Abstract
Objectives
To examine whether it is more efficacious to commence exercise medicine in men with prostate cancer at the onset of androgen‐deprivation therapy (ADT) rather than later on during treatment to preserve bone and soft‐tissue composition, as ADT results in adverse effects including: reduced bone mineral density (BMD), loss of muscle mass, and increased fat mass (FM).
Patients and methods
In all, 104 patients with prostate cancer, aged 48–84 years initiating ADT, were randomised to immediate exercise (IMEX, n = 54) or delayed exercise (DEL, n = 50) conditions. The former consisted of 6 months of supervised resistance/aerobic/impact exercise and the latter comprised 6 months of usual care followed by 6 months of the identical exercise programme. Regional and whole body BMD, lean mass (LM), whole body FM and trunk FM, and appendicular skeletal muscle (ASM) were assessed by dual X‐ray absorptiometry, and muscle density by peripheral quantitative computed tomography at baseline, and at 6 and 12 months.
Results
There was a significant time effect (P < 0.001) for whole body, spine and hip BMD with a progressive loss in the IMEX and DEL groups, although lumbar spine BMD was largely preserved in the IMEX group at 6 months compared with the DEL group (−0.4% vs −1.6%). LM, ASM, and muscle density were preserved in the IMEX group at 6 months, declined in the DEL group at 6 months (−1.4% to −2.5%) and then recovered at 12 months after training. FM and trunk FM increased (P < 0.001) over the 12‐month period in the IMEX (7.8% and 4.5%, respectively) and DEL groups (6.5% and 4.3%, respectively).
Conclusions
Commencing exercise at the onset of ADT preserves lumbar spine BMD, muscle mass, and muscle density. To avoid treatment‐related adverse musculoskeletal effects, exercise medicine should be prescribed and commenced at the onset of ADT.
Keywords: exercise, androgen‐deprivation therapy, #PCSM, #ProstateCancer
Abbreviations
- ADT
androgen‐deprivation therapy
- ALP
alkaline phosphatase
- ASM
appendicular skeletal muscle
- BMD
bone mineral density
- BMI
body mass index
- DXA
dual X‐ray absorptiometry
- IQR
interquartile range
- LM
lean mass
- LSI
Leisure Score Index
- NTX
N‐terminal telopeptide of type 1 collagen
- P1NP
procollagen type 1 N‐terminal propeptide
- RCT
randomised controlled trial
- RPE
Rating of Perceived Exertion
- RT
radiation treatment
Introduction
The use of androgen‐deprivation therapy (ADT) as an adjuvant, neoadjuvant or stand‐alone treatment for men with localised and advanced prostate cancer is accompanied by a range of adverse effects that impact on a patient's well‐being, risk of comorbidities, and quality of life 1, 2. Principal amongst these are the musculoskeletal toxicities of reduced bone mass 3, leading to osteoporosis and an increased risk of skeletal fracture 4; a loss of lean mass (LM) or muscle mass leading to sarcopaenia 5; and a reduced muscle attenuation or muscle density leading to myosteatosis 6, the fatty infiltration of skeletal muscle resulting in reduced muscle quality. In addition, these changes are accompanied by an increase in whole body fat mass (FM) and trunk FM, which may result in the patient being at an increased risk of cardiovascular and metabolic complications 7. In addressing musculoskeletal toxicities, pharmacological agents in the form of bisphosphonates can be used to increase bone mineral density (BMD), although the effects on fracture incidence are less clear 8 and they are also associated with potential adverse effects 9. Similarly, denosumab, a receptor activator of nuclear factor‐κΒ ligand (RANKL) inhibitor, results in an increase in BMD in men receiving ADT for non‐metastatic prostate cancer and is also associated with a reduction in the incidence of new vertebral fractures 10. However, as with the bisphosphonates, there are potential adverse effects such as an increase in the incidence and prevalence of osteonecrosis of the jaw 11, as well as musculoskeletal pain 10. Moreover, the loss of muscle mass is not addressed by these agents nor is muscle quality, and loss of muscle mass and muscle quality in older and less physically robust patients may compromise functioning and independence 12.
We and others have demonstrated the effectiveness of targeted exercise, primarily consisting of resistance and/or aerobic training, in reversing several ADT‐related adverse effects including: reduced muscle mass and strength 13, 14, physical function 15, aerobic fitness 16, fatigue 17, sexual health 18, and disease‐specific quality of life 19, in men on existing ADT regimens including those of a long‐term nature. In contrast, less successful has been the effectiveness of exercise in reversing the ADT‐related increases in FM 6, 14, 16 and only a few studies have recently been undertaken in addressing bone loss in men on existing ADT regimens with minimal benefit 20, 21.
However, these studies have been undertaken with rehabilitative intent and a more opportune time to intervene may be when ADT is initiated to mitigate or completely prevent the adverse effects from hormone suppression occurring in the first place. To this end, we previously undertook a 3‐month exercise trial comprised of resistance and aerobic training in men commencing ADT and found that treatment toxicity was significantly reduced compared with those undergoing usual care 22. In the present study, we extend those findings by asking the question: is it more efficacious to prevent ADT musculoskeletal toxicities from the outset rather than trying to rehabilitate the patient after the development of toxicities? The present study reports on the effects of a year‐long randomised trial, in which men initiating ADT were assigned exercise at the onset compared to 6 months later on during their treatment for prostate cancer. Specifically, we investigated whether the musculoskeletal toxicities could be prevented by a 6‐month exercise programme concurrently undertaken with the onset of ADT. As a result, we implemented an osteogenic exercise regimen that comprised impact loading activities combined with resistance and aerobic exercise.
Patients and methods
In all, 219 patients were screened for participation from August 2013 to April 2015 in Perth, Western Australia, and their progress through the study is shown in Fig. 1. Potential participants were referred by invitation of their treating radiation oncologist/urologist to the study coordinator to assess eligibility and describe the study. Inclusion criteria included: beginning treatment for prostate cancer involving ADT and intending to remain on it for at least the next 6 months, no regular exercise (structured aerobic or resistance training ≥2 sessions/week) in the past 3 months, able to walk 400 m, and had obtained medical clearance from their physician. Exclusion criteria included: prior exposure to ADT; established metastatic disease; established osteoporosis 23; taking medications known to effect bone metabolism, such as bisphosphonates; acute illness; or any musculoskeletal, cardiovascular or neurological disorder that could inhibit or put them at risk from exercising, as determined by their physician. The study was approved by the Human Research Ethics Committee, and all participants provided written informed consent.
Figure 1.
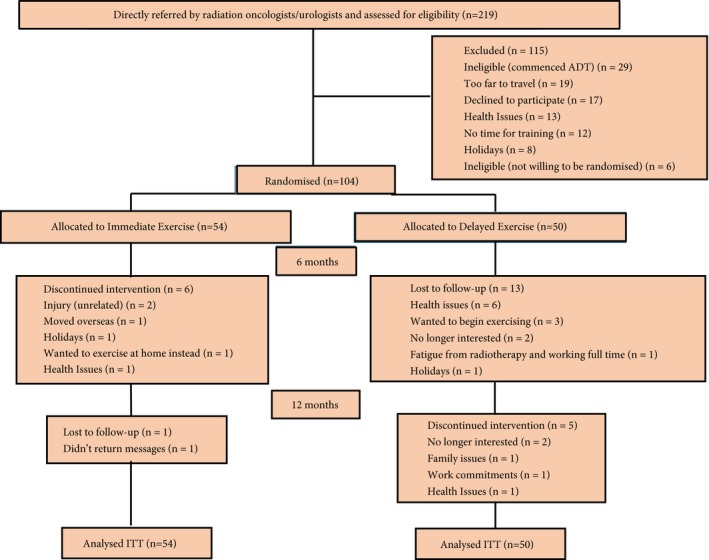
Consolidated Standards of Reporting Trials (CONSORT) diagram. ITT, intention‐to‐treat.
Study design
This was a single‐blinded randomised controlled trial (RCT; investigators and testing personnel blinded to group allocation) with partial crossover, with the primary study endpoint being BMD 24. Following familiarisation and baseline assessments, 104 men were randomly assigned using a computer random assignment program to either immediate exercise (IMEX) or delayed exercise (DEL), and stratified according to age (≤70 and >70 years) and smoking status (yes/no). The IMEX group undertook a multicomponent programme that combined resistance + aerobic + impact‐loading exercise in the initial 6 months with no formal intervention in the second 6 months, whilst the DEL group had 6 months of usual care followed by 6 months of the identical resistance + aerobic + impact‐loading exercise programme. All participants received standard daily supplementation with calcium (1000 mg/day) and vitamin D3 (800 IU/day). Measurements were performed at baseline, and at 6 and 12 months.
Exercise programme
The exercise programme was undertaken thrice weekly in several exercise clinics in the Perth metropolitan area, Western Australia. Sessions were conducted in small groups of 6–10 participants supervised by accredited exercise physiologists. The sessions were ~60 min in duration and consisted of a combination of impact loading, aerobic and resistance exercise. The frequency of aerobic and resistance exercise was alternated weekly such that two aerobic/impact loading and one resistance/impact loading session were performed in 1 week and two resistance/impact loading and one aerobic/impact loading session were performed in the subsequent week. Detailed description of the exercise programme and progression is described elsewhere 24. Briefly, the impact‐loading component consisted of a series of bounding (over soft hurdles), hopping, skipping, leaping, and drop jumping activities that resulted in peak ground reaction forces of 3.4–5.2 times body weight, with the volume and intensity progressive in nature. For the first 8 weeks, two rotations were performed of skipping (30 s), bounding (15 cm hurdles), and jumping (10 times), with jumping replaced in weeks 5–8 by drop jumping (15 cm, 10 times). For the second 8 weeks, three rotations were performed of bounding (15–30 cm hurdles), drop jumping (15–20 cm, 10 times), and skipping (30 s), with skipping replaced in weeks 13–16 by hopping/leaping (10 times). Thereafter, four rotations of hopping/leaping (10 times), bounding (30 cm hurdles), and drop jumping (20 cm, 10 times) were performed. Resistance training consisted of upper and lower body exercises for the main muscle groups and included the leg press, leg extension, leg curl, chest press, seated row, lat pulldown, and biceps curl. Intensity was set at 6–12 repetition maximum (RM; the maximal weight lifted 6–12 times) using 2–4 sets/exercise. The aerobic‐based component consisted of various modes and included walking/jogging on a treadmill and cycling or rowing on a stationary ergometer at an intensity of 60–85% estimated maximum heart rate for 25–40 min, with heart rate monitored using heart rate watches (Polar Electra Oy, Finland). All sessions commenced with a warm‐up comprising low‐level aerobic activities and concluded with a cooldown of stretching activities. In addition to clinic‐based exercise, the men were encouraged to undertake twice weekly home‐based training consisting of aerobic activities such as walking or cycling and a modified version of the impact‐loading programme consisting of hopping, leaping, and drop jumping.
Primary and secondary endpoints
The primary endpoints of lumbar spine, total hip and whole body BMD (g/cm2) were assessed by dual X‐ray absorptiometry (DXA, Hologic Discovery A, Waltham, MA, USA). The Instant Vertebral Assessment and Quantitative Morphometry program was used to determine the presence or absence of vertebral fractures before initiation of the study. Secondary endpoints included soft‐tissue composition and muscle density. Whole body LM and FM, percentage fat, trunk FM, and appendicular skeletal muscle (ASM) were derived from the whole body DXA scan, with ASM the sum of upper‐ and lower‐limb bone‐free LM 25. Muscle density of the lower leg was determined by peripheral quantitative CT (XCT3000, Stratec, Pforzheim, Germany), with measurement and analysis undertaken at the tibia 66% site.
Other measures
Demographic and clinical data were collected by self‐report and medical records, respectively. Height and weight were assessed using a stadiometer and electronic scales, respectively, with body mass index (BMI, kg/m2) calculated from weight in kg divided by height in metres squared. Physical activity was assessed by the Leisure Score Index (LSI) of the Godin Leisure‐Time Exercise Questionnaire 26. Testosterone and PSA, and markers of bone formation [procollagen type 1 N‐terminal propeptide (P1NP) and alkaline phosphatase (ALP)], and bone resorption [N‐terminal telopeptide of type 1 collagen (NTX)] were measured commercially by an accredited Australian National Association of Testing Authorities (NATA) laboratory (Pathwest Diagnostics, Perth, WA, Australia). Exercise intensity of each training session was assessed using Borg's Rating of Perceived Exertion (RPE) 6–20 scale 27.
Statistical analyses and sample size calculations
The sample size estimate was based on our previous study 3 of the 36‐week changes in BMD after initiation of ADT and the projected differences between groups with the undertaking of exercise 24, resulting in the requirement of 51 participants per group to achieve 80% power at an α level of 0.05 (two‐tailed). Data were analysed using the IBM Statistical Package for the Social Sciences (SPSS®) version 24 (SPSS Inc., IBM Corp., Armonk, NY, USA). Normality of the distribution was assessed using the Kolmogorov–Smirnov test. Between‐group differences in baseline characteristics were assessed using independent t‐tests or the Mann–Whitney U‐test, as appropriate, for continuous data and chi‐squared for categorical data, and a two‐way (group × time) repeated‐measures anova for change over time (baseline, 6 and 12 months) in the primary and secondary outcome variables. FM was not normally distributed and was log transformed (ln) for analysis. Follow‐up tests were performed if the interaction or main effect for time was significant. Where appropriate, the Bonferroni post hoc procedure for multiple comparisons was used to locate the source of the significant differences. Intention‐to‐treat was used for analyses of primary and secondary endpoints using maximum‐likelihood imputation of missing values (expectation maximisation). Freidman's anova with Bonferroni‐adjusted Wilcoxon signed‐ranked test, as the follow‐up test, was used for other measures. Tests were two‐tailed with statistical significance set at an α level of 0.05.
Results
There were no significant differences between groups at baseline (Table 1). The men were aged 48–84 years with a mean (SD) BMI of 27.9 (4.2) kg/m2, most were married, non‐smokers, with one or more comorbidities, and classed as insufficiently physically active based on the Godin LSI. Based on spine and/or hip BMD, 42 patients (77.8%) in the IMEX group and 34 (68.0%) in the DEL group had normal bone densities, 11 (20.4%) in the IMEX group and 15 (30.0%) in the DEL group were osteopaenic, and one (1.9%) in the IMEX group and one (2.0%) in the DEL group were osteoporotic (P = 0.521). Baseline measures were undertaken between 1 and 15 days, with a mean of 6 days after the first treatment injection (Lucrin or Zoladex). In the first 6 months of the study, six men in the IMEX group and 13 in the DEL group withdrew from the study, with an additional six men by 12 months for a total of 25 men withdrawing from the study (Fig. 1). In addition, during the initial 6 months, 10 men in the IMEX group and five in the DEL group ceased ADT and 40 men in the IMEX group and 30 in the DEL group commenced radiation treatment (RT). Of the men undergoing RT, the treatment regimen for 11 men in the IMEX group and eight in the DEL group continued into the initial portion of the second 6‐month period. During months 7–12, an additional 19 men from the IMEX group and 17 from the DEL group ceased ADT, whilst one man in the IMEX group recommenced ADT. For RT during this time period, four additional men in the IMEX group and two in the DEL group initiated treatment, whilst eight men in the IMEX group and four in the DEL group who previously had external beam RT underwent brachytherapy. At 6 months, there was no significant difference in PSA levels between those who ceased ADT and those who remained on treatment [ADT, median (interquartile range, IQR) PSA level of 0.07 (0.0–0.3) ng/mL; non‐ADT, 0.04 (0.0–0.2) ng/mL; P = 0.868], although the testosterone level was higher for those ceasing ADT (ADT, median (IQR) testosterone level of 0.5 (0.4–0.7) nmol/L; non‐ADT, 0.9 (0.5–15.8) nmol/L; P = 0.009]. At 12 months, there remained no significant difference in PSA levels (ADT, median (IQR) PSA level of 0.02 (0.0–0.1) ng/mL; non‐ADT, 0.04 (0.0–0.2) ng/mL; P = 0.497]; however, the testosterone level was significantly greater in those not on ADT (ADT, median (IQR) testosterone level of 1.0 (0.5–8.2) nmol/L; non‐ADT, 7.8 (3.2–14.5) nmol/L; P = 0.001]. At 6 months of ADT in the DEL group there was a loss in BMD (spine −1.6%, hip −1.1%, whole body −1.0%), LM (−1.4%), ASM (−2.5%), and muscle density (−1.5%), and an increase in FM (6.5%), trunk FM (5.7%), and percentage fat (5.6%). Patients in the IMEX group attended 79% of the scheduled exercise sessions and those in the DEL group attended 69% of the scheduled sessions.
Table 1.
Participant characteristics
| Variable | IMEX group | DEL group | P |
|---|---|---|---|
| Number of patients | 54 | 50 | |
| Mean (SD): | |||
| Age, years | 69.0 (6.3) | 67.5 (7.7) | 0.266 |
| Height, cm | 173.4 (7.1) | 172.7 (6.2) | 0.602 |
| Weight, kg | 82.9 (16.4) | 84.6 (12.9) | 0.551 |
| BMI, kg/m2 | 27.5 (4.4) | 28.3 (3.9) | 0.282 |
| Gleason score | 7.6 (1.0) | 7.6 (0.8) | 0.829 |
| N (%): | |||
| Married | 42 (77.8) | 41 (82.0) | 0.914 |
| Currently employed | 13 (24.1) | 17 (34.0) | 0.264 |
| Tertiary education | 13 (24.1) | 11 (22.0) | 0.802 |
| Current smoker | 5 (9.3) | 3 (6.0) | 0.533 |
| Number of medications, mean (sd) | 3.7 (2.3) | 3.6 (2.6) | 0.955 |
| Median (IQR): | |||
| Godin LSI | 10.0 (0.0–24.5) | 10.0 (0.0–27.0) | 0.872 |
| PSA level, ng/mL | 3.4 (0.7–6.4) | 3.9 (0.2–10.0) | 0.599 |
| Testosterone level, nmol/L | 8.0 (2.0–16.0) | 4.6 (1.9–15.8) | 0.413 |
| PINP, μg/L | 31.0 (22.0–40.0) | 36.0 (27.5–52.0) | 0.060 |
| ALP, U/L | 61.5 (51.8–78.3) | 68.0 (58.8–83.5) | 0.171 |
| NTX, nmol BCE/mmol creatine | 29.0 (24.0–37.0) | 32.0 (26.0–42.0) | 0.095 |
| Time since ADT injection, days, mean (sd) | 6.4 (2.1) | 5.7 (1.9) | 0.110 |
| N (%): | |||
| Prostatectomy | 15 (27.7) | 17 (34.0) | 0.532 |
| RT | 4 (7.4) | 3 (6.0) | 0.775 |
| Other conditions | |||
| Cardiovascular disease | 11 (21.1) | 10 (20.0) | 0.885 |
| Hypertension | 28 (51.9) | 30 (60.0) | 0.403 |
| Dyslipidaemia | 26 (48.1) | 25 (50.0) | 0.771 |
| Diabetes | 11 (20.4) | 8 (16.0) | 0.564 |
BCE, bone collagen equivalents. A moderate to strenuous LSI score of ≥24 classed as ‘active’ and ≤23 classed as ‘insufficiently active’.
BMD
There were no differences between groups for spine (P = 0.292), hip (P = 0.625) or whole body BMD (P = 0.228) at baseline. Over the 12‐month study period, there was no significant interaction but a significant effect of time (P ≤ 0.001) at the spine, hip, and whole body (Table 2). Spine BMD decreased at 12 months compared with baseline in both the IMEX and DEL groups; however, the BMD was largely preserved by exercise in both groups such that at 6 months, the change in the IMEX group was −0.4% compared with −1.6% in the DEL group, and in the second 6‐month period when the DEL group exercised and the IMEX group did not engage in the exercise programme the changes were 0.3% and −2.2%, respectively. As a result, over the 12‐month period, the loss in both groups was similar at −1.5% in the IMEX group and −1.3% in the DEL group. For the total hip and whole body, there was a progressive decrease in BMD in both groups, such that by 12 months, the loss at the hip was −2.1% and −2.3% in the IMEX and DEL groups, respectively, and −1.4% for the whole body.
Table 2.
Regional and whole body BMD at baseline, 6 and 12 months
| Baseline (0), mean (SD) | 6 months, mean (SD) | 12 months, mean (SD) | P | |||
|---|---|---|---|---|---|---|
| Time | Group × time | Comparison*, months | ||||
| Lumbar spine, g/cm2 | ||||||
| IMEX | 1.193 (0.197) | 1.188 (0.194) | 1.175 (0.185) | 0.001 | 0.111 | 0 > 12 |
| DEL | 1.154 (0.173) | 1.136 (0.175) | 1.139 (0.176) | 0 > 12 | ||
| Total hip, g/cm2 | ||||||
| IMEX | 1.013 (0.145) | 1.002 (0.141) | 0.992 (0.143) | <0.001 | 0.848 | 0 > 6 > 12 |
| DEL | 1.000 (0.122) | 0.989 (0.130) | 0.977 (0.122) | 0 > 6 > 12 | ||
| Whole body, g/cm2 | ||||||
| IMEX | 1.189 (0.115) | 1.179 (0.116) | 1.172 (0.110) | <0.001 | 0.827 | 0 > 6 > 12 |
| DEL | 1.161 (0.121) | 1.149 (0.118) | 1.144 (0.117) | 0 > 6 > 12 | ||
*Within‐group multiple comparisons for baseline (0), 6 and 12 months, with a Bonferroni‐corrected P < 0.05.
Soft‐tissue composition and muscle density
There were no differences between groups for soft‐tissue composition or muscle density at baseline (P = 0.159–0.897). IMEX preserved LM and ASM; there was a significant time effect (P < 0.001) for LM with no change between baseline and 6 months, whilst it was reduced in the DEL group by −0.8 kg at 6 months and then recovered (1.4 kg) with training during months 7–12, such that by 12 months, it was 1.4% and 1.1% higher in the IMEX and DEL groups, respectively (Table 3). For ASM, there was a group × time interaction (P = 0.009) with no significant change over 12 months in the IMEX group but with the 12‐month measure (after training) in the DEL group greater than at baseline and 6 months. The net differences in ASM compared with baseline in the IMEX and DEL groups at 12 months were 1.3% and 0.8%, respectively. There was a significant effect of time (P = 0.016) on muscle density, which was preserved in the IMEX group at 6 months and reduced in the DEL group by −1.5%. For FM, there was a significant time effect (P < 0.001), with FM progressively increasing in the IMEX group by 1 kg at 6 months and a further 0.9 kg at 12 months, but in the DEL group increasing during the non‐exercise period by 1.7 kg with no change thereafter with training. As a result, body fat percentage was greater at 6 and 12 months in both groups compared with baseline. There was a significant interaction (P = 0.025) for trunk FM, with little change between baseline and 6 months in the IMEX group but at 12 months greater than baseline; whilst in the DEL group, 6 and 12 months were greater than baseline, with no significant change in trunk FM in the DEL group after training. The net change in trunk FM over the 12‐month study period in both the IMEX and DEL groups was 0.6 kg.
Table 3.
Soft‐tissue composition and muscle density at baseline, 6 and 12 months
| Baseline (0), mean (SD) | 6 months, mean (SD) | 12 months, mean (SD) | P | |||
|---|---|---|---|---|---|---|
| Time | Group × time | Comparison*, months | ||||
| LM, kg | ||||||
| IMEX | 55.6 (9.0) | 55.6 (8.7) | 56.4 (9.2) | <0.001 | 0.068 | 0, 6 < 12 |
| DEL | 55.8 (7.2) | 55.0 (6.8) | 56.4 (6.2) | 6 < 0, 12 | ||
| FM, kg | ||||||
| IMEX | 24.5 (8.4) | 25.5 (8.0) | 26.4 (8.2) | <0.001† | 0.101† | 12 > 6 > 0 |
| DEL | 26.1 (7.6) | 27.8 (7.1) | 27.8 (7.5) | 6, 12 > 0 | ||
| Body fat, % | ||||||
| IMEX | 29.0 (5.1) | 29.4 (4.7) | 30.4 (4.6) | <0.001 | 0.013 | 6, 12 > 0 |
| DEL | 30.4 (5.2) | 32.1 (4.6) | 31.6 (4.7) | 6, 12 > 0 | ||
| ASM, kg | ||||||
| IMEX | 23.6 (4.1) | 23.6 (3.9) | 23.9 (4.3) | <0.001 | 0.009 | |
| DEL | 23.9 (3.4) | 23.3 (3.3) | 24.1 (3.1) | 0, 6 < 12 | ||
| Trunk FM, kg | ||||||
| IMEX | 13.4 (5.2) | 13.7 (4.9) | 14.0 (5.0) | <0.001 | 0.025 | 12 > 0 |
| DEL | 14.0 (4.5) | 14.8 (4.2) | 14.6 (4.3) | 6, 12 > 0 | ||
| Muscle density, mg/cm3 | ||||||
| IMEX | 72.6 (4.4) | 72.3 (5.1) | 72.0 (4.7) | 0.016 | 0.107 | |
| DEL | 72.9 (3.0) | 71.8 (3.4) | 72.3 (3.6) | 0 > 6 | ||
*Within‐group multiple comparisons for baseline (0), 6 and 12 months, with a Bonferroni‐corrected P < 0.05. †Statistical analysis based on log‐transformed data.
Other measures and adverse events
After initiation of ADT, the PSA level was reduced (P < 0.001) to negligible levels at 6 [IMEX group, median (IQR) PSA level of 0.1 (0.0–0.2) ng/mL; DEL group, 0.1 (0.0–0.4) ng/mL] and 12 months [IMEX group, median (IQR) PSA level of 0.0 (0.0–0.1) ng/mL; DEL group, 0.1 (0.0–0.2) ng/mL], as was the testosterone level at 6 months (P < 0.001) [IMEX group, median (IQR) testosterone level of 0.5 (0.5–0.7) nmol/L; DEL group, 0.5 (0.4–0.7) nmol/L] but not at 12 months when recovery took place due to a number of patients ceasing hormone treatment [IMEX group, median (IQR) testosterone level 6.4 (0.6–11.8) nmol/L; DEL group, 7.1 (1.0–13.5) nmol/L]. Physical activity level based on the Godin LSI increased (P = 0.001) at 6 and 12 months in the IMEX group, and there was a significant change in the DEL group (P = 0.033), although this was not detected in post hoc testing. There was also an increase in markers of bone turnover (P < 0.001) over the 12‐month period in the IMEX and DEL groups. P1NP progressively increased at 6 and 12 months in both groups, ALP significantly increased in the IMEX and DEL groups by 12 months, and NTX increased in both groups at 6 months with a further increase in the DEL group by 12 months. The mean (range) RPE of the training sessions was 13.3 (12.1–17.80), indicating ‘somewhat hard’ to ‘very hard’. There were no major adverse events related to the training programme.
Discussion
The present year‐long trial comparing immediate (initial 6 months) vs delayed (second 6‐month period) exercise in men with prostate cancer commencing ADT produced three important findings: (i) commencing exercise that incorporates impact loading, resistance and aerobic training at the onset of ADT largely preserves spinal BMD, as well as whole body LM, ASM, and muscle density; (ii) gains in whole body and trunk FM still occurred in patients undertaking exercise at the onset of ADT, although they were attenuated compared to the delayed group; (iii) by 12 months, the benefits from immediate exercise did not persist such that there were no differences between those undergoing immediate or delayed exercise for BMD, muscle mass or muscle quality, or for FM.
It is now becoming well recognised that exercise is beneficial for patients with cancer 28, 29, 30, with the objectives and potential benefits of exercise varying across the cancer continuum 31. Moreover, men with prostate cancer recognise the need for integrating exercise support as part of routine care 32. In the present study, we examined the timing of exercise for patients on hormone suppression treatment, with the goal of preventing or attenuating adverse effects on bone and body composition by undertaking exercise at the initiation of treatment instead of exercise being of rehabilitative intent after the development of treatment‐related adverse effects. To this end, prescribing exercise at the onset of ADT mitigated or attenuated musculoskeletal adverse effects. The decline in BMD at the clinically relevant site of the spine was attenuated, although this was not the case at the hip (also a clinically relevant site), or the whole body where the loss in the IMEX and DEL groups was similar.
In somewhat of a similar fashion, Winters‐Stone et al. 20 reported some preservation of BMD at L4 in men with prostate cancer on established regimens of ADT (~2–3 years) undertaking a year‐long combined impact loading and resistance training programme compared to usual care, with no effect at the hip. In the only other RCT in this patient group, Uth et al. 21, using recreational football (soccer) as a skeletal loading stimulus in men undergoing ADT, reported that 32‐week training resulted in an increase in BMD at the total hip of ~1%, which was significantly different to the control group, whilst the increase at the lumbar spine of 0.6% was not statistically different to the non‐exercisers. It is likely that the frequent accelerations and decelerations and changes in direction with football provide a novel osteogenic stimulus at the hip in this patient group 21. In a recent meta‐analysis of exercise in patients with cancer, a subgroup analysis indicated a positive benefit of combined resistance and impact exercise on lumbar spine BMD but not at the hip 33, which likely reflects both the site‐specific loading characteristics and the higher bone turnover rate at the spine due to a greater proportion of trabecular bone 34. With exercise, the DEL group similarly preserved their spine BMD between 6 and 12 months, whereas, with cessation of the structured exercise programme the IMEX group lost 2.2% in BMD during this period. For the whole body, given the site‐specific adaptive response of bone to an appropriate stimulus 35, it is not surprising that no change was detected in the IMEX or DEL groups over the 12‐month period as is often seen with resistance or resistance and impact loading exercise in the adult population without cancer 36, 37, 38.
However, concurrently initiating exercise with androgen suppression preserved whole body LM and ASM. In comparison, with 6 months of ADT, the delayed group lost 0.8 kg in whole body LM and 0.6 kg in ASM. Preventing the loss in skeletal muscle is clinically relevant, as the development of sarcopaenia is related to falls and fractures, disability, reduced ability to perform daily tasks and loss of independence 39, 40, and mortality in patients with cancer 41. However, with training, the loss incurred by DEL was recouped by 12 months, such that little difference existed at this time point between those where exercise was initiated as a preventative strategy or with rehabilitative intent. There was also a modest decline in muscle density with ADT, which was attenuated with exercise in the IMEX group, with the loss in the DEL group partially recouped once training was undertaken, so that there was no difference between groups at 12 months. The significance of a decline in muscle density in this patient population is unclear, although in the elderly population, lower muscle attenuation is associated with hip fracture risk, insulin resistance, and mobility loss 12, 42, 43. As in the present study, exercise that contains a resistance training component appears to be an effective strategy in the non‐cancer population to counter declines in muscle density 44.
FM was not as responsive to exercise as muscle mass, with at best an attenuation of the increase in whole body FM and trunk FM in the IMEX group, such that instead of a 6.5% and 5.7% increase that occurred in the DEL group the gains at 6 months were only 4.1% and 2.2%, respectively. Interestingly, with exercise in the DEL group, there were no further gains in whole body FM and trunk FM, whilst gains continued to occur in the IMEX group without supervised training. An increase in abdominal obesity in men undergoing ADT is associated with increased metabolic and cardiovascular disease risk, which may result in cardiovascular mortality 45. Moreover, the combined effects of preserving muscle mass and attenuating the increase in FM may be important in preventing the development of sarcopaenic obesity, where sarcopaenia and obesity coexist and are associated with surgical complications, disability, and mortality in those with cancer 46.
The present study has several strengths and limitations that are worthy of comment. This is the first study to compare the timing of exercise to counter ADT treatment‐related adverse effects, that is, exercise initiated with prevention as the goal vs exercise with rehabilitative intent. We examined the clinically relevant fracture sites of the spine and hip, as well as whole body BMD, and in addition to muscle mass, we examined muscle quality. However, a number of men also underwent RT during the initial 6‐month period, as well as the second 6‐month period (a result of patients being referred from radiation oncologists) and this may have influenced the outcomes. In addition, although patients were to remain on ADT for at least 6 months, some patients ceased therapy during the initial 6 months and others during the second 6‐month period, which is reflected in changes in testosterone levels with testosterone recovery taking place by 12 months, which may have also influenced the outcomes. Nevertheless, this also represents what occurs in clinical practice where treatments will vary based on patient responses and disease characteristics/progression. Lastly, our exclusion criteria included men with established metastatic disease. As a result, our present results do not apply to men with metastatic disease; however, we have recently reported on the findings from an exercise trial in men with bone metastases that utilised a modular multimodal exercise programme that resulted in the preservation of physical function with no adverse skeletal complications 47.
In conclusion, implementing exercise in patients with prostate cancer initiating ADT largely preserves spinal BMD, as well as muscle mass and muscle quality, thereby offsetting treatment‐related musculoskeletal toxicities. Consequently, although undertaking exercise with rehabilitative intent after ADT‐related adverse effects occur is beneficial for the patient with prostate cancer, exercise medicine at the onset of treatment should be prescribed in order to prevent or attenuate the development of musculoskeletal toxicities.
Clinical Trial Registry
Can exercise ameliorate treatment toxicity during the initial phase of testosterone deprivation in prostate cancer patients? Is this more effective than delayed rehabilitation? ACTRN12612000097842.
Conflict of Interest
The authors have no conflict of interests to disclose.
Acknowledgments
This study was funded by Cancer Australia, Prostate Cancer Foundation of Australia and Beyond Blue (NHMRC# 1029901). Daniel A. Galvão is funded by a Cancer Council Western Australia Research Fellowship. Suzanne Chambers is supported by an Australian Research Council Professorial Future Fellowship.
References
- 1. Nguyen PL, Alibhai SM, Basaria S et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol 2015; 67: 825–36 [DOI] [PubMed] [Google Scholar]
- 2. Spry NA, Kristjanson L, Hooton B et al. Adverse effects to quality of life arising from treatment can recover with intermittent androgen suppression in men with prostate cancer. Eur J Cancer 2006; 42: 1083–92 [DOI] [PubMed] [Google Scholar]
- 3. Galvão DA, Spry NA, Taaffe DR et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int 2008; 102: 44–7 [DOI] [PubMed] [Google Scholar]
- 4. Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 2005; 352: 154–64 [DOI] [PubMed] [Google Scholar]
- 5. Smith MR, Saad F, Egerdie B et al. Sarcopenia during androgen‐deprivation therapy for prostate cancer. J Clin Oncol 2012; 30: 3271–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen D, Joseph DJ, Ebert MA et al. Effect of androgen deprivation therapy on muscle attenuation in men with prostate cancer. J Med Imaging Radiat Oncol 2014; 58: 223–8 [DOI] [PubMed] [Google Scholar]
- 7. Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 2006; 24: 4448–56 [DOI] [PubMed] [Google Scholar]
- 8. Alibhai SM, Zukotynski K, Walker‐Dilks C et al. Bone health and bone‐targeted therapies for nonmetastatic prostate cancer: a systematic review and meta‐analysis. Ann Intern Med 2017; 167: 341–50 [DOI] [PubMed] [Google Scholar]
- 9. Saylor PJ, Smith MR. Bone health and prostate cancer. Prostate Cancer Prostatic Dis 2010; 13: 20–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith MR, Egerdie B, Hernández Toriz N et al. Denosumab in men receiving androgen‐deprivation therapy for prostate cancer. N Engl J Med 2009; 361: 745–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khan AA, Morrison A, Hanley DA et al. Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res 2015; 30: 3–23 [DOI] [PubMed] [Google Scholar]
- 12. Visser M, Goodpaster BH, Kritchevsky SB et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well‐functioning older persons. J Gerontol A Biol Sci Med Sci 2005; 60: 324–33 [DOI] [PubMed] [Google Scholar]
- 13. Segal RJ, Reid RD, Courneya KS et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol 2003; 21: 1653–9 [DOI] [PubMed] [Google Scholar]
- 14. Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol 2010; 28: 340–7 [DOI] [PubMed] [Google Scholar]
- 15. Galvão DA, Nosaka K, Taaffe DR et al. Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc 2006; 38: 2045–52 [DOI] [PubMed] [Google Scholar]
- 16. Segal RJ, Reid RD, Courneya KS et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol 2009; 27: 344–51 [DOI] [PubMed] [Google Scholar]
- 17. Taaffe DR, Newton RU, Spry N et al. Effects of different exercise modalities on fatigue in prostate cancer patients undergoing androgen deprivation therapy: a year‐long randomised controlled trial. Eur Urol 2017; 72: 293–9 [DOI] [PubMed] [Google Scholar]
- 18. Cormie P, Newton RU, Taaffe DR et al. Exercise maintains sexual activity in men undergoing androgen suppression for prostate cancer: a randomized controlled trial. Prostate Cancer Prostatic Dis 2013; 16: 170–5 [DOI] [PubMed] [Google Scholar]
- 19. Bourke L, Gilbert S, Hooper R et al. Lifestyle changes for improving disease‐specific quality of life in sedentary men on long‐term androgen‐deprivation therapy for advanced prostate cancer: a randomised controlled trial. Eur Urol 2014; 65: 865–72 [DOI] [PubMed] [Google Scholar]
- 20. Winters‐Stone KM, Dobek JC, Bennett JA, Maddalozzo GF, Ryan CW, Beer TM. Skeletal response to resistance and impact training in prostate cancer survivors. Med Sci Sports Exerc 2014; 46: 1482–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uth J, Hornstrup T, Christensen JF et al. Efficacy of recreational football on bone health, body composition, and physical functioning in men with prostate cancer undergoing androgen deprivation therapy: 32‐week follow‐up of the FC prostate randomised controlled trial. Osteoporos Int 2016; 27: 1507–18 [DOI] [PubMed] [Google Scholar]
- 22. Cormie P, Galvão DA, Spry N et al. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen‐deprivation therapy: a randomised controlled trial. BJU Int 2015; 115: 256–66 [DOI] [PubMed] [Google Scholar]
- 23. WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser 1994; 843: 1–129 [PubMed] [Google Scholar]
- 24. Newton RU, Taaffe DR, Spry N et al. Can exercise ameliorate treatment toxicity during the initial phase of testosterone deprivation in prostate cancer patients? Is this more effective than delayed rehabilitation? BMC Cancer 2012; 12: 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heymsfield SB, Smith R, Aulet M et al. Appendicular skeletal muscle mass: measurement by dual‐photon absorptiometry. Am J Clin Nutr 1990; 52: 214–8 [DOI] [PubMed] [Google Scholar]
- 26. Godin G, Shephard RJ. A simple method to assess exercise behaviour in the community. Can J Appl Sport Sci 1985; 10: 141–6 [PubMed] [Google Scholar]
- 27. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14: 377–81 [PubMed] [Google Scholar]
- 28. Hayes SC, Spence RR, Galvao DA, Newton RU. Australian Association for Exercise and Sport Science position stand: optimising cancer outcomes through exercise. J Sci Med Sport 2009; 12: 428–34 [DOI] [PubMed] [Google Scholar]
- 29. Schmitz KH, Courneya KS, Matthews C et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sport Exer 2010; 42: 1409–26 [DOI] [PubMed] [Google Scholar]
- 30. Newton RU, Taaffe DR, Chambers SK, Spry N, Galvão DA. Effective exercise interventions for patients and survivors of cancer should be supervised, targeted, and prescribed with referrals from oncologists and general physicians. J Clin Oncol 2018; 36: 927–8 [DOI] [PubMed] [Google Scholar]
- 31. Courneya KS, Friedenreich CM. Physical activity and cancer control. Semin Oncol Nurs 2007; 23: 242–52 [DOI] [PubMed] [Google Scholar]
- 32. Chambers SK, Hyde MK, Laurie K et al. Experiences of Australian men diagnosed with advanced prostate cancer: a qualitative study. BMJ Open 2018; 8: e019917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dalla Via J, Daly RM, Fraser SF. The effect of exercise on bone mineral density in adult cancer survivors: a systematic review and meta‐analysis. Osteoporos Int 2018; 29: 287–303 [DOI] [PubMed] [Google Scholar]
- 34. Erikson EF, Axelrod DW, Melsen F. Bone Histomorphometry. New York, NY: Raven Press, 1994. [Google Scholar]
- 35. Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am 1977; 59: 204–8 [PubMed] [Google Scholar]
- 36. Milliken LA, Going SB, Houtkooper LB et al. Effects of exercise training on bone remodeling, insulin‐like growth factors, and bone mineral density in postmenopausal women with and without hormone replacement therapy. Calcif Tissue Int 2003; 72: 478–84 [DOI] [PubMed] [Google Scholar]
- 37. Whiteford J, Ackland TR, Dhaliwal SS et al. Effects of a 1‐year randomized controlled trial of resistance training on lower limb bone and muscle structure and function in older men. Osteoporos Int 2010; 21: 1529–36 [DOI] [PubMed] [Google Scholar]
- 38. Bemben DA, Bemben MG. Dose–response effect of 40 weeks of resistance training on bone mineral density in older adults. Osteoporos Int 2011; 22: 179–86 [DOI] [PubMed] [Google Scholar]
- 39. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr 1997; 127: 990S–1S [DOI] [PubMed] [Google Scholar]
- 40. Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int 2010; 21: 543–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gonzalez MC, Pastore CA, Orlandi SP, Heymsfield SB. Obesity paradox in cancer: new insights provided by body composition. Am J Clin Nutr 2014; 99: 999–1005 [DOI] [PubMed] [Google Scholar]
- 42. Lang T, Cauley JA, Tylavsky F et al. Computed tomographic measurements of thigh muscle cross‐sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res 2010; 25: 513–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE . Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 1997; 46: 1579–85 [DOI] [PubMed] [Google Scholar]
- 44. Taaffe DR, Henwood TR, Nalls MA, Walker DG, Lang TF, Harris TB. Alterations in muscle attenuation following detraining and retraining in resistance‐trained older adults. Gerontology 2009; 55: 217–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Braga‐Basaria M, Dobs AS, Muller DC et al. Metabolic syndrome in men with prostate cancer undergoing long‐term androgen‐deprivation therapy. J Clin Oncol 2006; 24: 3979–83 [DOI] [PubMed] [Google Scholar]
- 46. Carneiro IP, Mazurak VC, Prado CM. Clinical implications of sarcopenic obesity in cancer. Curr Oncol Rep 2016; 18: 62 [DOI] [PubMed] [Google Scholar]
- 47. Galvão DA, Taaffe DR, Spry N et al. Exercise preserves physical function in prostate cancer patients with bone metastases. Med Sci Sports Exerc 2018; 50: 393–9 [DOI] [PMC free article] [PubMed] [Google Scholar]


