Abstract
Aging is one of the biggest risk factors for the major prevalent diseases such as cardiovascular diseases, neurodegeneration and cancer, but due to the complex and multifactorial nature of the aging process, the molecular mechanisms underlying age-related diseases are not yet fully understood. Research has been intensive in the last years aiming to characterize the pathophysiology of aging and develop therapies to fight age-related diseases. In this context advanced glycation end products (AGEs) have received attention. AGEs, when accumulated in tissues, significantly increase the level of inflammation in the body which has long been associated with the development of cancer. Here we discuss the classical settings promoting AGE formation, as well as reduction strategies, occurrence and relevance of AGEs in cancer tissues and the role of AGE-interaction with the receptor for advanced glycation end products (RAGE) in cancer initiation and progression.
Keywords: Advanced glycation end products, cancer, aging, inflammation, RAGE, cardiovascular diseases
1. INTRODUCTION
Aging is a complex and multifactorial process characterized by a progressive accumulation of damage to an organism over time and is considered as one of the biggest single risk factors for the development of a multitude of diseases such as type-II diabetes [1, 2], neurodegenerative diseases [3, 4], and cancer [5, 6]. Furthermore, it is widely accepted that these age-related diseases result from a combination of various endogenous, genetic, lifestyle, and environmental factors [7-9]. Due to the complex and multifactorial nature of aging, the molecular mechanisms underlying the initiation and progression of age-related diseases are not yet fully understood. Research has been intensive in the last years aiming to characterize the pathophysiology of aging and developing therapies to fight age-related diseases. In this context advanced glycation end products (AGEs) have received attention [10-12]. AGEs are a group of heterogeneous compounds formed via non-enzymatic glycation/ glycoxidation of reducing sugars with free amino groups of proteins. Not only proteins but also lipids and nucleic acids exposed to reducing sugars could suffer glycation or glycoxidation reactions. Formation of AGEs occurs exogenously in food, especially by thermal processing, but also endogenously in the body.
In vivo glycation of proteins occurs under physiological conditions and represents a type of post-translational modification taking place slowly but continuously throughout the life span, promoting AGE accumulation during aging. Thus, to some extent the accumulation of AGEs is inevitably linked to aging and age-related accumulation of AGEs was shown to exist in human cartilage, skin collagen and pericardial fluid [13-15]. Increased protein glycation is also associated with the pathogenesis of several age-related and chronic inflammatory diseases such as cardiovascular diseases [13], Alzheimer's disease [16], stroke [17], as well as the general decline in health associated with old age. Under hyperglycemic conditions,
such as that found in diabetes, AGE accumulation is accelerated. Hyperglycemia is known to be responsible for high rates of protein glycation and the gradual build-up of AGEs is furthermore involved in the pathogenesis and development of long-term diabetic complications such as retinopathy, nephropathy, neuropathy, and cardiomyopathy [18].
Protein glycation interferes with normal protein function by disrupting molecular conformation, or altering enzyme or receptor functionality and activity. The effects of AGEs are exerted on the one hand by direct damage to protein structures and extracellular matrix modification, and on the other hand by binding the receptor for advanced glycation end products (RAGE). A multitude of signaling cascades are activated via RAGE causing multiple pathological effects associated with oxidative stress and inflammation [19].
Since it is generally accepted that chronic inflammation, oxidative stress, and cancer are intrinsically linked [20], a potential contribution of AGEs to malignant cell transformation and the development and progression of cancer also seems to be conclusive. In addition, cancer cells are generally characterized by an inclination towards anaerobic metabolism of glucose, a phenotype that was first noted by Otto Warburg, called Warburg effect [21]. To meet the energy requirements and to compensate for this inefficient energy supply, tumors are characterized by an increased glucose uptake and a high rate of glycolysis. Consequently, as a by-product of enhanced glycolytic flux, this could lead to an elevated level of glycation and increased formation of AGEs. In this review, we tried to summarize the current knowledge on AGE formation as well as reduction strategies, occurrence and relevance in cancer tissues, the role of RAGE in cancer initiation and progression and the potential of AGEs as cancer biomarkers.
2. ENDOGENOUS GLYCATION AND EXOGENOUS SOURCES OF AGES/ALES
AGEs are formed endogenously via complex heterogeneous chemical reactions. The underlying mechanism is the so-called Maillard reaction, occurring at different rates depending on temperature, pH value and the respective sugar reactant. Most commonly, the formation of highly reactive α-dicarbonyls (e.g. 3-deoxyglucosone (3-DG), methylglyoxal (MGO) and glyoxal (GO)) initiates the formation of AGEs [22, 23]. These compounds arise from different pathways including the non-oxidative cleavage of Amadori compounds [24], oxidative fission of the Schiff base (Namiki pathway) [25], hexose autoxidation (Wolff pathway) [26], as by-product of the glycolytic or polyol pathway [27] as well as ketone body catabolism [28], or from lipid oxidation [29]. The latter pathway, in addition, initiates the formation of short chain reactive carbonyl species (RCS), i.e. α,β-unsaturated aldehydes, dialdehydes, keto-aldehydes, and phospholipid-based RCS which react with free amino groups of proteins to form advanced lipoxidation end products (ALEs) or in case of dialdehydes such as glyoxal or keto-aldehydes such as methylglyoxal so-called EAGLEs (either advanced glycation or lipoxidation end products) [22]. AGEs and ALEs precursors are prone to react with nucleophiles; moreover, they readily react with free amino moieties of proteins and peptides as well as nucleic acids and phospholipids. Lysine and arginine residues are the most susceptible glycation sites resulting in covalent modifications and/or cross-linked proteins with irreversibly modified biological activity. In addition, cysteine, tryptophan and histidine residues were shown to be favorable glycation sites [30]. The formed products can be divided into fluorescent and non-fluorescent, as well as cross-linking and non-cross-linking AGEs, with AGEs exhibiting both properties, while others show none of these characteristics. Prominent AGEs discussed to adversely affect the progression of hyperglycemic, inflammatory diseases and cancer are 3-DG derived pyrraline and pentosidine, MGO-derived Nε-(carboxylethyl)-l-lysine and Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-l-ornithine (MG-H1), GO-derived Nε-(carboxylmethyl)-l-lysine (CML) and S-carboxymethylcysteine as well as the cross-linking AGEs glyoxal-lysine dimer (GOLD) and methylglyoxal-lysine dimer (MOLD). It has been shown that the level of these compounds is high in plasma and tissues during aging, in various cancers as well as under hyperglycemic conditions such as diabetes mellitus [31-35]. ALE formation has been shown to be dependent on the oxidative status of individuals and is therefore connected to chronic inflammation and oxidative stress [36]. Furthermore, lipid oxidation might be initiated by glycosylation of phospholipids, indicating that AGE formation can trigger the formation of ALEs [29].
In combination, these pathways might increase the severity of inflammatory diseases, and the initiation and progression of malignant tumors. Up to now the quantification of AGEs in tissues and serum of humans is most commonly assessed by ELISA or immunohistochemical staining [35, 37]. However, some studies used liquid chromatography methods, which have been shown to be more reliable in terms of validity at least in the AGE analytics of food [38, 39]. Human studies show that baseline levels of MGO and GO range from upper nanomolar to lower micromolar, depending on the analytical set-up [40-43]. The discrepancies among the different baseline levels of e.g. MGO concentrations were investigated by Chaplen et al. showing that different analytic approaches give significantly different results [44]. To the best of our knowledge, so far no routine analytical method for serum and tissue levels of AGEs precursors exists. This lack of a reliable and reproducible method is even more pronounced for AGEs and ALEs levels. However, all studies have reported that elevated serum and tissue levels of AGEs and their precursors can be found in patients suffering from hyperglycemia, diabetes mellitus and uremia as well as individuals undergoing peritoneal dialysis [32, 45-48], suggesting that the endogenous pool of AGEs is inherently connected to the health status of an individual.
Besides being endogenously formed, AGEs and ALEs occur as a result of food processing. The dietary burden of AGEs and ALEs contributes to the accumulation of these compounds in vivo. Several studies show that every processed food contains AGEs in a certain amount and that the AGEs content of food relates to the processing conditions [38, 49, 50]. Most commonly AGEs concentrations are determined via monoclonal antibodies e.g. for CML contents [49, 51]. However, when analyzing the same food by means of chromatographic methods significant differences in concentrations are observed [38, 39, 49, 51]. Generally, high temperature processing of food leads to high amounts of AGEs while moderate processing like steam blanching limits the formation of AGEs. Raw products contain only negligible AGEs concentrations. The highest AGE concentrations are found in cereals, bakery products and processed meat. Fruits, vegetables, and high-fat or high moisture food like butter/ oil and yoghurt, respectively, provide the lowest concentrations. The overall daily intake of food-derived AGEs from a conventional diet is estimated to be between 25-75 mg [45]. In addition, smoking was shown to significantly elevate AGEs in vivo and should not be neglected when estimating an individual’s AGEs burden. Regarding the heterogeneity of the chemical structures of AGEs, absorption rates differ significantly, e.g. Urribarri and co-workers found that about 30% of ingested AGEs accumulate in the human body [46], while Koschinsky et al. concluded that up to 10% of food-borne AGEs (mainly CML) might be absorbed and undergo metabolic transition, with only 30% of the ingested AGEs being retrievable in the urine of healthy patients [52, 53]. In contrast, other studies found that 60-80% of food-borne pyrraline and pentosidine were absorbed, with 50-60% of the free consumed AGEs being excreted via the kidney [54, 55]. The urinary excretion rates might further be influenced by the health status of the individual being lowered to less than 5% in patients with diabetes mellitus or renal failure [37, 52]. The formation and accumulation of AGEs and ALEs in vivo seem to be inherently connected to lifestyle choices (e.g. diet and smoking), and the individual’s oxidative status and metabolism.
3. INFLAMMATION, RAGE AND CANCER
In contrast to several receptors such as scavenger receptors class A and B (SR-Ai, SRAII, CD36 and SR-BI)[19] described to be responsible to detoxify or remove AGEs from circulation or tissues, RAGE is a signal transduction receptor for AGEs, mediating diverse cellular responses. RAGE is a multiligand single transmembrane receptor and a member of the immunoglobulin superfamily of cell surface molecules [56], binding in addition to AGEs several other molecules such as β-amyloid peptides and β-sheet fibrils, high-mobility group box 1 (HMGB1), several members of the S100 protein family, and prions [57]. By binding to the receptor these molecules stimulate signal transduction via a multitude of pathways including Ras-extracellular signal-regulating kinase 1/2 [58], CDC42/Rac [59], p38 mitogen-activating protein kinase [60], NADPH-oxidase [61], and JAK1/2 [62]. Downstream signaling activates members of the STAT (signal transducers and activators of transcription) family [63], AP-1 (activator protein-1) [57] and NFκB [64], a key target of RAGE signaling. NFκB is a transcription factor for a large group of genes which is involved in several different pathways, transducing a multitude of pro- or antiapoptotic and inflammatory signals. The activation of NF-κB induces the expression of adhesion molecules, growth factors (e.g. transforming growth factor-β), and proinflammatory cytokines (such as IL-6 and TNF-α) [65]. In addition, the interaction of AGEs with RAGE activates NADPH oxidase, leading to increased intracellular oxidative stress. This sudden reactive oxygen species (ROS) increase will again activate NF-κB [61, 66]. Furthermore, it is important to note that RAGE also exhibits a functional NF-κB binding site and has been shown to be a direct target gene of NF-κB signaling. Therefore NF-κB activation increases RAGE expression, creating a positive feedback cycle, able to convert a transient proinflammatory reaction into a chronic pathophysiological state [67, 68]. Thus it is not surprising that the AGE-RAGE interaction is implicated in aging and considered as a major contributor to the initiation, progression and clinical severity of diseases by triggering oxidative stress and chronic inflammatory responses Fig. (1). Numerous studies have shown a strong contribution of chronic inflammation to tumor development [69, 70]. The generation of an inflammatory, pro-tumorigenic microenvironment largely depends on the activation of several transcription factors, mainly NF-κB, regulating the expression of tumor promoting cytokines, but also survival genes such as Bcl-XL. These soluble factors then recruit and activate immune cells of myeloid and lymphoid origin and trigger signaling pathways leading to the production of a large number of pro-inflammatory mediators in a positive feed-forward manner [53]. In the initial phase, inflammatory mediators such as cytokines and reactive oxygen and nitrogen species (ROS/RNS) derived from infiltrating immune cells induce epigenetic changes in pre-malignant lesions and silence tumor suppressor genes [71]. During tumor promotion, cytokines and chemokines are secreted from immune cells acting as survival and proliferation factors for malignant cells. The onset of angiogenesis is critical for an adequate supply of tumor cells with oxygen, nutrients, growth- and survival factors and the removal of waste products [72]. During progression and metastasis, both tumor and immune cells produce cytokines and chemokines leading to an increase in cell survival, motility, and invasiveness [73]. Through its ability to activate NF-κB, RAGE signaling and up-regulation provides a critical link between the accumulation of AGEs and cancer, and has been implicated in the development of many cancers [74-78]. The occurrence of AGEs (CML and argpyrimidine) in human tumors was first shown in squamous cell carcinomas of larynx, adenocarcinomas of the breast, adenocarcinomas of the colon, and leiomyosarcomas by immunohistochemical staining, with a great variation between different types of tumors [35]. AGE treatment of immortalized prostate [79] and breast [80] cancer cell lines showed promotion of cell growth, migration and invasion. A comparison of fresh-frozen breast cancer samples and corresponding noncancerous tissue samples indicate a high expression of the RAGE-encoding gene in cancerous tissues [81]. RAGE expression was up-regulated in high-stage, metastatic, and aggressive breast cancer tumors, suggesting that RAGE may contribute to the malignant potential of tumors. RAGE gene overexpression was also shown in ovarian cancer tissue compared to adjacent noncancerous tissue. A significant association between RAGE expression and tumor size, depth of stromal invasion, lymphovascular invasion and stage of cancer was observed [82]. RAGE is overexpressed in human pancreatic tumors but not in adjacent normal ducts. Furthermore, RAGE is a critical promoter in the transition of premalignant epithelial precursors to invasive pancreatic cancer [83]. Longstanding type II diabetes is an established risk factor for several cancers, especially pancreatic cancer and the mechanistic link might be the accumulation of AGEs, especially under poor glycemic control [84, 85]. In mice prone to pancreatic cancer, exogenous AGE administration (CML) induced RAGE upregulation in pancreatic intraepithelial neoplasias and markedly accelerated progression to invasive pancreatic cancer [86]. Overall, there is accumulating evidence that AGEs, via interaction with RAGE, could play a role in various types of cancer and contribute to tumor development, migration and metastasis. However, further studies are needed to shed more light on the physiological relevance of AGE-RAGE signaling in cancer biology.
Fig. (1).
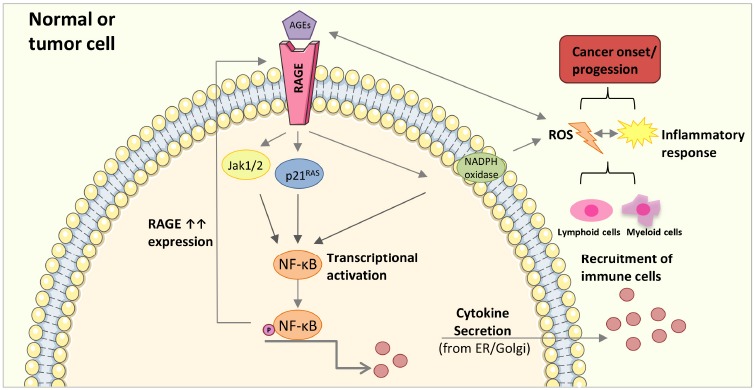
Simplified AGE-mediated activation of an inflammatory response leading to cancer onset and/or progession. AGE-RAGE interaction triggers the activation of transcription factors transducing inflammatory signals, mainly NF-κB, leading to an increased cytokine secretion, followed by lymphoid and myeloid immune cells recruitment into the tumor microenvironment, elevated ROS production, and an inflammatory response. NF-κB activation also increases RAGE expression, creating a positive feedback cycle. In addition, reactive intermediates generated during AGE formation can directly increase ROS production promoting the immune response and the formation of further AGEs creating a chronic inflammatory state. Membrane and RAGE receptor illustration were used from Servier Medical Art under creative commons license 3.0 (https://smart.servier.com/).
4. STRATEGIES FOR AGES REDUCTION
Due to the adverse effects of AGEs and the direct link to a multitude of diseases including cancer, strategies aiming to prevent or reduce AGEs accumulation could provide clear health benefits. Strategies to reduce AGEs/ALEs formation in vivo Fig. (2) include metal chelators and antioxidants able to interfere with oxidative pathways such as Namiki and Wolff pathway as well as radical-based pathways such as lipid oxidation. A diet rich in antioxidants (e.g. polyphenols and α-tocopherol as found in the Mediterranean diet) was shown to positively impact on oxidative stress and inflammatory diseases by decreasing AGE formation in food and in vivo [87, 88]. The connection between the consumption of polyphenol-rich diets and protection against cancer as well as other chronic diseases was profoundly reviewed by Vauzour et al. [89].
Fig. (2).
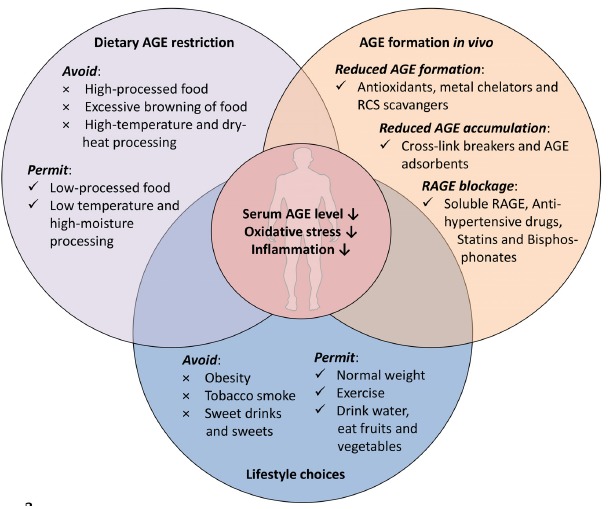
The paradigm of AGE restriction, inhibition of AGE formation and accumulation.
In addition, several metal-chelators such as diethylenetria-minepentaacidic acid (DETAPAC), desferrioxamine (DFO) and triethylenetetraamine (TETA) might inhibit the formation of AGES in vitro and in vivo as conclusively reviewed by Aldini et al. [90]. Furthermore, RCS quenching compounds reduce AGE/ALEs formation and accumulation by scavenging the RCS before they react with proteins. Compounds that were shown to possess RCS scavenging activity include thiol-containing compounds (such as glutathione, N-acetylcysteine and cysteamine etc.), guanidine/hydra-zine derivatives (such as aminoguanidine, β-resorcylidene aminoguanidine and ALT-946 (N-(2-acetamidoethyl)-hydrazine-carboxi-midamide hydrochloride)) and heterocycle-based compounds (such as pyridoxamine, carnosine and polyphenols (e.g. theaflavin and genistein)) as already reviewed in detail [89-91]. Other strategies to reduce AGE accumulation and RAGE activation in vivo comprise cross-link breakers (e.g. Alagebrium (ALT-711)[92]), AGE adsorbents (AST-120 (Kremezin) and galectin-3) [93, 94], anti-hypertensive drugs, statins and bisphosphonates [95], respectively. All of the mentioned pharmaceuticals, however, may have individual drawbacks in humans and are not yet established for clinical applications.
The most common way to reduce AGE accumulation is the dietary restriction of AGE consumption [34, 37, 51]. Generally, food preparation and processing determines the rate of dietary AGE formation. Therefore, lower temperature, shorter cooking times and lowering the pH lead to a decreased AGE formation rate in food. In addition, the water content is important for AGE formation; therefore, changing the cooking style from frying or grilling to boiling or steam-blanching will significantly reduce the AGE contents of food. Finally, avoiding obesity (by exercise) and tobacco smoke might serve as treatments to lower AGE accumulation in the human body.
5. AGES AS CANCER BIOMARKERS
The connection between elevated AGE accumulation in vivo and development and disparity of cancer is highlighted by numerous recent studies and reviews all emphasizing the elevated susceptibility to cancer initiation and progression by an increase in AGE-RAGE-dependent stress response, resulting in increased oxidative stress and chronic inflammation. While, indeed, cancer patients show overall increased AGE levels in their plasma as well as in tumor tissue [96-99], using specific AGE levels of tissues as biomarkers for the development of specific cancers is questionable. This is due to the systemic impact of AGE accumulation on several other metabolic disorders and chronic diseases such as type-II diabetes. Increased AGE levels are, therefore, intrinsically connected to the lifestyle of an individual, and can be considered as an individual’s oxidative status and risk for the development of metabolic disorders. The glycoxidation of specific defense and DNA-repair enzymes might lead to an elevated risk of cancer initiation and progression, but also contributes to diabetic complications [100]. Overall, the development of metabolic disorders is facilitated by increased AGE levels in vivo and clearly highlights the inter-connection among these diseases in the human body. Thus, restriction of AGE intake and accumulation might serve as a tool to counteract the progression and severity of cancer as well as several diseases connected to Western-style diets and lifestyle.
Also, it has to be mentioned that there are limitations regarding the routine assessment of specific AGEs. Accessibility to blood/plasma for repeated measurements of AGEs is much easier than tissue-requiring biopsies, but so far plasma AGE assays have not been shown to be directly related to tissue AGE content.
On the other hand, and as described above, RAGE is overexpressed in multiple cancers. In addition, the secreted isoform of RAGE, termed soluble RAGE (sRAGE) was found to be released from cells to bind the ligands of RAGE, and to be capable of preventing the adverse effects of RAGE-signaling, therefore representing a naturally occurring competitive inhibitor of RAGE-mediated events. Treatment with exogenous sRAGE might be able to block deleterious effects caused by RAGE ligands. Furthermore, sRAGE levels may provide a novel biomarker for tracking inflammatory diseases including cancer, potentially helping to assess the severity of diseases, but also the response to therapeutic intervention [101].
In summary, AGE/sRAGE levels might serve as a factor to assess the individual’s risk to develop cancer associated with early recognition. But currently, there is no evidence that measurement of both AGEs and sRAGE will become accepted and applicable for clinical use.
CONCLUSION AND PERSPECTIVES
There is ample evidence that AGEs play an important role in cancer development and progression. Many studies proposed that the cancer-promoting effect of AGEs is predominantly mediated by AGE-RAGE interaction leading to increased ROS formation and inflammatory processes. Therapeutic inhibition of AGEs, such as cross-link breakage, has yet to yield clinically viable and applicable therapeutics. The development of reliable analytical methods to detect plasma and tissue AGE levels would facilitate the applicability of novel medications. Currently, the simple application of dietary restriction and raising dietary awareness (e.g. polyphenol-rich, low processed food) may be useful in restricting AGEs and might have a potential for both cancer prevention and therapy. Education on the consequences of lifestyle choices, e.g. smoking or exercise, and home cooking behavior represents affordable prevention of AGE formation and accumulation, both for the individual and from a societal perspective. Following the paradigm of AGE restriction might not only be useful for cancer prevention but to counteract the development of a multitude of diseases connected to western lifestyle and aging.
ACKNOWLEDGEMENTS
This work was supported by a grant from the German Ministry of Education and Research (BMBF) and the State of Brandenburg (DZD grant 82DZD00302).
LIST OF ABBREVIATIONS
- AGEs
Advanced glycation end products
- ALEs
Advanced lipidoxidation end products
- CML
Nε-(carboxylmethyl)-l-lysine
- DETAPAC
Diethylenetriaminepentaacidic acid
- DFO
Desferrioxamine
- 3-DG
3-Deoxyglucosone
- GO
Glyoxal
- GOLD
Glyoxal-lysine dimer
- HMGB1
High-mobility group box 1
- MG-H1
Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-l-ornithine
- MGO
Methylglyoxal
- MOLD
Methylglyoxal-lysine dimer
- RAGE
Receptor for advanced glycation end products
- RCS
Reactive carbonyl species
- TETA
Triethylenetetraamine
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Andres R, Pozefsky T, Swerdloff RS, Tobin JD. Effect of aging on carbohydrate metabolism. Adv Metab Disord. 1970;1(Suppl. 1):1–349. doi: 10.1016/b978-0-12-027361-4.50041-7. [DOI] [PubMed] [Google Scholar]
- 2.Singh R., Barden A., Mori T., Beilin L. Advanced glycation end-products: A review. Diabetologia. 2001;44(2):129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 3.Morimoto R.I. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22(11):1427–1438. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foltynie T., Brayne C.E., Robbins T.W., Barker R.A. The cognitive ability of an incident cohort of Parkinson’s patients in the UK. The CamPaIGN study. Brain. 2004;127(Pt 3):550–560. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- 5.Aunan J.R., Cho W.C., Søreide K. The Biology of Aging and Cancer: A Brief Overview of Shared and Divergent Molecular Hallmarks. Aging Dis. 2017;8(5):628–642. doi: 10.14336/AD.2017.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 7.Steves C.J., Spector T.D., Jackson S.H. Ageing, genes, environment and epigenetics: what twin studies tell us now, and in the future. Age Ageing. 2012;41(5):581–586. doi: 10.1093/ageing/afs097. [DOI] [PubMed] [Google Scholar]
- 8.Strehler B.L. Environmental factors in aging and mortality. Environ. Res. 1967;1(1):46–88. doi: 10.1016/0013-9351(67)90005-9. [DOI] [PubMed] [Google Scholar]
- 9.J vB Hjelmborg, I Iachine, A Skytthe. Genetic influence on human lifespan and longevity. Hum Genet. 2006;119:312–21. doi: 10.1007/s00439-006-0144-y. [DOI] [PubMed] [Google Scholar]
- 10.Simm A., Müller B., Nass N., et al. Protein glycation - Between tissue aging and protection. Exp. Gerontol. 2015;68:71–75. doi: 10.1016/j.exger.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Höhn A., König J., Grune T. Protein oxidation in aging and the removal of oxidized proteins. J. Proteomics. 2013;92:132–159. doi: 10.1016/j.jprot.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Baynes J.W. The Maillard hypothesis on aging: time to focus on DNA. Ann. N. Y. Acad. Sci. 2002;959:360–367. doi: 10.1111/j.1749-6632.2002.tb02107.x. [DOI] [PubMed] [Google Scholar]
- 13.Simm A., Wagner J., Gursinsky T., et al. Advanced glycation endproducts: A biomarker for age as an outcome predictor after cardiac surgery? Exp. Gerontol. 2007;42(7):668–675. doi: 10.1016/j.exger.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Dyer D.G., Dunn J.A., Thorpe S.R., Lyons T.J., McCance D.R., Baynes J.W. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. Ann. N. Y. Acad. Sci. 1992;663:421–422. doi: 10.1111/j.1749-6632.1992.tb38687.x. [DOI] [PubMed] [Google Scholar]
- 15.Verzijl N., DeGroot J., Oldehinkel E., et al. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem. J. 2000;350(Pt 2):381–387. [PMC free article] [PubMed] [Google Scholar]
- 16.Perrone L., Grant W.B. Observational and ecological studies of dietary advanced glycation end products in national diets and Alzheimer’s disease incidence and prevalence. J. Alzheimers Dis. 2015;45(3):965–979. doi: 10.3233/JAD-140720. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerman G.A., Meistrell M., III, Bloom O., et al. Neurotoxicity of advanced glycation endproducts during focal stroke and neuroprotective effects of aminoguanidine. Proc. Natl. Acad. Sci. USA. 1995;92(9):3744–3748. doi: 10.1073/pnas.92.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamagishi S., Ueda S., Okuda S. Food-derived advanced glycation end products (AGEs): A novel therapeutic target for various disorders. Curr. Pharm. Des. 2007;13(27):2832–2836. doi: 10.2174/138161207781757051. [DOI] [PubMed] [Google Scholar]
- 19.Ott C., Jacobs K., Haucke E., Navarrete Santos A., Grune T., Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–429. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zinger A., Cho W.C., Ben-Yehuda A. Cancer and Aging - the Inflammatory Connection. Aging Dis. 2017;8(5):611–627. doi: 10.14336/AD.2016.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warburg O. The metabolism of carcinoma cells. J. Cancer Res. 1925;9(1):148–163. [Google Scholar]
- 22.Vistoli G., De Maddis D., Cipak A., Zarkovic N., Carini M., Aldini G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013;47(Suppl. 1):3–27. doi: 10.3109/10715762.2013.815348. [DOI] [PubMed] [Google Scholar]
- 23.Henning C., Glomb M.A. Pathways of the Maillard reaction under physiological conditions. Glycoconj. J. 2016;33(4):499–512. doi: 10.1007/s10719-016-9694-y. [DOI] [PubMed] [Google Scholar]
- 24.Hodge J.E., Rist C.E. The Amadori Rearrangement under New Conditions and its Significance for Non-enzymatic Browning Reactions2. J. Am. Chem. Soc. 1953;75:316–322. [Google Scholar]
- 25.Namiki M. Chemistry of Maillard reactions: recent studies on the browning reaction mechanism and the development of antioxidants and mutagens. Elsevier; 1988. pp. 115–184. [DOI] [PubMed] [Google Scholar]
- 26.Wolff S.P., Dean R.T. Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochem. J. 1987;245(1):243–250. doi: 10.1042/bj2450243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamada Y., Araki N., Koh N., Nakamura J., Horiuchi S., Hotta N. Rapid formation of advanced glycation end products by intermediate metabolites of glycolytic pathway and polyol pathway. Biochem. Biophys. Res. Commun. 1996;228(2):539–543. doi: 10.1006/bbrc.1996.1695. [DOI] [PubMed] [Google Scholar]
- 28.Thornalley P.J. Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification--a role in pathogenesis and antiproliferative chemotherapy. Gen. Pharmacol. 1996;27(4):565–573. doi: 10.1016/0306-3623(95)02054-3. [DOI] [PubMed] [Google Scholar]
- 29.Bucala R., Makita Z., Koschinsky T., Cerami A., Vlassara H. Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc. Natl. Acad. Sci. USA. 1993;90(14):6434–6438. doi: 10.1073/pnas.90.14.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Münch G., Schicktanz D., Behme A., et al. Amino acid specificity of glycation and protein-AGE crosslinking reactivities determined with a dipeptide SPOT library. Nat. Biotechnol. 1999;17(10):1006–1010. doi: 10.1038/13704. [DOI] [PubMed] [Google Scholar]
- 31.Luevano-Contreras C., Chapman-Novakofski K. Dietary advanced glycation end products and aging. Nutrients. 2010;2(12):1247–1265. doi: 10.3390/nu2121247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uribarri J., Cai W., Peppa M., et al. Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62(4):427–433. doi: 10.1093/gerona/62.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vlassara H., Cai W., Crandall J., et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc. Natl. Acad. Sci. USA. 2002;99(24):15596–15601. doi: 10.1073/pnas.242407999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vlassara H., Cai W., Tripp E., et al. Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: A randomised controlled trial. Diabetologia. 2016;59(10):2181–2192. doi: 10.1007/s00125-016-4053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Heijst J.W., Niessen H.W., Hoekman K., Schalkwijk C.G. Advanced glycation end products in human cancer tissues: detection of Nepsilon-(carboxymethyl)lysine and argpyrimidine. Ann. N. Y. Acad. Sci. 2005;1043:725–733. doi: 10.1196/annals.1333.084. [DOI] [PubMed] [Google Scholar]
- 36.Negre-Salvayre A., Coatrieux C., Ingueneau C., Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol. 2008;153(1):6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uribarri J., Peppa M., Cai W., et al. Restriction of dietary glycotoxins reduces excessive advanced glycation end products in renal failure patients. J. Am. Soc. Nephrol. 2003;14(3):728–731. doi: 10.1097/01.asn.0000051593.41395.b9. [DOI] [PubMed] [Google Scholar]
- 38.Scheijen J.L.J.M., Clevers E., Engelen L., et al. Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database. Food Chem. 2016;190:1145–1150. doi: 10.1016/j.foodchem.2015.06.049. [DOI] [PubMed] [Google Scholar]
- 39.Niquet-Léridon C., Tessier F.J. Quantification of Nε-carboxymethyl-lysine in selected chocolate-flavoured drink mixes using high-performance liquid chromatography–linear ion trap tandem mass spectrometry. Food Chem. 2011;126:655–663. [Google Scholar]
- 40.Rabbani N., Thornalley P.J. Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease. Biochem. Biophys. Res. Commun. 2015;458(2):221–226. doi: 10.1016/j.bbrc.2015.01.140. [DOI] [PubMed] [Google Scholar]
- 41.Dhar A., Desai K., Liu J., Wu L. Methylglyoxal, protein binding and biological samples: Are we getting the true measure? J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009;877(11-12):1093–1100. doi: 10.1016/j.jchromb.2009.02.055. [DOI] [PubMed] [Google Scholar]
- 42.Lapolla A., Reitano R., Seraglia R., Sartore G., Ragazzi E., Traldi P. Evaluation of advanced glycation end products and carbonyl compounds in patients with different conditions of oxidative stress. Mol. Nutr. Food Res. 2005;49(7):685–690. doi: 10.1002/mnfr.200400093. [DOI] [PubMed] [Google Scholar]
- 43.McLellan A.C., Phillips S.A., Thornalley P.J. The assay of methylglyoxal in biological systems by derivatization with 1,2-diamino-4,5-dimethoxybenzene. Anal. Biochem. 1992;206(1):17–23. doi: 10.1016/s0003-2697(05)80005-3. [DOI] [PubMed] [Google Scholar]
- 44.Chaplen F.W., Fahl W.E., Cameron D.C. Evidence of high levels of methylglyoxal in cultured Chinese hamster ovary cells. Proc. Natl. Acad. Sci. USA. 1998;95(10):5533–5538. doi: 10.1073/pnas.95.10.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henle T. AGEs in foods: do they play a role in uremia? Kidney Int. Suppl. 2003;63(84):S145–S147. doi: 10.1046/j.1523-1755.63.s84.16.x. [DOI] [PubMed] [Google Scholar]
- 46.Negre-Salvayre A, Salvayre R, Augé N, Pamplona R, Portero-Otin M. Hyperglycemia and glycation in diabetic complications Antioxidants & redox signaling. 2009;11:3071–109. doi: 10.1089/ars.2009.2484. [DOI] [PubMed] [Google Scholar]
- 47.Park M.S., Lee H.A., Chu W.S., Yang D.H., Hwang S.D. Peritoneal accumulation of AGE and peritoneal membrane permeability. Perit. Dial. Int. 2000;20(4):452–460. [PubMed] [Google Scholar]
- 48.Singh V.P., Bali A., Singh N., Jaggi A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014;18(1):1–14. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldberg T., Cai W., Peppa M., et al. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004;104(8):1287–1291. doi: 10.1016/j.jada.2004.05.214. [DOI] [PubMed] [Google Scholar]
- 50.Piroddi M., Palazzetti I., Quintaliani G., et al. Circulating levels and dietary intake of the advanced glycation end-product marker carboxymethyl lysine in chronic kidney disease patients on conservative predialysis therapy: A pilot study. J. Ren. Nutr. 2011;21(4):329–339. doi: 10.1053/j.jrn.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 51.Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet Journal of the American Dietetic Association. 2010;110:911–6.e12. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koschinsky T., He C-J., Mitsuhashi T., et al. Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. USA. 1997;94(12):6474–6479. doi: 10.1073/pnas.94.12.6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allavena P., Garlanda C., Borrello M.G., Sica A., Mantovani A. Pathways connecting inflammation and cancer. Curr. Opin. Genet. Dev. 2008;18(1):3–10. doi: 10.1016/j.gde.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Förster A., Kühne Y., Henle T. Studies on absorption and elimination of dietary maillard reaction products. Ann. N. Y. Acad. Sci. 2005;1043:474–481. doi: 10.1196/annals.1333.054. [DOI] [PubMed] [Google Scholar]
- 55.Hellwig M., Geissler S., Peto A., Knütter I., Brandsch M., Henle T. Transport of free and peptide-bound pyrraline at intestinal and renal epithelial cells. J. Agric. Food Chem. 2009;57(14):6474–6480. doi: 10.1021/jf901224p. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt A.M., Yan S.D., Yan S.F., Stern D.M. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J. Clin. Invest. 2001;108(7):949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rojas A., Delgado-López F., González I., Pérez-Castro R., Romero J., Rojas I. The receptor for advanced glycation end-products: A complex signaling scenario for a promiscuous receptor. Cell. Signal. 2013;25(3):609–614. doi: 10.1016/j.cellsig.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 58.Ishihara K., Tsutsumi K., Kawane S., Nakajima M., Kasaoka T. The receptor for advanced glycation end-products (RAGE) directly binds to ERK by a D-domain-like docking site. FEBS Lett. 2003;550(1-3):107–113. doi: 10.1016/s0014-5793(03)00846-9. [DOI] [PubMed] [Google Scholar]
- 59.Hudson B.I., Kalea A.Z., Del Mar Arriero M., et al. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J. Biol. Chem. 2008;283(49):34457–34468. doi: 10.1074/jbc.M801465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Origlia N., Righi M., Capsoni S., et al. Receptor for advanced glycation end product-dependent activation of p38 mitogen-activated protein kinase contributes to amyloid-beta-mediated cortical synaptic dysfunction. J. Neurosci. 2008;28(13):3521–3530. doi: 10.1523/JNEUROSCI.0204-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wautier M.P., Chappey O., Corda S., Stern D.M., Schmidt A.M., Wautier J.L. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am. J. Physiol. Endocrinol. Metab. 2001;280(5):E685–E694. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 62.Grimm S., Ott C., Hörlacher M., Weber D., Höhn A., Grune T. Advanced-glycation-end-product-induced formation of immunoproteasomes: involvement of RAGE and Jak2/STAT1. Biochem. J. 2012;448(1):127–139. doi: 10.1042/BJ20120298. [DOI] [PubMed] [Google Scholar]
- 63.Huang J.S., Guh J.Y., Chen H.C., Hung W.C., Lai Y.H., Chuang L.Y. Role of receptor for advanced glycation end-product (RAGE) and the JAK/STAT-signaling pathway in AGE-induced collagen production in NRK-49F cells. J. Cell. Biochem. 2001;81(1):102–113. doi: 10.1002/1097-4644(20010401)81:1<102::aid-jcb1027>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 64.Yan S.D., Schmidt A.M., Anderson G.M., et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J. Biol. Chem. 1994;269(13):9889–9897. [PubMed] [Google Scholar]
- 65.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu. Rev. Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 66.Lin L., Park S., Lakatta E.G. RAGE signaling in inflammation and arterial aging. Front. Biosci. 2009;14:1403–1413. doi: 10.2741/3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andrassy M., Igwe J., Autschbach F., et al. Posttranslationally modified proteins as mediators of sustained intestinal inflammation. Am. J. Pathol. 2006;169(4):1223–1237. doi: 10.2353/ajpath.2006.050713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heidland A., Sebekova K., Schinzel R. Advanced glycation end products and the progressive course of renal disease. Am. J. Kidney Dis. 2001;38(4) Suppl. 1:S100–S106. doi: 10.1053/ajkd.2001.27414. [DOI] [PubMed] [Google Scholar]
- 69.Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 70.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 71.Grivennikov S.I., Karin M. Inflammation and oncogenesis: A vicious connection. Curr. Opin. Genet. Dev. 2010;20(1):65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nishida N., Yano H., Nishida T., Kamura T., Kojiro M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006;2(3):213–219. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeNardo D.G., Johansson M., Coussens L.M. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27(1):11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 74.Nguyen A.H., Detty S.Q., Agrawal D.K. Clinical Implications of High-mobility Group Box-1 (HMGB1) and the Receptor for Advanced Glycation End-products (RAGE) in Cutaneous Malignancy: A Systematic Review. Anticancer Res. 2017;37(1):1–7. doi: 10.21873/anticanres.11282. [DOI] [PubMed] [Google Scholar]
- 75.Nguyen A.H., Bhavsar S.B., Riley E.M., Caponetti G.C., Agrawal D.K. Association of high mobility group BOX-1 and receptor for advanced glycation endproducts with clinicopathological features of haematological malignancies: A systematic review. Contemp. Oncol. (Pozn.) 2016;20(6):425–429. doi: 10.5114/wo.2016.65600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao D.C., Lu H.W., Huang Z.H. Association between the receptor for advanced glycation end products gene polymorphisms and cancer risk: A systematic review and meta-analysis. J. BUON. 2015;20(2):614–624. [PubMed] [Google Scholar]
- 77.Takino J., Nagamine K., Hori T., Sakasai-Sakai A., Takeuchi M. Contribution of the toxic advanced glycation end-products-receptor axis in nonalcoholic steatohepatitis-related hepatocellular carcinoma. World J. Hepatol. 2015;7(23):2459–2469. doi: 10.4254/wjh.v7.i23.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ishiguro H., Nakaigawa N., Miyoshi Y., Fujinami K., Kubota Y., Uemura H. Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate. 2005;64(1):92–100. doi: 10.1002/pros.20219. [DOI] [PubMed] [Google Scholar]
- 79.Rodriguez-Teja M., Gronau J.H., Breit C., et al. AGE-modified basement membrane cooperates with Endo180 to promote epithelial cell invasiveness and decrease prostate cancer survival. J. Pathol. 2015;235(4):581–592. doi: 10.1002/path.4485. [DOI] [PubMed] [Google Scholar]
- 80.Sharaf H., Matou-Nasri S., Wang Q., et al. Advanced glycation endproducts increase proliferation, migration and invasion of the breast cancer cell line MDA-MB-231. Biochim. Biophys. Acta. 2015;1852(3):429–441. doi: 10.1016/j.bbadis.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 81.Nankali M., Karimi J., Goodarzi M.T., et al. Increased Expression of the Receptor for Advanced Glycation End-Products (RAGE) Is Associated with Advanced Breast Cancer Stage. Oncol. Res. Treat. 2016;39(10):622–628. doi: 10.1159/000449326. [DOI] [PubMed] [Google Scholar]
- 82.Rahimi F., Karimi J., Goodarzi M.T., et al. Overexpression of receptor for advanced glycation end products (RAGE) in ovarian cancer. Cancer Biomark. 2017;18(1):61–68. doi: 10.3233/CBM-160674. [DOI] [PubMed] [Google Scholar]
- 83.Kang R., Loux T., Tang D., et al. The expression of the receptor for advanced glycation endproducts (RAGE) is permissive for early pancreatic neoplasia. Proc. Natl. Acad. Sci. USA. 2012;109(18):7031–7036. doi: 10.1073/pnas.1113865109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ben Q., Xu M., Ning X., et al. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur. J. Cancer. 2011;47(13):1928–1937. doi: 10.1016/j.ejca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 85.Zechner D., Radecke T., Amme J., et al. Impact of diabetes type II and chronic inflammation on pancreatic cancer. BMC Cancer. 2015;15:51. doi: 10.1186/s12885-015-1047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Menini S., Iacobini C., de Latouliere L., et al. The advanced glycation end-product Nϵ -carboxymethyllysine promotes progression of pancreatic cancer: implications for diabetes-associated risk and its prevention. J. Pathol. 2018;245(2):197–208. doi: 10.1002/path.5072. [DOI] [PubMed] [Google Scholar]
- 87.Lopez-Moreno J., Quintana-Navarro G.M., Delgado-Lista J., et al. Mediterranean Diet Reduces Serum Advanced Glycation End Products and Increases Antioxidant Defenses in Elderly Adults: A Randomized Controlled Trial. J. Am. Geriatr. Soc. 2016;64(4):901–904. doi: 10.1111/jgs.14062. [DOI] [PubMed] [Google Scholar]
- 88.Harris C.S., Cuerrier A., Lamont E., et al. Investigating wild berries as a dietary approach to reducing the formation of advanced glycation endproducts: chemical correlates of in vitro antiglycation activity. Plant Foods Hum. Nutr. 2014;69(1):71–77. doi: 10.1007/s11130-014-0403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vauzour D., Rodriguez-Mateos A., Corona G., Oruna-Concha M.J., Spencer J.P. Polyphenols and human health: prevention of disease and mechanisms of action. Nutrients. 2010;2(11):1106–1131. doi: 10.3390/nu2111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aldini G., Vistoli G., Stefek M., et al. Molecular strategies to prevent, inhibit, and degrade advanced glycoxidation and advanced lipoxidation end products. Free Radic. Res. 2013;47(Suppl. 1):93–137. doi: 10.3109/10715762.2013.792926. [DOI] [PubMed] [Google Scholar]
- 91.Palimeri S., Palioura E., Diamanti-Kandarakis E. Current perspectives on the health risks associated with the consumption of advanced glycation end products: recommendations for dietary management. Diabetes Metab. Syndr. Obes. 2015;8:415–426. doi: 10.2147/DMSO.S63089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coughlan M.T., Forbes J.M., Cooper M.E. Role of the AGE crosslink breaker, alagebrium, as a renoprotective agent in diabetes. Kidney Int. Suppl. 2007;72(106):S54–S60. doi: 10.1038/sj.ki.5002387. [DOI] [PubMed] [Google Scholar]
- 93.Ueda S., Yamagishi S., Takeuchi M., et al. Oral adsorbent AST-120 decreases serum levels of AGEs in patients with chronic renal failure. Mol. Med. 2006;12(7-8):180–184. doi: 10.2119/2005-00034.Ueda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vlassara H., Li Y.M., Imani F., et al. Identification of galectin-3 as a high-affinity binding protein for advanced glycation end products (AGE): A new member of the AGE-receptor complex. Mol. Med. 1995;1(6):634–646. [PMC free article] [PubMed] [Google Scholar]
- 95.Yamagishi S., Nakamura K., Matsui T., Ueda S., Fukami K., Okuda S. Agents that block advanced glycation end product (AGE)-RAGE (receptor for AGEs)-oxidative stress system: A novel therapeutic strategy for diabetic vascular complications. Expert Opin. Investig. Drugs. 2008;17(7):983–996. doi: 10.1517/13543784.17.7.983. [DOI] [PubMed] [Google Scholar]
- 96.Chen Y., Filipov N.M., Guo T.L. Dietary Glycation Products Regulate Immune Homeostasis: Early Glycation Products Promote Prostate Cancer Cell Proliferation through Modulating Macrophages. Mol. Nutr. Food Res. 2018;62(3):1700641. doi: 10.1002/mnfr.201700641. [DOI] [PubMed] [Google Scholar]
- 97.Kong S.Y., Takeuchi M., Hyogo H., et al. The association between glyceraldehyde-derived advanced glycation end-products and colorectal cancer risk. Cancer Epidemiol. Biomarkers Prev. 2015;24(12):1855–1863. doi: 10.1158/1055-9965.EPI-15-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Turner D.P. Advanced glycation end-products: A biological consequence of lifestyle contributing to cancer disparity. Cancer Res. 2015;75(10):1925–1929. doi: 10.1158/0008-5472.CAN-15-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tantalaki E., Piperi C., Livadas S., et al. Impact of dietary modification of advanced glycation end products (AGEs) on the hormonal and metabolic profile of women with polycystic ovary syndrome (PCOS). Hormones (Athens) 2014;13(1):65–73. doi: 10.1007/BF03401321. [DOI] [PubMed] [Google Scholar]
- 100.Nowotny K., Jung T., Höhn A., Weber D., Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5(1):194–222. doi: 10.3390/biom5010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tesarova P., Cabinakova M., Mikulova V., Zima T., Kalousova M. RAGE and its ligands in cancer - culprits, biomarkers, or therapeutic targets? Neoplasma. 2015;62(3):353–364. doi: 10.4149/neo_2015_061. [DOI] [PubMed] [Google Scholar]


