Abstract
Cholestasis is a condition that impairs bile flow, resulting in retention of bile fluid in the liver. It may cause significant morbidity and mortality due to pruritus, malnutrition, and complications from portal hypertension secondary to biliary cirrhosis. The zebrafish (Danio rerio) has emerged as a valuable model organism for studying cholestasis that complements with the in vitro systems and rodent models. Its main advantages include conserved mechanisms of liver development and bile formation, rapid external development, ease of monitoring hepatobiliary morphology and function in live larvae, and accessibility to genetic and chemical manipulations. In this chapter, we provide an overview of the existing zebrafish models of cholestatic liver diseases. We discuss the strengths and limitations of using zebrafish to study cholestasis. We also provide step-by-step descriptions of the methodologies for analyzing cholestatic phenotypes in zebrafish.
Keywords: Hepatocyte, Cholangiocyte, Bile duct, Bile canaliculi, Bile salt transporters, BODIPY fluorescent fatty acid analog, Fluorescent bile acid derivative, Confocal microscopy, Transmission electron microscopy
1. Introduction
Cholestasis is a pathology defined by impaired bile flow due to defects in hepatocyte bile acid uptake, conjugation, or excretion (hepatocellular cholestasis), or biliary tract obstruction (obstructive cholestasis) (Fig. 1). Environmental factors, such as viral infection, toxic insults, and hormonal changes, account for 75% of the conditions that lead to cholestasis. Genetic disorders cause the remaining 25% cases [1]. If untreated, retention of bile acids in the liver and serum results in pruritus, which is often refractory to medical or surgical therapy. Biliary fibrosis frequently progresses to end-stage liver disease with portal hypertension and its life-threatening complications including ascites, infections, and catastrophic bleeding from esophageal varices. On the other hand, lack of luminal bile acids leads to fat malabsorption, malnutrition, and deficiency in the fat-soluble vitamins.
Fig. 1.

Common causes of cholestatic liver diseases. Diseases that have been modeled in zebrafish are shown in black. PFIC progressive familial intrahepatic cholestasis, BRIC benign recurrent intrahepatic cholestasis, PSC primary sclerosing cholangitis, PBC primary biliary cholangitis
In the past two decades, rodent models have provided in vivo tools to investigate the pathogenesis of cholestasis and test the efficacy of new treatment strategies [2]. However, no single mouse model can strictly reproduce all the features of human cholestatic liver diseases. Incorporating multiple animal models is necessary to gain complementary insights into the pathophysiology. The teleost zebrafish (Danio rerio) has emerged as a popular animal model for studying liver development and diseases. Its general strengths include rapid external development, transparent larvae, accessibility to genetic and chemical manipulations, inexpensive maintenance, and ease of collecting hundreds of embryos on a weekly base. Unlike mice, laboratory zebrafish strains are not inbred and its genetic background does not impose a significant impact on the phenotype. Zebrafish liver becomes functional by 4 days postfertilization and contains counterparts of mammalian liver cell types, despite some architectural differences (Fig. 2) [3]. Similar to mammals, zebrafish hepatocytes secrete bile salts through bile canaliculus on the apical side. Bile fluid is drained to a network of intrahepatic bile ducts that is connected to extrahepatic bile duct. Zebrafish has been used to model cholestatic liver diseases, including progressive familial intrahepatic cholestasis type 2 (PFIC2) [4], Alagille syndrome [5, 6], biliary atresia [7–13], arthrogryposis-renal dysfunction-cholestasis syndrome [14, 15], North American Indian childhood cirrhosis [16], and choledochal cysts [17]. Some of these zebrafish models overcome the limitations of the in vitro systems and rodent models and have brought new knowledge to the disease pathogenesis.
Fig. 2.
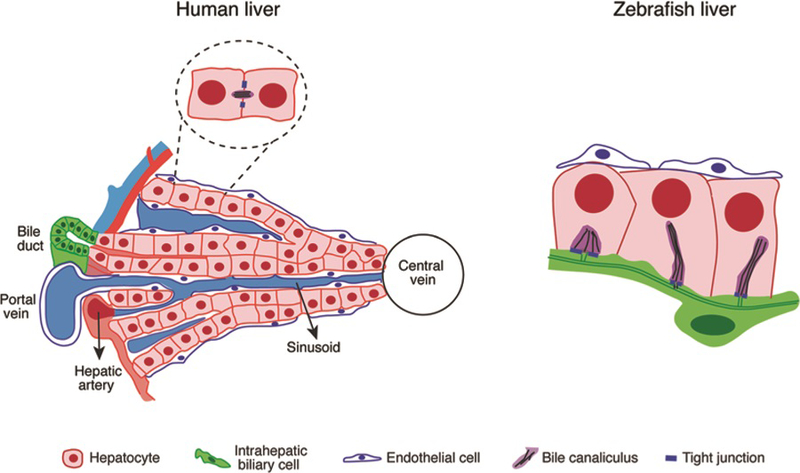
Schematic comparison of liver architecture in human and zebrafish. The most evident difference is that zebrafish liver does not have typical portal triads. The hepatocytes are arranged in tubules, and the intrahepatic bile ducts are located in the center. Similar to mammals, zebrafish hepatocytes secrete bile salts into bile ducts through bile canaliculus on the apical side. Blood vessels are located on the basal side of the hepatocytes, resembling the sinusoid capillaries in mammalian liver
Biliary atresia (BA) is the disease of fibro-inflammatory destruction of the extrahepatic bile ducts leading to obstructive cholestasis [18]. The most commonly used BA rodent model is newborn Balb/C mouse infected with rhesus rotavirus (RRV) [19, 20]. These animals develop extrahepatic obstruction and inflammatory response that resemble patients within 1 week of RRV infection. Compared to the rodent model, zebrafish offers easier and more cost-effective ways to manipulate gene function, including morpholino oligonucleotide-mediated gene knockdown, CRISPR/Cas9 genome editing, and chemical treatment. The subsequent impact on hepatobiliary morphology and function can be directly assessed in live larvae. Zebrafish was used to validate biological function of BA susceptible factors discovered through patient genome-wide association studies (GWAS) and to identify molecular pathways altered by the genetic variants [8, 11, 12, 21]. Pharmacological and genetic manipulations of DNA methylation in zebrafish revealed the role of DNA hypomethylation in initiating molecular cascades leading to BA [7, 10]. By combining chemical screen and fluorescent lipid analog feeding assay in zebrafish, Lorent and colleagues isolated a plant isoflavonoid, biliatresone, from the extracts of Dysphania species that caused the BA-like symptoms in Australian livestock [9]. They showed that biliatresone specifically destructs the extrahepatic biliary system of larval zebrafish and that glutathione antioxidant pathway activity determines the susceptibility of liver cells to biliatresone insults [9, 13]. While the RRV mouse model is useful for examining inflammation and immune responses in BA, the zebrafish models permit investigation of both environmental and genetic contributing factors in BA.
Bile salt export pump BSEP (ABCB11) controls excretion of bile acids across the hepatocyte canalicular membrane and serves as the rate-limiting factor of bile secretion [22, 23]. Human mutations in the ABCB11 gene cause PFIC2, which is characterized by early-onset cholestasis and rapid progression to end-stage liver failure and hepatocellular carcinoma [24–26]. Studies of ABCB11 mutations have been mostly conducted in vitro. However, the cultured cells are not exposed to an environment mimicking the liver, which may influence the expression and function of BSEP [27]. Abcb11 knockout mice do not replicate the patient phenotypes as they maintain substantial amount of bile flow and develop relatively mild cholestasis [28–30]. We generated a zebrafish null mutant of abcb11b, the ortholog of human ABCB11 [4]. These animals exhibit histological and ultrastructural features of hepatocyte injury similar to those observed in patients with PFIC2 and are prematurely lethal. It is by far the first animal model in which depleting BSEP results in an almost complete blockage of bile excretion. We discovered that multidrug resistance protein 1 (MDR1), which is thought to serve as a compensatory bile salt transporter in Abcb11 knockout mice [28], becomes mislocalized in the BSEP-deficient hepatocytes in both human and zebrafish. Treatment with mTOR inhibitor rapamycin restores bile excretion in abcb11b mutant zebrafish and improves their survival, correlating with the recovery of canalicular MDR1 localization. Thus our zebrafish work has uncovered novel pathogenic mechanisms and therapeutic strategies for PFIC2.
The zebrafish BA and PFIC2 models provide critical proofs of concept to show that zebrafish is a valid in vivo system for studying both obstructive and hepatocellular cholestasis. It is noteworthy that zebrafish bile salt is predominantly composed of C27 5α-bile alcohol sulfates, differing from human [31, 32]. Whether the different bile composition results in different regulation of bile synthesis and transport between fish and mammals is to be determined. The zebrafish genome is predicted to possess orthologs of the mammalian genes that encode various bile salt transporters, bile synthesis enzymes, and their upstream regulators. However, it is not clear which ones are the true functional orthologs of their mammalian counterparts. Due to the small sizes of larval and adult zebrafish, it is challenging to measure the speed of bile flow. So far, the studies of cholestasis are mainly conducted in larval fish. The phenotypes likely reflect what occurs during early stages of cholestasis but offer limited information on disease progression. In fact, none of the current zebrafish cholestasis models have been reported to develop advanced fibrosis and inflammation as seen in patients. Larval zebrafish only have innate immune responses because their adaptive immune system does not mature until after 4–6 weeks postfertilization [33]. Therefore, hepatobiliary injury does not induce similar immune responses in larval zebrafish as in patients. It is important to take these facts into account when considering using zebrafish in a particular study.
In this chapter, we describe the methodologies to characterize cholestasis in zebrafish. We use BODIPY™ FL C5 fatty acid analog and fluorescent bile acid derivative cholylglycylamidofluorescein (CGamF) feeding assays to track bile flow in larval and juvenile zebrafish (Fig. 3) [4, 34]. We measure total bile salts in whole larva and adult zebrafish liver by commercial kits that are commonly used in rodent models (Fig. 4e). We examine the morphology of biliary system by immunofluorescence and transgenic reporter zebrafish: Alcam antibody [36] and Tg(EPV.Tp1-Mmu.Hbb:EGFP) transgenic fish [37, 38] (Fig. 4a–d) mark intrahepatic bile ducts; Annexin A4 [39, 40] and cytokeratin [41] antibodies, and Tg(krt18:EGFP) transgenic fish [42] label both intrahepatic and extrahepatic biliary systems. We perform immunofluorescence to detect canalicular transporters BSEP [23, 36] and MDR1 [17, 37]. We also conduct transmission electron microscopy to examine ultrastructural features of cholestasis (Fig. 5) [4] (see Note 1).
Fig. 3.
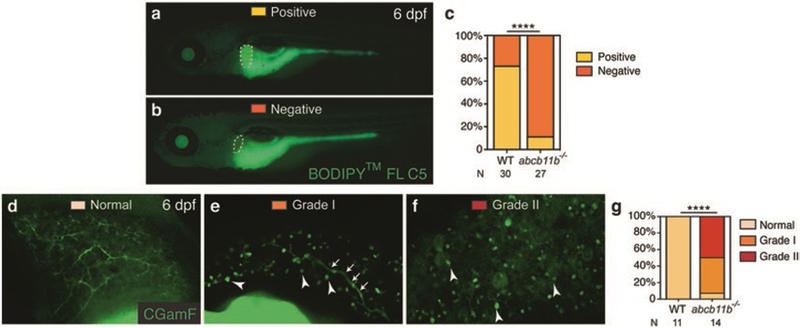
Assessment of bile secretion and transport in wild-type and abcb11b mutant zebrafish larvae. (a, b) Fluorescent micrographs of live larvae after BODIPY™ FL C5 feeding at 6 days postfertilization (dpf). The larva in (a) showed filling of the gallbladder (positive) and the one in (b) had no fluorescence signal in the gallbladder (negative). Lateral views, anterior to the left. Dashed lines outline the gallbladder. (c) Percentages of wild-type and abcb11b mutant larvae with either positive or negative BODIPY filling of the gallbladder. (d–f) Confocal images showing three grades of CGamF distribution in 6 dpf wild-type and abcb11b mutant larval liver, ranging from throughout the intrahepatic biliary network (normal grade in d) to only in the large bile ducts (grade I in e), and to no bile duct distribution at all (grade II in f). Arrowheads in (e, f) mark the CGamF signal close to the bile canaliculi. Arrows in (e) point to the large bile duct connecting to the extrahepatic duct. Lateral views, anterior to the top. (g) Percentages of larvae showing different grades of CGamF distribution. Statistical significance in (c) was calculated by Fisher’s exact test, and in (g) by Ridit test. ****p < 0.0001. The numbers of animals analyzed for each genotype are indicated (reproduced from ref. 4 with permission from John Wiley & Sons Press)
Fig. 4.

sox9b mutants exhibit obstructive cholestasis and elevated total liver bile salt levels. (a, b) Confocal projections of wild-type and sox9b mutant larvae at 5 dpf. Tg(EPV.Tp1-Mmu.Hbb:GFP) transgene expression marks the intrahepatic bile ducts. sox9b mutant exhibits cholestasis associated with hepatic and pancreatic duct proliferation, cyst formation, and fibrosis due to a point mutation in the sox9b gene encoding an SRY-related transcription factor [35]. In wild-type fish (a), the cell bodies of intrahepatic biliary cells are separated from one another and interconnected via cellular processes. In sox9b mutants (b), the biliary cells are clustered together. Ventral views, anterior to the top. (c, d) Cryosections of 1-month-old wild-type and sox9b mutant fish expressing Tg(EPV.Tp1-Mmu.Hbb:GFP). Compared to the wild-type biliary cells that form a complex intrahepatic biliary network, the mutant biliary cells remain clustered and some areas of the liver show bile duct paucity. Dashed lines in (a–d) label the livers. (e) Amount of total liver bile salts normalized to liver weight (average ± S.E.M.) in 2-month-old fish. Each dot represents one liver. Statistical significance was calculated by one-way ANOVA and Tukey’s post hoc test. *p < 0.05
Fig. 5.
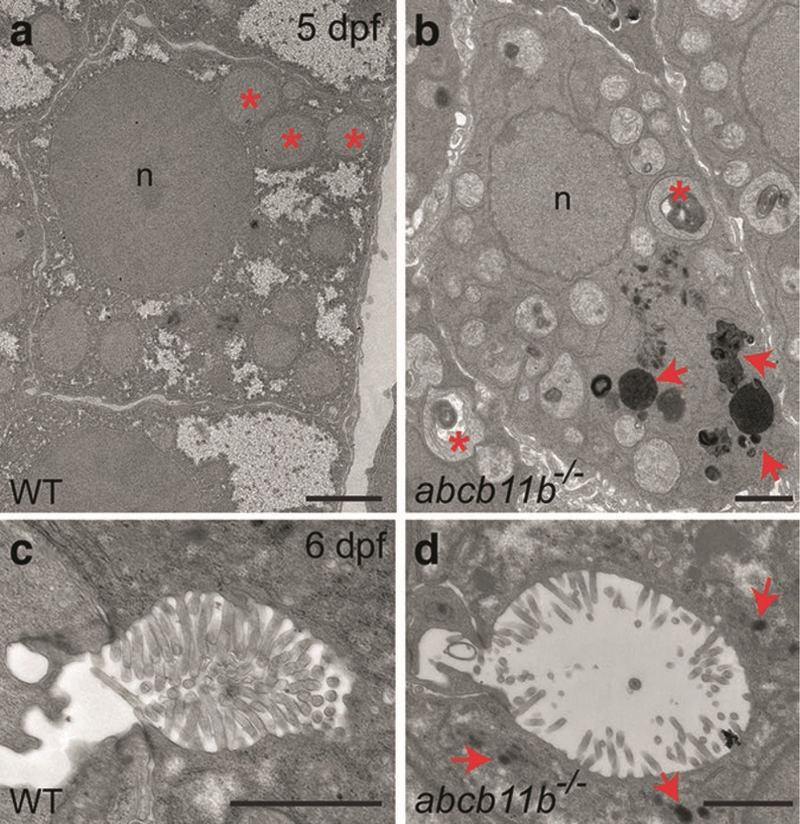
Ultrastructural features of hepatocellular cholestasis in zebrafish revealed by TEM. (a, b) TEM images of the hepatocytes in wild-type and abcb11b mutant zebrafish at 5 dpf. Red asterisks mark the mitochondria. The mutant mitochondria are pleomorphic with irregularities in arrangement of cristae and often contain electron-dense whorled membranes. Red arrows in (b) point to electron-dense amorphous bile-like granular deposits that are absent in the wild-type hepatocytes. n, nucleus. (c, d) TEM images of the bile canaliculi in wild-type and abcb11b mutant zebrafish at 6 dpf. The mutant shows dilatation of bile canaliculus and effacement of canalicular microvilli. Red arrows in (d) point to amorphous bile-like deposits near the bile canaliculus. Scale bars: 2 μm (reproduced from ref. 4 with permission from John Wiley & Sons Press)
2. Materials
2.1. Assessment of Bile Flow Using Fluorescent Fatty Acid Analog BODIPY™ FL C5 (See Note 2)
Chicken egg yolk: Store 1 mL aliquots in microcentrifuge tubes at −20 °C.
Embryo medium: Prepare 60 μg/μL Instant Ocean sea salts in distilled water.
Prepare 0.5 μg/μL BODIPY™ FL C5 (4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoic acid) stock solution in 200-proof ethanol. Store 20.3 μL aliquots of stock solution in microcentrifuge tubes at −20 °C. Wrap the tubes with parafilm to prevent evaporation.
3% Methyl cellulose solution: Dissolve 1.2 g of methyl cellulose in 40 mL distilled water in a 50 mL Falcon tube (see Note 3).
0.4% Tricaine (ethyl 3-aminobenzoate methanesulfonate) solution: Dissolve 2 g of tricaine powder in 489.5 mL of distilled water and add 10.5 mL of Tris–HCl pH 9 solution. Adjust to pH 7.0 (see Note 4). Store the solution at −20 °C until use.
Small weighing spatula.
Handheld homogenizer.
Stainless steel mini strainer.
Pasteur transfer pipettes, glass, short tip.
Standard disposable transfer pipettes, plastic.
50 mL Falcon tubes.
15 mL Round-bottom tubes.
Vortex mixer.
6-Well clear cell culture plate.
Aluminum foil.
Benchtop orbital shaker.
Nutating mixer.
Stereo fluorescence microscope.
2.2. High-Resolution Imaging of Intrahepatic Bile Secretion and Transport After Administering CGamF (See Note 5)
10 mM CGamF stock solution: Dissolve 4 mg of CGamF powder in 60 μL 200-proof ethanol. Add 100 μL DMSO at a time to prevent precipitation until a final volume of 503 μL. Store aliquots of stock solution in microcentrifuge tubes at −20 °C. Wrap the tubes with parafilm to prevent evaporation.
Embryo medium.
3% Methyl cellulose solution.
0.4% Tricaine solution.
Pasteur transfer pipettes, glass, short tip.
Standard disposable transfer pipettes, plastic.
Vortex mixer.
Multi-well clear cell culture plate.
Aluminum foil.
Benchtop orbital shaker.
24 mm × 60 mm Rectangular No. 1 glass coverslips.
U-100 29G × ½″ 0.3 cc Lo-dose insulin syringe.
Inverted confocal microscope.
2.3. Measurement of Total Bile Salts in Whole Larva and Adult Zebrafish Liver
Total bile acid detection kit—enzyme cycling method (see Note 6).
Standard bile acids.
Chloroform:methanol solution at 1:1 (volume/volume) ratio.
Dumont #5 and #55 forceps.
Stainless steel fine scissors, sharp tips, 21 mm.
Student Vannas Spring Scissors.
100 mm Petri dishes.
0.4% Tricaine solution.
Ice.
Analytical balance.
Bright-field dissecting microscope.
Benchtop microcentrifuge.
Evaporator.
96-Well clear tissue culture flat-bottom plate.
Sterile glass beads, 5 mm in diameter.
Bead ruptor.
Microplate spectrophotometer.
2.4. Transmission Electron Microscopy (TEM)
0.15 M Sodium cacodylate buffer: Dissolve 29.4 g of sodium cacodylate trihydrate in 1 L distilled water. Adjust to pH 7.4.
TEM fixative: 3% Glutaraldehyde in 0.15 M sodium cacodylate buffer, pH 7.4.
Post-fixative solution: 1% Osmium tetroxide in 0.15 M sodium cacodylate buffer, pH 7.4.
Ethanol.
LX-112 resin.
Pyramid tip mold.
Ultramicrotome.
Mounting cylinders.
Toluidine blue.
200 mesh grids for TEM.
2% Aqueous uranyl acetate (commercially available).
Reynolds’ lead citrate (commercially available).
Transmission electron microscope equipped with an AMT BioSprint 16 High Definition CCD Camera and AMT image V700 software.
3. Methods
3.1. Assessment of Bile Flow Using Fluorescent Fatty Acid Analog BODIPY™ FL C5 [34]
Raise the larvae to 6 days postfertilization (see Note 7). Prescreen to remove the larvae that show apparent developmental delay. Transfer the larvae to 6-well plate with no more than 30 larvae per well.
Prepare fresh 5% egg yolk solution: Thaw a 1 mL aliquot of frozen chicken egg yolk and bring it to room temperature. Use a small sterile spatula to carefully transfer egg yolk to 19 mL of embryo medium in a 50 mL Falcon tube. Vortex egg yolk/embryo medium mixture until yolk appears evenly dispersed. Clean homogenizer probe by immersing it 2–3 inches into a 50 mL Falcon tube filled with distilled water. Run homogenizer for 10 s and dry probe with clean tissue paper. Immerse homogenizer probe into egg yolk/embryo medium mixture 2–3 inches down into the solution. Run homogenizer for about 1 min until no chunks of egg yolk are visible. Strain the mixture with a mini strainer into a new Falcon tube to collect any aggregates that have not dispersed. Place the Falcon tube on a nutating mixer to mix continuously at room temperature.
Prepare 2.03 μg/mL BODIPY™ C5 working solution: Use a plastic transfer pipette to quickly add 5 mL of egg yolk mixture to a 15 mL round-bottom tube. Take one aliquot of BODIPY™ C5 stock solution from the freezer. Use a P100 micropipette to add 70 μL of egg yolk mixture to BODIPY stock solution and pipette up and down several times to mix. Transfer the entire 90 μL solution to the 15 mL round-bottom tube containing egg yolk mixture. Vortex the tube immediately for 20 s. Cover BODIPY™ C5 working solution with aluminum foil to protect from light and place the tube on a nutating mixer to mix continuously until use.
Add BODIPY™ C5 working solution to the larvae in 6-well plate, 5 mL per well, and cover the plate with aluminum foil to protect from light.
Shake the plate on a benchtop orbital shaker at slow speed and let larvae feed for 4–6 h at room temperature.
After feed, start with the first well, carefully remove BODIPY solution with a glass Pasteur pipette, and wash the larvae with embryo medium 2–3 times or until the solution is clear.
Anesthetize larvae by adding 0.4% tricaine solution to egg medium at 1/10 (volume/volume) ratio.
Immediately observe the larvae under a stereo fluorescence microscope using the GFP filter. Score them based on whether there is green fluorescence signal in the gallbladder (see Notes 8 and 9).
Move to the next well and repeat steps 5–7.
Perform quantification and statistical analyses (see Note 10).
3.2. High-Resolution Imaging of Intrahepatic Bile Secretion and Transport After Administering CGamF
Raise the larvae to 6 days postfertilization (see Note 11). Prescreen to remove the larvae that show apparent developmental delay. Transfer the larvae to multi-well cell culture plate depending on the number of larvae to be examined: 24-well plate: 0–10 larvae/well; 12-well plate: 11–30 larvae/well; and 6-well plate: 31–50 larvae/well.
Prepare fresh CGamF working solution: Take an aliquot of CGamF stock solution from the freezer. Add 0.8 μL of the stock solution per 1 mL egg medium to make the working solution. Vortex to mix.
At a density of ten larvae per mL, add CGamF working solution to each well. Cover the cell culture plate with aluminum foil to protect from light.
Shake the plate on a benchtop orbital shaker at slow speed for a minimum of 3 h at room temperature.
After incubation, start with the first well, and carefully remove the CGamF solution with a glass Pasteur pipette. Wash the larvae with embryo medium 2–3 times or until the solution is no longer yellow.
Anesthetize larvae by adding 0.4% tricaine solution to egg medium at 1/10 (volume/volume) ratio.
Mount the larvae on a 24 mm × 60 mm rectangular No. 1 glass coverslip in a small drop of 3% methyl cellulose. Use an insulin syringe to adjust the position of each larva so that its left side faces against the bottom of the coverslip (see Note 12).
Image the liver using an inverted confocal microscope. Use a 20× air objective and 2× zoom, or a 40× water objective and 1× zoom, to collect a 20 μm z stack from the surface of the liver at the step size of 1 μm (see Note 13).
Move to the next well and repeat steps 5–8.
Grade the CGamF distribution patterns and perform quantification and statistical analyses (see Notes 14 and 15).
3.3. Measurement of Total Bile Salts in Whole Larva and Adult Zebrafish Liver [43]
- Sample collection:
- Whole larva: Place a single larva in a 1.5 mL microcentrifuge tube. Remove all the liquid. Keep the sample on ice for immediate analysis, or snap freeze the sample in liquid nitrogen and store at −80 °C for later use (see Note 16).
- Adult zebrafish liver: Euthanize adult zebrafish by submersion in ice water (5 parts ice/1 part water, 0–4 °C) for at least 10 min following cessation of opercular movement (see Note 17). Place the fish in a 100 mm petri dish. Dissect the liver using forceps and fine scissors under a bright-field dissecting microscope. Transfer the liver to a 1.5 mL microcentrifuge tube and remove all the liquid. Weigh the liver using an analytical balance. Keep the sample on ice for immediate analysis, or snap freeze in liquid nitrogen and store at −80 °C for later use.
Tissue homogenization: Add 500 μL of chloroform:methanol solution to each sample. Add one sterile glass bead into each tube. Homogenize the tissue by shaking the microcentrifuge tube on a bead ruptor for 30 s at high speed. Incubate the sample at room temperature for 60 min. Centrifuge at 20,000 × g at room temperature for 15 min. Transfer the supernatant to a new microcentrifuge tube and keep on ice.
Prepare fresh bile acid standards by diluting in deionized water in series of 300, 150, 100, 75, 50, 25, 10, 5, and 0 pmol/tube (see Note 18).
Vacuum dry samples and standard bile acids using an evaporator at low level at room temperature for 50 min.
Add 150 μL of R1 buffer supplied with the total bile acid assay kit to each sample and standard.
Incubate at 37 °C for 5 min.
Transfer the samples and standards to a 96-well clear plate.
Immediately add 50 μL of R2 buffer supplied with the kit.
Incubate at room temperature for 10 min.
Immediately monitor the absorbance at 405 nm using a microplate spectrophotometer.
Graph the standard curve based on the OD values of the standards.
Calculate the quantity of bile salts present in each sample based on the standard curve (see Note 19).
3.4. Transmission Electron Microscopy (TEM) [44]
Fix larval zebrafish or dissected adult zebrafish liver in TEM fixative at 4 °C overnight.
Wash the samples in 0.15 M sodium cacodylate buffer three times, 10 min each, at room temperature.
Postfix in 1% osmium tetroxide in 0.15 M sodium cacodylate buffer at 4 °C for 1 h (see Note 20).
Wash in 0.15 M sodium cacodylate buffer three times, 10 min each, at room temperature.
-
Sample dehydration and infiltration with serial changes into the following solutions at room temperature:
25% Ethanol for 15 min
50% Ethanol for 15 min
75% Ethanol for 15 min
95% Ethanol for 15 min
100% Ethanol for 15 min, two times
Ethanol:LX-112 (3:1) for 30 min
Ethanol:LX-112 (1:1) for 30 min
Ethanol:LX-112 (1:3) for 30 min
LX-112 for 60 min, two times
Transfer the samples to pyramid tip mold and polymerize at 60 °C for 72 h.
Attach pyramid on mounting cylinders and cut 1 μm semi-thin sections using an ultramicrotome.
Stain the sections with toluidine blue to identify the position of the liver.
Cut 70–100 nm ultrathin sections and collect them on 200 mesh grids.
Stain the grids with 2% aqueous uranyl acetate at room temperature for 10 min.
Stain the grids with Reynolds’ lead citrate at room temperature for 5 min.
Examine the sections with a Hitachi model H-7650 transmission electron microscope equipped with an AMT Biosprint 16 High Definition CCD camera and AMT image V700 software (see Notes 21 and 22).
Acknowledgments
We thank Drs. Steve Farber and Jessica Otis for sharing the original BODIPY protocol, Dr. Alan Hofmann for providing CGamF and C-NBD-L bile acid derivatives, Dr. Shinpei Kawaoka for advice on total bile salt measurement, and Dr. Kevin Bove and Ms. Georgianne Ciraolo for assistance with TEM. This work was supported by NIH grant R00AA020514 and American Gastroenterological Association AGA-Elsevier Pilot Research Award to C.Y., the Center for Pediatric Genomics at Cincinnati Children’s Hospital Medical Center, and NIH grant P30DK078392 to the Integrative Morphology Core of the Digestive Disease Research Core Center in Cincinnati.
4 Notes
In our opinion, hematoxylin and eosin (H&E) stain is not very informative for assessing cholestasis in zebrafish. It is difficult to observe intrahepatic bile ducts as zebrafish liver does not form portal triad and majority of the cholangiocytes do not adopt a columnar epithelial morphology like the mammalian cholangiocytes (Fig. 2). Whereas accumulation of bile acids can be revealed by H&E in mammalian cholestatic livers, we have not been able to detect bile accumulation in the zebrafish cholestatic models by routine H&E.
BODIPY™ FL C5 lipid analog consists of saturated acyl chain of five carbons tagged with the BODIPY fluorophore. It is metabolized in the intestine after being ingested, delivered to the liver, and secreted into bile by hepatocytes. Subsequently, the intrahepatic bile ducts and gallbladder exhibit bright green fluorescence signal. An absence of fluorescence signal in the gallbladder suggests bile flow blockage (Fig. 3a, b).
To prepare 3% methyl cellulose solution, rock the solution on a nutating mixer for 4 days at 4 °C. Inspect and shake several times daily. After the particles seem to be dissolved uniformly, centrifuge the tube at 4500 × g at room temperature for 15 min to spin down any undissolved particles. Store the solution at room temperature.
Tricaine solution should be buffered with sodium bicarbonate to a neutral pH before immersing fish. Non-buffered tricaine is acidic and causes an aversive reaction in unanesthetized fish.
CGamF is used as a surrogate for natural cholylglycine to monitor bile acid transport in mammalian hepatocytes. It is a known substrate of human bile salt export pump (BSEP) [45]. Zebrafish BSEP protein is capable of transporting CGamF [4]. Confocal imaging of zebrafish larvae after administering CGamF reveals bile excretion and transport at cellular resolution in real time.
The protocol is based on the total bile acid detection kit from Diazyme, but can be applied to other enzyme cycling method-based commercial kits.
BODIPY feeding assay can be applied to larval and juvenile zebrafish between 5 days and 3 weeks of age as long as the gallbladder is visible under the stereo fluorescence microscope.
Recording of gallbladder fluorescent signal should be performed immediately upon anesthesia as tricaine triggers the larva to expel the yolk mixture.
BODIPY assay represents a straightforward and noninvasive approach to detect bile flow impairment that can be applied in high-throughput chemical and genetic screens. However, it does not reveal the cause of cholestasis. Further investigation is needed to determine whether bile flow is blocked due to impaired hepatocyte bile uptake or excretion, or obstruction and malformation of the intrahepatic and extrahepatic biliary systems. Moreover, an absence of gallbladder fluorescence is not a definitive indicator of cholestasis as it can also be caused by defects in BODIPY uptake and/or processing in the intestine.
Percentages of animals showing positive and negative fluorescence signal in the gallbladder are calculated and compared between the control and experimental groups. Fisher’s exact test followed by a pairwise comparison on R software is used to calculate statistical significance. Power analysis is performed to determine the sample size. Figure 3c provides a representative example of the result.
CGamF assay can be performed on larval and juvenile zebrafish at 5 days postfertilization and beyond as long as it is feasible to image the liver through the skin on a confocal microscope. Fish that are older than 6 days postfertilization must be fed daily prior to the experiment as fasting interferes with hepatocyte bile secretion.
The gallbladder accumulates a large amount of bile salts and the resulting strong fluorescence signal masks the dimmer fluorescence in the hepatocytes and intrahepatic bile ducts. Therefore, it is recommended to image from the left side of the animal to avoid the gallbladder.
Confocal imaging should be performed immediately after mounting as CGamF gets expelled from the liver rapidly. Because the liver is not a homogeneous organ and has a multicellular organization, it is recommended to collect a z stack rather than one single plane snapshot in order to obtain a more comprehensive understanding of the phenotype.
CGamF distribution pattern is graded first based on if there is fluorescence signal throughout the intrahepatic biliary network. If not, the grades are based on whether there is signal in the large intrahepatic bile ducts, bile canaliculi, or hepatocytes. Percentages of animals showing different grades of CGamF distribution are calculated and compared between the control and experimental groups. Ridit test followed by a pairwise comparison on R software is used to calculate statistical significance. Power analysis is performed to determine the sample size. Figure 3g provides a representative example of the result.
In addition to CGamF, this method can be applied to other fluorescent bile acid analogs such as cholyl-Nε-nitrobenzoxadiazol-lysine (C-NBD-L) and cholyl-lysyl-fluorescein (CLF) to evaluate the function of different bile transporters.
Whole-animal assay is recommended for zebrafish younger than 1 month of age as dissecting liver from larval fish is technically difficult.
Anesthesia can also be achieved by overdose of tricaine (200– 300 mg/L) immersion. Fish should be left in the solution for at least 10 min following cessation of opercular movement.
Each bile acid standard should be assayed in duplicates. A freshly prepared standard curve should be used each time the assay is performed.
For whole larval bile salt measurement, because the larval body weight is difficult to measure and there is no correlation between the larval body length and total bile salt levels [43], the total bile salt amount of each animal is plotted directly without normalization. For adult liver bile salt measurement, the total bile salt amount is normalized to the liver weight. Figure 4e provides a representative example of the result.
This postfix step provides an enhanced contrast to TEM images.
Ultrathin sections can be stored at room temperature for up to 1 year.
Ultrastructural features of cholestasis include hepatocyte bile accumulation, bile canaliculi dilatation and microvilli effacement, impairment of hepatocyte and cholangiocyte cell-cell junctions, as well as other subcellular defects reflecting hepatocyte and cholangiocyte injury. Figure 5 provides representative TEM images of wild-type and abcb11b mutant hepatocytes.
References
- 1.Matte U, Mourya R, Miethke A et al. (2010) Analysis of gene mutations in children with cholestasis of undefined etiology. J Pediatr Gastroenterol Nutr 51:488–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mariotti V, Strazzabosco M, Fabris L et al. (2018) Animal models of biliary injury and altered bile acid metabolism. Biochim Biophys Acta 1864:1254–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pham DH, Zhang C, Yin C (2017) Using zebrafish to model liver diseases-where do we stand? Curr Pathobiol Rep 5:207–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellis JL, Bove KE, Schuetz EG et al. (2018) Zebrafish abcb11b mutant reveals strategies to restore bile excretion impaired by bile salt export pump deficiency. Hepatology 67:1531–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorent K, Yeo SY, Oda T et al. (2004) Inhibition of Jagged-mediated Notch signaling disrupts zebrafish biliary development and generates multi-organ defects compatible with an Alagille syndrome phenocopy. Development 131:5753–5766 [DOI] [PubMed] [Google Scholar]
- 6.Zhang D, Gates KP, Barske L et al. (2017) Endoderm jagged induces liver and pancreas duct lineage in zebrafish. Nat Commun 8:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cofer ZC, Cui S, EauClaire SF et al. (2016) Methylation microarray studies highlight PDGFA expression as a factor in biliary atresia. PLoS One 11:e0151521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui S, Leyva-Vega M, Tsai EA et al. (2013) Evidence from human and zebrafish that GPC1 is a biliary atresia susceptibility gene. Gastroenterology 144:1107–1115.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorent K, Gong W, Koo KA et al. (2015) Identification of a plant isoflavonoid that causes biliary atresia. Sci Transl Med 7:286ra267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews RP, Eauclaire SF, Mugnier M et al. (2011) DNA hypomethylation causes bile duct defects in zebrafish and is a distinguishing feature of infantile biliary atresia. Hepatology 53:905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ningappa M, So J, Glessner J et al. (2015) The role of ARF6 in biliary atresia. PLoS One 10:e0138381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang V, Cofer ZC, Cui S et al. (2016) Loss of a candidate biliary atresia susceptibility gene, add3a, causes biliary developmental defects in zebrafish. J Pediatr Gastroenterol Nutr 63:524–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X, Lorent K, Wilkins BJ et al. (2016) Glutathione antioxidant pathway activity and reserve determine toxicity and specificity of the biliary toxin biliatresone in zebrafish. Hepatology 64:894–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cullinane AR, Straatman-Iwanowska A, Zaucker A et al. (2010) Mutations in VIPAR cause an arthrogryposis, renal dysfunction and cholestasis syndrome phenotype with defects in epithelial polarization. Nat Genet 42:303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews RP, Plumb-Rudewiez N, Lorent K et al. (2005) Zebrafish vps33b, an ortholog of the gene responsible for human arthrogryposis-renal dysfunction-cholestasis syndrome, regulates biliary development downstream of the onecut transcription factor hnf6. Development 132:5295–5306 [DOI] [PubMed] [Google Scholar]
- 16.Wilkins BJ, Lorent K, Matthews RP et al. (2013) p53-mediated biliary defects caused by knockdown of cirh1a, the zebrafish homolog of the gene responsible for North American Indian Childhood Cirrhosis. PLoS One 8:e77670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadler KC, Amsterdam A, Soroka C et al. (2005) A genetic screen in zebrafish identifies the mutants vps18, nf2 and foie gras as models of liver disease. Development 132:3561–3572 [DOI] [PubMed] [Google Scholar]
- 18.Asai A, Miethke A, Bezerra JA (2015) Pathogenesis of biliary atresia: defining biology to understand clinical phenotypes. Nat Rev Gastroenterol Hepatol 12:342–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen C, Biermanns D, Kuske M et al. (1997) New aspects in a murine model for extrahepatic biliary atresia. J Pediatr Surg 32:1190–1195 [DOI] [PubMed] [Google Scholar]
- 20.Petersen C, Grasshoff S, Luciano L (1998) Diverse morphology of biliary atresia in an animal model. J Hepatol 28:603–607 [DOI] [PubMed] [Google Scholar]
- 21.Omenetti A, Bass LM, Anders RA (2011) Hedgehog activity, epithelial-mesenchymal transitions, and biliary dysmorphogenesis in biliary atresia. Hepatology 53:1246–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Childs S, Yeh RL, Georges E, Ling V (1995) Identification of a sister gene to P-glycoprotein. Cancer Res 55:2029–2034 [PubMed] [Google Scholar]
- 23.Gerloff T, Stieger B, Hagenbuch B et al. (1998) The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem 273:10046–10050 [DOI] [PubMed] [Google Scholar]
- 24.Strautnieks SS, Bull LN, Knisely AS et al. (1998) A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet 20:233–238 [DOI] [PubMed] [Google Scholar]
- 25.Jansen PL, Strautnieks SS, Jacquemin E et al. (1999) Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology 117:1370–1379 [DOI] [PubMed] [Google Scholar]
- 26.Knisely AS, Strautnieks SS, Meier Y et al. (2006) Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology 44:478–486 [DOI] [PubMed] [Google Scholar]
- 27. Telbisz A, Homolya L (2016) Recent advances in the exploration of the bile salt export pump (BSEP/ABCB11) function. Expert Opin Ther Targets 20:501–514 [DOI] [PubMed] [Google Scholar]
- 28. Lam P, Wang R, Ling V (2005) Bile acid transport in sister of P-glycoprotein (ABCB11) knockout mice. Biochemistry 44:12598–12605 [DOI] [PubMed] [Google Scholar]
- 29.Wang R, Salem M, Yousef IM et al. (2001) Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci U S A 98:2011–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Li F, Patterson AD, Wang Y et al. (2012) Abcb11 deficiency induces cholestasis coupled to impaired beta-fatty acid oxidation in mice. J Biol Chem 287:24784–24794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagey LR, Moller PR, Hofmann AF et al. (2010) Diversity of bile salts in fish and amphibians: evolution of a complex biochemical pathway. Physiol Biochem Zool 83:308–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reschly EJ, Ai N, Ekins S et al. (2008) Evolution of the bile salt nuclear receptor FXR in vertebrates. J Lipid Res 49:1577–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novoa B, Figueras A (2012) Zebrafish: model for the study of inflammation and the innate immune response to infectious diseases. Adv Exp Med Biol 946:253–275 [DOI] [PubMed] [Google Scholar]
- 34.Carten JD, Bradford MK, Farber SA (2011) Visualizing digestive organ morphology and function using differential fatty acid metabolism in live zebrafish. Dev Biol 360:276–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delous M, Yin C, Shin D et al. (2012) Sox9b is a key regulator of pancreaticobiliary ductal system development. PLoS Genet 8:e1002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakaguchi TF, Sadler KC, Crosnier C et al. (2008) Endothelial signals modulate hepatocyte apicobasal polarization in zebrafish. Curr Biol 18:1565–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorent K, Moore JC, Siekmann AF et al. (2010) Reiterative use of the notch signal during zebrafish intrahepatic biliary development. Dev Dyn 239:855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parsons MJ, Pisharath H, Yusuff S et al. (2009) Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech Dev 126:898–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crosnier C, Vargesson N, Gschmeissner S et al. (2005) Delta-Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development 132:1093–1104 [DOI] [PubMed] [Google Scholar]
- 40.Dong PD, Munson CA, Norton W et al. (2007) Fgf10 regulates hepatopancreatic ductal system patterning and differentiation. Nat Genet 39:397–402 [DOI] [PubMed] [Google Scholar]
- 41.Matthews RP, Lorent K, Russo P et al. (2004) The zebrafish onecut gene hnf-6 functions in an evolutionarily conserved genetic pathway that regulates vertebrate biliary development. Dev Biol 274:245–259 [DOI] [PubMed] [Google Scholar]
- 42.Wilkins BJ, Gong W, Pack M (2014) A novel keratin18 promoter that drives reporter gene expression in the intrahepatic and extrahepatic biliary system allows isolation of cell-type specific transcripts from zebrafish liver. Gene Expr Patterns 14:62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Enya S, Kawakami K, Suzuki Y et al. (2018) A novel zebrafish intestinal tumor model reveals a role for cyp7a1-dependent tumor-liver cross-talk in causing adverse effects on the host. Dis Model Mech 11:pii: dmm032383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar S, Ciraolo G, Hinge A et al. (2014) An efficient and reproducible process for transmission electron microscopy (TEM) of rare cell populations. J Immunol Methods 404:87–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holzinger F, Schteingart CD, Ton-Nu HT et al. (1998) Transport of fluorescent bile acids by the isolated perfused rat liver: kinetics, sequestration, and mobilization. Hepatology 28:510–520 [DOI] [PubMed] [Google Scholar]


