Abstract
Identifying the signaling mechanisms that regulate adult neurogenesis is essential to understanding how the brain may respond to neuro‐inflammatory events. P2X7 receptors can regulate pro‐inflammatory responses, and in addition to their role as cation channels they can trigger cell death and mediate phagocytosis. How P2X7 receptors may regulate adult neurogenesis is currently unclear. Here, neural progenitor cells (NPCs) derived from adult murine hippocampal subgranular (SGZ) and cerebral subventricular (SVZ) zones were utilized to characterize the roles of P2X7 in adult neurogenesis, and assess the effects of high extracellular ATP, characteristic of inflammation, on NPCs. Immunocytochemistry found NPCs in vivo and in vitro expressed P2X7, and the activity of P2X7 in culture was demonstrated using calcium influx and pore formation assays. Live cell and confocal microscopy, in conjunction with flow cytometry, revealed P2X7+ NPCs were able to phagocytose fluorescent beads, and this was inhibited by ATP, indicative of P2X7 involvement. Furthermore, P2X7 receptors were activated with ATP or BzATP, and 5‐ethynyl‐2′‐deoxyuridine (EdU) used to observe a dose‐dependent decrease in NPC proliferation. A role for P2X7 in decreased NPC proliferation was confirmed using chemical inhibition and NPCs from P2X7−/− mice. Together, these data present three distinct roles for P2X7 during adult neurogenesis, depending on extracellular ATP concentrations: (a) P2X7 receptors can form transmembrane pores leading to cell death, (b) P2X7 receptors can regulate rates of proliferation, likely via calcium signaling, and (c) P2X7 can function as scavenger receptors in the absence of ATP, allowing NPCs to phagocytose apoptotic NPCs during neurogenesis. stem cells 2018;36:1764–1777
Keywords: P2X7, Adult neurogenesis, Adult neural stem cells, Hippocampus, ATP, Neuro‐inflammation
Significance Statement.
High concentrations of extracellular ATP are a hallmark of neuroinflammatory and neurodegenerative disorders. By activating P2X7 receptors, ATP can exacerbate the initial insult, furthering cell death. Results of this study demonstrated that P2X7 has multiple roles in neural progenitor cells. As a calcium channel, P2X7 signaling may negatively regulate neural progenitor cell proliferation, while retaining its canonical role as a death receptor via the formation of transmembrane pores. P2X7 may also contribute to niche maintenance by facilitating phagocytosis of apoptotic cell bodies. The presence of P2X7 within the adult neurogenic niches is of importance given the therapeutic potential of some antagonists following ischemic injury.
Introduction
Adult neurogenesis occurs in at least two stem cell niches, the subgranular zone (SGZ) of the hippocampal dentate gyrus and the subventricular zone (SVZ) that lines the walls of the anterior lateral ventricles 1. Neural progenitor cells (NPCs) in both niches proliferate to generate large progenitor pools. In the hippocampus, these NPCs mature into granule neurons, and the newly integrated neurons exhibit an enhanced plasticity for a short period that is vitally important for the formation and retrieval of memories 2, 3. In the SVZ, NPCs give rise to neuroblasts that migrate via the rostral migratory stream to the olfactory bulb and differentiate into GABAergic interneurons that participate in olfaction 4. Neurogenesis requires tight regulation of cell numbers and proliferation is balanced by programmed cell death (PCD), allowing selection of only the most appropriate cells to participate in synaptic competition and integration. Apoptotic cells must be removed to prevent buildup and inflammation in the neurogenic environment 5, 6.
Purinergic signaling has emerged as an important regulator of proliferation, PCD, and lineage maturation during adult neurogenesis 7, 8. P2X7 is unique among the P2X receptors in its ability to subserve a number of these physiological needs: (a) as an ion channel, P2X7 is able to regulate proliferation, (b) as a transmembrane pore, it can induce cell death, and (c) as a scavenger receptor, it is able to remove cell corpses by facilitating phagocytosis 9. In addition to these roles, P2X7 is well known to mediate inflammatory responses and cytokine release 10, 11.
Embryonic stem cells can utilize P2X7 receptors to regulate cell cycle progression, alter the expression of neuronal markers, and play a role in differentiation 12, 13. Cultured neurons also had increased axonal growth and branching in conditions of P2X7 inhibition, highlighting the negative influence of extracellular ATP, and P2X7 activation, on neurons in the hippocampus 14. In response to higher concentrations of ATP (≥1 mM, such as those found following inflammatory events), P2X7 receptors form large transmembrane pores permeable to molecules up to 800 Da, leading to osmotic dysregulation and cell death 15, 16. P2X7 pore formation also plays a significant role in inflammatory processes during ischemic events, such as a stroke, where the large amounts of ATP released from dying cells can activate the P2X7 receptors on neighboring cells and exacerbate the initial infarct 17.
P2X7 receptors have been shown to facilitate phagocytosis in the strict absence of ATP 18, 19. Disulfide bonds in the extracellular domain distinct from agonist binding sites have been identified as important for the recognition and binding of apoptotic cells 18. Uptake occurs via P2X7 C‐terminus association with the nonmuscle myosin complex in the membrane cytoskeleton. Agonist binding results in dissociation of the C‐terminus from the cytoskeleton, thus inhibiting P2X7 mediated phagocytosis 20, 21. We recently demonstrated that cultured human fetal NPCs utilize P2X7 to phagocytose cellular debris, possibly as a means of cell removal and maintenance in the developing neurogenic niches 6. Interestingly, P2X7+ NPCs and neuroblasts appear earlier in the developing brain than microglia, the professional phagocytes, suggesting progenitor cells can self‐regulate their niches by acting as nonprofessional phagocytes, a process that may persist into adulthood 22.
From these reports, P2X7 receptors have the potential to play a number of regulatory roles depending on the extracellular environment within the neurogenic niche. This in vitro study characterizes P2X7 receptors in adult NPCs to elucidate their possible involvement in proliferation, cell death, and phagocytosis in neurogenic niches in the mammalian brain. Identifying the signaling mechanisms that regulate adult neurogenesis is an essential step towards understanding how pathological conditions resulting in high extracellular ATP may influence the neurogenic niches and more generally, the pathology of the adult central nervous system.
Materials and Methods
Dissection and Cell Culture
Primary cultures of adult NPCs were derived from the hippocampi and SVZ of female C57BL/6 or Pfizer P2X7−/− 10 mice between 8 and 12 weeks of age. The tissue was minced using a scalpel blade (30 seconds), trypsinized (30 minutes) and triturated as previously described 23, 24. The resulting single cell suspension was cultured in NeuroCult Basal Medium with NeuroCult Proliferation Supplement (StemCell Technologies), glutamine (2 mM, Invitrogen), EGF and bFGF (10 ng/ml, Peprotech), and heparin (2 μg/ml). Neurospheres could be visualized by ≈7 days in vitro and the initial culture was passaged after 2–3 weeks. Cell cultures were maintained at 37°C, 5% CO2 and subsequently passaged every 7–10 days. Cultures used for experiments were between passages 2 and 6. Genotyping of P2X7−/− NPC cultures confirmed the expected deletion in the P2X7−/− gene, and the presence of the neomycin resistance gene used to disrupt the P2X7 gene. Primers were as follows: P2X7 forward, GCA GCC CAG CCC TGA TAC AGA CAT T; P2X7 reverse, TCG GGA CAG CAC GAG CTT ATG GA; neomycin forward, TGC TCC TGC CGA GAA AGT ATC CAT CAT GGC; neomycin reverse, CGC CAA GCT CTT CAG CAA TAT CAC GGG TAG. Reaction master mix was prepared with GoTaq Hot Start Polymerase (Promega) and reaction conditions were 35 cycles of 94°C for 60 seconds, 58°C for 72 seconds and 72°C for 75 seconds, followed by 72°C for 10 minutes. All reagents were obtained from Sigma‐Aldrich unless otherwise stated.
Multi‐Marker Immunochemistry of Adult Mouse Hippocampal and SVZ Neural Progenitor Cells
Adult NPCs were plated on poly‐l‐ornithine (5 μg/ml) and laminin (2.5 μg/ml, Thermo Fisher Scientific) coated glass coverslips and cultured for 3 days before fixation with 4% paraformaldehyde (PFA) followed by multiple‐marker immunohistochemistry, modified from Weible and Chan‐Ling 23. Differentiated cultures were seeded at low density and 50% proliferation medium was supplemented with differentiation medium (NeuroCult Basal Media with NeuroCult Differentiation Supplement) daily for 7 days. For the detection of cytoplasmic antigens, cells were permeabilized with 0.5% Triton X‐100 and blocked with 3% normal goat serum. Primary antibodies were applied for 16 hours at 4°C and included those raised against P2X7 (rabbit, 1:1000, Alomone Labs, Jerusalem, Israel intracellular: APR‐004; and extracellular: APR‐008, epitopes), PROX1 (mouse, 1:200), glial fibrillary acidic protein (GFAP, mouse conjugate, 1:1000), nestin (rabbit, 1:200, Abcam, Cambridge, UK); SRY‐related HMG BOX gene 2 (SOX2, rabbit, 1:2000, Abcam), vimentin (mouse, 1:1000), mammalian achaete‐scute homolog 1 (MASH1, mouse, 1:500, BD Pharmingen, San Jose, CA), brain lipid‐binding protein (BLBP, rabbit, 1:1000), and doublecortin (DCX, rabbit, 1:200, Cell Signaling Technology, Danvers, MA). Immune complexes were detected with goat antibodies to mouse IgG H + L, IgG1, IgM or rabbit IgG H + L, conjugated to Alexa‐Fluor 488, Cyanine3 or Cyanine5 (Thermo Fisher Scientific, Waltham, MA). Nuclei were counterstained with 4′,6‐diamidino‐2‐phenylindole dihydrochloride (DAPI). Isotype control and/or omission of secondary antibodies served as negative controls. Images were captured with an Olympus FV1000 confocal microscope. For immunohistochemistry, mice were perfused with 4% PFA and brains drop‐fixed for 4 hours at 4°C. Sections were permeabilized with 0.5% Triton X‐100 for 30 minutes and blocked for 1 hour in 10% normal goat serum and 0.1% saponin before antibody application. Western blotting membranes were blocked with 5% skim milk and probed as above, and immune complexes were detected with horseradish peroxidase (HRP).
Click‐it EdU to Identify Proliferating Cells
The thymidine analog 5‐ethynyl‐2′‐deoxyuridine (EdU) was used to quantify proliferating cells as per the manufacturer's protocol (Click‐it EdU Imaging Kit; Life Technologies, Carlsbad, CA). For in vitro assays, single cells were plated as above. Treatments, including purines and P2X7 inhibitors, were applied for 18 hours followed by a 4‐hour incubation with EdU (10 μM). For live cell proliferation assays, treatments were applied for 24 hours and recorded using live cell microscopy. Cells were fixed, blocked and permeabilized as previously described. The click‐it EdU reaction cocktail [consisting of (in mM): PBS (137), CuSO4 (4), AlexaFluor‐488 azide dye (0.02), ascorbic acid (11.4)] was applied for 30 minutes at room temperature 25. DAPI was used as a nuclear counterstain. At least 10 randomly selected fields of view (FOV) per treatment were imaged and threshold intensity analysis was utilized to calculate the percentage of EdU positive cells per FOV. For immunohistochemistry, mice were given three intraperitoneal injections of EdU (50 mg/kg) and sacrificed on the 4th day. Sections were permeabilized and blocked as previously described, then incubated with click‐it EdU cocktail for 30 minutes.
Flow Cytometry
All flow cytometry assays were performed on a Becton Dickinson FACS Calibur flow cytometer (Florey Institute, University of Melbourne) or a Beckman Coulter CyAn flow cytometer (Griffith University).
Calcium Assays
NPCs were suspended in 1 ml of Ca2+ free Na+ buffer (in mM: NaCl [140], NaOH [5], KCl [5], HEPES [10], glucose [5], BSA [0.1%]) and loaded with Fluo8‐AM (2 ng/ml) and 5% pluronic acid (10 μl) for 30 minutes at 37 °C with gentle shaking. NPCs were washed twice with Ca2+‐free Na+ buffer and allowed to de‐esterify on ice for 30 minutes before resuspension in K+ buffer (in mM: KCl [145], KOH [5], HEPES [10], glucose [5], CaCl2 [0.1], BSA [1%]) with or without preincubation with the P2X7 specific inhibitors AZ10606120 (15 minutes, 1 μM, Tocris Bioscience) or A438079 (30 minutes, 10 μM, Tocris Biosciences, Bristol). CaCl2 (3 mM) was added prior to analysis by flow cytometry, followed by the addition of ATP (1 mM) or BzATP (100 μM) after 40 seconds of acquisition. Assays were run for 3 minutes.
Ethidium Assays
NPCs were resuspended in K+ buffer with or without AZ10606120 preincubation. EtBr (25 μM) was added before analysis by flow cytometry, followed by the addition of ATP or BzATP at the 40 seconds mark. Assays were run for 6 minutes.
Apoptosis Assays
NPCs were cultured overnight in the presence of ATP (100 μM), BzATP (100 μM), and staurosporine (0.2 μM). The cells were harvested, washed and resuspended at 1 × 106 cells/mL in Annexin binding buffer (in mM: HEPES [10], NaCl [140], CaCl2 [2.5]) according to the manufacturer's protocol (Annexin V staining kit; Thermo Fisher Scientific). Annexin V‐APC was incubated for 15 minutes at room temperature. NPCs were washed and resuspended in binding buffer, before the addition of 7‐AAD and analysis by flow cytometry. Fluorescence intensity of ethidium bromide reaches a plateau at about 4–6 minutes following the addition of ATP. Necrotic or dead cells are excluded automatically as the fluorescence intensity of ethidium bromide in these cells reaches the maximum (256 channel). Baseline cell leakage was seen in all types of cells due to membrane blebbing and/or macropinocytosis, and is subtracted when calculating area under the ethidium uptake curve.
Calcium Microscopy
NPCs were loaded with 2 ng/ml Fluo‐8 AM dye with 10 μl 5% pluronic acid in 250 μl of medium for 20 minutes and allowed to de‐esterify for 10 minutes. Phenol red‐free Neurobasal medium was used for imaging. ATP was applied at various concentrations and images were captured at 100 ms intervals over a 1‐minute period. Approximately 30–50 regions of interest were selected at random from monolayer areas and the change in fluorescence (F/F0) was recorded.
Phagocytosis Assays
Cellular engulfment of 1 μm carboxylated yellow‐green (YG) beads was used to measure innate phagocytosis via P2X7 in a method adapted from Lovelace et al. 6. For live cell microscopy, cells were labeled with LysoTracker Red (1 μM, Molecular Probes, Eugene, OR) to identify lysosomes or CellTracker Red (1 μM, Molecular Probes) to identify the cell body. Cultures were incubated with YG beads overnight in a Zeiss AxioObserver under physiological live cell conditions. Confocal microscopy was used to confirm intracellular location. For flow cytometry analysis, NPCs were resuspended in Na+ buffer. YG beads were added to the FACS tube 20 sec following the start of acquisition and samples were run for 6 minutes. Treatments included ATP (1 mM; 15 minutes preincubation), Cytochalasin D (20 μM; 20 minutes preincubation), PFA (4%, 20 minutes preincubation), or serum (5%).
Statistical Analysis
Data were presented as mean values ± SEM. Statistical significance was determined by one‐way ANOVA and post hoc analysis was carried out using Tukey HSD. In cases where Levene's test for homogeneity showed an effect of variance, Welch's one‐way ANOVA was carried out and Games Howell post hoc test used. The alpha value was set to 0.05. Unless otherwise stated, for each experiment at least three biological repeats (N) were conducted with a minimum of three technical repeats, and (n) refers to the total number of cells counted. Effect size was determined by partial eta‐squared (η2) analysis where 0.01–0.09 was small, 0.09–0.25 medium, and ≥ 0.25 was regarded as substantially large 26.
Results
EdU Positive Adult NPCs Express P2X7 Receptors In Vivo
EdU incorporation in combination with immunohistochemistry (N = 3) was used to determine the presence of proliferative P2X7+ NPCs within the adult dentate gyrus and SVZ neurogenic niches. Hippocampal NPCs of the SGZ were identified by MASH1 immunoreactivity, indicative of NPC entry into the proliferative phase of neurogenesis 27 and were observed to express P2X7 receptors (Fig. 1A–1G). P2X7 immunoreactivity (N = 4) was primarily located on the membrane or within the cytoplasm (Fig. 1B, 1C) but was also found in the nucleus of some MASH1+/EdU+ dividing precursors (Fig. 1D–1G). These findings are consistent with previous reports showing P2X7 receptors mostly localize within the cytoplasm 28, but may also be observed at the nucleus 29. Using human‐specific antibodies, we previously found P2X7 in the cytoplasm of human NPCs 6. Immunohistochemistry conducted on SVZ sections revealed similar findings, with P2X7 expression in both the ventricular and the proliferative subventricular layers (Fig. 1H–1J). P2X7 expression on ciliated ependymal cells lining the lateral ventricles has previously been described 30. Our staining results show a layer of intense P2X7 immunoreactivity in cells immediately adjacent to lateral ventricles, in agreement with their study. NPCs in the SVZ were identified by EdU incorporation, and had a relatively higher percentage of total EdU+ cells (23.0 ± 3.9%, n = 628, Supporting Information Figure S1A–S1D), than did NPCs of the dentate gyrus (2.5 ± 1.2%, n = 500).
Figure 1.

Adult hippocampal and SVZ NPCs express P2X7 receptors in vivo. NPCs were identified in sections of the hippocampal dentate gyrus (A, with insets B–C and D–G) by immunohistochemical staining for MASH1 in combination with EdU to label actively dividing cells. P2X7 immunoreactivity was detected in the cytoplasm and membrane of these NPCs (B,C), and occasionally in the nucleus of actively dividing cells (D–G). DAPI was used to label the nuclei. NPCs were also identified in sections of the subventricular zone (H–J) by EdU incorporation to label actively dividing cells. P2X7 immunoreactivity was identified in the ventricular layer and in EdU+ cells of the subventricular layer, separated by the short dashed line in I and J. The boundary with the lateral ventricles (LV) is also indicated, and the rostral migratory stream (RMS) can be seen in H. Scale bars represent 10 μm.
P2X7+ NPCs Were Identified as Type 2/Type C Intermediate Progenitors
Immuno‐characterization of SGZ‐ and SVZ‐derived NPCs cultured on glass coverslips found the majority of cells were GFAP−, nestin+, SOX2+, vimentin+, MASH1+, and BLBP+. SGZ NPCs were additionally Prox1+ and were identified as intermediate type 2 progenitors (Fig. 2A–2L), similar to previously described 31, whereas SVZ NPCs were defined as type C progenitors 32. At 3 DIV, a small number of SGZ cells, 3.8 ± 0.8% (n = 1,887), were identified as possible type 1 NPCs by co‐expression of GFAP and nestin (Fig. 2A–2C). MASH1 was observed to be negative in 13.9 ± 2.3% of cells (Fig. 2G, 2I; n = 507). When DCXhigh was used to identify type 3 neuroblasts they comprised <0.1% of the cells at 3 DIV (Supporting Information Fig. S1E–S1G). At 3 DIV, no cells were observed to express immature neuronal markers βIII tubulin, Map2a/b, or NeuN. When NPCs were maintained in differentiation media for 7 days, 10.1 ± 0.8% expressed βIII tubulin and 16.7 ± 0.9% expressed MAP2a/b (Supporting Information Fig. S1H–S1M, n > 7,000 each). The postmitotic neuronal marker NeuN was also observed in the nucleus of all cells (Supporting Information Fig. S1N–S1P) demonstrating the capability of the NPC cultures to differentiate into cells of the neuronal lineage. GFAP+ vimentin− cells in the differentiated culture also indicated the presence of astrocytic precursor cells and demonstrated multipotency, a key feature of NPCs (Supporting Information Fig. S1Q–S1S). O4 staining to detect oligodendrocyte precursor cells was negative in differentiated hippocampal cells, whereas 2.6 ± 0.86% of differentiated SVZ cells were positive (Supporting Information Fig. S1T, S1U, respectively).
Figure 2.

Adult NPCs were characterized as type 2/type C intermediate progenitor cells and express P2X7 receptors in vitro. Representative NPCs derived from the hippocampus and cultured in vitro were stained for GFAP and nestin (A–C) to identify type 1 NPCs. NPCs were also positive for the markers SOX2, vimentin (D–F), and BLBP (G–H). MASH1 was used to identify type 2 NPCs (G, I) and had a variable expression from low (indicated by hash) to high (asterisk). NPCs derived from the hippocampus expressed Prox1, a marker for granule cell neurons, and all NPCs expressed intracellular P2X7 receptors (J–L). Approximately 10% of representative hippocampal NPCs displayed negative P2X7 immunoreactivity under nonpermeabilized conditions, for example, they did not express P2X7 receptors on the membrane surface, indicated by an asterisk (M–O). Dual labeling against intracellular P2X7 and extracellular P2X7 revealed cells with negative surface expression retained intracellular stores (P–R). N ≥ 3; scale bars represent 10 μm.
NPCs from both the SGZ and SVZ were observed to express P2X7 receptors in all cells when probed with antibodies against an intracellular epitope (Fig. 2J, 2K). NPCs examined without permeabilization displayed positive surface expression of P2X7 in 90.1 ± 2.5% of cells (n = 1,465, Fig. 2M–2O), whereas cells with absent surface expression (indicated by asterisks) retained intracellular P2X7 stores that were detected following permeabilization (Fig. 2P–2R). No staining was detected following exclusion of primary antibody (Supporting Information Fig. S1V). Immunochemical analysis of cryosectioned neurospheres found P2X7 was expressed homogenously throughout the spheres, confirming the presence in flask cultures (Supporting Information Fig. S2A). Western blotting also identified a band at ~85 kDa, consistent with the glycosylated form of P2X7 (Supporting Information Fig. S2B).
Full Length P2X7 Receptors on Adult NPCs Function as Calcium Channels and Can Form Transmembrane Pores
P2X7 receptor function as a calcium channel was assessed using time resolved flow cytometry. Adult NPCs were loaded with Fluo‐8 calcium indicator dye, and calcium influx was observed upon P2X7 activation. Application of the general P2 agonist ATP (1 mM) and P2X7 agonist BzATP (100 μM) evoked calcium influx in hippocampal (Fig. 3A, N = 4) and SVZ (Fig. 3B, N = 4) derived NPCs. Calcium influx was reduced by preincubation with the P2X7 specific inhibitors AZ10606120 (1 μM, an allosteric antagonist) and A438079 (10 μM, a competitive antagonist), indicating presence of functional P2X7. Calcium microscopy was used to verify ATP‐induced calcium influx in hippocampal NPCs (Supporting Information Fig. S3A–S3H), and a dose‐dependent increase in cytosolic calcium concentration in response to increasing ATP concentration was observed [Welch's F(8, 650) = 455.0, p < 0.01; Supporting Information Fig. S3I].
Figure 3.
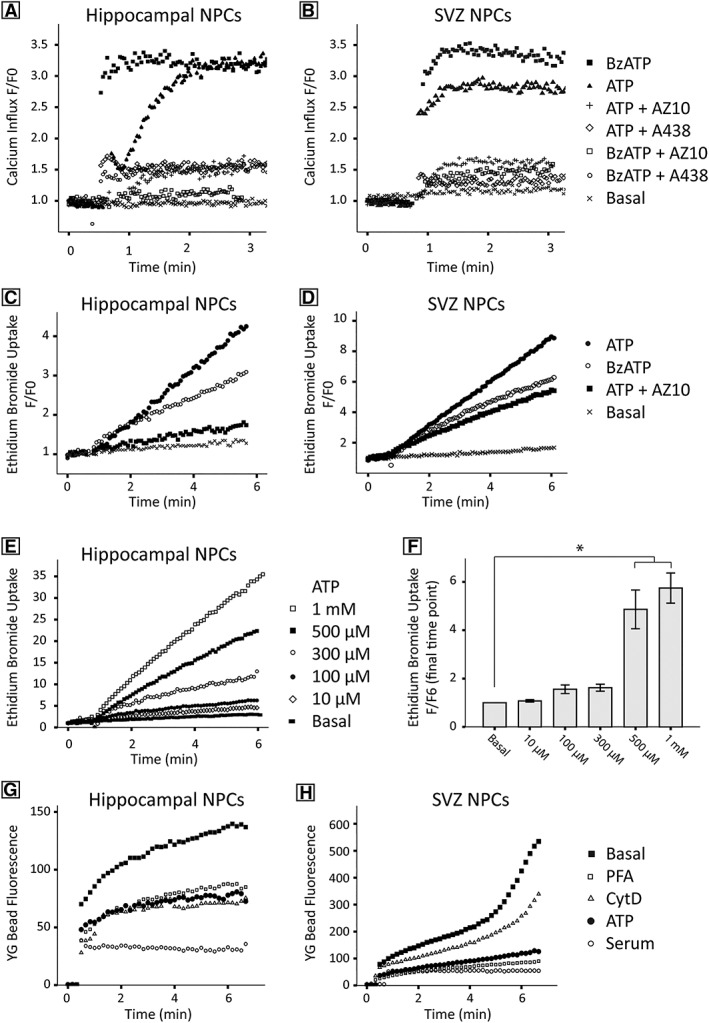
Hippocampal and SVZ derived NPCs express functional P2X7 receptors. P2X7 receptor calcium channel function was demonstrated in hippocampal (A) and SVZ (B) derived NPCs by a change in relative Fluo‐8 fluorescence over time following application of the general P2X agonist ATP and the P2X7 agonist BzATP. Using real‐time flow cytometry, both agonists were observed to result in calcium influx that could be blocked with the P2X7‐specific inhibitors AZ10606120 (AZ10) and A438079 (A438). P2X7 transmembrane pore formation was demonstrated by ethidium bromide uptake for both hippocampal (C) and SVZ (D) derived NPC, and was assessed by real‐time flow cytometry. ATP and BzATP elicited P2X7 receptor pore formation, allowing ethidium bromide uptake, and the P2X7 inhibitor AZ10606120 attenuated this phenomenon. ATP dose response assays (E) demonstrated significant pore formation at 500 μM and 1 mM (F; N = 8). Flow cytometry was also used to assess the involvement of P2X7 receptors in phagocytosis. YG bead uptake in both hippocampal (G) and SVZ (H) derived NPCs was measured, and phagocytosis was notably higher in SVZ derived NPCs. Application of ATP blocked P2X7 mediated phagocytosis in NPCs derived from both niches, and similar findings were produced when using nonspecific inhibitors of phagocytosis included PFA, cytochalasin D (CytD), whereas 5% serum abolished all bead uptake (N > 3).
Time‐resolved flow cytometry was also used to assess the pore‐forming capabilities of P2X7 receptors in adult NPCs. Ethidium bromide was used to measure pore formation and is excluded from cells lacking the large transmembrane pore. Application of the agonists ATP and BzATP evoked pore formation in both hippocampal (Fig. 3C) and SVZ (Fig. 3D) derived NPCs. This effect was attenuated by the P2X7 specific inhibitor AZ10606120. Dose‐response assays (Fig. 3E) demonstrated that pore formation occurred in response to concentrations of 500 μM ATP and above (quantified in Fig. 3F; N = 4 each of SVZ and SGZ (Welch's F[5,27] = 15.6, p = .002, partial η2 = 0.80). The ethidium bromide uptake assays demonstrate the expression of functional P2X7 with a full‐length C‐terminus, as opposed to splice variants lacking the complete C‐terminus, which are unable to associate with the membrane cytoskeleton and do not form transmembrane pores 33.
P2X7 Receptors Facilitate Phagocytosis by Adult NPCs within the Neurogenic Niches
In the absence of ATP, P2X7 can also function as a scavenger receptor to facilitate phagocytosis 18, 19, 21, 34. The carboxyl terminus of P2X7 is associated with the heavy chain of nonmuscle myosin IIA (NMMHC‐IIA); it is the activity of this motor protein that enables the cytoskeletal rearrangements required for phagosome formation and particle engulfment. Our group has previously demonstrated that activation of P2X7 by extracellular ATP results in conformational changes which open the selective cation channel/pore and also dissociate the P2X7 C‐terminus from the NMMHC‐IIA in the underlying actin cytoskeleton, thereby abolishing its phagocytic function 9, 20, 21.
To determine the potential of adult NPCs to phagocytose, and if P2X7 can facilitate this process, we used live cell flow cytometry, as well as live cell and confocal microscopy, to measure uptake of fluorescent YG latex beads. NPCs from the hippocampus (Fig. 3G) and SVZ (Fig. 3H, N = 2) were observed to phagocytose beads over a time period of 6 minutes. NPCs derived from the SVZ displayed a greater capacity to phagocytose than that observed for hippocampal cultures. Preincubation with ATP inhibited phagocytosis of YG beads to the same extent as PFA and actin polymerization inhibitor Cytochalasin D, whereas 5% serum completely abolished all innate phagocytosis. These data strongly suggest P2X7 receptors can play a role in facilitating phagocytosis by adult NPCs within the neurogenic niches.
To confirm the intracellular localization of phagocytosed beads, hippocampal NPC cultures were stained with LysoTracker (red) to identify lysosomes and incubated overnight with YG beads (green). Live cell imaging captured NPCs phagocytosing YG beads in real‐time, showing internalization, followed by docking with lysosomes and trafficking inside the cell (Fig. 4A, 4B, frame rate is 5 minutes). Engulfed beads could be identified as yellow (indicated by white arrowheads and indicative of co‐localization in lysosomes) whereas non‐engulfed beads were green. The total percentage of NPCs containing beads was 62 ± 3.3% with an average of 4.5 ± 0.3 beads per cell (n = 491). Orthogonal reconstruction using confocal microscopy further exemplified intracellular localization of YG beads in NPCs stained red with CellTracker (Fig. 4C, planes of projection indicated by yellow cross hairs). NPC cultures were probed for microglial marker Iba1 to confirm the absence of professional phagocytes. No Iba1+ cells were observed in NPC cultures (Fig. 4D, 4E), whereas Iba1+ microglia is shown in Figure 4F as a positive control.
Figure 4.

Intracellular localisation of YG beads in adult NPCs was confirmed by microscopy. Representative cultured hippocampal NPCs loaded with LysoTracker red were observed to phagocytose 1 μm YG beads (green) using live‐cell microscopy (A–B, frame rate 5 minutes). White arrow heads indicate beads being engulfed by the cell and trafficked inside lysosomes, and green beads appear yellow once incorporated in red lysosomes. Orthogonal reconstruction of z‐stack confocal microscopy (C) demonstrated the cytosolic location of YG beads using NPCs stained with CellTracker red. Yellow cross hairs indicate the plane of projection. NPC cultures were negative for microglia, as determined by negative Iba1 immunochemistry (D–E; positive Iba1 control in microglial culture F). Scale bars represent 5 μm.
Activation of Hippocampal and SVZ P2X7 Receptors Negatively Regulates Proliferation of NPCs: Evidence from Inhibition and Knock‐Out Studies
The effects of purinergic signaling on NPC proliferation were investigated using EdU incorporation to identify proliferative cells, with DAPI used as a nuclear counterstain. A negative dose‐dependent correlation was demonstrated between increasing exogenous ATP concentrations and percentage of total EdU+ proliferating NPCs (Fig. 5A) with a large effect size (Welch's F[9,236] = 125.0, p < .001, partial η2 = 0.70, N = 6). Post hoc analysis revealed the maximal response to be observed at 100 μM with a decrease of 24.9 ± 2.7% (p < 0.001). A small reversal of the decreasing trend in proliferation was observed at 500 μM, possibly due to the recruitment of multiple purinergic signaling pathways. Purines ADP, UTP, and UDP (100 μM) were tested in comparison to ATP, to gauge the possible involvement of multiple purinergic receptors in regulating proliferation (Fig. 5B). Application of ADP also resulted in a decrease (23.6 ± 2.8%, p < .001) in NPC proliferation, though UTP and UDP showed no effect, indicating other ionotropic or metabotropic purinergic receptors may be involved. Cell death was not observed upon application of ATP (100 μM) using live cell microscopy (Supporting Information Movie S1) and recovery assays demonstrated NPC proliferation returned to basal level following ATP washout and overnight recovery, indicating the reduction in proliferation was not due to activation of irreversible apoptotic or necrotic pathways (Fig. 5C).
Figure 5.

P2X7 receptor signaling decreases adult NPC proliferation. EdU labeling was used to gauge effects of purinergic signaling on proliferation in representative hippocampal NPC cultures. Increasing concentrations of extracellular ATP were applied overnight and a dose‐dependent decrease in proliferation was observed between 10 and 500 μM, where * indicates p < .001 (A). ADP demonstrated a similar effect as ATP, whereas UTP and UDP did not impact proliferation, * p < .001 (B). The decrease in proliferation caused by extracellular ATP was rescued upon washout and overnight recovery and indicated loss of proliferation was not due to cell death, * p < .001 (C). P2X7 agonist BzATP also demonstrated a dose‐dependent decrease in proliferation up to 500 μM, * indicates p = .002, whereas ** indicates p < .001 (D). Preincubating NPCs with P2X7 inhibitor A438079 prior to ATP application had a protective effect against an ATP‐induced reduction in proliferation, * p = .001 and ** p < .001 (E). NPC cultures generated from P2X7−/− (KO) mice were confirmed by PCR to lack P2X7 receptors, instead of demonstrating the insertion of the neomycin resistance gene utilized in the creation of the KO mouse line (F). Increasing concentrations of ATP applied to hippocampal KO cultures did not result in as significant a decrease in proliferation as did the wild‐type cultures (G), confirming our observations using the inhibitor, * p < .001. ATP treatment of WT NPC cultures over 3 days resulted in an increase of DCX positive cells, * p < .001 (H).
To determine any specific involvement of P2X7 signaling in the regulation of NPC proliferation, P2X7 agonists and antagonists were utilized. BzATP application (Fig. 5D) also produced a significant negative dose–response relationship [F(7,143) = 130.1, p < .001, partial η2 = 0.86, N = 4]. The negative effects of ATP on the proliferative potential of NPCs were significantly blocked by preincubation with the P2X7 antagonist A438079 (10 and 25 μM) prior to the application of ATP, Fig. 5E (Welch's F[5,108] = 85.5, p < .001, partial η2 = 0.68). Finally, hippocampal NPC cultures generated from knock‐out mice (P2X7−/−) were also utilized to confirm P2X7 receptor involvement in proliferation and were confirmed to be lacking P2X7 receptors using PCR (Fig. 5F). P2X7−/− hippocampal NPC cultures had significantly higher proliferation rates when cultured in the presence of ATP, compared with the wild‐type NPCs (Fig. 5G [Welch's F(9,298) = 182.9, p < .001, partial η2 = 0.68]). Similar results were observed for SVZ P2X7−/− cultures (Supporting Information Fig. S3; [Welch's F(3,125) = 189.2, p < 0.001, partial η2 = 0.75]). P2X7−/− NPC culture proliferation was still negatively impacted by exogenous ATP application, indicating the involvement of other purinergic receptors in modulating proliferation and aligning with data presented in Figure 5B.
Purinergic Signaling in Adult NPCs Can Influence Neuronal Differentiation
P2X7 receptors have previously been shown to regulate differentiation of embryonic NPCs 13. We investigated if purinergic signaling in adult NPCs could also influence neuronal differentiation. Lineage investigations following application of ATP over a 3‐day period induced a small but significant increase in the number of DCX+ neuroblasts generated in wild‐type hippocampal NPC cultures (Welch's F[1,78] = 38.3, p < .001, partial η2 = 0.33, n = 41,389) correlating with decreased proliferation (Fig. 5H).
ATP Treatment Negatively Regulates NPC Proliferation without Increasing Cell Death
Application of ATP at 100 μM overnight decreased cell proliferation without significant effects on cell death (N = 10). NPCs were treated with ATP (100 μM), BzATP (100 μM), and staurosporine (0.2 μM), and were assessed by flow cytometry for initiation of apoptosis, as detected by Annexin V binding. Viability marker 7‐AAD was used to distinguish early apoptosis (7‐AAD negative) from late apoptosis or nonviable cells (7‐AAD positive). Cell populations were gated (Fig. 6A) and Annexin V fluorescence was plotted against 7‐AAD, to identify region 1 (R1; Annexin V positive, 7‐AAD positive) and region 2 (R2; Annexin V positive, 7‐AAD negative). Staurosporine significantly increased apoptosis, but not cell death, and increased Annexin V binding above control, whereas ATP and BzATP remained unchanged (Fig. 6B). Importantly, there was no significant difference between control and ATP or BzATP in the percent of late apoptotic or nonviable cells (R1; [Welch's F(2,27) = 1.85, p = .20]; Fig. 6C) or early apoptotic cells (R2; [F(2,27) = 1.57, p = .23]; Fig. 6D).
Figure 6.
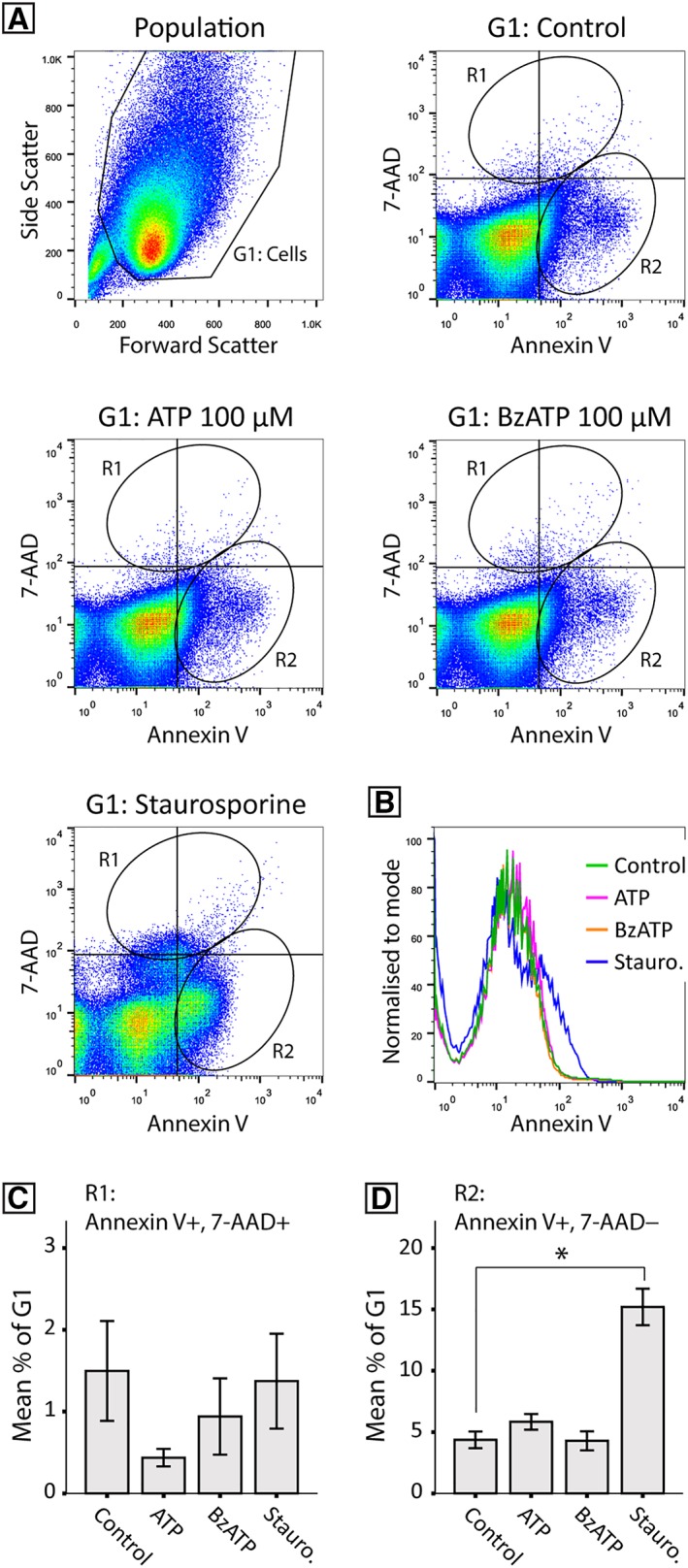
ATP does not cause significant cell death at 100 μM. Flow cytometry was used to determine if ATP‐induced decreases in proliferation were resulting from undetected cell death. NPCs were cultured overnight with ATP (100 μM), BzATP (100 μM), and staurosporine (0.2 μM). NPCs were harvested and assessed for Annexin V binding. 7‐AAD was used as a viability marker. The NPC population (A) was gated (G1) and 7‐AAD fluorescence was plotted against Annexin V fluorescence. Region 1 (R1) gated 7‐AAD positive Annexin positive cells and represents late apoptotic and nonviable cells. Region 2 (R2) gated 7‐AAD negative Annexin positive cells and represents cells in the early stages of apoptosis. Histogram of Annexin V fluorescence (B) shows an increase in Annexin V binding following staurosporine treatment, but no difference following ATP or BzATP treatment. Quantification of the mean percentage of G1 for regions 1 and 2 (C and D respectively; N = 10) found no effect of ATP or BzATP treatment (p = .196 and p = .227 for R1 and R2 respectively), whereas staurosporine‐induced early apoptosis (D, * indicates p = .018), but not late apoptosis (C, p = 1.00).
Discussion
High concentrations of extracellular ATP are a hallmark of neuroinflammation resulting from various ischemic and neurodegenerative disorders 8, 35. Our studies demonstrated P2X7 receptor signaling may have significant effects on adult neurogenesis, particularly in the aftermath of inflammatory events. Using immunohistochemistry, we found EdU+ cells were evident in both adult neurogenic niches and displayed positive immunoreactivity against P2X7 receptors. P2X7 was mostly observed on the membrane or within the cytoplasm of NPCs but was also detected at the nucleus of some MASH1+/EdU+ dividing precursors (Fig. 1D–1G). This nuclear staining was similar to observations made of mouse retinal ganglion cells 29 and is congruent with our work on postmortem human brain tissue where P2X7 can be detected on the nuclear envelope of microglia and astrocytes, but not mature neurons (unpublished observations). In vitro cultures derived from these niches were characterized as intermediate neural progenitors (type 2 from the SGZ of the dentate gyrus and type C from the SVZ), and were also P2X7 receptor immunoreactive.
Functional analysis by calcium influx and ethidium bromide uptake utilized the P2X7 agonist BzATP and the specific P2X7 inhibitor AZ10606120 and found that P2X7 receptors expressed in NPC cultures were able to form both cation‐selective channels and transmembrane pores. This confirmed the presence of a full‐length functional receptor, as opposed to the Δ‐C splice variant displaying a deleted C‐terminus that is also present in the brain 33. Expression of multiple variants may account for a number of observational discrepancies in previous studies, such as the absence of pore functionality in embryonic NPCs 36. Our data aligns with a study by Messemer and colleagues, who used patch clamping to report functional P2X7 in SVZ NPCs 16.
Numerous studies report the existence of P2X7 receptors in mature neurons 37, although this remains debated 38. Currently, however, evidence of receptor presence in adult hippocampal NPCs is limited to reports of immunochemistry and membrane current recordings 39, 40. Our study confirms and builds on these observations. Our functional investigations demonstrate P2X7 receptors could subserve a number of physiological functions in the NPCs within both the hippocampal and subventricular neurogenic niches, depending on the concentrations of extracellular ATP. Through its function as a calcium channel, P2X7 signaling may govern the size of the adult progenitor pools by negatively regulating cell proliferation, whereas retaining its canonical role as a death receptor via the formation of transmembrane pores. P2X7 receptors may also contribute to niche maintenance by facilitating phagocytic clearance of apoptotic cell bodies. Figure 7 is a schematic representation of the roles subserved by P2X7 based on findings from the present study as well as earlier investigators 6, 9, 13, 15, 16, 21. The presence of P2X7 receptors within the adult neurogenic niches is of importance given the therapeutic potential of some antagonists following ischemic injury 41, 42, 43.
Figure 7.
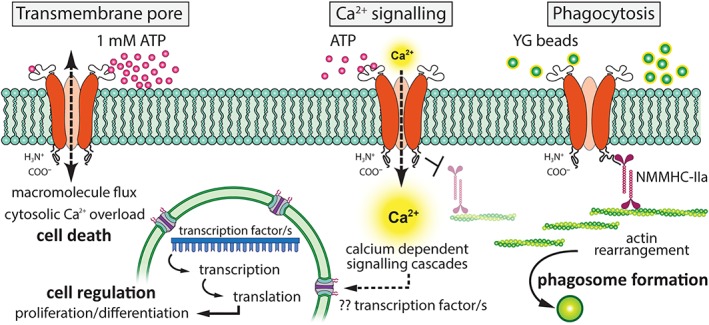
Diagrammatic depiction of the three signaling roles of P2X7 receptors in adult NPCs identified in this study. P2X7 receptors can play at least three main roles in adult NPCs, the first of these being the formation of a large transmembrane pore, which has significant implications following inflammatory events. The transmembrane pore allows macromolecule exchange and an overload in cytosolic calcium concentrations, resulting in cell death. The second role of P2X7 is as a cation channel. The influx of calcium in response to ATP signaling can activate downstream calcium‐dependent signaling cascades and result in possible transcription factor activation to regulate proliferation and differentiation. The third function of P2X7 receptors is that of phagocytosis. P2X7 receptors can act as a scavenger receptor in the absence of their agonist, and likely engulf particles by modulating the actin cytoskeleton by the C‐terminus association with NMMHC‐IIa. Activation of the receptor by agonist binding dissociates NMMHC‐IIa and inhibits phagocytosis 21.
P2X7 Receptors Facilitate Phagocytosis by Adult NPCs in the Absence of ATP
During neurogenesis, cell proliferation is balanced by large amounts of cell death both in the niche (target‐independent PCD) and at the site of neural integration (target‐dependent PCD), allowing for the refinement of new neurons and synapses 44. These apoptotic cells must be removed to prevent buildup and inflammation. Both professional phagocytes (microglia) and nonprofessional phagocytes contribute to this process; resident NPCs and neuroblasts have been demonstrated to engulf the debris of neighboring apoptotic cells via innate phagocytosis. In 2011, Lu and colleagues demonstrated DCX positive adult NPCs to have a phagocytic role within both the hippocampal and subventricular neurogenic niches 22. Recently, human neuroblasts with high expression of both DCX and P2X7 had the greatest phagocytic capability of developing CNS cells, and DCXlow NPCs a lesser capability 6. P2X7 receptors were identified as the scavenger receptor responsible for phagocytosis of beads, apoptotic neuroblasts, and apoptotic ReN line neural stem cells 6. Traditionally, the role of phagocyte belonged exclusively to microglia, generally requiring “activation” by an inflammatory stimuli 45. The presence of scavenger receptors on nonprofessional phagocytes, such as NPCs and astrocytes alleviates, the responsibility of niche maintenance from microglia alone, and allows the clearance of debris without activation of resident microglia.
In the present study, we also demonstrate that adult NPCs derived from both the hippocampus and SVZ were capable of phagocytosis via P2X7 receptors, despite low levels of DCX expression. NPCs isolated from the SVZ had greater basal rates of proliferation than those derived from the SGZ; this potentially reflects the need for the greater phagocytic capacity that was also observed in the SVZ, to remove cell corpses generated by the higher basal proliferation rates. Phagocytosis was inhibited by the presence of ATP or serum as previously observed in human NPCs 6. This is of physiological significance as though P2X7 receptors are best known for their roles in inflammation, under normal conditions ATP levels in the cerebrospinal fluid are between 8 and 16 nM 46. Even in the event of astrocyte stimulation, which can result in ATP release, luciferase probe assays report values no higher than 80 nM 47. Given that micromolar concentrations of extracellular ATP are required to activate the P2X7 receptor 15 it is unlikely that ATP could reach the required concentrations except under inflammatory or pathological conditions such as trauma or infection, where high levels of ATP can result in further damage and hinder innate repair mechanisms. This permits a potential role for P2X7 in adult NPCs under normal physiological conditions, which is to facilitate the phagocytosis of apoptotic progenitors in the neurogenic niches. Although other scavenger receptors, for example MerTK, have been identified within SVZ cells, MerTK blocking antibody did not completely abrogate phagocytosis 48. This suggests that scavenger receptors like P2X7 could operate cooperatively within the SVZ microenvironment. Furthermore, it is possible that MerTK actively removes apoptotic cells in inflammatory environments where high extracellular ATP levels do not allow P2X7 to operate as a scavenger receptor.
P2X7 Receptor Regulation of NPCs in the Presence of High Extracellular ATP: Relevance to Neuroinflammation
P2X7 function in ischemic brain injury, epilepsy, and stroke has recently gained momentum in the light of a number of studies demonstrating conferral of neuroprotection by modulation of P2X7 activity, alleviating the detrimental effects of excess extracellular ATP 11, 42, 49. Antagonism of P2X7 receptors assisted functional recovery in spinal cord injury, possibly by decreasing secondary cell death caused by excessive ATP release, and was also shown to decrease pro‐inflammatory mediators and increase neuronal survival rates in the striatum 50, 51. Supporting this observation, status epilepticus (prolonged seizures) increased levels of P2X7 in the granule neurons of the dentate gyrus, and antagonizing the receptor reduced both seizure duration and subsequent neuronal death 52. P2X7 receptors have also been associated with numerous neurodegenerative diseases including Alzheimer's disease 53, 54, Multiple Sclerosis 55, 56, age‐related macular degeneration 57, and were shown to be significantly increased in the hippocampus of rats suffering from cognitive dysfunction 58. Subsequently, the neuroprotective effects of P2X7 antagonists have led to drug candidate studies 42, 43. Given the potential P2X7 receptors hold as a therapeutic target, their function in SGZ‐ and SVZ‐derived NPCs was confirmed. We demonstrated adult NPCs exposed to high ATP concentrations (500 μM and 1 mM) formed P2X7‐mediated transmembrane pores. This was reduced by preincubation with the specific P2X7 inhibitor AZ10606120 and highlights the vulnerability of adult NPCs to cell death following neuro‐inflammatory events.
P2X7 Receptor as a Negative Regulator of Proliferation in SVZ and Hippocampal: Evidence from ATP or BzATP Stimulation
ATP has pleiotropic physiological effects and its actions may converge at multiple purinergic receptors to derive an overall phenotype. Recently, ATP release from hippocampal astrocytes has been shown to positively regulate neural stem cell proliferation 59, however this occurs via P2Y1 receptors. There currently exists a lack of consensus in the literature as to the nature of P2X7 involvement in the regulation of proliferation and differentiation. P2X7 signaling was found to maintain survival in mouse embryonic stem cells 60, as well as proliferation in neuroblastoma cells 61, 62 and glial cells 63. Alternatively, ATP and BzATP application was observed to decrease proliferation in murine luteal cells, via P2X7 modulation of the p38 MAP kinase pathway, and this occurred without inducing apoptosis 64. In embryonic NPCs, BzATP application also decreased cell counts and BrdU+ cell numbers and enhanced neuronal differentiation 12. P2X7 inhibition in embryonic NPCs was further demonstrated to increase expression of the proliferation marker Ki‐67 13. These differences may be attributed to agonist concentration or exposure times, as well as P2X7 expression levels or the presence of various splice variants. Cell type and function may also contribute to differences, for example, proliferation in response to inflammation is a general function of glial cell populations 65. In the current study, the application of exogenous ATP and BzATP decreased proliferation and preincubation with P2X7 inhibitor A438079 partially attenuated the effects of ATP on proliferation. P2X7−/− cultures treated with ATP did not demonstrate as substantial a reduction in proliferation as was observed in the wild‐type cultures. This suggests P2X7 receptors can contribute to negative regulation of adult NPC proliferation in the neurogenic niches, particularly in the context of neuroinflammation, and align with findings by Tsao et al. 12.
It is of note that ATP application still resulted in a decrease in proliferation in NPCs derived from P2X7−/− mice. This in combination with the finding that ADP also decreased proliferation in wild‐type cultures, as well as ATP having a greater potency than BzATP at inhibiting proliferation, indicates that P2X7 receptors are not the only purinergic receptor present on the surface of adult NPCs that may exert regulatory effects over proliferation. A decrease in NPC proliferation following an inflammatory event may have severe implications in terms of adult neurogenesis, particularly in the hippocampus where new neurons are vital for memory formation 3. Decreased hippocampal neurogenesis is associated with impaired memory, as well as major depression 66, whereas rodents provided with an enriched environment and/or exercise consistently show increased rates of neurogenesis and improved learning and memory performance 67, 68, 69, 70.
In addition, we reported an increase in DCX positive NPCs following ATP application over a 3‐day period. This aligns with findings that long‐term BzATP application increased TUJ1 and MAP2 expression via P2X7 activation 12. This may be a physiological response of NPCs to a cell death event, where neighboring cells (who might only experience a decrease in proliferation) initiate lineage elaboration pathways as a compensatory mechanism to replace those cells lost. Alternatively, maturation may be a default state for NPCs that cease to proliferate. As our studies were undertaken in vitro in the absence of surrounding cells in the intact niche, further work could explore if NPC counterparts in the intact niche behave similarly as reported in this study.
Conclusion
Our data shows P2X7 receptors have the ability to subserve at least three distinct physiological roles in NPCs derived from the adult neurogenic niches, depending on extracellular ATP conditions in vitro. The first, in the presence of low to moderate levels of ATP, results in calcium influx and a decrease in NPC proliferation. The second function of P2X7 receptors is cell death via transmembrane pore formation in response to the high concentrations of ATP that may be present during an inflammatory event. The third role for P2X7 receptors in the absence of ATP is phagocytosis, which may contribute to the maintenance of the neurogenic niche by removal of apoptotic bodies. Our study demonstrates the complex roles of P2X7 receptors in adult hippocampal and subventricular NPCs and is an important step towards understanding how inflammation may regulate adult neurogenesis.
Author Contributions
H.L.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; M. K.: collection and assembly of data, final approval of manuscript; T.C.‐L.: provision of study materials, manuscript writing, final approval of manuscript; M.L.: manuscript writing, final approval of manuscript; J.B.: manuscript writing, final approval of manuscript; K.T.: collection of data, final approval of manuscript; B.G.: financial support, provision of study materials, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; M.W.: conception and design, financial support, administrative support, provision of study materials, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
T.C.‐L. declared advisory role with Eyeco. The other authors indicated no potential conflicts of interest.
Supporting information
Supplementary Figure 1. Additional characterization shows SVZ NPCs have greater proliferation and adult NPCs retain pluripotency in culture. NPCs were identified in in vivo sections of the subventricular zone by EdU incorporation to label actively dividing cells. The SVZ contained a greater population of dividing NPCs (A‐C) than the dentate gyrus, this was quantified by EdU incorporation, * p = 0.007 (D). When cultured in proliferation medium for three DIV, representative hippocampal NPC cultures contained less than 0.1% DCX+ neuroblasts (E‐G). Morphological changes were observed in NPC maintained in differentiation medium for seven DIV; processes became elongated and ramified and the soma more compact (H‐S). Differentiated cultures expressed immature neuron marker βIII tubulin (H‐J) and the mature neuron markers MAP2a/b (K‐M) and NeuN (N‐P). Differentiated cultures also contained astrocytic progenitor cells identified by GFAP+/vimentin− as indicated by the numeral 1 (Q‐S). An example of co‐expression is numbered 2, while a vimentin+/GFAP− stain indicates a more neuronal phenotype and is numbered 3. Oligodendrocyte precursor cells were stained for O4 in hippocampal and SVZ cultures (T, U respectively). Hippocampal cultures were negative whereas SVZ cultures were 2.6 ± 0.86% positive. Secondary controls (V) demonstrated no non‐specific binding for Alexa‐488, Cy3, or Cy5. Scale bars represent 10 μm.
Supplementary Figure 2. P2X7 receptor expression in spheroid cultures was confirmed by immunochemistry and Western blot. P2X7 receptor expression in the neurosphere culture was confirmed by immunochemical analysis of cryosectioned spheres (A). Scale bar represents 20 μm. Antibody observations were confirmed by Western blot, which identified a band at approximately 85 kDa, consistent with the glycosolated form of P2X7 (B).
Supplementary Figure 3. ATP induced calcium influx in NPCs. NPCs were loaded with Fluo‐8 AM calcium indicator dye and calcium channel opening was assessed using live cell microscopy. Application of ATP evoked calcium influx (A‐H, frame rate 0.5 seconds) in a dose‐dependent manner. Scale bar represents 50 μm. Between 30 and 50 regions of interest were selected at random and the maximum cytosolic calcium concentrations (F/F0) from three biological repeats were quantified (I). A Welch ANOVA determined significance at concentrations ≥ 0.1 μM (Welch's F[8, 650] = 455.00, p < 0.01), error bars ±1 SE, * p < 0.01.
Supplementary Figure 4. SVZ NPCs derived from P2X7 −/− mice had a reduced response to the inhibitory effects of ATP on proliferation. EdU incorporation was used to measure the effect of purinergic signaling on proliferation in P2X7 KO compared with WT NPC cultures from the SVZ. ATP applied to KO cultures did not result in as significant a decrease in proliferation as did the WT cultures, *p < 0.001.
Supplementary Movie 1. Live cell microscopy recording of NPCs following the addition of 100 μM ATP. NPCs were plated on glass and cultured for 24 hours with or without ATP. Images were captured every 10 minutes and examined for signs of cell stress and death. Live cell recordings showed NPCs exposed to 100 μM ATP continued to thrive in culture similar to control.
Acknowledgments
This work was supported by grants from the Rebecca L. Cooper Medical Research Foundation to M.W., T.C.L., and M.L., and to T.C.L. from the National Health and Medical Research Council (NHMRC) of Australia (571100 and 1048082) and the Baxter Charitable Foundation (Sydney, Australia). B.G. was supported by ARC Future Fellowship (BG, FT120100581), NHMRC Project Grants (1048082, 1061419, and 1120095) and the Victorian Government's Operational Infrastructure Support Grant to the Florey Institute. M.L. was supported by a Charles D. Kelman, M.D. Postdoctoral Award (2010) from the International Retinal Research Foundation (USA). Microglial cultures were kindly donated by the Clem Jones Centre for Neurobiology and Stem Cell Research, Brisbane Australia. The authors thank Maria Nguyen (Griffith Institute for Drug Discovery) for advice regarding microscopy, and Dr Xin Huang (Florey Institute of Neuroscience and Mental Health) for assistance with flow cytometry experiments.
Contributor Information
Ben J. Gu, Email: ben.gu@florey.edu.au.
Michael W. Weible, II, Email: m.weible@griffith.edu.au.
REFERENCES
- 1. Ming GL, Song HJ. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011;70:687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Praag H, Schinder AF, Christie BR et al. Functional neurogenesis in the adult hippocampus. Nature 2002;415:1030–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gu Y, Arruda‐Carvalho M, Wang J et al. Optical controlling reveals time‐dependent roles for adult‐born dentate granule cells. Nat Neurosci 2012;15:1700–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ming GL, Song HJ. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 2005;28:223–250. [DOI] [PubMed] [Google Scholar]
- 5. Sierra A, Encinas JM, Deudero JJ et al. Microglia shape adult hippocampal neurogenesis through apoptosis‐coupled phagocytosis. Cell Stem Cell 2010;7:483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lovelace MD, Gu BJ, Eamegdool SS et al. P2X7 receptors mediate innate phagocytosis by human neural precursor cells and neuroblasts. Stem Cells 2015;33:526–541. [DOI] [PubMed] [Google Scholar]
- 7. Ulrich H, Abbracchio MP, Burnstock G. Extrinsic purinergic regulation of neural stem/progenitor cells: Implications for CNS development and repair. Stem Cell Rev 2012;8:755–767. [DOI] [PubMed] [Google Scholar]
- 8. Burnstock G. An introduction to the roles of purinergic signalling in neurodegeneration, neuroprotection and neuroregeneration. Neuropharmacology 2016;104:4–17. [DOI] [PubMed] [Google Scholar]
- 9. Gu BJ, Lovelace MD, Weible MW 2nd et al. P2X7 is an archaic scavenger receptor recognizing apoptotic neuroblasts in early human neurogenesis. Receptor Clin Invest 2015;2:e699. [Google Scholar]
- 10. Solle M, Labasi J, Perregaux DG et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem 2001;276:125–132. [DOI] [PubMed] [Google Scholar]
- 11. Skaper SD, Debetto P, Giusti P. The P2X7 purinergic receptor: From physiology to neurological disorders. FASEB J 2010;24:337–345. [DOI] [PubMed] [Google Scholar]
- 12. Tsao HK, Chiu PH, Sun SH. PKC‐dependent ERK phosphorylation is essential for P2X7 receptor‐mediated neuronal differentiation of neural progenitor cells. Cell Death Dis 2013;4:e751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Glaser T, de Oliveira SL, Cheffer A et al. Modulation of mouse embryonic stem cell proliferation and neural differentiation by the P2X7 receptor. PLoS One 2014;9:e96281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diaz‐Hernandez M, del Puerto A, Diaz‐Hernandez JI et al. Inhibition of the ATP‐gated P2X7 receptor promotes axonal growth and branching in cultured hippocampal neurons. J Cell Sci 2008;121:3717–3728. [DOI] [PubMed] [Google Scholar]
- 15. Surprenant A, Rassendren F, Kawashima E et al. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 1996;272:735–738. [DOI] [PubMed] [Google Scholar]
- 16. Messemer N, Kunert C, Grohmann M et al. P2X7 receptors at adult neural progenitor cells of the mouse subventricular zone. Neuropharmacology 2013;73:122–137. [DOI] [PubMed] [Google Scholar]
- 17. Domercq M, Perez‐Samartin A, Aparicio D et al. P2X7 receptors mediate ischemic damage to oligodendrocytes. Glia 2010;58:730–740. [DOI] [PubMed] [Google Scholar]
- 18. Gu BJ, Saunders BM, Petrou S et al. P2X(7) is a scavenger receptor for apoptotic cells in the absence of its ligand, extracellular ATP. J Immunol (Baltimore, MD:1950) 2011;187:2365–2375. [DOI] [PubMed] [Google Scholar]
- 19. Yamamoto M, Kamatsuka Y, Ohishi A et al. P2X7 receptors regulate engulfing activity of non‐stimulated resting astrocytes. Biochem Biophys Res Commun 2013;439:90–95. [DOI] [PubMed] [Google Scholar]
- 20. Gu BJ, Rathsam C, Stokes L et al. Extracellular ATP dissociates nonmuscle myosin from P2X(7) complex: This dissociation regulates P2X(7) pore formation. Am J Physiol Cell Physiol 2009;297:C430–C439. [DOI] [PubMed] [Google Scholar]
- 21. Gu BJ, Saunders BM, Jursik C et al. The P2X7‐nonmuscle myosin membrane complex regulates phagocytosis of nonopsonized particles and bacteria by a pathway attenuated by extracellular ATP. Blood 2010;115:1621–1631. [DOI] [PubMed] [Google Scholar]
- 22. Lu Z, Elliott MR, Chen Y et al. Phagocytic activity of neuronal progenitors regulates adult neurogenesis. Nat Cell Biol 2011;13:1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weible MW 2nd, Chan‐Ling T. Phenotypic characterization of neural stem cells from human fetal spinal cord: Synergistic effect of LIF and BMP4 to generate astrocytes. Glia 2007;55:1156–1168. [DOI] [PubMed] [Google Scholar]
- 24. Walker TL, Kempermann G. One mouse, two cultures: Isolation and culture of adult neural stem cells from the two neurogenic zones of individual mice. J Vis Exp 2014;84:e51225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chehrehasa F, Meedeniya AC, Dwyer P et al. EdU, a new thymidine analogue for labelling proliferating cells in the nervous system. J Neurosci Methods 2009;177:122–130. [DOI] [PubMed] [Google Scholar]
- 26. Levine TR, Hullett CR. Eta squared, partial eta squared, and misreporting of effect size in communication research. Hum Commun Res 2002;28:612–625. [Google Scholar]
- 27. Kim EJ, Ables JL, Dickel LK et al. Ascl1 (Mash1) defines cells with long‐term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PLoS One 2011;6:e18472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gu BJ, Zhang WY, Bendall LJ et al. Expression of P2X(7) purinoceptors on human lymphocytes and monocytes: Evidence for nonfunctional P2X(7) receptors. Am J Physiol Cell Physiol 2000;279:C1189–C1197. [DOI] [PubMed] [Google Scholar]
- 29. Vessey KA, Fletcher EL. Rod and cone pathway signalling is altered in the P2X7 receptor knock out mouse. PLoS One. 2012;7:e29990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Genzen JR, Platel JC, Rubio ME et al. Ependymal cells along the lateral ventricle express functional P2X(7) receptors. Purinergic Signal 2009;5:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kempermann G, Jessberger S, Steiner B et al. Milestones of neuronal development in the adult hippocampus. Trends Neurosci 2004;27:447–452. [DOI] [PubMed] [Google Scholar]
- 32. Zhang J, Jiao J. Molecular biomarkers for embryonic and adult neural stem cell and neurogenesis. Biomed Res Int 2015;2015:727542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheewatrakoolpong B, Gilchrest H, Anthes JC et al. Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem Biophys Res Commun 2005;332:17–27. [DOI] [PubMed] [Google Scholar]
- 34. Ousingsawat J, Wanitchakool P, Kmit A et al. Anoctamin 6 mediates effects essential for innate immunity downstream of P2X7 receptors in macrophages. Nat Commun 2015;6:6245. [DOI] [PubMed] [Google Scholar]
- 35. Abbracchio MP, Burnstock G, Verkhratsky A et al. Purinergic signalling in the nervous system: An overview. Trends Neurosci 2009;32:19–29. [DOI] [PubMed] [Google Scholar]
- 36. Delarasse C, Gonnord P, Galante M et al. Neural progenitor cell death is induced by extracellular ATP via ligation of P2X7 receptor. J Neurochem 2009;109:846–857. [DOI] [PubMed] [Google Scholar]
- 37. Miras‐Portugal MT, Sebastian‐Serrano A, de Diego GL et al. Neuronal P2X7 receptor: Involvement in neuronal physiology and pathology. J Neurosci 2017;37:7063–7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Illes P, Khan TM, Rubini P. Neuronal P2X7 receptors revisited: Do they really exist? J Neurosci 2017;37:7049–7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hogg RC, Chipperfield H, Whyte KA et al. Functional maturation of isolated neural progenitor cells from the adult rat hippocampus. Eur J Neurosci 2004;19:2410–2420. [DOI] [PubMed] [Google Scholar]
- 40. Rozmer K, Gao P, Araujo MGL et al. Pilocarpine‐induced status epilepticus increases the sensitivity of P2X7 and P2Y1 receptors to nucleotides at neural progenitor cells of the juvenile rodent hippocampus. Cereb Cortex 2017;27:3568–3585. [DOI] [PubMed] [Google Scholar]
- 41. Hu F, Xing F, Zhu G et al. Rhein antagonizes P2X7 receptor in rat peritoneal macrophages. Sci Rep 2015;5:14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sperlagh B, Illes P. P2X7 receptor: An emerging target in central nervous system diseases. Trends Pharmacol Sci 2014;35:537–547. [DOI] [PubMed] [Google Scholar]
- 43. Le Feuvre R, Brough D, Rothwell N. Extracellular ATP and P2X7 receptors in neurodegeneration. Eur J Pharmacol 2002;447:261–269. [DOI] [PubMed] [Google Scholar]
- 44. Ryu JR, Hong CJ, Kim JY et al. Control of adult neurogenesis by programmed cell death in the mammalian brain. Mol Brain 2016;9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kettenmann H, Hanisch U‐KK, Noda M et al. Physiology of microglia. Physiol Rev 2011;91:461–553. [DOI] [PubMed] [Google Scholar]
- 46. Pegg CC, He C, Stroink AR et al. Technique for collection of cerebrospinal fluid from the cisterna magna in rat. J Neurosci Methods 2010;187:8–12. [DOI] [PubMed] [Google Scholar]
- 47. Joseph SM, Buchakjian MR, Dubyak GR. Colocalization of ATP release sites and ecto‐ATPase activity at the extracellular surface of human astrocytes. J Biol Chem 2003;278:23331–23342. [DOI] [PubMed] [Google Scholar]
- 48. Ginisty A, Gely‐Pernot A, Abaamrane L et al. Evidence for a subventricular zone neural stem cell phagocytic activity stimulated by the vitamin K‐dependent factor protein S. Stem Cells 2015;33:515–525. [DOI] [PubMed] [Google Scholar]
- 49. Yu Q, Guo Z, Liu X et al. Block of P2X7 receptors could partly reverse the delayed neuronal death in area CA1 of the hippocampus after transient global cerebral ischemia. Purinergic Signal 2013;9:663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang X, Arcuino G, Takano T et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med 2004;10:821–827. [DOI] [PubMed] [Google Scholar]
- 51. Choi HB, Ryu JK, Kim SU et al. Modulation of the purinergic P2X7 receptor attenuates lipopolysaccharide‐mediated microglial activation and neuronal damage in inflamed brain. J Neurosci 2007;27:4957–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Engel T, Gomez‐Villafuertes R, Tanaka K et al. Seizure suppression and neuroprotection by targeting the purinergic P2X7 receptor during status epilepticus in mice. FASEB J 2012;26:1616–1628. [DOI] [PubMed] [Google Scholar]
- 53. Delarasse C, Auger R, Gonnord P et al. The purinergic receptor P2X7 triggers alpha‐secretase‐dependent processing of the amyloid precursor protein. J Biol Chem 2011;286:2596–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gu BJ, Huang X, Ou A et al. Innate phagocytosis by peripheral blood monocytes is altered in Alzheimer's disease. Acta Neuropathol 2016;132:377–389. [DOI] [PubMed] [Google Scholar]
- 55. Oyanguren‐Desez O, Rodriguez‐Antiguedad A, Villoslada P et al. Gain‐of‐function of P2X7 receptor gene variants in multiple sclerosis. Cell Calcium 2011;50:468–472. [DOI] [PubMed] [Google Scholar]
- 56. Gu BJ, Field J, Dutertre S et al. A rare P2X7 variant Arg307Gln with absent pore formation function protects against neuroinflammation in multiple sclerosis. Hum Mol Genet 2015;24:5644–5654. [DOI] [PubMed] [Google Scholar]
- 57. Gu BJ, Baird PN, Vessey KA et al. A rare functional haplotype of the P2RX4 and P2RX7 genes leads to loss of innate phagocytosis and confers increased risk of age‐related macular degeneration. FASEB J 2013;27:1479–1487. [DOI] [PubMed] [Google Scholar]
- 58. Liu Y, Chen GQ, Liu BY et al. P2X7 receptor in the hippocampus is involved in gp120‐induced cognitive dysfunction. Genet Mol Res 2017;16:1. [DOI] [PubMed] [Google Scholar]
- 59. Cao X, Li LP, Qin XH et al. Astrocytic adenosine 5′‐triphosphate release regulates the proliferation of neural stem cells in the adult hippocampus. Stem Cells 2013;31:1633–1643. [DOI] [PubMed] [Google Scholar]
- 60. Thompson BA, Storm MP, Hewinson J et al. A novel role for P2X7 receptor signalling in the survival of mouse embryonic stem cells. Cell Signal 2012;24:770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Raffaghello L, Chiozzi P, Falzoni S et al. The P2X7 receptor sustains the growth of human neuroblastoma cells through a substance P‐dependent mechanism. Cancer Res 2006;66:907–914. [DOI] [PubMed] [Google Scholar]
- 62. Wu PY, Lin YC, Chang CL et al. Functional decreases in P2X7 receptors are associated with retinoic acid‐induced neuronal differentiation of Neuro‐2a neuroblastoma cells. Cell Signal 2009;21:881–891. [DOI] [PubMed] [Google Scholar]
- 63. Zou J, Vetreno RP, Crews FT. ATP‐P2X7 receptor signaling controls basal and TNFalpha‐stimulated glial cell proliferation. Glia 2012;60:661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang J, Liu S, Nie Y et al. Activation of P2X7 receptors decreases the proliferation of murine luteal cells. Reprod Fertil Dev 2015;27:1262–1271. [DOI] [PubMed] [Google Scholar]
- 65. Donegan M, Kernisant M, Cua C et al. Satellite glial cell proliferation in the trigeminal ganglia after chronic constriction injury of the infraorbital nerve. Glia 2013;61:2000–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jaako‐Movits K, Zharkovsky A. Impaired fear memory and decreased hippocampal neurogenesis following olfactory bulbectomy in rats. Eur J Neurosci 2005;22:2871–2878. [DOI] [PubMed] [Google Scholar]
- 67. Nilsson M, Perfilieva E, Johansson U et al. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol 1999;39:569–578. [DOI] [PubMed] [Google Scholar]
- 68. Bruel‐Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long‐term memory following environmental enrichment. Eur J Neurosci 2005;21:513–521. [DOI] [PubMed] [Google Scholar]
- 69. Iso H, Simoda S, Matsuyama T. Environmental change during postnatal development alters behaviour, cognitions and neurogenesis of mice. Behav Brain Res 2007;179:90–98. [DOI] [PubMed] [Google Scholar]
- 70. Kim YM, Ji ES, Kim SH et al. Treadmill exercise improves short‐term memory by enhancing hippocampal cell proliferation in quinolinic acid‐induced Huntington's disease rats. J Exerc Rehabil 2015;11:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Additional characterization shows SVZ NPCs have greater proliferation and adult NPCs retain pluripotency in culture. NPCs were identified in in vivo sections of the subventricular zone by EdU incorporation to label actively dividing cells. The SVZ contained a greater population of dividing NPCs (A‐C) than the dentate gyrus, this was quantified by EdU incorporation, * p = 0.007 (D). When cultured in proliferation medium for three DIV, representative hippocampal NPC cultures contained less than 0.1% DCX+ neuroblasts (E‐G). Morphological changes were observed in NPC maintained in differentiation medium for seven DIV; processes became elongated and ramified and the soma more compact (H‐S). Differentiated cultures expressed immature neuron marker βIII tubulin (H‐J) and the mature neuron markers MAP2a/b (K‐M) and NeuN (N‐P). Differentiated cultures also contained astrocytic progenitor cells identified by GFAP+/vimentin− as indicated by the numeral 1 (Q‐S). An example of co‐expression is numbered 2, while a vimentin+/GFAP− stain indicates a more neuronal phenotype and is numbered 3. Oligodendrocyte precursor cells were stained for O4 in hippocampal and SVZ cultures (T, U respectively). Hippocampal cultures were negative whereas SVZ cultures were 2.6 ± 0.86% positive. Secondary controls (V) demonstrated no non‐specific binding for Alexa‐488, Cy3, or Cy5. Scale bars represent 10 μm.
Supplementary Figure 2. P2X7 receptor expression in spheroid cultures was confirmed by immunochemistry and Western blot. P2X7 receptor expression in the neurosphere culture was confirmed by immunochemical analysis of cryosectioned spheres (A). Scale bar represents 20 μm. Antibody observations were confirmed by Western blot, which identified a band at approximately 85 kDa, consistent with the glycosolated form of P2X7 (B).
Supplementary Figure 3. ATP induced calcium influx in NPCs. NPCs were loaded with Fluo‐8 AM calcium indicator dye and calcium channel opening was assessed using live cell microscopy. Application of ATP evoked calcium influx (A‐H, frame rate 0.5 seconds) in a dose‐dependent manner. Scale bar represents 50 μm. Between 30 and 50 regions of interest were selected at random and the maximum cytosolic calcium concentrations (F/F0) from three biological repeats were quantified (I). A Welch ANOVA determined significance at concentrations ≥ 0.1 μM (Welch's F[8, 650] = 455.00, p < 0.01), error bars ±1 SE, * p < 0.01.
Supplementary Figure 4. SVZ NPCs derived from P2X7 −/− mice had a reduced response to the inhibitory effects of ATP on proliferation. EdU incorporation was used to measure the effect of purinergic signaling on proliferation in P2X7 KO compared with WT NPC cultures from the SVZ. ATP applied to KO cultures did not result in as significant a decrease in proliferation as did the WT cultures, *p < 0.001.
Supplementary Movie 1. Live cell microscopy recording of NPCs following the addition of 100 μM ATP. NPCs were plated on glass and cultured for 24 hours with or without ATP. Images were captured every 10 minutes and examined for signs of cell stress and death. Live cell recordings showed NPCs exposed to 100 μM ATP continued to thrive in culture similar to control.


