Abstract
Background
The aim of the present study was to develop a risk calculator predictive of postoperative pneumonia in patients undergoing gastroenterological surgery.
Methods
We analyzed data from 382 124 patients undergoing eight main gastroenterological surgeries between 2011 and 2013 using the National Clinical Database in Japan. A risk model was developed using multivariate logistic regression analysis with patient data from 2011 to 2012 (n = 247 604) and validated using data from 2013 (n = 134 520).
Results
Pneumonia was observed in 11 105 patients (2.9%). After the input of significant primary disease and surgical procedures, 18 patient characteristics including gender, chronic obstructive pulmonary disease, sepsis, and need for any assistance in the activities of daily living, six laboratory parameters, and two intraoperative factors were used for risk calculation. Area under the receiver‐operating characteristic curve was 0.822 (95% confidence interval, 0.817‐0.826) in the derivation group and 0.826 (0.819‐0.832) in the validation group.
Conclusion
The risk calculator accurately predicted the occurrence of pneumonia following gastroenterological surgery.
Keywords: gastroenterological surgery, National Clinical Database, pneumonia, postoperative complication, risk model
1. INTRODUCTION
Pneumonia is the third leading cause of postoperative infectious complications, following surgical site infections (SSI) and urinary tract infections.1 Incidence of postoperative pneumonia after cardiac surgery has been reported to be between 2.1% and 3.3%.2, 3, 4 This is higher than in non‐cardiac surgery, which has a postoperative pneumonia incidence of between 0.6% and 1.8%.5, 6, 7, 8 Patients having abdominal surgery are also particularly vulnerable to developing postoperative pneumonia, with an estimated incidence of 3.2% to 10.7%.9, 10, 11, 12, 13, 14, 15 Postoperative pneumonia is associated with increased mortality, length of hospital stay and cost of care. Thompson et al9 reported that postoperative pneumonia increased the risk of in‐hospital mortality ninefold, resulting in a mean increase of 11 days in length of hospital stay and a $28 000 US dollar increase in total hospital costs per patient. A better understanding of which patients are at an increased risk of postoperative pneumonia is important to help prioritize the introduction of enhanced preventive interventions.6
In a study of pulmonary complications specific to pneumonia, Kinlin et al2 reported a clinical prediction rule for use after coronary artery bypass graft surgery. Gupta et al5 developed a risk calculator in patients who underwent multiple surgical subspecialties (cardiac surgery 0.3%). Seven independent predictors were identified: age; American Society of Anesthesiologists (ASA) class; chronic obstructive pulmonary disease; dependent functional status; preoperative sepsis; smoking; and type of operation. Arozullah et al8 also developed a multifactorial risk index for predicting postoperative pneumonia after major non‐cardiac surgery. Pneumonia rates were 0.2% among those with 0‐15 risk points; 1.2% for those with 16‐25 points; 4.0% for those with 26‐40 points; 9.4% for those with 41‐55 points; and 15.3% for those with >55 points. Several models for wide‐spectrum pulmonary complications, including pulmonary collapse or pulmonary embolism post‐abdominal surgery, have been reported;10, 13, 16 however, a risk calculator to predict pneumonia after abdominal surgery is not currently available.
Enhanced preventive interventions are required in patients who are assessed as high risk for postoperative pneumonia. Pneumonia‐prevention programs have been successfully implemented not only in intensive care unit (ICU) settings but also in surgical wards.6, 17 The program in surgical wards consists of ambulation, breathing exercises, oral care, and bedhead elevation.6 However, the pneumonia‐prevention program has not been implemented routinely in patients undergoing gastroenterological surgery because of the lack of a distribution of the standard approach in non‐critical care settings and the limited number of medical staff in Japan. In addition, a concerted effort is needed to maintain compliance with the program. To become a widely adopted program, identification of high‐risk patients for postoperative pneumonia is required.
The aim of the present study was to develop and validate a risk calculator for predicting postoperative pneumonia after gastroenterological surgery. We used data from the gastroenterological section of the National Clinical Database (NCD) of Japan, which was established in April 2010, with 10 surgical subspecialty societies on the board of the Japan Surgical Society.18 The NCD collaborates with the American College of Surgeons' National Surgical Quality Improvement Program (ACS‐NSQIP),19 which shares a similar goal of developing a standardized surgical database for quality improvement.20
2. METHODS
2.1. Data set
The NCD is a nationwide project administered in conjunction with the board certification system for surgery in Japan. Data were extracted from the 2011‐2013 NCD data files. There were 2158 participant hospitals. The NCD continuously recruits individuals who approve the data, and members of various departments are in charge of cases and data entry officers, and a web‐based data management system ensures data traceability. The staff also validate data consistency through random inspections of the institutions. Surgical cases from each department were registered in the gastroenterological surgery section of the NCD, which required detailed input of eight selected main procedures. All variables and definitions, as well as the inclusion criteria for the NCD, are accessible on the NCD web site (http://www.ncd.or.jp/). The NCD supports an E‐learning system to ensure consistent data entry.
2.2. Patients
Inclusion criteria for the study were patients who underwent the following operations: (i) esophagectomy; (ii) total gastrectomy, (iii) distal gastrectomy; (iv) right colectomy; (v) low anterior resection; (vi) hepatectomy with >1 segment except for the lateral segment; (vii) pancreaticoduodenectomy; and (viii) surgery for acute diffuse peritonitis. Patients agreed for their data to be included in the research projects by using presumed consent with an opt‐out through the web page and/or a notice from each hospital. The NCD project was approved by the Japan Surgical Society Ethics Committee on November 2010. Patients who declined to have their records entered into the NCD were excluded from our analysis. Records with missing data on patient age, gender, or the occurrence of postoperative pneumonia were excluded. In the 2011‐12 dataset for the development of the risk calculator, 247 604 records were used; in the 2013 dataset for the validation of the model, 134 520 records were used.
2.3. Outcome measures
Primary outcome of interest was postoperative pneumonia. Postoperative pneumonia was defined as pneumonia occurring within 30 days post‐surgery in patients with no evidence of pneumonia preoperatively.
The registry defines postoperative pneumonia as having met one of the following conditions:
-
1
Rales or dullness to percussion on physical examination of the chest and any of the following:
New onset of purulent sputum or change in character of sputum.
Positive growth in blood culture.
Isolation of pathogen from specimen obtained by transtracheal aspirate, bronchial brushing, or biopsy.
-
2
Chest radiography showing new or progressive infiltrate, consolidation or pleural effusion, and any of the following:
New onset of purulent sputum or change in character of sputum.
Positive growth in blood culture.
Isolation of pathogen from specimen obtained by transtracheal aspirate, bronchial brushing, or biopsy.
Isolation of virus or detection of viral antigen in respiratory secretions.
Diagnostic single antibody titer (IgM) or fourfold increase in paired serum samples (IgG) for pathogen.
Histopathological evidence of pneumonia.
In addition, we evaluated 30‐day mortality and operative mortality in patients with pneumonia. The latter was defined within the index hospitalization period, regardless of the length of hospital stay (up to 90 days), as well as any death after discharge, within 30 days of surgery. Surgical site‐related morbidities that occurred within 30 days of surgery (superficial/deep incisional SSI, organ space SSI, wound dehiscence, and anastomotic leak) were also assessed.
2.4. Preoperative and intraoperative variables
NCD variables including patient demographics, pre‐existing comorbidities, preoperative laboratory values and perioperative data are almost identical to those applied in ACS‐NSQIP.21 In addition to these, intraoperative factors such as prolonged surgery and severe blood loss were also evaluated. The definition of prolonged surgery is an operation lasting over the 75th percentile of the distribution of operation time for a specific category of procedures. The definition of severe blood loss is blood loss that is over the 75th percentile of the distribution for a specific category of procedures.
2.5. Statistical analysis
IBM SPSS Statistics for Windows (Version 20; IBM Corp, Armonk, NY, USA) was used for all data analysis. Univariate analysis of the data was carried out using Fisher's exact test, unpaired Student's t test, and Mann‐Whitney U test. Data were assigned to one of two sets: model development was based on the 2011‐12 dataset; and the model was validated using the 2013 dataset.
Logistic regression models were constructed using stepwise selection of the predictors. Discriminatory ability of the prediction rule in the derivation group was quantified using the area under the receiver‐operating characteristic (ROC) curve. Model calibration was examined by comparing the observed and predicted means with 10 equally sized subgroups, which were arranged in order of increasing patient risk. Model validation applied the developed model to estimate pneumonia probabilities for all patients in the 2013 dataset. A value of P < 0.05 was considered statistically significant.
3. RESULTS
Among 382 124 patients in the 2011‐13 dataset, postoperative pneumonia was observed in 11 105 patients (2.9%). Patients who experienced pneumonia had a significantly higher operative mortality (23.1% vs 2.1%, P < 0.001). Operative mortality in patients with pneumonia was 12.5% in esophagectomy, 20.2% in total gastrectomy, 16.6% in distal gastrectomy, 28.4% in right colectomy, 17.8% in low anterior resection, 35.6% in hepatectomy, 27.6% in pancreaticoduodenectomy, and 43.0% in surgery for acute diffuse peritonitis.
Patients with postoperative pneumonia had more surgical site‐related complications compared with those without postoperative pneumonia. Specifically, 23.1% of patients with postoperative pneumonia also had a superficial incisional SSI; 25.1% had an organ/space SSI; and 20.4% had an anastomotic leak. The most common types of surgery were gastrectomy [total gastrectomy: n = 58 809 (15.4%); and distal gastrectomy: n = 112 867 (29.5%)] and colorectal resection [right colectomy: n = 60 738 (15.9%); and lower anterior resection: n = 58 401 (15.3%)] followed by pancreaticoduodenectomy [n = 27 702 (7.2%)], operation for acute diffuse peritonitis [n = 27 377 (7.2%)], hepatectomy [n = 23 610 (6.2%)] and esophagectomy [n = 16 556, (4.3%)]. Cut‐off values for prolonged surgery and severe intraoperative blood loss are shown in Table 1.
Table 1.
Cut‐off values of prolonged surgery and severe intraoperative blood loss in eight selected gastroenterological surgeries
| Surgical procedure | Duration of surgery, 75th percentile (min) | Intraoperative blood loss, 75th percentile (mL) |
|---|---|---|
| Esophagectomy | 573 | 684 |
| Distal gastrectomy | 305 | 323 |
| Total gastrectomy | 338 | 635 |
| Right hemicolectomy | 245 | 246 |
| Low anterior resection | 335 | 375 |
| Hepatectomy with >1 segment except for the lateral segment | 477 | 1570 |
| Pancreaticoduodenectomy | 547 | 1300 |
| Operation for acute diffuse peritonitis | 160 | 247 |
The highest incidence of postoperative pneumonia was observed in esophagectomy (13.7%). Surgery for acute diffuse peritonitis was next highest, with an incidence of 7.5%. Gastrectomy (total/distal), pancreaticoduodenectomy and hepatectomy had incidences of 2.5% (3.6/1.9), 2.5% and 2.2%, respectively. The lowest incidence of postoperative pneumonia was observed in low anterior resection (0.9%) and right hemicolectomy (1.6%).
Univariate analysis for predictors of postoperative pneumonia is shown in Table 2. We identified 38 independent predictors of postoperative pneumonia, and the multivariate logistic regression model with odds ratios (OR), 95% confidence intervals (CI) and β coefficient is shown in Table 3. After the input of significant primary disease and surgical procedures for a given patient (shown in Table 4), characteristics of 18 patients including gender, chronic obstructive pulmonary disease, sepsis and need for any assistance in the activities of daily living (ADL), six preoperative laboratory data and two intraoperative factors were used for risk calculation (Table 5). The scoring system for the postoperative pneumonia risk models based on the logistic regression equation was as follows:
where βi is the coefficient of the variable X i in the logistic regression equation provided in Table 4. If a categorical risk factor is present, the X i value is 1 (0 if it is absent). For the age categories, X i is defined according to the following definitions (<60 years = 1, 60‐64 years = 2, 65‐69 years = 3, 70‐74 years = 4, 75‐79 years = 5, ≥80 years = 6). Total risk score for a given patient was calculated by summing (βi × X i) of the primary diagnosis and surgical procedures (Table 4) and patients' characteristics, laboratory data and intraoperative factors (Table 5). (−6.444 + Σ [βi × X i]) represents the log of the odds of pneumonia and −6.444 is the intercept of the multivariate model. Risk of postoperative pneumonia can also be approximated from a graph of predicted probability versus calculated risk score (Σ [βi × X i]) (Figure 1).
Table 2.
Univariate analysis of risk factors for postoperative pneumonia in 382 124 patients undergoing eight main gastroenterological surgeries between 2011 and 2013 using the NCD in Japan
| Risk factors | No. of patients with postoperative pneumonia (%) | ||
|---|---|---|---|
| Corresponding factor (+) | Corresponding factor (−) | P value | |
| Demographics | |||
| Age category (<60, 60‐64, 65‐69, 70‐74, 75‐79, ≥80 y) | – | <0.0001 | |
| Male | 8864/248 341 (3.6%) | 2241/133 783 (1.7%) | <0.0001 |
| Comorbidity | |||
| Chronic obstructive pulmonary disease | 1245/13 166 (9.5%) | 9860/368 958 (2.7%) | <0.0001 |
| Cerebrovascular disease | 1048/13 979 (7.5%) | 10 057/368 145 (2.7%) | <0.0001 |
| Diabetes | 2352/6671 (3.5%) | 8753/315 413 (2.8%) | <0.0001 |
| Ascites | 1018/12 871 (7.9%) | 10 087/369 253 (2.7%) | <0.0001 |
| Hypertension | 4896/132 237 (3.7%) | 6209/249 887 (2.5%) | <0.0001 |
| Congestive heart failure | 320/3369 (9.5%) | 10 785/378 755 (2.8%) | <0.0001 |
| Myocardial infarction | 125/2101 (5.9%) | 10 980/380 023 (2.9%) | <0.0001 |
| Angina | 246/5095 (4.8%) | 10 859/377 029 (2.9%) | <0.0001 |
| Peripheral vascular disease | 136/1365 (10.0%) | 10 969/380 759 (2.9%) | <0.0001 |
| Hemodialysis | 239/3564 (6.7%) | 10 866/378 560 (2.9%) | <0.0001 |
| Bleeding disorder | 1129/15 090 (7.5%) | 9976/367 034 (2.7%) | <0.0001 |
| ASA Physical Status classification >grade 2 | 3549/50 276 (7.1%) | 7566/331 848 (2.3%) | <0.0001 |
| Medical history | |||
| Previous cardiac surgery | 266/4354 (6.1%) | 10 839/377 770 (2.9%) | <0.0001 |
| Previous percutaneous coronary intervention | 532/8906 (6.0%) | 10 573/373 218 (2.8%) | <0.0001 |
| Chronic steroid use | 309/3950 (7.8%) | 10 796/378 174 (2.9%) | <0.0001 |
| Preoperative chemotherapy | 692/11 627 (6.0%) | 10 413/370 497 (2.8%) | <0.0001 |
| Preoperative radiotherapy | 204/3381 (6.0%) | 10 901/378 743 (2.9%) | <0.0001 |
| Lifestyle, activity | |||
| ADL need for any assistance | 2505/25 384 (9.7%) | 8600/356 290 (2.4%) | <0.0001 |
| Tobacco use, Brinkman index >400 | 4635/109 161 (4.2%) | 6470/272 963 (2.4%) | <0.0001 |
| Alcohol habitually | 3525/97 146 (3.6%) | 7580/284 978 (2.7%) | <0.0001 |
| Preoperative particular condition | |||
| Body weight loss >10% | 1182/20 154 (5.9%) | 9923/361 970 (2.7%) | <0.0001 |
| Preoperative transfusions | 676/10 387 (6.5%) | 10 429/371 737 (2.8%) | <0.0001 |
| Disseminated cancer | 534/11 954 (4.5%) | 10 571/370 170 (2.9%) | <0.0001 |
| SIRS with infection | 1322/9798 (13.5%) | 9783/372 326 (2.6%) | <0.0001 |
| Emergent surgery | 2410/35 199 (6.8%) | 8695/346 925 (2.5%) | <0.0001 |
| Preoperative ventilation | 214/1491 (14.4%) | 10 891/380 633 (2.9%) | <0.0001 |
| Primary disease | |||
| Gastric cancer | 4233/164 858 (2.6%) | 6872/217 266 (3.2%) | <0.001 |
| Cholangiocarcinoma (extrahepatic bile ducts perihilar) | 75/1838 (4.1%) | 11 030/380 286 (2.9%) | 0.0031 |
| Cholangiocarcinoma (extrahepatic bile ducts distal) | 204/5817 (3.5%) | 10 901/376 307 (2.9%) | 0.0067 |
| Gallbladder cancer | 49/1198 (4.1%) | 11 056/380 926 (2.9%) | 0.0163 |
| No tumor | 2073/34 530 (6.0%) | 9032/347 594 (2.6%) | <0.001 |
| Primary surgery | |||
| Operation for acute diffuse peritonitis (JSGS15) | 2045/27 377 (7.5%) | 9060/354 747 (2.6%) | <0.001 |
| Hepatectomy with >1 segment except for the lateral segment (JSGS39) | 515/23 610 (2.2%) | 10 590/358 514 (3.0%) | <0.001 |
| Pancreaticoduodenectomy (JSGS91) | 692/27 702 (2.5%) | 10 413/354 422 (2.9%) | <0.001 |
| Esophagectomy (JSGS103) | 2268/16 556 (13.7%) | 8837/365 568 (2.4%) | <0.001 |
| Low anterior resection (JSGS 3) | 524/58 401 (0.9%) | 10 581/323 723 (3.3%) | <0.001 |
| Right hemicolectomy (JSGS 31) | 1000/60 738 (1.6%) | 10 105/321 386 (3.1%) | <0.001 |
| Total gastrectomy (JSGS47) | 2105/58 809 (3.6%) | 9000/323 315 (2.8%) | <0.001 |
| Distal gastrectomy (JSGS49) | 2192/112 867 (1.9%) | 8913/269 257 (3.3%) | <0.001 |
| Associated surgery | |||
| Splenectomy (JSGS69) | 225/5873 (3.8%) | 10 880/376 251 (2.9%) | <0.001 |
| Enterostomy/closure with bowel resection (JSGS73) | 353/5640 (6.3%) | 10 752/376 484 (2.9%) | <0.001 |
| Enterostomy/closure without bowel resection (JSGS74) | 685/10 066 (6.8%) | 10 420/372 058 (2.8%) | <0.001 |
| Preoperative laboratory examination | |||
| Albumin <2.5 g/dL | 1283/13 542 (9.5%) | 9822/368 582 (2.7%) | <0.001 |
| AST >35 IU/L | 2100/48 875 (4.3%) | 9005/333 249 (2.7%) | <0.001 |
| ALP >340 IU/L | 1877/48 829 (3.8%) | 9228/333 295 (2.8%) | <0.001 |
| BUN >25 mg/dL | 2103/27 217 (7.7%) | 9002/354 907 (2.5%) | <0.001 |
| Na <138 mEq/dL | 2636/48 885 (5.4%) | 8469/333 239 (2.5%) | <0.001 |
| Creatinine >3 mg/dL | 396/5575 (7.1%) | 10 709/376 549 (2.8%) | <0.001 |
| Hematocrit <21% | 150/2886 (5.2%) | 10 955/379 238 (2.9%) | <0.001 |
| Hematocrit: male >48%, female >42% | 234/101 866 (2.3%) | 10 871/371 938 (2.9%) | 0.002 |
| Total bilirubin >3 mg/dL | 275/6210 (4.4%) | 10 830/375 914 (2.9%) | <0.001 |
| C‐reactive protein >10 mg/dL | 1401/17 665 (7.9%) | 9704/364 459 (2.7%) | <0.001 |
| White blood cell count <3500/μL | 1025/23 487 (4.4%) | 10 090/358 637 (2.8%) | <0.001 |
| White blood cell count >12 000/μL | 945/16 817 (5.6%) | 10 160/365 307 (2.8%) | <0.001 |
| Platelet count <15 × 104/μL | 1695/32 917 (5.1%) | 9410/349 207 (2.7%) | <0.001 |
| Prothrombin time‐INR >1.1 | 2742/51 603 (5.3%) | 8363/330 521 (2.5%) | <0.001 |
| Intraoperative factors | |||
| Prolonged surgery (>75th percentile) | 3706/95 328 (3.9%) | 7399/286 796 (2.6%) | <0.001 |
| Severe blood loss (>75th percentile) | 4319/94 332 (4.6%) | 6786/287 792 (2.4%) | <0.001 |
ADL, activities of daily living; ALP, alkaline phosphatase; ASA, American Society of Anesthesiologists; AST, aspartate aminotransferase; BUN, blood urea nitrogen; INR, international normalized ratio; NCD, National Clinical Database; SIRS, systemic inflammatory response syndrome.
Table 3.
Multivariate logistic regression model of risk factors for postoperative pneumonia in 247 604 patients between 2011 and 2012 using the NCD in Japan
| Risk factors | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Adjusted odds ratio | 95% CI | P value | β coefficient | ||
| Lower | Upper | ||||
| Demographics | |||||
| Age category | 1.293 | 1.272 | 1.314 | <0.001 | 0.257 |
| Male | 1.740 | 1.632 | 1.856 | <0.001 | 0.554 |
| Comorbidity | |||||
| Chronic obstructive pulmonary disease | 2.041 | 1.877 | 2.219 | <0.001 | 0.713 |
| Cerebrovascular disease | 1.607 | 1.469 | 1.758 | <0.001 | 0.474 |
| Diabetes | 1.073 | 1.009 | 1.140 | 0.024 | 0.070 |
| Ascites | 1.123 | 1.014 | 1.244 | 0.026 | 0.116 |
| Hypertension | 1.114 | 1.059 | 1.172 | <0.001 | 0.108 |
| Bleeding disorder | 1.129 | 1.030 | 1.237 | 0.010 | 0.121 |
| ASA >grade 2 | 1.417 | 1.329 | 1.511 | <0.001 | 0.348 |
| Medical history | |||||
| Previous percutaneous coronary intervention | 1.301 | 1.154 | 1.467 | <0.001 | 0.263 |
| Chronic steroid use | 1.626 | 1.391 | 1.901 | <0.001 | 0.486 |
| Lifestyle, activity | |||||
| ADL need for any assistance | 2.066 | 1.921 | 2.222 | <0.001 | 0.726 |
| Tobacco use, Brinkman index >400 | 1.305 | 1.236 | 1.379 | <0.001 | 0.266 |
| Preoperative particular condition | |||||
| Body weight loss >10% | 1.286 | 1.183 | 1.397 | <0.001 | 0.251 |
| Preoperative transfusions | 1.204 | 1.079 | 1.343 | 0.001 | 0.185 |
| SIRS with infection | 1.777 | 1.594 | 1.981 | <0.001 | 0.575 |
| Emergent surgery | 1.609 | 1.423 | 1.818 | <0.001 | 0.476 |
| Disseminated cancer | 1.187 | 1.056 | 1.333 | 0.004 | 0.171 |
| Primary disease | |||||
| Gastric cancer (total/distal gastrectomy, JSGS47, 49) | 1.843 | 1.722 | 1.973 | <0.001 | 0.612 |
| Cancer of small intestine | 1.606 | 1.163 | 2.218 | 0.004 | 0.474 |
| Cholangiocarcinoma (extrahepatic bile ducts perihilar) | 1.655 | 1.255 | 2.184 | <0.001 | 0.504 |
| Cholangiocarcinoma (extrahepatic bile ducts distal) | 1.284 | 1.043 | 1.580 | 0.018 | 0.250 |
| Gallbladder cancer | 1.655 | 1.165 | 2.351 | 0.005 | 0.504 |
| Primary surgery | |||||
| Operation for acute diffuse peritonitis (JSGS15) | 1.740 | 1.524 | 1.986 | <0.001 | 0.554 |
| Hepatectomy with >1 segment except for lateral segment (JSGS39) | 1.700 | 1.499 | 1.927 | <0.001 | 0.530 |
| Pancreaticoduodenectomy (JSGS91) | 1.809 | 1.590 | 2.057 | <0.001 | 0.593 |
| Esophagectomy (JSGS103) | 13.727 | 12.640 | 14.907 | <0.001 | 2.619 |
| Associated surgery | |||||
| Splenectomy (JSGS69) | 1.363 | 1.138 | 1.632 | 0.001 | 0.310 |
| Enterostomy/closure with bowel resection (JSGS73) | 1.279 | 1.094 | 1.495 | 0.002 | 0.246 |
| Enterostomy/closure without bowel resection (JSGS74) | 1.228 | 1.097 | 1.375 | <0.001 | 0.206 |
| Preoperative laboratory examination | |||||
| Hematocrit: male >48%, female >42% | 1.178 | 1.006 | 1.379 | 0.042 | 0.164 |
| Albumin <2.5 g/dL | 1.365 | 1.244 | 1.498 | <0.001 | 0.311 |
| AST >35 IU/L | 1.130 | 1.056 | 1.210 | <0.001 | 0.123 |
| ALP >340 IU/L | 1.146 | 1.066 | 1.232 | <0.001 | 0.136 |
| BUN >25 mg/dL | 1.232 | 1.145 | 1.325 | <0.001 | 0.208 |
| Na <138 mEq/dL | 1.168 | 1.098 | 1.241 | <0.001 | 0.155 |
| Intraoperative factors | |||||
| Prolonged surgery (>75th percentile) | 1.368 | 1.295 | 1.444 | <0.001 | 0.313 |
| Severe blood loss (>75th percentile) | 1.461 | 1.387 | 1.539 | <0.001 | 0.379 |
95% CI, 95% confidence interval; ADL, activities of daily living; ALP, alkaline phosphatase; ASA, American Society of Anesthesiologists; AST, aspartate aminotransferase; BUN, blood urea nitrogen; NCD, National Clinical Database; SIRS, systemic inflammatory response syndrome.
Table 4.
Calculation of risk score of primary diagnosis and surgical procedures
| Risk factors | X i | βi | |
|---|---|---|---|
| Yes | No | ||
| 1. Disseminated cancer | 1 | 0 | 0.171 |
| 2. Gastric cancer | 1 | 0 | 0.612 |
| 3. Cancer of small intestine | 1 | 0 | 0.474 |
| 4. Cholangiocarcinoma (extrahepatic bile ducts perihilar) | 1 | 0 | 0.504 |
| 5. Cholangiocarcinoma (extrahepatic bile ducts distal) | 1 | 0 | 0.250 |
| 6. Gallbladder cancer | 1 | 0 | 0.504 |
| 7. Operation for acute diffuse peritonitis | 1 | 0 | 0.554 |
| 8. Hepatectomy with >1 segment except for lateral segment | 1 | 0 | 0.530 |
| 9. Pancreaticoduodenectomy | 1 | 0 | 0.593 |
| 10. Esophagectomy | 1 | 0 | 2.619 |
| 11. Splenectomy | 1 | 0 | 0.310 |
| 12. Enterostomy/closure with bowel resection | 1 | 0 | 0.246 |
| 13. Enterostomy/closure without bowel resection | 1 | 0 | 0.206 |
Risk score of primary diagnosis and surgical procedures, Σ [βi × X i].
βi is the coefficient of the variable X i in the logistic regression equation. If a categorical risk factor is present, the X i value is 1 (0 if it is absent). A total risk score of primary diagnosis and surgical procedures for a given patient is calculated by summing βi × X i.
Table 5.
Calculation of the total risk score
| Risk factors | X i | βi | |
|---|---|---|---|
| Yes | No | ||
| Patient characteristics | |||
| 1. Age category, y | <60 = 1, 60‐64 = 2, 65‐69 = 3, 70‐74 = 4, 75‐79 = 5, ≥80 = 6 | 0.257 | |
| 2. Male | 1 | 0 | 0.554 |
| 3. Chronic obstructive pulmonary disease | 1 | 0 | 0.713 |
| 4. Cerebrovascular disease | 1 | 0 | 0.474 |
| 5. Diabetes | 1 | 0 | 0.070 |
| 6. Ascites | 1 | 0 | 0.116 |
| 7. Hypertension | 1 | 0 | 0.108 |
| 8. Bleeding disorder | 1 | 0 | 0.121 |
| 9. ASA >grade 2 | 1 | 0 | 0.348 |
| 10. Previous percutaneous coronary intervention | 1 | 0 | 0.263 |
| 11. Chronic steroid use | 1 | 0 | 0.486 |
| 12. ADL need for any assistance | 1 | 0 | 0.726 |
| 13. Tobacco use, Brinkman index >400 | 1 | 0 | 0.266 |
| 14. Body weight loss >10% | 1 | 0 | 0.251 |
| 15. Preoperative transfusions | 1 | 0 | 0.185 |
| 16. SIRS with infection | 1 | 0 | 0.575 |
| 17. Emergent surgery | 1 | 0 | 0.476 |
| Preoperative laboratory data | |||
| 1. Hematocrit: male >48%, female >42% | 1 | 0 | 0.164 |
| 2. Albumin <2.5 g/dL | 1 | 0 | 0.311 |
| 3. AST >35 IU/L | 1 | 0 | 0.123 |
| 4. ALP >340 IU/L | 1 | 0 | 0.136 |
| 5. BUN >25 mg/dL | 1 | 0 | 0.208 |
| 6. Na <138 mEq/dL | 1 | 0 | 0.155 |
| Intraoperative factors | |||
| 1. Prolonged surgery (>75th percentile) | 1 | 0 | 0.313 |
| 2. Severe blood loss (>75th percentile) | 1 | 0 | 0.379 |
Risk score of patients' characteristics, laboratory data and intraoperative factors, Σ [βi × X i].
Total risk score = risk score of primary diagnosis and surgical procedures + risk score of patients' characteristics, laboratory data and intraoperative factors.
βi is the coefficient of the variable X i in the logistic regression equation. If a categorical risk factor is present, the X i value is 1 (0 if it is absent). For the age categories, X i is defined according to the definition. Total risk score for a given patient is calculated by summing βi × X i.
ADL, activities of daily living; ALP, alkaline phosphatase; ASA, American Society of Anesthesiologists; AST, aspartate aminotransferase; BUN, blood urea nitrogen; SIRS, systemic inflammatory response syndrome.
Figure 1.
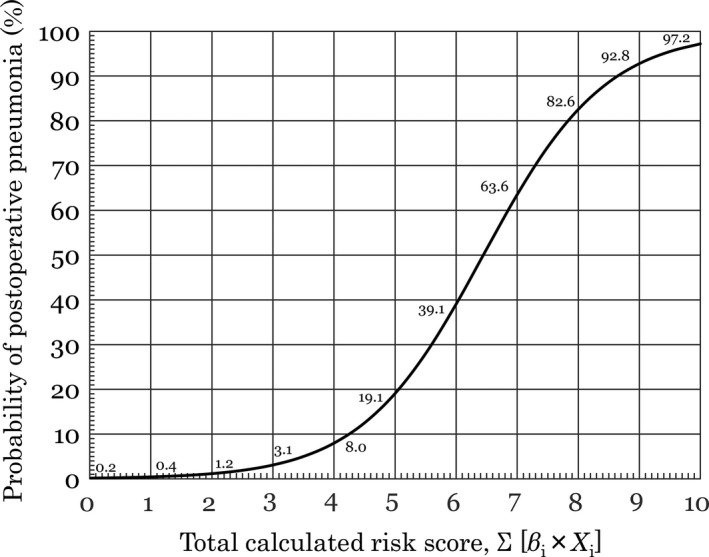
Approximated predicted risk for postoperative pneumonia. Risk for postoperative pneumonia is approximated from a nomogram of predicted probability vs calculated total risk score. βi is the coefficient of the variable Xi in the logistic regression equation provided in Table 4
Area under the ROC curve was 0.822 (95% CI, 0.817‐0.826; P < 0.001) in the derivation group, indicating good discrimination ability (Figure 2). In the analysis of the 2013 dataset for the validation of the model (n = 134 520), the area under the ROC curve was 0.826 (95% CI, 0.819‐0.832) (Figure 2). Figure 3 shows the calibration of the models. The rate for the predicted events corresponded reasonably well with those for the observed events in each risk‐stratified subgroup.
Figure 2.
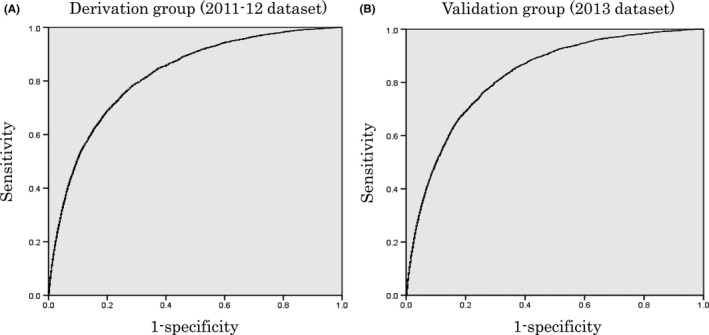
Receiver‐operating characteristic (ROC) curve for postoperative pneumonia in the derivation and validation groups. Area under the ROC curve was 0.822 (95% CI, 0.817‐0.826) in the derivation subset of the study sample and 0.826 (95% CI, 0.819‐0.832) in the validation subset of the study sample
Figure 3.
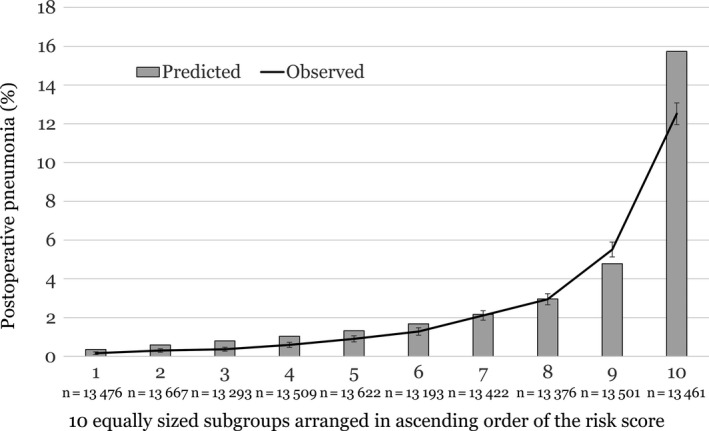
Calibration of the postoperative pneumonia model. Bar charts represent mean rate for the predicted events. Line chart represents those for the observed events, and the error bars represent 95% CI of the observed events. The risk score is calculated using Σ [βi × X i]. βi is the coefficient of the variable Xi in the logistic regression equation. This figure represents how well the rates of the predicted events matched those of the observed events according to the patient risk subgroups
4. DISCUSSION
Estimations of the incidence of postoperative pneumonia vary widely in the literature, from 0.6% to 17.5%.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 22 The variability is primarily because of the diagnosis criteria used and differing surgical procedures. In the present study, the overall incidence of postoperative pneumonia within 30 days of gastroenterological surgery was 2.9%, which is similar to the incidence reported by Yang et al10 (3.2%) in a sample of 165 196 patients who underwent major abdominal surgery. Thompson et al9 reported that hospital‐acquired pneumonia occurred in 10.7% of patients following intra‐abdominal surgery. They analyzed all patients with a discharge diagnosis of pneumonia, including late‐onset pneumonia.
In the present study, the highest incidence of postoperative pneumonia was observed after esophagectomy (13.7%) and the lowest incidence was observed after low anterior resection and right hemicolectomy (0.9% and 1.6%, respectively). Yang et al10 described similar incidences as follows; esophagectomy 16.2%; gastrectomy 6.4%; pancreatectomy 4.8%; hepatectomy 3.3%; and colectomy/proctectomy 2.4%. In Japan, transthoracic excision of the esophagus with extended lymph node dissection is the standard procedure for patients with esophageal cancer. In one report from Japan, the incidence of pneumonia was 8.7% in patients who underwent subtotal esophagectomy.14 Molena et al15 reported a relatively low incidence of postoperative pneumonia (5.9%), possibly because a considerable number of patients underwent transhiatal esophagectomy. In a study of pulmonary complications, those who had esophagectomy remained prevalent despite advances in perioperative management.14, 15 In addition, upper abdominal surgical location has been consistently identified as an independent risk factor for postoperative pulmonary complications.13, 16, 23
In the multivariate logistic regression analysis, a high β coefficient was observed in males, patients with chronic obstructive pulmonary disease (COPD), patients with a need for any assistance in ADL, patients with preoperative systemic inflammatory response syndrome with infection, three diseases including gastric cancer, gallbladder cancer and cholangiocarcinoma (extrahepatic bile ducts perihilar) and four operative procedures including esophagectomy, pancreaticoduodenectomy, operation for acute diffuse peritonitis and hepatectomy with >1 segment except for the lateral segment. These were consistent with previously described risk factors for postoperative pneumonia. Gupta et al5 reported that preoperative variables associated with an increased risk of postoperative pneumonia included age, ASA class, COPD, dependent functional status, preoperative sepsis, smoking and type of operation; all these risk factors were also included in our risk model. Arozullah et al8 also developed a postoperative pneumonia risk index that included type of surgery, age, functional status, weight loss, COPD, general anesthesia, impaired sensorium, cerebral vascular accident, blood urea nitrogen level, transfusion, emergency surgery, steroid use, smoking and alcohol use. Using 38 variables, our risk calculator indicated good discrimination ability and was well calibrated. The model also showed reasonable discrimination in the validation subset.
As a practical use of our risk model, tentative probability of postoperative pneumonia is estimated by the preoperative risk score, calculated by summing the βi × X i of primary diagnosis and surgical procedures, patient characteristics and preoperative laboratory data. Using these data, an attending physician explains the risk of postoperative pneumonia to patients and the indication for a pneumonia‐prevention program is discussed among medical staff. After surgery, definitive probability of postoperative pneumonia is assessed by adding the risk score of intraoperative factors to the preoperative risk score.
Our risk model for postoperative pneumonia addresses several limitations of previous studies. Previous studies have had relatively small sample sizes. The Japanese NCD contains data from 2158 participant hospitals, including both community and teaching hospitals. We developed the risk model limited to patients undergoing gastroenterological surgery. Previous studies included patients undergoing a wide range of non‐cardiac operations.5, 7, 8 Most of the previous studies have included a wide array of postoperative pulmonary complications such as atelectasis, pulmonary embolism and respiratory failure.10, 13, 16, 22 Varying definitions of postoperative pulmonary complications cause variability in risk estimates. We evaluated preoperative risk factors as well as intraoperative factors (prolonged surgery and severe hemorrhage). Several studies have identified that these two intraoperative factors were independently related to postoperative pneumonia7, 21 or respiratory complication.15, 24
Despite the many strengths of our study, there are several limitations. First, although our model was validated by the 2013 dataset, external validation on independent datasets from other countries is required before the index can be widely applied in clinical practice. Second, because a substantial number of cancer patients were registered in gastroenterological surgery, influence of cancer stage and extent of lymphadenectomy should also be considered. Third, pulmonary function test results were not available in this study. Fourth, laparoscopic approach could reduce pulmonary complications in several procedures.25, 26, 27 However, we did not evaluate laparoscopic surgery as a potential predictor. Fifth, postoperative pneumonia was defined as pneumonia occurring within 30 days post‐surgery. Pneumonia rate will be underestimated if no post‐discharge surveillance is carried out. Adherence of post‐discharge surveillance might be different in each institution. Finally, information on hospital volume is not contained in the database.
5. CONCLUSIONS
We developed a risk calculator for predicting postoperative pneumonia after gastroenterological surgery using a large dataset from the NCD in Japan. Performance of the model was good in terms of discrimination and calibration, and the model was validated using datasets submitted to the NCD in different years. In patients who were assessed as high risk for postoperative pneumonia, enhanced preventive interventions should be considered. This risk model is also useful in counseling and for obtaining informed consent from patients.
DISCLOSURE
Conflicts of Interest: Authors declare no conflicts of interest for this article.
ACKNOWLEDGEMENTS
The authors would like to thank all data managers and hospitals participating in the NCD project for their great efforts in entering the data, and also thank the members and the working members of the Japanese Society of Gastroenterological Surgery (JSGS) database committee.
Takesue Y, Miyata H, Gotoh M, et al. Risk calculator for predicting postoperative pneumonia after gastroenterological surgery based on a national Japanese database. Ann Gastroenterol Surg. 2019;3:405–415. 10.1002/ags3.12248
REFERENCES
- 1. Watanabe T, Miyata H, Konno H, Kawai K, Ishihara S, Sunami E, et al. Prediction model for complications after low anterior resection based on data from 33,411 Japanese patients included in the National Clinical Database. Surgery. 2017;161:1597–608. [DOI] [PubMed] [Google Scholar]
- 2. Kinlin LM, Kirchner C, Zhang H, Daley J, Fisman DN. Derivation and validation of a clinical prediction rule for nosocomial pneumonia after coronary artery bypass graft surgery. Clin Infect Dis. 2010;50:493–501. [DOI] [PubMed] [Google Scholar]
- 3. Strobel RJ, Liang Q, Zhang M, Wu X, Rogers MA, Theurer PF, et al. Michigan Society Of Thoracic And Cardiovascular Surgeons Quality Collaborative.: a preoperative risk model for postoperative pneumonia after coronary artery bypass grafting. Ann Thorac Surg. 2016;102:1213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allou N, Bronchard R, Guglielminotti J, Dilly MP, Provenchere S, Lucet JC, et al. Risk factors for postoperative pneumonia after cardiac surgery and development of a preoperative risk score. Crit Care Med. 2014;42:1150–6. [DOI] [PubMed] [Google Scholar]
- 5. Gupta H, Gupta PK, Schuller D, Fang X, Miller WJ, Modrykamien A, et al. Development and validation of a risk calculator for predicting postoperative pneumonia. Mayo Clin Proc. 2013;88:1241–9. [DOI] [PubMed] [Google Scholar]
- 6. Kazaure HS, Martin M, Yoon JK, Wren SM. Long‐term results of a postoperative pneumonia prevention program for the inpatient surgical ward. JAMA Surg. 2014;149:914–8. [DOI] [PubMed] [Google Scholar]
- 7. Diez‐Sebastian J, Herruzo R, Garcia‐Caballero J. Prevention of early‐onset pneumonia in surgical patients by chemoprophylaxis. Am J Surg. 2012;204:441–6. [DOI] [PubMed] [Google Scholar]
- 8. Arozullah AM, Khuri SF, Henderson WG, Daley J. Participants in the National Veterans Affairs Surgical Quality Improvement Program: development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med. 2001;135:847–57. [DOI] [PubMed] [Google Scholar]
- 9. Thompson DA, Makary MA, Dorman T, Pronovost PJ. Clinical and economic outcomes of hospital acquired pneumonia in intra‐abdominal surgery patients. Ann Surg. 2006;243:547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang CK, Teng A, Lee DY, Rose K. Pulmonary complications after major abdominal surgery: National Surgical Quality Improvement Program analysis. J Surg Res. 2015;198:441–9. [DOI] [PubMed] [Google Scholar]
- 11. Mohri Y, Tonouchi H, Miki C, Kobayashi M, Kusunoki M, Mie Surgical Infection Research Group . Incidence and risk factors for hospital‐acquired pneumonia after surgery for gastric cancer: results of prospective surveillance. World J Surg. 2008; 32:1045–50. [DOI] [PubMed] [Google Scholar]
- 12. Miki Y, Makuuchi R, Tokunaga M, Tanizawa Y, Bando E, Kawamura T, et al. Risk factors for postoperative pneumonia after gastrectomy for gastric cancer. Surg Today. 2016;46:552–6. [DOI] [PubMed] [Google Scholar]
- 13. Ntutumu R, Liu H, Zhen L, Hu YF, Mou TY, Lin T, et al. Risk factors for pulmonary complications following laparoscopic gastrectomy: a single‐center study. Medicine (Baltimore). 2016;95:e4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoshida N, Watanabe M, Baba Y, Iwagami S, Ishimoto T, Iwatsuki M, et al. Risk factors for pulmonary complications after esophagectomy for esophageal cancer. Surg Today. 2014;44:526–32. [DOI] [PubMed] [Google Scholar]
- 15. Molena D, Mungo B, Stem M, Lidor AO. Incidence and risk factors for respiratory complications in patients undergoing esophagectomy for malignancy: a NSQIP analysis. Semin Thorac Cardiovasc Surg. 2014;26:287–94. [DOI] [PubMed] [Google Scholar]
- 16. Brooks‐Brunn JA. Predictors of postoperative pulmonary complications following abdominal surgery. Chest. 1997;111:564–71. [DOI] [PubMed] [Google Scholar]
- 17. Dodek P, Keenan S, Cook D, Heyland D, Jacka M, Hand L, et al. Evidence‐based clinical practice guidelines for the prevention of ventilator‐associated pneumonia [clinical guideline]. Ann Intern Med. 2004;141:305–13. [DOI] [PubMed] [Google Scholar]
- 18. Gotoh M, Miyata H, Hashimoto H, Wakabayashi G, Konno H, Miyakawa S, et al. National Clinical Database feedback implementation for quality improvement of cancer treatment in Japan: from good to great through transparency. Surg Today. 2016;46:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hall BL, Hamilton BH, Richards K, Bilimoria KY, Cohen ME, Ko CY. Does surgical quality improve in the American College of Surgeons National Surgical Quality Improvement Program: an evaluation of all participating hospitals. Ann Surg. 2009;250:363–76. [DOI] [PubMed] [Google Scholar]
- 20. Miyata H, Gotoh M, Hashimoto H, Motomura N, Murakami A, Tomotaki A, et al. Challenges and prospects of a clinical database linked to the board certification system. Surg Today. 2014;44:1991–9. [DOI] [PubMed] [Google Scholar]
- 21. American College of Surgeons. ACS National Surgical Quality Improvement Program. Accessed February 12, 2017. Available from http://site.acsnsqip.org/wp-content/uploads/2013/10/ACSNSQIP.PUF_UserGuide.2012.pdf#search=%2Cuser+guide+for+the+2012+ACS+NSQIP
- 22. Garibaldi RA, Britt MR, Coleman ML, Reading JC, Pace NL. Risk factors for postoperative pneumonia. Am J Med. 1981;70:677–80. [DOI] [PubMed] [Google Scholar]
- 23. Hall JC, Tarala RA, Hall JL, Mander J. A multivariate analysis of the risk of pulmonary complications after laparotomy. Chest. 1991;99:923–7. [DOI] [PubMed] [Google Scholar]
- 24. Canet J, Sabaté S, Mazo V, Gallart L, de Abreu MG, Belda J, et al. Development and validation of a score to predict postoperative respiratory failure in a multicentre European cohort: a prospective, observational study. Eur J Anaesthesiol. 2015;32:458–70. [DOI] [PubMed] [Google Scholar]
- 25. Nozaki I, Mizusawa J, Kato K, Igaki H, Ito Y, Daiko H, et al. Impact of laparoscopy on the prevention of pulmonary complications after thoracoscopic esophagectomy using data from JCOG0502: a prospective multicenter study. Surg Endosc. 2018;32:651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pan Y, Chen K, Yu WH, Maher H, Wang SH, Zhao HF, et al. Laparoscopic gastrectomy for elderly patients with gastric cancer: a systematic review with meta‐analysis. Medicine (Baltimore). 2018;97:e0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fuks D, Cauchy F, Ftériche S, Nomi T, Schwarz L, Dokmak S, et al. Laparoscopy decreases pulmonary complications in patients undergoing major liver resection: a propensity score analysis. Ann Surg. 2016;263:353–61. [DOI] [PubMed] [Google Scholar]


