Abstract
Essentials.
FcγRIIa mediates life‐threatening heparin‐induced thrombocytopenia (HIT).
Most anti‐platelet factor (PF)4‐heparin IgGs are not pathogenic so diagnosis of HIT is challenging.
Dimeric rsFcγRIIa was used to quantify receptor‐binding activity of anti‐PF4‐heparin antibodies.
Dimeric rsFcγRIIa binding specifically correlated with occurrence of HIT.
Summary
Background
Heparin‐induced thrombocytopenia (HIT) is a major and potentially fatal consequence of antibodies produced against platelet factor 4 (PF4)–heparin complexes following heparin exposure. Not all anti‐PF4–heparin antibodies are pathogenic, so overdiagnosis can occur, with resulting inappropriate use of alternative anticoagulation therapies that have associated risks of bleeding. However, definitive platelet functional assays are not widely available for routine analysis.
Objectives
To assess the utility of dimeric recombinant soluble FcγRIIa (rsFcγRIIa) ectodomains for detecting HIT antibodies.
Patients/Methods
Plasma from 27 suspected HIT patients were tested for pathogenic anti‐PF4–heparin antibodies by binding of a novel dimeric FcγRIIa ectodomain probe. Plasmas were also tested by the use of PF4–heparin IgG ELISA, the HemosIL AcuStar HIT IgG‐specific assay, and a serotonin release assay (SRA).
Results
The dimeric rsFcγRIIa test produced no false positives and excluded four samples that were positive by IgG ELISA. In this small patient cohort, the novel assay correctly assigned 93% of the suspected HIT patients, with two of the HIT patients being scored as false negatives. The improved discrimination of the novel assay over the IgG ELISA, which scored four false positives, supports the mechanistic interpretation that binding of dimeric rsFcγRIIa detects pairs of closely spaced IgG antibodies in PF4–heparin immune complexes.
Conclusions
This study found the cell‐free, function‐based dimeric rsFcγRIIa assay to be convenient, simple, and potentially predictive of HIT. The assay had improved specificity over the IgG ELISA, and correlated strongly with the AcuStar HIT IgG‐specific assay, warranting further evaluation of its potential to identify HIT in larger patient cohorts.
Keywords: enzyme immunoassay, heparin, platelet factor 4, thrombocytopenia, thrombosis
Introduction
Heparin‐induced thrombocytopenia (HIT) occurs when antibodies form immune complexes (ICs) with platelet factor 4 (PF4) bound to heparin or glycosaminoglycans 1, 2, 3. The pathogenic ICs bind to FcγRIIa, which is the only FcγR on platelets, triggering their activation and aggregation, leading to thrombosis. Binding to FcγRIIa on monocytes also causes both prothrombotic production of thrombin and tissue factor 4 and the clearance of platelets and thrombocytopenia 5. Many patients treated with heparin develop antibodies against PF4–heparin, but the presence of antibody–PF4–heparin complexes does not necessarily result in clinical manifestations of thrombosis/thrombocytopenia. Antigen recognition‐based methods (e.g. ELISA) detect anti‐PF4–heparin antibodies, but fail to distinguish pathogenic from non‐pathogenic antibodies. Thus, platelet functional assays, such as the serotonin release assay (SRA), are the most reliable for confirming HIT 2, 6, 7, but require access to appropriate donor platelets that are sensitive to activation, and are not easily replicated between many clinical laboratories.
The mAb KKO binds the PF4–heparin complex and activates human platelets in an FcγRIIa‐dependent manner 8; it causes HIT in a human FcγRIIa/PF4 transgenic mouse model 9, 10. A recent X‐ray crystallography analysis showed the KKO mAb bound to a conformation‐dependent epitope on heparin‐related pentasaccharide (fondaparinux)‐bound PF4 tetramers, promoting the formation of higher‐order complexes 11, 12. In contrast, a non‐pathogenic antibody bound an overlapping epitope, but only in the PF4 monomer. Plate‐based ELISAs present a heterogeneous mixture of PF4 forms, and so do not distinguish innocuous antibodies from those forming complexes capable of activating FcγRs.
Pathological HIT antibodies engage FcγRIIa, and trigger platelet activation and clearance 3 and tissue factor production 4. The pathology depends, in part, on an R131H polymorphism within FcγRIIa, which does not alter the expression levels of the receptor but does significantly alter the affinity of FcγRIIa for its ligand 13. We recently described the use of dimeric recombinant soluble FcγRIIa (rsFcγRIIa) to determine the proximity of pairs of IgG antibodies in immune cell‐activating ICs 14. The binding of dimeric rsFcγRIIa in this assay is correlated with the capacity of IgG ICs to activate FcγR‐dependent cellular responses 14, 15. In this study, we tested the capacity of this unique dimeric rsFcγRIIa to distinguish pathogenic antibodies, which recognize PF4–heparin complexes and are able to activate platelets, from clinically irrelevant, non‐pathogenic antibodies.
Materials and methods
Plasma samples were obtained from 27 medical and surgical inpatients based at a tertiary hospital, the Royal Adelaide Hospital, in Adelaide, Australia, in whom HIT was suspected. Local ethics committee approval was obtained prior to the commencement of the study. The collection of samples conformed to institutional guidelines. Both plasma from citrated blood and sera were prepared for analysis. For the purposes of this study, and to ensure that HIT cases reflected the integration of both clinical and laboratory criteria, a diagnosis of HIT was defined as a 4T score of ≥ 4 and a positive SRA result (> 20% at 0.1 U mL−1 heparin, and suppression at 100 U mL−1 heparin) 16. Levels of PF4–heparin autoantibodies were analyzed with an IgG‐specific solid‐phase ELISA (GTI, Waukesha, WI, USA) 17 and with the HemosIL AcuStar HIT IgG‐specific assay (Instrumentation Laboratory, Bedford, MA, USA) 18 under standardized laboratory conditions. High specificity with the HemosIL AcuStar HIT IgG‐specific assay has been previously reported 17.
The production and use of dimeric rsFcγRIIa (His131 allelic form) has been described previously 14. To assess the ability of dimeric rsFcγRIIa to differentially bind pathogenic versus non‐pathogenic HIT antibodies, patient plasma was used in a PF4–heparin IgG ELISA kit (Diagnostica Stago, Melbourne, Victoria, Australia) at a plasma dilution of 1 : 40, and with incubation and washing steps according to the manufacturers’ instructions. The bound anti‐PF4–heparin antibodies were then reacted with dimeric rsFcγRIIa–biotin (0.2 μg mL−1) in phosphate‐buffered saline (PBS) diluent containing 1 mm EDTA, 0.05% (v/v) Tween‐20 and 1% (w/v) bovine serum albumin for 1 h at 37 °C, and this was followed by five cycles of filling and aspirating wells with wash buffer (PBS, 0.05% v/v Tween‐20). Bound receptor was then detected by the incubation with a 1 : 10 000 dilution of high‐sensitivity horseradish peroxidase–streptavidin (Pierce, Rockford, IL, USA), for 1 h at 37 °C, washed 10 times, and incubated with TMB Single solution (Life Technologies, Mulgrave, Victoria, Australia). The reaction was terminated with an equal volume of 1 m HCl, and absorbance at 450 nm was determined. An in‐house plasma sample from a patient with confirmed HIT (4T score of 8, an SRA release of 94.2% with suppression of serotonin release with high‐dose heparin, and an anti‐heparin–PF4 antibody OD of > 3.0 by IgG ELISA) was used as a standard to define a nominal 100% response, to which absorbance values were normalized.
Statistical analysis was performed with graphpad prism version 6.05 (GraphPad Software, San Diego, CA, USA). The optimal OD cut‐off for the dimeric rsFcγRIIa assay (normalized value = 3) was confirmed by receiver operating characteristic analysis (not shown). Cut‐off values for the PF4–heparin IgG ELISA (OD = 0.4) and for the HemosIL AcuStar HIT IgG‐specific assay (1 U mL−1) were defined according to the manufacturers’ instructions. SRA positivity was defined as > 20% serotonin release with low‐dose heparin at 0.1 U mL−1, and < 20% release with a high heparin concentration of 100 U mL−1.
Results and discussion
A cohort of 27 patients with suspected HIT were evaluated, of whom 13 were considered to be HIT‐positive because of a positive SRA finding and a 4Ts score of ≥ 4 6. Samples were also evaluated for anti‐PF4–heparin antibodies with PF4–heparin IgG ELISA and the HemosIL AcuStar HIT IgG‐specific assay. The ability of a novel engineered dimeric rsFcγRIIa (His131) to detect pathogenic anti‐PF4–heparin antibodies was evaluated by comparison with these established assays.
The novel dimeric rsFcγRIIa assay, AcuStar HIT IgG‐specific assay and PF4–heparin IgG ELISA all showed significantly higher mean activity for HIT‐positive samples than for HIT‐negative samples (all assays, Mann–Whitney U‐test P < 0.0001; Figure 1A). The performance of the assays in distinguishing HIT patients is summarized in Table 1. The dimeric rsFcγRIIa assay correctly assigned 93% of the patients (25/27) and did not detect two HIT samples (i.e. two false negatives). This was similar to the performance of the AcuStar HIT IgG‐specific assay, which correctly assigned 96%, with only one false negative. These two assays contrasted with the more sensitive PF4–heparin IgG ELISA, which had a lower specificity (71%), with four false positives from this cohort. Larger patient groups that include differing clinical groupings will be necessary to establish robustly the predictive value of this approach.
Figure 1.
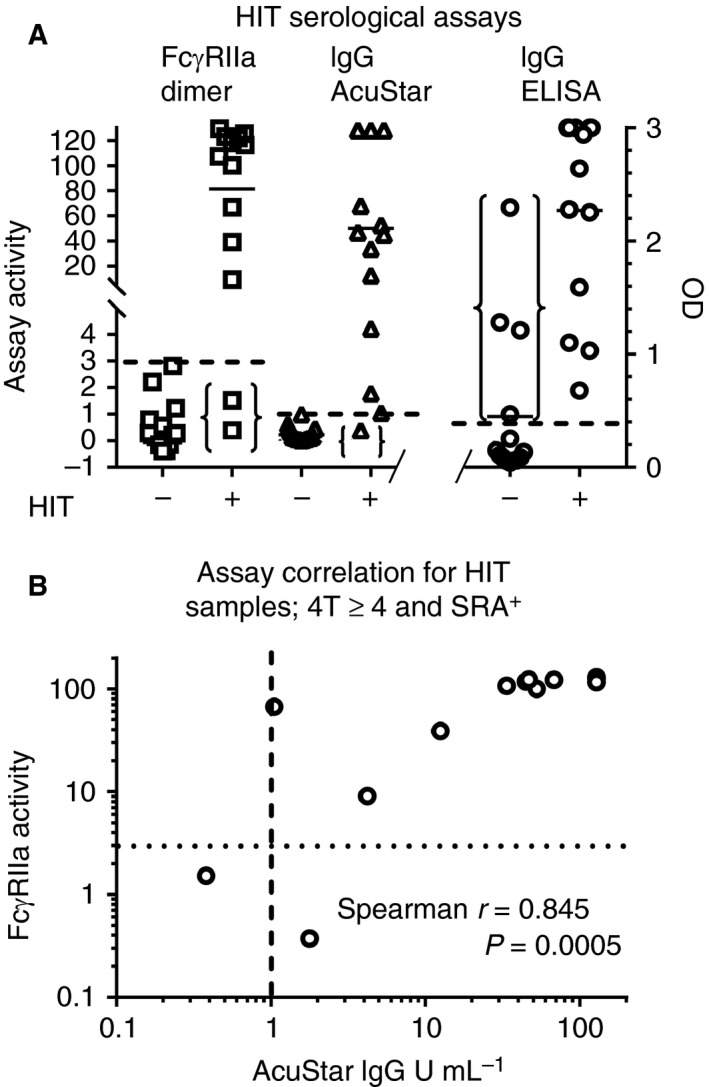
Comparison of the novel dimeric recombinant soluble FcγRIIa (rsFcγRIIa) assay with existing serological assays for the detection of heparin‐induced thrombocytopenia (HIT) antibodies. (A) Suspected HIT patients were segregated on the basis of a positive serotonin release assay (SRA) result and a 4Ts score of ≥ 4. The three dashed lines indicate the cut‐off values for a positive result with each assay. These thresholds were 3% of the normalized signal for the dimeric rsFcγRIIa assay: 1 U mL −1 for the AcuStar HIT IgG‐specific assay, and an OD of 0.4 (right y‐axis) for the platelet factor 4 (PF4)–heparin IgG ELISA. Two sets of parentheses mark the false negatives from the assays: two from the dimeric rsFcγRIIa assay, and one from the AcuStar HIT IgG‐specific assay. The third set of parentheses marks the four false positives from the PF4–heparin IgG ELISA. (B) The dimeric rsFcγRIIa and AcuStar HIT IgG‐specific assays were correlated for the HIT‐positive (i.e. 4T score of ≥ 4 and SRA‐positive) samples. Cut‐off values for the two assays are shown as dotted and dashed lines, respectively. HIT samples with low antibody levels are more sensitively identified by combining both the dimeric rsFcγRIIa and AcuStar HIT IgG‐specific assays. When HIT positivity is defined as rsFcγRII a positivity and/or AcuStar HIT IgG‐specific assay positivity, sensitivity is improved over that with either assay used alone.
Table 1.
Immunoassays of patient plasma (n = 27) with or without heparin‐induced thrombocytopenia (HIT) defined by a positive serotonin release assay result and a 4Ts score of ≥ 4
| Assay | Positive test | Negative test | Specificity | Sensitivity | ||
|---|---|---|---|---|---|---|
| True | False | True | False | |||
| Dimeric rsFcγRIIa | 11 | 0 | 14 | 2 | 1.00 | 0.85 |
| PF4–heparin IgG | 13 | 4 | 10 | 0 | 0.71 | 1.00 |
| AcuStar HIT IgG‐specific | 12 | 0 | 14 | 1 | 1.00 | 0.92 |
PF4, platelet factor 4; rsFcγRIIa, recombinant soluble FcγRIIa.
A comparison of the dimeric rsFcγRIIa and AcuStar HIT IgG‐specific assays for the HIT‐positive samples showed that these two assays correlated more strongly with each other (Spearman's correlation r = 0.845, P = 0.0005) than either did with the PF4–heparin IgG ELISA (ELISA versus AcuStar assay, r = 0.813, P = 0.0015; ELISA versus dimeric rsFcγRIIa assay, r = 0.673, P = 0.015).
As the SRA is a definitive functional assay for the diagnosis of HIT, and the main limitation of the PF4–heparin IgG ELISA is that it gives false positives, we examined the samples that were positive with the PF4–heparin IgG ELISA (Figure 2A) and the relationship between their activities in the SRA and in the other assays (Figure 2B,C). Both the AcuStar HIT IgG‐specific and dimeric rsFcγRIIa assays appropriately assigned low, below cut‐off, values to the SRA‐negative samples, although some were very near the cut‐off values of both assays. Furthermore, the AcuStar HIT IgG‐specific and dimeric rsFcγRIIa assays also correctly assigned as negative a sample (see filled blue star; Figure 2) that had ~ 50% release at both high and low heparin doses, and was weakly positive with the PF4–heparin IgG ELISA. The AcuStar HIT IgG‐specific and dimeric rsFcγRIIa assays, although correlating strongly (Figure 1B), showed some important differences (Figure 2B,C). One HIT patient plasma sample was weakly AcuStar HIT IgG‐specific assay‐positive (1.78 U mL−1; cut‐off of 1.0 U mL−1) and dimeric rsFcγRIIa assay‐negative (0.4% response; cut‐off of 3%; open triangle, Figure 2). Another HIT patient was weakly positive with the AcuStar HIT IgG‐specific assay (1.05 U mL−1) but scored strongly in the dimeric rsFcγRIIa assay (67% response; open square, Figure 2). Thus, defining samples as HIT‐positive according to AcuStar HIT IgG‐specific assay‐positive and/or dimeric rsFcγRIIa assay‐positive criteria may be an approach for improving HIT prediction with either assay alone.
Figure 2.
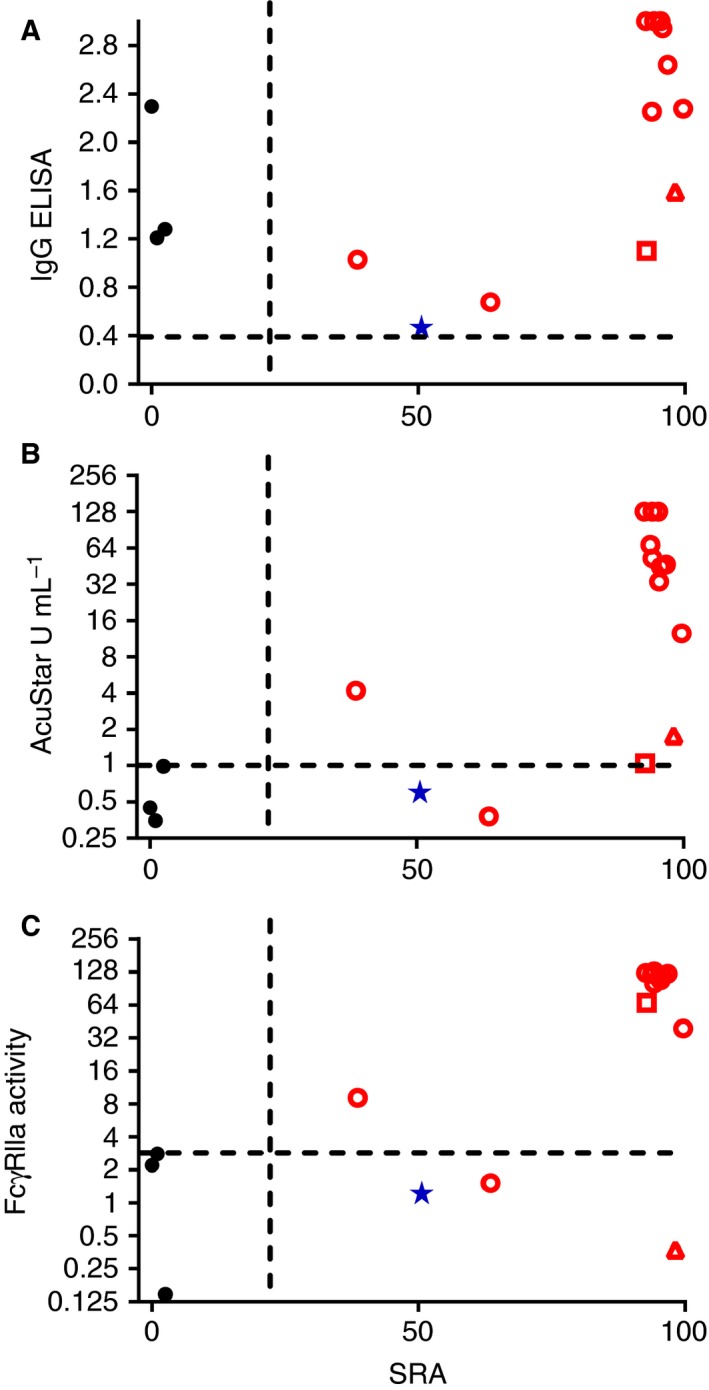
Correlation of the novel dimeric recombinant soluble FcγRIIa (rsFcγRIIa) assay and the existing serological assays with serotonin release. Results from all the serological assays of samples positive in the IgG ELISA, including four HIT false positives (filled blue star and black circles), were compared against the SRA results. The SRA (release at 0.1 U mL−1 heparin) was compared with (A) the PF4–heparin IgG ELISA, (B) the AcuStar HIT IgG‐specific assay, and (C) the novel dimeric rsFcγRIIa assay. The dashed lines indicate the cut‐off values for a positive result with each assay. Open symbols represent HIT‐diagnosed patients (positive SRA result and a 4Ts score of ≥ 4). Filled symbols represent PF4–heparin IgG ELISA‐positive HIT‐negative patients, with the blue star symbol indicating one SRA‐negative patient with equivalent levels of serotonin release with both high‐dose and low‐dose heparin stimulation. The open triangle indicates an HIT patient plasma sample that was weakly AcuStar HIT IgG‐specific assay‐positive and dimeric rsFcγRIIa assay‐negative, and the open square indicates an HIT patient plasma sample that was weakly AcuStar HIT IgG‐specific assay‐positive and strongly dimeric rsFcγRIIa assay‐positive. [Color figure can be viewed at wileyonlinelibrary.com]
In HIT, pathogenic PF4–heparin IgG complexes engage and aggregate FcγRIIa on platelets 1, 3, 5, and possibly other FcγRs on monocytes 4, 19. The size and stoichiometry of IgG ICs are major determinants of both the avidity of interaction with the low‐affinity FcγRs 20 and the capacity of anti‐PF4–heparin antibodies to activate cells. In a large proportion of laboratories, the initial clinical suspicion of HIT is followed by the performance of standard HIT IgG ELISAs. However, the diagnosis of HIT and subsequent decisions regarding patient anticoagulation and management are made challenging by the prevalence of non‐pathogenic anti‐PF4–heparin antibodies that are unable to activate FcγRs. Although the levels of anti‐PF4–heparin antibodies as detected by HIT IgG ELISA provide useful information, this measurement alone does not provide sufficient specificity for the diagnosis of HIT 7, 21.
Thus, a platelet functional HIT assay, such as platelet aggregation or the SRA, is crucial for diagnosis. The SRA, however, involves handling of radioactive isotopes and requires specialized personnel, specialized equipment and rapid access to functional ‘high‐responder’ platelets that may not be routinely available. Use of the SRA is also limited to major reference laboratories. Refinements to improve the specificity of the HIT anti‐PF4–heparin antibody ELISA have included raising the OD cut‐off for positive results and the addition of a high‐dose heparin confirmatory step 16, 22. However, there is still a need for improved serological HIT assays.
We investigated the potential of dimeric rsFcγRIIa, a probe of the FcγR‐mediated effector functionality of IgG complexes, to improve the specificity of a PF4–heparin IgG assay. The novel assay had superior specificity to the detection of PF4–heparin IgG by ELISA, and performed similarly to the HemosIL AcuStar HIT IgG‐specific assay, a test in which high specificity has been reported previously 18. The combined use of both the dimeric rsFcγRIIa and HemosIL AcuStar HIT IgG‐specific assays to assign HIT correctly identified some HIT samples that would have been false negative with the use of either assay alone. The detection of HIT antibodies with the dimeric rsFcγRIIa assay adds a functional qualitative component to the assay that mechanistically underlies the possible improved specificity and utility.
Dimeric rsFcγRIIa binding requires closely spaced pairs of antibodies, as occurs in ICs 14, and has been used to detect FcγR functional antibodies against influenza virus and HIV envelope proteins 14, 15, 23, 24, 25, 26, 27, 28. HIT pathogenesis requires Fc‐mediated clustering of platelet FcγRIIa, but two SRA‐positive samples were not detected with the dimeric rsFcγRIIa‐binding assay. Studies of HIT complexes have shown that the formation of the PF4–heparin–IgG complex is dynamic and, importantly, the proximity of the IgG Fcs in the pathogenic complexes is influenced by the binding of heparin, and the epitope specificity and avidity of the bound IgG 11, 12, 29, 30. The failure of the dimeric rsFcγRIIa assay to detect these two HIT samples suggests that the Fcs were not appropriately presented in the current ELISA format. Thus, the assay may need further optimization to a format that mimics the dynamic behavior of the PF4 complexes that cluster platelet FcγRIIa in vivo. Nonetheless, our proof‐of‐concept study has shown that the dimeric rsFcγRIIa assay has the potential to be predictive of HIT and warrants further investigation.
This study has established a novel FcγRIIa probe as a powerful surrogate for evaluating HIT antibody functionality. The use of dimeric rsFcγRIIa also offers the advantages that it is simple, scalable, and adaptable to any format, such as multiplexing 25 and flow cytometry.
Addendum
B. D. Wines, E. E. Gardiner, and P. M. Hogarth designed the study, reviewed data, and drafted the manuscript. B. D. Wines, S. Esparon, E. E. Gardiner, and C. W. Tan performed experiments. All authors analyzed and interpreted the data, and critically reviewed the manuscript.
Disclosure of Conflict of Interests
P. M. Hogarth and B. D. Wines report receiving grants from the Australian Centre for HIV and Hepatitis Virology Research during the conduct of the study, and have a patent issued (to the Burnet Institute): ‘Binding assays and method for probing antibody function with fc binding multimers WO 2017054033 A1’. S. Esparon reports receiving grants from the Australian Centre for HIV and Hepatitis Virology Research during the conduct of the study. R. Baker reports providing clinical trial support for Biogen Idec, Boehringer Ingelheim, Bayer, Shire, Pfizer, Daiichi Sankyo, Portola, Alexion Pharmaceuticals, Astellas, and CSL Behring, serving on clinical advisory boards of Bayer, Shire, Pfizer, and Amgen, and receiving research support from Bristol‐Meyers Squibb, Shire, and Bayer. E. Duncan reports receiving personal fees from Novo Nordisk. The other authors state that they have no conflict of interest.
Acknowledgements
This work was funded by the National Health and Medical Research Council of Australia and the Victorian Operational Infrastructure Scheme. The authors thank T. Brighton for generously providing the SRA data, J. Jing for expert technical assistance, and A. Chenoweth for reading the manuscript.
Wines BD, Tan CW, Duncan E, McRae S, Baker RI, Andrews RK, Esparon S, Gardiner EE, Hogarth PM. Dimeric FcγR ectodomains detect pathogenic anti‐platelet factor 4–heparin antibodies in heparin‐induced thromobocytopenia. J Thromb Haemost 2018; 16: 2520–5.
Manuscript handled by: W. Bergmeier
Final decision: P. H. Reitsma, 24 September 2018
References
- 1. Arepally GM. Heparin‐induced thrombocytopenia. Blood 2017; 129: 2864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fabris F, Luzzatto G, Stefani PM, Girolami B, Cella G, Girolami A. Heparin‐induced thrombocytopenia. Haematologica 2000; 85: 72–81. [PubMed] [Google Scholar]
- 3. Greinacher A. Heparin‐induced thrombocytopenia. N Engl J Med 2015; 373: 1883–4. [DOI] [PubMed] [Google Scholar]
- 4. Tutwiler V, Madeeva D, Ahn HS, Andrianova I, Hayes V, Zheng XL, Cines DB, McKenzie SE, Poncz M, Rauova L. Platelet transactivation by monocytes promotes thrombosis in heparin‐induced thrombocytopenia. Blood 2016; 127: 464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qiao J, Al‐Tamimi M, Baker RI, Andrews RK, Gardiner EE. The platelet Fc receptor, FcγRIIa. Immunol Rev 2015; 268: 241–52. [DOI] [PubMed] [Google Scholar]
- 6. Vianello F, Sambado L, Scarparo P, Lombardi A, Bernardi D, Plebani M, Fabris F. Comparison of three different immunoassays in the diagnosis of heparin‐induced thrombocytopenia. Clin Chem Lab Med 2015; 53: 257–63. [DOI] [PubMed] [Google Scholar]
- 7. Nagler M, Bachmann LM, ten Cate H, ten Cate‐Hoek A. Diagnostic value of immunoassays for heparin‐induced thrombocytopenia: a systematic review and meta‐analysis. Blood 2016; 127: 546–57. [DOI] [PubMed] [Google Scholar]
- 8. Arepally GM, Kamei S, Park KS, Kamei K, Li ZQ, Liu W, Siegel DL, Kisiel W, Cines DB, Poncz M. Characterization of a murine monoclonal antibody that mimics heparin‐induced thrombocytopenia antibodies. Blood 2000; 95: 1533–40. [PubMed] [Google Scholar]
- 9. Reilly MP, Sinha U, Andre P, Taylor SM, Pak Y, Deguzman FR, Nanda N, Pandey A, Stolla M, Bergmeier W, McKenzie SE. PRT‐060318, a novel Syk inhibitor, prevents heparin‐induced thrombocytopenia and thrombosis in a transgenic mouse model. Blood 2011; 117: 2241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reilly MP, Taylor SM, Hartman NK, Arepally GM, Sachais BS, Cines DB, Poncz M, McKenzie SE. Heparin‐induced thrombocytopenia/thrombosis in a transgenic mouse model requires human platelet factor 4 and platelet activation through FcγRIIA. Blood 2001; 98: 2442–7. [DOI] [PubMed] [Google Scholar]
- 11. Cai Z, Yarovoi SV, Zhu Z, Rauova L, Hayes V, Lebedeva T, Liu Q, Poncz M, Arepally G, Cines DB, Greene MI. Atomic description of the immune complex involved in heparin‐induced thrombocytopenia. Nat Commun 2015; 6: 8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cai Z, Zhu Z, Greene MI, Cines DB. Atomic features of an autoantigen in heparin‐induced thrombocytopenia (HIT). Autoimmun Rev 2016; 15: 752–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rollin J, Pouplard C, Sung HC, Leroux D, Saada A, Gouilleux‐Gruart V, Thibault G, Gruel Y. Increased risk of thrombosis in FcγRIIA 131RR patients with HIT due to defective control of platelet activation by plasma IgG2. Blood 2015; 125: 2397–404. [DOI] [PubMed] [Google Scholar]
- 14. Wines BD, Vanderven HA, Esparon SE, Kristensen AB, Kent SJ, Hogarth PM. Dimeric FcγR ectodomains as probes of the Fc receptor function of anti‐influenza virus IgG. J Immunol 2016; 197: 1507–16. [DOI] [PubMed] [Google Scholar]
- 15. Kristensen AB, Lay WN, Ana‐Sosa‐Batiz F, Vanderven HA, Madhavi V, Laurie KL, Carolan L, Wines BD, Hogarth M, Wheatley AK, Kent SJ. Antibody responses with Fc‐mediated functions after vaccination of HIV‐infected subjects with trivalent influenza vaccine. J Virol 2016; 90: 5724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cuker A, Rux AH, Hinds JL, Dela Cruz M, Yarovoi SV, Brown IA, Yang W, Konkle BA, Arepally GM, Watson SP, Cines DB, Sachais BS. Novel diagnostic assays for heparin‐induced thrombocytopenia. Blood 2013; 121: 3727–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tan CW, Ward CM, Morel‐Kopp MC. Evaluating heparin‐induced thrombocytopenia: the old and the new. Semin Thromb Hemost 2012; 38: 135–43. [DOI] [PubMed] [Google Scholar]
- 18. Althaus K, Hron G, Strobel U, Abbate R, Rogolino A, Davidson S, Greinacher A, Bakchoul T. Evaluation of automated immunoassays in the diagnosis of heparin induced thrombocytopenia. Thromb Res 2013; 131: e85–90. [DOI] [PubMed] [Google Scholar]
- 19. Kasthuri RS, Glover SL, Jonas W, McEachron T, Pawlinski R, Arepally GM, Key NS, Mackman N. PF4/heparin‐antibody complex induces monocyte tissue factor expression and release of tissue factor positive microparticles by activation of FcγRI. Blood 2012; 119: 5285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daeron M. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 2009; 113: 3716–25. [DOI] [PubMed] [Google Scholar]
- 21. Greinacher A, Juhl D, Strobel U, Wessel A, Lubenow N, Selleng K, Eichler P, Warkentin TE. Heparin‐induced thrombocytopenia: a prospective study on the incidence, platelet‐activating capacity and clinical significance of antiplatelet factor 4/heparin antibodies of the IgG, IgM, and IgA classes. J Thromb Haemost 2007; 5: 1666–73. [DOI] [PubMed] [Google Scholar]
- 22. McFarland J, Lochowicz A, Aster R, Chappell B, Curtis B. Improving the specificity of the PF4 ELISA in diagnosing heparin‐induced thrombocytopenia. Am J Hematol 2012; 87: 776–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Madhavi V, Kulkarni A, Shete A, Lee WS, McLean MR, Kristensen AB, Ghate M, Wines BD, Hogarth PM, Parsons MS, Kelleher A, Cooper DA, Amin J, Emery S, Thakar M, Kent SJ; ENCORE1 Study Group . Effect of combination antiretroviral therapy on HIV‐1‐specific antibody‐dependent cellular cytotoxicity responses in subtype B‐ and subtype C‐infected cohorts. J Acquir Immune Defic Syndr 2017; 75: 345–53. [DOI] [PubMed] [Google Scholar]
- 24. Madhavi V, Wines BD, Amin J, Emery S, ENCORE1 Study Group , Lopez E, Kelleher A, Sydney LTNP Study Group , Center RJ, Hogarth PM, Chung AW, Kent SJ, Stratov I. HIV‐1 Env‐ and Vpu‐specific antibody‐dependent cellular cytotoxicity responses associated with elite control of HIV. J Virol 2017; 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McLean MR, Madhavi V, Wines BD, Hogarth PM, Chung AW, Kent SJ. Dimeric Fcγ receptor enzyme‐linked immunosorbent assay to study HIV‐specific antibodies: a new look into breadth of Fcγ receptor antibodies induced by the RV144 vaccine trial. J Immunol 2017; 199: 816–26. [DOI] [PubMed] [Google Scholar]
- 26. Parsons MS, Lloyd SB, Lee WS, Kristensen AB, Amarasena T, Center RJ, Keele BF, Lifson JD, LaBranche CC, Montefiori D, Wines BD, Hogarth PM, Swiderek KM, Venturi V, Davenport MP, Kent SJ. Partial efficacy of a broadly neutralizing antibody against cell‐associated SHIV infection. Sci Transl Med 2017; 9. [DOI] [PubMed] [Google Scholar]
- 27. Vanderven HA, Liu L, Ana‐Sosa‐Batiz F, Nguyen TH, Wan Y, Wines B, Hogarth PM, Tilmanis D, Reynaldi A, Parsons MS, Hurt AC, Davenport MP, Kotsimbos T, Cheng AC, Kedzierska K, Zhang X, Xu J, Kent SJ. Fc functional antibodies in humans with severe H7N9 and seasonal influenza. JCI Insight 2017; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wines BD, Billings H, McLean MR, Kent SJ, Hogarth PM. Antibody functional assays as measures of Fc receptor‐mediated immunity to HIV – new technologies and their impact on the HIV vaccine field. Curr HIV Res 2017; 15: 202–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nguyen TH, Medvedev N, Delcea M, Greinacher A. Anti‐platelet factor 4/polyanion antibodies mediate a new mechanism of autoimmunity. Nat Commun 2017; 8: 14945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nguyen TH, Greinacher A. Platelet factor 4/heparin complexes present epitopes differently on solid‐phase vs platelet surfaces. Blood 2017; 129: 3498–501. [DOI] [PubMed] [Google Scholar]


