Abstract
For the past several decades, disease‐related outcomes, particularly local recurrence rate, in patients with locally advanced rectal cancer have significantly improved as a result of advancement of surgical technique and implementation of neoadjuvant chemoradiation. However, distant metastasis remains unresolved, being a significant cause of cancer death. To focus on micrometastases early in the course of multimodal treatment, delivering systemic chemotherapy in the neoadjuvant setting is emerging. Also, driven by patient demand and interest in preserving quality of life, upfront chemotherapy prior to surgery serves as a strategy for organ preservation in the management of rectal cancer. Herein, currently available literature on different methods and strategies of the multimodal approach is critically appraised.
Keywords: chemoradiation, chemotherapy, neoadjuvant therapy, organ preservation, rectal cancer
1. INTRODUCTION
The multidisciplinary approach of neoadjuvant chemoradiotherapy (nCRT) followed by total mesorectal excision (TME) and postoperative adjuvant chemotherapy has been accepted worldwide as the standard treatment for patients with locally advanced rectal cancer. This therapeutic approach markedly reduced the local recurrence rate from 35% to 5%‐10% and significantly improved overall survival.1, 2, 3, 4 Local recurrence now seems less of a concern, whereas distant metastasis is now the leading cause of cancer death in rectal cancer.5 Although adjuvant chemotherapy has been recommended in patients with locally advanced rectal cancer treated with nCRT and TME, it does not show clear benefit in improving overall survival or cancer‐specific survival.6 Poor patient compliance with adjuvant chemotherapy is also a serious concern in clinical practice.6, 7 Therefore, the efficacy of adjuvant chemotherapy in rectal cancer treatment remains controversial.
With the purpose of improving patient survival, delivery of chemotherapy before surgery had been proposed to treat occult micrometastases early and increase treatment compliance.8 Multiple trials evaluating various modes of incorporating both chemotherapy and CRT in the neoadjuvant setting, referred to as “total neoadjuvant therapy (TNT),” have reported optimistic results. This review discusses the rationale for TNT and available evidence from clinical trials, appraising the emerging literature on the conception of organ preservation to the current therapeutic paradigm.
2. CHRONOLOGY OF THE MULTIMODAL APPROACH IN RECTAL CANCER TREATMENT
In the 1980s, reports by Heald et al, including a report on the “Holy Plane” by Heald alone, were acclaimed in the history of rectal cancer surgery.9, 10 Dissecting the entire rectum through the “Holy Plane,” meaning the surgical plane based on the embryological development of the hindgut, resulted in removal of the entire circumferential perirectal tissue without leaving mesorectal tissue.10 This artful surgical technique reduced the rate of positive circumferential resection margin; consequently, the local recurrence rate dropped dramatically from 30%‐50% to a single digit, and the survival outcome ultimately improved up to 80%.1 TME also allowed patients to undergo a sphincter‐saving procedure.11 Now, the prime role of TME in rectal cancer management is indisputable.
At a similar time, the effectiveness of chemotherapy and radiation therapy (RT) was eagerly investigated for the treatment of locally advanced rectal cancer as shown in Figure 1. Before TME had gained popularity and general application in rectal cancer surgery, early clinical trials, such as NSABP R‐01, NCCTG or GITSG G‐7175, were conducted to test whether postoperative RT, chemotherapy, or combined chemoradiotherapy could improve survival benefit with effective local control.12, 13, 14 These trials indicated that adjuvant radiotherapy could improve local control and, in a combined setting with chemotherapy, possible improvement in survival was suggested. Based on these findings, adjuvant chemotherapy with radiotherapy was recommended in the management of rectal cancer.15
Figure 1.
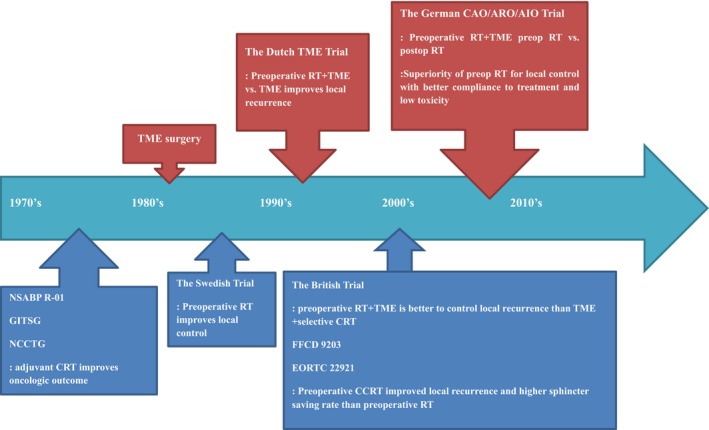
Chronology of major research results in rectal cancer management. CRT, chemoradiotherapy; EORTC, European Organisation for Research and Treatment of Cancer Radiotherapy Group; FFCD, The Fédération Francophone de Cancérologie Digestive; GITSG, Gastrointestinal Tumor Study Group; NCCTG, North Central Cancer Treatment Group; NSABP, National Surgical Adjuvant Breast and Bowel Project; RT, Radiation therapy; TME, total mesorectal excision
Although the trials of adjuvant therapy in a postoperative setting were mainly conducted in the USA, preoperative radiation therapy was common in European countries. The Swedish Trial evaluating preoperative short‐course RT combined with conventional rectal resection demonstrated that both the 5‐year local recurrence rate and the 5‐year survival rate significantly improved with preoperative short‐course RT, 9% vs 23% and 58% vs 48% respectively, among the curatively treated patients.16 Along with implementation of TME, the Dutch TME trial showed a significant benefit of preoperative RT in reducing the local recurrence rate for patients with TNM stage III.5 However, the overall survival or cancer‐specific survival was similar when compared with the patients who received TME surgery only.
In terms of sequencing multimodal therapy, the German trial clearly showed the superiority of preoperative RT over postoperative therapy.17 Similar trials indicated that preoperative radiotherapy combined with TME effectively lowered the risk of pelvic local recurrence, while high patient compliance with and low toxicity of the treatment were obtained.18, 19, 20 Subsequent trials showed that concurrent chemotherapy with fluorouracil and leucovorin, acting as a radiosensitizer, significantly boosted the effect of preoperative radiotherapy.2, 3, 17, 21 Consequently, the rate of local recurrence reached as low as 5.3%, and sphincter‐saving surgery was possible in the majority of patients. Thus, the standard of care in most countries has been preoperative chemoradiotherapy followed by TME surgery. Nonetheless, the 10‐year cumulative incidence of distant metastases was 30% and the 10‐year disease‐free survival was 68% overall.17 It is clear that better systemic control is necessary.
3. LIMITATION OF POSTOPERATIVE ADJUVANT CHEMOTHERAPY
Adjuvant chemotherapy has been a standard part of the multimodal approach in the management of locally advanced rectal cancer and has been recommended in the US National Comprehensive Cancer Network (NCCN) guidelines. This recommendation for patients with stage II or III rectal cancer is based on extrapolation of results from phase III trials of adjuvant treatment for colon cancer22, 23 and from data of patients with rectal cancer treated without preoperative radiotherapy or CRT.24 According to a systematic review and meta‐analysis of adjuvant chemotherapy after preoperative radiotherapy and surgery in patients with rectal cancer, adjuvant chemotherapy for rectal cancer does not improve overall survival, disease‐free survival, or distant recurrences.6, 25 However, in a subgroup analysis of patients with tumors 10‐15 cm from the anal verge, there was improved disease‐free survival and fewer distant metastases with adjuvant therapy.6 Another meta‐analysis suggested that patients with a pathological complete response (pCR) after chemoradiotherapy might not benefit from adjuvant chemotherapy, whereas patients with residual tumor showed superior outcomes when adjuvant chemotherapy was given.26 Such conflicting interpretation of available data in the benefit of adjuvant chemotherapy has resulted in endless debates on its clinical application.
In addition, serious concerns regarding patient compliance with adjuvant therapy was raised. Nearly 30% of eligible patients had never initiated adjuvant chemotherapy27 and less than half of them had received the full treatment without interruption or delays.7, 18, 28 Postoperative complications including leakage, poor general condition and slow recovery, problems with temporary stoma, or refusal of treatment were the main reasons for withdrawal from adjuvant therapy.29 Evaluation on the timing and efficacy of postoperative adjuvant chemotherapy showed that each 4‐week delay in treatment correlated with a 14% drop in overall survival.30 It is clear that poor treatment compliance with adjuvant chemotherapy, regardless of the reasons, impedes patient survival. Therefore, other modes of delivering chemotherapy that can increase compliance are desperately needed. Hence, the concept of delivering chemotherapy before surgery is proposed to treat occult micrometastases early and increase treatment compliance, ultimately improving survival outcome.8 Different methods and schedules to carry out systemic chemotherapy before surgery are now of particular interest in clinical trials of rectal cancer management.
4. CLINICAL IMPORTANCE OF PATHOLOGICAL COMPLETE RESPONSE AFTER nCRT
It is well known that nCRT not only results in significant downsizing or downstaging of tumor, but also in pathological complete response (pCR) defined as the complete absence of cancer cells in the resected specimen. In trials evaluating nCRT in locally advanced rectal cancer, pCR was reported in 10%‐32% of patients.31, 32, 33 In general, patients who were treated with nCRT and TME did not show significant improvement in overall survival despite the excellent local control rate.12, 34 However, the prognosis in patients who obtain pCR is usually excellent, so that it is often used as a surrogate marker for good oncological outcome.35
Analysis of the factors that might increase the rate of pCR showed that the use of continuous infusion of 5‐fluorouracil, the addition of a second cytotoxic drug and high radiation dose were associated with a higher rate of pCR.31, 32 Noninferiority, phase III randomized trials comparing capecitabine to 5‐fluorouracil in CRT showed that capecitabine could be an alternative in neoadjuvant or adjuvant CRT regimens.36 Given that capecitabine functions equally as a radiosensitizer, addition of oxaliplatin to either continuous 5‐fluorouracil or capecitabine and radiotherapy during chemoradiation was attempted.37, 38, 39 Unfortunately, these trials failed to show improvement of tumor response but only increased toxicity. In terms of the long‐term oncological outcomes, conflicting results were reported. In the ACCORD 12 trial, the addition of oxaliplatin did not show significant improvement in 5‐year disease free survival or overall survival.40 However, in the German CAO/ARO/AIO‐04 trial, 3‐year disease‐free survival was significantly enhanced.41 Nevertheless, at this time, the addition of oxaliplatin to standard nCRT is not supported. Finally, although the rate of pCR was higher with increasing radiation dose, a dose‐response effect beyond 45 Gy was not recommended due to a lack of data.31, 32 Therefore, 5‐fluoropyrimidine‐based nCRT remains the current standard treatment, and a way of intensifying the effect of nCRT is the primary focus of ongoing clinical trials.
One problem related to the identification of patients who have achieved pCR is the optimal interval from the end of chemoradiation to surgery. It is known that the effect of chemoradiation takes time for a result to be seen, and complete clinical regression of tumor could be achieved when surgery occurred after a longer time interval.42 The Lyon R90‐01 trial showed that patients undergoing surgery at 6‐8 weeks after completion of nCRT had significantly increased rates of pCR compared to those operated at 2 weeks after the completion of nCRT, 26% vs 10% respectively, supporting the currently accepted timing of surgery.43 The 17‐year follow‐up report indicated that the long‐term oncological outcome was similar in the two groups: local recurrence was 14% in the 6‐ to 8‐week group and 12% in the 2‐week group, and the overall survival was 42% and 40%, respectively.33 With a longer interval after therapy, tumor shrinkage and downstaging seemed to be more apparent. Other retrospective studies and meta‐analyses also support that tumor response to nCRT is time‐dependent and suggest that an extended interval between completion of nCRT and surgery is an important determinant in achieving a pCR.44, 45, 46, 47 However, the GRECCAR‐6 trial, a phase III randomized, multicenter trial evaluating the effect of interval (7 or 11 weeks) between nCRT and surgery on pCR, failed to show a significant difference in the pCR rate between the two groups.48 The authors concluded that the longer interval resulted in increased postoperative morbidity and poor quality of mesorectal resection without increasing pCR rate. Furthermore, the possibility of preventing disease progression and distant metastasis by many clinicians and surgeons is not yet fully addressed. The optimal interval of the waiting period and the timing of surgery remain to be clarified. Further research is needed to determine the risk and benefit of a prolonged interval between nCRT and surgery. Nevertheless, to improve pCR rates, upfront chemotherapy prior to surgery is under active investigation. The question of timing of surgery may receive clues from the period of upfront chemotherapy.
5. MODE OF DELIVERING SYSTEMIC CHEMOTHERAPY: CONSOLIDATION CHEMOTHERAPY
In order to address the risk of disease progression and distant metastasis during a longer interval between nCRT and surgery and to improve tumor regression, delivering systemic chemotherapy after nCRT, called consolidation chemotherapy, was suggested. Currently available reports on consolidation chemotherapy are listed in Table 1.49, 50, 51, 52 A prospective, multicenter, phase II trial was conducted to evaluate the effect of adding cycles of chemotherapy in between chemoradiation and surgery, extending the waiting period.49 This study showed that the pCR rate increased with the length of chemoradiation‐to‐surgery interval and with increased number of chemotherapy cycles. Surgical complications or surgical technical difficulty after delaying surgery for up to 20 weeks was not increased, showing surgical safety. Despite long‐term oncological outcome not being reported, disease progression or distant metastasis was not observed, which implies that consolidation chemotherapy is oncologically safe. Treatment compliance with neoadjuvant chemotherapy was only as low as 77%, which is much better compared to 40%‐60% for the adjuvant chemotherapy.6, 7 What is uncertain in this trial is the relative contribution of chemotherapy and waiting period to the rate of pCR. This ambiguity is expected to be clarified when the results from the RAPIDO phase III randomized trial, comparing short‐course radiotherapy followed by capecitabine and oxaliplatin chemotherapy before surgery to standard long‐course nCRT, are received.53
Table 1.
Studies investigating consolidation chemotherapy
| Study | Design | N | CRT regimen | NAC regimen | Adjuvant CTx | pCR rate (%) | Compliance | R0 resection rate (%) | Surgical complication rate (%) | Survival outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Garcia‐Aguilar49 | Phase II nonrandomized four‐arm | 259 | CRT+5FU | None | mFOLFOX (8×) | 18 | Not reported | 98 | 15 | Not reported |
| CRT+5FU | mFOLFOX6 (2×) | mFOLFOX (6×) | 25 | 82% completed NAC | 100 | 6 | Not reported | |||
| CRT+5FU | mFOLFOX6 (4×) | mFOLFOX (4×) | 30 | 81% completed NAC | 96 | 4 | Not reported | |||
| CRT+5FU | mFOLFOX6 (6×) | mFOLFOX (2×) | 36 | 77% completed NAC | 100 | 9 | Not reported | |||
| Polish II trial50 | Phase III randomized two‐arm | 515 | RT (5 × 5 Gy) | FOLFOX4 (3 cycles) | Not reported | 16 | 63% completed NAC | 77 | 29 | 3‐y DFS = 53% 3‐y OS = 73% |
| CRT+5FU / leucovorin / oxaliplatin | None | Not reported | 12 | 66% completed CRT | 71 | 25 | 3‐y DFS = 52% 3‐y OS = 65% | |||
| Gao51 | Prospective single‐arm | 36 | CRT+CAPOX | CAPOX (1×) | Not reported | 36 | 94% completed NAC | 100 | 36 | Not reported |
| Zhu52 | Phase II single‐arm | 42 | CRT+CAPOX | Cape (1×) | CAPOX (6‐8×) | 17 | 100% completed NAC | 92 | 16 | 3‐y DFS = 57% 3‐y OS = 66% |
5‐FU, 5‐fluorouracil; Cape, capecitabine; CAPOX, capecitabine/oxaliplatin; CRT, chemoradiotherapy; CTx, chemotherapy; DFS, disease‐free survival; Gy, gray; mFOLFOX6, 5‐fluorouracil, leucovorin, and oxaliplatin; NAC, neoadjuvant chemotherapy; OS, overall survival; pCR, pathological complete response; R0, microscopically clear resection; RT, radiotherapy.
A large phase III Polish trial provides valuable information regarding the long‐term oncological outcome after consolidation chemotherapy.50 In this trial, patients were randomly assigned to two treatment groups: one with short‐course radiotherapy followed by three cycles of chemotherapy with fluorouracil and oxaliplatin and the other with the standard CRT with oxaliplatin and boluses of 5‐fluorouracil and leucovorin. Both groups had similar intervals between the start of irradiation and surgery, approximately 12 weeks. The R0 resection rate and postoperative surgical complication rate were similar in both groups. Also, there was no difference in disease‐free survival, local failure rate, and distant metastases rate in the two groups. However, the patients given short‐course radiotherapy followed by consolidation chemotherapy showed better treatment compliance and lower acute toxicity than the long‐course CRT group. Interestingly, the 3‐year overall survival was better in the patients with short‐course radiotherapy followed by consolidation chemotherapy, despite the rate of distant metastasis or local recurrence being similar in the two groups. The authors presumed that large irradiation doses result in activation of antitumor immune responses during the waiting period before surgery. Observation on longer follow ups will probably clarify this phenomenon. So far, these studies suggest that consolidation chemotherapy can be a safe and feasible option in the multimodal approach, encouraging implementation in future trials.
6. MODE OF DELIVERING SYSTEMIC CHEMOTHERAPY: INDUCTION CHEMOTHERAPY
Another way of delivering chemotherapy before surgery is dividing adjuvant chemotherapy and delivering a limited number of cycles before nCRT, and then delivering the remaining cycles postoperatively, named induction therapy.54 Studies assessing the effect of induction chemotherapy are listed in Table 2.55, 56, 57, 58, 59, 60, 61, 62 This approach is particularly appealing for locally far advanced diseases showing high‐risk features of distant metastasis or difficult local resection, such as extramural venous invasion or lateral pelvic lymph node metastasis. Theoretically, upfront chemotherapy allows the chemotherapeutic agents to reach the primary tumor directly when the vasculature is not disrupted by either radiation or surgery. Therefore, the tumor may optimally respond to the chemotherapeutic agent. Clinical trials of induction chemotherapy show no adverse effect that would delay treatment, increased pCR rate, and early identification of nonresponders along with excellent treatment compliance.8, 31, 35, 54 Regarding long‐term oncological outcome, the Spanish GCR‐3 phase II trial reported that local recurrence rate and survival outcomes at 5 years of follow up were not different from the control group, although the experimental group showed lower toxicity and better compliance.55 The CONTRE trial notably demonstrated a similar pCR rate to the consolidation chemotherapy trial conducted by Garcia‐Aguilar et al.57 This prospective single‐arm study reported the outcome of patients who received eight cycles of mFOLFOX6 before CRT. Again, the compliance rate was over 90%, and the R0 resection rate was 100%. It appears that approximately 6‐8 cycles of induction chemotherapy are needed to achieve a similar rate of pCR. In a future trial, the optimal duration and number of cycles of induction chemotherapy would be an important topic to address.
Table 2.
Studies investigating induction chemotherapy
| Study | Design | N | NAC regimen | CRT regimen | Adjuvant CTx | pCR rate (%) | Compliance | R0 resection rate (%) | Survival outcome |
|---|---|---|---|---|---|---|---|---|---|
| Spanish GCR‐355 | Phase II RCT | 108 | CAPOX (4×) | CRT+CAPOX | 14 | 94% completed NAC; 85% completed CRT | 86 | 5‐y DFS = 64% 5‐y OS = 74% | |
| CAPOX (4×) | 13 | 57% completed adjuvant CTx; 80% completed RT | 87 | 5‐y DFS = 62% 5‐y OS = 77% | |||||
| EXPERT56 | Phase II single‐arm | 105 | CAPOX (4×) | CRT+Cape | Cape (12 wks) | 20 | 89% complete NAC; 91% complete CRT | 98 | 5‐y DFS = 64% 5‐y OS = 75% |
| CONTRE57 | Prospective single‐arm | 39 | mFOLFOX6 (8×) | CRT+5FU or Cape | None | 33 | 92% completed NAC; 90% completed CRT | 100 | Not reported |
| COPERNICUS58 | Phase II single‐arm | 60 | Oxali+5‐FU (4×) | SC‐RT (5 Gy × 5) | Oxali+5‐FU (8×) | 12 | 95% completed NAC; 97% completed CRT | 98 | 2‐y PFS = 86.2% |
| Dueland59 | Phase II single‐arm | 97 | FLOX (2×) | CRT+CAPOX | None | 17 | 98% completed NAC; 95% completed RT | 90 | 5‐y DFS = 61% 5‐y OS = 83% |
| Schou60 | Prospective single‐arm | 84 | CAPOX (2×) | CRT+Cape | Not reported | 23 | 91% completed 21 NAC cycles; 93% completed CRT | 94 | 5‐y DFS = 63% 5‐y OS = 67% |
| EXPERT‐C61 | Phase II RCT | 165 | CAPOX+cetuximab (4×) | CRT+Cape+ cetuximab | CAPOX+cetuximab (4×) | 11 | 95% completed NAC; 91% completed CRT | 96 | Not reported |
| CAPOX (4×) | CRT+Cape | CAPOX (4×) | 7 | 93% completed NAC; 90% completed CRT | 92 | Not reported | |||
| AVACROSS62 | Phase II single‐arm | 47 | CAPOX1 Bevacizumab (4×) | CRT+Cape+ bevacizumab | CAPOX (4×) | 34 | 85% completed NAC; 83% completed CRT | 98 | Not reported |
5‐FU, 5‐fluorouracil; Cape, capecitabine; CAPOX, capecitabine/oxaliplatin; CRT, chemoradiotherapy; CTx, chemotherapy; DFS, disease‐free survival; Gy, gray; mFOLFOX6, 5‐fluorouracil, leucovorin, and oxaliplatin; NAC, neoadjuvant chemotherapy; OS, overall survival; pCR, pathological complete response; PFS, progression‐free survival; R0, microscopically clear resection; RT, radiotherapy; SC‐RT, short‐course radiotherapy.
Two clinical studies included target agents in the induction therapy. EXPERT‐C, a branching trial of EXPERT, investigated the effect of adding cetuximab to capecitabine‐based induction chemotherapy followed by chemoradiotherapy, then again adding cetuximab in adjuvant chemotherapy.61 Similar to other studies, this randomized trial indicated that compliance was over 90% in both groups, but either pCR rate or R0 resection in the two groups was not different. AVACROSS was a phase II single‐arm study evaluating the effect of bevacizumab to induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiotherapy.62 Along with high compliance with the treatment, the pCR rate was as high as 34%, and the R0 resection rate was 98%. However, postoperative morbidity occurred in 58% of patients, and 24% required surgical reintervention. The safety of adding bevacizumab to induction chemotherapy should be addressed. Nevertheless, the role of target agent in induction chemotherapy is not yet fully investigated.
7. RISK‐ADAPTED APPROACH AND ORGAN PRESERVATION
Based on previous reports, it is quite certain that TNT, consolidation or induction chemotherapy with nCRT, provides several benefits in the treatment of locally advanced rectal cancer. It not only improves pCR rate and treatment compliance but also delivers early systemic control for possible micrometastasis. Beyond these benefits, TNT gives the opportunity to assess chemosensitivity and tumor response prior to surgery. This can lead to risk stratification and identification of patients who may not require surgery or radiation therapy. In fact, several studies reporting a nonoperative approach have suggested that with robust follow ups, a certain subgroup of patients who achieved a complete clinical response could be safely left with the rectum and have good long‐term oncological outcomes.63, 64, 65 However, an important concern arising from this “watch and wait” approach is how to accurately access tumor regression and identify patients with no residual disease.
Reflecting back to the past, pelvic magnetic resonance imaging (MRI) enormously contributed to the development of a multidisciplinary therapeutic approach, providing accurate clinical staging. Pelvic MRI has been used as a tool to guide preoperative decision on treatment modality, essentially determining which patients undergo nCRT before surgery.66, 67 Also, previous studies show that high‐resolution MRI can predict the survival outcome based on the assessment of tumor regression grade and circumferential resection margin status prior to surgery, enabling identification of poor responders and good responders.68, 69 Thus, MRI can help to appropriately stratify risk and identify potential candidates for organ preservation. The TRIGGER trial, a multicenter, open, interventional, randomized control feasibility study, is ongoing to validate assessment of tumor response based on an MRI‐derived tumor regression grading system named mrTRG for short.70 Shown in Figure 2, two prospective subtrials comprise the intervention arm based on mrTRG: the good‐response group and the poor response group. The good response group follows a nonoperative approach with additional systemic chemotherapy, whereas the poor response group is planned for upfront systemic chemotherapy before surgery. Results from this study will provide much vital information regarding the role of MRI in risk‐adapted approach.
Figure 2.
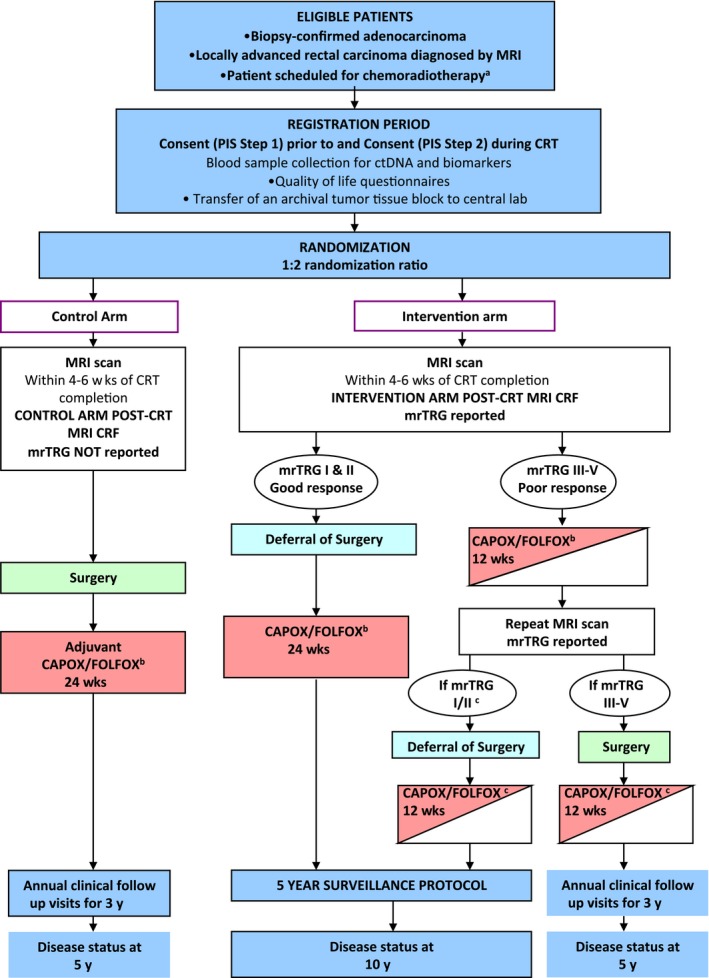
The TRIGGER trial study schema. The TRIGGER trial is a multicenter, open, interventional, randomized control feasibility study to validate assessment of tumor response based on a magnetic resonance imaging (MRI)‐derived tumor regression grading system, named mrTRG.67 CRF, clinical report form; CRT, chemoradiotherapy; PIS, patient information sheet
A large phase II multicentered randomized trial is also ongoing investigating the efficacy of TNT and a selective nonoperative approach in locally advanced rectal cancer.71 In this trial, patients are randomly assigned to either an induction chemotherapy or a consolidation chemotherapy group. Upon completion of neoadjuvant therapy, restaging with digital rectal exam, endoscopy, and MRI will be undertaken to measure tumor regression and apply risk stratification to determine whether patients should undergo standard TME surgery or nonoperative management. This study will provide information on 3‐year recurrence‐free survival in patients undergoing selective nonoperative management.
Apart from nonoperative management, there is an attempt to omit radiotherapy in the course of TNT. Several small, single‐arm studies reported that neoadjuvant chemotherapy alone could result in relatively comparable oncological outcome and, in some patients, even tumor downstaging and complete regression occurred.72, 73, 74 A phase II pilot study of induction chemotherapy without radiotherapy in locally advanced rectal cancer showed that R0 resection was fully achieved in all patients, and the pCR rate to chemotherapy alone was 25%.75 Furthermore, the oncological outcome was not compromised: 0% for the 4‐year local recurrence rate and 84% for the 4‐year disease‐free survival rate. A multicentered, phase III randomized controlled trial, Preoperative Radiation or Selective Preoperative Radiation and Evaluation Before Chemotherapy and TME (PROSPECT) is under investigation to validate the findings of the pilot study.
Several clinical trials, listed in Table 3, are ongoing, investigating the effectiveness and efficacy of the TNT approach.53, 70, 71, 75, 76, 77, 78 All these trials uniformly show the immense effort that is being made to intensify neoadjuvant therapy and to improve survival outcome. Outcomes of the trials will help understand tumor characteristics and provide essential information on optimization of the TNT approach. The results of these studies are earnestly anticipated.
Table 3.
Ongoing trials investigating the TNT approach and organ preservation
| TNT type | Trial | Design | N | Arms | 1° endpoint | 2° endpoint |
|---|---|---|---|---|---|---|
| Consolidation | RAPIDO trial53 (NCT01558921) | Phase III RCT | 842 | Standard long course CRT → surgery → optional adjuvant CAPOX (8×) | 3‐y DFS | Toxicity, R0 resection rate, pCR QOL, functional outcome OS |
| SC‐RT (5 Gy × 5) → CAPOX (6×) → surgery | ||||||
| Consolidation | TRIGGER trial70 (NCT02704520) | Phase III RCT | 633 | Refer to Figure 2 | Rate of patient recruitment and randomization | Rate of unit recruitment, toxicity, reproducibility of mrTRG reporting, surgical morbidity, pCR, residual tumor density, surgical quality rates |
| Consolidation or induction | Smith et al71 (NCT02008656) | Phase II RCT | 202 | Induction CTx+CRT | 3‐y RFS | Organ preservation rate, compliance, toxicity, functional outcome, QOL |
| CRT+consolidation CTx | ||||||
| Consolidation | KONCLUDE76 (NCT02843191) | Phase III RCT | 358 | Standard CRT→ surgery → mFOLFOX6 (8×) | pCR 3‐y DFS | Toxicity, R0 resection rate, tumor response rate, postoperative morbidity, peripheral neuropathy at 3 y after surgery |
| Standard CRT → mFOLFOX6 (3×) → surgery → mFOLFOX (5×) | ||||||
| TNT without RT | PROSPECT75 (NCT01515787) | Phase III RCT | 1060 | 5‐FU +CRT → surgery → FOLFOX (8×) | R0 rate DFS LRR | pCR OS Toxicity Rate of CRT |
| FOLFOX (6×) → tumor response assessment → TME or CRT Adjuvant therapy if R0 → FOLFOX (6×) R1+ → FOLFOX (4×)+CRT | ||||||
| TNT without RT | BACCHUS77 (NCT01650428) | Phase II RCT | 60 | FOLFOX + bev | pCR rate | Response rate CRM negative resection T and N downstaging PFS, DFS, OS, LRR 1‐y colostomy rate Toxicity, compliance |
| FOLFOXIRI + bev | ||||||
| TNT with or without RT | FOWARC78 (NCT01211210) | Phase II RCT | 495 | Standard CRT | 3‐y DFS | pCR, R0, LRR, OS Predictive biomarkers QOL, toxicity |
| FOLFOX + CRT | ||||||
| FOLFOX alone |
5‐FU, 5‐fluorouracil; bev, bevacizumab; Cape, capecitabine; CAPOX, capecitabine/oxaliplatin; CRM, circumferential resection margin; CRT, chemoradiotherapy; CTx, chemotherapy; DFS, disease‐free survival; FOLFOXIRI, oxaliplatin/5‐FU/irinotecan; Gy, gray; LRR, local recurrence rate; mFOLFOX6, 5‐fluorouracil, leucovorin, and oxaliplatin; mrTRG, magnetic resonance imaging‐derived tumor regression grading system; OS, overall survival; pCR, pathological complete response; PFS, progression‐free survival; QOL, quality of life; R0, microscopically clear resection; RCT, randomized controlled trial; RT, radiotherapy; SC‐RT, short‐course radiotherapy; TME, total mesorectal excision; TNT, total neoadjuvant therapy.
8. CONCLUSION
Total neoadjuvant therapy offers a chance to deliver aggressive treatment against the development and progression of micrometastases, potentially increasing survival rates in locally advanced rectal cancer. Furthermore, there is tremendous interest and desire for organ preservation in rectal cancer partly driven by patients who want to preserve a decent quality of life in the modern era. The total neoadjuvant therapy approach may facilitate a greater number of patients having the potential for organ preservation. Upcoming results from multiple ongoing and future trials will assist the clinical decisions that fulfil optimal oncological outcomes as well as quality of life.
DISCLOSURE
Conflicts of Interest: Authors declare no conflicts of interest for this article.
Author Contribution: Hyung Jin Kim and Ri Na Yoo conceived of the presented idea. Hyung Jin Kim developed the theory. Ri Na Yoo drafed the manuscript. Hyung Jin Kim and Ri Na Yoo both discussed the final draft of manuscript.
Yoo RN, Kim HJ. Total neoadjuvant therapy in locally advanced rectal cancer: Role of systemic chemotherapy. Ann Gastroenterol Surg. 2019;3:356–367. 10.1002/ags3.12253
REFERENCES
- 1. Havenga K, Enker WE, Norstein J, Moriya Y, Heald RJ, van Houwelingen HC, et al. Improved survival and local control after total mesorectal excision or D3 lymphadenectomy in the treatment of primary rectal cancer: an international analysis of 1411 patients. Eur J Surg Oncol. 1999;25(4):368–74. [DOI] [PubMed] [Google Scholar]
- 2. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic‐Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–23. [DOI] [PubMed] [Google Scholar]
- 3. Gerard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon‐Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3‐4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24(28):4620–5. [DOI] [PubMed] [Google Scholar]
- 4. Sebag‐Montefiore D, Stephens RJ, Steele R, Steele R, Monson J, Grieve R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC‐CTG C016): a multicentre, randomised trial. Lancet. 2009;373(9666):811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, et al. The TME trial after a median follow‐up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246(5):693–701. [DOI] [PubMed] [Google Scholar]
- 6. Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L, et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta‐analysis of individual patient data. Lancet Oncol. 2015;16(2):200–7. [DOI] [PubMed] [Google Scholar]
- 7. Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic‐Rundic S, Bensadoun RJ, et al. Fluorouracil‐based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long‐term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15(2):184–90. [DOI] [PubMed] [Google Scholar]
- 8. Glynne‐Jones R, Grainger J, Harrison M, Ostler P, Makris A. Neoadjuvant chemotherapy prior to preoperative chemoradiation or radiation in rectal cancer: should we be more cautious? Br J Cancer. 2006;94(3):363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery–the clue to pelvic recurrence? Br J Surg. 1982;69(10):613–6. [DOI] [PubMed] [Google Scholar]
- 10. Heald RJ. The ‘Holy Plane’ of rectal surgery. J Royal Soc Med. 1988;81(9):503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ruo L, Guillem JG. Major 20th‐century advancements in the management of rectal cancer. Dis Colon Rectum. 1999;42(5):563–78. [DOI] [PubMed] [Google Scholar]
- 12. Fisher B, Wolmark N, Rockette H, Redmond C, Deutsch M, Wickerham DL, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R‐01. J Natl Cancer Inst. 1988;80(1):21–9. [DOI] [PubMed] [Google Scholar]
- 13. Thomas PR, Lindblad AS. Adjuvant postoperative radiotherapy and chemotherapy in rectal carcinoma: a review of the Gastrointestinal Tumor Study Group experience. Radiother Oncol. 1988;13(4):245–52. [DOI] [PubMed] [Google Scholar]
- 14. Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, et al. Effective surgical adjuvant therapy for high‐risk rectal carcinoma. N Engl J Med. 1991;324(11):709–15. [DOI] [PubMed] [Google Scholar]
- 15. NIH Consensus Conference . Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264(11):1444–50. [PubMed] [Google Scholar]
- 16. Cedermark B, Dahlberg M, Glimelius B, Pahlman L, Rutqvist LE, Wilking N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336(14):980–7. [DOI] [PubMed] [Google Scholar]
- 17. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO‐94 randomized phase III trial after a median follow‐up of 11 years. J Clin Oncol. 2012;30(16):1926–33. [DOI] [PubMed] [Google Scholar]
- 18. van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12‐year follow‐up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12(6):575–82. [DOI] [PubMed] [Google Scholar]
- 19. Pahlman L, Glimelius B. Pre‐ or postoperative radiotherapy in rectal and rectosigmoid carcinoma. Report from a randomized multicenter trial. Ann Surg. 1990;211(2):187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345(9):638–46. [DOI] [PubMed] [Google Scholar]
- 21. Guillem JG, Chessin DB, Cohen AM, Shia J, Mazumdar M, Enker W, et al. Long‐term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg. 2005;241(5):829–36; discussion 36‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322(6):352–8. [DOI] [PubMed] [Google Scholar]
- 23. Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–16. [DOI] [PubMed] [Google Scholar]
- 24. Petersen SH, Harling H, Kirkeby LT, Wille‐Jorgensen P, Mocellin S. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev. 2012;3:Cd004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bujko K, Glimelius B, Valentini V, Michalski W, Spalek M. Postoperative chemotherapy in patients with rectal cancer receiving preoperative radio(chemo)therapy: a meta‐analysis of randomized trials comparing surgery +/‐ a fluoropyrimidine and surgery + a fluoropyrimidine +/‐ oxaliplatin. Eur J Surg Oncol. 2015;41(6):713–23. [DOI] [PubMed] [Google Scholar]
- 26. Maas M, Nelemans PJ, Valentini V, Crane CH, Capirci C, Rödel C, et al. Adjuvant chemotherapy in rectal cancer: defining subgroups who may benefit after neoadjuvant chemoradiation and resection: a pooled analysis of 3,313 patients. Int J Cancer. 2015;137(1):212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taal BG, Van Tinteren H, Zoetmulder FA. Adjuvant 5FU plus levamisole in colonic or rectal cancer: improved survival in stage II and III. Br J Cancer. 2001;85(10):1437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Engelen SM, Maas M, Lahaye MJ, Leijtens JW, van Berlo CL, Jansen RL, et al. Modern multidisciplinary treatment of rectal cancer based on staging with magnetic resonance imaging leads to excellent local control, but distant control remains a challenge. Eur J Cancer. 2013;49(10):2311–20. [DOI] [PubMed] [Google Scholar]
- 29. Khrizman P, Niland JC, ter Veer A, Milne D, Bullard Dunn K, Carson WE 3rd, et al. Postoperative adjuvant chemotherapy use in patients with stage II/III rectal cancer treated with neoadjuvant therapy: a national comprehensive cancer network analysis. J Clin Oncol. 2013;31(1):30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta‐analysis. JAMA. 2011;305(22):2335–42. [DOI] [PubMed] [Google Scholar]
- 31. Hartley A, Ho KF, McConkey C, Geh JI. Pathological complete response following pre‐operative chemoradiotherapy in rectal cancer: analysis of phase II/III trials. Br J Radiol. 2005;78(934):934–8. [DOI] [PubMed] [Google Scholar]
- 32. Sanghera P, Wong DW, McConkey CC, Geh JI, Hartley A. Chemoradiotherapy for rectal cancer: an updated analysis of factors affecting pathological response. Clin Oncol. 2008;20(2):176–83. [DOI] [PubMed] [Google Scholar]
- 33. Cotte E, Passot G, Decullier E, Maurice C, Glehen O, François Y, et al. Pathologic response, when increased by longer interval, is a marker but not the cause of good prognosis in rectal cancer: 17‐year follow‐up of the Lyon R90‐01 randomized trial. Int J Radiat Oncol Biol Phys. 2016;94(3):544–53. [DOI] [PubMed] [Google Scholar]
- 34. Gastrointestinal Tumor Study Group . Prolongation of the disease‐free interval in surgically treated rectal carcinoma. N Engl J Med. 1985;312(23):1465–72. [DOI] [PubMed] [Google Scholar]
- 35. Park IJ, You YN, Agarwal A, Skibber JM, Rodriguez‐Bigas MA, Eng C, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30(15):1770–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non‐inferiority, phase 3 trial. Lancet Oncol. 2012;13(6):579–88. [DOI] [PubMed] [Google Scholar]
- 37. Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR‐01 randomized phase III trial. J Clin Oncol. 2011;29(20):2773–80. [DOI] [PubMed] [Google Scholar]
- 38. Gerard JP, Azria D, Gourgou‐Bourgade S, Martel‐Laffay I, Hennequin C, Etienne PL, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405‐Prodige 2. J Clin Oncol. 2010;28(10):1638–44. [DOI] [PubMed] [Google Scholar]
- 39. O'Connell MJ, Colangelo LH, Beart RW, Petrelli NJ, Allegra CJ, Sharif S, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R‐04. J Clin Oncol. 2014;32(18):1927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Azria D, Doyen J, Jarlier M, Martel‐Lafay I, Hennequin C, Etienne P, et al. Late toxicities and clinical outcome at 5 years of the ACCORD 12/0405‐PRODIGE 02 trial comparing two neoadjuvant chemoradiotherapy regimens for intermediate‐risk rectal cancer. Ann Oncol. 2017;28(10):2436–42. [DOI] [PubMed] [Google Scholar]
- 41. Rodel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, et al. Oxaliplatin added to fluorouracil‐based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO‐04 study): final results of the multicentre, open‐label, randomised, phase 3 trial. Lancet Oncol. 2015;16(8):979–89. [DOI] [PubMed] [Google Scholar]
- 42. Cummings BJ, Rider WD, Harwood AR, Keane TJ, Thomas GM. Radical external beam radiation therapy for adenocarcinoma of the rectum. Dis Colon Rectum. 1983;26(1):30–6. [DOI] [PubMed] [Google Scholar]
- 43. Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter‐sparing surgery for rectal cancer: the Lyon R90‐01 randomized trial. J Clin Oncol. 1999;17(8):2396. [DOI] [PubMed] [Google Scholar]
- 44. Kalady MF, de Campos‐Lobato LF, Stocchi L, Geisler DP, Dietz D, Lavery IC, et al. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg. 2009;250(4):582–9. [DOI] [PubMed] [Google Scholar]
- 45. Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer: a meta‐analysis of published studies. Ann Surg. 2016;263(3):458–64. [DOI] [PubMed] [Google Scholar]
- 46. Probst CP, Becerra AZ, Aquina CT, Tejani MA, Wexner SD, Garcia‐Aguilar J, et al. Extended intervals after neoadjuvant therapy in locally advanced rectal cancer: the key to improved tumor response and potential organ preservation. J Am Coll Surg. 2015;221(2):430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Foster JD, Jones EL, Falk S, Cooper EJ, Francis NK. Timing of surgery after long‐course neoadjuvant chemoradiotherapy for rectal cancer: a systematic review of the literature. Dis Colon Rectum. 2013;56(7):921–30. [DOI] [PubMed] [Google Scholar]
- 48. Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, de Chaisemartin C, et al. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR‐6). J Clin Oncol. 2016;34(31):3773–80. [DOI] [PubMed] [Google Scholar]
- 49. Garcia‐Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16(8):957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bujko K, Wyrwicz L, Rutkowski A, Malinowska M, Pietrzak L, Kryński J, et al. Long‐course oxaliplatin‐based preoperative chemoradiation versus 5 x 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27(5):834–42. [DOI] [PubMed] [Google Scholar]
- 51. Gao YH, An X, Sun WJ, Cai J, Cai MY, Kong LH, et al. Evaluation of capecitabine and oxaliplatin administered prior to and then concomitant to radiotherapy in high risk locally advanced rectal cancer. J Surg Oncol. 2014;109(5):478–82. [DOI] [PubMed] [Google Scholar]
- 52. Zhu J, Gu W, Lian P, Sheng W, Cai G, Shi D, et al. A phase II trial of neoadjuvant IMRT‐based chemoradiotherapy followed by one cycle of capecitabine for stage II/III rectal adenocarcinoma. Radiat Oncol. 2013;8:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nilsson PJ, van Etten B, Hospers GA, Påhlman L, van de Velde CJ, Beets‐Tan RG, et al. Short‐course radiotherapy followed by neo‐adjuvant chemotherapy in locally advanced rectal cancer–the RAPIDO trial. BMC Cancer. 2013;13:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cercek A, Goodman KA, Hajj C, Weisberger E, Segal NH, Reidy‐Lagunes DL, et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw. 2014;12(4):513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fernandez‐Martos C, Garcia‐Albeniz X, Pericay C, Maurel J, Aparicio J, Montagut C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long‐term results of the Spanish GCR‐3 phase II randomized trial. Ann Oncol. 2015;26(8):1722–8. [DOI] [PubMed] [Google Scholar]
- 56. Chua YJ, Barbachano Y, Cunningham D, Oates JR, Brown G, Wotherspoon A, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI‐defined poor‐risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11(3):241–8. [DOI] [PubMed] [Google Scholar]
- 57. Perez K, Safran H, Sikov W, Vrees M, Klipfel A, Shah N, et al. Complete neoadjuvant treatment for rectal cancer: The Brown University Oncology Group CONTRE Study. Am J Clin Oncol. 2017;40(3):283–7. [DOI] [PubMed] [Google Scholar]
- 58. Gollins S, West N, Sebag‐Montefiore D, Susnerwala S, Falk S, Brown N, et al. A prospective phase II study of pre‐operative chemotherapy then short‐course radiotherapy for high risk rectal cancer: COPERNICUS. Br J Cancer. 2018;119(6):697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dueland S, Ree AH, Groholt KK, Saelen MG, Folkvord S, Hole KH, et al. Oxaliplatin‐containing preoperative therapy in locally advanced rectal cancer: local response, toxicity and long‐term outcome. Clin Oncol. 2016;28(8):532–9. [DOI] [PubMed] [Google Scholar]
- 60. Schou JV, Larsen FO, Rasch L, Linnemann D, Langhoff J, Høgdall E, et al. Induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiotherapy before total mesorectal excision in patients with locally advanced rectal cancer. Ann Oncol. 2012;23(10):2627–33. [DOI] [PubMed] [Google Scholar]
- 61. Dewdney A, Cunningham D, Tabernero J, Capdevila J, Glimelius B, Cervantes A, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high‐risk rectal cancer (EXPERT‐C). J Clin Oncol. 2012;30(14):1620–7. [DOI] [PubMed] [Google Scholar]
- 62. Nogue M, Salud A, Vicente P, Arriví A, Roca JM, Losa F, et al. Addition of bevacizumab to XELOX induction therapy plus concomitant capecitabine‐based chemoradiotherapy in magnetic resonance imaging‐defined poor‐prognosis locally advanced rectal cancer: the AVACROSS study. Oncologist. 2011;16(5):614–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Habr‐Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr, Silva e Sousa AH Jr, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long‐term results. Ann Surg. 2004;240(4):711–7; discussion 7‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maas M, Beets‐Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, et al. Wait‐and‐see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29(35):4633–40. [DOI] [PubMed] [Google Scholar]
- 65. Smith JD, Ruby JA, Goodman KA, Saltz LB, Guillem JG, Weiser MR, et al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg. 2012;256(6):965–72. [DOI] [PubMed] [Google Scholar]
- 66. MERCURY Study Group . Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333(7572):779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. MERCURY Study Group . Extramural depth of tumor invasion at thin‐section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243(1):132–9. [DOI] [PubMed] [Google Scholar]
- 68. Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, et al. Magnetic resonance imaging‐detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29(28):3753–60. [DOI] [PubMed] [Google Scholar]
- 69. Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease‐free survival and local recurrence: 5‐year follow‐up results of the MERCURY study. J Clin Oncol. 2014;32(1):34–43. [DOI] [PubMed] [Google Scholar]
- 70. Battersby NJ, Dattani M, Rao S, Cunningham D, Tait D, Adams R, et al. A rectal cancer feasibility study with an embedded phase III trial design assessing magnetic resonance tumour regression grade (mrTRG) as a novel biomarker to stratify management by good and poor response to chemoradiotherapy (TRIGGER): study protocol for a randomised controlled trial. Trials. 2017;18(1):394 10.1186/s13063-017-2085-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Smith JJ, Chow OS, Gollub MJ, Nash GM, Temple LK, Weiser MR, et al. Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3‐year disease‐free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Uehara K, Hiramatsu K, Maeda A, Sakamoto E, Inoue M, Kobayashi S, et al. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor‐risk rectal cancer: N‐SOG 03 Phase II trial. Jpn J Clin Oncol. 2013;43(10):964–71. [DOI] [PubMed] [Google Scholar]
- 73. Ishii Y, Hasegawa H, Endo T, Okabayashi K, Ochiai H, Moritani K, et al. Medium‐term results of neoadjuvant systemic chemotherapy using irinotecan, 5‐fluorouracil, and leucovorin in patients with locally advanced rectal cancer. Eur J Surg Oncol. 2010;36(11):1061–5. [DOI] [PubMed] [Google Scholar]
- 74. Koike J, Funahashi K, Yoshimatsu K, Yokomizo H, Kan H, Yamada T, et al. Efficacy and safety of neoadjuvant chemotherapy with oxaliplatin, 5‐fluorouracil, and levofolinate for T3 or T4 stage II/III rectal cancer: the FACT trial. Cancer Chemother Pharmacol. 2017;79(3):519–25. [DOI] [PubMed] [Google Scholar]
- 75. Schrag D, Weiser MR, Goodman KA, Gonen M, Hollywood E, Cercek A, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32(6):513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kim CW, Kang BM, Kim IY, Kim JY, Park SJ, Park WC, et al. Korean Society of Coloproctology (KSCP) trial of cONsolidation Chemotherapy for Locally advanced mid or low rectal cancer after neoadjUvant concurrent chemoraDiothErapy: a multicenter, randomized controlled trial (KONCLUDE). BMC Cancer. 2018;18(1):538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Glynne‐Jones R, Hava N, Goh V, Bosompem S, Bridgewater J, Chau I, et al. Bevacizumab and Combination Chemotherapy in rectal cancer Until Surgery (BACCHUS): a phase II, multicentre, open‐label, randomised study of neoadjuvant chemotherapy alone in patients with high‐risk cancer of the rectum. BMC Cancer. 2015;15:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open‐label, randomized three‐arm phase III trial. J Clin Oncol. 2016;34(27):3300–7. [DOI] [PubMed] [Google Scholar]


