Abstract
Essentials.
Procoagulant platelets can be detected using GSAO in human whole blood.
Stable coronary artery disease is associated with a heightened procoagulant platelet response.
Agonist‐induced procoagulant platelet response is not inhibited by aspirin alone.
Collagen plus thrombin induced procoagulant platelet response is partially resistant to clopidogrel.
Summary
Background
Procoagulant platelets are a subset of highly activated platelets with a critical role in thrombin generation. Evaluation of their clinical utility in thrombotic disorders, such as coronary artery disease (CAD), has been thwarted by the lack of a sensitive and specific whole blood assay.
Objectives
We developed a novel assay, utilizing the cell death marker, GSAO [(4‐(N‐(S‐glutathionylacetyl)amino)phenylarsonous acid], and the platelet activation marker, P‐selectin, to identify procoagulant platelets in whole blood by flow cytometry.
Patients/Methods
Using this assay, we characterized the procoagulant platelet population in healthy controls and a cohort of patients undergoing elective coronary angiography.
Results
In patients with CAD, compared with patients without CAD, there was a heightened procoagulant platelet response to thrombin (25.2% vs. 12.2%), adenosine diphosphate (ADP) (7.8% vs. 2.7%) and thrombin plus collagen (27.2% vs. 18.3%). The heightened procoagulant platelet potential in CAD patients was not associated with other markers of platelet function, including aggregation, dense granule release and activation of α2bβ3 integrin. Although dual antiplatelet therapy (DAPT) was associated with partial suppression of procoagulant platelets, this inhibitory effect on a patient level could not be predicted by aggregation response to ADP and was not fully suppressed by clopidogrel.
Conclusions
We report for the first time that procoagulant platelets can be efficiently detected in a few microliters of whole blood using the cell death marker, GSAO, and the platelet activation marker, P‐selectin. A heightened procoagulant platelet response may provide insight into the thrombotic risk of CAD and help identify a novel target for antiplatelet therapies in CAD.
Keywords: coronary artery disease, flow cytometry, platelet activation, platelet aggregation inhibitors, platelet function studies, procoagulant platelets
Introduction
Procoagulant platelets are an important functional platelet subset in thrombotic disorders. These platelets provide a membrane surface for the assembly of coagulation factors, an essential step for generation of the thrombin burst and fibrin formation, thereby linking primary and secondary hemostasis 1, 2, 3. In animal models, procoagulant platelets localize preferentially at sites of collagen exposure and are maximally generated by exposure to a combination of thrombin and collagen 4.
Acute coronary syndrome is caused by a sudden decrease in coronary blood flow, usually as a result of occlusion of a coronary artery by a thrombus developing in response to rupture or erosion of an atheromatous plaque 5, 6. Platelets are crucial cellular determinants of the blood prothrombotic potential 7, 8, 9, which together with the plaque properties influences the magnitude of intraluminal thrombus (partial vs. occlusive). Despite their pivotal role in the pathogenesis of atherothrombosis 6, 10, there are no reports evaluating the procoagulant platelet subset in patients with coronary artery disease (CAD).
Clinical interest in measuring procoagulant platelets has been hampered by a lack of sensitive and specific assays. Phosphatidylserine (PS) exposure is a prerequisite for platelet procoagulant function; however, beyond PS exposure, the identity of procoagulant platelets remains debated 11, 12, 13, 14, 15. We recently published a flow cytometry method for washed platelets using a combination of a cell death marker, GSAO [(4‐(N‐(S‐glutathionylacetyl)amino)phenylarsonous acid], and a platelet activation marker, P‐selectin 16. When tagged with a reporter compound such as the fluorophore AF647 at the γ‐glutamyl residue, tagged GSAO is taken up by procoagulant platelets and retained in their cytoplasm by covalent bonding to proteins containing closely spaced di‐thiols 17. Platelets demonstrating intracellular GSAO uptake and exposure of P‐selectin (indicating degranulation) were shown to undergo cyclophilin D‐mediated necrosis and provide a procoagulant surface supportive of thrombin generation 16. Therefore, we refer to this GSAO+/P‐selectin+ platelet subset as procoagulant platelets.
In the present study we establish a method for detection of GSAO+/P‐selectin+ procoagulant platelets in whole blood and investigate its application in patients with stable CAD. We demonstrate that patients with CAD have heightened procoagulant platelet responses and hypersensitivity to thrombin and adenosine diphosphate (ADP) compared with either healthy controls and patients without CAD, and that this novel response is not predicted by other markers of platelet activation.
Methods
Patients and controls
Study approval
Human studies were approved by the University of New South Wales (UNSW) (HC13191), the St Vincent's Hospital (SVH) and Concord Repatriation General Hospital (CRGH) Human Research Ethics Committees (HREC/12/SVH/11, HREC/14/CRGH/21 and HREC/15/CRGH/54) and conducted according to the Declaration of Helsinki. All healthy volunteers and patients gave written informed consent.
Study populations
In the CAD derivation cohort, clinically stable patients over 18 years of age with suspected CAD presenting for coronary angiography were prospectively recruited from St Vincent's Hospital (SVH) (Sydney, Australia). Exclusion criteria were pregnancy, known hematological disorder affecting platelet number or function, and use of GPIIb/IIIa inhibitors. For the validation cohort, patients with the same criteria were recruited from Concord Repatriation General Hospital (CRGH) (Sydney, Australia) and evaluated for stable versus unstable CAD. In both cohorts, CAD was defined by presence of luminal stenosis on angiography. Healthy control subjects were enrolled from staff and students at the UNSW, ANZAC Research Institute and CRGH (Sydney, Australia). Healthy control subjects abstained from antiplatelet therapies including NSAIDs for 14 days.
Materials
GSAO [4‐(N‐((S‐glutathionyl) acetyl)amino)benzoic acid] and control compound GSCA were synthesized and conjugated to amine‐reactive succinimidyl ester Alexa Fluor 647‐NHS (AF647) (Life Technologies, Carlsbad, CA, USA) as described previously 17, 18, 19. Bovine thrombin and Gly‐Pro‐Arg‐Pro (GPRP) were purchased from Sigma Aldrich (Sydney, NSW, Australia), equine tendon collagen (type I) Collagen Reagens HORM from Takeda Pharmaceuticals (Linz, Austria), and adenosine 5‐diphosphate (ADP) from Helena Laboratories (Mt Waverly, VIC, Australia).
Blood collection
Blood for procoagulant platelet assays was collected into 3.2% citrate tubes. Healthy control samples were collected from the antecubital fossa vein via a 21G butterfly needle. In the derivation cohort, multiple arterial blood samples were collected from 60 patients. The first blood sample was taken from the femoral or radial artery immediately following the sheath insertion and before intra‐arterial heparin administration. Paired arterial and peripheral venous blood samples gave similar procoagulant platelet responses (Figure S1). Blood from patients in the validation cohort was collected via a 20G intravenous cannula inserted into a cubital fossa vein after discarding the first 3 mL.
Whole blood procoagulant platelet assay
Our previously described assay using combination GSAO/P‐selectin to identify procoagulant platelet subsets in washed platelets 16 was modified for detection in whole citrated blood. Briefly, 15 μL of whole blood was diluted with Hank's balanced salt solution (HBSS, pH adjusted to 7.35), final concentrations of GPRP, (2.5 mm) (Sigma‐Aldrich) and calcium, (2.5 mm) ‐ with and without agonists. Reactions were stopped after 10 min of incubation at room temperature by a 20‐fold dilution with HBSS, aliquots labeled for 15 min with fluorescent tagged antibodies to CD62P (Psel.KO2.3) (eBioscience, San Diego, CA, USA), CD41a (HIP8) (BD Biosciences, San Diego, CA, USA), CD45 (HI30) (StemCell Technologies, Vancouver, BC, Canada) and GSAO conjugated to AF647. Samples were then fixed with two volumes of PAMFix (Platelet Solutions Ltd, Nottingham, UK), transferred to FACS tubes, washed once, resuspended and stored at room temperature in the dark for 1 h prior to analysis on a BD FACS Canto II or LSRFortessa with acquisition of 10 000 platelet events.
Whole blood aggregometry and flow cytometry assays of platelet activation
Whole blood aggregometry and standard flow cytometry assays 20, 21, 22 are described in Data S1.
Statistical analyses
Statistical analyses were performed using statistical software (either PRISM 6.05, GraphPad Software, San Diego, CA, USA, or S‐PLUS 8, TIBCO Software, Palo Alto, CA, USA). Differences were considered statistically significant at P < 0.05. Further details can be found in Data S1.
Results
Procoagulant platelets can be detected using GSAO in human whole blood
We have previously determined that GSAO identifies the coagulation factor‐binding platelet population in human platelet‐rich plasma (PRP), whereas annexin V had poorer specificity 16 (Figure S2). To identify this population in whole blood, platelets were defined as events within the CD41a‐positive and CD45‐negative region (platelet gate) (Fig. 1A). P‐selectin and GSAO thresholds delimiting the procoagulant platelet (GSAO+/P‐selectin+) region were determined on agonist‐treated samples using isotype‐matched antibody and GSCA controls, respectively (Fig. 1B). Procoagulant platelets are minimal in healthy human whole blood in the absence of an activating factor (Fig. 1C). Following agonist stimulation, procoagulant (GSAO+/P‐selectin+) platelets are generated (Fig. 1D). The strongest single agonist for procoagulant platelet formation in whole blood was thrombin (Fig. 1E). Stimulation with collagen alone resulted in generation of few procoagulant platelets, but a combination of collagen and thrombin was synergistic (Fig. 1E, F) as was previously demonstrated in washed platelets 16. As expected, the weaker platelet agonist, ADP, had little effect on the generation of procoagulant platelets in healthy controls (Fig. 1E). A small proportion of platelets takes up cell death marker GSAO without evidence of activation (P‐selectin negative) (Fig. 1B), and is referred to as apoptotic as per our previous characterization 16, and did not increase in response to agonist stimulation (Fig. 1G).
Figure 1.
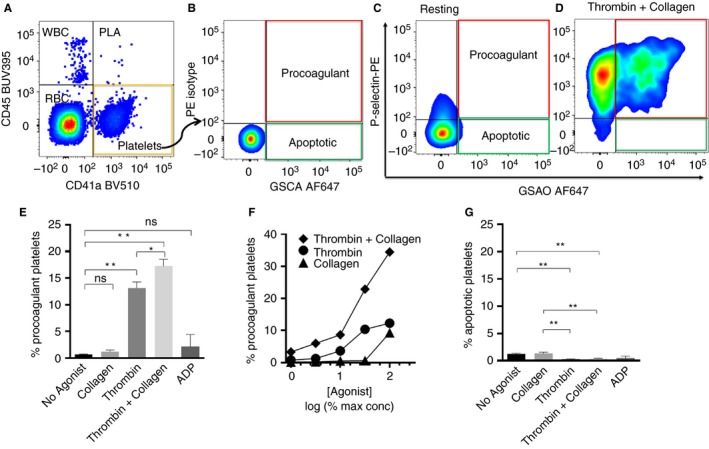
Gating strategy for detection of procoagulant platelets in human whole blood using the cytoplasmic cell death marker (GSAO). (A) Platelets are defined as CD41a positive but CD45 negative events. (B) Threshold values for P‐selectin and GSAO‐positive regions are set using a thrombin plus collagen‐stimulated sample stained with IgG isotype control and control GSCA, respectively. GSAO+/P‐selectin+ (Procoag) quadrant (outlined in red) contains procoagulant platelets. GSAO+/P‐selectin− (Apopt) quadrant (outlined in green) contains apoptotic platelets. (C) Very few platelets show the procoagulant phenotype (P‐selectin+/GSAO+) in unstimulated whole blood. (D) Following stimulation with thrombin plus collagen a significant proportion of platelets exhibited the procoagulant phenotype. (E) Characterization of the stimulus‐induced procoagulant platelet response in human whole blood. (F) Synergistic interaction between thrombin and collagen occurred at all levels of individual agonist concentrations. Normalized agonist dose (maximal [100%] for collagen [100 μg mL−1] and thrombin [6.3 U mL−1]) expressed as percent maximal is shown on a logarithmic scale. A representative example is shown. (G) Apoptotic platelets are a very small subpopulation that does not increase with agonist stimulation. (E, G) Mean ± SEM, n = 16–35. One‐way anova with Sidak's correction for multiple comparisons, *P < 0.05, **P < 0.0001. ADP, adenosine diphosphate; GSAO, [(4‐(N‐(S‐glutathionylacetyl)amino)phenylarsonous acid]; PLA, platelet leukocyte aggregates; WBC, white blood cells. [Color figure can be viewed at wileyonlinelibrary.com]
The procoagulant platelet response to physiological agonists thrombin, collagen and thrombin plus collagen was measured in 10 young healthy adults (Table 1). Unstimulated blood contained 0.66% (95% confidence interval [CI], 0.22–0.74) procoagulant platelets, which increased to 13.2% (95% CI, 10.8–15.15) with thrombin stimulation (P < 0.0001) and 17.2% (95% CI, 14.5–19.9) with thrombin/collagen (P < 0.0001).
Table 1.
Procoagulant platelet response to physiological agonists in healthy humans
| Agonist | Mean (n = 10) | Range (10th–90th percentile) |
|---|---|---|
| No agonist (resting) | 0.66% | 0.09–1.7% |
| Collagen (10 μg mL−1) | 1.2% | 0.27–2.9% |
| Thrombin (2 U mL−1) | 13.15% | 6.1–21.5% |
| Thrombin (2 U mL−1) and Collagen (10 μg mL−1) | 17.3% | 8.1–26.8% |
The combination of GSAO/P‐selectin has advantages over other flow cytometry markers for identification of procoagulant platelets in human whole blood
To evaluate matrix impact on agonist‐induced formation of procoagulant platelets, the procoagulant platelet response was compared between whole blood, PRP and washed platelets from the same individuals. Equal volumes of each substrate were processed in parallel according to the whole blood protocol described above. The proportion of procoagulant platelets was significantly higher in whole blood compared with either PRP or washed platelets (Fig. 2A).
Figure 2.
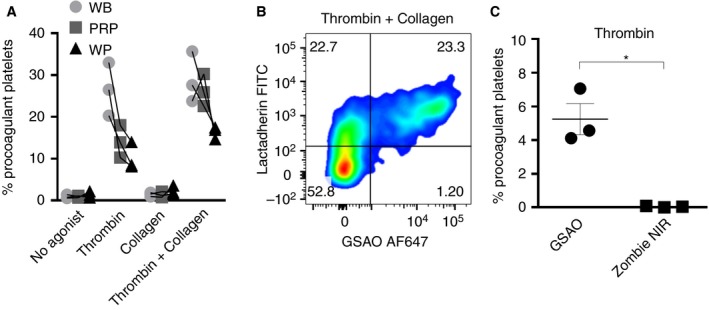
GSAO‐based assay has advantages over other markers of cell death for identification of procoagulant platelets in human whole blood. (A) Whole blood is the preferred substrate for demonstration of procoagulant platelet potential. Citrated blood samples from individuals were prepared as whole blood (WB), platelet‐rich plasma (PRP) or washed platelet (WP) preparations. Each preparation was treated with thrombin (2 U mL−1), collagen (10 μg mL−1), or a combination of both, then stained with GSAO/P‐selectin. WB demonstrated higher levels of GSAO+/P‐selectin+ platelets compared with PRP or WP from the same draw. (B) Most GSAO+ platelets externalized PS (lactadherin+); however, a significant proportion of lactadherin+ platelets did not take up GSAO. Pseudocolors are used to indicate population density. (C) Thrombin (2 U mL−1)‐treated WB samples were stained either with a standard panel of markers or a modified panel where GSAO was replaced with the amine reactive dye Zombie NIR, fixed and analyzed by flow cytometry. Procoagulant platelets identified by GSAO were not identified by Zombie NIR (n = 3, paired‐t‐test, *P < 0.05). GSAO, [(4‐(N‐(S‐glutathionylacetyl)amino)phenylarsonous acid]. [Color figure can be viewed at wileyonlinelibrary.com]
Although PS exposure is a prerequisite for coagulation factor binding 1, 15, 23, PS exposure alone is not considered sufficient for formation of procoagulant platelets 14, 15, 24. We demonstrate that all GSAO+ platelets have PS exposure by either lactadherin or annexin V binding, thus confirming the capacity of this platelet subset to bind coagulation factors. The same experiments demonstrated a strong correlation between GSAO uptake and surface binding of activated coagulation factor Xa (F Xa) (Fig. 2B, Figure S2A, S2B, S8). Imperfect correlation between PS and coagulation factor binding has previously been reported 4, 25 and under the conditions of our assay, a proportion of PS‐exposing platelets did not appear to bind FXa (Fig. 2B, Figure S2C). Significantly, GSAO uptake was limited to the subpopulation of PS‐exposing platelets that showed evidence of surface FXa binding. We compared GSAO with an alternative cytoplasmic cell death marker, Zombie NIR™, which enters cells with compromised membranes but binds cytoplasmic ligands via amine rather than dithiol linkage. Although Zombie NIR™ labeled necrotic nucleated mammalian cells in control experiments (Figure S3), it failed to detect procoagulant platelets in thrombin‐treated whole blood (Fig. 2C).
Coronary artery disease is associated with a heightened procoagulant platelet potential
Using the GSAO‐based whole blood assay we examined the procoagulant platelet potential of a derivation cohort of 60 patients undergoing routine coronary angiography (Table 2) (Figure S4). Of 54 eligible patients with no history of heparin exposure, 10 patients (18%) had no history of exposure to antiplatelet agents, whereas 33% were treated with dual antiplatelet therapy (DAPT). A P2Y12 inhibitor (clopidogrel) as a sole antiplatelet agent was taken by only one (2%) patient. Angiography confirmed abnormal coronary arteries in 29 (54%) patients. However, in those with no exposure to P2Y12 antagonists, angiographically confirmed CAD was observed less frequently (41%).
Table 2.
Derivation cohort patient demographics
| Clinical variables | All patients | No heparin before sample collection | No P2Y12 inhibitors | |||
|---|---|---|---|---|---|---|
| Number of patients | 60 | 54 | 27 | |||
| Age, median (IQR), years | 65 (56–75) | 66 (56–75) | 63 (56–75) | |||
| Gender | ||||||
| Male, no. (%) | 38 (63) | 33 (61) | 14 (52) | |||
| Female, no. (%) | 22 (37) | 21 (39) | 13 (48) | |||
| Smoking | ||||||
| Yes, no. (%) | 7 (12) | 7 (13) | 4 (15) | |||
| No, no. (%) | 47 (78) | 42 (78) | 22 (81) | |||
| Unknown, no. (%) | 6 (10) | 5 (9) | 1 (4) | |||
| Diabetes | ||||||
| Yes, no. (%) | 17 (28) | 15 (28) | 7 (26) | |||
| No, no. (%) | 37 (62) | 33 (61) | 19 (70) | |||
| Unknown, no. (%) | 6 (10) | 6 (11) | 1 (4) | |||
| Hypertension | ||||||
| Yes, no. (%) | 39 (65) | 35 (65) | 16 (59) | |||
| No, no. (%) | 17 (28) | 15 (28) | 10 (37) | |||
| Unknown, no. (%) | 4 (7) | 4 (7) | 1 (4) | |||
| Dyslipidemia | ||||||
| Yes, no. (%) | 43 (72) | 39 (72) | 18 (67) | |||
| No, no. (%) | 14 (23) | 13 (24) | 9 (33) | |||
| Unknown, no. (%) | 3 (5) | 2 (4) | – | |||
| Type of procedure | ||||||
| Elective, no. (%) | 48 (80) | 48 (89) | 27 (100) | |||
| Urgent, no. (%) | 12 (20) | 6 (11) | – | |||
| CAD confirmed by angiogram, no. (%) |
Yes 33 (55) |
No 22 (37) |
Yes 29 (54) |
No 20 (37) |
Yes 11 (41) |
No 16 (59) |
| Antiplatelet therapy, no. (%) | ||||||
| Aspirin | 27 (82) | 12 (55) | 23 (79) | 11 (55) | 9 (82) | 8 (50) |
| Clopidogrel | 18 (55) | 1 (5) | 15 (52) | 1 (5) | – | – |
| Prasugrel | – | 1 (5) | – | – | – | – |
| Ticagrelor | 6 (18) | 3 (14) | 2 (7) | 2 (10) | – | – |
| Aspirin + P2Y12 inhibitor | 17 (52) | 4 (18) | 14 (48) | 3 (15) | – | |
CAD, coronary artery disease; IQR, interquartile range.
Few patients undergoing angiography are not on antiplatelet agents, thus comparison was performed to determine if patients on aspirin could be analyzed together with patients without antiplatelet therapy. Consistent with our previous observations in healthy volunteers 16, exposure to aspirin was not associated with inhibition of a procoagulant platelet subset in response to either thrombin (odds ratio [OR], 1.1; P = 0.966; 95% CI, 0.7–1.7) or thrombin plus collagen (OR, 0.9; P = 0.554; 95% CI, 0.6–1.4) (Fig. 3A, B).
Figure 3.
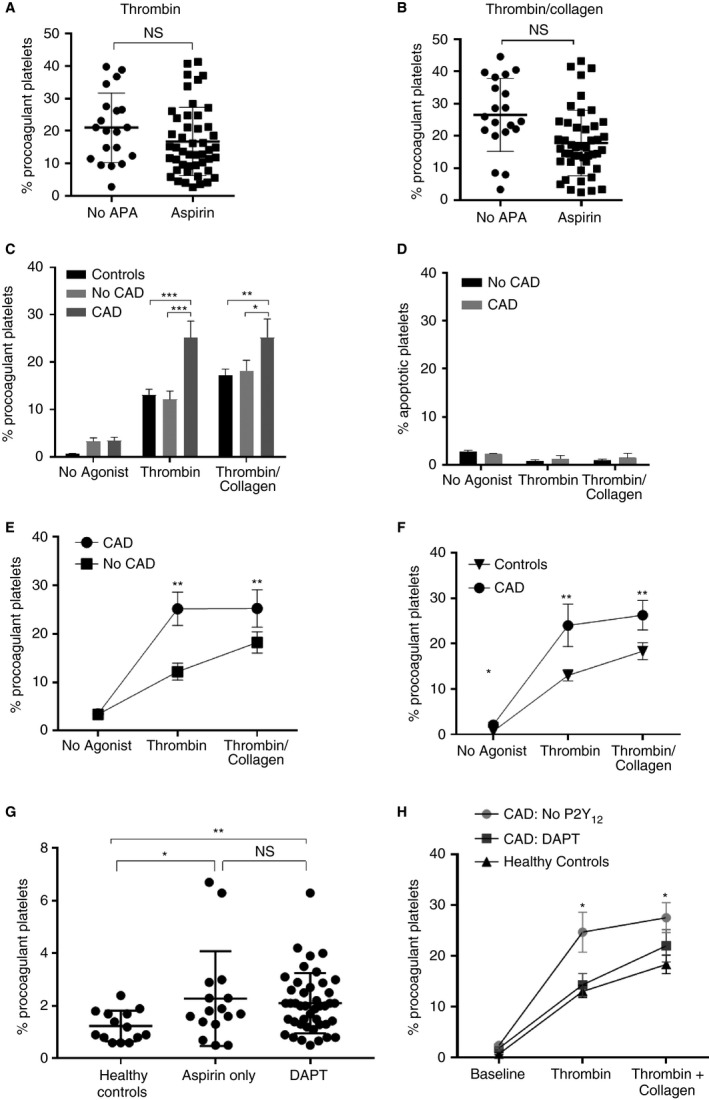
CAD is associated with a heightened procoagulant platelet potential and loss of synergism between thrombin and collagen that is not affected by aspirin. DAPT ameliorates thrombin hypersensitivity in CAD but only partially suppresses thrombin and collagen. (A, B) Blood samples (1–4 per individual) were collected during coronary angiography from 60 patients on either aspirin or no antiplatelet agents. Procoagulant platelets were assayed in whole blood samples treated with thrombin (2 U mL−1) or thrombin (2 U mL−1) plus collagen (10 μg mL−1). Data are shown as the percentage of platelets with the procoagulant phenotype (GSAO+/ P‐selectin+). Linear mixed effects models, two‐tailed tests, *P < 0.05. (C) Platelets from patients with CAD showed increased stimulus‐induced procoagulant potential compared to those without CAD and healthy controls. Two‐way anova analysis with Tukey correction for multiple comparisons. Mean ± SEM, n = 11 (CAD), n = 16 (no CAD) and n = 10 (healthy controls). P < 0.0001. (D) Apoptotic platelet subset (GSAO+/P‐selectin−) did not vary according to the presence of CAD. Two‐way anova analysis with Tukey correction for multiple comparisons. Mean ± SEM, n = 11 (CAD), n = 17 (no CAD). (E) Platelets from CAD patients showed increased procoagulant response to thrombin and thrombin collagen compared with angiographically normal patients (**P < 0.01). However, CAD patients failed to respond to further stimulation with addition of collagen to thrombin (thrombin vs. thrombin plus collagen, paired t‐test, P = 0.9684). (F) Validation of heightened procoagulant platelet response to thrombin and thrombin collagen in an independent cohort of CAD patients not treated with P2Y12 antagonists. Mean ± SEM, n = 11 (CAD) and n = 10 (healthy controls), t‐test **P < 0.01. (G) Without stimulation, circulating procoagulant platelets were elevated in CAD patients compared with healthy controls (t‐test *P < 0.05, **P < 0.01)). Levels of circulating procoagulant platelets were not statistically different between patients on aspirin or DAPT. (H) DAPT reduced the hypersensitivity to thrombin and restored the synergism between thrombin and collagen stimulation compared with thrombin alone (t test **P < 0.01) in the validation CAD cohort. DAPT (n = 22), no P2Y12 (n = 11). APA, antiplatelet agents; CAD, coronary artery disease; DAPT, dual antiplatelet therapy; GSAO, [(4‐(N‐(S‐glutathionylacetyl)amino)phenylarsonous acid]; NS, not significant.
To examine the effects of CAD on procoagulant platelets, data were analyzed from non‐heparinized blood samples from patients on no antiplatelet therapy or aspirin alone, with and without angiographic CAD (Table 2) (Figure S4). Patients with no evidence of CAD demonstrated a similar stimulus‐induced procoagulant platelet response to healthy controls (Fig. 3C). Compared with either healthy controls or patients without CAD, patients with CAD showed a heightened platelet procoagulant potential in response to thrombin (12.2 ± 3.4% vs. 25.2 ± 1.7%, P < 0.001) or thrombin plus collagen (18.2 ± 2.2 vs. 25.2 ± 3.9%, P < 0.05) (Fig. 3C). The apoptotic (GSAO+/P‐selectin−) platelet subset failed to demonstrate any differential effect according to CAD status (Fig. 3D). Patients without CAD had the expected synergistic increase in procoagulant platelets when collagen was added to thrombin (thrombin vs. thrombin plus collagen 12.2 ± 3.4% vs. 18.2 ± 2.2% *P < 0.05); however, platelets from CAD patients reached maximal procoagulant potential with thrombin stimulation alone and no further increment occurred with addition of collagen, suggesting hypersensitivity to thrombin (thrombin vs. thrombin plus collagen in CAD patients 25.2 ± 3.5% vs. 25.2 ± 3.8%, P = 0.9684) (Fig. 3E).
A validation and extension study was performed in an independent cohort of CAD patients undergoing coronary angiography (n = 33) from a second institution (Table 3, Figure S4). Eleven patients had no P2Y12 exposure. Because only two patients had no angiographic evidence of CAD, a comparison was made with healthy controls. This analysis confirmed the significant increase in procoagulant platelets in patients with CAD without P2Y12 exposure in response to thrombin (13 ± 1.2% vs. 25.6 ± 4.6%, P < 0.001) and thrombin plus collagen (18.3 ± 1.5% vs. 27.5 ± 2.9%, P < 0.01) (Fig. 3F). In addition, a small but significant increase in circulating procoagulant platelets was detected in the CAD patients, regardless of antiplatelet therapy (Fig. 3G).
Table 3.
Validation cohort patient demographics
| Clinical variables | All patients | No P2Y12 inhibitors | P2Y12 inhibitors |
|---|---|---|---|
| Number of patients | 33 | 11 | 22 |
| Age, median (IQR), years | 64 (56–70) | 64 (55–75) | 65 (58–68) |
| Gender | |||
| Male, no. (%) | 30 (91) | 10 (91) | 20 (91) |
| Female, no. (%) | 3 (9) | 1 (9) | 2 (9) |
| Smoking | |||
| Yes, no. (%) | 10 (30) | 3 (27) | 7 (32) |
| No, no. (%) | 23 (70) | 8 (73) | 15 (68) |
| Unknown, no. (%) | – | – | – |
| Diabetes | |||
| Yes, no. (%) | 13 (39) | 4 (36) | 9 (41) |
| No, no. (%) | 20 (61) | 7 (64) | 13 (59) |
| Unknown, no. (%) | – | – | – |
| Hypertension | |||
| Yes, no. (%) | 26 (79) | 9 (82) | 17 (77) |
| No, no. (%) | 7 (21) | 2 (18) | 5 (23) |
| Unknown, no. (%) | – | – | – |
| Dyslipidemia | |||
| Yes, no. (%) | 21 (64) | 6 (55) | 15 (68) |
| No, no. (%) | 12 (36) | 5 (45) | 7 (32) |
| Unknown, no. (%) | – | – | – |
| Type of procedure | |||
| Elective, no. (%) | 28 (85) | 11 (100) | 17 (77) |
| Urgent, no. (%) | 5 (15) | – | 5 (23) |
| Antiplatelet therapy, no. (%) | |||
| Aspirin | 28 (85) | 7 (64) | 21 (96) |
| Clopidogrel | 13 (39) | – | 13 (59) |
| Prasugrel | 1 (3) | – | 1 (5) |
| Ticagrelor | 8 (24) | – | 8 (36) |
| Aspirin + P2Y12 inhibitor | 21 (64) | – | 21 (96) |
| CAD confirmed by angiography, no. (%) | |||
| Yes | 31 (94) | 9 (82) | 22 (100) |
| No | 2 (6) | 2 (18) | 0 (0) |
| Unknown | – | – | – |
CAD, coronary artery disease; IQR, interquartile range.
Stimulus‐induced platelet procoagulant potential is favorably modified by dual antiplatelet therapy
In the validation CAD cohort, we analyzed the effect of DAPT (aspirin plus either clopidogrel, ticagrelor or prasugrel) vs. aspirin alone. Four study participants met criteria for unstable CAD (Table S1); however, comparison of stable and unstable DAPT patients showed no statistical difference in procoagulant platelet response (Figure S5), thus the DAPT cohort was analyzed as a whole. After thrombin stimulation, DAPT‐treated CAD patients were similar to healthy controls (13.0 ± 1.2% vs. 14.3 ± 2.2%, P = 0.68), losing the hypersensitization to thrombin seen in CAD with no exposure to P2Y12 inhibitors (Fig. 3H). In contrast, DAPT did not suppress the increased procoagulant platelet response to thrombin and collagen (27.6 ± 3.1% vs. 22.0 ± 2.9, P = 0.24) (Fig. 3H). The increase in circulating procoagulant platelets in CAD versus healthy controls was present regardless of aspirin or DAPT therapy (2.3 ± 0.45 vs. 1.2 ± 0.15%, P < 0.05 [aspirin]; 2.1 ± 0.17 vs. 1.2 ± 0.15, P < 0.01 [DAPT]; Fig. 3G).
Patients with CAD demonstrate an abnormal procoagulant platelet response to ADP
CAD patients in the validation cohort demonstrated a significantly increased response to 5 μm ADP compared with healthy controls (7.5 ± 1.0% vs. 2.7 ± 0.9%, P < 0.05), which was attenuated in DAPT‐treated patients (7.5 ± 1.0% vs. 4.1 ± 0.5%, P < 0.01) (Fig. 4A). A dose–response curve in a subset of patients with CAD (n = 3) demonstrated the abnormal response to ADP at concentrations as low as 0.625 μm (Fig. 4B). Of note, procoagulant platelet formation in healthy controls remained relatively insensitive to ADP stimulation, even at supra‐physiological doses (Figure S6). To confirm this unexpected finding, a third cohort of CAD patients was recruited, who demonstrated the same pattern of response (Figure S7). Comparison was also performed between parallel whole blood samples anticoagulated with either citrate or hirudin in a subset of angiogram patients on aspirin or no antiplatelet therapy, but not P2Y12 inhibitors. As expected, thrombin stimulation showed increased procoagulant platelets in citrate, but reduction to non‐stimulated numbers in hirudin (12.2 ± 4.7% vs. 3.9 ± 1.5%, n = 4, *P = 0.02). In contrast, ADP‐stimulated procoagulant platelet numbers were similar in citrate versus hirudin (5.8 ± 1.3% vs. 6.1 ± 1.3%, n = 4, P = 0.78), indicating that the heightened ADP response in CAD was not mediated by endogenous thrombin (data not shown).
Figure 4.
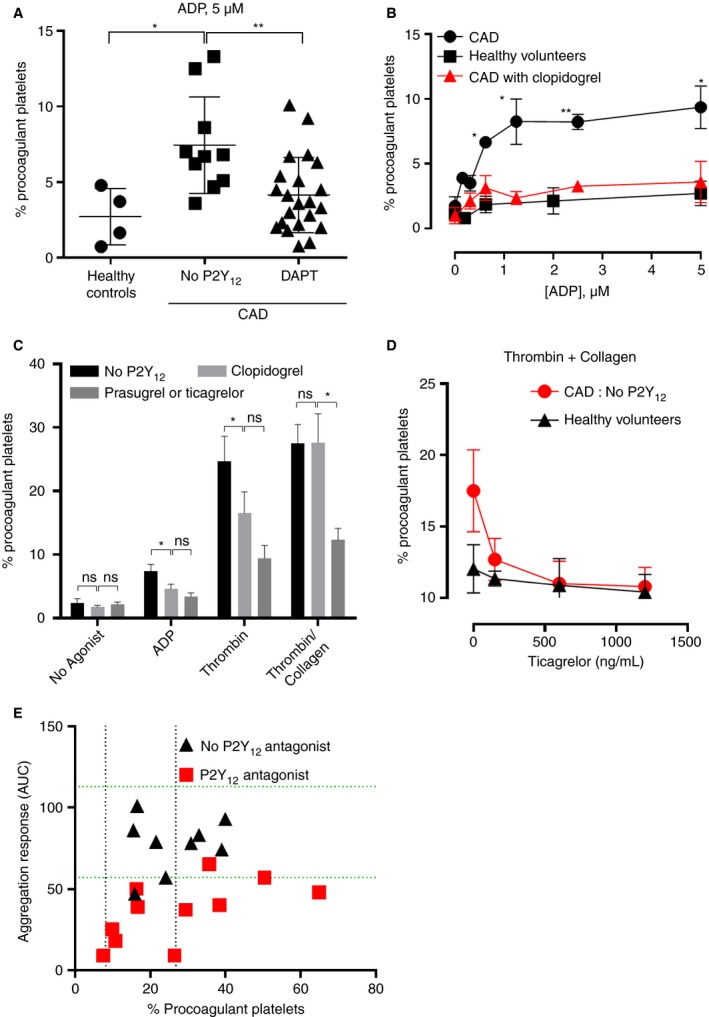
Clopidogrel abrogates the abnormal response to ADP and thrombin but not to a thrombin and collagen combination in patients with CAD, and shows a discordant inhibitory effect on platelet aggregation and procoagulant potential. (A) CAD patients without P2Y12 exposure show an increased procoagulant response to ADP 5 μm (2.7% ± 1.8% vs. 7.6% ± 3.2%, *P < 0.05) that is abrogated in patients on DAPT (7.6% ± 3.2% vs. 4.1% ± 2.5%, t‐test **P < 0.01). (B) Platelets from patients with CAD showed heightened procoagulant response to ADP (≥ 0.625 μm) compared with healthy controls. Treatment with clopidogrel restored the pattern of response. n = 3, t‐test *P < 0.05, **P < 0.01. (C) Compared with patients without P2Y12 antagonists, patients on clopidogrel showed significant inhibition of procoagulant platelet formation in response to ADP 5 μm (*P < 0.05) or thrombin 2 U mL−1 (*P < 0.05), but not thrombin 2 U mL−1 and collagen 10 μg mL−1 (t‐test P = 0.99). Patients on prasugrel or ticagrelor demonstrated significant inhibition of thrombin/collagen‐induced procoagulant platelets compared with patients on clopidogrel (27.2 ± 4.5% vs. 12.0 ± 1.8%, n = 8–22, t‐test P < 0.05); however, there was no additional inhibition of procoagulant response to ADP (P = 0.3) or thrombin (P = 0.13) in patients on prasugrel or ticagrelor vs. clopidogrel. (D) Ex‐vivo spiking of ticagrelor into subjects’ blood stimulated with thrombin (2 U mL−1) and collagen (10 mg mL−1) showed that ticagrelor reduced procoagulant platelet formation in CAD patients on aspirin only (n = 5), to the same level as in healthy controls (n = 6). (E) The GSAO‐based assay allows identification of clopidogrel‐treated patients with heightened procoagulant platelet response despite adequate suppression of aggregation. Correlation between ADP‐induced platelet aggregation responses (y‐axis) measured in whole blood and the procoagulant platelet response to thrombin plus collagen (x‐axis). Dotted horizontal green lines represent the published normal range for ADP‐induced aggregation in healthy blood donors 43; dotted vertical black lines represent the 10th and 90th percentile range for thrombin plus collagen‐induced procoagulant platelets in healthy controls. Treatment with P2Y12 antagonists is associated with suppression of ADP‐induced aggregation; however, a significant proportion of procoagulant responses remain above the 90th percentile. ADP, adenosine diphosphate; AUC, area under curve; CAD, coronary artery disease; DAPT, dual antiplatelet therapy; GSAO, [(4‐(N‐(S‐glutathionylacetyl)amino)phenylarsonous acid]. [Color figure can be viewed at wileyonlinelibrary.com]
To investigate the procoagulant platelet response in relation to the potency of P2Y12 inhibition, we subdivided patients in the validation cohort into those without P2Y12 inhibition (n = 11), those on aspirin plus P2Y12 inhibition (clopidogrel, n = 12) and those on more potent inhibitors (ticagrelor or prasugrel, n = 9) (Figure S4). The single patient on clopidogrel alone was analyzed with the aspirin + clopidogrel group. Addition of clopidogrel to aspirin decreased the excess ADP and thrombin responses but did not inhibit the response to thrombin and collagen (P = 0.99), whereas patients on prasugrel and ticagrelor demonstrated marked reduction in thrombin and collagen response compared with clopidogrel (4.5 ± 27.2% prasugrel or ticagrelor vs. 1.8 ± 12.0% clopidogrel, n = 8–22, P < 0.05) and no P2Y12 inhibitors (3.1 ± 27.6% prasugrel or ticagrelor vs. 1.8 ± 12.0% no P2Y12 inhibitor, P < 0.01), (Fig. 4C). Ex vivo addition of increasing doses of ticagrelor to blood from CAD patients on aspirin alone resulted in suppression of procoagulant platelet formation in response to thrombin + collagen stimulation to levels of healthy volunteers (Fig. 4D). Of note, healthy controls showed minimal suppression of procoagulant platelet formation with ex vivo addition of ticagrelor.
P2Y12‐treated patients demonstrated inhibition of platelet aggregation as measured by Multiplate aggregation response to ADP. Despite this, they showed a range of procoagulant platelet responses from 7.5% to 65%, with half of P2Y12‐treated patients generating more procoagulant platelets than the 90th percentile for healthy controls (Fig. 4E).
Measures of platelet aggregation do not predict procoagulant platelet potential
Platelet aggregation and flow cytometric measures of platelet activation have been proposed as biomarkers for CAD 26. Thus, to assess whether the GSAO‐defined procoagulant responses are independent of other markers of platelet activation, additional blood samples were collected from the validation cohort and markers of platelet activation, aggregation, α‐granule release (P‐selectin), dense granule release (CD63) and α2bβ3 integrin activation (PAC‐1) were measured in parallel with procoagulant platelet potential. There was no significant correlation between procoagulant platelet formation and whole blood platelet aggregation in response to stimulation with ADP or arachidonic acid, or flow cytometric detection of P‐selectin, CD63 and PAC‐1 exposure in response to ADP, SFLLRN, thrombin and thrombin plus collagen (Table 4). This suggests that measurement of procoagulant platelets in addition to platelet activation is not redundant.
Table 4.
Correlation between traditional markers of platelet activation and procoagulant platelet potential
| Activation parameter | Agonist | Procoagulant (GSAO+/P‐selectin+) platelets (%) | |||
|---|---|---|---|---|---|
| Thrombin (2 U mL−1) plus collagen (10 μg mL−1) | Thrombin (2 U mL−1) | ||||
| r 2 | Significance | r 2 | Significance | ||
| Aggregation | ADP (6.7 μm) | 0.06 | NS | 0.07 | NS |
| AA (1 mm) | 0.02 | NS | 0.35 | NS | |
| Alpha granule release (P‐ selectin) | ADP (5 μm) | 0.05 | NS | 0.08 | NS |
| SFLLRN (20 μm) | 0.01 | NS | 0.00 | NS | |
| Thrombin (2 U mL−1) | <0.01 | NS | 0.01 | NS | |
| Thrombin (2 U mL−1) plus collagen (10 μg mL−1) | 0.04 | NS | 0.04 | NS | |
| Dense granule release (CD63) | ADP (5 μm) | 0.01 | NS | 0.18 | <0.05 |
| SFLLRN (20 μm) | 0.00 | NS | 0.02 | NS | |
| Thrombin (2 U mL−1) | 0.06 | NS | 0.00 | NS | |
| Thrombin (2 U mL−1) /collagen (10 μg mL−1) | 0.04 | NS | 0.00 | NS | |
| Integrin activation (PAC‐1) | ADP (5 μm) | 0.08 | NS | 0.08 | NS |
| SFLLRN (20 μm) | 0.11 | NS | 0.01 | NS | |
| Thrombin (2 U mL−1) | 0.08 | NS | 0.01 | NS | |
| Thrombin (2 U mL−1) /collagen (10 μg mL−1) | 0.21 | NS | 0.07 | NS | |
SFLLRN indicates Ser‐Phe‐Leu‐Leu‐Arg‐Asn (protease‐activated receptor 1 activating peptide). Correlation was determined by Pearson's test for normally distributed data and Spearman Rho for non‐parametric data. Data presented are pooled for all 33 patients from the validation cohort as no differences were found when correlation analysis was performed separately for the no P2Y12 and P2Y12 subgroups. AA, arachidonic acid; ADP, adenosine diphosphate adenosine diphosphate; GSAO, [(4‐(N‐(S‐glutathionylacetyl)amino)phenylarsonous acid].
Discussion
A subpopulation of platelets can become procoagulant in response to strong agonists, support coagulation and generate fibrin. Fresh coronary thrombi consist of erythrocytes, neutrophils and aggregated platelets within a fibrin meshwork. This composition suggests that fibrin stabilization of the atheroplatelet aggregate is a key feature of occlusive coronary thrombosis 27, 28. Standard platelet assays and antiplatelet therapies measure and target, respectively, aggregation and platelet activation rather than procoagulant function. Selective inhibition of platelet procoagulant function may provide a targeted approach to coronary thrombosis by specifically inhibiting thrombin generation within a thrombus without affecting endothelial fibrin formation 29.
We have shown recently that the necrosis marker, GSAO, together with the platelet activation marker, P‐selectin, can identify agonist‐induced procoagulant platelets in vitro in a washed platelet assay and in in vivo in models of mouse thrombosis. GSAO identified that procoagulant platelets positively correlated with platelet thrombus size and fibrin formation 16. Although PS exposure was detected on all GSAO+ platelets, in keeping with other studies 4, 14, 15, 23, 24, 25, the correlation between PS exposure and binding of FXa was not perfect. The current study shows that procoagulant platelets can also be identified using GSAO and P‐selectin within a few microliters of whole blood. Compared with a washed‐platelet system, the magnitude of agonist‐induced procoagulant platelets was lower with the whole‐blood method that uses dilution rather than with washing to stop the agonist stimulation, thus minimizing ex vivo activation. This assay enables assessment of procoagulant platelet potential in healthy humans and patients with CAD. Alternative assays for procoagulant platelets, or for the closely related subset known as coated platelets 4, have largely been performed in PRP 30 or washed platelets 31. These preparations have a well‐documented requirement for relatively large volumes of blood, and reliance on skilled operators and specialized blood collection procedures, which hampers the study of procoagulant platelets in clinical settings. Our assay uses agonist stimulation within a whole‐blood matrix and results in minimal manipulation. In keeping with another study of the procoagulant platelet response in whole blood 32, our results show that thrombin rather than collagen was the most potent inducer of procoagulant platelets in healthy controls, with a preserved characteristic synergistic response of the thrombin/collagen combined stimulation. Previous studies show that platelet adhesion to immobilized collagen causes PS exposure, and co‐stimulation with other agonists such as thrombin is needed to obtain substantial fractions of PS‐exposing platelets 1, 33. Our data suggest that in a soluble whole‐blood assay, collagen is likely to have a potentiating rather than primary inducing role in the formation of a procoagulant phenotype.
Using this assay in patients with angiographically confirmed CAD, we identified a heightened procoagulant platelet response to ADP, thrombin and thrombin/collagen and a small but expanded population of circulating procoagulant platelets in CAD, indicating that measures of procoagulant platelets have potential utility as a marker of atherothrombotic risk. The degree of the procoagulant platelet response in CAD patients was not associated with traditional markers used to measure platelet response, including aggregation, dense granule release and activation of α2bβ3 integrin, indicating that information provided by this assay is not redundant and may facilitate identification of patients who will benefit from therapeutic targeting of this functionally important platelet phenotype.
Importantly, our results reveal that the presence of CAD is associated with an altered biological response to agonist stimulation. In particular, CAD patients demonstrate a markedly heightened response to P2Y12 receptor stimulation, with quantifiable procoagulant platelet formation observed at low‐dose ADP (0.625 μm), and a marked response at a standard agonist dose (5 μm). Two independent cohorts showed a clear hyperreactivity to thrombin in patients with CAD compared with healthy controls. In addition to an absolute increase in agonist procoagulant platelets, a maximal procoagulant response could be attained in CAD in response to thrombin alone, without a need for co‐stimulation with a GPVI agonist (collagen), and lastly a small increase in circulating procoagulant platelets could be detected. These patterns indicate that platelets in patients with CAD may be primed to form procoagulant platelets with minimal stimuli, perhaps through chronic exposure to low‐level agonists associated with atheroma or stenosis.
Currently marketed antiplatelet drugs do not specifically suppress platelet procoagulant function, reflecting a potential therapeutic opportunity in the management of CAD 34, 35, 36. Similar to other studies 16, 37, 38, aspirin, the most frequently prescribed antiplatelet agent targeting the TXA2 pathway, had no effect on the agonist‐induced procoagulant platelet subset in our patient cohort, regardless of CAD status. The effect of P2Y12 antagonists on procoagulant platelet formation in CAD has not previously been studied, although inhibition of coated platelets after in vivo administration of clopidogrel has been shown in patients undergoing elective coronary catheterization 39. Our study shows clear amelioration of the thrombin hypersensitivity and abnormal ADP response in patients with CAD treated with P2Y12 inhibitors, with strong P2Y12 inhibitors ticagrelor and prasugrel demonstrating a greater inhibitory effect compared with clopidogrel. We speculate that the thrombin hypersensitivity may be related to the autocrine feed‐forward action of granule‐released ADP on the P2Y12 receptor.
Importantly, the procoagulant response to a combination of collagen/thrombin was not significantly suppressed in patients on clopidogrel, even in individuals with a demonstrated inhibition of ADP‐induced aggregation. However, although a heightened thrombin/collagen response was not abrogated by clopidogrel, more potent P2Y12 antagonists ticagrelor and prasugrel were associated with a significant reduction in the collagen potentiation effect. This novel action of P2Y12 antagonism in reducing procoagulant platelet potential may offer an additional mechanistic insight into the observed benefits of DAPT over aspirin alone in preventing ischemic events, both short and long‐term, after myocardial infarction and coronary artery stenting, as well as providing insights into differences in clinical outcomes seen with clopidogrel versus prasugrel or ticagrelor 40, 41, 42.
Although antiplatelet agents (aspirin, P2Y12 antagonists) have resulted in a significant reduction of cardiovascular mortality over recent decades, patients on DAPT continue to experience significant rates of major adverse coronary events (MACE) and use of existing platelet function testing to adjust P2Y12 inhibitor therapy has not improved outcomes to date. Our assay identified 40% of P2Y12‐treated CAD patients who remained hyper‐responsive to thrombin and collagen on procoagulant platelet testing (Fig. 4E), despite showing marked suppression of ADP‐induced platelet aggregation. These findings suggest that use of the GSAO‐based assay may identify patients on DAPT who remain at a heightened risk of thrombotic complications despite an adequately inhibited aggregation response. Therefore, standard aggregation assays would not be useful in detecting this additional facet of biological resistance to clopidogrel, which adds an increased impetus to development of novel therapies that target hyper‐responsive procoagulant platelets for patients with CAD.
This study is subject to limitations that apply to all proof‐of‐concept studies. We cannot determine causality and it remains unknown if platelets become hyperreactive in response to atherosclerotic milieu, or if individuals with increased propensity to form procoagulant platelets are predisposed to develop CAD; longitudinal and family studies in patients with early CAD are needed to determine this. The study population was relatively heterogeneous and predominantly characterized by stable CAD. Thus, we have not yet been able to explore the association between procoagulant platelet formation and MACE outcome. Future studies are planned to evaluate procoagulant platelet responses in more diverse healthy populations to allow establishment of age and/or gender‐specific reference ranges. Finally, because of the novel nature of the GSAO‐based assay, all statistical analyses were performed with the attendant limitations of uncontrolled variables.
In conclusion, we show that the GSAO‐based whole‐blood assay is a robust and reproducible method for evaluation of procoagulant platelets using a few microliters of whole blood. We demonstrated the assay's utility as a potential clinical tool in the setting of CAD by showing, for the first time, the heightened procoagulant platelet potential in these patients; platelets are sensitized to thrombin and ADP, giving a potential insight into CAD pathophysiology. Furthermore, the fact that DAPT modulates the platelet procoagulant response differentially according to the agonist provides additional insight into the mechanism of P2Y12 inhibition in CAD, and has potential therapeutic implications. The GSAO‐based assay for measurement of procoagulant platelets in whole blood should facilitate translational and clinical research into the pathophysiological role of this platelet functional subpopulation, identify individuals that require more potent P2Y12 inhibition and perhaps point to future potential therapeutic targets in thrombotic diseases.
Addendum
L. Pasalic conceived the study, performed experiments, analyzed data and wrote the manuscript. E. Wing‐Lun and H. Campbell performed experiments and collected data. J. Lau performed experiments, analyzed data and contributed to the manuscript. G. J. Pennings, E Lau, D Connor and H. P. Liang performed experiments and analyzed data. D. Muller and L. Kritharides revised the manuscript and contributed to design. P. J. Hogg conceived the study and revised the manuscript, and V. M. Chen conceived the study, analyzed data and wrote the manuscript.
Disclosure of Conflict of Interests
L. Pasalic, V. M. Chen and P. J. Hogg have patent PCT/AU2015/000638 pending. The other authors state that they have no conflict of interest.
Supporting information
Data S1. Supplemental methods.
Table S1. Troponin T levels in the validation cohort patients with CAD according to antiplatelet therapy.
Fig. S1. Arterial and venous blood samples give comparable measurement of procoagulant platelet subset.
Fig. S2. GSAO identifies the coagulation factor‐binding platelet population while annexin V has poorer specificity.
Fig. S3. Zombie NIR™ labelling of necrotic mammalian cells.
Fig. S4. Study schema.
Fig. S5. Procoagulant platelets according to clinical status: unstable vs. stable CAD patients.
Fig. S6. Healthy controls show minimal formation of procoagulant platelets in response to ADP.
Fig. S7. Additional cohort of CAD patients confirms sensitization of patients with CAD to ADP stimulation.
Fig. S8. Time course of annexin V staining and GSAO uptake.
Acknowledgements
L. Pasalic was supported by a PhD scholarship from the University of New South Wales and was the recipient of doctoral training grants from Prince of Wales and Westmead Hospitals (Sydney, Australia). The study was supported by grants from the National Health and Medical Research Council of Australia, Ramaciotti Establishment Grant, SEALS Haematology Research Fund and donors to the Coagulation in Cancer Research Fund, University of New South Wales. L. Kritharides was supported by National Health and Medical Research Council of Australia Program grant 1037903. Flow cytometry was performed at the Biological Resources Laboratory, University of New South Wales, St Vincent's Centre for Applied Medical Research, and the Diagnostic Haematology Laboratory, Concord Repatriation and General Hospital, Sydney, Australia.
Pasalic L, Wing‐Lun E, Lau JK, Campbell H, Pennings GJ, Lau E, Connor D, Liang HP, Muller D, Kritharides L, Hogg PJ, Chen VM. Novel assay demonstrates that coronary artery disease patients have heightened procoagulant platelet response. J Thromb Haemost 2018; 16: 1198–1210.
Manuscript handled by: W. Bergmeier
Final decision: P. H. Reitsma, 5 March 2018
References
- 1. Heemskerk JWM, Mattheij NJA, Cosemans JMEM. Platelet‐based coagulation: different populations, different functions. J Thromb Haemost 2013; 11: 2–16. [DOI] [PubMed] [Google Scholar]
- 2. McFadyen JD, Jackson SP. Differentiating haemostasis from thrombosis for therapeutic benefit. Thromb Haemost 2013; 110: 859–67. [DOI] [PubMed] [Google Scholar]
- 3. Bouchard BA, Silveira JR, Tracy PB. Chapter 21 ‐ interactions between platelets and the coagulation system In: Michelson AD, ed. Platelets (3rd Edn). Cambridge: Academic Press, 2013: 425–51. [Google Scholar]
- 4. Munnix ICA, Kuijpers MJE, Auger J, Thomassen CMLGD, Panizzi P, van Zandvoort MAM, Rosing J, Bock PE, Watson SP, Heemskerk JWM. Segregation of platelet aggregatory and procoagulant microdomains in thrombus formation: regulation by transient integrin activation. Arterioscler Thromb Vasc Biol 2007; 27: 2484–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation 2005; 111: 3481–8. [DOI] [PubMed] [Google Scholar]
- 6. Freynhofer MK, Bruno V, Wojta J, Huber K. The role of platelets in athero‐thrombotic events. Curr Pharm Des 2012; 18: 5197–214. [DOI] [PubMed] [Google Scholar]
- 7. Versteeg HH, Heemskerk JWM, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev 2013; 93: 327–58. [DOI] [PubMed] [Google Scholar]
- 8. Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arterioscler Thromb Vasc Biol 2002; 22: 1381–9. [DOI] [PubMed] [Google Scholar]
- 9. Monroe DM, Hoffman M. What does it take to make the perfect clot? Arterioscler Thromb Vasc Biol 2006; 26: 41–8. [DOI] [PubMed] [Google Scholar]
- 10. Monaco C, Mathur A, Martin JF. What causes acute coronary syndromes? Applying Koch's postulates. Atherosclerosis 2005; 179: 1–15. [DOI] [PubMed] [Google Scholar]
- 11. Hua VM, Chen VMY. Procoagulant platelets and the pathways leading to cell death. Semin Thromb Hemost 2015; 41: 405–12. [DOI] [PubMed] [Google Scholar]
- 12. Fager AM, Wood JP, Bouchard BA, Feng P, Tracy PB. Properties of procoagulant platelets: defining and characterizing the subpopulation binding a functional prothrombinase. Arterioscler Thromb Vasc Biol 2010; 30: 2400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alberio L, Safa O, Clemetson KJ, Esmon CT, Dale GL. Surface expression and functional characterization of α‐granule factor V in human platelets: effects of ionophore A23187, thrombin, collagen, and convulxin. Blood 2000; 95: 1694–702. [PubMed] [Google Scholar]
- 14. Kempton CL, Hoffman M, Roberts HR, Monroe DM. Platelet heterogeneity: variation in coagulation complexes on platelet subpopulations. Arterioscler Thromb Vasc Biol 2005; 25: 861–6. [DOI] [PubMed] [Google Scholar]
- 15. London FS, Marcinkiewicz M, Walsh PN. A subpopulation of platelets responds to thrombin‐ or SFLLRN‐stimulation with binding sites for factor IXa. J Biol Chem 2004; 279: 19854–9. [DOI] [PubMed] [Google Scholar]
- 16. Hua VM, Abeynaike L, Glaros E, Campbell H, Pasalic L, Hogg PJ, Chen VM. Necrotic platelets provide a procoagulant surface during thrombosis. Blood 2015; 126: 2852–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park D, Don AS, Massamiri T, Karwa A, Warner B, MacDonald J, Hemenway C, Naik A, Kuan KT, Dilda PJ, Wong JW, Camphausen K, Chinen L, Dyszlewski M, Hogg PJ. Noninvasive imaging of cell death using an Hsp90 ligand. J Am Chem Soc 2011; 133: 2832–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Don AS, Kisker O, Dilda P, Donoghue N, Zhao X, Decollogne S, Creighton B, Flynn E, Folkman J, Hogg PJ. A peptide trivalent arsenical inhibits tumor angiogenesis by perturbing mitochondrial function in angiogenic endothelial cells. Cancer Cell 2003; 3: 497–509. [DOI] [PubMed] [Google Scholar]
- 19. Park D, Xie BW, Van Beek ER, Blankevoort V, Que I, Lowik CW, Hogg PJ. Optical imaging of treatment‐related tumor cell death using a heat shock protein‐90 alkylator. Mol Pharm 2013; 10: 3882–91. [DOI] [PubMed] [Google Scholar]
- 20. Pasalic L, Pennings GJ, Connor D, Campbell H, Kritharides L, Chen VM. Flow cytometry protocols for assessment of platelet function in whole blood. Methods Mol Biol 2017; 1646: 369–89. [DOI] [PubMed] [Google Scholar]
- 21. Yong AS, Pennings GJ, Chang M, Hamzah A, Chung T, Qi M, Brieger D, Behnia M, Krilis SA, Ng MK, Lowe HC, Kritharides L. Intracoronary shear‐related up‐regulation of platelet P‐selectin and platelet‐monocyte aggregation despite the use of aspirin and clopidogrel. Blood 2011; 117: 11–20. [DOI] [PubMed] [Google Scholar]
- 22. Pennings GJ, Yong AS, Kritharides L. Expression of EMMPRIN (CD147) on circulating platelets in vivo. J Thromb Haemost 2010; 8: 472–81. [DOI] [PubMed] [Google Scholar]
- 23. Shi J, Heegaard CW, Rasmussen JT, Gilbert GE. Lactadherin binds selectively to membranes containing phosphatidyl‐L‐serine and increased curvature. Biochim Biophys Acta 2004; 1667: 82–90. [DOI] [PubMed] [Google Scholar]
- 24. Butenas S, van't Veer C, Mann KG. “Normal” thrombin generation. Blood 1999; 94: 2169–78. [PubMed] [Google Scholar]
- 25. Berny MA, Munnix IC, Auger JM, Schols SE, Cosemans JM, Panizzi P, Bock PE, Watson SP, McCarty OJ, Heemskerk JW. Spatial distribution of factor Xa, thrombin, and fibrin(ogen) on thrombi at venous shear. PLoS ONE 2010; 5: e10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pasalic L, Wang SS, Chen VM. Platelets as biomarkers of coronary artery disease. Semin Thromb Hemost 2016; 42: 223–33. [DOI] [PubMed] [Google Scholar]
- 27. Silvain J, Collet J‐P, Nagaswami C, Beygui F, Edmondson KE, Bellemain‐Appaix A, Cayla G, Pena A, Brugier D, Barthelemy O, Montalescot G, Weisel JW. Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol 2011; 57: 1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sadowski M, Zabczyk M, Undas A. Coronary thrombus composition: links with inflammation, platelet and endothelial markers. Atherosclerosis 2014; 237: 555–61. [DOI] [PubMed] [Google Scholar]
- 29. Kaplan ZS, Jackson SP. The role of platelets in atherothrombosis. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program 2011; 2011: 51–61. [DOI] [PubMed] [Google Scholar]
- 30. Mohammed BM, Contaifer D Jr, Lastrapes KK, Martin EJ, Mazepa MA, Hoffman M, Monroe DM, Brophy DF. Coated platelet assay: a feasible approach to a complicated science. Haemophilia 2016; 22: e67–70. [DOI] [PubMed] [Google Scholar]
- 31. Remenyi G, Szasz R, Friese P, Dale GL. Role of mitochondrial permeability transition pore in coated‐platelet formation. Arterioscler Thromb Vasc Biol 2005; 25: 467–71. [DOI] [PubMed] [Google Scholar]
- 32. Frelinger AL 3rd, Jakubowski JA, Li Y, Barnard MR, Linden MD, Tarnow I, Fox ML, Sugidachi A, Winters KJ, Furman MI, Michelson AD. The active metabolite of prasugrel inhibits adenosine diphosphate‐ and collagen‐stimulated platelet procoagulant activities. J Thromb Haemost 2008; 6: 359–65. [DOI] [PubMed] [Google Scholar]
- 33. Bevers EM, Williamson PL. Getting to the outer leaflet: physiology of phosphatidylserine exposure at the plasma membrane. Physiol Rev 2016; 96: 605–45. [DOI] [PubMed] [Google Scholar]
- 34. Antithrombotic Trialists’ Collaboration . Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324: 71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta‐analysis of individual participant data from randomised trials. Lancet 2009; 373: 1849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. Low‐dose aspirin for the prevention of atherothrombosis. N Engl J Med 2005; 353: 2373–83. [DOI] [PubMed] [Google Scholar]
- 37. Lecut C, Schoolmeester A, Kuijpers MJ, Broers JL, van Zandvoort MA, Vanhoorelbeke K, Deckmyn H, Jandrot‐Perrus M, Heemskerk JW. Principal role of glycoprotein VI in alpha2beta1 and alphaIIbbeta3 activation during collagen‐induced thrombus formation. Arterioscler Thromb Vasc Biol 2004; 24: 1727–33. [DOI] [PubMed] [Google Scholar]
- 38. Vanschoonbeek K, Feijge MAH, Van Kampen RJW, Kenis H, Hemker HC, Giesen PLA, Heemskerk JWM. Initiating and potentiating role of platelets in tissue factor‐induced thrombin generation in the presence of plasma: subject‐dependent variation in thrombogram characteristics. J Thromb Haemost 2004; 2: 476–84. [DOI] [PubMed] [Google Scholar]
- 39. Norgard NB, Saya S, Hann CL, Hennebry TA, Schechter E, Dale GL. Clopidogrel attenuates coated‐platelet production in patients undergoing elective coronary catheterization. J Cardiovasc Pharmacol 2008; 52: 536–9. [DOI] [PubMed] [Google Scholar]
- 40. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation. N Engl J Med 2001; 345: 494–502. [DOI] [PubMed] [Google Scholar]
- 41. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, Bengtsson O, Oude Ophuis T, Budaj A, Theroux P, Ruda M, Hamm C, Goto S, Spinar J, Nicolau JC, Kiss RG, et al Long‐term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372: 1791–800. [DOI] [PubMed] [Google Scholar]
- 42. Mauri L, Kereiakes DJ, Yeh RW, Driscoll‐Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, et al Twelve or 30 months of dual antiplatelet therapy after drug‐eluting stents. N Engl J Med 2014; 371: 2155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. ADPtest Package Insert (06673686001V2). Roche Diagnostics Gmbh, 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.
Table S1. Troponin T levels in the validation cohort patients with CAD according to antiplatelet therapy.
Fig. S1. Arterial and venous blood samples give comparable measurement of procoagulant platelet subset.
Fig. S2. GSAO identifies the coagulation factor‐binding platelet population while annexin V has poorer specificity.
Fig. S3. Zombie NIR™ labelling of necrotic mammalian cells.
Fig. S4. Study schema.
Fig. S5. Procoagulant platelets according to clinical status: unstable vs. stable CAD patients.
Fig. S6. Healthy controls show minimal formation of procoagulant platelets in response to ADP.
Fig. S7. Additional cohort of CAD patients confirms sensitization of patients with CAD to ADP stimulation.
Fig. S8. Time course of annexin V staining and GSAO uptake.


