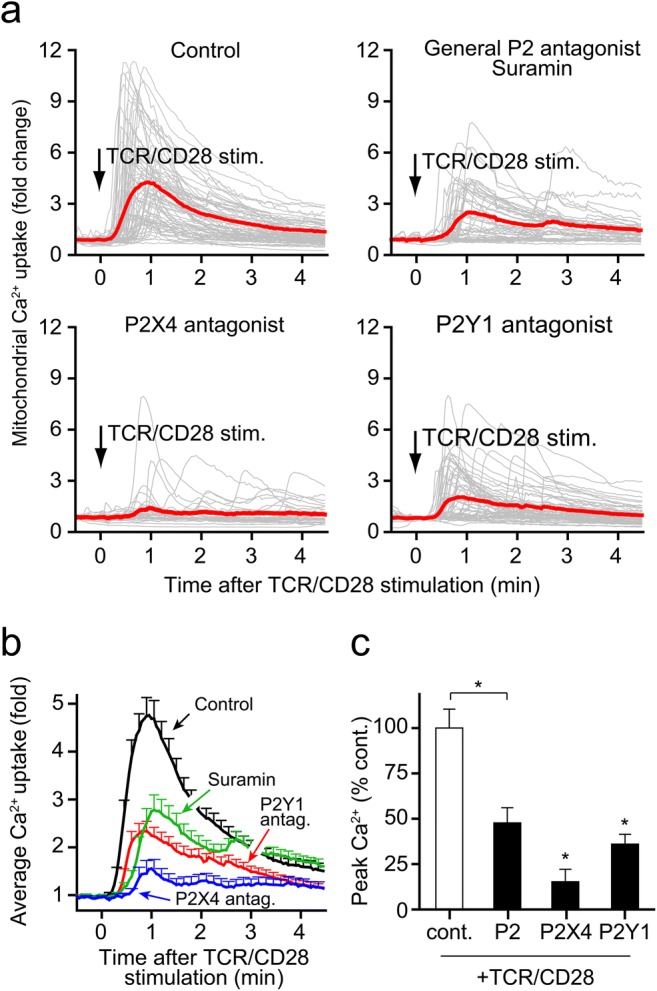
P2Y1 and P2X4 receptors activate mitochondria in response to TCR/CD28 stimulation. a Jurkat cells expressing the mitochondrial Ca2+ indicator mito-CAR-GECO1 were pretreated for 10 min with specific antagonists of P2X4 (5-BDBD, 10 μM) or P2Y1 (MRS2279, 10 μM) receptors or with the general P2 receptor antagonist suramin (100 μM). Then, cells were stimulated by TCR/CD28 cross-linking, and changes in mitochondrial Ca2+ uptake were recorded over time using fluorescence microscopy. Traces of individual cells are shown in gray, and the averages of all cells acquired (n = 30–80) are shown in red. Data shown are representative of independent experiments (n ≥ 3). b Averaged mean fluorescence values (+ SEM) of cells (n = 30-80) from different experiments (n ≥ 3 experiments). c Peak mitochondrial Ca2+ levels following TCR/CD28 stimulation; mean ± SEM; *p < 0.05 vs. control, one-way ANOVA
P2Y1 receptors promote IL-2 expression in response to TCR/CD28 stimulation
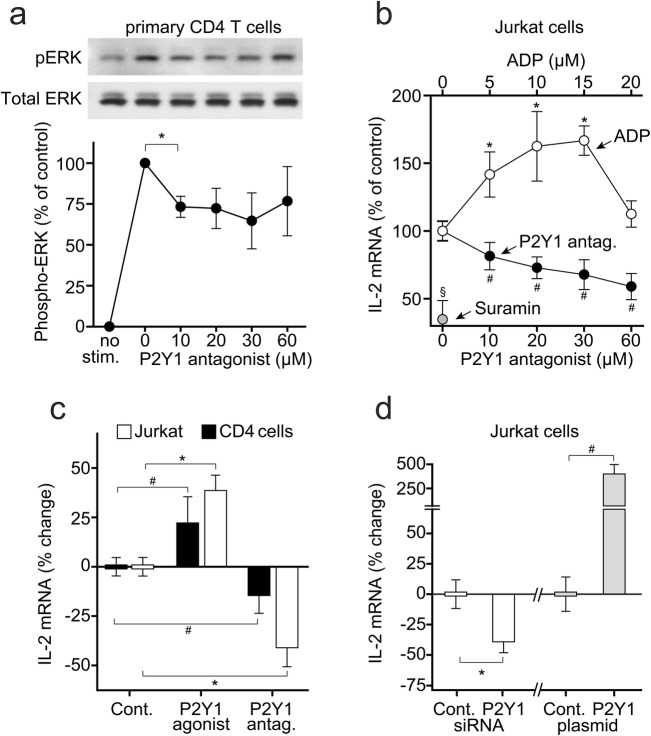
Activation of MAPK signaling is involved in IL-2 expression and T cell proliferation [25]. Pretreatment of CD4 T cells with the P2Y1 receptor antagonist MRS2279 reduced the phosphorylation of the ERK1/2 MAPK in response to T cell stimulation (Fig. 5a). These findings suggest that autocrine stimulation of P2Y1 receptors is involved in T cell activation by contributing to ERK1/2 MAPK activation.
Fig. 5.
P2Y1 receptors promote IL-2 expression in response to TCR/CD28 stimulation. a CD4 T cells were treated for 10 min with the indicated concentrations of the P2Y1 antagonist MRS2279 and stimulated for 5 min with anti-CD3/CD28 antibody-coated microbeads, and ERK1/2 MAPK activation was determined by immunoblotting with phosphospecific anti-ERK1/2 antibodies. Antibodies recognizing total ERK1/2 were used to verify equal protein loading. Data represent mean values ± SD of separate experiments (n = 3); *p < 0.05, one-way ANOVA. b Jurkat cells were stimulated for 4 h with anti-CD3/CD28 antibody-coated beads in the presence of the indicated concentrations of ADP, the P2Y1 antagonist MRS2279, or the general P2 receptor inhibitor suramin (500 μM). IL-2 mRNA levels were determined with qPCR. Data represent mean values ± SD of independent experiments (n = 3–8); §p < 0.05, t test; *,#p < 0.05, one-way ANOVA, compared with untreated controls. c Jurkat cells or primary human CD4 T cells were treated with the non-hydrolysable ADP analogs ADPβS (10 μM; Jurkat cells) or Me-S-ADP (100 nM; T cells) or with the P2Y1 receptor antagonist MRS2279 (60 μM). Then, cells were stimulated with anti-CD3/CD28 antibody-coated beads, and IL-2 mRNA transcription was determined after 4 h. Data are shown as mean ± SD of independent experiments with cells from different donors (n = 3); *, #p < 0.05, one-way ANOVA. d Jurkat cells were transfected with siRNA to silence P2Y1 receptor expression (n = 6) or with an expression plasmid to overexpress P2Y1 receptors (n = 9). Then, cells were stimulated with anti-CD3/CD28 antibody-coated beads, and IL-2 mRNA transcription was measured after 4 h (mean ± SD; *p < 0.05, one-way ANOVA)
IL-2 production is a key event in the signaling cascade that leads to T cell proliferation [26]. Treatment of Jurkat cells with exogenous ADP at concentrations ranging from 5 to 15 μM increased IL-2 mRNA expression in response to TCR/CD28 stimulation (Fig. 5b). At higher concentrations, ADP lost its costimulatory effect, possibly due to increased formation of adenosine. Treatment of Jurkat cells or primary human CD4 T cells with the P2Y1 receptor antagonist MRS2279 dose-dependently reduced IL-2 mRNA expression in response to TCR/CD28 stimulation (Fig. 5b, c). Treating T cells with the stable ADP analogs ADPβS or Me-S-ADP also significantly increased IL-2 mRNA expression of TCR/CD28-stimulated Jurkat cells and of primary human CD4 T cells (Fig. 5c). Silencing of P2Y1 receptors reduced IL-2 mRNA expression in response to cell stimulation. Conversely, overexpression of P2Y1 receptors increased IL-2 mRNA expression (Fig. 5d).
Taken together, our results demonstrate that extracellular ADP and autocrine as well as paracrine stimulation of P2Y1 receptors act in synergy with ATP and P2X receptors to promote cell activation and to regulate the functional T cell responses involved in inflammation and host defense.
Discussion
The concentration of extracellular nucleotides is a function of cellular ATP release, ATP hydrolysis, and cellular reuptake of ATP breakdown products. These processes are thought to maintain low extracellular nucleotide levels that prevent uncontrolled purinergic signaling [27]. Unstimulated T cells release low levels of ATP that fuels autocrine signaling mechanisms that involve P2X1 receptors and maintain a basal metabolic activity profile that enables immune surveillance of T cells [24]. We have shown that mitochondria deliver the ATP that is required for this process and that mitochondrial dysfunction in sepsis patients impairs T cell responses [11, 24]. When T cells are stimulated by chemokines or antigens, they rapidly upregulate cell metabolism by increasing mitochondrial ATP synthesis through mechanisms that involve autocrine stimulation of P2X4 receptors. P2X4 receptors colocalize with mitochondria to deliver Ca2+ ions that mitochondria need to increase their metabolic rate [18].
In order to respond to antigens, T cells form an immune synapse with APCs, which facilitates intercellular communications of T cells and APCs. This immune synapse is the physical location at which complex purinergic signaling processes take place that orchestrate TCR/CD28 receptor stimulation and facilitate intercellular communication between T cells and APCs. Panx1, P2X4 receptors, and activated mitochondria converge at the immune synapse, where these signaling components promote mitochondrial metabolism and T cell activation [6, 11]. Panx1-mediated ATP release and synchronized P2X4 receptor-mediated Ca2+ influx upregulate purinergic signaling to the level necessary for successful TCR/CD28 stimulation and the induction of functional T cell responses [4–6].
Compared with resting T cells, activated T cells have significantly higher energy demands that are satisfied by rapid upregulation of ATP synthesis and ultimately by a metabolic switch from mitochondrial ATP synthesis to glycolytic ATP production [28, 29]. We and others have demonstrated that autocrine feedback through P2X1, P2X4, and P2X7 receptors is needed for the transition of T cells from their resting states to fully activated effector T cells that contribute to inflammation and host defense.
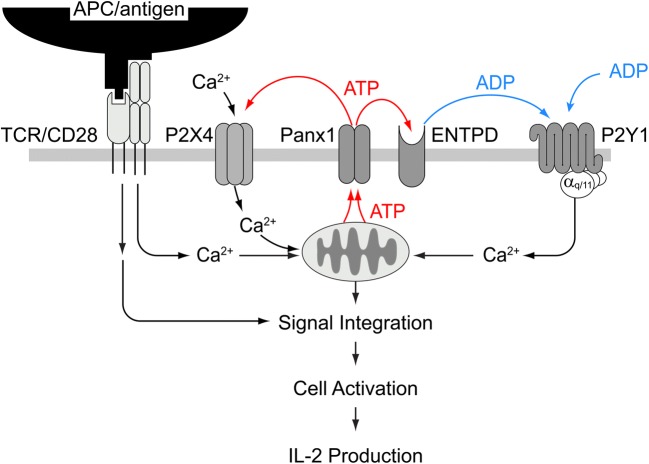
Our current data show, to our knowledge for the first time, that the ATP breakdown product ADP and autocrine feedback via P2Y1 receptors are also required for T cell activation. We propose that ADP is generated from released ATP by ectonucleotidases that are present on the cell surface of T cells. However, we cannot exclude the possibility that ADP is directly released from stimulated T cells as was previously proposed for hepatic cells [30]. Either way, our findings show that stimulation of P2Y1 receptors participates in the autocrine purinergic signaling mechanisms that ramp up mitochondrial ATP production and cytosolic Ca2+ signaling in response to TCR/CD28 stimulation (Fig. 6). P2Y1 receptors are Gq/11 coupled receptors known to stimulate the release of Ca2+ from intracellular stores, whereas P2X4 receptors promote Ca2+ influx from the extracellular space [8, 9]. The finding that blockade of either P2Y1 or P2X4 receptors downregulates mitochondrial Ca2+ uptake and cytosolic Ca2+ signaling suggests that both these P2 receptor subtypes have non-redundant functions in T cell activation and that their combined actions are necessary to define the downstream signaling events that regulate IL-2 production, T cell activation, and other cellular processes that are involved in immune defense.
Fig. 6.
Proposed mechanisms by which P2X4 and P2Y1 receptors synergize to regulate T cells. Stimulation of their T cells via T cell receptor (TCR) and CD28 coreceptors triggers mitochondrial ATP production and ATP release through pannexin-1 channels (Panx1). The released ATP activates P2X4 receptors that promote Ca2+ influx. Hydrolysis of extracellular ATP by ectonucleotide triphosphate diphosphohydrolases (ENTPD) generates ADP that activates P2Y1 receptors that contribute to the increase in intracellular Ca2+ concentrations. Increased mitochondrial activity, P2X4 and P2Y1 receptor Ca2+ signaling increases ATP release and fuel an autocrine feed-forward signaling mechanism that enhances T cell activation, and subsequent downstream signaling pathways that culminate in IL-2 transcription and effector functions involved in inflammation and host defense
P2Y1, P2Y12, and P2Y13 receptors are designated ADP receptors [19]. However, we did not detect any P2Y13 receptor mRNA transcripts in primary CD4 T cells and only negligible expression levels in Jurkat T cells. Although P2Y12 receptor mRNA was present in CD4 T cells, protein expression levels were considerably lower when compared to P2Y1 receptors. These findings suggest that P2Y1 receptors are the primary ADP receptor subtype that is expressed in CD4 T cells. In addition to regulating IL-2 expression, P2Y1 receptors may also regulate T cell migration as suggested by the finding that inhibition of P2Y1 receptors reduced the motility of effector T cells (Supplemental Fig. 1 and video 1).
While little is known about their functions in T cells, P2Y1 and P2Y12 receptors have well-known roles in platelet activation [31, 32]. Both receptors promote platelet aggregation and P2Y12 receptors are the primary targets of modern antithrombotic therapies [33, 34]. However, P2Y1 and P2Y12 receptors may also have pro-inflammatory effects that are unrelated to platelet activation [35–38]. We show that P2Y1 receptors contribute to T cell activation, suggesting that antiplatelet drugs could influence T cell activation, which may ameliorate inflammatory processes but may also attenuate T cell-mediated immune defenses. This notion is supported by findings that P2Y1 receptor deletion prevents atherosclerosis-associated vascular inflammation [39, 40].
We conclude that ADP and ATP receptors jointly regulate the purinergic signaling mechanism that control T cell activation and that P2X4 and P2Y1 receptors have prominent roles in these processes. P2Y1 receptors may represent potential therapeutic targets to modulate inflammation and T cell function in inflammatory and infectious diseases. However, more work is needed to fully define how P2Y1 receptors regulate CD4 T cells and the other T cell subsets involved in inflammatory diseases and host defense.
Electronic supplementary material
(PDF 231 kb)
(AVI 2669 kb)
Acknowledgements
This work was funded in part by grants from the National Institutes of Health (GM-51477, GM-60475, GM-116162, AI-080582, and T32 GM-103702) and from the Friedrich-Baur Foundation (25/11; to T.W.). The authors thank Ms. Katharina Strasser for her excellent technical assistance.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tobias Woehrle and Carola Ledderose contributed equally to this work.
Contributor Information
Carola Ledderose, Phone: (617) 667-7415, Email: cleddero@bidmc.harvard.edu.
Wolfgang G. Junger, Phone: (617) 667-7415, Email: wjunger@bidmc.harvard.edu
References
- 1.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnstock G, Boeynaems JM. Purinergic signalling and immune cells. Purinergic Signal. 2014;10:529–564. doi: 10.1007/s11302-014-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16:177–192. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 4.Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y, Ferrari V, Insel PA, Junger WG. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 2009;23:1685–1693. doi: 10.1096/fj.08-126458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 6.Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, Junger WG. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood. 2010;116:3475–3484. doi: 10.1182/blood-2010-04-277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 8.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011;63:641–683. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quintana A, Schwindling C, Wenning AS, Becherer U, Rettig J, Schwarz EC, Hoth M. T cell activation requires mitochondrial translocation to the immunological synapse. Proc Natl Acad Sci U S A. 2007;104:14418–14423. doi: 10.1073/pnas.0703126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ledderose C, Bao Y, Lidicky M, Zipperle J, Li L, Strasser K, Shapiro NI, Junger WG. Mitochondria are gate-keepers of T cell function by producing the ATP that drives purinergic signaling. J Biol Chem. 2014;289:25936–25945. doi: 10.1074/jbc.M114.575308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8:437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Jacobsen SEW, Bengtsson A, Erlinge D. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol. 2004;5:16. doi: 10.1186/1471-2172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sumi Y, Woehrle T, Chen Y, Bao Y, Li X, Yao Y, Inoue Y, Tanaka H, Junger WG. Plasma ATP is required for neutrophil activation in a mouse sepsis model. Shock. 2014;42:142–147. doi: 10.1097/SHK.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J, Liu L, Matsuda T, Zhao Y, Rebane A, Drobizhev M, Chang YF, Araki S, Arai Y, March K, Hughes TE, Sagou K, Miyata T, Nagai T, Li WH, Campbell RE. Improved orange and red Ca2+ indicators and photophysical considerations for optogenetic applications. ACS Chem Neurosci. 2013;4:963–972. doi: 10.1021/cn400012b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ledderose C, Woehrle T, Ledderose S, Strasser K, Seist R, Bao Y, Zhang J, Junger WG. Cutting off the power: inhibition of leukemia cell growth by pausing basal ATP release and P2X receptor signaling? Purinergic Signal. 2016;12:1–13. doi: 10.1007/s11302-015-9480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledderose C, Liu K, Kondo Y, Slubowski CJ, Dertnig T, Denicoló S, Arbab M, Hubner J, Konrad K, Fakhari M, Lederer JA, Robson SC, Visner GA, Junger WG. Purinergic P2X4 receptors and mitochondrial ATP production regulate T cell migration. J Clin Invest. 2018;128:3583–3594. doi: 10.1172/JCI120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Kügelgen I, Hoffmann K. Pharmacology and structure of P2Y receptors. Neuropharmacology. 2016;104:50–61. doi: 10.1016/j.neuropharm.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 20.Waldo GL, Harden TK. Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol Pharmacol. 2004;65:426–436. doi: 10.1124/mol.65.2.426. [DOI] [PubMed] [Google Scholar]
- 21.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 22.Pathak T, Trebak M. Mitochondrial Ca(2+) signaling. Pharmacol Ther. 2018;192:112–123. doi: 10.1016/j.pharmthera.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adinolfi E, Callegari MG, Ferrari D, Bolognesi C, Minelli M, Wieckowski MR, Pinton P, Rizzuto R, Di Virgilio F. Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum-independent growth. Mol Biol Cell. 2005;16:3260–3272. doi: 10.1091/mbc.e04-11-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ledderose C, Bao Y, Ledderose S, Woehrle T, Heinisch M, Yip L, Zhang J, Robson SC, Shapiro NI, Junger WG. Mitochondrial dysfunction, depleted purinergic signaling, and defective T cell vigilance and immune defense. J Infect Dis. 2016;213:456–464. doi: 10.1093/infdis/jiv373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 26.Crispín JC, Tsokos GC. Transcriptional regulation of IL-2 in health and autoimmunity. Autoimmun Rev. 2009;8:190–195. doi: 10.1016/j.autrev.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dwyer KM, Deaglio S, Gao W, Friedman D, Strom TB, Robson SC. CD39 and control of cellular immune responses. Purinergic Signal. 2007;3:171–180. doi: 10.1007/s11302-006-9050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buck MD, O'Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee C, Sparks DL. P2X receptors regulate adenosine diphosphate release from hepatic cells. Purinergic Signal. 2014;10:587–593. doi: 10.1007/s11302-014-9419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fabre JE, Nguyen M, Latour A, Keifer JA, Audoly LP, Coffman TM, Koller BH. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat Med. 1999;5:1199–1202. doi: 10.1038/13522. [DOI] [PubMed] [Google Scholar]
- 32.Turner NA, Moake JL, McIntire LV. Blockade of adenosine diphosphate receptors P2Y(12) and P2Y(1) is required to inhibit platelet aggregation in whole blood under flow. Blood. 2001;98:3340–3345. doi: 10.1182/blood.V98.12.3340. [DOI] [PubMed] [Google Scholar]
- 33.Dorsam RT, Kunapuli SP. Central role of the P2Y12 receptor in platelet activation. J Clin Invest. 2004;113:340–345. doi: 10.1172/JCI20986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wijeyeratne YD, Heptinstall S. Anti-platelet therapy: ADP receptor antagonists. Br J Clin Pharmacol. 2011;72:647–657. doi: 10.1111/j.1365-2125.2011.03999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abele S, Spriewald BM, Ramsperger-Gleixner M, Wollin M, Hiemann NE, Nieswandt B, Weyand M, Ensminger SM. Attenuation of transplant arteriosclerosis with clopidogrel is associated with a reduction of infiltrating dendritic cells and macrophages in murine aortic allografts. Transplantation. 2009;87:207–216. doi: 10.1097/TP.0b013e3181938913. [DOI] [PubMed] [Google Scholar]
- 36.Preidl RHM, Eckl S, Ramsperger-Gleixner M, Koch N, Spriewald BM, Weyand M, Ensminger SM. Clopidogrel reduces post-transplant obliterative bronchiolitis. Transpl Int. 2013;26:1038–1048. doi: 10.1111/tri.12163. [DOI] [PubMed] [Google Scholar]
- 37.Suh DH, Trinh HKT, Liu JN, Pham le D, Park SM, Park HS, Shin YS. P2Y12 antagonist attenuates eosinophilic inflammation and airway hyperresponsiveness in a mouse model of asthma. J Cell Mol Med. 2016;20:333–341. doi: 10.1111/jcmm.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hechler B, Gachet C. Purinergic receptors in thrombosis and inflammation. Arterioscler Thromb Vasc Biol. 2015;35:2307–2315. doi: 10.1161/ATVBAHA.115.303395. [DOI] [PubMed] [Google Scholar]
- 39.Hechler B, Freund M, Ravanat C, Magnenat S, Cazenave JP, Gachet C. Reduced atherosclerotic lesions in P2Y1/apolipoprotein E double-knockout mice: the contribution of non-hematopoietic-derived P2Y1 receptors. Circulation. 2008;118:754–763. doi: 10.1161/CIRCULATIONAHA.108.788927. [DOI] [PubMed] [Google Scholar]
- 40.Zerr M, Hechler B, Freund M, Magnenat S, Lanois I, Cazenave JP, Léon C, Gachet C. Major contribution of the P2Y1 receptor in purinergic regulation of TNFα-induced vascular inflammation. Circulation. 2011;123:2404–2413. doi: 10.1161/CIRCULATIONAHA.110.002139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 231 kb)
(AVI 2669 kb)