Abstract
In recent years, there has been interest in evaluating the morbidity and mortality risk of circadian, diurnal, or noctur-nal blood pressure variation. Variation is a normative property of blood pressure, necessary for survival. Like many physio-logical functions, blood pressure undergoes allostasis, meaning that the body does not defend a particular blood pressure value, but rather blood pressure maintains bodi-ly stability through continual change that is initiated by constantly fluctuating internal and external environmental stimuli. Be-cause of its allostatic and adaptive properties, the blood pressure response to unusual situations like a visit to the clinic can lead to misdiagnosis of hypertension. However, blood pressure variation is mostly ignored when evaluating hypertension, which is an arbitrary dichotomy. Whether variation is indicative of pathology should be determined by assessing its appro-priateness for the circumstance, which requires quantification of the sources and extent of normative blood pressure respons-es to everyday living. These responses will vary among populations due to evolutionary genetic differences. The incon-sistency of reports regarding aspects of ambulatory blood pressure variation as cardiovascular risk factors likely results from the fact that the measures used do not reflect the actual nature of blood pressure allostasis.
Keywords: Allostasis, blood pressure variability, ambulatory blood pressure, white coat hypertension, masked hypertension, human evolution
1. INTRODUCTION
Since the report of the first measurement of blood pressure by Stephen Hales in 1733, its inherent variability has been well known [1, 2] but largely ignored [1, 3]. Practitioners of cardiovascular medicine even today are largely at a loss regarding how to deal with the continuous variation of blood pressure clinically, primarily because, as George Pickering noted early on, the entire medical focus on blood pressure is differentiating a dichotomy of normalcy (normotension) and pathology (hypertension) [4].
Blood pressure whether characterized as a continuously distributed measurement or as a dichotomized diagnosis, is a statistical risk factor for adverse cardiovascular related outcomes [5, 6]. However, treating blood pressure as simple numbers that put people at risk misses its true nature. Blood pressure is a property of blood flow. When a bolus of blood is squeezed with force out of the rhythmically contracting left ventricle into the aorta, pulsatile waves of blood are produced. Pressure develops because the aortic walls and smaller downstream arterial and capillary space resist the pulsatile flow. The numbers analyzed in risk assessment represent the maximum pressure of the ejecting force (systolic pressure) and the ambient pressure when there is no blood bolus being pushed into the aorta (diastolic pressure), which can be visualized as the maximum and minimum of a tracing of the arterial pressure pulse. Blood pressure, then, is an indicator of two things: the strength of the contracting left ventricle and the extent of the vascular resistance. It varies because the rate and strength of ventricular contractions and vascular resistance are not constant due to neural and hormonal inputs that are regulated by the brain to meet changing internal and external demands [7].
Nikolai Korotkoff, a field surgeon during the Russo-Japanese war discovered the auscultatory technique of blood pressure measurement by listening for blood flow in injured limbs using a stethoscope placed distal to Riva-Rocci’s recently developed sphygmomanometer [8]. He reported on the sounds that bear his name to the Imperial Military Medical Academy in St. Petersburg, Russia in 1905 [8]. His insight was that the appearance and disappearance of sound represented the blood pulse maxima and minima (systole and diastole) and corresponded to the pressure displayed on the mercury column when they happened. From his observation, a reliable and reproducible means of ascertaining blood pressure level and variation was educed. It wasn’t until 1939 that the American Heart Association and the Cardiac Society of Great Britan and Ireland officially recommended Korotkoff ’s method as the standard technique for arterial blood pressure measurement [9].
Following the acceptance of the technique, a variety of observations regarding blood pressure variation both inside and outside the clinic was made. First, laboratory studies demonstrated that aspects of physiological habitus such as respiration, exercise, and posture and external environmental factors like experienced temperature all substantially influenced blood pressure variability [1, 10]. There were also rare studies showing that there was substantial variation in “resting blood pressure” by setting and over time. One often cited is the 1940 report by Ayman and Goldshine [11] who trained hypertensive patients or their family members in how to take blood pressures at home. They found that these measurements were substantially lower from those taken in the office by as much as 70/36 mmHg, a difference that persisted over 6 months. Other contemporaneous studies suggested that a person’s psychological state could affect the reliability of resting ausculted blood pressure measurements [12, 13]. Data later emerged that pressure levels could be influenced by the familiarity between the person taking the pressure and the patient [14] as well as the gender of the person taking the pressure [15]. During this time, the variation in blood pressure measurements related to the patient’s response to the circumstances was seen medically as something that confounded accurate clinical assessment and thus needed to be minimalized.
In the 1950’s, a controversy also arose regarding the definition of hypertension (high blood pressure) which came to be known as the Pickering-Platt debate. The dispute centered on whether there were inherited hypertensive and normotensive genotypes (Platt position) or whether blood pressure was a varying quantity between and within individuals such that hypertension per se was an arbitrary distributional cut point determined by the medical community that defined when treatment was necessary (Pickering position) [4, 16]. As data accumulated about CVD mortality, it became apparent that hypertensive patients with the same level of pressure did not have the same outcomes, and so researchers like Maurice Sokolow wondered whether the difference in outcome was related to the heretofore largely ignored blood pressure levels and variation measured outside the clinic [1]. Consequently, in the in the early 1960’s, the variability of arterial blood pressure began to be studied using ambulatory monitors that could take blood pressures during typical daily circumstances and conditions [3]. These studies emerged with the technical development of the Remler® ambulatory blood pressure recorder, which required that subjects manually inflate the cuff [17-19] and with the development of intra-arterial devices that measured pressure continuously [20]. The results of several studies showed the extensive situational variation of arterial pressure, convincingly demonstrating that blood pressure was a continuously distributed and variable function [19-21], but also that office blood pressure measurements were often unrepresentative of pressure outside that venue [4, 16].
As automatic ambulatory blood pressure monitoring technology emerged in the late 1970’s and improved through the 2000’s, the effects of various typical behaviors on blood pressures were evaluated, first using intra-arterial devices and later using monitors that employed either auscultatory or oscillometric technology [1, 22]. These studies, mostly undertaken by biobehavioral researchers, were designed to quantify the intraindividual variation in pressure associated with psychological, sociological, and environmental sources [3, 23]. Their purposes were to evaluate how the things that people do, think and experience as part of their typical lifestyle relate to the development of sustained high blood pressure and subsequent cardiovascular pathology and to assess how people adapt to the strains of everyday living [22]. The upshot of the results of these studies is that the extent of circadian arterial pressure variation and its relation to pathology are affected by both the mix and psychological appraisal of circumstances, activities and interpersonal relationships that are experienced during the course of a day, and also by the duration and frequency of the experience of these factors over a lifespan [3, 22].
These studies, which focus on the external sources of blood pressure variation, are mostly ignored in the clinical assessment of blood pressure pathology. When circadian blood pressure variation is considered in a diagnostic process, the focus is on either the difference between blood pressure measured in the clinic and an average pressure outside the clinic or three derived measures from an ambulatory monitoring: 1) the difference between an average of pressures taken while awake and those taken while sleeping, 2) statistical measures of variance of all or a portion of the pressures taken during a day and 3) the “average real variability” [4, 24-27]. None of these clinically used measures reflects the adaptive allostatic nature of blood pressure. The purpose of this brief overview is to critically examine how blood pressure variability is treated with regard to cardiovascular pathology and health, and to introduce allostasis as a paradigm for understanding blood pressure variation and its diagnostic utility.
2. HOMEOSTASIS, ALLOSTASIS, AND BLOOD PRESSURE VARIATION
Homeostasis as a model of physiological regulation has dominated the study of chronic metabolic diseases including cardiovascular disease for nearly a century. In this model, vital processes have one main objective: to preserve relatively constant conditions in the internal body environment, which has been interpreted medically to mean that the purpose of physiological regulation is to preserve internal parameters at a “setpoint” [8, 28]. The logical consequence of this interpretation is that when a measurement is not within a relatively narrow range around the setpoint, some internal mechanism must be broken which indicates disease; hence therapy will be designed to get the offending value back to the setpoint (i.e. a normal healthy state) [8]. There are many bodily processes that maintain homeostasis and fit this model reasonably well including core body temperature, water content, and various aspects of blood chemistry, including blood pH and ion balance [28].
However, there are also physiological processes that do not fit the model, and vary considerably [8]. Walter Cannon who coined the term “homeostasis”, also described the alarm reaction, better known as “fight or flight” that ensues from the fear, anger and anxiety responses to perceived threats. The physiological functions involved in this response, such as arterial blood pressure, change to meet the external environmental demands, and because these demands themselves continuously vary, these functions do not really settle at a specific setpoint and are not maintained in the classic sense of homeostasis. Rather they continuously vary to keep the physiological system stable, adapting the body to unpredictable external stimuli [8].
In 1988, Peter Sterling and Joseph Eyre [29] introduced the physiological concept of allostasis, which literally means “stability through change” to describe the behavior of fluctuating physiological parameters that do not center on a single setpoint. The idea is that levels in these parameters fluctuate to adapt the individual to ever changing circumstances, so that there is a connection between external conditions and the body’s ability to meet the demands imposed by them, which is all regulated by the brain [3]. These measurable physiological systems anticipate demand and have a multitude of stable states that occur as responses to continuously changing environmental strains, either real or perceived. In introducing the concept of allostasis, Sterling and Eyre [29] used arterial blood pressure as an exemplar, because of its inherent variability and its well-known relationships with external psychosocial factors. In fact, variation is what gives blood pressure its adaptive value, and is perhaps the single most important normative property of blood pressure, for without it humans could not survive [22].
3. CURRENT EVALUATIONS OF BLOOD PRESSURE VARIABILITY
3.1. The Inside Clinic-Outside Clinic Blood Pressure Difference
The medical evaluation of blood pressure mostly centers on seated blood pressure measurements that are made in the clinic or office, and there is a certain logic to that, particularly seen from a historical perspective. However, under the principles of allostasis, the measurement in the office reflects only an adaptation to that circumstance. From the perspective of most patients, purposely going to a medical clinic is a major event in their lives, something that is a relatively rare occurrence [3]. The environment of the clinic, office, or hospital is unique and different from every other place that they go. The whole purpose of the visit is to get an evaluation of their health, so there is likely trepidation on their part. One should expect that a blood pressure taken during the event (being in the clinic) would reflect the patient’s allostatic adaptive response to it, which is going to depend upon how that setting is perceived (fearfully or otherwise) [3]. However, for the typical clinician, adaptive variation is not part of the diagnostic equation. Rather, the interpretation of office blood pressure measurements follows a homeostatic paradigm, in which the question to be answered is whether the patient is normotensive or hypertensive, and that assessment is based on whether measurements exceed a cut point (e.g. 140/90) (Fig. 1).
Fig. (1).
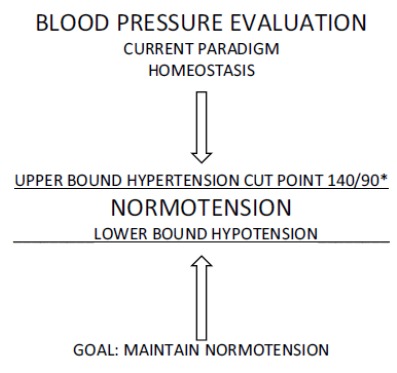
The use of homeostasis as the paradigm for treating blood pressure.
There are also allostatic issues related to the measurement itself. In describing the sphygmomanometric method of measurement he developed, Riva-Rocci noted that arterial occlusion is enough of a stimulus to initiate an increase in blood pressure [1]. Because the taking of an arterial blood pressure measurement is also a unique social interaction involving the patient and a medical professional, there will also be allostatic variation initiated by the perceptions of the patient connected to that interaction. Even if an automatic device takes measurements when the patient is alone, that situation still requires an adaptive response [3].
If the allostatic response to the atypical environment of the office exceeds the average response to all other daily environments (e.g. average ambulatory waking pressure), the patient is described as showing a white coat effect, but if that response leads to measurements that exceed 140/90 (hypertension cut point) the patient is diagnosed with white coat hypertension [3]. It is well known that a blood pressure measurement can be profoundly increased by the perceptions of the patient, as was dramatically demonstrated by Mancia and colleagues [30] in their classic study in which intra-arterial blood pressure measurements were continuously taken on one arm while a nurse or physician took an ausculted blood pressure from the other. The intra-arterial measurements showed that relative to the pressure prior to the ausculted measurement interaction with the physician, there was an increase of some 23/18 mmHg when the physician took the ausculted pressure. Further, the increase in pressure by the physician was about twice that when a nurse took the pressure. A study by Jhalani et al. [31] provides some answers regarding why there is this increase. They examined the acute effects of anxiety and expectancy on pressures measured in the clinic, and found that when assessed as a specific office related effect, anxiety had a substantial influence on increasing pressure in the office [3]. They assessed anxiety before, during, and after pressure was taken by the physician. They also demonstrated that there is also an increasing effect related to the patients' expectations that their measurements will be elevated. Their findings suggest that prior experience can trigger anxiety regarding this peculiar environment of the office and the relationships within it, so that the blood pressure response is elevated, and that it will remain elevated because the patient expects it to be.
Masked hypertension as a diagnosis is just the opposite of white coat hypertension. In these patients, the allostatic adaptive response to the clinical environment is less than an average of the responses to all other events outside the clinic [3]. Rather than being made anxious, masked hypertensives may be calmed by the interpersonal interactions and setting. Interestingly, masked hypertension is often seen as emerging from behaviors that elevate pressure outside the clinic, such as alcohol consumption, smoking, or contraceptive use [32].
A problem with the concepts of white coat and masked hypertension is that they rely on the allostatic blood pressure response to the unique environment of the clinic for a referent, as if it is a standardized reproducible condition. The office pressure hypertension Rubicon of 140/90 mmHg is treated as if it is an upper bound of the “homeostatic range” of a healthy steady state of normotension. But a more important problem with diagnosing these conditions is the fact that in order to define them, it is necessary to also determine an upper bound of the homeostatic range for normotension based on the ambulatory pressures outside the clinic. Consensus is that it must be less than 140/90 mmHg, and there is an emerging belief that the level should be 135/85 mmHg [6] intimating that a non-office healthy steady state has a different upper bound than an office healthy steady state. It is as if office and ambulatory blood pressure are actually two distinct phenomenon, which they are not. They are simply two samples (one larger and one smaller) of the 100,000 or so measureable allostatically varying pressures during the day which differ due to circumstances and adaptive needs [3, 33].
In drawing attention to the fact that office pressures are likely biased due to the medical setting, our research group, headed by Tom Pickering, in our seminal paper examining the prevalence of white coat hypertension provided a conservative cut point for normal ambulatory blood pressure [34]. The division was necessary to make the point that office pressures lacked sensitivity for diagnosing hypertension. However, in hindsight, by using the cut point to make our argument, we inadvertently created the same dichotomy problem that Sir George Pickering criticized with respect to continuous blood pressure measurements some 25-30 years earlier [e.g. 4]. As a result of our paper, a vast literature has grown regarding white coat and masked hypertension that is based on arbitrary cut points that clouds the issue of high blood pressure and when to treat it. The recent ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guidelines in some ways actually clouds the issue further, because it creates new cut points and furthers the use of a homeostatic paradigm that does not adequately describe the nature of arterial pressure [6]. Stergiou and colleagues [35] have noted in their response to the guidelines, that average circadian ambulatory blood pressure over 24 hours should be used for the diagnosis of hypertension (high blood pressure). While better than the pressures measured in the unique situation of the clinic, this average masks circadian variation and the adaptive function of arterial pressure with respect to changing daily circumstances which is relevant with regard to determining pathology [33].
3.2. Derived Ambulatory Blood Pressure Variation Parameters
Recently [3], I evaluated the validity of the various means of expressing ambulatory pressure variation outside the clinic in light of the allostatic nature of blood pressure, and it is worth paraphrasing several of the main points of that discussion here. First, given that blood pressure changes to meet the demands of fluctuating of conditions, it would make sense that an assessment of the link between circadian variation and morbidity or mortality would incorporate a determination of the appropriateness of the pressure responses to the changing external and internal circumstances [36]. However, nearly every study that investigates blood pressure variation as a risk factor for cardiovascular events ignores the dynamic interplay between the demands an individual faces during everyday life and blood pressure. Instead, studies of vascular risk focus on the event predictability of dichotomous indicators such as the difference between an average of pressures taken while awake and those taken while sleeping (either “dipping”-the difference between average waking pressure and average sleep pressure, or the “morning surge”- the difference between various pressures prior to and just after morning awakening), statistical measures of variance of all or a portion of the pressures taken during a day, or a value defined from the cumulative differences of intermittent pressure measurements taken with a non-invasive ambulatory monitor over the course of one 24-hour period [25-27, 37, 38]. These measures are examined only with regard to whether risk is related to either too much or too little variation. The inconsistent results from these studies, where some suggest variability is an important risk factor and others find little or no effect has spurred a controversy as to whether blood pressure variation is important with regard to cardiovascular risk [26, 38, 39]. Before determining whether this type of issue is resolvable, it is useful to examine what each indicator is measuring. Are these parameters meaningful measures of allostatic blood pressure variation?
3.2.1. Dipping and the Morning Surge
Dipping and the morning surge are measures of blood pressure change between the state of waking and the state of sleep. Operationally, there is really no consistent characterization for either measure across studies, although with dipping, a 10% decline, particularly for systolic pressure is a popular demarcation for normalcy and pathology, but there is no definitive reason why this value is a clinically relevant cut point [24, 26, 38]. The waking average that is used to determine dipping is based on blood pressure measurements that are varying non-randomly in tandem with the conditions experienced during the day of study. So depending upon whether a person had a difficult day or an easy day, the waking average could be higher or lower. There are also ample data showing that excessive psychological stress during the day can carry over and increase pressure during sleep [40]. Thus, non-dipping may occur with a given monitoring simply because it was a stressful waking day. Another problem is that the dipping/non-dipping dichotomy assumes that all people experience only two contiguous periods in the day (waking and sleep) that correspond to day and night. This presumption is demonstrably false as there are plentiful data showing that waking-sleep patterns can change with age and differ by culture [41, 42]. These factors affect the circadian patterns of adaptive blood pressure responses in ways that confound the determination of who dips and who does not. Similarly, whatever pressure(s) chosen to define the post- awakening point and the low pre-awakening point in defining the morning surge are also allostatic adaptive responses to the circumstances, so that the magnitude of the difference between the points is likely a function of any number of factors affecting both sets of measurements. Aside from the obvious allostatic concerns, there may also be technical issues relating to the measurements. For example, “sleep” pressures taken by a cuff occlusion method can also vary considerably due to the changing position of the cuff relative to the heart when a person rolls over or moves during sleep [1, 43].
It is not surprising that waking-sleep transition measures vary in their effects on cardiovascular health across populations and have poor day-to-day reproducibility [24, 26, 39]. Patterns of behavior, stress, and sleep quality vary from day to day, and all these are factors are affected by cultural background and occupation [23]. While there may be theoretical reasons to believe that the transition in blood pressure between wakefulness and sleep ought to have health implications, the operationalization of the concepts using non-invasive ambulatory measurements fail to embrace the allostatic and adaptive nature of blood pressure, which makes it impossible to define what normative transitions ought to be. Without a clear definition of normalcy, there is no way to rationally employ these parameters for treatment purposes [3, 24].
3.2.2. Statistical Measures of Variance
Standard deviations (SD) or coefficients of variation (CV) are parameters that describe the spread of a normal distribution, nominally generated from random sampling. They are substantially affected by sample size, biased sampling, and outlying measurements, such that if the distribution is not normal and the sample from which they are calculated is small and unrepresentative, these measures will not accurately describe the distributional dispersion [44]. Over the course of one 24-hour period, the pumping heart will generate 100,000 or more measureable arterial blood pressures. Non-invasive ambulatory monitors sample perhaps 50 of those (5/100ths of 1% of all those generated) which vary with non-randomly experienced conditions (pressures change to adapt the person to continuously changing circumstances). Because the sample of pressures from intermittent monitoring is small and biased it is highly unlikely that the SD or CV calculated from it will validly describe the circadian dispersion of blood pressure measurements. Furthermore, Parati et al. [2] some 25 years ago noted that these measures do not tell you anything about how single values, as collected, are actually distributed around the daily mean. Do the pressures spread out or is there perhaps a bimodal shape? Many odd distributions could provide the same calculated SD or CV. The bottom line is that these measures of dispersion do not provide any information about the pattern and extent of individual pressure responses, and because what needs to be evaluated is the appropriateness of allostatic blood pressure changes, they are unsuitable for examining morbidity risk or determining treatment. Lastly, because of the various sampling issues, it is also not surprising that SD and CV measures as indicators of 24-hour blood pressure variation are also poorly reproducible over 24-hours [26, 39].
3.2.3. Average Real Variability
Finally, another measure, the “average real variability” (ARV24) has also been used to describe blood pressure variation. It is defined as the mean of the absolute differences of consecutive non-invasive ambulatory measurements [37]. Bearing in mind that each of the sequential blood pressures is an adaptive response to the specific conditions when it is taken, the ARV24 can go up or down depending upon the variability or stability of conditions during the day. What this quantity really represents is a summary score of the differences between blood pressure responses to some indeterminate number of sequential unknown stressors. Ultimately, the magnitude of this parameter depends solely upon the variability of the environments experienced and the lability of behavioral/emotional responses [22, 45]. Thus, someone who is monitored on a day where they are inactive, remain at home and are emotionally stable will have low ARV24, whereas one that performs multiple varying tasks, transitions through many daily microenvironments (goes to work, out to dinner, etc.) and experiences an array of emotions will have a high ARV24 [3]. Since the blood pressure changes are adaptive and are a normative response to the tribulations of everyday living, it is not clear from the studies that have used this parameter why high (or low) values of ARV24 would be indicative of pathology or health. Ultimately, it is not surprising that in epidemiological evaluations, the effects of this measure on outcomes is small or inconclusive [26, 38]. It most certainly does not evaluate the appropriateness of blood pressure variation associated with environmental change.
In sum, after assessing the nature of variation indicators that have been employed in large international and community based morbidity and mortality risk studies, it is apparent that none of them assess the appropriateness of the allostatic changes in blood pressure. Thus, based on the analysis of these measures, it is unclear as to what the true role of allostatic variation is with regard to cardiovascular pathology or clinical decision-making.
4. EVALUATING ALLOSTATIC BLOOD PRESSURE VARIATION
Variation is perhaps the single most important normative property of blood pressure, but it is not clear what normal allostatic blood pressure variation looks like. Non-medical researchers, who have employed ambulatory blood pressure monitors to evaluate the physiological effects of stress, and emotional and environmental change, have reported on average patterns of circadian blood pressure variation in a variety of population groups. Over the past three decades, a number of studies show that many psychological, social and behavioral parameters contribute substantially to increasing ambulatory blood pressure variation. These effects have been summarized in a number of reviews [22, 23, 33, 36, 46]. In brief, mood changes, postural changes, location changes, and activity variation all induce allostatic blood pressure variation. These effects are further moderated by sodium intake [47]), seasonal (temperature) influences [48], alcohol consumption, smoking, specific social interactions (such as with spouses) and among employed people, the appraisal of job strain [22, 33, 36, 46]. A given blood pressure is a response to all the factors that are present when the measurement occurs, and research shows that the effects of the factors are additive [22]. Interestingly, most behavioral studies of circadian blood pressure variation were not undertaken to evaluate why blood pressure allostasis occurs. Rather, studies focused on either defining the sources of circadian pressure variation, or evaluating whether people with specific attributes differed in their responses to similar lifestyle factors [3].
Research designed to evaluate the allostasis of blood pressure takes two general forms. The first is one where each blood pressure measurement is assessed with regard to concurrent conditions and habitus that are reported in a diary (referred to as ecological momentary data) [49-54]. Using inferential statistical models, the proportion of variation associated with each reported circumstance is quantified, as is the number of mmHg the alternative levels of each (such as location-work or home) contribute to either increasing or decreasing the magnitude of the blood pressure measurement. With this approach, the choice of diary reporting alternatives is key. The sources of variation chosen to be reported in the diary and the manner in which they are coded determine how allostatic changes in blood pressure get estimated [22, 23].
Fig. 2 shows a standard diary that has been used in ambulatory studies.
Fig. (2).
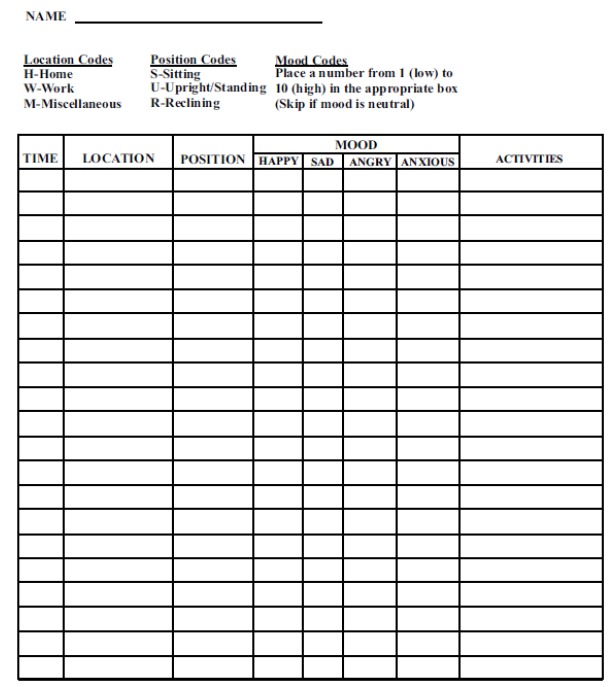
A typical blood pressure diary for use in evaluating the sources of blood pressure variation during an ambulatory blood pressure monitoring (from reference [23]).
Evaluation of ecological momentary blood pressure measurements has been conducted using standardized [41, 49, 55] or the actual [50, 52, 56] measurements. The estimated effects of various factors, while similar, do vary among studies, due in part to the statistical modeling assumptions, but also because of the demographic, health status, and cultural diversity of the groups studied [22, 23]. Fig. 3 shows the additive allostatic effects of posture, location and mood on the blood pressures of middle aged men in New York City.
Fig. (3).
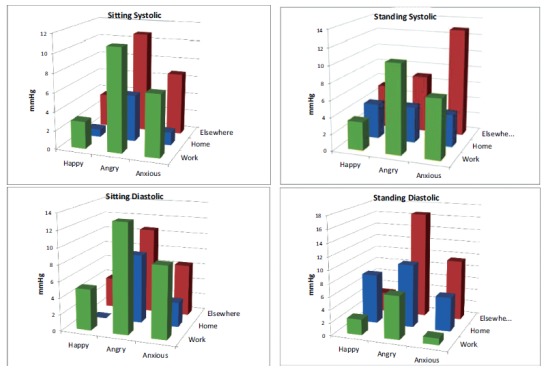
The additive effects of posture, location and mood on the allostatic blood pressure variation of middle aged working men (data from reference [49]).
The second utilizes a “natural experimental” approach [22, 23, 45, 57]. In brief, natural experiments are studies that have design elements that contrast naturally occurring predictable conditions that occur during a typical day [22]. This approach is most often used by anthropologists and human population biologists, and it has its roots in psychological and psychophysiological research designs where blood pressure reactivity to specific stressful tasks are compared in a laboratory setting [56, 58, 59] In laboratory experiments, a baseline condition is established and then subjects undertake a series of standardized tasks designed to elicit a response. The difference between the baseline measurements and those taken during or after the tasks define the magnitude of blood pressure reactivity [22]. Because these experiments are conducted under laboratory circumstances, many controls can be introduced in the procedures so that specific effects can be isolated, systematic measurements can be taken, and all the participants experience the exact same protocol. Control groups can also be included in the experiment. Moving this framework into real life and outside the laboratory requires a modification because it is not possible to establish a true baseline in real life. However, a “natural experiment” frames contrasts in which blood pressure can be evaluated as people move through differing daily conditions (such as their work and home environments) during the course of their everyday lives. For example, a person who resides in a suburb and travels to an urban workplace every day has a structured work environment where economic related activities occur and social interactions take place with non-relative co-workers, and where an occupational hierarchy determines the nature of social relationships [22]. The features of this circumstance differ substantially with that of the home setting, where household tasks and leisure activities occur in a social milieu where interactions are largely with family members and neighbors [22]. The allostatic blood pressure response required to adapt to these relatively predictable conditions can be assessed by comparing the average blood pressure while in them with that during overnight sleep, or more specifically, while the person is quietly recumbent in a dark room acting as a pseudo-baseline. Fig. 4 shows a comparison of laboratory and natural experimental designs. Studies employing this approach have shown that the perceptions of how stressful daily microenvironments are perceived to be profoundly affect the patterns of circadian blood pressure variation (Fig. 5).
Fig. (4).
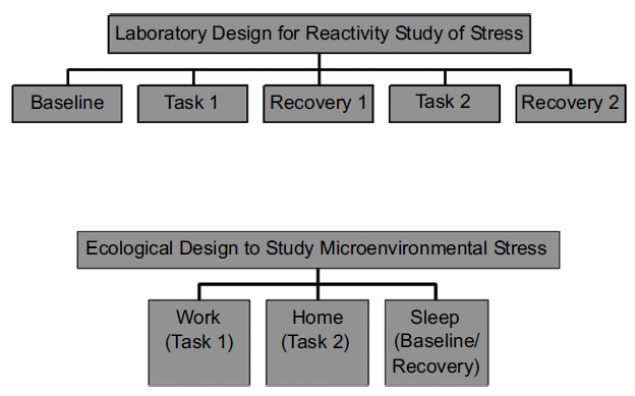
Natural experimental research design used for evaluating circadian microenvironmental (work, home, sleep) blood pressure variation as contrasted with a laboratory reactivity research design (from reference [57]).
Fig. (5).
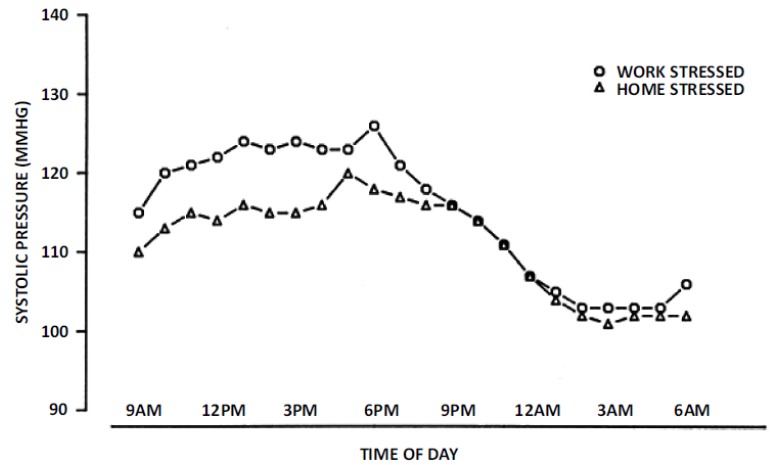
Average hourly circadian systolic blood pressure variation among women employed outside the home as secretaries based upon whether they perceived greater stress at work or home on the day their blood pressures were monitored (from reference [72]).
In assessing intra-individual blood pressure variability, it is important to recognize that the array of measurements collected will be related since they are determined from a single vascular system that has specific structural and functional properties. That is, the average and dispersion of the sample of pressures measured over the course of 24-hours on the same person will be related [1]. Hence, people with lower 24-hour average blood pressure will tend to have a narrower range of blood pressures diurnally than those with higher average pressures. Pickering [1] has noted that this heteroscedasticity is probably related to underlying arterial structural differences such as stiffness and/or other functional factors such as differences in vasoactive hormone receptor density or sensitivity [60]. When assessed within a given patient over time, an increase in daily pressure coupled with an increase in allostatic variation would suggest that there has been an increase in vascular resistance which needs to be treated. Simply lowering the pressure is not going to improve the underlying pathology.
5. HUMAN POPULATION VARIATION AFFECTS BLOOD PRESSURE ALLOSTASIS
Biological evolution in our species over the past 100,000-200,000 years driven by diet and climate has resulted in physiological variation between populations that can affect allostatic blood pressure responses [3, 22, 45, 61, 62]. These heritable differences likely arose from natural selection and are reflected in population differences in arterial blood pressure responses to environmental factors such as and dietary salt and cold temperature.
Current evolutionary evidence suggests that all modern human populations are descended from tropical “heat adapted” ancestors in Africa, and it is also true that modern sub-Saharan African populations retain a physiology adapted to a mostly hot, wet environment [3, 22, 61-64] There are two relevant aspects of adaptation to heat in humans: the ability to 1) profusely sweat and 2) retain salt (sodium). The former is important because humans lose salt while sweating, and the latter is important because salt availability is limited in tropical ecosystems [61, 62, 64]. Young et al. [64] has reported that there is a geographic cline from the equator to the poles of “heat adapted” allelic variants from 5 functional genetic sites that affect salt retention and blood vessel tone. They evaluated 53 geographically dispersed populations and found that native populations living within 10 degrees of the equator (hot, salt poor environments) had an average 74% “heat adapted” allelic variants, while those within 10 degrees of the arctic (cold, salt rich environments) had only 43% “heat adapted” variants. They hypothesized that the frequency of “heat adapted” alleles declined as our African ancestors colonized ecosystems that were cooler and salt rich and then rose again among groups that migrated from those areas back to more salt poor tropical climates [61, 64]. They further argued that since the “heat adapted” alleles facilitate salt retention and excessive dietary salt intake can contribute to the development of hypertension, populations
with an increased number of “heat adapted” alleles are more susceptible to developing hypertension, particularly if they have moved to salt rich environments or who have had an increase in salt in their diets [64]. These genetic findings may partially explain the higher prevalence of hypertension and cardiovascular morbidity among African-American populations, [22, 61]. What this means more broadly, however, is that allostatic blood pressure variation that is moderated by salt intake may be different depending upon the evolutionary history of the people being evaluated.
Many human populations also migrated to ecosystems characterized by temperate and freezing climates, and have survived there for millennia. When cold conditions are experienced in the unprotected human, there is a sympathetically driven constriction of peripheral arteries, particularly in the hands and feet that is designed to conserve core body temperature, which, if left unchecked, will lead to tissue damage [62]. To minimize this injury, populations who migrated out of the warm African climate to more temperate and cold environments developed a peripheral cold induced vasodilatory (CIVD) response [65]. CIVD is a periodic release of the sympathetically driven arterial constriction in the appendages, which suffuses them with blood, rewarming the tissues to keep them functional [3, 62, 66]. However, those populations who remained in Africa did not develop this response since it was unnecessary in the tropical conditions [62]. Numerous studies have found that African-Americans, whose ancestors journeyed to the colder climate environments of North America over the past 500 years, have a generally more intense vasoconstrictive response to peripheral cold stress, with either inadequate or no CIVD [62, 65]. The increased cold pressor response is most often demonstrated in studies of hand or foot emersion in cold water. Interestingly, cold to the face also elicits the accentuated pressor response [67, 68]. Research has also shown that African-American subjects may have heightened myocardial and vasoconstrictive reactivity during passive exposure to temperatures from eight to ten degrees centigrade [69, 70]. Taken together, the findings from these cold stress studies suggest that the typical outside exposure of the face and hands during the winter may be sufficient to elicit enhanced vasoconstrictive responses from African-Americans.
Why this physiological response is important from the perspective of blood pressure allostasis is that the sympathetically driven peripheral vasoconstriction induced by cold also increases arterial blood pressure [58]. Thus, it is possible that African Americans living in the temperate and freezing climates of North America who experience a chronic cold stress through the winter months also suffer chronic vasoconstriction due the their enhanced cold pressor response and inadequate CIVD, which in turn, increases pressure during cold exposure and heightens the variability of their diurnal pressure [22, 62]. This possibility is supported by research showing that sympathetic hormone receptors among African-Americans may be more sensitive than those of European-Americans [70], and other data that show the day-night increases in blood pressure relative to changes in catecholamines is accentuated among African-Americans compared to European-Americans, which intimates that blood pressure is more reactive to epinephrine among African-Americans [60].
Because of these evolutionary-based physiological differences in salt and fluid retention and peripheral vascular responses to cold, it is likely that the allostatic blood pressure responses of patients from different ethnic groups vary in different ways. The extent to which blood pressure may vary, or move to presumptively adaptive states in response to challenges may depend upon how natural selection has shaped physiology. Underlying physiological differences should be considered when evaluating allostatic blood pressure variation in studies that examine ethnically diverse groups.
DISCUSSION AND CONCLUSION
A cornerstone of medical diagnostic is the inductive logic of choosing alternatives to arrive at a conclusion. Given this framework, diagnostic decisions about continuously varying physiological phenomena are problematic as they do not readily lend themselves to bifurcation. Using a homeostatic paradigm, phenomena such as blood pressure are evaluated as either falling within or outside the upper or lower bounds of normal limits, so that if a measured value falls outside the proscribed limits, treatment is rendered. The idea is that the body is defending a setpoint value, and if it loses the ability to do that, treatment will be given to maintain that value.
Measurements taken in the clinic/office have been employed as a standardized means of determining the limits of blood pressure homeostasis, with 140/90 being considered the upper bound of normalcy, although recent guidelines have lowered that value, creating controversy. This cut point defines a clinical malady, hypertension which has been shown to be a statistical risk factor for cardiovascular mortality. Because of this association, there is a fixation on lowering clinic blood pressure to a range below the cut point. Unfortunately, many clinical studies as well as the development and use of ambulatory monitors have revealed that office measurements are not appropriate standardized values for defining the homeostatic limit for a variety of reasons [71].
While this above description defines the current state of blood pressure management, in needs to be said that using homeostasis as the physiological paradigm for evaluating blood pressure is incorrect. Blood pressure is a property of blood flow which varies in an allostatic fashion and as such does not have a value that the body defends. If blood pressure becomes too elevated for the circumstance in which it is measured, and circadian variation increases, it suggests that there is some other underlying flow related vascular issue, so that lowering the systemic pressure does not solve the actual problem with the patient. Since it is untreated, that actual underlying issue goes unchecked and will likely be the cause of later morbidity or mortality.
There is overwhelming evidence that the average level of the continuous pulsatile pressures that are generated by the contracting heart is related to the variability among them, as their values change to meet environmental demands. Information regarding that variability can be used to evaluate possible sources of pathology, particularly if evaluated in a patient over time.
Indicators of variation that might be used for diagnostic purposes need to take account of the adaptive allostatic changes in pressure during the day. Those that have been used, (dipping, morning surge, SD or CV of the sample, and ARV24) ignore the allostatic variation of blood pressure entirely, which is probably why they lack reproducibility and are of limited value in predicting cardiovascular outcomes.
Allostasis is the paradigm to understand blood pressure, and because of that, there really needs to be a reconceptualization of hypertension. Rather than being a diagnosis of a pathological condition defined by a distributional cut point which requires lifelong perpetual remediation, hypertension should be a term reserved for a blood flow related problem that is defined by the appropriateness of circadian allostatic blood pressure variability and changes in the magnitude of the pressure pulse. Giving drugs to lower pressure would be a short term means of stabilizing the system while the actual pathology is determined and treated. Long term drug use to keep blood pressure low would only be necessary when the problem is unfixable and there is a need to protect target organs from damage that could result from perpetually high pressure. The determination of high pressure should be based on the patient, and not some arbitrary fixed number [4].
In sum, a homeostatic framework with fixed cut points is inappropriate for evaluating blood pressure. Blood pressure is an allostatic property of blood flow, which means that determination of health needs to evaluate the appropriateness of the blood pressure variation and the characteristics of the system that is generating blood flow.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Pickering T.G. Ambulatory monitoring and blood pressure variability. London: Science Press; 1991. [Google Scholar]
- 2.Parati G., Mutti E., Omboni S., Mancia G. How to deal with blood pressure variability. In: Brunner H, B. Waeber B, editors. Ambulatory blood pressure recording. New York: Raven Press, Ltd; 1992. pp. 71–99. [Google Scholar]
- 3.James G.D. Understanding blood pressure variation and variability: Biological importance and clinical significance. Adv. Exp. Med. Biol. 2017;956:3–19. doi: 10.1007/5584_2016_83. [DOI] [PubMed] [Google Scholar]
- 4.Pickering G. Hyperpiesis: High blood-pressure without evident cause: Essential hypertension. BMJ. 1965;2:959–968. doi: 10.1136/bmj.2.5469.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James P.A., Oparil S., Carter B.L., et al. Evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 6.Whelton P.K., Carey R.M., Wilbert S., et al. 2017 ACC/AHA/AAPA/ ABC/ ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 7.Sterling P. Principles of allostasis: Optimal design, predictive regulation, pathophysiology, and rational therapeutics. In: Schulkin J., editor. Allostasis, homeostasis, and the costs of physiological adaptation. Cambridge: Cambridge University Press; 2004. pp. 17–64. [Google Scholar]
- 8.Paskalev D., Kircheva A., Krivoshiev S. A centenary of auscultatory blood pressure measurement: A tribute to Nikolai Korotkoff. Kidney Blood Press. Res. 2005;2:259–263. doi: 10.1159/000090084. [DOI] [PubMed] [Google Scholar]
- 9.Joint Recommendations of the American Heart Association and Cardiac Society of Great Britain and Ireland. Standardization of blood pressure readings. Am. Heart J. 1939;18:95–101. [Google Scholar]
- 10.Rowell L.B. Human circulation: Regulation during physical stress. New York: Oxford University Press; 1986. [Google Scholar]
- 11.Ayman D., Goldshine A.D. Blood pressure determinations by patients with essential hypertension: The difference between clinic and home readings before treatment. Am. J. Med. Sci. 1940;200:465–474. [Google Scholar]
- 12.Levy R.L., Hillman C.C., Stroud W.D., White P.D. Transient hypertension: Its significance in terms of later development of sustained hypertension and cardiovascular-renal disease. J Am Med Assn. 1944;126:829–833. [Google Scholar]
- 13.Rogers W.F., Palmer R.S. Transient hypertension as a military risk: Its relation to essential hypertension. N. Engl. J. Med. 1944;230:39–42. [Google Scholar]
- 14.Shapiro A., Meyers T., Reier M.F., Ferris E.B. Comparison of blood pressure response to Veriloid and to the doctor. Psychosom. Med. 1954;16:478–488. doi: 10.1097/00006842-195411000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Comstock G.W. An epidemiological study of blood pressure levels in a biracial community in the southern United States. Am. J. Hyg. 1957;65:271–315. doi: 10.1093/oxfordjournals.aje.a119870. [DOI] [PubMed] [Google Scholar]
- 16.Pickering G. Hyperpiesis: High blood-pressure without evident cause: Essential hypertension. BMJ. 1965;2:1021–1026. doi: 10.1136/bmj.2.5469.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kain H.K., Hinman A.T., Sokolow M. Arterial blood pressure measurements with a portable recorder in hypertensive patients: Variability and correlation with ‘casual’ pressure. Circulation. 1964;30:882–892. doi: 10.1161/01.cir.30.6.882. [DOI] [PubMed] [Google Scholar]
- 18.Hinman A.T., Engel B.T., Bickford A.F. Portable blood pressure recorder: accuracy and preliminary use in evaluating intra-daily variation in pressure. Am. Heart J. 1962;63:663–668. doi: 10.1016/0002-8703(62)90011-x. [DOI] [PubMed] [Google Scholar]
- 19.Sokolow M., Werdegar D., Kain H.K., Hinman A.T. Relationship between level of blood pressure measured casually and by portable recorders and severity of complications in essential hypertension. Circulation. 1966;34:279–298. doi: 10.1161/01.cir.34.2.279. [DOI] [PubMed] [Google Scholar]
- 20.Richardson D.W., Honour A.J., Fenton G.W., Stott F.H., Pickering G.W. Variation in pressure throughout the day and night. Clin. Sci. 1964;26:445–460. [PubMed] [Google Scholar]
- 21.Bevan A.T., Honor A.J., Stott F.H. Direct arterial pressure recording in unrestricted man. Clin. Sci. 1969;36:329–344. [PubMed] [Google Scholar]
- 22.James G.D. Ambulatory blood pressure variation: Allostasis and adaptation. Autonom Neuro Basic Clin. 2013;177:87–94. doi: 10.1016/j.autneu.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 23.James G.D. Evaluation of journals, diaries, and indexes of worksite and environmental stress. In: White W.H., editor. Clinical hypertension and vascular disease: Blood pressure monitoring in cardiovascular medicine and therapeutics. 2nd ed. Totowa, N.J: The Humana Press; 2007. pp. 39–58. [Google Scholar]
- 24.Flores J.S. Blood pressure variability: A novel and important risk factor. Can. J. Cardiol. 2013;29:557–563. doi: 10.1016/j.cjca.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Palatini P., Reboldi G., Beilin L.J., et al. Added predictive value of night-time blood pressure variability for cardiovascular events and mortality: The Ambulatory Blood Pressure International Study. Hypertension. 2014;64:487–493. doi: 10.1161/HYPERTENSIONAHA.114.03694. [DOI] [PubMed] [Google Scholar]
- 26.Asayama K., Fang-Fei W., Hara A., Hansen T.W., Li Y., Staessen J.A. Prognosis in relation to blood pressure variability; con side of the argument. Hypertension. 2015;65:1170–1179. doi: 10.1161/HYPERTENSIONAHA.115.04808. [DOI] [PubMed] [Google Scholar]
- 27.Parati G., Ochoa J.E., Lombardi C., Bilo G. Blood pressure variability: Assessment, predictive value, and potential as a therapeutic target. Curr. Hypertens. Rep. 2015;17:23. doi: 10.1007/s11906-015-0537-1. [DOI] [PubMed] [Google Scholar]
- 28.Cannon WB. The wisdom of the body. New York: The Norton Library, Norton WW & Company, reprint 1963; 1939. [Google Scholar]
- 29.Sterling P., Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher J., Reason J., editors. Handbook of life stress. New York: John Wiley; 1988. pp. 629–649. [Google Scholar]
- 30.Mancia G., Parati G., Pomidossi G., Grassi G., Casadei R., Zanchetti A. Alerting reaction and rise in pressure during management by physician and nurse. Hypertension. 1987;9:209–215. doi: 10.1161/01.hyp.9.2.209. [DOI] [PubMed] [Google Scholar]
- 31.Jhalani J., Goyala T., Clemow L., Schwartz J.E., Pickering T.G., Gerin W. Anxiety and outcome expectations predict the white-coat effect. Blood Press. Monit. 2005;10:317–319. doi: 10.1097/00126097-200512000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Longo D., Dorigatti F., Palatini P. Masked hypertension in adults. Blood Press. Monit. 2005;10:307–310. doi: 10.1097/00126097-200512000-00004. [DOI] [PubMed] [Google Scholar]
- 33.James G.D. Continuous blood pressure variation: Hidden adaptability. In: Sievert L.L., Brown D.E., editors. Biological measures of human experience across the lifespan: Making visible the invisible. New York: Springer, Inc.; 2016. pp. 143–169. [Google Scholar]
- 34.Pickering T.G., James G.D., Boddie C., Harshfield G.A., Blank S.G., Laragh J.H. How common is white coat hypertension? JAMA. 1988;259:225–228. [PubMed] [Google Scholar]
- 35.Stergiou G., Palatini P., Asmar R., et al. Blood pressure measurement and hypertension diagnosis in the 2017 US guidelines: First things first. Hypertension. 2018;71:963–965. doi: 10.1161/HYPERTENSIONAHA.118.10853. [DOI] [PubMed] [Google Scholar]
- 36.Zanstra Y.J., Johnston D.W. Cardiovascular reactivity in real life settings: Measurement, mechanisms and meaning. Biol. Psychol. 2011;86:98–105. doi: 10.1016/j.biopsycho.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen T.W., Thijs L., Li Y., et al. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55:1049–1057. doi: 10.1161/HYPERTENSIONAHA.109.140798. [DOI] [PubMed] [Google Scholar]
- 38.Taylor K.S., Heneghan C.J., Stevens R.J., Adams E.C., Nunan D., Ward A. Heterogeneity of prognostic studies of 24-hour blood pressure variability: Systematic review and meta-analysis. PLoS One. 2015;10(5):e0126375. doi: 10.1371/journal.pone.0126375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.James G.D., Pickering T.G., Schlussel Y.R., Clark L.A., Denby L., Pregibon D. Measures of reproducibility of blood pressure variability measured by noninvasive ambulatory blood pressure monitors. J Ambul Monit. 1990;3(2):139–147. [Google Scholar]
- 40.James G.D., Cates E.M., Pickering T.G., Laragh J.H. Parity and perceived job stress elevate blood pressure in young normotensive working women. Am. J. Hypertens. 1989;2:637–639. doi: 10.1093/ajh/2.8.637. [DOI] [PubMed] [Google Scholar]
- 41.Ice G.H., James G.D., Crews D.E. Blood pressure variation in the institutionalized elderly. Coll. Antropol. 2003;27:47–55. [PubMed] [Google Scholar]
- 42.Ice G.H., James G.D. Human biology and stress. In: Stinson S., Bogin B., O’Rourke D., editors. Human biology: An evolutionary and biocultural perspective. 2nd ed. New York: Wiley-Blackwell Publishing; 2012. pp. 459–512. [Google Scholar]
- 43.James G.D., Bovbjerg D.H., Hill L.A. Daily environmental differences in blood pressure and heart rate variability in healthy premenopausal women. Am. J. Hum. Biol. 215(27):136–138. doi: 10.1002/ajhb.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cochran W.G. Sampling techniques. New York: John Wiley & Sons; 1977. [Google Scholar]
- 45.James G.D. Blood pressure response to the daily stressors of urban environments: methodology, basic concepts, and significance. Yearb. Phys. Anthropol. 1991;34:189–210. [Google Scholar]
- 46.Gerin W., James G.D. Psychosocial determinants of hypertension: Laboratory and field models. Blood Press. Monit. 2010;15:93–99. doi: 10.1097/MBP.0b013e3283380e0a. [DOI] [PubMed] [Google Scholar]
- 47.James G.D., Pecker M.S., Pickering T.G., et al. Extreme changes in dietary sodium effect the daily variability and level of blood pressure in borderline hypertensive patients. Am. J. Hum. Biol. 1994;6:283–291. doi: 10.1002/ajhb.1310060303. [DOI] [PubMed] [Google Scholar]
- 48.Modesti P.A., Moriabito M., Bertolozzi I. et al. Weather-related changes in 24-hour blood pressure profile: Effects of age and implications for hypertension management. Hypertension. 2006;47:155–161. doi: 10.1161/01.HYP.0000199192.17126.d4. [DOI] [PubMed] [Google Scholar]
- 49.James G.D., Yee L.S., Harshfield G.A., Blank S., Pickering T.G. Sex differences in factors affecting the daily variation of blood pressure. Soc. Sci. Med. 1988;26:1019–1023. doi: 10.1016/0277-9536(88)90219-5. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz J.E., Warren K., Pickering T.G. Mood, location and physical position as predictors of ambulatory blood pressure and heart rate: application of a multilevel random effects model. Ann. Behav. Med. 1994;16:210–220. [Google Scholar]
- 51.Kamarck T.W., Schiffman S.M., Smithline L., et al. Effects of task strain, social conflict, on ambulatory cardiovascular activity: Life consequences of recurring stress in a multiethnic adult sample. Health Psychol. 1998;17:17–29. doi: 10.1037//0278-6133.17.1.17. [DOI] [PubMed] [Google Scholar]
- 52.Brondolo E., Karlin W., Alexander K., Bubrow A., Schwartz J. Workday communication and ambulatory blood pressure: Implications for the reactivity hypothesis. Psychophysiology. 1999;36:86–94. doi: 10.1017/s0048577299961565. [DOI] [PubMed] [Google Scholar]
- 53.Gump B.B., Polk D.E., Kamarck T.W., Shiffman S. Partner interactions are associated with reduced blood pressure in the natural environment: Ambulatory blood pressure monitoring evidence from a healthy, multiethnic adult sample. Psychosom. Med. 2001;63:423–433. doi: 10.1097/00006842-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 54.Kamarck T.W., Janicki D.L., Shiffman S., et al. Psychosocial demands and ambulatory blood pressure: A field assessment approach. Physiol. Behav. 2002;77:699–704. doi: 10.1016/s0031-9384(02)00921-6. [DOI] [PubMed] [Google Scholar]
- 55.Brown D.E., James G.D., Nordloh L. Comparison of factors affecting daily variation of blood pressure in Filipino-American and Caucasian nurses in Hawaii. Am. J. Phys. Anthropol. 1998;106:373–383. doi: 10.1002/(SICI)1096-8644(199807)106:3<373::AID-AJPA9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 56.Kamarck T.W., Schwartz J.E., Janiki D.L., Schiffman S., Raynor D.A. Correspondence between laboratory and ambulatory measures of cardiovascular reactivity: A multilevel modeling approach. Psychophysiology. 2003;40:675–683. doi: 10.1111/1469-8986.00069. [DOI] [PubMed] [Google Scholar]
- 57.James G.D. Measuring changes in the cardiovascular system: Ambulatory blood pressure. In: Ice G.H., James G.D., editors. Measuring stress in humans: A practical guide for the field. Cambridge: Cambridge University Press; 2007. pp. 158–180. [Google Scholar]
- 58.Pickering T.G., Gerin W. Cardiovascular reactivity in the laboratory and the role of behavioral factors in hypertension: A critical review. Ann. Behav. Med. 1990;12:3–16. [Google Scholar]
- 59.Linden W., Gerin W., Davidson K. Cardiovascular reactivity: Status quo and a research agenda for the new millennium. Psychosom. Med. 2003;65:5–8. doi: 10.1097/01.psy.0000046076.93591.ad. [DOI] [PubMed] [Google Scholar]
- 60.Van Berge-Landry HM, Bovbjerg DH, James GD. The relationship between waking-sleep blood pressure and catecholamine changes in African American and European American women. Blood Pres Monit. 2018;13:257–62. doi: 10.1097/MBP.0b013e3283078f45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.James G.D. Climate-related morphological variation and physiological adaptations in Homo Sapiens. In: Larsen C.S., editor. A companion to biological anthropology. Malden, MA: Wiley-Blackwell; 2010. pp. 153–166. [Google Scholar]
- 62.James G.D., Baker P.T. Human population biology and blood pressure: Evolutionary and ecological considerations and interpretations of population studies. In: Laragh J.H., Brenner B.M., editors. Hypertension: Pathophysiology, diagnosis and management. New York: Raven Press, Ltd.; 1995. pp. 115–126. [Google Scholar]
- 63.Hanna J.M., Brown D.A. Human heat tolerance: Biological and cultural adaptations. Yearb. Phys. Anthropol. 1979;22:163–186. [Google Scholar]
- 64.Young J.H., Chang Y.C., Kim J.D., et al. Differential susceptibility to hypertension is due to selection during the out-of-Africa expansion. PLoS Genet. 2005;1(6):e82. doi: 10.1371/journal.pgen.0010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steegmann A.G. Human adaptation to cold. In: Damon A., editor. Physiological anthropology. New York: Oxford University Press; 1975. pp. 130–166. [Google Scholar]
- 66.Beall C.M., Jablonski N.G., Steegmann A.T. Human adaptation to climate: Temperature, ultraviolet radiation, and altitude. In: Stinson S., Bogin B., O’Rourke D., editors. Human biology: An evolutionary and biocultural perspective. 2nd ed. New York: Wiley-Blackwell Publishing; 2012. pp. 177–250. [Google Scholar]
- 67.Anderson N.B., Lane L.D., Muranaka M., Williams R.B., Jr, Houseworth S.J. Racial differences in blood pressure and forearm vascular responses to the cold face stimulus. Psychosom. Med. 1988;50:57–63. doi: 10.1097/00006842-198801000-00007. [DOI] [PubMed] [Google Scholar]
- 68.Treiber F.A., Musante L., Braden D., et al. Racial differences in hemodynamic responses to the cold face stimulus in children and adults. Psychosom. Med. 1990;52:286–296. doi: 10.1097/00006842-199005000-00003. [DOI] [PubMed] [Google Scholar]
- 69.Kelsey R.M., Alpert B.S., Patterson S.M., Barnard M. Racial differences in hemodynamic responses to environmental thermal stress among adolescents. Circulation. 2000;101:2284–2289. doi: 10.1161/01.cir.101.19.2284. [DOI] [PubMed] [Google Scholar]
- 70.Mills P.J., Dimsdale J.E., Ziegler M.G., Nelesen R.A. Racial differences in epinephrine and beta 2-adrenergic receptors. Hypertension. 1995;25:88–91. doi: 10.1161/01.hyp.25.1.88. [DOI] [PubMed] [Google Scholar]
- 71.Parati G., Ochoa J.E., Bilo G. Moving beyond office blood pressure to achieve a personalized and more precise hypertension management. Which way to go? Hypertension. 2017;70:e20–e31. doi: 10.1161/HYPERTENSIONAHA.117.08250. [DOI] [PubMed] [Google Scholar]
- 72.James G.D., Moucha O.P., Pickering T.G. The normal hourly variation of blood pressure in women: average patterns and the effect of work stress. J. Hum. Hypertens. 1991;5:505–509. [PubMed] [Google Scholar]


