Abstract
Background: Venous thromboembolism (VTE) is a common complication in patients with cancer. Direct oral anticoagulants (DOACs) have been proved to be effective on anticoagulation therapy in many diseases. However, the efficacy and the safety of DOACs in the secondary prevention of cancer-associated thrombosis (CAT) remain unclear. To assess the value of DOACs in patients with CAT, we performed a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies.
Methods: Medline, Embase, and the Cochrane Library were searched from their earliest date through to June 2018. Two investigators independently assessed eligibility. Data were extracted by one investigator and verified by the second investigator. The efficacy outcome of this study was recurrent VTE, whereas the safety outcome was major and clinically relevant nonmajor bleeding. Relative risks (RRs) and their corresponding 95% confidence interval (CI) were determined. To pool the results, the Mantel–Haenszel fixed-effects or random-effects models were used.
Results: A total of nine articles (six randomized controlled trials and three prospective studies) involving 2,697 patients with CAT who were prescribed DOACs (apixaban, edoxaban, rivaroxaban, or dabigatran) and 2,852 patients who were prescribed traditional anticoagulants [vitamin K antagonists (VKAs), low molecular weight heparin (LMWH), dalteparin, or enoxaparin] were compared. VTE recurrence in the DOAC group was significantly lower than that observed in the traditional anticoagulant group (RR: 0.60; 95%CI: 0.49–0.75; I 2: 0%; p < 0.00001). No significant difference in bleeding risk between both groups was found (RR: 0.95; 95%CI: 0.67–1.36; I 2: 75%; p = 0.79).
Conclusions: Our findings showed that anticoagulant therapy with DOACs may be more effective than traditional anticoagulants to prevent recurrent VTE in patients with CAT, while the safety of DOACs may be equal to that of traditional anticoagulants. These findings support the use of DOACs as the first-line therapy for secondary prevention of CAT in most cancer patients.
Keywords: direct oral anticoagulants (DOACs), cancer-associated thrombosis (CAT), secondary prevention, meta-analysis, randomized controlled trials (RCTs), prospective cohort studies
Introduction
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common complication in patients with cancer. Prior studies have suggested that the risk of VTE in cancer patients could be elevated four to seven times (Timp et al., 2013). Unfortunately, the management of cancer-associated thrombosis (CAT) is challenging, as these patients have higher risks of both recurrent VTE and major bleeding (MB) events after treatment (Prandoni et al., 2002). Nowadays, low molecular weight heparin (LMWH) is suggested to be the most useful anticoagulant for CAT (Akl et al., 2014; Farge et al., 2016), but long-term subcutaneous administration is inconvenient for many patients. Nevertheless, most clinical guidelines (2016 ACCP: American College of Chest Physicians; 2013 ASCO: American Society of Clinical Oncology; 2015 BCSH: British Committee for Standards in Haematology; 2014 ESC: European Society of Cardiology; 2011 ESMO: European Society for Medical Oncology) prefer LMWHs as the initial treatment for CAT (Lee and Peterson, 2013; Lee et al., 2015; Bach and Bauersachs, 2016; Kearon et al., 2016; Khorana et al., 2016). Vitamin K antagonists (VKAs) are also effective, but their efficacy is influenced by many external factors, requiring repeated blood taking to assess clotting.
In recent years, direct oral anticoagulants (DOACs), including factor IIa inhibitors and factor Xa inhibitors, have been proved to be effective on anticoagulation therapy in many diseases. Compared with LMWH and VKAs, these drugs have advantages such as convenience, as no therapeutic monitoring is required. Although many randomized controlled trials (RCTs) and observational studies have examined the efficacy and safety of DOACs for the secondary prevention of CAT, there are inconsistencies regarding the results of these studies, and thus, the efficacy and safety of DOACs for the secondary prevention of CAT remain unclear. Currently, DOACs are recommended as the second-line therapy for patients who are unable or unwilling to use long-term parenteral therapy (Lee and Peterson, 2013; Lee et al., 2015; Bach and Bauersachs, 2016; Kearon et al., 2016; Khorana et al., 2016). Therefore, the aim of this systematic review and meta-analysis of RCTs and prospective cohort studies is to assess the efficacy and safety of DOACs for secondary prevention of CAT.
Materials and Methods
Search Strategy
A holistic review of the published articles (through the end of June 2018) with limitation to humans was performed by using Medline (PubMed), Embase, and the Cochrane Library database. The search strategies and keywords were as follow: (“new oral anticoagulants” or “direct oral anticoagulants” or “DOAC” or “NOAC” or “new oral anticoagul” or “direct oral anticoagul” or “factor Xa inhibitors” or “rivaroxaban” or “apixaban” or “edoxaban” or “antithrombins” or “direct thrombin inhibitors” or “dabigatran”) and (“cancer” or “neoplasm” or “carcinoma” or “adenoma” or “adenocarcinoma” or “lymphoma” or “leukemia”) and (“venous thrombosis” or “venous thromboembolism” or “venous thrombos” or “deep venous thrombosis” or “deep venous thrombos” or “pulmonary embolism” or “pulmonary thromboembolism” or “VTE” or “DVT” or “PE”). All our search terms are applied for anywhere in the text.
Study Eligibility
Two authors (YW and HL) independently identified studies eligible for inclusion based on an initial screen of reference titles and abstracts. Studies were considered potentially eligible for this systematic review if they met the following inclusion criteria: 1) Adult patients (age > 18 years) developed CAT and received anticoagulant therapy with DOACs (apixaban, edoxaban, rivaroxaban, or dabigatran); 2) The outcomes were recurrent VTE or major bleeding (MB) or clinically relevant nonmajor bleeding (CRNMB); 3) We only included RCTs and prospective cohort studies and excluded case reports, review articles, guidelines, editorials, meta-analyses, and retrospective studies; 4) Only articles in English were selected for the final meta-analysis; 5) Only manuscripts with extractable primary data among patients with CAT were included in the final analysis.
Outcome Definition
The efficacy outcome of this study was defined as recurrent VTE, and the safety outcome was MB and CRNMB during patients receiving anticoagulant treatment. These recurrent VTE events could be asymptomatic or symptomatic, including deep vein thrombosis (DVT) and pulmonary embolism (PE). DVT and PE occurring in the same patient was recorded as single event. MB and CRNMB episodes were defined according to the criteria of the International Society on Thrombosis and Haemostasis (Schulman and Kearon, 2005; Kaatz et al., 2015).
Data Extraction and Quality Assessment
Data were extracted into a standardized collection form by one investigator (YW) and verified by a second (HL). Data collected from each study included author, year of publication, study design, duration of patient follow-up, sample size, type of anticoagulation, mean age, male gender, and endpoint definition and incidence. Risk of bias for each study was using the Cochrane risk of bias tool for RCTs (Higgins et al., 2011) ( Table 1 ); the evaluated domains included random sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. The quality of the prospective cohort studies were assessed using the Newcastle–Ottawa scale ( Table 2 ); the evaluated domains included representativeness of the exposed cohort, selection of the unexposed cohort, ascertainment of exposure, outcome of interest not present at start of study, control for important factor or additional factor, assessment of outcome, follow-up long enough for outcomes to occur, and adequacy of follow-up of cohorts. The degree of bias found in the individual studies were categorized into high, moderate, or low risk of bias according to the Cochrane risk of bias tool and the Newcastle–Ottawa scale.
Table 1.
Methodological quality of randomized controlled trials (RCTs) included in the meta-analysis*.
| First author, publication year | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other sources of bias | Quality |
|---|---|---|---|---|---|---|---|---|
| Raskob et al. (2018) | + | + | − | + | + | + | + | Low risk of bias |
| Raskob et al. (2016) | + | + | + | + | + | + | + | Low risk of bias |
| Agnelli et al. (2015) | ? | ? | + | + | + | + | + | Moderate risk of bias |
| Schulman et al. (2015) | − | + | + | + | + | + | + | Low risk of bias |
| Prins et al. (2014) | + | − | − | + | + | + | + | High risk of bias |
| Young et al. (2017) | + | + | − | + | + | + | + | Low risk of bias |
*Cochrane Risk of Bias Tool for randomized controlled trials is used to assess bias for RCTs.
Low risk is represented by “+”,
high risk is represented by “−”,
unclear risk/insufficient information is represented by “?”.
Table 2.
Methodological quality of cohort studies included in the meta-analysis*.
| First author, publication year | Representativeness of the exposed cohort | Selection of the unexposed cohort | Ascertainment of exposure | Outcome of interest not present at start of study | Control for important factor or additional factor† | Assessment of outcome | Follow-up long enough for outcomes to occur‡ | Adequacy of follow-up of cohorts§ | Quality |
|---|---|---|---|---|---|---|---|---|---|
| McBane et al. (2016) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | Low risk of bias |
| Angelini et al. (2018) | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | — | ☆ | Moderate risk of bias |
| Ageno et al. (2016) | ☆ | ☆ | ☆ | ☆ | ☆☆ | — | ☆ | ☆ | Low risk of bias |
*A study could be awarded a maximum of one star for each item except for the item control for important factor or additional factor. The definition/explanation of each column of the Newcastle–Ottawa scale is available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
†A maximum of two stars could be awarded for this item. Studies that controlled for anticoagulation treatment for at least 3 months received one star, whereas studies that controlled for other important confounders received an additional star.
‡A cohort study with a follow-up time >6 months was assigned one star.
§A cohort study with a follow-up rate >75% was assigned one star.
Statistical Analysis
We performed meta-analysis for the efficacy and safety outcomes, assessed by relative risk (RR), and associated 95% confidence interval (CI), with a P < 0.05 considered statistically significant. We used the software Review Manager (RevMan, version 5.3, The Cochrane Collaboration, Copenhagen, Denmark) (DerSimonian and Laird, 1986) to create forest plots comparing RRs using the Mantel–Haenszel fixed-effects or random effects model. The Cochrane P value and the I 2 statistics was used to quantify the heterogeneity across the studies (Higgins et al., 2003). Statistically, heterogeneity was considered to be present when Cochrane P value ≤0.05, and the I 2 statistics <25%, 25–75%, and >75% to represent low, moderate, and high degree of heterogeneity, respectively. Funnel plots were used to assess for publication bias. Subgroup analyses were conducted separately for the type of factor Xa inhibitors (e.g., rivaroxaban and edoxaban) and study type (RCTs vs. prospective cohort studies). Finally, considering heterogeneity between some studies, we carried out a sensitivity analysis by excluding one study at a time sequentially to evaluate the impact of individual data set on the overall effect estimate.
Results
Study Selection and Characteristics
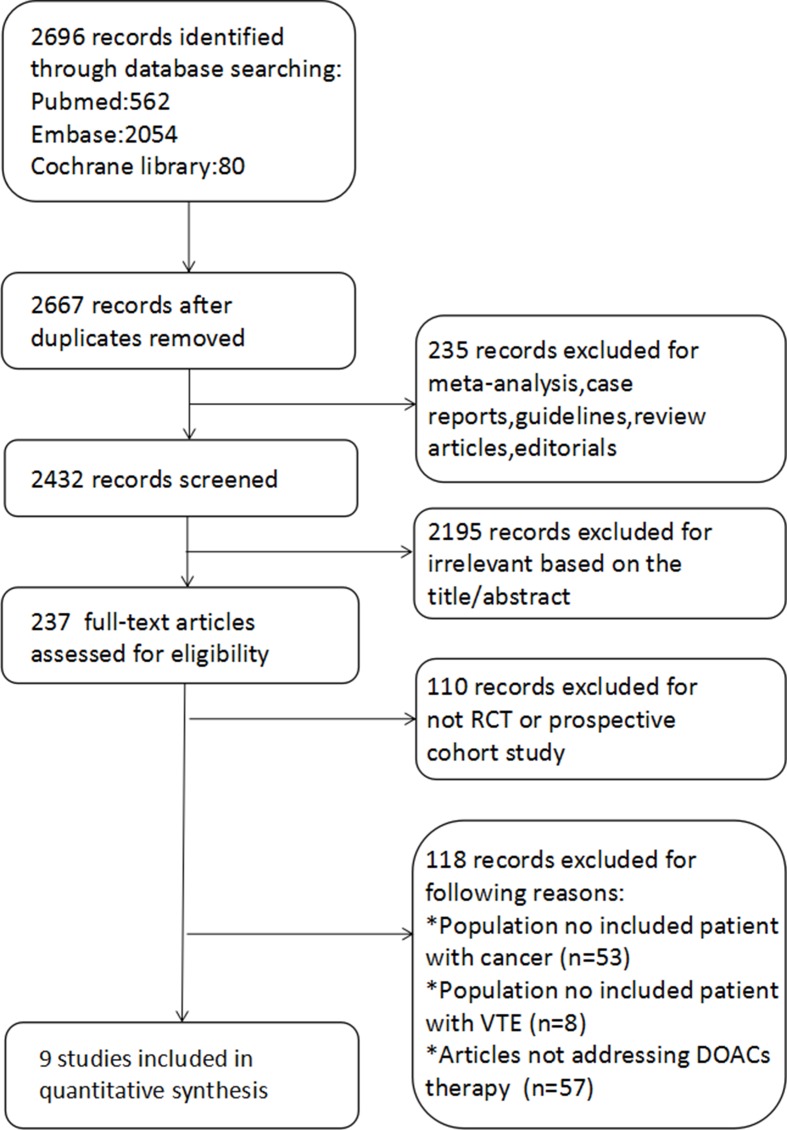
The flow diagram of the evaluation process is shown in Figure 1 . The literature search yielded a total of 2,696 related articles. After duplicates were removed, 2,667 entries were screened further. Further 235 records were excluded, as they were case reports, review articles, guidelines, editorials, or meta-analyses. Moreover, 2,195 records were excluded, as they were not relevant to our study aim based on the title and abstract. Full-text screening led to exclusion of 110 records, as these did not take the RCT or prospective cohort study designs and 118 records as these were found to be irrelevant to our study aim.
Figure 1.
The flow diagram.
In the end, nine articles [six RCTs (Prins et al., 2014; Agnelli et al., 2015; Schulman et al., 2015; Raskob et al., 2016; Young et al., 2017; Raskob et al., 2018) and three prospective cohort studies (Ageno et al., 2016; McBane et al., 2016; Angelini et al., 2018)] were included. A total of 2,697 patients with CAT received anticoagulant therapy with DOACs (apixaban, edoxaban, rivaroxaban, or dabigatran), and 2,852 patients received anticoagulant therapy with traditional anticoagulants (VKAs, LMWH, dalteparin, or enoxaparin). Five of the included studies recruited patients receiving anticoagulant therapy with rivaroxaban (Prins et al., 2014; Ageno et al., 2016; McBane et al., 2016; Young et al., 2017; Angelini et al., 2018), two of the studies with edoxaban (Raskob et al., 2016; Raskob et al., 2018), one with apixaban (Agnelli et al., 2015), and one with dabigatran (Schulman et al., 2015). The baseline characteristics of the studies included in this systematic review are shown in Table 3 , while the drugs used in the assessed studies are shown in Table 4 .
Table 3.
Baseline characteristics.
| Study | Design | Duration of study design follow-up (years) | Intervention | Outcome | ||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | DOACs | nDOACs | Endpoint | DOACs | nDOACs | |||
| Raskob et al. (2018) | RCT | 12 months | Number of subjects | 522 | 524 | Recurrent VTE | 41/522 | 59/524 |
| Mean age | 64.3 ± 11.0 years | 63.7 ± 11.7 years | MB or CRNMB | 97/522 | 73/524 | |||
| Male gender | 53.1% | 50.2% | ||||||
| Raskob (2016) | RCT | 3–12 months | Number of subjects | 650 | 666 | Recurrent VTE | 33/650 | 61/666 |
| Mean age | 66y | 67y | MB or CRNMB | 83/572 | 119/569 | |||
| Male gender | 48% | 52% | ||||||
| Agnelli et al. (2015) | RCT | 6 months | Number of subjects | 284 | 260 | Recurrent VTE | 5/260 | 16/253 |
| Mean age | 65.5y | 65.1y | MB or CRNMB | 3/271 | 9/259 | |||
| Male gender | 56.8% | 60.5% | ||||||
| Schulman et al. (2015) | RCT | 6 months | Number of subjects | 173 | 162 | Recurrent VTE | 9/173 | 12/162 |
| Mean age | 63.5 ± 12.1 years | 65.3 ± 12.0 years | MB or CRNMB | 23/159 | 20/152 | |||
| Male gender | 51% | 55% | ||||||
| Prins et al. (2014) | RCT | 3–12 months | Number of subjects | 584 | 534 | Recurrent VTE | 21/584 | 25/534 |
| Mean age | N/A | N/A | MB or CRNMB | 73/584 | 71/534 | |||
| Male gender | N/A | N/A | ||||||
| Young et al. (2017) | RCT | 6 months | Number of subjects | 203 | 203 | Recurrent VTE | 8/203 | 22/203 |
| Mean age | 67y | 67y | MB or CRNMB | 11/203 | 34/203 | |||
| Male gender | 53% | 48% | ||||||
| McBane et al. (2016) | CS | 6 months | Number of subjects | 135 | 121 | Recurrent VTE | 4/135 | 2/121 |
| Mean age | 65 ± 14 years | 66 ± 12 years | MB or CRNMB | 3/135 | 7/121 | |||
| Male gender | 50% | 62% | ||||||
| Angelini et al. (2018) | CS | N/A | Number of subjects | 24 | 166 | Recurrent VTE | 0/24 | 17/166 |
| Mean age | N/A | N/A | MB or CRNMB | N/A | N/A | |||
| Male gender | N/A | N/A | ||||||
| Ageno et al. (2016) | CS | 12 months | Number of subjects | 146 | 223 | Recurrent VTE | 5/146 | 10/223 |
| Mean age | 69y | 68y | MB or CRNMB | 2/146 | 8/223 | |||
| Male gender | 52% | 47% | ||||||
*RCT, randomized controlled trial; CS, cohort study; VTE, venous thromboembolism; MB, major bleeding; CRNMB, clinically relevant nonmajor bleeding; DOACs, direct oral anticoagulants; nDOACS, not direct oral anticoagulants.
Table 4.
The drugs used in the assessed studies.
| Study | Drugs | |
|---|---|---|
| DOACs | nDOACs | |
| Raskob et al. (2018) | Edoxaban | Dalteparin |
| Raskob et al. (2016) | Edoxaban | Warfarin |
| Agnelli et al. (2015) | Apixaban | Enoxaparin/warfarin |
| Schulman et al. (2015) | Dabigatran | Warfarin |
| Prins et al. (2014) | Rivaroxaban | Enoxaparin/warfarin |
| Young et al. (2017) | Rivaroxaban | LMWH |
| McBane et al. (2016) | Rivaroxaban | LMWH |
| Angelini et al. (2018) | Rivaroxaban | Enoxaparin |
| Ageno et al. (2016) | Rivaroxaban | Dalteparin |
*RCT, randomized controlled trial; CS, cohort study; LMWH, low molecular weight heparin.
VTE Recurrence
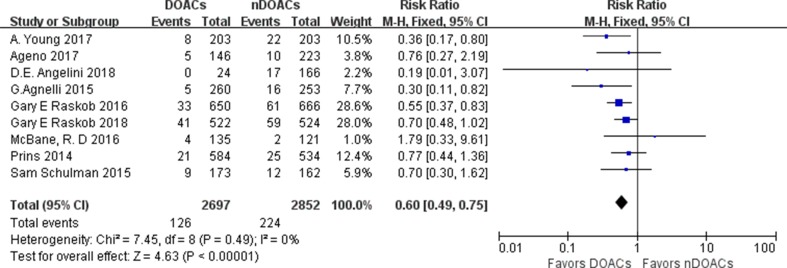
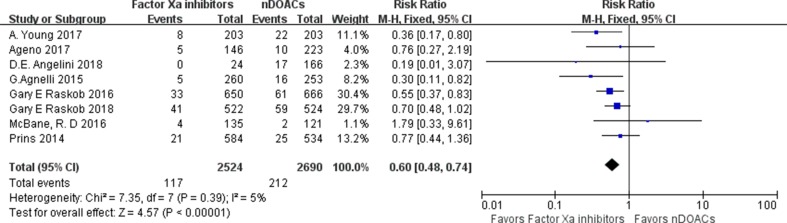
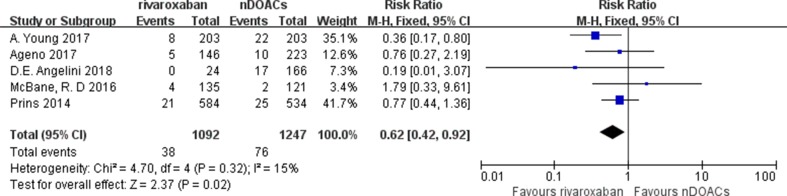
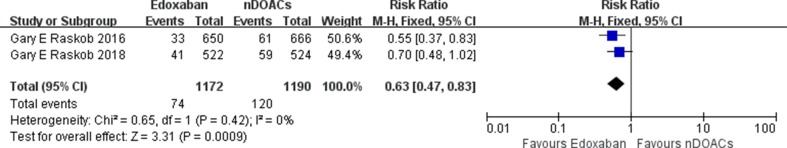
VTE recurrence was evaluated in nine studies. VTE recurrence occurred in 126 of 2,697 patients (4.7%) treated with DOACs and in 224 of 2,852 patients (7.9%) treated with traditional anticoagulants. The incidence rates of VTE recurrence in survivor treated with a DOACs varied from 0 to 7.85%, and those with CAT treated with traditional anticoagulants varied from 1.6% to 11.26%. The recurrence rate of VTE in DOACs group was significantly lower than that of the traditional anticoagulant group [relative risk (RR): 0.60; 95% confidence interval (CI): 0.49–0.75; I 2: 0%; p < 0.00001] ( Figure 2 ). Inspection of the funnel plot did not reveal publication bias ( Supplementary Figure 1 ). A subgroup analysis on studies comparing factor Xa inhibitors (apixaban, edoxaban, and rivaroxaban) with traditional anticoagulants was performed, demonstrating a lower recurrence for the factor Xa Inhibitors group (RR: 0.60; 95%CI: 0.48–0.74; I 2: 5%; p < 0.00001) ( Figure 3 ), rivaroxaban group (RR: 0.62; 95%CI: 0.42–0.92; I 2: 15%; p = 0.02) ( Figure 4 ), and edoxaban group (RR: 0.63; 95%CI: 0.47–0.83; I 2: 0%; p = 0.0009) ( Figure 5 ). Subgroup analysis on RCTs showed a lower recurrence for the DOACs group ( Supplementary Figure 2 ) while that of the prospective cohort studies did not demonstrate a statistical significance ( Supplementary Figure 3 ).
Figure 2.
Recurrence venous thromboembolism (VTE) forest plot.
Figure 3.
Factor Xa inhibitors recurrent VTE forest plot.
Figure 4.
Rivaroxaban recurrent VTE forest plot.
Figure 5.
Edoxaban recurrent VTE forest plot.
Bleeding Events
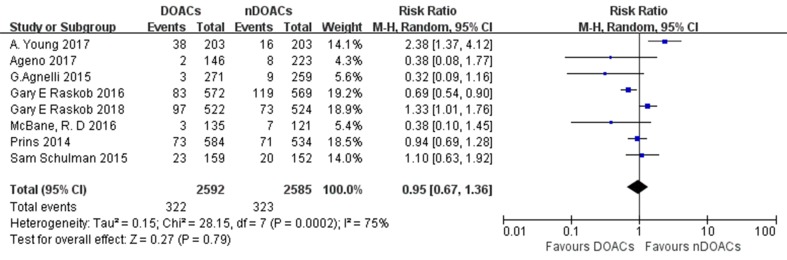
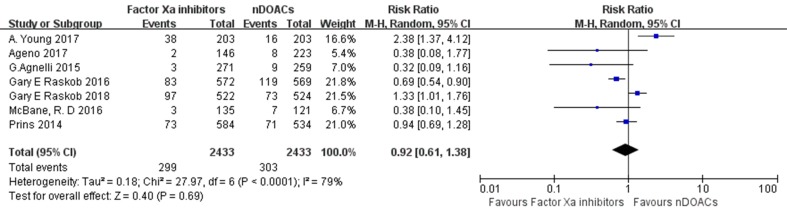
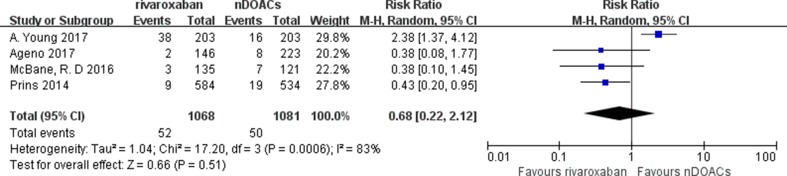
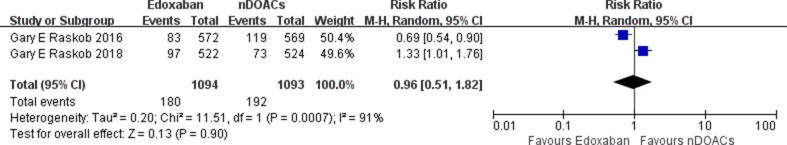
MB and CRNMB were evaluated in eight studies. Bleeding events occurred in 322 of the 2,592 patients (12.4%) treated with DOACs and in 323 of the 2,585 patients (12.5%) treated with traditional anticoagulants. The incidence rates of bleeding in patients with CAT treated with DOACs varied from 1.11 to 18.58%, and those in patients with CAT treated with traditional anticoagulants varied from 3.47 to 20.91%. No significant difference in bleeding risk was found between both groups (RR: 0.95; 95%CI: 0.67–1.36; I 2: 75%; p = 0.79) ( Figure 6 ). There did not appear to be a publication bias across studies based on visual inspection of the funnel plots ( Supplementary Figure 4 ). Three subgroup analysis on studies comparing factor Xa inhibitors (apixaban, edoxaban, and rivaroxaban), rivaroxaban, or edoxaban with not direct oral anticoagulants (nDOACs) was performed. There was no significant difference in bleeding risk between Factor Xa inhibitors group (RR: 0.92; 95%CI: 0.61–1.38; I 2: 79%; p = 0.69) ( Figure 7 ), rivaroxaban group (RR: 0.68; 95%CI: 0.22–2.12; I 2: 83%; p = 0.51) (Figure 8 ), or edoxaban group (RR: 0.96; 95%CI: 0.51–1.82; I 2: 91%; p = 0.90) ( Figure 9 ) and nDOACs group. Subgroup analysis based on study type did not reach statistical significance (RCTs: Supplementary Figure 5 ; prospective cohort studies: Supplementary Figure 6 ). Our meta-analyses demonstrated substantial heterogeneity between the studies (I 2: 75–91%).
Figure 6.
Major bleeding (MB) or clinically relevant nonmajor bleeding (CRNMB) forest plot.
Figure 7.
Factor Xa inhibitors MB or CRNMB forest plot.
Figure 8.
Rivaroxaban MB or CRNMB forest plot.
Figure 9.
Edoxaban MB or CRNMB forest plot.
Sensitivity Analysis
Sensitivity analysis by the leave-one-out method was conducted to evaluate the impact of individual data set on the overall outcome. No individual study, when excluded, resulted in significant alterations in any of the study outcomes.
Discussion
The main findings of this systematic review and meta-analysis are that anticoagulant therapy with DOACs is more effective than traditional anticoagulants to prevent recurrent VTE in patients with CAT, while the safety of DOACs is equal to traditional anticoagulants.
Over the past years, the use of DOACs has increased significantly, offering alternative choices to traditional anticoagulants for a wide range of therapeutic indications, including non-valvular atrial fibrillation (Bhardwaj et al., 2018) and VTE (Tse et al., 2018). The prevalence of VTE in cancer patients is higher than that in patients who are not suffering from cancer. Cancer patients with VTE are also at a higher risk of recurrent VTE after anticoagulant therapy. However, the efficacy and safety of DOACs in cancer patients are still unclear.
A subgroup analysis of RCTs (EINSTEIN-DVT and EINSTEIN-PE) by Prins (Prins et al., 2014) published in 2014 found that rivaroxaban had a similar efficacy to prevent recurrence of VTE and reduced MB events compared with enoxaparin and VKAs in patients with CAT. A meta-analysis of RCTs, prospective and retrospective cohort studies (Li et al., 2018), found that DOACs were more effective than LMWHs for the prevention of recurrent VTE but were associated with increased risk of bleeding. A network meta-analysis (Vedovati et al., 2018), which included 12 RCTs with DOACs, VKAs, and LMWH, found that DOACs are more effective than VKAs and equal to LMWHs without significant differences in MB. However, another network meta-analysis of 13 RCTs (Sobieraj et al., 2018) found that DOACs are more effective than VKAs and LMWH in CAT but were associated with increased MB risk compared with LMWH. These different results may be due to the differences in the number, type, and quality of the studies included. Thus, there remains no consensus on the efficacy and safety of DOACs for the secondary prevention of CAT compared with traditional anticoagulants. Therefore, we conducted this study aiming to assess the value of DOACs for the secondary prevention of patients with CAT.
To our knowledge, this is the first meta-analysis summarizing the efficacy and safety of DOACs for the secondary prevention of patients with CAT including only RCTs and prospective cohort studies. RCT and prospective studies have a lower likelihood of selection bias and recall bias compared with retrospective studies. This is the reason why the latter study type was excluded. Our meta-analysis quantitatively assessed the value of DOACs in patients with CAT compared with all the traditional anticoagulants (VKAs, LMWH, dalteparin, and enoxaparin). Our results found that the recurrence rate of VTE in DOACs group was significantly lower than that in the traditional anticoagulant group and there was no significant difference in bleeding risk between the DOAC and traditional anticoagulant groups. In addition, of the nine studies we eventually included, only one assessed dabigatran (Schulman et al., 2015). Hence, we performed a subgroup analysis concerning on factor Xa inhibitors (apixaban, edoxaban, and rivaroxaban) only. Five studies evaluated the use of rivaroxaban (Prins et al., 2014; Ageno et al., 2016; McBane et al., 2016; Young et al., 2017; Angelini et al., 2018), and two studies assessed the use of edoxaban (Raskob et al., 2016; Raskob et al., 2018). There were some differences between the assessed studies, including study designs (RCTs and prospective cohort studies), drugs used, and cancer type. All of these factors could have contributed to the heterogeneity observed between the studies. Subgroup analyses were also conducted separately for RCTs and prospective cohort studies. The results of efficacy and safety were roughly the same as the main analyses, and the heterogeneity was also acceptable. Finally, sensitivity analysis by leave-one-out method did not demonstrate significant effects by any single study.
The pharmacological actions of DOACs are well established, involving the inhibition of either factor IIa or factor Xa directly. The clearance of DOACs is less affected by other factors unlike vitamin K antagonists, and DOACs are generally eliminated from the body quickly. Their onset is relatively rapid, and reversal agents are available in case of uncontrolled bleeding. Finally, the oral route of DOACs offers advantages over subcutaneous administration for patients’ convenience. In summary, these potential mechanisms make DOACs an advantage for long-term secondary prevention of VTE recurrence in patients with CAT. Thus, a number of expert consensus are now recommending DOACs for the secondary prevention of patients with CAT. Together, our systematic review and meta-analysis contributes to the literature by providing clinicians and policymakers with new insight to aid decision making for patients with CAT. Future research studies should explore the roles of DOACs for primary prevention of CAT.
Limitations
Several limitations of this study should be noted. Firstly, only a small number of studies have been included in this systematic review and meta-analysis, with considerable differences in drugs assessed, which may have different efficacies and safety profiles. By grouping these diverse drugs into only two groups (DOACs vs traditional anticoagulants), differences between individual drugs might have been concealed, resulting in potentially skewed results. Secondly, it should be acknowledged that there were differences in baseline characteristics of the patients enrolled into each study, such as follow-up duration, sample size, age, and gender. Moreover, information on the type of cancer and previous patient medical histories were not available in all of the studies, making meta-regression analysis not possible. Thirdly, our meta-analysis demonstrated substantial heterogeneity between the studies in terms of safety (I 2: 75–91%). We have not identified a source of heterogeneity but carried out a sensitivity analysis by excluding each included study individually, sequentially to evaluate the impact of individual data set on the overall outcome. The heterogeneity in terms of safety might have reduced the robustness of our conclusion. Finally, we have not performed a meta-analysis on DOACs for primary prevention of CAT because even a fewer number of studies were published when compared with secondary prevention. This is nevertheless an important topic, which remains to be explored in future studies.
Conclusions
Our findings showed that anticoagulant therapy with DOACs may be more effective than traditional anticoagulants to prevent recurrent VTE in patients with CAT, while the safety of DOACs may be equal to that of traditional anticoagulants. These findings support the use of DOACs as the first-line therapy for secondary prevention of CAT in most cancer patients.
Author Contributions
This study was conceived and designed by YW and HL, and YX, YW, and HL did the data collection and data analysis and wrote the main manuscript text along with preparing the tables and figures. YX and GT supervised the data collection and data analysis and critically revised the manuscript. All authors reviewed the manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (no. 81570313, no. 81700245) and the Chang Jiang Scholars Program of China (no. T2017124).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Materials
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2019.00773/full#supplementary-material
Recurrent VTE funnel plot.
RCT recurrent VTE forest plot.
Prospective cohort studies recurrent VTE forest plot.
MB or CRNMB funnel plot.
RCT MB or CRNMB forest plot.
Prospective cohort studies MB or CRNMB forest plot.
References
- Ageno W., Mantovani L. G., Haas S., Kreutz R., Monje D., Schneider J., et al. (2016). Safety and effectiveness of oral rivaroxaban versus standard anticoagulation for the treatment of symptomatic deep-vein thrombosis (xalia): an international, prospective, non-interventional study. Lancet Haematol. 3, e12–21. 10.1016/S2352-3026(15)00257-4 [DOI] [PubMed] [Google Scholar]
- Agnelli G., Buller H. R., Cohen A., Gallus A. S., Lee T. C., Pak R., et al. (2015). Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the amplify trial. J. Thromb. Haemost. 13, 2187–2191. 10.1111/jth.13153 [DOI] [PubMed] [Google Scholar]
- Akl E. A., Kahale L., Barba M., Neumann I., Labedi N., Terrenato I., et al. (2014). Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst. Rev. 12, Cd006650. 10.1002/14651858.CD006652.pub4 [DOI] [PubMed] [Google Scholar]
- Angelini D. E., Rahman S., Poudel S., Radivoyevitch T., Wilks M., Pinkava V., et al. (2018). Treatment outcomes of cancer associated venous thrombosis; the cleveland clinic experience. Thromb. Res. 164, S205–S206. 10.1016/j.thromres.2018.02.058 [DOI] [Google Scholar]
- Bach M., Bauersachs R. (2016). Spotlight on advances in vte management: Callisto and Einstein choice. Thromb. Haemost. 116, S24–S32. 10.1160/TH16-06-0486 [DOI] [PubMed] [Google Scholar]
- Bhardwaj A., Sawant A. C., Tetewsky S., McCray W., Mammen M., Khan S., et al. (2018). Superior survival with direct oral anticoagulants compared to warfarin in patients with atrial fibrillation and underlying cancer: veterans affairs database study. Circulation 138. 10.1161/circ.138.suppl_1.12775 [DOI] [PMC free article] [PubMed]
- DerSimonian R., Laird N. (1986). Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- Farge D., Bounameaux H., Brenner B., Cajfinger F., Debourdeau P., Khorana A. A., et al. (2016). International clinical practice guidelines including guidance for direct oral anticoagulants in the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 17, e452–e466. 10.1016/S1470-2045(16)30369-2 [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Altman D. G., Gotzsche P. C., Juni P., Moher D., Oxman A. D., et al. (2011). The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J., Altman D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327, 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz S., Ahmad D., Spyropoulos A. C., Schulman S. (2015). Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the ssc of the isth. J. Thromb. Haemost. 13, 2119–2126. 10.1111/jth.13140 [DOI] [PubMed] [Google Scholar]
- Kearon C., Akl E. A., Ornelas J., Blaivas A., Jimenez D., Bounameaux H., et al. (2016). Antithrombotic therapy for vte disease: chest guideline and expert panel report. Chest 149, 315–352. 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- Khorana A. A., Yannicelli D., McCrae K. R., Milentijevic D., Crivera C., Nelson W. W., et al. (2016). Evaluation of us prescription patterns: are treatment guidelines for cancer-associated venous thromboembolism being followed? Thromb. Res. 145, 51–53. 10.1016/j.thromres.2016.07.013 [DOI] [PubMed] [Google Scholar]
- Lee A. Y. Y., Kamphuisen P. W., Meyer G., Bauersachs R., Janas M. S., Jarner M. F., et al. (2015). Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA 314, 677–686. 10.1001/jama.2015.9243 [DOI] [PubMed] [Google Scholar]
- Lee A. Y., Peterson E. A. (2013). Treatment of cancer-associated thrombosis. Blood 122, 2310–2317. 10.1182/blood-2013-04-460162 [DOI] [PubMed] [Google Scholar]
- Li A., Garcia D. A., Lyman G. H., Carrier M., (2018). Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (cat): a systematic review and meta-analysis. Thromb. Res. 173, 158–163. 10.1016/j.thromres.2018.02.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBane R. D., Simmons B., Saadiq R., Wysokinski W., Bott-Kitslaar D., Lenz C., et al. (2016). Rivaroxaban compared to low molecular weight heparin in treatment of malignancy associated venous thromboembolism. J. Am. Coll. Cardiol. 67, 2257. 10.1016/S0735-1097(16)32258-6 [DOI] [Google Scholar]
- Prandoni P., Lensing A. W., Piccioli A., Bernardi E., Simioni P., Girolami B., et al. (2002). Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 100, 3484–3488. 10.1182/blood-2002-01-0108 [DOI] [PubMed] [Google Scholar]
- Prins M. H., Lensing A. W. A., Brighton T. A., Bernardi E., Simioni P., Girolami B., et al. (2014). Oral rivaroxaban versus enoxaparin with vitamin k antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (einstein-dvt and einstein-pe): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 1, e37–e46. 10.1016/S2352-3026(14)70018-3 [DOI] [PubMed] [Google Scholar]
- Raskob G. E., van Es N., Segers A., Angchaisuksiri P., Oh D., Boda Z., et al. (2016). Edoxaban for venous thromboembolism in patients with cancer: results from a non-inferiority subgroup analysis of the hokusai-vte randomised, double-blind, double-dummy trial. Lancet Haematol. 3, e379–387. 10.1016/S2352-3026(16)30057-6 [DOI] [PubMed] [Google Scholar]
- Raskob G. E., van Es N., Verhamme P., Carrier M., Di Nisio M., Garcia D., et al. (2018). Edoxaban for the treatment of cancer-associated venous thromboembolism. N. Engl. J. Med. 378, 615–624. 10.1056/NEJMoa1711948 [DOI] [PubMed] [Google Scholar]
- Schulman S., Kearon C. (2005). Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 3, 692–694. 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- Schulman S., Goldhaber S. Z., Kearon C., Kakkar A. K., Schellong S., Eriksson H., et al. (2015). Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb. Haemost. 114, 150–157. 10.1160/TH14-11-0977 [DOI] [PubMed] [Google Scholar]
- Sobieraj D. M., Baker W. L., Smith E., et al. (2018). Anticoagulation for the treatment of cancer-associated thrombosis: a systematic review and network meta-analysis of randomized trials 1076029618800792 [DOI] [PMC free article] [PubMed]
- Timp J. F., Braekkan S. K., Versteeg H. H., Cannegieter S. C. (2013). Epidemiology of cancer-associated venous thrombosis. Blood 122, 1712–1723. 10.1182/blood-2013-04-460121 [DOI] [PubMed] [Google Scholar]
- Tse G., Gong M., Li G., Wong S. H., Wu W. K. K., Wong W. T., et al. (2018). Genotype-guided warfarin dosing vs. Br. J. Clin. Pharmacol. 84, 1868–1882. 10.1111/bcp.13621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedovati M. C., Giustozzi M., Bonitta G., Agnelli G., Becattini C. (2018). Efficacy and safety of anticoagulant agents in patients with venous thromboembolism and cancer: a network meta-analysis. Thromb. Res. 170, 175–180. 10.1016/j.thromres.2018.08.023 [DOI] [PubMed] [Google Scholar]
- Young A., Marshall A., Thirlwall J., Hill C., Hale D., Dunn J., et al. (2017). Anticoagulation therapy in selected cancer patients at risk of recurrence of venous thromboembolism: results of the select-d™ pilot trial. Blood 130, 194. 10.1016/j.thromres.2018.02.036 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Recurrent VTE funnel plot.
RCT recurrent VTE forest plot.
Prospective cohort studies recurrent VTE forest plot.
MB or CRNMB funnel plot.
RCT MB or CRNMB forest plot.
Prospective cohort studies MB or CRNMB forest plot.