Abstract
Background
The natural history of lymphangioleiomyomatosis (LAM) is mainly derived from retrospective cohort analyses, and it remains incompletely understood. A National Institutes of Health LAM Registry was established to define the natural history and identify prognostic biomarkers that can help guide management and decision-making in patients with LAM.
Methods
A linear mixed effects model was used to compute the rate of decline of FEV1 and to identify variables affecting FEV1 decline among 217 registry patients who enrolled from 1998 to 2001. Prognostic variables associated with progression to death/lung transplantation were identified by using a Cox proportional hazards model.
Results
Mean annual decline of FEV1 was 89 ± 53 mL/year and remained remarkably constant regardless of baseline lung function. FEV1 decline was more rapid in those with greater cyst profusion on CT scanning (P = .02) and in premenopausal subjects (118 mL/year) compared with postmenopausal subjects (74 mL/year) (P = .003). There were 26 deaths and 43 lung transplantations during the evaluation period. The estimated 5-, 10-, 15-, and 20-year transplant-free survival rates were 94%, 85%, 75%, and 64%, respectively. Postmenopausal status (hazard ratio, 0.30; P = .0002) and higher baseline FEV1 (hazard ratio, 0.97; P = .008) or diffusion capacity of lung for carbon monoxide (hazard ratio, 0.97; P = .001) were independently associated with a lower risk of progression to death or lung transplantation.
Conclusions
The median transplant-free survival in patients with LAM is > 20 years. Menopausal status, as well as structural and physiologic markers of disease severity, significantly affect the rate of decline of FEV1 and progression to death or lung transplantation in LAM.
Key Words: menopause, natural history, pulmonary function tests, tuberous sclerosis complex, VEGF-D
Abbreviations: Dlco, diffusion capacity of lung for carbon monoxide; LAM, lymphangioleiomyomatosis; mTOR, mechanistic target of rapamycin; NHLBI, National Heart, Lung, and Blood Institute; NIH, National Institutes of Health; PFT, pulmonary function test; TSC, tuberous sclerosis complex; VEGF-D, vascular endothelial growth factor-D
FOR EDITORIAL COMMENT, SEE PAGE 249
Lymphangioleiomyomatosis (LAM) is a progressive, female-predominant, low-grade neoplasm driven by mutations in the tuberous sclerosis complex (TSC) genes.1, 2, 3 These mutations lead to constitutive activation of the mechanistic target of rapamycin (mTOR) pathway, which promotes chaotic lymphatic channel development through expression of lymphangiogenic growth factors such as vascular endothelial growth factor-C and vascular endothelial growth factor-D (VEGF-D).4, 5 It also drives the metastasis of neoplastic smooth muscle-like cells (LAM cells) leading to progressive cystic remodeling of the pulmonary parenchyma.1, 3
The Multicenter International LAM Efficacy of Sirolimus (MILES) trial showed that treatment with sirolimus, an mTOR inhibitor, could stabilize lung function decline and improve quality of life in patients with LAM.6 Based on these results, sirolimus was approved by the US Food and Drug Administration for the treatment of patients with LAM in the United States in 2015.7 Optimal selection of patients for treatment requires a clear understanding of the natural history of LAM, as well as the factors that influence disease progression and treatment response.
Almost 20 years ago, the National Heart, Lung, and Blood Institute (NHLBI) established a national LAM registry.8 For a period of 3 years (1998-2001), a total of 246 women with LAM were enrolled at six different US sites and followed up for a period of up to 5 years, through 2003. High-resolution CT scans were performed at enrollment, and longitudinal data, including pulmonary function test (PFT) results, were collected every 12 months. Serum specimens were obtained at regular intervals and stored for future analysis. The baseline characteristics of the patients enrolled in this registry have been published previously, but the longitudinal analysis of this cohort was not completed due to a lapse in funding. Subsequently, the patient data and serum specimens were transferred to the National Disease Research Interchange and placed in the public domain. The passage of time while the data lay fallow offered a unique opportunity to understand the natural history of LAM in the pre-sirolimus era.
Materials and Methods
We retrieved de-identified data from the National Disease Research Interchange; which were linked to serum specimens collected during the NHLBI LAM registry patient visits. An imaging core consisting of expert thoracic radiologists calculated the radiologic disease severity based on the profusion of cystic changes seen on high-resolution CT scans (e-Table 1). Serum VEGF-D measurements were performed on archival serum specimens in the Translational Trials Laboratory at Cincinnati Children's Hospital Medical Center, a College of American Pathologists/Clinical Laboratory Improvement Act-certified facility. Menopausal status of the participants was determined at the baseline visit based on history if clearly naturally or surgically menopausal, and in other cases by using measurements of serum follicle-stimulating hormone and estradiol levels.
To determine which of the patients in this cohort had either died or undergone lung transplantation in the interval between registry enrollment and our analysis, Social Security numbers for all registry participants were submitted to the National Death Index and the United Network for Organ Sharing databases. The National Death Index is a centralized database of death record information established by the Centers for Disease Control and Prevention and the National Center for Health Statistics to aid investigators in mortality ascertainment activities. The United Network for Organ Sharing, established by the US Congress in 1984, is a nonprofit organization that maintains a robust database containing information for every transplant that occurs in the United States.
PFTs were performed in a standard manner across all sites with strict monitoring and quality control.8 The rate of decline of FEV1, and the association between FEV1 slope and various clinical characteristics and serum biomarkers, was estimated by using a linear mixed effects model with random intercept and random slope. The model included time since the first measurement, age at the first measurement, the factor of interest, and the interaction between time and the factor of interest as fixed effects. The regression parameter for the interaction term was used to examine the association. Unstructured within-subject correlation was assumed for the random error.
A Cox proportional hazards model for the incidence of lung transplantation or death was used to examine the association with clinical characteristics. Time to event was calculated as the number of years between the date of LAM diagnosis to the date of lung transplantation or date of death, whichever came first. Right-censored time was based on the interval from the LAM diagnosis to the data censor date (December 31, 2014). For the multiple Cox regression model, we used stepwise selection by applying a significance level of 0.25 threshold for entering a variable into the model and a 0.15 threshold for retaining the variable in the model. The supremum test on martingale residuals was used for checking the proportional hazards assumption (no indication of violation was noted). All analyses were performed by using SAS version 9.4 (SAS Institute, Inc).
Results
Baseline Characteristics
A total of 246 patients had enrolled in the NHLBI LAM registry. Sixteen patients were excluded because the diagnosis of LAM was incompletely substantiated or unconfirmed. Another 13 patients were excluded because they had undergone lung transplantation prior to enrollment in the registry. Data from the remaining 217 patients were analyzed (Tables 1, 2).
Table 1.
Baseline Characteristics of the NHLBI LAM Registry Cohort (N = 217)
| Characteristic | Mean ± SD or No. (%) |
|---|---|
| Age at initial registry visit, y | 44 ± 10 |
| Age at disease diagnosis, y | 41 ± 10 |
| VEGF-D, pg/mL | 1,572 ± 1,696 |
| CT score | 11 ± 5 |
| No. of patients with sporadic LAM | 180 (83) |
| No. of patients with TSC-LAM | 36 (17) |
| No. of postmenopausal patients | 135 (62) |
| No. of patients with renal AML(s) | 88 (41) |
| No. of patients with at least one spontaneous pneumothorax | 120 (55) |
| No. of patients with a bronchodilator response | 48 (22) |
| No. of patients with supplemental oxygen use | 70 (32) |
AML = angiomyolipoma; LAM = lymphangioleiomyomatosis; NHLBI = National Heart, Lung, and Blood Institute; TSC = tuberous sclerosis complex; VEGF-D = vascular endothelial growth factor-D.
Table 2.
Baseline Pulmonary Function Test Values of the NHLBI LAM Registry Cohort
| Characteristic | Mean Value | SD | % Predicted | SD |
|---|---|---|---|---|
| FEV1 | 2.1 L | 0.79 | 69 | 24 |
| FVC | 3.2 L | 0.73 | 86 | 17 |
| Dlco | 15.4 mL/mmHg/min | 6.3 | 63 | 24 |
Dlco = diffusion capacity for carbon monoxide. See Table 1 legend for expansion of other abbreviations.
Analysis of PFTs
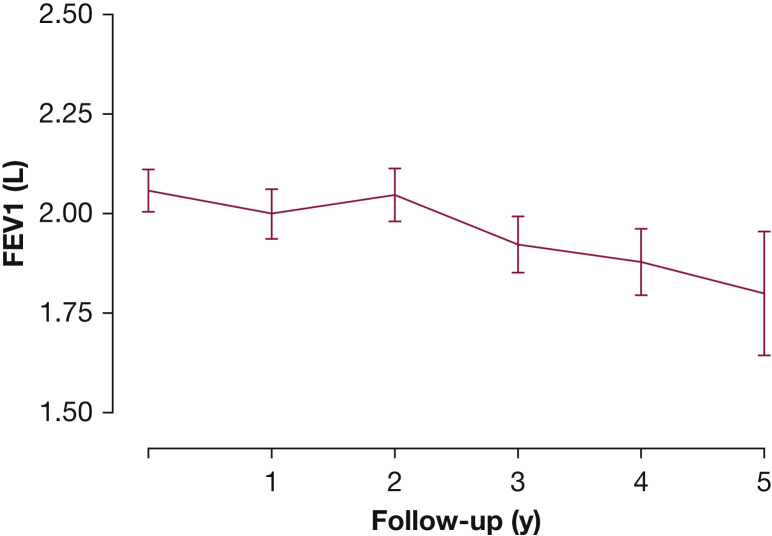
Among the 217 patients in the study cohort, 188 had at least two PFTs. Of these 188 patients, 161 patients had at least three PFTs, and 116 patients had at least four PFTs. Adequate serum specimens to allow VEGF-D measurement were available in 159 patients, and 165 patients had a baseline CT score computed. Longitudinal analysis of the PFTs revealed an average FEV1 decline rate of 89 mL/year (Fig 1, Table 3).
Figure 1.
FEV1 decline over time in the National Heart, Lung, and Blood Institute (NHLBI) Registry Cohort.
Table 3.
Age-adjusted Rate of Decline in Pulmonary Function Measures During the 5-Year Follow-up
| Variable | Mean Slope | SD | Mean Slope (% Predicted/Year) | SD |
|---|---|---|---|---|
| FEV1 | –89.2 mL/y | 53.4 | –2.79 | 3.49 |
| FVC | –71.3 mL/y | 57.5 | –1.88 | 3.21 |
| Dlco | –0.81 mL/mmHg/min/y | 0.20 | –3.05 | 0.15 |
See Table 2 legend for expansion of abbreviation.
Baseline disease severity was associated with the rate of FEV1 decline, with faster rates of decline seen among patients with a higher initial FEV1 (P < .0001), FVC (P = .007), and CT score (P = .02). Each one-unit increase in baseline FEV1 % predicted, FVC % predicted, and CT score translated to acceleration in the rate of FEV1 decline of 0.31 mL/year, 0.78 mL/year, and 3.53 mL/year, respectively. FEV1 decline rates decreased with older age at diagnosis (P = .05), with the rate of decline of FEV1 reduced by 1.39 mL/year with every 1-year increase in the age at diagnosis. Menopausal status at enrollment had a major impact on the FEV1 decline rates, with postmenopausal women declining 44 mL/year slower than the premenopausal women (74 mL/year vs 118 mL/year; P = .003). Patients with a positive bronchodilator response on spirometry tended to decline faster than patients without a bronchodilator response (113 mL/year vs 83 mL/year; P = .09). Other baseline variables had no significant effect on the rate of decline of FEV1 (Table 4).
Table 4.
Relationship Between Demographic, Clinical, Radiologic, and Serologic Characteristics and the Rate of Change of FEV1
| Characteristic | Parameter Estimate (SE) | P Value |
|---|---|---|
| Age at diagnosisa | 1.39 (0.70) | .05 |
| FEV1 % predicteda | –0.31 (0.05) | < .0001 |
| FVC % predicteda | –0.78 (0.29) | .007 |
| Dlco % predicteda | 0.48 (0.29) | .10 |
| CT scorea | –3.53 (1.53) | .02 |
| Log2 (VEGF-D)a | –6.55 (5.01) | .19 |
| Serum VEGF-D > 600 pg/mL | .32 | |
| No | –78.6 (11.6) | |
| Yes | –93.0 (8.3) | |
| Bronchodilator response | .09 | |
| No | –82.8 (7.5) | |
| Yes | –113.2 (16.4) | |
| AMLs | .13 | |
| No | –97.5 (8.9) | |
| Yes | –76.2 (10.5) | |
| Menopausal status | .003 | |
| No | –118 (12.4) | |
| Yes | –73.7 (8.1) | |
| History of pneumothorax | .98 | |
| No | –89.8 (10.2) | |
| Yes | –90.1 (9.2) | |
| Supplemental oxygen use | .61 | |
| No | –86.4 (8.2) | |
| Yes | –94.0 (12·5) | |
| No. of pneumothoracesa | 2.2 (1.7) | .20 |
| Sporadic LAM | –85.9 (7.1) | .68 |
| TSC-LAM | –93.5 (16.8) |
For binary factors, the parameter estimates refer to the mean rate of decline of FEV1 in each subgroup. See Table 1 and 2 legends for expansion of abbreviations.
For the continuous variables, the parameter estimates reflect the effect on the rate of decline of FEV1 per unit increase of the factor being studied. For example, the rate of decline of FEV1 increases by 0.31 mL/year per 1% increase in the baseline FEV1 % predicted.
Progression to Death or Transplantation
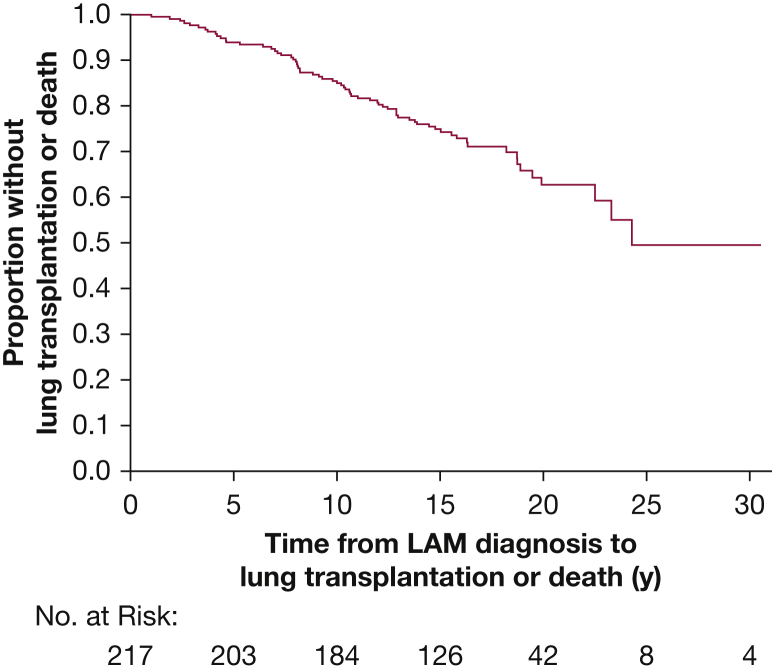
From the study cohort of 217 patients, 26 patients had died and 43 patients had undergone lung transplantation as of the data censor date of December 31, 2014. This outcome equates to 5-, 10-, 15-, and 20-year survival rates of 94%, 85%, 75%, and 64%, respectively, based on the composite endpoint of death or transplantation (Fig 2).
Figure 2.
Kaplan-Meier curve showing death/transplantation as outcome from the time of diagnosis. LAM = lymphangioleiomyomatosis.
Using a Cox proportional hazards model adjusted for age at diagnosis, we examined the characteristics that may affect disease progression. The following factors were associated with an increased risk of progression to death or transplantation: baseline disease severity as measured according to pulmonary physiology (lower % predicted FEV1, FVC, and diffusion capacity of lung for carbon monoxide [Dlco]) and higher CT score; tempo of disease progression as measured by the rate of decline in FEV1, FVC, and Dlco; and the rate of increase in serum VEGF-D, supplemental oxygen use, and bronchodilator responsiveness on spirometry. Other clinical variables such as history of angiomyolipomas, spontaneous pneumothorax, or TSC association (ie, TSC-LAM vs sporadic LAM) had no effect on future progression to death/lung transplant (e-Figs 1-3, e-Table 2).
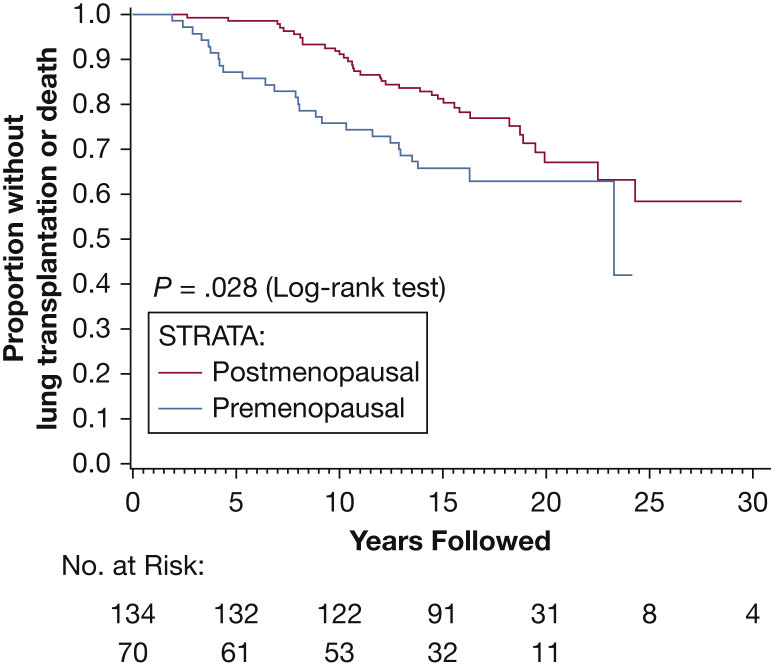
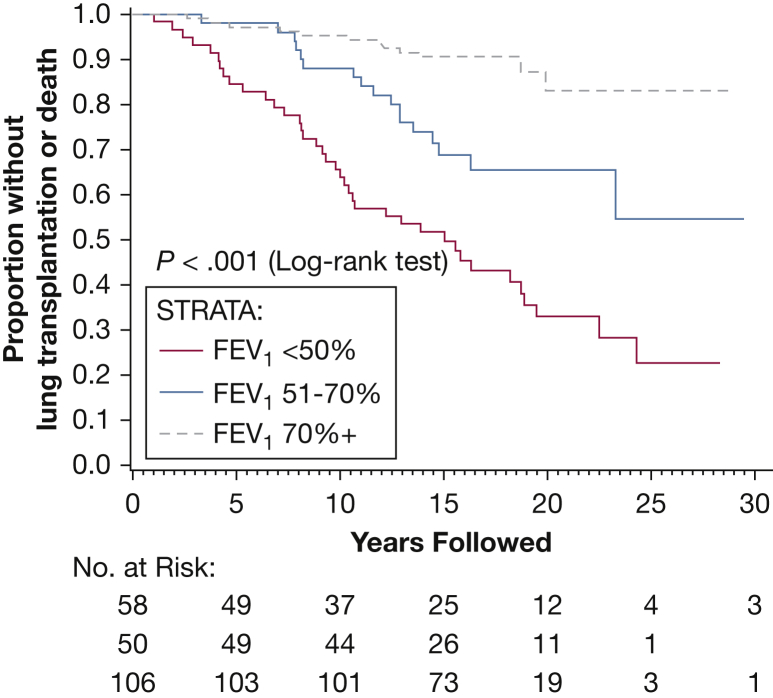
Finally, a multiple Cox regression model was used with stepwise variable selection to determine the combination of risk factors that were most predictive of outcome. The final model showed that menopausal status and baseline disease severity based on initial FEV1 and Dlco are the major determinants of progression to death or transplantation (Figure 3, Figure 4, Figure 5, Table 5).
Figure 3.
Kaplan-Meier curve showing death/transplantation as outcome after segregating patients into premenopausal and postmenopausal status at their baseline visit. Postmenopausal subjects had a lower risk of progression to death or lung transplantation compared with the premenopausal subjects.
Figure 4.
Kaplan-Meier curve showing death/transplantation as an outcome after segregating patients on the basis of their initial FEV1 values. Higher baseline FEV1 was associated with a decreased risk of progression to death or lung transplantation.
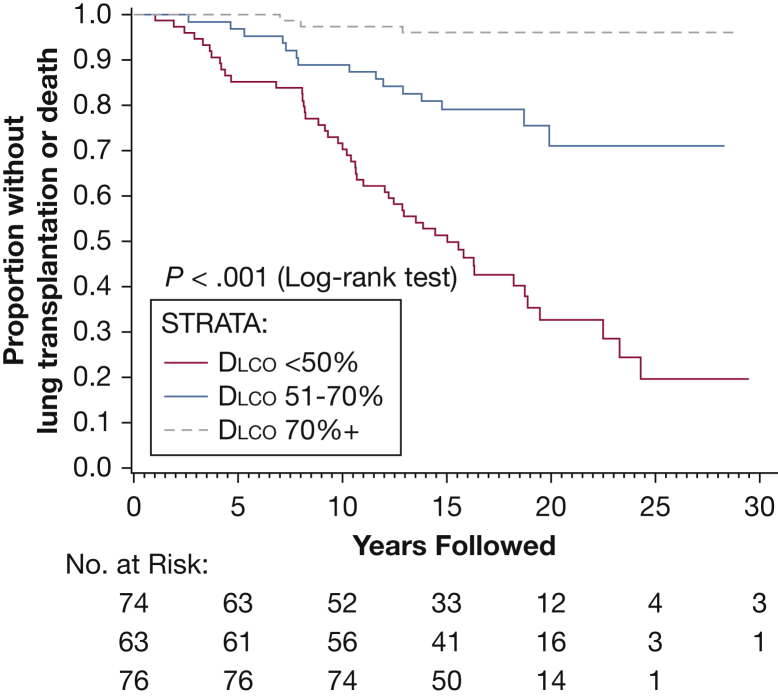
Figure 5.
Kaplan-Meier curve showing death/transplantation as outcome after segregating patients based on their initial Dlco values. Higher baseline Dlco was associated with a decreased risk of progression to death or lung transplantation. Dlco = diffusion capacity of lung for carbon monoxide.
Table 5.
Multiple Cox Regression Model for Progression to Death or Lung Transplantation (Adjusted for Age at Diagnosis)
| Characteristic | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| FEV1 % predicted | 0.97 (0.96-0.99) | .008 |
| Dlco % predicted | 0.97 (0.95-0.99) | .001 |
| Postmenopausal status | .0002 | |
| No | Reference | |
| Yes | 0.30 (0.16-0.57) |
See Table 2 legend for expansion of abbreviation.
Discussion
The natural history of lung function decline in LAM has been defined primarily by retrospective cohort analyses and the placebo groups of randomized controlled clinical trials, yielding wide-ranging estimates of FEV1 decline between 47 and 134 mL/year.6, 9, 10, 11, 12 The divergent FEV1 decline rates among these cohorts can largely be explained by ascertainment bias and varying baseline disease severity. Baseline PFT results have previously been reported to affect the future rate of decline,9, 11 with the slowest decline reported in patients with mild disease (FEV1 > 70% predicted).11 Although our data also indicate a relationship between baseline FEV1 and the future rate of FEV1 decline, the magnitude of effect is very small and opposite in direction (ie, higher baseline FEV1 predicts faster rate of decline) to previous reports. Subsequently, we divided the study cohort into quartiles on the basis of initial FEV1 and determined that the rate of FEV1 decline was similar across all groups (Table 6). We therefore concluded that the association between baseline FEV1 and future rate of decline is clinically insignificant, and we assert that rate of FEV1 decline is largely independent of baseline FEV1. This finding has important implications for treatment decisions in patients with mild but progressive disease and for the design of early intervention trials.
Table 6.
Rate of Decline of FEV1 According to Quartiles of Initial FEV1 Values at Enrollment
| FEV1 Values (% Predicted) | No. of Patients | No. of Patients With at Least 2 PFTs | FEV1 Decline Rate (mL/y) | SE |
|---|---|---|---|---|
| > 70% | 106 | 100 | 88.3 | 9.2 |
| 51%-70% | 50 | 42 | 92.7 | 14.8 |
| 36%-50% | 42 | 33 | 85.5 | 16.4 |
| ≤ 35% | 17 | 13 | 85.4 | 33.2 |
There was no statistically significant difference in the rate of decline of FEV1 after dividing patients into different categories based on their initial FEV1 (P = .99). PFT = pulmonary function test.
The rate of decline of FEV1 was also higher in patients with greater profusion of parenchymal cysts on CT scanning. This finding suggests that CT assessment of disease burden can be helpful for prognostication, and it also highlights the need to develop automated and widely accessible methods to quantify radiologic disease burden as a useful prognostic biomarker.
Hormonal influences clearly play a role in the pathogenesis of LAM based on the near absolute restriction of symptomatic disease to female subjects,1 expression of estrogen and progesterone receptors by LAM cells,13 and reports of disease worsening with estrogen supplementation14, 15 and pregnancy.16, 17 Attenuation in the rate of FEV1 decline after menopause as seen in our analysis further supports a role for estrogen in the pathogenesis of LAM.
We found that patients with a positive bronchodilator response on spirometry exhibited a trend toward a faster rate of decline of FEV1 and an increased risk of progression to death or transplantation (e-Fig 1). A positive bronchodilator response was previously reported to be associated with more severe obstruction and a greater rate of FEV1 decline in patients with LAM.18 We found that patients with a positive bronchodilator response had a lower baseline FEV1 (1.7 L, 57% predicted) compared with those without a bronchodilator response (2.2 L, 73% predicted) (P = .0007) (e-Table 3). These data suggest that positive bronchodilator response may be a surrogate for more advanced airway involvement with LAM19 and may be a potential prognostic biomarker.
Several traditional prognostic biomarkers of disease progression in LAM did not correlate with the rate of decline of FEV1 in the present study. For example, baseline serum VEGF-D was not significantly associated with the rate of FEV1 decline. Several groups have validated the value of VEGF-D as a prognostic biomarker,20 while others have failed to show an association between baseline VEGF-D levels and future decline.21, 22 Although the reasons for these divergent results are not known, several explanations are possible for the lack of a correlation in our study, including the following: (1) the accuracy of serum VEGF-D measurement may have been affected by storage at –80°C for > 15 years; (2) the VEGF-D relation with rate of lung function decline may only apply to patients with more severe disease; or (3) the extent of lymphatic involvement in the two cohorts may have been different. With respect to the last point, in an analysis of 111 patients with LAM at the National Institutes of Health (NIH), elevated levels of serum VEGF-D were shown to correlate with the presence of lymphangioleiomyomas and/or lymphadenopathy,22 and studies from the NIH23 and Japan24 have shown that response to therapy is better in patients with lymphatic involvement. It is possible that the correlation between serum VEGF-D and the rate of decline of FEV1 is limited to the subset of LAM patients with a lymphatic phenotype. Unfortunately, the presence of lymphangioleiomyomas and/or lymphadenopathy was not recorded during the registry visits, and we were unable to conduct a subgroup analysis to investigate this hypothesis.
Some reports suggest that patients with TSC-LAM have milder and less progressive disease compared with those with sporadic LAM.25, 26 More recently, the NIH Intramural group has reported that the rate of lung function decline is similar when TSC-LAM and patients with sporadic LAM are matched for baseline disease severity.27 Ascertainment bias may certainly play a role in these differing results because screening can identify TSC-LAM in earlier stages than sporadic LAM is typically discovered. In our study cohort, there was no difference between patients with TSC-LAM and sporadic LAM in the rate of decline of FEV1, in keeping with the conclusions of the NIH Intramural study,27 or in the future risk of progression to death/transplantation.
Pneumothorax can be a sentinel event that leads to early diagnosis of LAM and has been reported to identify a cohort with a more favorable prognosis,28, 29 likely reflecting a lead-time bias. In the present cohort, 55% of the patients (120 of 217) had at least one spontaneous pneumothorax. The mean age at LAM diagnosis was 37.6 years for women in the pneumothorax subgroup compared with 44.5 years in the non-pneumothorax subgroup (P < .0001), consistent with a role for pneumothorax in bringing the diagnosis to light earlier. However, neither the occurrence of spontaneous pneumothorax nor the number of spontaneous pneumothoraces had an impact on the rate of decline of FEV1 or progression to death/lung transplantation in our study.
Prognostic information with regard to mortality in LAM is limited and is based exclusively on retrospective cohort studies. The initial reports cited 10-year mortality rates of up to 80% in patients with LAM.30 Kitaichi et al31 reviewed 46 Asian patients with LAM and reported that 62% of the patients had died by 8.5 years following their diagnosis. More recent studies suggest much better survival, with reported 10-year survival rates of 79%, 76%, and 86% across patients with LAM in France, Japan, and the United States, respectively.28, 29, 32 The results from our analysis support these more recent findings, with 5-, 10-, 15-, and 20-year survival rates of 94%, 85%, 75%, and 64%.
Relatively little is known about the variables that predict mortality in LAM; lower FEV1/FVC ratios,31 worse histologic findings,33 presentation with dyspnea,28 and the need for supplemental oxygen29 are the major reported factors that are associated with an increased risk of death. Our data further illustrate that lower lung function is associated with decreased survival. In addition, our study shows for the first time that postmenopausal women with LAM have a better survival rate than premenopausal women. The improved survival, coupled with a reduced rate of FEV1 decline among postmenopausal women, suggests that menopause is a milestone that segregates patients with LAM into two different subgroups with markedly different outcomes.
Strengths
This analysis was the largest prospective natural history study of women with LAM, and the only one to include rigorously validated PFT, survival, and transplantation endpoints. In addition, these data represent perhaps the last opportunity to understand disease progression in the pre-sirolimus era and will be a useful comparator for future studies to determine the impact of mTOR therapy on survival. All PFTs were conducted in a limited number of predefined, American Thoracic Society/European Respiratory Society-compliant laboratories, using postbronchodilator measurements and strict quality control. The serum VEGF-D assays were performed in a College of American Pathologists/Clinical Laboratory Improvement Act-certified laboratory. This dataset can aid in the development of a LAM-specific disease severity score that will be useful for prognostication and treatment decisions. In addition, this publicly available, prospective natural history dataset and linked serum and plasma specimens can be used by the LAM research community as a resource for the discovery and development of novel LAM-specific biomarkers.
Limitations
Our study has several limitations. The MILES trial showing the efficacy of sirolimus in LAM was published in 2011.6 The impact of sirolimus on progression to death/lung transplantation has not been studied. Because our data censor date (December 2014) overlapped with the early use of sirolimus in LAM, it is possible that some of the estimates affecting progression to death/lung transplantation were skewed by treatment with sirolimus. To address this concern, we performed a sensitivity analysis with a data censor date of December 31, 2010 (prior to publication of MILES). Excluding death/transplantation beyond 2010 resulted in the exclusion of 15 events of a total of 69 events, and had no impact on the overall survival or the prognostic variables.
The majority of patients enrolled in the NHLBI LAM registry were enrolled at the NIH, a group that has published extensively on the natural history of LAM.9, 18, 27, 33 Although there is clearly some overlap in the present cohort and populations reported in previous NIH studies, the longitudinal analysis of the LAM registry data has never been reported, and these previous analyses did not include the biochemical assessment of menopausal status, analysis of VEGF-D as a long-term prognostic biomarker, or prospective assessment of survival and transplantation outcomes.
Finally, menopausal status was obtained only at baseline, and transitions to menopause during the study were not recorded. In addition, although better outcomes in postmenopausal women seem biologically plausible, given the known hormonal influences on the pathogenesis of LAM, the potential influence of survival bias on this finding cannot be completely excluded.
Conclusions
The present study highlights that LAM is chronic, progressive disease with a rate of lung function decline that is roughly three times higher than that of healthy subjects. Postmenopausal women decline at a significantly lower rate than premenopausal women. The median transplant-free survival in untreated women with LAM is > 20 years and is significantly affected by the baseline PFT results and menopausal status.
Acknowledgments
Author contributions: N. G., J. M., and F. X. M. conceived the study design; H. S. L., G. J. B., and J. C. L. conducted the data analysis; J. H. R., A. M. T., G. A. F., K. K. B., S. J. R., and J. M. were the site investigators and helped with patient enrollment and data collection; K. M. provided oversight and quality control for all PFTs during the registry visits; and N. A. A. reviewed all CT scans conducted as part of the registry and devised and calculated the CT cyst score. The data interpretation and manuscript writing were conducted by a writing group (F. X. M., J. M., and N. G.). All listed authors contributed substantially in editing the manuscript. N. G. and H. S. L. had full access to all the data in this study, and N. G., J. M., and F. X. M. had final responsibility for the decision to submit for publication.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: F. X. M. owns a patent for performing the diagnostic assay to measure serum VEGF-D. All royalties from this patent are waived to the parent institution (the University of Cincinnati). None declared: (N. G., H. S. L., J. H. R., G. J. B., J. C. L., K. M., N. A. A., A. M. T., J. M.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, or any of the other study sponsors.
Other contributions: The authors express their sincere gratitude to all the patients with LAM who participated in the NHLBI LAM registry for their efforts and their enduring dedication and commitment to research.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Moss and McCormack contributed equally as co-senior authors.
FUNDING/SUPPORT: This study was supported by a National Heart, Lung and Blood Institute [Grant U01HL58440], the Halis Gorgulu Research Fund, the Carespring Foundation, and the National Institutes of Health/National Heart, Lung, and Blood Institute Intramural Research Program.
Supplementary Data
References
- 1.Gupta N., Vassallo R., Wikenheiser-Brokamp K.A., McCormack F.X. Diffuse cystic lung disease. Part I. Am J Respir Crit Care Med. 2015;191(12):1354–1366. doi: 10.1164/rccm.201411-2094CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smolarek T.A., Wessner L.L., McCormack F.X., Mylet J.C., Menon A.G., Henske E.P. Evidence that lymphangiomyomatosis is caused by TSC2 mutations: chromosome 16p13 loss of heterozygosity in angiomyolipomas and lymph nodes from women with lymphangiomyomatosis. Am J Hum Genet. 1998;62(4):810–815. doi: 10.1086/301804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henske E.P., McCormack F.X. Lymphangioleiomyomatosis—a wolf in sheep's clothing. J Clin Invest. 2012;122(11):3807–3816. doi: 10.1172/JCI58709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumasaka T., Seyama K., Mitani K. Lymphangiogenesis in lymphangioleiomyomatosis: its implication in the progression of lymphangioleiomyomatosis. Am J Surg Pathol. 2004;28(8):1007–1016. doi: 10.1097/01.pas.0000126859.70814.6d. [DOI] [PubMed] [Google Scholar]
- 5.Seyama K., Kumasaka T., Kurihara M., Mitani K., Sato T. Lymphangioleiomyomatosis: a disease involving the lymphatic system. Lymphat Res Biol. 2010;8(1):21–31. doi: 10.1089/lrb.2009.0018. [DOI] [PubMed] [Google Scholar]
- 6.McCormack F.X., Inoue Y., Moss J. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364(17):1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCormack F.X., Gupta N., Finlay G.R. Official American Thoracic Society/Japanese Respiratory Society clinical practice guidelines: lymphangioleiomyomatosis diagnosis and management. Am J Respir Crit Care Med. 2016;194(6):748–761. doi: 10.1164/rccm.201607-1384ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryu J.H., Moss J., Beck G.J. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173(1):105–111. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taveira-DaSilva A.M., Stylianou M.P., Hedin C.J., Hathaway O., Moss J. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest. 2004;126(6):1867–1874. doi: 10.1378/chest.126.6.1867. [DOI] [PubMed] [Google Scholar]
- 10.Chang W.Y., Cane J.L., Kumaran M., Lewis S., Tattersfield A.E., Johnson S.R. A 2-year randomised placebo-controlled trial of doxycycline for lymphangioleiomyomatosis. Eur Respir J. 2014;43(4):1114–1123. doi: 10.1183/09031936.00167413. [DOI] [PubMed] [Google Scholar]
- 11.Hayashida M., Yasuo M., Hanaoka M. Reductions in pulmonary function detected in patients with lymphangioleiomyomatosis: an analysis of the Japanese National Research Project on Intractable Diseases database. Respir Investig. 2016;54(3):193–200. doi: 10.1016/j.resinv.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Johnson S.R., Tattersfield A.E. Decline in lung function in lymphangioleiomyomatosis: relation to menopause and progesterone treatment. Am J Respir Crit Care Med. 1999;160(2):628–633. doi: 10.1164/ajrccm.160.2.9901027. [DOI] [PubMed] [Google Scholar]
- 13.Gao L., Yue M.M., Davis J., Hyjek E., Schuger L. In pulmonary lymphangioleiomyomatosis expression of progesterone receptor is frequently higher than that of estrogen receptor. Virchows Arch. 2014;464(4):495–503. doi: 10.1007/s00428-014-1559-9. [DOI] [PubMed] [Google Scholar]
- 14.Yano S. Exacerbation of pulmonary lymphangioleiomyomatosis by exogenous oestrogen used for infertility treatment. Thorax. 2002;57(12):1085–1086. doi: 10.1136/thorax.57.12.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen A., Iseman M.D., Waldron J.A., King T.E. Exacerbation of pulmonary lymphangioleiomyomatosis by exogenous estrogens. Chest. 1987;91(5):782–785. doi: 10.1378/chest.91.5.782. [DOI] [PubMed] [Google Scholar]
- 16.Brunelli A., Catalini G., Fianchini A. Pregnancy exacerbating unsuspected mediastinal lymphangioleiomyomatosis and chylothorax. Int J Gynaecol Obstet. 1996;52(3):289–290. doi: 10.1016/0020-7292(95)02619-3. [DOI] [PubMed] [Google Scholar]
- 17.Yockey C.C., Riepe R.E., Ryan K. Pulmonary lymphangioleiomyomatosis complicated by pregnancy. Kans Med. 1986;87(10):277–278. 293. [PubMed] [Google Scholar]
- 18.Taveira-DaSilva A.M., Hedin C., Stylianou M.P. Reversible airflow obstruction, proliferation of abnormal smooth muscle cells, and impairment of gas exchange as predictors of outcome in lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2001;164(6):1072–1076. doi: 10.1164/ajrccm.164.6.2102125. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi T., Kumasaka T., Mitani K. Bronchial involvement in advanced stage lymphangioleiomyomatosis: histopathologic and molecular analyses. Hum Pathol. 2016;50:34–42. doi: 10.1016/j.humpath.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Young L., Lee H.S., Inoue Y. Serum VEGF-D a concentration as a biomarker of lymphangioleiomyomatosis severity and treatment response: a prospective analysis of the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) trial. Lancet Respir Med. 2013;1(6):445–452. doi: 10.1016/S2213-2600(13)70090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang W.Y., Cane J.L., Blakey J.D., Kumaran M., Pointon K.S., Johnson S.R. Clinical utility of diagnostic guidelines and putative biomarkers in lymphangioleiomyomatosis. Respir Res. 2012;13:34. doi: 10.1186/1465-9921-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glasgow C.G., Avila N.A., Lin J.P., Stylianou M.P., Moss J. Serum vascular endothelial growth factor-D levels in patients with lymphangioleiomyomatosis reflect lymphatic involvement. Chest. 2009;135(5):1293–1300. doi: 10.1378/chest.08-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taveira-DaSilva A.M., Jones A.M., Julien-Williams P., Stylianou M., Moss J. Long-term effect of sirolimus on serum VEGF-D levels in patients with lymphangioleiomyomatosis. Chest. 2018;153(1):124–132. doi: 10.1016/j.chest.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takada T., Mikami A., Kitamura N. Efficacy and safety of long-term sirolimus therapy for Asian patients with lymphangioleiomyomatosis. Ann Am Thorac Soc. 2016;13(11):1912–1922. doi: 10.1513/AnnalsATS.201605-335OC. [DOI] [PubMed] [Google Scholar]
- 25.Avila N.A., Dwyer A.J., Rabel A., Moss J. Sporadic lymphangioleiomyomatosis and tuberous sclerosis complex with lymphangioleiomyomatosis: comparison of CT features. Radiology. 2007;242(1):277–285. doi: 10.1148/radiol.2421051767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taveira-DaSilva A.M., Pacheco-Rodriguez G., Moss J. The natural history of lymphangioleiomyomatosis: markers of severity, rate of progression and prognosis. Lymphat Res Biol. 2010;8(1):9–19. doi: 10.1089/lrb.2009.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taveira-DaSilva A.M., Jones A.M., Julien-Williams P., Yao J., Stylianou M., Moss J. Severity and outcome of cystic lung disease in women with tuberous sclerosis complex. Eur Respir J. 2015;45(1):171–180. doi: 10.1183/09031936.00088314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashida M., Seyama K., Inoue Y. The epidemiology of lymphangioleiomyomatosis in Japan: a nationwide cross-sectional study of presenting features and prognostic factors. Respirology. 2007;12(4):523–530. doi: 10.1111/j.1440-1843.2007.01101.x. [DOI] [PubMed] [Google Scholar]
- 29.Oprescu N., McCormack F.X., Byrnes S., Kinder B.W. Clinical predictors of mortality and cause of death in lymphangioleiomyomatosis: a population-based registry. Lung. 2013;191(1):35–42. doi: 10.1007/s00408-012-9419-3. [DOI] [PubMed] [Google Scholar]
- 30.Corrin B., Liebow A.A., Friedman P.J. Pulmonary lymphangiomyomatosis. A review. Am J Pathol. 1975;79(2):348–382. [PMC free article] [PubMed] [Google Scholar]
- 31.Kitaichi M., Nishimura K., Itoh H., Izumi T. Pulmonary lymphangioleiomyomatosis: a report of 46 patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med. 1995;151(2 pt 1):527–533. doi: 10.1164/ajrccm.151.2.7842216. [DOI] [PubMed] [Google Scholar]
- 32.Urban T., Lazor R., Lacronique J. Pulmonary lymphangioleiomyomatosis. A study of 69 patients. Groupe d'Etudes et de Recherche sur les Maladies "Orphelines" Pulmonaires (GERM"O"P) Medicine (Baltimore) 1999;78(5):321–337. doi: 10.1097/00005792-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Matsui K., Beasley M.B., Nelson W.K. Prognostic significance of pulmonary lymphangioleiomyomatosis histologic score. Am J Surg Pathol. 2001;25(4):479–484. doi: 10.1097/00000478-200104000-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.