Abstract
Healthcare transition from childhood to adulthood is required to ensure continuity of care of an increasing number of individuals with chronic conditions surviving into adulthood. The transition for patients with tuberous sclerosis complex (TSC) is complicated by the multisystemic nature of this condition, age‐dependent manifestations, and high clinical variability and by the presence of intellectual disability in at least half of the individuals. In this article, we address the medical needs regarding each TSC‐related manifestation in adulthood, and the services and support required. We review existing models of transition in different chronic conditions, discuss our experience in transitioning from the pediatric to the adult TSC Clinic at our Institution, and propose general rules to follow when establishing a transition program for TSC. Although a generalizable transition model for TSC is likely not feasible for all Institutions, a multidisciplinary TSC clinic is probably the best model, developed in accordance with the resources available and country‐specific healthcare systems. Coordination of care and education of the adult team should be always sought regardless of the transition model.
Keywords: adult care, adult manifestations, healthcare needs, pediatric care, transition, transitional program, TSC, tuberous sclerosis complex
1. INTRODUCTION
Healthcare transition is defined as the “purposeful, planned movement of adolescents and young adults with chronic physical and medical conditions from child‐centered to adult‐oriented health care systems” (Blum et al., 1993). Quality healthcare should be continuous during transition for all young people, but for individuals with rare diseases and intellectual disability (ID), methods of transition differ from standard pathways.
The process of transition for people with special needs was designated as a top‐10 priority by the 2007 American Academy of Pediatrics Annual Leadership Forum. It begins in childhood/early adolescence and has the goal of helping children become as independent as possible as adults, and of seeking to meet their individual needs as they move into adulthood (American Academy of Pediatrics, American Academy of Family Physicians, & American College of Physicians‐American Society of Internal Medicine, 2002).
Unlike decades ago when a significant number of children with ID and/or birth defects died during childhood or adolescence, a larger number of these individuals now survive into adulthood thanks to improved diagnostic and therapeutic resources, and support services. Mortality during childhood in tuberous sclerosis complex (TSC) is low (Amin et al., 2017), thus warranting that adult clinics be ready to provide long‐term care to these individuals. However, these patients and their families often face significant barriers in transitioning from the multidisciplinary pediatric healthcare system to an adult setting, which is generally individually based and less familiar with rare disorders (Royal College of Paediatrics and Child Health, 2003).
The importance of transition is being increasingly recognized and has become a matter of discussion in the scientific community and among patient support organizations, including those focused on TSC (Both et al., 2018). However, there are only a few articles in the literature that address transition in individuals with rare diseases (Waldboth, Patch, Mahrer‐Imhof, & Metcalfe, 2016), and international or national guidelines have not yet been delineated. A few pediatric centers have tried to establish transition programs in their practices, but no consensus on specific models for certain rare diseases have been obtained to date (American Academy of Pediatrics et al., 2011; Carrizosa, An, Appleton, Camfield, & Von Moers, 2014; Reiss, Gibson, & Walker, 2005). Patient‐centered transition planning should be considered an aspect of standard healthcare and possibly become an integral part of medical training to bring about a change in attitudes in future generations of healthcare professionals (Van Lierde et al., 2013). As a matter of fact, a consensus statement created by the Child Neurology Foundation in 2015 and endorsed by the American Academy of Neurology, the Child Neurology Society and the American Academy of Pediatrics proposed eight common principles that should be part of any transition model, including medical, legal, and reproductive issues (Brown et al., 2016).
As for other medical conditions with multisystem involvement and ID, the ideal care for adult individuals with TSC should consider multidisciplinary specialty clinics designed to provide comprehensive care. Many of the medical issues change in adults with TSC: epilepsy may persist, as well as ID and mental health issues, but renal and/or pulmonary problems may become the most concerning issues, in terms of morbidity and occasionally mortality (Thiele, Granata, Matricardi, & Chugani, 2014). Indeed, dissemination of knowledge to the medical audience about TSC manifestations at different ages will improve the clinical management of adults with TSC.
Given the extremely wide clinical variability in TSC, it is impossible to generalize when discussing transition of care from childhood to adulthood in affected individuals. Some children have ID and will require a caregiver's assistance for their entire lives, while others have medical problems without cognitive impairment and will likely become independent in managing their own health. In this article, we will focus on the medical needs for each clinical manifestation in adulthood, and the services and support required. We will review existing models of transition in different conditions, discuss our experience, and propose general rules to follow when establishing a transition program for TSC. In the interest of conciseness, topics related to educational systems, employment and higher education, trust funds, respite care and residential options will not be covered here, but nevertheless remain important to consider when transitioning patients.
2. CLINICAL MANIFESTATIONS OF TSC IN ADULTHOOD
2.1. Intellectual disability
The process of transition in individuals with ID can be especially challenging (Camfield et al., 2017). About 50% of individuals with TSC have normal intellectual ability, and the remainders have varying levels of ID (Curatolo, Moavero, & de Vries, 2015), with epidemiological data reporting an over‐representation of severe and profound ID (Joinson et al., 2003). Genotype–phenotype correlations show that ID is less frequent in patients with no mutation identified (NMI) compared to patients carrying demonstrable TSC2/TSC1 mutations (Kothare et al., 2014; Peron et al., 2018). Also in individuals with normal intellectual abilities, specific learning, and attention/memory/executive function deficits can interfere with academic achievements and social functioning (Tierney, McCartney, Serfontein, & de Vries, 2011).
When addressing transition in individuals with ID, healthcare practitioners ought to also consider the life changes both patients and parents will encounter, and it is strongly advised that practical aspects such as guardianship, options for residential living facilities, aging parents, and future caregivers when parents pass away be addressed (Thiele et al., 2014).
2.2. Epilepsy
In a recent article, parents of patients with TSC identified epilepsy as one of the major concerns for their children and a limitation to their independent life (e.g., if seizures are drug resistant, a driver's license cannot be issued; Both et al., 2018). Indeed, 90% of individuals with TSC have epilepsy at some point during their life, and two thirds of them develop medically refractory epilepsy (Thiele et al., 2014). Regular neurological follow‐up and constant update of therapeutic strategies are mandatory for these patients. Moreover, chronic use of antiepileptic drugs (AEDs) and/or m‐TOR inhibitors should be monitored to minimize short and long‐term side effects.
The onset of epilepsy during adolescence and adulthood is uncommon, but may occur. It is also possible that adolescents and young adults experience a single seizure. In our experience, AEDs are not constantly required for these patients (Vignoli et al., 2013). It also is important to note that some individuals with TSC experience remission of their epilepsy, and tapering off medications can be considered (Chu‐Shore, Major, Camposano, Muzykewicz, & Thiele, 2010; Vignoli et al., 2013).
2.3. Other neuropsychiatric disorders
Although often present since childhood, a wide range of psychological and behavioral manifestations (Tuberous sclerosis‐Associated Neuropsychiatric Disorders [TAND]) may appear, persist, or become more significant during adulthood (Curatolo et al., 2015; Thiele et al., 2014). During their lifetime, several individuals with TSC will present with behavioral issues, such as aggression, tantrums, self‐injury, and sleep difficulties (Eden, de Vries, Moss, Richards, & Oliver, 2014; Wilde et al., 2017). Anxiety and depressive disorders are often identified from early adolescence and in adulthood, in contrast with psychotic disorders, occurring as commonly as in the general population (about 1%) (Leclezio & de Vries, 2015).
Neurodevelopmental disorders, such as autism spectrum disorder (ASD, 40%–50%) and attention deficit hyperactivity disorder (30%–50%), are common in TSC (Curatolo et al., 2015). ASD has demonstrated to be related to epilepsy, infantile spasms, and mutations in TSC2 (Vignoli et al., 2015) and may result in a major burden of care especially in adulthood when rehabilitation facilities are often no longer available. Furthermore, transition into adulthood often involves loss of school support and child and adolescence mental health support, known to be a challenge in ASD in general (Lai, Lombardo, & Baron‐Cohen, 2014).
Whenever diagnosed, TAND should be treated with specific symptomatic therapies. The potential role of mTOR inhibitors for improving neurocognitive functioning and behavior is still debated (Kilincaslan et al., 2017; Krueger et al., 2017).
Transition may therefore be the right time to investigate all levels of TAND, especially if they have been under‐recognized during childhood and particularly in individuals with normal intelligence, as they prepare to finds jobs and live independent lives.
2.4. Renal manifestations
Renal cysts and angiomyolipomas are the main manifestations of TSC affecting the kidneys, although angiomyolipomas can be found also in other organs (i.e., the liver) (Northrup & Krueger, 2013). While cysts are a major concern in children, especially those who have a contiguous gene deletion involving both TSC2 and PKD1, renal angiomyolipomas represent the main renal cause of morbidity and mortality in adult individuals with TSC (Shepherd, Gomez, Lie, & Crowson, 1991). The occurrence of renal angiomyolipomas is age dependent, with more than 80% affected by age 18 years (Hamer et al., 2018). Although angiomyolipomas are benign tumors, they are highly vascular and can encounter spontaneous bleeding, usually presenting with abrupt abdominal pain and hematuria. Sudden bleeding of an angiomyolipoma requires embolization, can be life threatening and sometimes lead to renal insufficiency requiring dialysis and/or renal transplant (Kingswood et al., 2016). Fortunately, the use of mTOR inhibitors is changing the natural history of angiomyolipomas, reducing the size of the lesions in the majority of patients, and preventing major complications in an increasing number of individuals (Bissler et al., 2017). TSC patients are also at risk of developing renal cell carcinoma at a younger age than the general population (Peron et al., 2016).
A recent European survey pointed out the limited knowledge of renal angiomyolipomas in TSC patients and caregivers, and the poor adherence to the surveillance guidelines (i.e., renal imaging every 1–3 years, assessment of renal function and blood pressure yearly, from Krueger & Northrup, 2013), especially in the individuals older than age 15 years (Cockerell et al., 2018). For these reasons, providers are encouraged to carefully instruct patients and families about the possible consequences of angiomyolipomas, the need to continue screening and/or treatment, and the available therapeutic options when transferring the care to an adult clinic.
2.5. Pulmonary manifestations
Pulmonary manifestations of TSC include: lymphangioleiomyomatosis (LAM), found in about 75% of women with TSC, and characterized by proliferation of bundles of smooth muscle cells, resultant in cystic changes in the lung parenchyma; multifocal micronodular pneumocytic hyperplasia (MMPH), caused by the growth of proliferating epithelial cells into the alveolar walls, present with or without LAM in up to 60% of patients with TSC; and rarely clear cell lung tumor. Clinically, LAM can manifest with exertional dyspnea, recurrent pneumothoraces, thoracic lymphadenopathy, and hemoptysis (Johnson et al., 2010), while the presence of MMPH is not associated with a specific clinical or functional profile (Di Marco et al., 2016). Even if in some cases LAM is the reason for diagnosis of TSC during infancy (Hancock, Tomkins, Sampson, & Osborne, 2002), usually the pulmonary manifestations of TSC are typically only seen in adult women, since “age” has been found to be the only independent condition associated with the development of LAM (Di Marco et al., 2016). In most cases, the diagnosis results from proactive screening, as CT scans are recommended for women with TSC at ages between 18 and 30 years, even if asymptomatic (Krueger & Northrup, 2013). Therefore, the process of transition is not expected, with few exceptions, to be challenging for pulmonary involvement.
2.6. Cutaneous manifestations
Hypomelanotic macules, poliosis and “confetti” skin lesions, facial angiofibromas, fibrous cephalic plaque, ungual fibromas, shagreen patch, dental enamel pits, and intraoral fibromas are the main dermatological and dental signs in patients with TSC (Northrup & Krueger, 2013). Most of them—except for ungual fibromas—develop in childhood and are not associated with major medical concerns, although they can be one of the most useful diagnostic findings. However, facial angiofibromas can increase in number and size over time or bleed, causing cosmetic and dysfunctional problems. Healthcare practitioners should not underestimate the impact of potentially disfiguring lesions, which may lead to psychological discomfort especially in adolescents and young adults, and it is advised to refer patients for evaluation of treatment as part of the transition process. Suggested useful interventions include surgical excision, lasers, and use of topical mTOR inhibitors (Koenig et al., 2018; Malissen et al., 2017; Wataya‐Kaneda et al., 2017). As there is no evidence on which intervention is more effective, the 2012 TSC Dermatology and Dentistry Subcommittee recommended that the choice be based on available recourses and clinician expertise, considering if the patient is or will soon be on systemic mTOR inhibitors (Teng et al., 2014).
2.7. Cardiac findings
Cardiac rhabdomyomas are early signs of TSC, often discovered at prenatal ultrasound, but they may also be detected later on in life as part of the diagnostic workup or after the TSC diagnosis, especially if they are asymptomatic. Unless the cardiac rhabdomyomas are symptomatic (in which case an echocardiogram should be performed until regression is documented), adult patients should receive a 12‐lead ECG every 3–5 years to monitor for conduction defects, according to the guidelines (Krueger & Northrup, 2013).
2.8. Ocular manifestations
TSC patients can exhibit multiple retinal hamartomas and/or retinal achromic patches, which are useful in the diagnostic process but do not usually cause problems with vision. An annual ophthalmologic evaluation is recommended (Krueger & Northup, 2013). However, in our opinion, this could be performed as part of the regular ophthalmologic screening as in the general population, especially if an adult ophthalmologist is not part of the TSC clinic.
2.9. Bone findings
Two main considerations should be made for bone‐related problems in adult TSC patients. The first one concerns bone manifestations in this condition, consisting of sclerotic bone lesions, bone cysts, and fibrous dysplasia. Sclerotic bone lesions are usually multiple and can be present in up to 90% of adult patients (Avila et al., 2010; Boronat, Barber, & Thiele, 2017). Although they might not be specific for TSC, recent evidence from a case–control study evaluating CT scans of 49 adults with TSC suggests that sclerotic bone lesions are found more frequently in TSC patients than in the general population (Brakemeier et al., 2018).
They are not of clinical concern, and the 2012 International Tuberous Sclerosis Complex Consensus Group concluded that routine evaluation of bone findings in TSC is not supported by sufficient evidence unless there are clinical symptoms (Krueger & Northrup, 2013). However, physicians caring for adult TSC patients—including family doctors and radiologists—need to be aware that these individuals can show bone lesions, so to recognize and distinguish them from more serious manifestations not related to TSC, that is, osteoblastic metastases, to avoid misdiagnoses of cancer, as happened in one of our patients (personal communication).
The second consideration regards bone health in TSC patients affected by seizures. Adolescents with epilepsy have a higher risk of reduced bone mineral density and consequent fractures, and a substantial number of AEDs are associated with adverse effects on the bone through cytochrome P450 enzyme‐induced increased vitamin D metabolism, or other mechanisms (Camfield et al., 2017). Although there are no specific guidelines for bone health assessment in individuals with TSC, neurologists in the TSC clinics should request screening of 25‐hydroxyvitamin D and possibly consider referring patients on polytherapy for DEXA scan, as suggested by Camfield et al. (2017), especially if their mobility is reduced.
2.10. Reproductive system and genetic counseling
During the transition period and/or after transition has been completed, there are two main points to consider regarding genetics.
The first one concerns molecular testing. Some of the patients diagnosed with TSC in early years may have only a clinical diagnosis, as genetic testing might have not been performed in the past. At this point of time, it is important to offer molecular analysis, as this will have clinical implications for genetic counseling especially related to reproductive choices. In our opinion, reports of genetic testing performed in the past and with a negative result or with variants of unknown significance should be reviewed, and a re‐evaluation of those results and/or testing with Next Generation Sequencing (NGS) techniques can be offered. As a matter of fact, a pathogenic variant—mosaic or in noncoding regions—is likely to be identified in more and more patients who had no mutation identified in the past, thanks to NGS (Nellist et al., 2015; Tyburczy et al., 2015).
The second point to consider is family planning and genetic counseling, especially for those individuals with normal or borderline cognitive functioning who are likely to reproduce. Assuming that the parents have knowledge about the genetics of TSC, we suggest that recurrence risk be gradually introduced to the patient when the transition process starts in teen ages. The best timing is probably when parents and providers agree that the adolescents have sufficient maturity to understand it. A genetics visit, a separate genetic counseling session or multiple sessions may be necessary based on the context. The provider will gradually address recurrence risk, clinical variability (even in members of the same family), any genotype–phenotype correlations if available for few specific mutations, possible options for preimplantation genetic diagnosis and prenatal diagnosis if the pathogenic variant is known, and other reproductive options, such as adoption (Northrup, Koenig, Pearson & Au, 1999). The three‐generation pedigree should also be updated to determine if additional family members are at risk of TSC. When appropriate, birth control options and/or changes in therapies should be discussed as well, especially with regard to possible teratogenicity of certain drugs (i.e., AEDs) and the effect of estrogen‐containing contraception and pregnancy itself on LAM.
3. MODELS OF TRANSITION IN OTHER DISEASES
Although the needs of patients and families during the transitional period have been recognized (Both et al., 2018) and the TSC community has dedicated an educational track to transition during the 2018 TSC World Conference held in Dallas, we are not aware of specific guidelines or proposed models of transition in TSC. However, several examples from other chronic conditions, both genetic and nongenetic, are available in the scientific literature, and policy documents support the need for transition models (Van Lierde et al., 2013; Couce et al., 2018; Chabrol et al., 2018). Although each transition program has its own characteristics and no formal evaluation has been made for most of them, the main models that have been utilized can be summarized as follows:
The model of “transfer with referral letter”: transition is planned at an interdisciplinary meeting with healthcare providers and patients/families, and a clinical summary written by the pediatric team is given to the adult team, who has access to the pediatric medical records of the patient. This model implies an adult clinic only. An example is the Phenylketonuria clinic in Leipzig, Germany (Mütze et al., 2011), with good clinical and social outcomes (Mütze et al., 2016). Similarly, a referral letter is written by the pediatric provider to the adult one, and the patient is seen in the adult setting with no further communication in some transition programs for epilepsy in Canada and France (Carrizosa et al., 2014).
The “joint clinic” transition model: in this model, patients and families are prepared to transition during the pediatric clinic, then the pediatric physician transfers all medical records to the adult team and attends the first adult clinic (1–4 visits), in addition to being available to support and educate the adult team. The joint visits can take place at either the pediatric or adult hospital. When the process is completed, the patient is seen in the adult setting. Examples of this model are the transition epilepsy clinics in the United Kingdom (Carrizosa et al., 2014) and the Cystic Fibrosis transition clinic at the University of Michigan (Chaudhry, Keaton, & Nasr, 2013). This model allows patients to meet and trust the new providers without feeling simply discharged by the pediatric ones. However, it requires time from both parties and enormous economic resources.
The “teenager/transition clinic” model: in this model, clinics dedicated to adolescents have been established. The clinic is held by professionals who have received specialized training, and takes place in appropriate spaces (Kerr, Kluger, & Philip, 2011). An example of this model is the Adolescence Epilepsy Transition Clinic at the University of Alberta Hospital in Canada (Jurasek, Ray, & Quigley, 2010). The aim of this model is to provide an adequate setting between the pediatric and adult ones.
Of note, although most of the transition programs described in the literature had statistically significant positive outcomes (Gabriel, McManus, Rogers, & White, 2017), some of them were discontinued when physicians retired or moved to different hospitals and others were too expensive to be maintained, thus highlighting the difficulties in maintaining such complex programs (Carrizosa et al., 2014).
4. OUR EXPERIENCE
Since 2001, at San Paolo University Hospital in Milan (Italy), a multidisciplinary group of physicians developed experience in caring for patients with TSC. The TSC clinic was developed following the need of pediatric and adult neurologists of the Epilepsy Center to involve other specialists in the care of TSC patients, and is directed by the Director of the Epilepsy Center. The list of healthcare practitioners involved in the TSC clinic is listed in Table 1, and a schematic overview is presented in Figure 1.
Table 1.
List of healthcare practitioners involved in the pediatric and adult TSC clinic of San Paolo University Hospital in Milan, Italy
| Pediatric TSC clinic | Adult TSC clinic |
|---|---|
| Pediatric neurology | Neurology |
| Pediatric psychiatry | Psychiatry |
| Medical genetics | Medical genetics |
| Pediatrics | DAMA (internal medicine/general surgery) |
| Nephrology | Nephrology |
| Pulmonology (from age 16 years) | Pulmonology |
| Dermatology | Dermatology |
| Ophthalmology | Ophthalmology |
| Radiology (affiliated children's hospital) | Radiology |
| Neurosurgery (affiliated hospital) | Neurosurgery (affiliated hospital) |
| Epilepsy surgery (affiliated hospital) | Epilepsy surgery (affiliated hospital) |
| Dentistry | Dentistry |
| Gynecology | |
| Psychology | Psychology |
| Social worker | Social worker |
| Molecular laboratory (NGS, MLPA) | Molecular laboratory (NGS, MLPA) |
Abbreviations: DAMA, disabled advanced medical assistance; MLPA, multiplex ligation‐dependent probe amplification; NGS, next generation sequencing.
Figure 1.
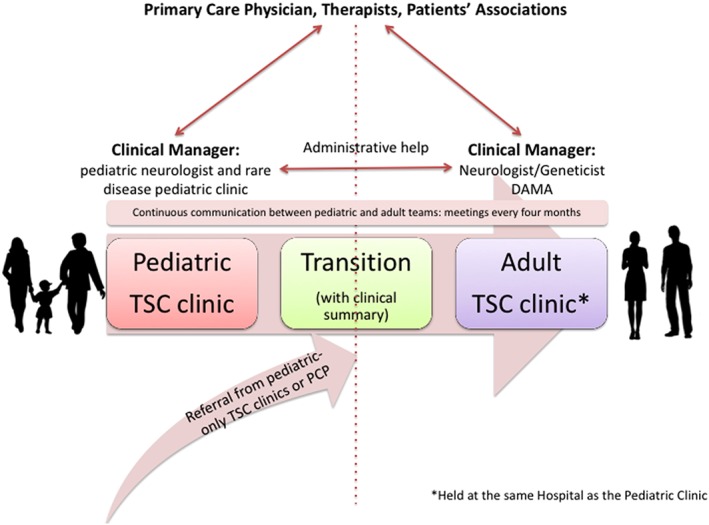
Schematic overview illustrating the healthcare transition process from childhood to adulthood at the pediatric and adult TSC clinic of San Paolo University Hospital in Milan, Italy. DAMA, disabled advanced medical assistance; PCP, primary care physician
Clinical care is coordinated by a pediatric neurologist and a pediatrician for children up to age 14 years, and by a neurologist for adolescents and adult patients. These physicians serve as clinical managers in addition to their clinical duties. They keep the contact with the families and/or the individual if able to self‐manage, and schedule appointments according to the individual needs and the local and international guidelines on surveillance and management (Krueger & Northrup, 2013; PDTA, 2015, http://malattierare.marionegri.it/images/downloads/PDTA/PDTA_schede/sclerosi_tuberosa.pdf), every 6 months or on an annual basis.
TSC individuals are seen as outpatients with the clinic spanning one or two different days. We usually schedule five patients per week, caring for about 250 patients overall (1/3 pediatric; 2/3 adults). Admittance as inpatients is reserved to those who need EEG monitoring for drug resistant seizures, or those who need renal surgery or preoperative assessment for epilepsy surgery, and rarely to patients with acute behavioral problems or intercurrent diseases. The pediatrician, neurologist, and/or geneticist perform the last visit, reviewing all notes by previous physicians, discussing the results, and making a follow‐up plan. Genetic test is available on site, allowing a direct dialogue between the laboratory and clinicians. In case of overload, because of the increased number of patients or limited resources, patients are advised to schedule specialty appointments in other facilities and then bring the results at the visit with the pediatrician, neurologist or geneticist. Matters related to legal competency (i.e., legal independence vs guardianship) are addressed by the pediatric neurologist before age 18 years, and those related to reproductive risk are discussed by the clinical geneticist during multiple sessions starting when both parents and providers agree that the adolescents have sufficient maturity to understand it. A social worker is available to address questions about housing, employment, welfare and disability benefits, when needed. The clinical manager is also responsible for keeping in contact with the primary care physician, therapists, and patient support organizations.
Physicians meet on a regular basis (generally three times a year), and discuss both recent advances in the scientific literature and selected patients of particular interest or clinical difficulty. All clinical information is stored in an ad hoc database used for scientific research.
To minimize the risk of loss at follow‐up, because of nonadherence, missed appointments, or refusal to manage care independently (Gray, Schaefer, Resmini‐Rawlinson, & Wagoner, 2017) the transition coordinator usually employs a structured plan, regularly calls the patients and reminds them to maintain the clinical appointments, with the support of an administrative person. This work is time consuming and receives no special reimbursement, but it is often necessary to guarantee adherence to care.
A medical unit called Disabled Advanced Medical Assistance (DAMA) has a space, phone line, and personnel dedicated to patients with ID and complex disabilities and helps the clinical manager in the care of patients with ID. DAMA is composed of a specialized team of internal medicine doctors, surgeons, and registered nurses trained to care for patients with complex disabilities. It also has a fast track for patients in the Emergency Department. The DAMA database helps provide better and faster diagnosis and care when time is an important factor for successful care.
Ours is currently the only adult TSC clinic in northern Italy, and a formal program for transition and transfer of care was developed as follows:
For patients referred by other pediatric TSC clinics: the referring physician contacts our clinic, and sends us a referral note with clinical summary; we then call the families to schedule an appointment. Each pediatric specialist addresses the issue of transition according to his/her own experience or local protocols, and we are not aware of a common transition plan. On average, we receive 20 new referrals for adult TSC patients per year.
For patients already known to our TSC clinic: the clinical manager starts addressing transition when TSC children are in their teens. The pediatrician or the child neurologist (with the support of residents) prepares a clinical summary for the new adult doctors (Table 1). All healthcare practitioners have access to both the pediatric and adult electronic medical records. The same specialists, who have developed expertise in this condition, often follow the TSC patients at all ages (i.e., nephrologist). Based on our experience and considering the limited resources, this ensures continuity of care and is appreciated by the patients.
The number of patients seen in our TSC clinic has increased from few dozens in 2001–2005 to more than 250 patients in 2018, and this model has allowed us to considerably reduce the number of days TSC individuals need to travel to the hospital in a year (from many to generally two), improving their quality of life, especially for those with significant disabilities. Funding by Regione Lombardia Healthcare System allowed us to organize this model of care.
Although we have not performed a study to formally evaluate the outcomes of our clinic and this approach certainly has several pitfalls, our model has developed over more than 15 years, adjusting to try and balance the patients' needs and the limited resources (both economic and availability of staff). We plan on creating a questionnaire for patients/families and healthcare providers in the near future, to assess the impact of this model, its cost efficiency, and how it could be ameliorated.
5. PROPOSAL OF HEALTHCARE TRANSITION IN TSC
Knowledge about the natural history of TSC‐related manifestations is a prerequisite for an effective transition. It is accepted that adult neurologists, nephrologists, pulmonologists, and psychiatrists should be involved when transitioning healthcare from childhood to adulthood in TSC (Thiele et al., 2014), but how to organize the transition process is less clear. As stated by Nabbout et al. (2017), a multidisciplinary clinic is probably the ideal mode of care for these individuals, and in our opinion it should be sought whenever possible.
Although a unifying and worldwide model of transition in TSC would be ideal, we conclude that it may not be applicable—and would likely not be successful—because of differences in regulations, costs, organization of healthcare systems, access to care, insurance systems, and so forth. This is supported by the fact that several models regarding different diseases have been reported in the literature, with no evidence that one is more effective than others (Crowley, Wolfe, Lock, & McKee, 2011).
We therefore think that each TSC clinic should find and endorse the best model that fits their practice and healthcare setting, be it a joint TSC clinic, a clinic that cares for patients throughout the life‐span, or other examples.
Transition (the dynamic process) is always desirable. However, transfer of care (the single event) may be required in some realities and have different timing then transition, whereas it may not be necessary in highly specialized and well‐established multidisciplinary TSC clinics that are used to caring for patients of any ages. Indeed, the latter (clinics that care for patients throughout the life‐span) may be the most suitable model, as suggested by Thiele et al. (2014) and seen in several TSC clinics in Europe and in the United States, where certain pediatric physicians are trained and certified to care also for adults, facilitating the provision of lifelong care. Such a model would ensure that healthcare practitioners have the best expertise possible and that care is easily accessible, in accordance with the needs pointed out by TSC families in a recent survey (Both et al., 2018).
Regardless of the model that is chosen, some topics should always be covered and some basic rules should be followed when transitioning young TSC patients into adulthood, with the aim to ensure continuity of care and patients' adherence, and to minimize the drop out of patients at follow‐up.
According to the general consensus on transition (American Academy of Pediatrics et al. 2011) and the consensus statement of the Child Neurology Foundation (Brown et al., 2016), healthcare providers should start discussing the process early, involving the teens with sufficient cognitive abilities, and educating them about the disease and treatment. This will allow the families to learn more about transition and discuss the best individual plan. The medical team should recapitulate the possible medical issues of TSC in adulthood, explaining the availability of resources and how transition at their Institution will take place.
We also propose that the neurologist and/or the geneticist be the lead clinicians and coordinators of care in the transition process in TSC, and that a referral letter to the adult physicians—in case of transfer of care—should always be obtained, as a starting point of the transfer process. In addition to attending physicians, residents, fellows, and nurses are invaluable resources in the transition process, as well as patient support organizations.
The ultimate goal is to provide the patients with medically appropriate care, ensuring an uninterrupted healthcare for as long as possible.
With this in mind, we propose a list of basic tips to guide healthcare professionals in establishing a transition process for TSC in their clinic based on best practice (Figure 2).
Figure 2.
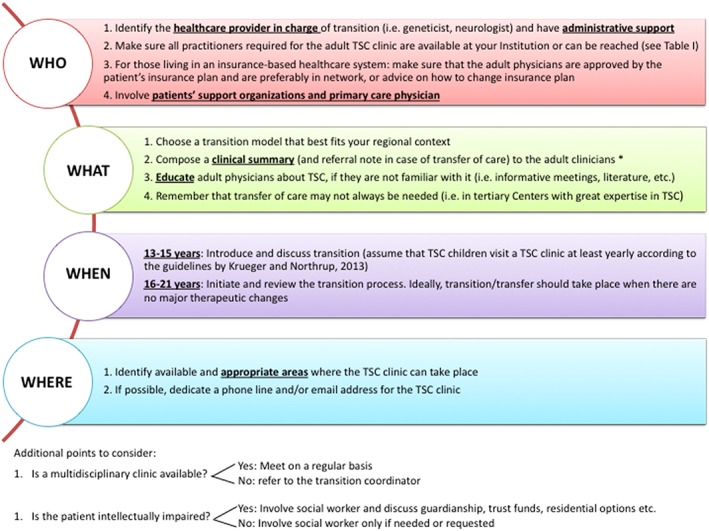
The four W's: Proposed list containing points to consider and basic tips when establishing a transition process for TSC. *the clinical summary should preferably include: cognitive status; molecular diagnosis; presence of clinical manifestations related to TSC, with date of the last examination performed for each; medicines and dosages; allergies; social status and concerns
Additional useful and practical resources can be obtain at:
https://www.gottransition.org/about/index.cfm, a website curated by the Maternal and Child Health Bureau and The National Alliance to Advance Adolescent Health of the United States, aimed at providing innovative strategies of transition for health professionals, youth, and families;
https://www.nice.org.uk/guidance/ng43, the website of the National Institute for Health and Care Excellence in the United Kingdom, providing national guidance and advice to improve health and social care for both professionals and the general public.
6. CONCLUSIONS
Healthcare transition from childhood to adulthood is a process aimed at assuring continuity of care and patients adherence to medical care, promoting active involvement of the patients, whenever possible. This process in TSC may be complicated by the multisystemic nature of this condition with age‐dependent manifestations and clinical variability, and by the presence of ID in at least half of the individuals.
In this article, we have discussed medical issues that should be addressed during transition, and have illustrated different possible approaches to transition. We conclude that there is probably no generalizable transition model for TSC, but transition in TSC should be flexible, patient oriented, and developed in accordance with the resources available. We have also provided some key points to consider when establishing a transition program for TSC, and highlight the importance of coordinating the process and educating the adult practitioners about this condition.
Finally, we would like to encourage TSC experts to engage in a panel discussion that will hopefully lead to a consensus statement on guidelines for healthcare transition from childhood to adulthood in TSC.
CONFLICT OF INTEREST
None.
ACKNOWLEDGMENTS
The authors would like to thank all the other healthcare professionals involved in the TSC clinic of the San Paolo Hospital in Milan for their valuable work and participation in the care of our patients: Francesca La Briola, Valentina Chiesa, Elena Zambrelli, Katherine Turner, Monica Saccani, Graziella Cefalo, Rosangela Arancio, Angela Volpi, Emanuele Montanari, Massimo Corona, Gianluca Imeri, Silvia Terraneo, Loredana Frattini, Chiara Bencini, Lorenzo Gualandri, Valentina Barbieri, Roberto Rozza, Luca Rossetti, Stefano Izzo, Giovanni Pompili, Federica Galli, Rosa Maria Alfano, Gaetano Bulfamante, and Gianpaolo Carrafiello. A.P. wishes to acknowledge the Foundation Blanceflor Ludovisi, née Bildt, for supporting her research abroad.
Biographies
A. Peron is a clinical geneticist and postdoctoral fellow at the University of Milan (Italy) and an adjunct assistant professor at the University of Utah. She serves as the geneticist of the pediatric and adult TSC clinic of San Paolo University Hospital in Milan, and her research interests focus on individuals with TSC and No Mutation Identified, and genotype–phenotype correlations. Her other research interests include dysmorphology, intellectual disability, genetics of epilepsy and autism spectrum disorder, and genomic medicine.

M. P. Canevini is an associate professor of Child Neurology and Psychiatry at the Università degli Studi di Milano, Italy. She is the head of the Child Neuropsychiatric Unit and Epilepsy Center and the director of the TSC Clinic at San Paolo University Hospital in Milan, Italy. Her main research interests focus on epilepsy and rare diseases associated with epilepsy.

F. Ghelma is a general surgeon and a researcher of the University of Milan (Italy). He is the chief of the Disabled Advanced Medical Assistance (DAMA) Unit of the San Paolo University Hospital in Milan. His main research interest is developing and testing new hospital organizational and care models for patients with severe intellectual disabilities. Dr. Ghelma's others research interests are treatment of functional pharingo‐oesophageal disorders and oncological surgery for people with severe intellectual disability.

F. Di Marco is an associate professor at the University of Milan (Italy) and the head of the Respiratory Unit of the Papa Giovanni XXIII Hospital in Bergamo (Italy). He served as the pulmonologist of the adult TSC/LAM clinic of San Paolo Hospital in Milan (Italy) until July 2018. His other research interests include pathophysiology of respiratory disease, including asthma and COPD.

A. Vignoli is a senior assistant professor in Child Neurology and Psychiatry at the Department of Health Sciences, University of Milan, Italy. She has published widely on seizures, treatment, and behavioral outcomes in children with epilepsy. Her research has focused on children with epilepsy and rare diseases, in particular with drug resistant seizures.

Peron A, Canevini MP, Ghelma F, Di Marco F, Vignoli A. Healthcare transition from childhood to adulthood in Tuberous Sclerosis Complex. Am J Med Genet Part C. 2018;178C:355–364. 10.1002/ajmg.c.31653
Funding information Foundation Blanceflor Ludovisi, née Bildt
REFERENCES
- American Academy of Pediatrics, American Academy of Family Physicians, & American College of Physicians‐American Society of Internal Medicine . (2002). A consensus statement on health care transitions for young adults with special health care needs. Pediatrics, 110(6), 1304–1306. [PubMed] [Google Scholar]
- American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians, Transitions Authoring Group , Cooley, W. C. , & Sagerman, P. J. (2011). Clinical report—Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics, 128(1), 182–200. 10.1542/peds.2011-0969 [DOI] [PubMed] [Google Scholar]
- Amin, S. , Lux, A. , Calder, N. , Laugharne, M. , Osborne, J. , & O'callaghan, F. (2017). Causes of mortality in individuals with tuberous sclerosis complex. Developmental Medicine and Child Neurololgy, 59(6), 612–617. 10.1111/dmcn.13352 [DOI] [PubMed] [Google Scholar]
- Avila, N. A. , Dwyer, A. J. , Rabel, A. , Darling, T. , Hong, C. H. , & Moss, J. (2010). CT of sclerotic bone lesions: Imaging features differentiating tuberous sclerosis complex with lymphangioleiomyomatosis from sporadic lymphangioleiomymatosis. Radiology, 254(3), 851–857. 10.1148/radiol.09090227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissler, J. J. , Kingswood, J. C. , Radzikowska, E. , Zonnenberg, B. A. , Belousova, E. , Frost, M. D. , … Budde, K. (2017). Everolimus long‐term use in patients with tuberous sclerosis complex: Four‐year update of the EXIST‐2 study. PLoS One, 12(8), e0180939 10.1371/journal.pone.0180939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum, R. W. , Garell, D. , Hodgman, C. H. , Jorissen, T. W. , Okinow, N. A. , Orr, D. P. , & Slap, G. B. (1993). Transition from child‐centered to adult health‐care systems for adolescents with chronic conditions. A position paper of the Society for Adolescent Medicine. Journal of Adolescent Health, 14(7), 570–576. [DOI] [PubMed] [Google Scholar]
- Boronat, S. , Barber, I. , & Thiele, E. A. (2017). Sclerotic bone lesions in tuberous sclerosis complex: A genotype‐phenotype study. American Journal of Medical Genetics Part A, 173, 1891–1895. 10.1002/ajmg.a.38260 [DOI] [PubMed] [Google Scholar]
- Both, P. , Ten Holt, L. , Mous, S. , Patist, J. , Rietman, A. , Dieleman, G. , … van Eeghen, A. (2018). Tuberous sclerosis complex: Concerns and needs of patients and parents from the transitional period to adulthood. Epilepsy and Behavior, 83, 13–21. 10.1016/j.yebeh.2018.03.012 [DOI] [PubMed] [Google Scholar]
- Brakemeier, S. , Vogt, L. , Adams, L. C. , Zukunft, B. , Diederichs, G. , Hamm, B. , … Makowski, M. R. (2018). Sclerotic bone lesions as a potential imaging biomarker for the diagnosis of tuberous sclerosis complex. Scientific Reports, 8(1), 953 10.1038/s41598-018-19399-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, L. W. , Camfield, P. , Capers, M. , Cascino, G. , Ciccarelli, M. , de Gusmao, C. M. , … Zupanc, M. (2016). The neurologist's role in supporting transition to adult health care: A consensus statement. Neurology, 87(8), 835–840. 10.1212/WNL.0000000000002965 [DOI] [PubMed] [Google Scholar]
- Camfield, P. , Camfield, C. , Busiah, K. , Cohen, D. , Pack, A. , & Nabbout, R. (2017). The transition from pediatric to adult care for youth with epilepsy: Basic biological, sociological, and psychological issues. Epilepsy and Behavior, 69, 170–176. 10.1016/j.yebeh.2016.11.009 [DOI] [PubMed] [Google Scholar]
- Carrizosa, J. , An, I. , Appleton, R. , Camfield, P. , & Von Moers, A. (2014). Models for transition clinics. Epilepsia, 55(Suppl 3), 46–51. 10.1111/epi.12716 [DOI] [PubMed] [Google Scholar]
- Chabrol, B. , Jacquin, P. , Francois, L. , Broué, P. , Dobbelaere, D. , Douillard, C. , … Belmatoug, N. (2018). Transition from pediatric to adult care in adolescents with hereditary metabolic diseases: Specific guidelines from the French network for rare inherited metabolic diseases (G2M). Archives de Pédiatrie. 10.1016/j.arcped.2018.05.009 [DOI] [PubMed] [Google Scholar]
- Chaudhry, S. R. , Keaton, M. , & Nasr, S. Z. (2013). Evaluation of a cystic fibrosis transition program from pediatric to adult care. Pediatric Pulmonology, 48(7), 658–665. 10.1002/ppul.22647 [DOI] [PubMed] [Google Scholar]
- Chu‐Shore, C. J. , Major, P. , Camposano, S. , Muzykewicz, D. , & Thiele, E. A. (2010). The natural history of epilepsy in tuberous sclerosis complex. Epilepsia, 51(7), 1236–1241. 10.1111/j.1528-1167.2009.02474.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerell, I. , Guenin, M. , Heimdal, K. , Bjørnvold, M. , Selmer, K. K. , & Rouvière, O. (2018). Renal manifestations of tuberous sclerosis complex: patients' and parents' knowledge and routines for renal follow‐up—A questionnaire study. BMC Nephrology, 19(1), 39 10.1186/s12882-018-0835-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couce, M. L. , Del Toro, M. , García‐Jiménez, M. C. , Gutierrez‐Solana, L. , Hermida‐Ameijeiras, Á. , López‐Rodríguez, M. , … Torralba, M. Á. (2018). Transition from paediatric care to adult care for patients with mucopolysaccharidosis. Revista Clinica Española, 218(1), 17–21. 10.1016/j.rce.2017.06.005 [DOI] [PubMed] [Google Scholar]
- Crowley, R. , Wolfe, I. , Lock, K. , & McKee, M. (2011). Improving the transition between paediatric and adult healthcare: A systematic review. Archives of Disease in Childhood, 96(6), 548–553. 10.1136/adc.2010.202473 [DOI] [PubMed] [Google Scholar]
- Curatolo, P. , Moavero, R. , & de Vries, P. (2015). Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurology, 14(7), 733–745. 10.1016/S1474-4422(15)00069-1 [DOI] [PubMed] [Google Scholar]
- Di Marco, F. , Terraneo, S. , Imeri, G. , Palumbo, G. , La Briola, F. , Tresoldi, S. , … Centanni, S. (2016). Women with TSC: Relationship between clinical, lung function and radiological features in a genotyped population investigated for Lymphangioleiomyomatosis. PLoS One, 11(5), e0155331 10.1371/journal.pone.0155331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden, K. E. , de Vries, P. J. , Moss, J. , Richards, C. , & Oliver, C. (2014). Self‐injury and aggression in tuberous sclerosis complex: Cross syndrome comparison and associated risk markers. Journal of Neurodevelopmental Disorders, 6(1), 10 10.1186/1866-1955-6-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel, P. , McManus, M. , Rogers, K. , & White, P. (2017). Outcome evidence for structured pediatric to adult health care transition interventions: A systematic review. Journal of Pediatrics, 188, 263–269.e15. 10.1016/j.jpeds.2017.05.066 [DOI] [PubMed] [Google Scholar]
- Gray, W. N. , Schaefer, M. R. , Resmini‐Rawlinson, A. , & Wagoner, S. T. (2017). Barriers to transition from pediatric to adult care: A systematic review. Journal of Pediatric Psychology, 43(5), 488–502. 10.1093/jpepsy/jsx142 [DOI] [PubMed] [Google Scholar]
- Hamer, H. M. , Pfäfflin, M. , Baier, H. , Bösebeck, F. , Franz, M. , Holtkamp, M. , … Brandt, C. (2018). Characteristics and healthcare situation of adult patients with tuberous sclerosis complex in German epilepsy centers. Epilepsy and Behavior, 82, 64–67. 10.1016/j.yebeh.2018.03.006 [DOI] [PubMed] [Google Scholar]
- Hancock, E. , Tomkins, S. , Sampson, J. , & Osborne, J. (2002). Lymphangioleiomyomatosis and tuberous sclerosis. Respiratory Medicine, 96(1), 7–13. [DOI] [PubMed] [Google Scholar]
- Johnson, S. R. , Cordier, J. F. , Lazor, R. , Cottin, V. , Costabel, U. , Harari, S. , … Kingswood, C. (2010). European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. European Respiratory Journal, 35(1), 14–26. 10.1183/09031936.00076209 [DOI] [PubMed] [Google Scholar]
- Kerr, M. , Kluger, G. , & Philip, S. (2011). Evolution and management of Lennox‐Gastaut syndrome through adolescence and into adulthood: Are seizures always the primary issue? Epileptic Disorders, 13(Suppl 1), S15–S26. 10.1684/epd.2011.0409 [DOI] [PubMed] [Google Scholar]
- Kilincaslan, A. , Kok, B. E. , Tekturk, P. , Yalcinkaya, C. , Ozkara, C. , & Yapici, Z. (2017). Beneficial effects of Everolimus on autism and attention‐deficit/hyperactivity disorder symptoms in a Group of Patients with tuberous sclerosis complex. Journal of Child and Adolescent Psychopharmacology, 27(4), 383–388. 10.1089/cap.2016.0100 [DOI] [PubMed] [Google Scholar]
- Kingswood, J. C. , Bissler, J. J. , Budde, K. , Hulbert, J. , Guay‐Woodford, L. , Sampson, J. R. , … Zonnenberg, B. A. (2016). Review of the tuberous sclerosis renal guidelines from the 2012 consensus conference: Current data and future study. Nephron, 134(2), 51–58. 10.1159/000448293 [DOI] [PubMed] [Google Scholar]
- Koenig, M.K. , Bell, C.S. , Hebert, A.A. , Roberson, J. , Samuels, J.A. , Slopis, J.M . … TREATMENT Trial Collaborators . (2018) Efficacy and safety of topical rapamycin in patients with facial Angiofibromas secondary to tuberous sclerosis complex: The TREATMENT randomized clinical trial JAMA Dermatology, 154(7), 773–780. 10.1001/jamadermatol.2018.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothare, S. V. , Singh, K. , Chalifoux, J. R. , Staley, B. A. , Weiner, H. L. , Menzer, K. , & Devinsky, O. (2014). Severity of manifestations in tuberous sclerosis complex in relation to genotype. Epilepsia, 55(7), 1025–1029. 10.1111/epi.12680 [DOI] [PubMed] [Google Scholar]
- Krueger, D. A. , Northrup, H. , & International Tuberous Sclerosis Complex Consensus Group . (2013). Tuberous sclerosis complex surveillance and management: Recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatric Neurology, 49(4), 255–265. 10.1016/j.pediatrneurol.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger, D. A. , Sadhwani, A. , Byars, A. W. , de Vries, P. J. , Franz, D. N. , Whittemore, V. H. , … Sahin, M. (2017). Everolimus for treatment of tuberous sclerosis complex‐associated neuropsychiatric disorders. Annals of Clinical and Translational Neurology, 4(12), 877–887. 10.1002/acn3.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joinson, C. , O'Callaghan, F. J. , Osborne, J. P. , Martyn, C. , Harris, T. , & Bolton, P. F. (2003). Learning disability and epilepsy in an epidemiological sample of individuals with tuberous sclerosis complex. Psychological Medicine, 33(2), 335–344. [DOI] [PubMed] [Google Scholar]
- Jurasek, L. , Ray, L. , & Quigley, D. (2010). Development and implementation of an adolescent epilepsy transition clinic. Journal of Neuroscience Nursing, 42(4), 181–189. [DOI] [PubMed] [Google Scholar]
- Lai, M. C. , Lombardo, M. V. , & Baron‐Cohen, S. (2014). Autism. Lancet, 383(9920), 896–910. 10.1016/S0140-6736(13)61539-1 [DOI] [PubMed] [Google Scholar]
- Leclezio, L. , & de Vries, P. J. (2015). Advances in the treatment of tuberous sclerosis complex. Current Opinion in Psychiatry, 28(2), 113–120. 10.1097/YCO.0000000000000136. [DOI] [PubMed] [Google Scholar]
- Malissen, N. , Vergely, L. , Simon, M. , Roubertie, A. , Malinge, M. C. , & Bessis, D. (2017). Long‐term treatment of cutaneous manifestations of tuberous sclerosis complex with topical 1% sirolimus cream: A prospective study of 25 patients. Journal of the American Academy of Dermatology, 77(3), 464–472.e3. 10.1016/j.jaad.2017.04.005 [DOI] [PubMed] [Google Scholar]
- Mütze, U. , Roth, A. , Weigel, J. F. , Beblo, S. , Baerwald, C. G. , Bührdel, P. , & Kiess, W. (2011). Transition of young adults with phenylketonuria from pediatric to adult care. Journal of Inherited Metabolic Disease, 34(3), 701–709. 10.1007/s10545-011-9284-x [DOI] [PubMed] [Google Scholar]
- Mütze, U. , Thiele, A. G. , Baerwald, C. , Ceglarek, U. , Kiess, W. , & Beblo, S. (2016). Ten years of specialized adult care for phenylketonuria ‐ a single‐Centre experience. Orphanet Journal of Rare Diseases, 11, 27 10.1186/s13023-016-0410-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabbout, R. , Andrade, D. M. , Bahi‐Buisson, N. , Cross, H. , Desquerre, I. , Dulac, O. , … Camfield, C. S. (2017). Outcome of childhood‐onset epilepsy from adolescence to adulthood: Transition issues. Epilepsy and Behavior, 69, 161–169. 10.1016/j.yebeh.2016.11.010 [DOI] [PubMed] [Google Scholar]
- Nellist, M. , Brouwer, R. W. , Kockx, C. E. , van Veghel‐Plandsoen, M. , Withagen‐Hermans, C. , Prins‐Bakker, L. , & van IJcken, W. F. (2015). Targeted next generation sequencing reveals previously unidentified TSC1 and TSC2 mutations. BMC Medical Genetics, 16, 10 10.1186/s12881-015-0155-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrup, H. , Koenig, M. K. , Pearson, D. A. , & Au, K. S. (1999). Tuberous sclerosis complex [updated July 18, 2018]. In Adam M. P., Ardinger H. H., Pagon R. A., Wallace S. E., Bean L. J. H., Stephens K., & Amemiya A. (Eds.), GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle. [Google Scholar]
- Northrup, H. , Krueger, D. A. , & International Tuberous Sclerosis Complex Consensus Group . (2013). Tuberous sclerosis complex diagnostic criteria update: Recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatric Neurology, 49(4), 243–254. 10.1016/j.pediatrneurol.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PDTA (2015). http://malattierare.marionegri.it/images/downloads/PDTA/PDTA_schede/sclerosi_tuberosa.pdf
- Peron, A. , Vignoli, A. , La Briola, F. , Volpi, A. , Montanari, E. , Morenghi, E. , … Canevini, M. P. (2016). Do patients with tuberous sclerosis complex have an increased risk for malignancies? American Journal of Medical Genetics Part A, 170(6), 1538–1544. 10.1002/ajmg.a.37644 [DOI] [PubMed] [Google Scholar]
- Peron, A. , Vignoli, A. , La Briola, F. , Morenghi, E. , Tansini, L. , Alfano, R. M. , … TSC Study Group of the San Paolo Hospital of Milan . (2018). Deep phenotyping of patients with tuberous sclerosis complex and no mutation identified in TSC1 and TSC2. European Journal of Medical Genetics, 61(7), 403–410. 10.1016/j.ejmg.2018.02.005 [DOI] [PubMed] [Google Scholar]
- Reiss, J. G. , Gibson, R. W. , & Walker, L. R. (2005). Health care transition: Youth, family, and provider perspectives. Pediatrics, 115(1), 112–120. [DOI] [PubMed] [Google Scholar]
- Royal College of Paediatrics and Child Health . (2003). Bridging the gap: Health care for adolescents. London: RCPCH. [Google Scholar]
- Shepherd, C. , Gomez, M. , Lie, J. , & Crowson, C. (1991). Causes of death in patients with tuberous sclerosis. Mayo Clinic Proceedings, 66(8), 792–796. [DOI] [PubMed] [Google Scholar]
- Teng, J. M. , Cowen, E. W. , Wataya‐Kaneda, M. , Gosnell, E. S. , Witman, P. M. , Hebert, A. A. , … Darling, T. N. (2014). Dermatologic and dental aspects of the 2012 international tuberous sclerosis complex consensus statements. JAMA Dermatology, 150(10), 1095–1101. 10.1001/jamadermatol.2014.938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele, E. A. , Granata, T. , Matricardi, S. , & Chugani, H. T. (2014). Transition into adulthood: Tuberous sclerosis complex, Sturge‐Weber syndrome, and Rasmussen encephalitis. Epilepsia, 55(Suppl 3), 29–33. 10.1111/epi.12722 [DOI] [PubMed] [Google Scholar]
- Tierney, K. M. , McCartney, D. L. , Serfontein, J. R. , & de Vries, P. J. (2011). Neuropsychological attention skills and related behaviours in adults with tuberous sclerosis complex. Behavior Genetics, 41(3), 437–444. 10.1007/s10519-010-9423-4 [DOI] [PubMed] [Google Scholar]
- Tyburczy, M. E. , Dies, K. A. , Glass, J. , Camposano, S. , Chekaluk, Y. , Thorner, A. R. , … Kwiatkowski, D. J. (2015). Mosaic and Intronic mutations in TSC1/TSC2 explain the majority of TSC patients with no mutation identified by conventional testing. PLoS Genetics, 11(11), e1005637 10.1371/journal.pgen.1005637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lierde, A. , Menni, F. , Bedeschi, M. F. , Natacci, F. , Guez, S. , Vizziello, P. , … Esposito, S. (2013). Healthcare transition in patients with rare genetic disorders with and without developmental disability: Neurofibromatosis 1 and Williams‐Beuren syndrome. American Journal of Medical Genetics Part A, 161A(7), 1666–1674. 10.1002/ajmg.a.35982 [DOI] [PubMed] [Google Scholar]
- Vignoli, A. , La Briola, F. , Turner, K. , Scornavacca, G. , Chiesa, V. , Zambrelli, E. , … Canevini, M. P. (2013). Epilepsy in TSC: Certain etiology does not mean certain prognosis. Epilepsia, 54(12), 2134–2142. 10.1111/epi.12430 [DOI] [PubMed] [Google Scholar]
- Vignoli, A. , La Briola, F. , Peron, A. , Turner, K. , Vannicola, C. , Saccani, M. , … Canevini, M. P. (2015). Autism spectrum disorder in tuberous sclerosis complex: Searching for risk markers. Orphanet Journal of Rare Diseases, 10, 154 10.1186/s13023-015-0371-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldboth, V. , Patch, C. , Mahrer‐Imhof, R. , & Metcalfe, A. (2016). Living a normal life in an extraordinary way: A systematic review investigating experiences of families of young people's transition into adulthood when affected by a genetic and chronic childhood condition. International Journal of Nursing Studies, 62, 44–59. 10.1016/j.ijnurstu.2016.07.007 [DOI] [PubMed] [Google Scholar]
- Wataya‐Kaneda, M. , Nakamura, A. , Tanaka, M. , Hayashi, M. , Matsumoto, S. , Yamamoto, K. , & Katayama, I. (2017). Efficacy and safety of topical Sirolimus therapy for facial Angiofibromas in the tuberous sclerosis complex: A randomized clinical trial. JAMA Dermatology, 153(1), 39–48. 10.1001/jamadermatol.2016.3545 [DOI] [PubMed] [Google Scholar]
- Wilde, L. , Eden, K. , de Vries, P. , Moss, J. , Welham, A. , & Oliver, C. (2017). Self‐injury and aggression in adults with tuberous sclerosis complex: Frequency, associated person characteristics, and implications for assessment. Research in Developmental Disabilities, 64, 119–130. 10.1016/j.ridd.2017.03.007 [DOI] [PubMed] [Google Scholar]


