Abstract
Extracellular adenosine triphosphate (ATP) regulates a broad variety of physiological functions in a number of tissues partly via ionotropic P2X receptors. Therefore, P2X receptors are promising targets for the development of therapeutically active molecules. Bile acids are cholesterol-derived amphiphilic molecules; their primary function is the facilitation of efficient nutrient fat digestion. However, bile acids have also been shown to serve as signaling molecules and as modulators of different membrane proteins and receptors including ion channels. In addition, some P2X receptors are sensitive to structurally related steroid hormones. In this study, we systematically analyzed whether rat P2X receptors are affected by micromolar concentrations of different bile acids. The taurine-conjugated bile acids TLCA, THDCA, and TCDCA potently inhibited P2X2, whereas other P2X receptors were only mildly affected. Furthermore, stoichiometry and species origin of the P2X receptors affected the modulation by bile acids: in comparison to rat P2X2, the heteromeric P2X2/3 receptor was less potently modulated and the human P2X2 receptor was potentiated by TLCA. In summary, bile acids are a new class of P2X receptor modulators, which might be of physiological relevance.
Keywords: P2X , Bile acid , Cation channel
Introduction
P2X receptors are Ca2+-permeable cation channels that are activated by extracellular ATP. Purinergic signaling via ionotropic P2X-receptors plays a pivotal role in many physiological processes, both in excitable and non-excitable mammalian cells. Modulation of synaptic transmission [1, 2] and of the vascular tone through initiation of smooth muscle contraction [3], fine tuning of immune responses [4], and platelet aggregation [4] exemplify the variety of physiological and pathophysiological settings P2X receptors are involved in. Because of their broad functions, P2X receptors are valuable targets for the development of therapeutically active molecules.
In vertebrates, the P2X receptor family comprises seven genes coding for seven receptors: P2X1 to P2X7. Functional P2X receptors assemble either as homo- or heterotrimers [5, 6]. Each subunit is composed of a large extracellular domain (ECD), which harbors the ATP binding site, and two transmembrane domains (TMDs). The N- and C-termini protrude into the cytosol. The overall shape of each subunit resembles that of a jumping dolphin with the head domain, the upper and lower body domains, and the fluke of the dolphin represented by the ECD and the two TMD helices, respectively [5]. The responses to prolonged application of ATP vary between different P2X receptors: while P2X1, P2X3, and P2X4 desensitize upon ATP application, P2X2 is characterized by a sustained non-desensitizing current [7, 8].
ATP binding at the interface of two adjacent subunits induces cleft closure between the head and dorsal fin domains and pushes the left flipper domain outward [9–11]. These initial conformational rearrangements are transmitted to the lower body, resulting in an outward flexing movement of the β strands directly coupled to the TM1 and TM2 helices, which eventually causes the helices to expand outward and thereby opening the ion channel pore [9, 10].
A common pharmacological feature of P2X receptors is their inhibition by the antiprotozoal molecule suramin [12]. Drugs that specifically act on P2X receptors, however, have not been approved yet [13, 14].
Together with P2X4 and P2X7, P2X2 is the predominant receptor type in neurons of the brain [15]. However, it is also found in neurons of the enteric nervous system, mainly in the myenteric plexus, where P2X2 is involved in fast synaptic transmission [16, 17].
Bile acids are derivatives of cholesterol, synthesized by hepatocytes in the liver and secreted into the gallbladder and subsequently released at millimolar concentrations into the duodenum to enable efficient solubilization and digestion of nutrient fats. Besides this classical physiological role of bile acids, they have more recently been shown to function as signaling molecules [18]. In the liver, bile acids mediate signaling cascades controlling their synthesis and secretion mainly via two receptors, the nuclear farnesoid receptor (FXR) and the G protein–coupled receptor TGR5 [19–21]. In other tissues like the intestinal tract or immune cells, TGR5 is also expressed and mediates bile acid–dependent responses [22, 23]. TGR5 is also found in the brain [24], and some data suggest that bile acids are present at low concentrations in the brain, both under physiological and pathophysiological conditions like liver failure [25–27]. In addition to the receptors FXR and TGR5, the bile acid–sensitive ion channel (BASIC) was recently identified as an ionotropic bile acid receptor [28, 29]. The physiological role of BASIC is, however, not clear [30]. Related ion channels like the epithelial Na+ channel (ENaC) and acid-sensing ion channels (ASIC) are also modulated by bile acids [31–33].
Since various P2X receptors are found in many tissues in mammals, several of them might be in direct or indirect contact with bile and its main constituent bile acids. Furthermore, some P2X receptors are sensitive to structurally related steroid hormones [8]. Therefore, we tested whether bile acids could affect ATP-gated currents of various P2X receptors. We found that rat P2X2 is strongly inhibited by lithocholic, chenodeoxycholic, and hyodeoxycholic acid. In contrast, P2X1, P2X3, P2X4, and P2X7 were only mildly affected. Taken together, these data suggest that bile acids represent a new class of modulators of P2X receptors, with P2X2 being the most susceptible representative.
Materials and methods
Molecular biology
The cDNAs of rat P2X1, rat P2X2, rat P2X3, and human P2X2 were cloned in the oocyte expression vector pRSSP [34]. Rat P2X4 and rat P2X7 were cloned in pNKS2 [35, 36]. Rat P2X4 and rat P2X7 contained a 6xHis-tag at the N-terminus. Capped cRNA was synthesized by SP6 RNA polymerase from linearized cDNA, using the SP6 mMessage mMachine kit (Ambion, USA).
Two-electrode voltage clamp in Xenopus laevis oocytes
Surgical removal of oocytes was performed as described previously [37]. Anesthetized frogs were killed after the final oocyte collection by decapitation. Animal care and surgery of frogs were conducted under protocols approved by the State Office for Nature, Environment and Consumer Protection (LANUV) of North Rhine-Westphalia (NRW), Germany, and were performed in accordance with LANUV NRW guidelines. cRNAs were injected into stage V or VI oocytes of Xenopus laevis at the following concentrations: rP2X2 0.04 ng, P2X2/P2X3 0.25 and 0.5 ng, hP2X2 0.04 ng, rP2X1 4 ng, rP2X3 4 ng, rP2X4 4 ng, and rP2X7 0.25 ng. Oocytes were incubated in OR-2 medium (in mM, 140 NaCl, 2.5 KCl, 1 Na2HPO4, 5.0 HEPES, 1.0 MgCl2, 1 CaCl2, and 0.5 mg/ml polyvinylpyrrolidone, pH 7.3) at 19 °C. Whole-cell currents were recorded 24–72 h post-injection at room temperature (20–25 °C) with a TurboTec 03X amplifier (npi electronic, Tamm, Germany). P2X2, P2X2/3, and P2X7 expressing oocytes were superfused using an automated, fast, pump-driven solution exchange system (npi electronic). Data acquisition and solution exchange were controlled using CellWorks 5.1.1 (npi electronic). Data were filtered at 20 Hz and acquired at 200 Hz. For oocytes expressing P2X1, P2X3, or P2X4, a solution exchange system based on a pipetting robot was used (npi screening tool). In this case, data were filtered at 20 Hz and acquired by CellWorks 6.1.2 (npi electronic) at 500 Hz. In both cases, the holding potential was − 70 mV. Standard bath solution for two-electrode voltage clamp measurements contained (in mM) 140 NaCl, 2.8 MgCl2, and 10 HEPES, pH 7.4. Free ATP concentrations reported were calculated online (http://maxchelator.stanford.edu/MgATP-TS.htm).
Chemicals
Taurolithocholic acid (TLCA), taurochenodeoxycholic acid (TCDCA), taurodeoxycholic acid (TDCA), taurocholic acid (TCA), taurohyodeoxycholic acid (THDCA), glycohyodeoxycholic acid (GHDCA), hyodeoxycholic acid (HDCA), and taurine were purchased from Sigma-Aldrich (Germany). Tauroursodeoxycholic acid was purchased from Merck (Germany). All other chemicals were purchased from Sigma-Aldrich (Germany). Bile acids and taurine were dissolved in water at a concentration of 20 mM and subsequently diluted in standard bath solution.
Data analysis and statistics
Data were visualized and quantified using the software CellWorks Reader 6.2.2 (npi electronic, Tamm, Germany). Subsequent statistical analysis was performed in Microsoft Excel. Data represented in figures were plotted using Igor Pro 5.0.3 (WaveMetrics, Portland, USA). All experiments were performed using at least two independent batches of oocytes. In experiments with rP2X2, hP2X2, or rP2X2/3 and a single long application of ATP, recordings in which the baseline at the end of the ATP application was shifted more than 30% relative to the ATP-activated current amplitude were excluded from analysis.
For rP2X2, hP2X2, or rP2X2/3, currents were quantified during long applications of ATP at the time points immediately before solution was exchanged. Ratios of current amplitudes in presence of bile acids and the preceding current amplitude in absence of bile acid were calculated for each experimental condition. These ratios were compared with the ratios of the control conditions by student’s unpaired t test. The p value was subsequently multiplied by the number of tests that were conducted against the control condition to correct for multiple testing (Bonferroni correction). For P2X1, P2X3, and P2X4, peak currents were quantified. Peak currents in the presence of bile acids were compared with the mean amplitude of the two peak currents preceding and following bile acid application. P values were determined by student’s paired t test and subsequently multiplied by two, the number of bile acids that were tested per oocyte (Bonferroni correction). Ratios of currents in the presence of bile acids divided by the mean of the preceding and following current are depicted for simplicity. For P2X7, we used a procedure like for P2X1, P2X3, and P2X4, but currents were quantified at the time points immediately before solution was exchanged. Data are reported as mean ± S.E.M, concentrations of half maximal activation (EC50) or inhibition (IC50) were determined by Hill fits. Values are reported as mean ± S.E.M.
Results
Various bile acids inhibit rat P2X2
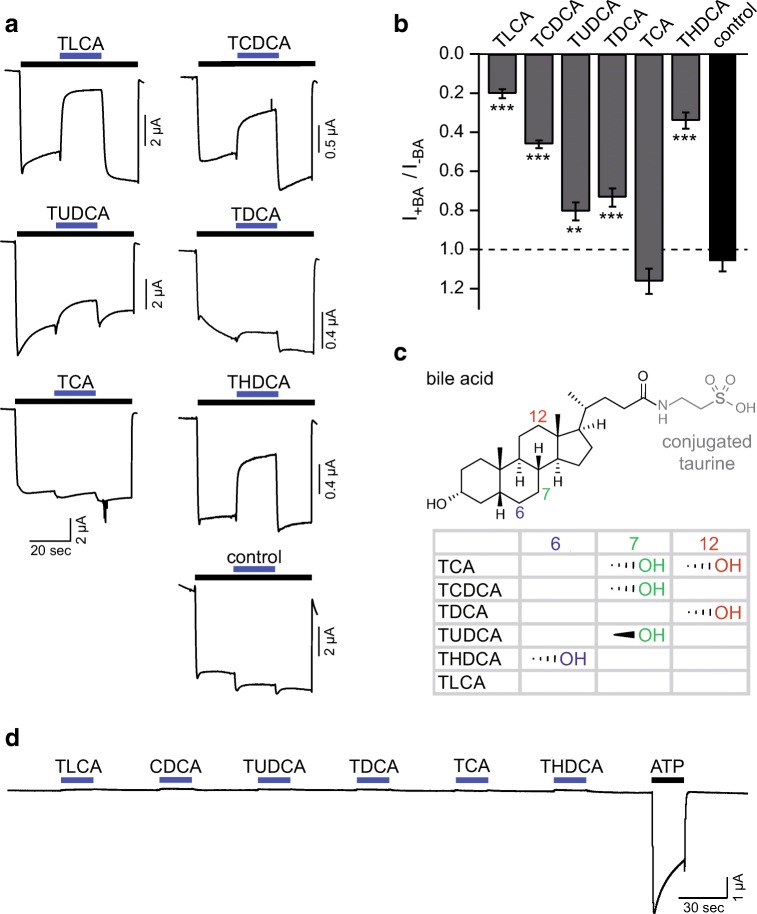
To test modulation of P2X2 by bile acids, we activated P2X2 heterologously expressed in oocytes by a long (60 s) application of ATP (10 μM) (Fig. 1a). At this concentration, P2X2 only slightly desensitized (Fig. 1a). Within the activation by ATP, we co-applied the following taurine-conjugated bile acids at a concentration of 20 μM: taurolithocholic acid (TLCA), taurochenodeoxycholic acid (TCDCA), tauroursodeoxycholic acid (TUDCA), taurodeoxycholic acid (TDCA), taurocholic acid (TCA), or taurohydodeoxycholic acid (THDCA) (Fig. 1). The co-application of bile acids reduced the ATP-induced currents with the following rank order (from strong to weak reduction): TLCA (21 ± 7%) > THDCA (34 ± 11%) > TCDCA (46 ± 6%) > TDCA (73 ± 12%) > TUDCA (81 ± 13%) (n = 7–11, p < 0.01; Fig. 1b); only TCA had no significant effect (p > 0.99). The two most effective bile acids were TLCA and THDCA (Fig. 1b). Control measurements, in which we exchanged only the ATP, resulted in a relative current of 107 ± 4% (n = 9). When applied alone, bile acids did not affect P2X2, ruling out that bile acids are partial agonists of P2X2 that inhibit it by competing with the full agonist ATP (Fig. 1d). Taken together, bile acids, in particular, TLCA and THDCA, potently reduced P2X2-activated currents.
Fig. 1.
Bile acids inhibit rat P2X2 at micromolar concentrations. a Representative current traces of Xenopus laevis oocytes expressing rat P2X2; P2X2 was activated by ATP (10 μM, black bar). Blue bars indicate the addition of 20 μM of different bile acids or control (solution exchange only). b Mean current amplitudes in the presence of the indicated substance relative to the ATP-activated current prior to substance application. Error bars represent standard error of the mean (S.E.M.), **p < 0.005, ***p < 0.001; n = 7–11. c Structure of TLCA with its conjugated taurine group indicated in gray. Hydroxy groups at the three carbon atoms highlighted by their IUPAC number discriminate the tested bile acids. The hydroxyl groups of each bile acid with their steric orientation are summarized in the table. TLCA, taurolithocholic acid; TCDCA, taurochenodeoxycholic acid; TUDCA, tauroursodeoxycholic acid; TDCA, taurodeoxycholic acid; TCA, taurocholic acid; THDCA, taurohydodeoxycholic acid. d Representative current trace of rat P2X2 activated by ATP (10 μM, black bar). Blue bars indicate the application of 20 μM of different bile acids alone
Since we used taurine-conjugated bile acids to inhibit P2X2 and taurine contains a sulfonic acid group (Fig. 2a), which is also present in the prototypical P2X inhibitor suramine [12], we tested if the sulfonic acid group might be essential for P2X inhibition by bile acids. We co-applied with 10 μM ATP either 20 μM of the glycine-conjugated HDCA (GHDCA) or 20 μM of the unconjugated bile acid hyodeoxycholic acid (HDCA) or 20 μM of taurine to P2X2-expressing oocytes. GHDCA had a similar effect as THDCA (reduction to 56 ± 8%, n = 14; p < 0.001), while the unconjugated bile acid HDCA strongly reduced the ATP-gated current to 6 ± 5% (n = 11, p < 0.001). In contrast, taurine did not significantly alter P2X2 current amplitude (n = 13, p = 0.21) (Fig. 2b and c). We conclude that bile acids alone are effective in inhibiting P2X2 and that the sulfonic group in the conjugated taurine is not required for inhibition.
Fig. 2.
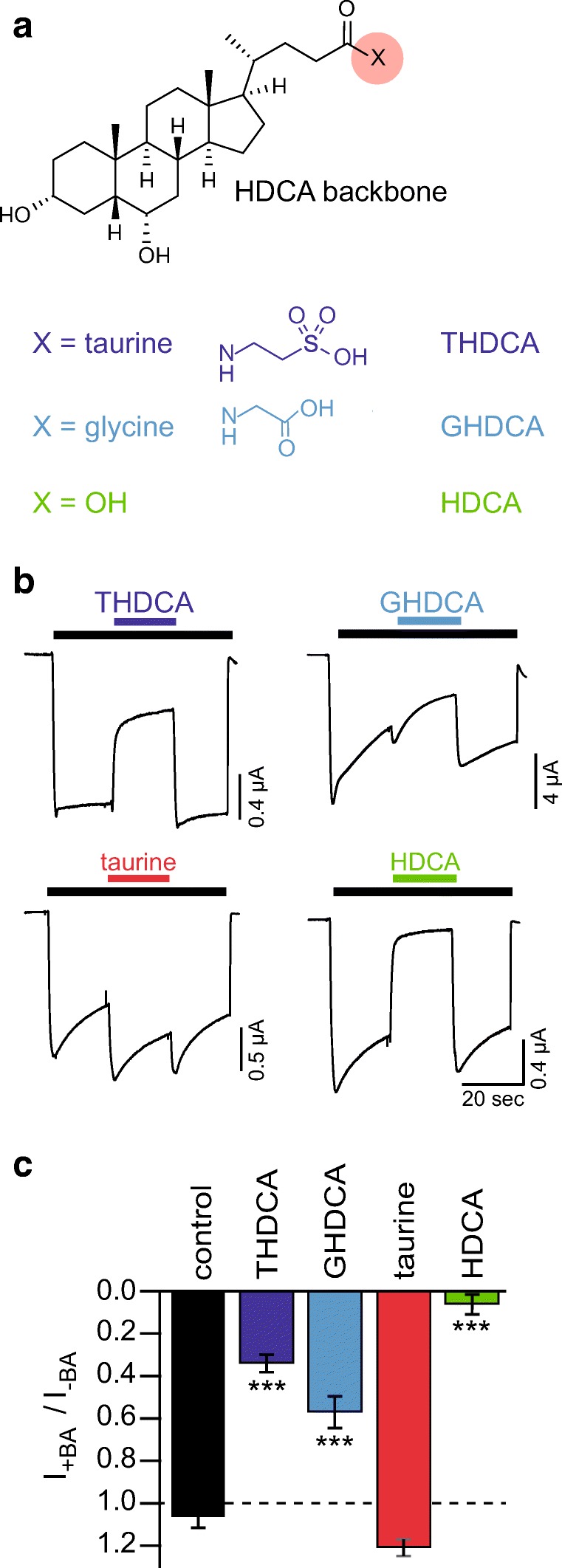
The sulfonic acid group of taurine is not required for the inhibitory effect of THDCA. a Structure of HDCA with possible taurine or glycine conjugates. b Representative current traces of Xenopus laevis oocytes expressing rat P2X2, which was activated by ATP (10 μM, black bar). Colored bars indicate the addition of THDCA, GHDCA, HDCA (20 μM), or 20 μM taurine. c Mean current amplitudes in the presence of the indicated bile acids relative to the ATP-activated current prior to substance application. Control measurements are same as in Fig. 1. Error bars represent standard error of the mean (S.E.M.). ***p < 0.001; n = 7–14
Effects of bile acids on rP2X1, rP2X3, rP2X4, and rP2X7
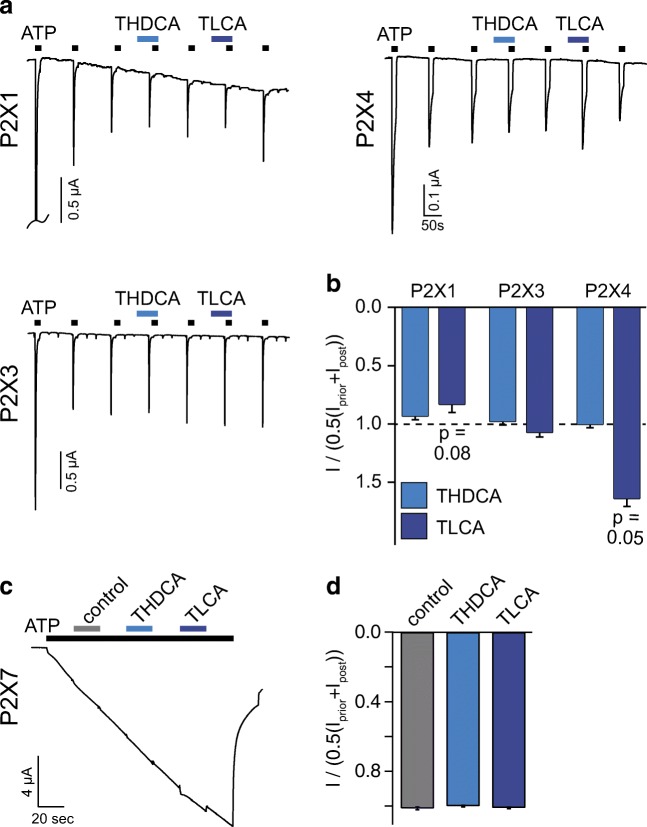
Next, we asked whether the inhibition of rP2X2 by bile acids is specific for this receptor or if other rP2X receptors are similarly inhibited. We tested the effect of 20 μM THDCA and 200 μM TLCA, the most potent inhibitors of P2X2, on P2X1, P2X3, P2X4, and P2X7. Since P2X1, P2X3, and P2X4 quickly desensitize, we activated them by repetitive application of ATP. We used 300 nM ATP for P2X1 and P2X3 and 3 μM for P2X4 in order to produce a low desensitization rate. Under these conditions, peak currents were constant after three activations (Fig. 3a). We then pre- and co-applied THDCA or TLCA with ATP (Fig. 3a). THDCA and TLCA had no significant effect on the amplitudes of ATP-activated currents of these three P2X receptors (Fig. 3b). Yet, the application of 200 μM TLCA weakly decreased P2X1 currents to 84 ± 7% (n = 13; p = 0.08) and increased P2X4 currents to 164 ± 6% (n = 8; p = 0.05) (Fig. 3b). To test if P2X7 is affected by bile acids, we activated P2X7 by applying 1.7 mM ATP (yielding 124 μM free ATP), which induced a typical slowly and continuously increasing inward current (Fig. 3c). Next, we co-applied THDCA (20 μM) or TLCA (200 μM) with ATP. When compared with the current amplitude before and after bile acid application, neither THDCA nor TLCA modulated P2X7 currents (n = 6–9) (Fig. 3d). Taken together, micromolar concentrations of several bile acids inhibited mainly P2X2 while P2X1 and P2X4 were slightly modulated by TLCA.
Fig. 3.
Only TLCA mildly modulated rP2X1 and rP2X4 currents. a Representative current traces of P2X1, P2X3, and P2X4 expressed in Xenopus laevis oocytes repetitively activated by ATP (P2X1, P2X3 0.3 μM; P2X4 3 μM). THDCA (20 μM) or TLCA (200 μM) (black bars) were pre- and co-applied as indicated by the blue bars. b Mean current amplitudes in the presence of the indicated bile acid relative to the average of the previous and the subsequent ATP-activated currents, n = 8–13. c Representative current trace of P2X7 expressed in Xenopus laevis oocytes activated by ATP (1.7 mM). Pre- and co-application of THDCA (20 μM) or TLCA (200 μM) (blue bars) or control (gray bar) did not affect current amplitude. d Mean current amplitudes in the presence of the indicated bile acid relative to the average of the current amplitude before and after bile acid coapplication. n = 6–9; error bars represent S.E.M.
The heteromeric rat P2X2/P2X3 receptor is also sensitive to bile acids
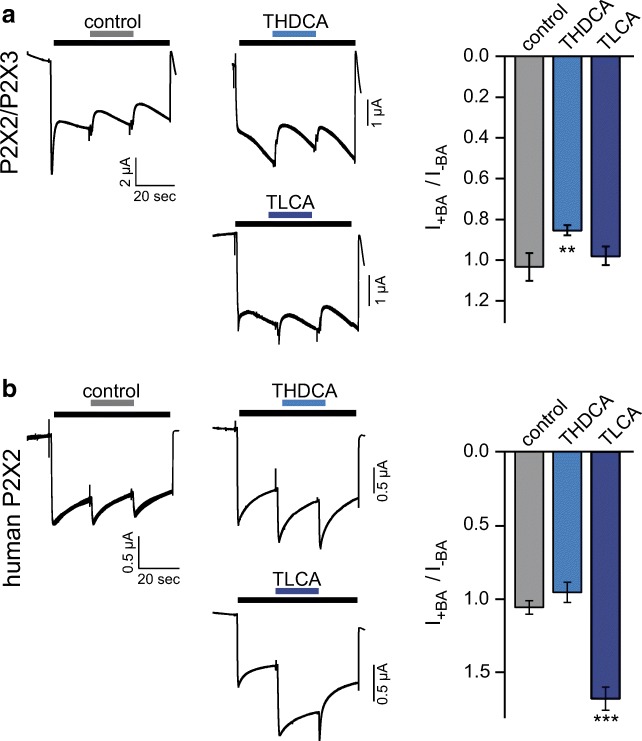
We tested whether bile acids also affected the P2X2/P2X3 heteromer. We activated oocytes co-expressing P2X2 and P2X3 with 1 μM of α,β-meATP alone or in combination with either 20 μM THDCA or 20 μM TLCA, the two bile acids with the most potent effect on the P2X2 homomeric receptor. Only THDCA decreased the current to 87 ± 3% (n = 10; p < 0.01), while the application of control solution or TLCA resulted in a relative current of 99 ± 2% or 100 ± 4% (n = 10–11, p = 0.8) (Fig. 4a). In conclusion, of the two bile acids THDCA and TLCA, only TLCA inhibited the P2X2/P2X3 heteromeric receptor significantly; however, the potency was strongly reduced compared with the P2X2 homomeric receptor.
Fig. 4.
THDCA weakly inhibits P2X2/P2X3 heteromers, while TLCA strongly potentiates human P2X2. a Left, representative current traces of Xenopus laevis oocytes expressing P2X2/P2X3, activated by of α,β-meATP (1 μM, black bar). Blue bars indicate the addition of THDCA or TLCA (20 μM); gray bar indicates control (solution exchange only). Right, mean currents in the presence of the indicated bile acids relative to the ATP-activated current prior to substance application, **p < 0.001; n = 10–11. b Left, representative current traces of Xenopus laevis oocytes expressing human P2X2, activated by ATP (1 μM, black bar). Colored bars indicate the addition of THDCA or TLCA (20 μM). Right, mean currents in the presence of the indicated bile acids relative to the ATP-activated current prior to substance application, ***p < 0.001; n = 7–8. Error bars represent standard error of the mean (S.E.M.)
The current of human P2X2 is increased by TLCA but not THDCA
We next asked whether bile acids also affect the human P2X2 ortholog. We applied the same experimental protocol for human P2X2 as for rat P2X2. When 20 μM of TLCA was co-applied with 10 μM of ATP, the current increased to 170 ± 8% of control (n = 7, p < 0.001). In contrast, control solution or 20 μM THDCA had no effects on the current amplitude (108 ± 5%, n = 8 or 98 ± 7%, n = 8) (Fig. 4b). These findings uncover a strong species dependence of P2X2 modulation by bile acids.
Concentration dependency of bile acid modulation of P2X2
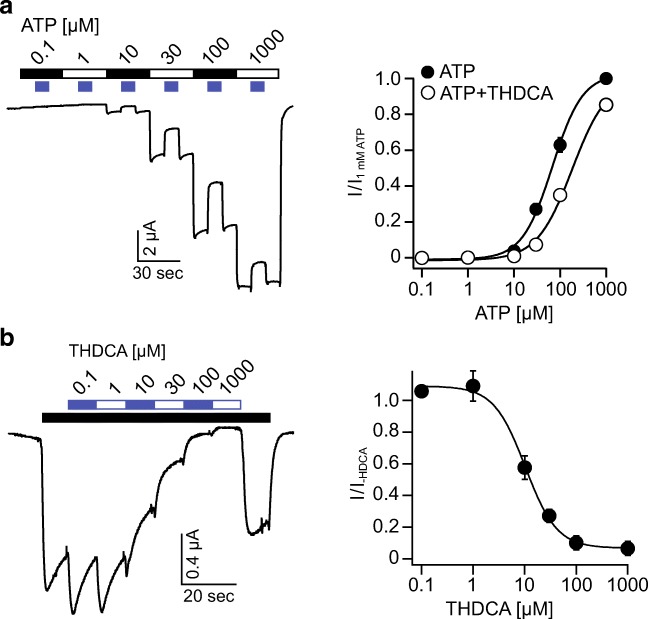
Bile acids may inhibit rat P2X2 by either reducing the potency or the efficacy of ATP. To address this question, we recorded ATP concentration-response curves in the absence or presence of 20 μM THDCA. Increasing concentrations of ATP induced responses with increasing amplitudes. During ATP application, we co-applied 20 μM THDCA, which decreased the current amplitude. The resulting concentration-response curve for ATP in the absence of THDCA resulted in an EC50 value of 63 ± 4 μM, which is in agreement with the literature [38]. In contrast, the presence of THDCA shifted the concentration-response curve resulting in an EC50 value of 122 ± 8 μM (n = 11, p < 0.001), showing that the potency for ATP was decreased by THDCA. In contrast, at the maximal concentration of ATP (1 mM), current amplitude was almost not decreased by THDCA (Fig. 5a), suggesting that the bile acid did not affect the efficacy of ATP to activate P2X2.
Fig. 5.
THDCA decreases the ATP affinity of rP2X2 and inhibits P2X2 currents in a concentration-dependent manner. a Left, representative current trace showing the application of increasing ATP concentrations and intermitted co-application of 20 μM THDCA to rP2X2 expressed in Xenopus laevis oocytes. Right, concentration-response curve for ATP in the absence or presence of 20 μM THDCA normalized to the application of 1 mM ATP alone, n = 12. b Left, representative current trace showing the activation of rP2X2 by ATP (10 μM) and the inhibitory effect of increasing concentrations of THDCA (white-blue bar). Right, concentration-response curve of THDCA in the presence of 10 μM ATP normalized to the current in absence of THDCA, n = 10. Error bars represent S.E.M.
We then determined the apparent affinity of P2X2 for THDCA when activated by 10 μM ATP. ATP was applied continuously and increasing THDCA concentrations were applied. The ATP-activated current was inhibited in a concentration-dependent manner with an IC50 value of 10.7 ± 1.1 μM (n = 10) (Fig. 5b). Taken together, these results show that bile acids represent a new class of P2X2 modulators.
Discussion
Bile acids were long considered natural detergents solely required for the efficient digestion of nutrient fats. Nowadays, they are more and more appreciated as enteroendocrine hormones, which play important roles for the regulation of various aspects of mammalian physiology [39]. In this study, we describe for the first time the effect of bile acids on different P2X receptors and show that some bile acids potently inhibit rat P2X2.
While a positive modulatory effect of bile acids on other channels like the bile acid–sensitive ion channel and the epithelial Na+ channel (ENaC) requires millimolar concentrations [29, 31], the effect of bile acids on P2X2 receptors described in the present study occurred at low micromolar concentrations. This concentration is reached not only in the intestinal tract but also in other tissues like the brain, where bile acids may function as hormone-like signaling molecules [27, 39, 40]. Two bile acid–sensitive receptors are known, the nuclear FXR receptor and the TGR5 receptor. Both require low micromolar concentrations of various bile acids for activation [41, 42]. P2X2 therefore represents a new receptor, which is a possible target for bile acid-signaling. Since P2X2 is involved in, for example, fast synaptic transmission in neurons of the brain and the enteric nervous system [16, 17], bile acids might modulate transmission at these synapses. Especially, neurons of the enteric nervous system could represent targets for a combined regulation by ATP and bile acids. Thus, our findings add another layer of regulatory complexity to P2X2 receptors.
P2X2 showed the most pronounced effects in our study and was inhibited by five of the six bile acids tested; only TCA did not affect P2X2. The secondary bile acids TLCA and THDCA were the most potent inhibitory bile acids tested followed by TCDCA, TDCA, and TUDCA. The effects of bile acids on the other P2X receptors (P2X1, P2X3, P2X4, and P2X7) were only mild and did not reach statistical significance after correcting for multiple testing by the Bonferroni procedure. TLCA inhibited P2X1 but, unlike P2X2, the inhibition was only subtle. P2X4 activity on the other hand was increased by TLCA, contrary to the effect of TLCA on P2X2, suggesting a subunit-specific effect of bile acids. The structural difference between the bile acids used in this study, is the number and steric orientation of the hydroxyl groups at the steroid backbone. While TCA has two hydroxyl groups at the steroid backbone, TCDCA, TDCA, TUDCA, and THDCA have one and TLCA has no hydroxyl group (see Fig. 1c). Because our results show clearly that the taurine conjugate is not required for the inhibitory effect, the differences of the inhibitory potency on P2X2 can only be attributed to location and orientation of the hydroxyl groups. Whether the hydroxyl groups are sterically relevant for binding to the channel or whether the degree of hydrophilicity that is conferred by the hydroxyl groups is important for the modulatory effect of bile acids on P2X receptors will be an interesting question to follow in further studies.
What is the mechanism of inhibition of rat P2X2 by bile acids? A previous study has shown that P2X2 is sensitive to alteration of the plasma membrane induced by membrane-active substances [43]. Bile acids are amphiphilic molecules and thus can affect the properties of the plasma membrane. One possible mechanism of P2X2 inhibition might therefore involve modulation of the plasma membrane by bile acids, which then indirectly influences the activity of the P2X receptor. However, a modulation of the plasma membrane of Xenopus oocytes by bile acids that has an impact on the activity of Deg/ENaC channels like BASIC probably requires higher concentrations [43]. Thus, either P2X2 is more sensitive to membrane alterations or bile acids directly bind to P2X2, either in the ECD or in the TMDs. Because THDCA affected ATP potency and did almost not inhibit P2X2 at high ATP concentrations (Fig. 5a), an open pore block by ATP can be excluded. Several P2X receptors are antagonized by suramin, the underlying mechanism being an inhibition of gating [44, 45]. Suramin is a polysulfonated multicyclic molecule, with larger dimensions than bile acids. Bile acids might act similarly via interference with gating processes. The potentiating effect of TLCA on P2X4 also supports the modulation of P2X receptor gating by bile acids. Since ATP and bile acids are structurally unrelated, it is unlikely that bile acids compete with ATP for its binding site. Thus, bile acids most likely allosterically affect ATP potency.
A surprising finding of our study is the striking difference between rat and human P2X2 with regard to their modulation by different bile acids. While THDCA and TLCA strongly inhibited rP2X2, THDCA had no effect on hP2X2 and TLCA even increased hP2X2 currents. This may represent an adaptation to the species-specific bile acid pool: THDCA is present in rodents and absent from human. However, this cannot explain why TLCA inhibits rP2X2 but increased hP2X2 currents. We have recently shown that, for the bile acid–sensitive ion channel BASIC, the cell system, which is used for studying the channel, strongly affects the biophysical properties of the channel and can account for strong differences between species orthologs [46]. This may also be the case for rat and human P2X2 expressed in Xenopus laevis oocytes. Further studies will be necessary to unravel the details of bile acid modulation of P2X receptors.
Under physiological conditions, bile acids are in direct contact with hepatocytes and cholangiocytes in the liver and with cells of the intestinal tract. Various P2X receptors, including P2X2, are found in these tissues, P2X4 being the predominant receptor in cholangiocytes and hepatocytes [47, 48]. Identifying bile acids as naturally occurring modulators of P2X receptors may open a new perspective on regulation and fine tuning of these receptors in their specific sites of expression. Under pathophysiological conditions, e.g., gallstones, bile acids may also reach the pancreas leading to pancreatitis. Since P2X1, P2X4, and P2X7 are also found in the pancreas [49], bile acid–mediated modulation of P2X activity may be involved in the pathogenesis of pancreatitis.
In summary, we have shown that bile acids are natural modulators of P2X receptors, in particular P2X2.
Acknowledgements
We thank A. Oslender-Bujotzek for her expert technical assistance.
Abbreviations
- BA
Bile acid
- BASIC
Bile acid–sensitive ion channel
- ENaC
Epithelial Na+ channel
- GHDCA
Glycohyodeoxycholic acid
- HDCA
Hyodeoxycholic acid
- TCA
Taurocholic acid
- TCDCA
Taurochenodeoxycholic acid
- TDCA
Taurodeoxycholic acid
- THDCA
Taurohyodeoxycholic acid
- TLCA
Taurolithocholic acid
- TUDCA
Tauroursodeoxycholic acid
Compliance with ethical standards
Conflict of interest
Axel Schmidt declares that he has no conflict of interest.
Sylvia Joussen declares that she has no conflict of interest.
Ralf Hausmann declares that he has no conflict of interest.
Stefan Gründer declares that he has no conflict of interest.
Dominik Wiemuth declares that he has no conflict of interest.
Ethical approval
Animal care and surgery of frogs were conducted under protocols approved by the State Office for Nature, Environment and Consumer Protection (LANUV) of North Rhine-Westphalia (NRW), Germany, and were performed in accordance with LANUV NRW guidelines.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khakh BS, North RA. Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron. 2012;76:51–69. doi: 10.1016/j.neuron.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnstock G. P2X receptors in sensory neurones. Br J Anaesth. 2000;84:476–488. doi: 10.1093/oxfordjournals.bja.a013473. [DOI] [PubMed] [Google Scholar]
- 3.Ralevic V. P2X receptors in the cardiovascular system and their potential as therapeutic targets in disease. Curr Med Chem. 2015;22:851–865. doi: 10.2174/0929867321666141215094050. [DOI] [PubMed] [Google Scholar]
- 4.Burnstock G. P2X ion channel receptors and inflammation. Purinergic Signal. 2016;12:59–67. doi: 10.1007/s11302-015-9493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawate T, Michel JC, Birdsong WT, Gouaux E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaczmarek-Hajek K, Lorinczi E, Hausmann R, Nicke A. Molecular and functional properties of P2X receptors--recent progress and persisting challenges. Purinergic Signal. 2012;8:375–417. doi: 10.1007/s11302-012-9314-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dal Ben D, Buccioni M, Lambertucci C, Marucci G, Thomas A, Volpini R. Purinergic P2X receptors: structural models and analysis of ligand-target interaction. Eur J Med Chem. 2015;89:561–580. doi: 10.1016/j.ejmech.2014.10.071. [DOI] [PubMed] [Google Scholar]
- 8.Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011;63:641–683. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hattori M, Gouaux E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature. 2012;485:207–212. doi: 10.1038/nature11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansoor SE, Lu W, Oosterheert W, Shekhar M, Tajkhorshid E, Gouaux E. X-ray structures define human P2X(3) receptor gating cycle and antagonist action. Nature. 2016;538:66–71. doi: 10.1038/nature19367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang R, Taly A, Lemoine D, Martz A, Cunrath O, Grutter T. Tightening of the ATP-binding sites induces the opening of P2X receptor channels. EMBO J. 2012;31:2134–2143. doi: 10.1038/emboj.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 13.Hausmann R, Schmalzing G. P2X1 and P2X2 receptors in the central nervous system as possible drug targets. CNS Neurol Disord Drug Targets. 2012;11:675–686. doi: 10.2174/187152712803581128. [DOI] [PubMed] [Google Scholar]
- 14.North RA, Jarvis MF. P2X receptors as drug targets. Mol Pharmacol. 2013;83:759–769. doi: 10.1124/mol.112.083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burnstock G, Kennedy C. P2X receptors in health and disease. Adv Pharmacol. 2011;61:333–372. doi: 10.1016/B978-0-12-385526-8.00011-4. [DOI] [PubMed] [Google Scholar]
- 16.Ren J, Bian X, DeVries M, Schnegelsberg B, Cockayne DA, Ford AP, Galligan JJ. P2X2 subunits contribute to fast synaptic excitation in myenteric neurons of the mouse small intestine. J Physiol. 2003;552:809–821. doi: 10.1113/jphysiol.2003.047944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galligan JJ, Bertrand PP. ATP mediates fast synaptic potentials in enteric neurons. J Neurosci. 1994;14:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang JY. Bile acid metabolism and signaling. Comp Physiol. 2013;3:1191–1212. doi: 10.1002/cphy.c120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 20.Chiang JY. Bile acid regulation of gene expression: roles of nuclear hormone receptors. Endocr Rev. 2002;23:443–463. doi: 10.1210/er.2000-0035. [DOI] [PubMed] [Google Scholar]
- 21.Deutschmann K, Reich M, Klindt C, Droge C, Spomer L, Haussinger D, Keitel V. Bile acid receptors in the biliary tree: TGR5 in physiology and disease. Biochim Biophys Acta. 2018;1864:1319–1325. doi: 10.1016/j.bbadis.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 23.Ward JB, Mroz MS, Keely SJ. The bile acid receptor, TGR5, regulates basal and cholinergic-induced secretory responses in rat colon. Neurogastroenterol Motil. 2013;25:708–711. doi: 10.1111/nmo.12148. [DOI] [PubMed] [Google Scholar]
- 24.Keitel V, Ullmer C, Haussinger D. The membrane-bound bile acid receptor TGR5 (Gpbar-1) is localized in the primary cilium of cholangiocytes. Biol Chem. 2010;391:785–789. doi: 10.1515/bc.2010.077. [DOI] [PubMed] [Google Scholar]
- 25.Zheng X, Chen T, Zhao A, Wang X, Xie G, Huang F, Liu J, Zhao Q, Wang S, Wang C, Zhou M, Panee J, He Z, Jia W. The brain metabolome of male rats across the lifespan. Sci Rep. 2016;6:24125. doi: 10.1038/srep24125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mano N, Goto T, Uchida M, Nishimura K, Ando M, Kobayashi N, Goto J. Presence of protein-bound unconjugated bile acids in the cytoplasmic fraction of rat brain. J Lipid Res. 2004;45:295–300. doi: 10.1194/jlr.M300369-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.McMillin M, Frampton G, Quinn M, Ashfaq S, de los Santos M, 3rd, Grant S, DeMorrow S. Bile acid signaling is involved in the neurological decline in a murine model of acute liver failure. Am J Pathol. 2016;186:312–323. doi: 10.1016/j.ajpath.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiemuth D, Sahin H, Lefevre CM, Wasmuth HE, Grunder S. Strong activation of bile acid-sensitive ion channel (BASIC) by ursodeoxycholic acid. Channels. 2013;7:38–42. doi: 10.4161/chan.22406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiemuth D, Sahin H, Falkenburger BH, Lefevre CM, Wasmuth HE, Grunder S. BASIC--a bile acid-sensitive ion channel highly expressed in bile ducts. FASEB J. 2012;26:4122–4130. doi: 10.1096/fj.12-207043. [DOI] [PubMed] [Google Scholar]
- 30.Wiemuth D, Assmann M, Grunder S. The bile acid-sensitive ion channel (BASIC), the ignored cousin of ASICs and ENaC. Channels. 2014;8:29–34. doi: 10.4161/chan.27493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiemuth D, Lefevre CM, Heidtmann H, Gründer S. Bile acids increase the activity of the epithelial Na+ channel. Pflugers Arch - Eur J Physiol. 2014;466:1725–1733. doi: 10.1007/s00424-013-1403-0. [DOI] [PubMed] [Google Scholar]
- 32.Ilyaskin AV, Diakov A, Korbmacher C, Haerteis S (2017) Bile acids potentiate proton-activated currents in Xenopus laevis oocytes expressing human acid-sensing ion channel (ASIC1a). Phys Rep 5 10.14814/phy2.13132 [DOI] [PMC free article] [PubMed]
- 33.Ilyaskin AV, Diakov A, Korbmacher C, Haerteis S. Activation of the human epithelial sodium channel (ENaC) by bile acids involves the degenerin site. J Biol Chem. 2016;291:19835–19847. doi: 10.1074/jbc.M116.726471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bässler EL, Ngo-Anh TJ, Geisler HS, Ruppersberg JP, Gründer S. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J Biol Chem. 2001;276:33782–33787. doi: 10.1074/jbc.M104030200. [DOI] [PubMed] [Google Scholar]
- 35.Hausmann R, Rettinger J, Gerevich Z, Meis S, Kassack MU, Illes P, Lambrecht G, Schmalzing G. The suramin analog 4,4′,4″,4″′-(carbonylbis(imino-5,1,3-benzenetriylbis (carbonylimino)))tetra-kis-benzenesulfonic acid (NF110) potently blocks P2X3 receptors: subtype selectivity is determined by location of sulfonic acid groups. Mol Pharmacol. 2006;69:2058–2067. doi: 10.1124/mol.106.022665. [DOI] [PubMed] [Google Scholar]
- 36.Schneider M, Prudic K, Pippel A, Klapperstuck M, Braam U, Muller CE, Schmalzing G, Markwardt F. Interaction of purinergic P2X4 and P2X7 receptor subunits. Front Pharmacol. 2017;8:860. doi: 10.3389/fphar.2017.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiemuth D, Gründer S. A single amino acid tunes Ca2+ inhibition of brain liver intestine Na+ channel (BLINaC) J Biol Chem. 2010;285:30404–30410. doi: 10.1074/jbc.M110.153064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- 39.Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. Bile acids as regulatory molecules. J Lipid Res. 2009;50:1509–1520. doi: 10.1194/jlr.R900007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mano N, Sato Y, Nagata M, Goto T, Goto J. Bioconversion of 3beta-hydroxy-5-cholenoic acid into chenodeoxycholic acid by rat brain enzyme systems. J Lipid Res. 2004;45:1741–1748. doi: 10.1194/jlr.M400157-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Huang C, Wang J, Hu W, Wang C, Lu X, Tong L, Wu F, Zhang W. Identification of functional farnesoid X receptors in brain neurons. FEBS Lett. 2016;590:3233–3242. doi: 10.1002/1873-3468.12373. [DOI] [PubMed] [Google Scholar]
- 42.Keitel V, Gorg B, Bidmon HJ, Zemtsova I, Spomer L, Zilles K, Haussinger D. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia. 2010;58:1794–1805. doi: 10.1002/glia.21049. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt A, Alsop RJ, Rimal R, Lenzig P, Joussen S, Gervasi NN, Khondker A, Grunder S, Rheinstadter MC, Wiemuth D. Modulation of DEG/ENaCs by amphiphiles suggests sensitivity to membrane alterations. Biophys J. 2018;114:1321–1335. doi: 10.1016/j.bpj.2018.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trujillo CA, Nery AA, Martins AH, Majumder P, Gonzalez FA, Ulrich H. Inhibition mechanism of the recombinant rat P2X(2) receptor in glial cells by suramin and TNP-ATP. Biochemistry. 2006;45:224–233. doi: 10.1021/bi051517w. [DOI] [PubMed] [Google Scholar]
- 45.El-Ajouz S, Ray D, Allsopp RC, Evans RJ. Molecular basis of selective antagonism of the P2X1 receptor for ATP by NF449 and suramin: contribution of basic amino acids in the cysteine-rich loop. Br J Pharmacol. 2012;165:390–400. doi: 10.1111/j.1476-5381.2011.01534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lenzig P, Wirtz M, Wiemuth D (2018) Comparative electrophysiological analysis of the bile acid-sensitive ion channel (BASIC) from different species suggests similar physiological functions. Arch Eur J Physiol. 471:329–336. 10.1007/s00424-018-2223-z [DOI] [PubMed]
- 47.Doctor RB, Matzakos T, McWilliams R, Johnson S, Feranchak AP, Fitz JG. Purinergic regulation of cholangiocyte secretion: identification of a novel role for P2X receptors. Am J Physiol Gastrointest Liver Physiol. 2005;288:G779–G786. doi: 10.1152/ajpgi.00325.2004. [DOI] [PubMed] [Google Scholar]
- 48.Emmett DS, Feranchak A, Kilic G, Puljak L, Miller B, Dolovcak S, McWilliams R, Doctor RB, Fitz JG. Characterization of ionotrophic purinergic receptors in hepatocytes. Hepatology. 2008;47:698–705. doi: 10.1002/hep.22035. [DOI] [PubMed] [Google Scholar]
- 49.Novak I. Purinergic receptors in the endocrine and exocrine pancreas. Purinergic Signal. 2008;4:237–253. doi: 10.1007/s11302-007-9087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]