Abstract
Background:
Due to the special nature of Chinese Herbal medicine and the complexity of its clinical use, it is difficult to identify and evaluate its toxicity and resulting herb induced liver injury (HILI).
Methods:
First, the database would provide full profile of HILI from the basic ingredients to clinical out-comes by the most advanced algorithms of artificial intelligence, and it is also possible that we can predict possibilities of HILI after patients taking Chinese herbs by individual patient evaluation and prediction. Second, the database would solve the chaos and lack of the relevant data faced by the current basic re-search and clinical practice of Chinese Herbal Medicine. Third, we can also screen the susceptible patients from the database and thus prevent the accidents of HILI from the very beginning.
Results:
The Roussel Uclaf Causality Assessment Method (RUCAM) is the most accepted method to evalu-ate DILI, but at present before using the RUCAM evaluation method, data resource collection and analysis are yet to be perfected. Based on existing research on drug-metabolizing enzymes mediating reactive me-tabolites (RMs), the aim of this study is to explore the possibilities and methods of building multidimen-sional hierarchical database composing of RMs evidence library, Chinese herbal evidence library, and indi-vidualized reports evidence library of herb induced liver injury HILI.
Conclusion:
The potential benefits lie in its ability to organize, use vast amounts of evidence and use big data mining techniques at the center for Chinese herbal medicine liver toxicity research, which is the most difficult key point of scientific research to be investigated in the next few years.
Keywords: Drug-induced Liver Injury (DILI), Herb-induced Liver Injury (HILI), Evidence-based Medicine, Reactive Metabolites (RMs), evidence library, hepatotoxicity individualized, clinical study, Chinese herbal recognition evidence library, RUCAM
1. INTRODUCTION
The liver is an important metabolic organ in the human body and is an important component throughout the human lifecycle. The decomposition and metabolism of any drug need to be carried out with the aid of liver function in order to develop smoothly [1]. Therefore, diseases arising from the liver as the primary metabolic organ have been a focal point throughout the medical world. Drug-induced Liver Injury (DILI) refers to toxic damage or an allergic reaction caused by the drug itself or its metabolites [2]. Drug hepatotoxicity refers to liver injury induced by all manner of prescriptions, over-the-counter drugs (e.g., chemical drugs, biological agents, traditional Chinese medicine, natural medicine, health care products, dietary supplements) and their metabolites [3-5]. In recent years, the global pharmaceutical industry has continued to expand, in addition to the aging of society and the increase in concomitant drug usage has also caused a sharp rise in the incidence of DILI worldwide. With a clinical incidence of approximately 10~15/100,000 annually [6], it has become one of the most difficult critical diseases to combat throughout the world. According to a report by the World Health Organization (WHO), DILI is the top cause of death among liver diseases worldwide. In 2002, France reported an annual incidence of approximately 13.9/100,000 and in 2013, Iceland reported an annual incidence of 19.1/100,000 [7]. The DILIs reported in China mainly originate from the outpatients, emergencies and inpatients of medical institutions. Due to the lack of DILI epidemiological data for large populations, the exact incidence of DILI in our population is as yet undetermined [8-10]. At present, the clinical diagnosis of DILI primarily employs the Roussel Uclaf Causality Assessment Method (RUCAM) scoring system and the recommended use of Structured Expert Opinion Process (SEOP) for hepatotoxicity assessment [11]. At present, RUCAM is the primary method by which Drug-induced Liver Injury (DILI) is evaluated.
Herb-induced Liver Injury (HILI) is an important aspect of hepatotoxicity, which refers to liver damage caused by Chinese herbal medicine, natural drugs and related preparations for disease treatment [12]. In recent years, with the widespread use of Chinese herbal medicine and related products across the world, adverse reactions caused by Chinese herbal medicines have also attracted worldwide attention [13]. Due to the complexity of Chinese herbal medicine, when RUCAM is used to evaluate DILI, the input data’s quality is much lower when compared with Western medicine, thereby giving significantly different diagnostic outcomes for HILI. Meanwhile, in regards to clinical diagnostics, there are very few medical records pertaining to the composition and dosage of Chinese Herbal Medicine, which results in a HILI diagnostic bias [14].
2. DRUG-METABOLIZING ENZYMES MEDIATING REACTIVE METABOLITES (RMs) ARE ONE OF THE MOST IMPORTANT ROUTES TO LIVER INJURY
2.1. The Electrophilic RMs of Drugs Produced by CYP450 Enzymes are an Important Mechanism Leading to Liver Injury
Drugs mediated by metabolizing enzymes causing hepatotoxicity mainly do so through two mechanisms: (1) Direct toxic effects of prototype drugs on the liver. Most of the drug-induced damages are associated with drug-accumulation in the liver and depend on the concentration, duration, as well as the susceptibility and tolerance of the liver towards the drug [15]. (2) The toxic effects of active intermediates (RMs) on liver [16, 17]. When exogenous substances (including drugs) enter organisms (including humans, animals, microorganisms, and plants), they may give rise to different biological reactions; the body's defense system works to dispose of such foreign substances, which in turn causes a series of reactions. Some exogenous substances are eliminated in their original form, but others are modified before they can be removed. For drugs, this bio-transformation process is called drug metabolism [18]. CYP450s are the most important metabolizing enzyme, accounts for the highest ratio of all the drug-metabolizing enzymes and is the most extensively studied [19-23]. Drugs are metabolized by CYP450s to produce electrophilic RMs and lipid peroxidation is stimulated by binding to the unsaturated fatty acids of cell membrane phospholipids, causing damage to the organelle membrane and thereby leading to mitochondrial damage and necrosis [24, 25]. Electrophilic RMs can also covalently bind to nucleophilic groups such as cellular cysteine residues, amino groups of lysine and lysine residues, triggering actin condensation and cytoskeletal destruction, causing the cell membrane to ultimately lose its chemical and physiological properties, resulting in cell stress or apoptosis [26].
2.2. Components of Certain Active Functional Groups in Chinese Herbal Medicine May be Potential Risk Factors for HILI
From the study of 207 commonly used oral drugs, Lammert et al. found that approximately 62-69% of the drugs producing RMs resulting in hepatotoxicity [27]. Fontana et al. [28] summarized the common reactive functional groups that may potentially produce RMs, and these functional groups are mainly comprised of: (1) terminal (ɷ) and ɷ-1 acetylenes; (2) furans and thiophenes; (3) epoxides; (4) dichloro- and trichloro-ethylenes; (5) secondary amines; (6) benzodioxoles (methylene dioxyphenyl compounds); (7) isothiocyanates; (8) thioamides; (9) dithiocarbamates; (10) conjugated structures; and (11) terminal alkenes. On this basis, the present research concluded that [29]: drugs (e.g., fluothane) containing dichloro- and trichloro-ethylenes reactive groups usually formed free radicals by oxidation-reduction reactions. The free radicals produced by the reduction reaction of fluothane can bind with CYP450s, destroy the enzyme’s structure and cause the cytochrome’s accumulation toxicity. Oxides produced from the oxidation reaction bind with CYP450s and produce antibodies based on the Mechanism-based Inhibition (MBI) and immunogenicity, thereby inducing idiosyncratic hepatotoxicity; furthermore, the oxide also binds to intracellular macromolecular proteins, leading to stress toxicity and ultimately resulting in hepatotoxicity. Drugs containing secondary amine functional groups produce quinones RMs by CYP450s. The RMs combine with CYP450s to produce an MBI effect; binding with mitochondrial/lysosomal membranes disrupts their function, resulting in increased activity of reactive oxygen species (ROS); binding with GSH and further depletion of GSH results in hepatotoxicity, which is characterized by elevated aminotransferase levels and hepatitis. To summarize, the common route for DILI is based on: Drugs which produce RMs by metabolizing enzymes, and RMs combine with GSH to deplete GSH; binding with large molecular proteins in cells, resulting in the formation of antibodies which cause autoimmune reactions, thereby inducing idiosyncratic hepatotoxicity; binding to cell membrane/organelle, leading to cell stress toxicity and ultimately liver damage.
Chinese Herbal medicines contain a large number of ingredients that may eventually lead to potentially dangerous HILI which could lead to fatal clinical outcomes. Such components also bind to the cytochrome P450 enzyme and form RMs. In our experiments, the traditional Chinese Herbal Medicine including psoralen, Polygonum multiflorum Thunb, Tripterygium wilfordii, asarum, xanthate, corydalis, and evodia fruit were used as research carriers (as shown in Fig. 1) to study the relationship between the production of active metabolites and hepatotoxicity: (1) Taking the main coumarins of psoralen and isopsoralen in Chinese Herbal Medicine psoralen as the research object, the mechanism of hepatotoxicity based on RMs was systematically studied. The results showed that both psoralen and isopsoralen could generate an MBI effect on CYP1A2, CYP2D6 and CYP3A4 activities; Psoralen and isopsoralen could generate the RMs of γ-ketoenal or furanoepoxide in HLM and MLM, and the produced RMs could then be captured by GSH or inactivated by hydrolysis; GSH’s BSO depletion agent could aggravate the hepatotoxicity of psoralen and isopsoralen in mice, but the hepatotoxicity could be relieved by CYP450’s ABT broad-spectrum inhibitor. The above research indicated that psoralen and isopsoralen could both cause hepatotoxicity through RMs. (2) Taking the main active ingredients of emodin and rhein in Polygonum multiflorum Thunb as the research object, the mechanism of hepatotoxicity based on RMs was systematically studied [30]. Findings of the present study showed that rhein was metabolized to the active intermediate product of epoxy in the liver, which was subsequently hydrolyzed into two mono hydroxy rheins. Cellular tests with CYP2C19 inhibitors in the primary liver cells of rats showed that the hepatotoxicity of rhein after adding the inhibitors was significantly alleviated, thereby proving rhein may cause hepatotoxicity based on the activation of CYP2C19 to RM. (3). The main active ingredient in Traditional Chinese medicine’s evodia, including evodiamine, rutecarpine, synephrine, evodin, obacunone and nomolin, were the study object in systematic research in the mechanism of hepatotoxicity based on RMs [31]. The results showed that evodiamine possessed strong inhibitory effects on CYP2C19, obacunone on CYP1A2 and 2E1, evodin on CYP3A4 and 2E1, evodin on CYP3A4, and nomolin on CYP3A4 and 2E1. The inhibitory effect of rutecarpine on CYP1A2 was an MBI, and rutecarpine was metabolized in the liver into an active intermediate of epoxy, RMs. In a cellular test plus CYP450 enzyme inhibitors in the primary liver cells of rats, it was found that the hepatotoxicity of rutecarpine was significantly alleviated after adding the inhibitor, which suggested that the hepatotoxicity of rutecarpine was based on the RMs. On the basis of the above tests, we concluded that [32], some herbal ingredients are proven to induce hepatotoxicity through their RMs, and the possible mechanism for this hepatotoxicity is the reaction of RMs with cellular components such as proteins, DNA, and membranes, resulting in ROS overproduction, respiratory chain dysfunction and cell stress. From the above research results, we found that the amine and furan heterocyclic groups might be the most potential substructures in mechanism-based inhibitors which cause hepatotoxicity, and when phytomedicine derived mechanism-based inhibitors containing amine or furan heterocyclic moieties are used as a monotherapy or concomitantly with other drugs, in vivo hepatotoxicity screening or the clinical use of herbs may give rise to interactive risks.
Fig. (1).
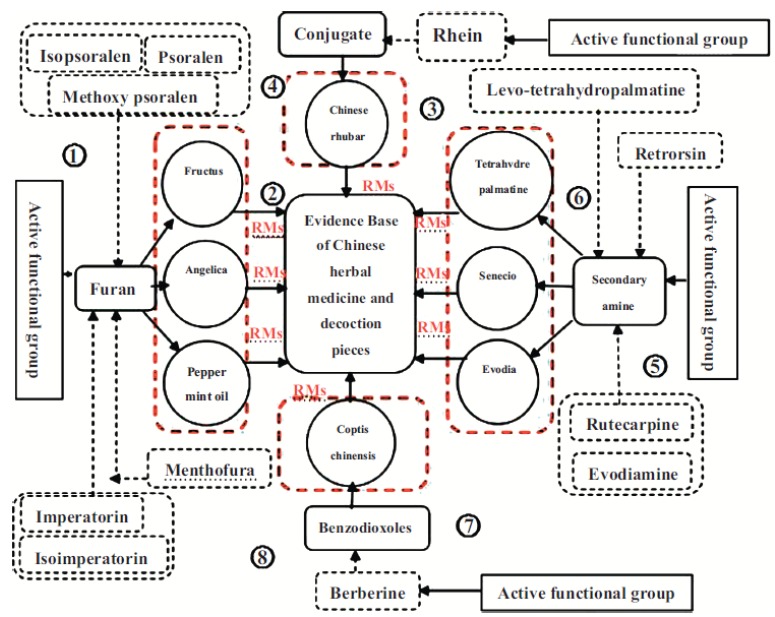
Multivariate evidence base of Chinese herbal medicine hepatotoxicity based on RMs.
3. PROBLEMS RECOGNIZING AND EVALUATING THE CLINICAL HEPATOTOXICITY OF CHINESE HERBAL MEDICINES
The diagnosis of drug-induced liver injuries is a scientific problem that has puzzled clinicians in the medical field. Due to the lack of specific indicators, there is no specific technique to recognize, or method to diagnose, the hepatotoxicity of suspected drugs. At present, clinicians can only rely on the “exclusion method” to determine the clinical symptoms of patients [33]. The diagnosis of drug hepatotoxicity mainly depends on the history of the disease and drug use, the time course and the clinical manifestation of the disease and excludes all other factors. So far, the primary methods used clinically are based on the diagnostic criteria and classification of Western medicine. Amongst which, the establishment of the Roussel Uclaf Causality Assessment Method (RUCAM) scale in 1993 to determine the relationship between a drug and its hepatotoxicity is one of the commonly accepted and widely used methods for recognizing drug-induced liver damage [34, 35]. However, the information needed by the method to evaluate HILI is often inaccurate and incomplete, and sometimes the methods and standards to acquire the information are not standardized. These two factors result in misleading evaluations through RUCAM. In 2010, studies published in the Journal of Hepatology revealed that [36, 37] the consensus rate of RUCAM scores for different clinicians was only 19%, the reasons for such errors are attributable to the sources from which the data is acquired and the means by which data is collected.
It is eminently critical to reduce or prevent the toxicity of Chinese herbal medicines, to discover the clinical symptoms of hepatotoxicity, as well as adjusting and applying specific interventions in a timely manner. Nevertheless, the identification methods of Chinese herbal medicine hepatotoxicity have considerable limitations: (1) The clinical manifestations of Chinese herbal medicine hepatotoxicity are diverse, complex and are often obscured the by underlying diseases. The clinical symptoms and biochemical tests often lack specific indexes, and furthermore, considerations regarding the patient’s quality of life are difficult to implement when there is significant trauma from liver biopsies [38]. 2) Combinations of Traditional Chinese Medicine and Western Medicine is widely used in clinics, which makes the confirmation and further study of hepatotoxicity specifically in Chinese herbal medicine extremely complicated, thus causing the false impression that Chinese herbal medicine may lead to hepatotoxicity. (3) The composition of Chinese herbal medicine is complex and there is a lack of reliable data on the hepatotoxic mechanism, and a lack of credible clinical data and toxic evaluation of these herbal medicines. The combination of all these factors amounts to significant difficulties for the accurate clinical diagnosis and treatment of diseases. Research on methods for the early recognition of Chinese Herbal Medicine hepatotoxicity is therefore of upmost importance. Research is therefore based on more standardized, data-centric, pre-emptive, sensitive and less invasive methods to identify hepatotoxicity and innovative biomarkers to compensate for the limitations of liver biopsies and clinical biochemical examinations. This would provide doctors with timely warning and rational indication for drug usage, to reduce the occurrence of drug-induced hepatotoxicity as far as possible and improve the level of medical treatment and the patients’ quality of life (Fig. 2).
Fig. (2).
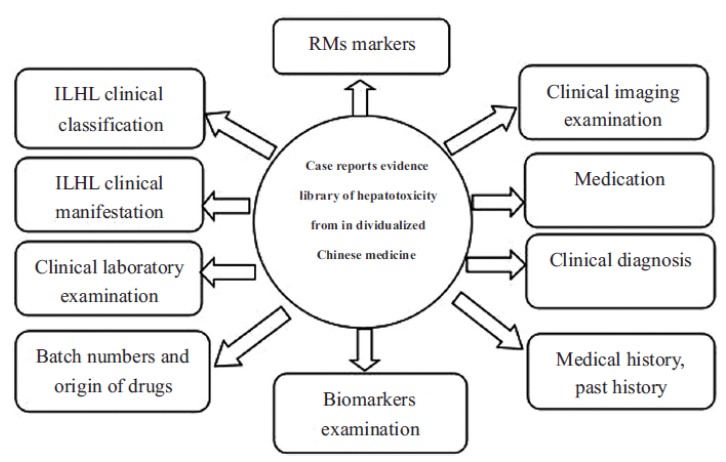
Case reports included in the database for clinical hepatotoxicity from Chinese herbal medicines.
4. ESTABLISHMENT OF THE RECOGNITION AND EVALUATION METHODS OF CHINESE HERBAL MEDICINE HEPATOTOXICITY BASED ON LARGE-SCALE MULTIPLE DATABASES
From the information detailed above, the author proposes, on the basis of existing research results on drug metabolism enzyme-mediated active metabolites, to identify basic biomarker information, individualized clinical patient report information and information pertaining to the characteristics of Traditional Chinese herbal medicine to identify Traditional Chinese medicine hepatoxicity, extract the relevant to form a library model structure of evidence sets, establish scientific assumptions based on evidence-based medicine for the evaluation and identification of Chinese herbal medicine hepatotoxicity and further produce and evaluate various joint evidence related to said hepatotoxicity from the perspective of evidence collection. Therefore, basic evidence will be introduced into clinical work to provide scientific research methods and methodological references for the evaluation of Chinese herbal medicine hepatotoxicity (Fig. 3).
Fig. (3).
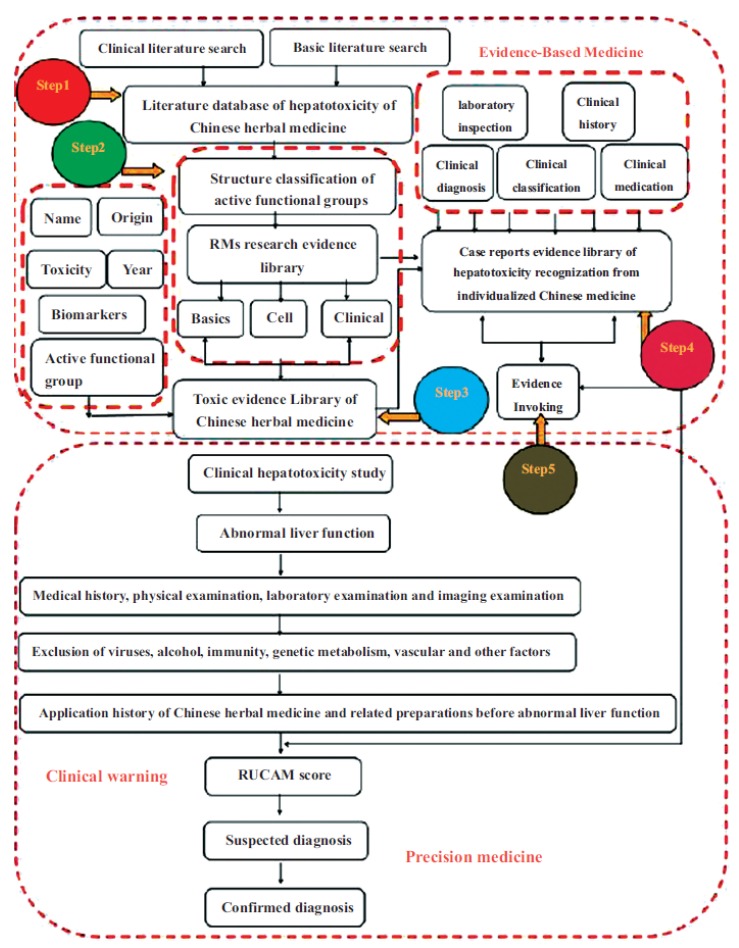
Recognization method of Chinese herbal medicine hepatotoxicity based on evidence.
Step 1: Establish Research Literature Database
Research technique: The design is based on the four sets of Traditional Chinese medicine theory diagnostic information combined with patient hepatotoxicity. Included amongst the four sets of dialectical information are the corresponding dialectical information for hepatotoxicity to ensure a comprehensive screening. At the same time, literature research, expert interviews and other methods were employed to enrich the content of literature screening. A BASE Database (BD) and Clinical Database (CD) for hepatotoxicity were established respectively [39-48].
Literature research: Key words in English and Chinese were explored, including “RMs”, “Active functional group”, “Chinese herbal medicine hepatotoxicity”, “Chinese herbal medicine liver injury”, “HILI”, “DILI”, “Biomarkers”, “CYP450”, “Metabolic enzyme”, “Drug hepatotoxicity diagnosis” and “Hepatotoxicity diagnosis of Chinese herbal medicine” etc. in the Chinese biomedical database, Chinese hospital digital library, Chinese CNKI database, VIP database, Wanfang database, PubMed/MEDLINE database, and Embase database.
Database construction: The retrieved literature was incorporated into the established literature database and indexed according to category, enabling researchers to trace the literature on hepatotoxicity.
Step 2: Construction and Classification of Active Functional Group Database
Irreversible inhibition of CYP450 is the most common cause of clinical drug interaction. It is hoped that through summarizing the structure-activity relationship of common factors, we can rapidly assist in the improvement and design of new drugs and rationally enhance the clinical use of drugs [49, 50]. Therefore, the database is used to find and classify the active functional groups reported in the literature. In addition, the MBI inhibitors of natural products and chemical drugs have been summarized and broadly classified into the following categories based on their structure: Alkyne, furane and thiophene, epoxide, dichloroethylene and chlorine, amine, benzodioxoles (methylene dioxyphenyl), isothiocyanates, thioacid amide, ester, conjugated structure and terminal olefins.
Compound-related information includes the Chinese and English names, molecular formula, and the inhibition and inhibitory capacity of structural CYP enzymes, including a quantitative description of the inactivation response of the three parameters: KI, Kinact and partition ratio. Their values are related to the compounds’ ability as an inhibitor. KI and kinact are the kinetic parameters describing the inactivation process: KI is the inhibitor concentration required to reach half the maximum inactivation; kinact represents the maximum inactivation rate constant. The partition coefficient represents the number of catalytic cycles a CYP must undergo before becoming fully inactivated; it is the quantity required to diminish the inhibitor by one molecule in order to achieve total inactivation [51]; RMs structural information and related research statistical databases include three parts: basic research, cell research, and clinical research. In basic studies which take into consideration the animal species, weight, number of animals, sex ratio and age, a series of biochemical parameters [52-56] are tracked, including ALT, AST and total bilirubin. Additionally, morphological changes of liver tissues are considered, including liver weight changes, liver index changes, cellular swelling, cellular necrosis, inflammatory cell infiltration, fatty degeneration and histological sections. In cell studies, cell type, transfer algebra, cell viability (%), biochemical parameters (ALT, AST, LDH, ROS), membrane potential changes, and cell growth status are often used as key indicators. Clinically, vital signs (gender, age, height, weight, disease history, medication history (drugs), clinically recommended dose, dosage, treatment period, concomitant medications), clinical manifestations (clinical symptoms, liver function, routine examination, imaging examination and clinical diagnosis) are the two sets of data which are recorded.
Step 3: Establishment of a Toxicity Database for Chinese Herbal Medicines Containing Toxic Enzymes after RM Enzyme Reaction
Included in the Chinese Pharmacopoeia published in 2010 are 2165 varieties of Chinese medicinal herbs and prepared decoctions; amongst which are 83 kinds of poisonous drugs (10 deadly poisonous medicinal materials, 42 poisonous herbs, 31 slightly poisonous herbs). However, the toxicity mentioned in Pharmacopoeia refers to acute toxicity, and there is a lack of research and results regarding the chronic pathogenic toxicity of Chinese herbal medicine [57].
According to the standards of Method II, taking active functional groups as the main indicator, Chinese herbal medicines which showed as toxic after enzymatic reactions were included in the database (referring to the standards of Chinese Pharmacopoeia). The active functional groups, RMs and Chinese herbal medicines were linked together to form a shared Chinese herbal medicine hepatotoxicity database as a multidimensional and multi-level network [58]. Several RMs biological target groups were formed by different active functional groups and Chinese herbal medicines which may lead to toxic effects when the target effect was magnified. With reference to the standards of network toxicology, the composition and causal relationship of the toxic chain of Chinese herbal medicine were illustrated from the point (active functional group) -line and (pathway)-surface (Chinese herbal medicine variety) levels. The databased constructed can be used to analyze the toxicity of Chinese herbal medicine by means of network topology mapping.
The database can incorporate various names and relevant evidence of Chinese herbal medicines caused by RMs, which have been confirmed clinically or in published literature, so as to provide data reference and evidence for follow-up toxicity studies and clinical applications.
Step 4: Establish a Chinese Herbal Medicine Clinical Hepatotoxicity Reporting Database
A Chinese herbal medicine clinical case report database has been constructed by the author based on the DILI clinical guidelines published by the American Gastroenterological Association (ACG) in 2014 [15, 59] and used guidelines for clinical diagnosis and treatment of Chinese herbal medicine related liver injury formulated by the standards of the Chinese Herbal Medicine Association (T/CACM 005-2016) as a reference standard [60].
Following the collection of patient case reports with hepatotoxicity in clinical studies, they were incorporated into the clinical case database and screened in the follow-up period.
Case reports included in the database for clinical hepatotoxicity from Chinese herbal medicines should all meet the unified standards [61-65], the content of which includes: ① Clinical classification of ILHL; ② Clinical manifestations of ILHL; ③ Clinical laboratory examinations; ④ Clinical imaging examinations; ⑤ Biomarker examinations ⑥ Clinical diagnosis; ⑦ Medication; ⑧ Liver injury outcome indices. Unlike clinical cases, clinical reports included in the database are focused primarily on the diagnosis and treatment of hepatotoxicity associated with Chinese herbal medicine [66, 67]. For example, in the collection of clinical symptoms, more specific symptoms of liver injury need to be described; In the collection of medical history, extra attention should be paid regarding the patient's past history (mainly related to the clinical history of liver damage) and medication history; In the laboratory results, more attention should be paid to the changes of transaminase in relation to the use of Chinese herbal medicines, in addition to concerns regarding the abnormal outcome of the patient's primary diseases; In clinical imaging examinations, the results of hepatobiliary and vascular examination should be emphasized; During diagnosis, the classification and grading of liver injury is the main indicator, and the differential diagnosis of drug-induced liver injury should be carefully considered. In addition to the above, the treatment options should be described in detail for all cases of drug-induced liver injury. The author will refer to the above guidelines, the format of the existing clinical medical records and related medical records reported to produce a database format that meets the database inclusion criteria, so as to facilitate clinical researchers to record and submit relevant medical records reports.
Step 5: Clinical Significance of RMs in the Recognition of Clinical Hepatotoxicity and the Objective Evidence for Individualized Diagnosis of Chinese Herbal Medicine Hepatotoxicity
Extract RMs biomarker evidence from basic and cellular RMs research databases.
Compare and correlate RM biomarkers with Chinese herbal medicine databases to identify Chinese herbal medicines that can produce toxic substances through RMs.
Extract clinical case reports that are suspected of being consistent with reported cases of hepatotoxicity associated with the same Chinese herbal medicine. After using RUCAM scores, investigations on the concomitant use of Chinese and Western medicines, and the identification of the qualities of Chinese herbal medicines etc., to recognize their hepatotoxicity, RMs were then used to predict whether the drugs patients have been taking would cause hepatotoxicity. Here, we take approaches of the deep learning frame work from the most advanced machine learning techniques such as neural networks and gradient boosting. These techniques can handle large scale data and find the most accurate patterns and interactions among RMS and patients’ profile. Such predictions will be the most reliable approaches available. If suspected recognition results are found, the RM biomarker library will be combined with the Chinese herbal database to identify suspected drugs that may cause hepatotoxicity. Subsequently, a joint analysis of suspected drugs and data from individualized case reports were conducted to discover the final diagnostic results corresponding to the hepatotoxicity of clinical medication [68-72].
The individual diagnostic results of Chinese Herbal Medicine will be incorporated into the clinical individualized recognition database for hepatotoxicity, so as to provide evidence for follow-up clinical studies.
5. DISCUSSION
In recent years, reports of adverse events in Chinese Herbal Medicine have been increasing. The problem of hepatotoxicity in traditional Chinese medicine has seriously affected its reputation and acceptance across the world and has become a bottleneck restricting further development in the international medical field. Due to a lack of specific indicators for hepatotoxicity recognition, the factors which cause hepatotoxicity through the use of Chinese herbal medicines are very complicated. In addition to the ingredients of the medicine themselves and toxicity caused by different sources, the complexity of the medicine’s decoction, compatibility, dosage, and human individual differences are important factors in the Chinese herbal medicine hepatoxicity. At present, there are no specific methods and standards for inputting data into the Roussel Uclaf Causality Assessment Method (RUCAM) to recognize Chinese herbal medicine hepatotoxicity, which makes it even more problematic for doctors to evaluate and identify, thereby resulting in an extremely high probability for misdiagnosis. At this stage, the study of Chinese herbal medicine hepatotoxicity is limited to a single study from pharmacological, chemical, toxicological, and clinical aspects etc., without close correlation with each other. Besides, there are insufficient standardized evaluation techniques and research methods under the guidance of traditional Chinese medicine theory. In this regard, the determination of traditional Chinese medicine hepatotoxicity needs to be scientifically standardized through ① the formation of a research standard, and ② the inclusion of adequate basic and clinical studies. Furthermore, there are still some issues to be addressed in regards to identification methods of HILI: it would be very complicated to extract sets of core factors from all related hepatotoxicity indicators. Further effort is needed from experts across multiple fields to elucidate such indicators, which must be accepted and unified by both basic researchers and clinical experts. Furthermore, the establishment of a standardized information system would require the support of multiple information sources such as hospitals, doctors, and patients. It requires the submission of evidential information provided to continuously improve the evidence library for hepatotoxicity identification research. Problems related to the above may well arise during the construction of said methods.
With the rapid development of modern science and technology in conjunction with bioinformatic derivative methods becoming accepted as a powerful tool for drug evaluation, a variety of new evaluation techniques and methods continue to develop further both at home and abroad for traditional Chinese medicine hepatotoxicity research [73-75]. As a branch of bioinformatics, data mining plays an important role in the analysis and prediction of clinical hepatotoxicity. Generally speaking, data mining refers to the process of searching for hidden information amongst a large quantity of data through the use of algorithms. In the future, the information for each drug can be correlated with the clinical reports of individual patients using big data mining techniques thereby allowing for an accurate prediction of hepatotoxicity. Hepatotoxicity biomarker techniques have made some progress. According to my team's latest findings,RM detection is considered the main mechanism for the prediction of hepatotoxicity, which can contribute to rapid detection and capture of drug metabolizing enzymes deactivation in the human body, resulting in various clinical phenomena, including the formation of immunogenicity of autoantibodies, idiosyncratic liver injury, and severe side effects in the human body. In regards to current research on Chinese herbal medicine hepatoxicity: The evidence obtained from basic and clinical research is unclear, there is a lack of standardized and multidimensional joint evidence between basic and clinical research.
CONCLUSION
Modern medical models are changing from disease medicine to health medicine, and the medical models for predictive, preventive and individualized treatment is proposed accordingly. Amongst which, individualized therapy is the basis of precision medicine, and fully embodies the “people-oriented” medical concept, which has something in common with the individual dialectics of Chinese Herbal Medicine [76, 77]. There is a great deal of potential for this research to continue; the potential benefits lie in its ability to organize, summarize, use vast amounts of evidence and use big data mining techniques at the center for Chinese herbal medicine liver toxicity research, which is the most difficult key point of scientific research to be investigated in the next few years. The establishment of a multidimensional evidence library model and method in accordance with evidence-based medicine for individual Chinese herbal medicine hepatotoxicity, RM biomarkers and a Chinese herbal evidence database can evaluate hepatotoxicity effectively, scientifically, objectively, solve the toxic mechanism which was unable to be understood in former toxicity evaluation systems, find possible toxic biomarkers and predict the possible population with individual hepatotoxicity, thereby providing a new method for the early evaluation Chinese herbal medicine hepatotoxicity.
ACKNOWLEDGEMENTS
The paper was supported by the National Natural Science Foundation of China (NSFC, No.81373890, No.81503456 and NO.81430096), and the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, No. IRT_14R41).
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
All authors have read and approved this version of the article, and due care has been taken to ensure the integrity of the work. Neither the entire paper nor any part of its content has been published or has been accepted elsewhere. It is not being submitted to any other journal.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
REFERENCES
- 1.Zhang S.T., Wan G.J., Li L. Research advances in the mechanism of drug-induced liver injury. Pharm. Care Res. 2017;17:39. [Google Scholar]
- 2.Qi Y.B., Qiu L., Jiang H.L. Clinical characteristics of drug-induced liver injury: An analysis of 394 cases. Clin. Hepatol. 2014;30:438–441. [Google Scholar]
- 3.Bjornsson E.S., Bergmann O.M., Bjornsson H.K., Kvaran R.B., Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in thegeneral population of ice land. Gastroenterology. 2013;144:1419–1425. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Fontana R.J., Watkins P.B., Bonkovsky H.L., Chalasani N., Davern T., Serrano J., Rochon J. Drug-induced Liver Injury Network (DILIN) prospective study: Rationale, design and conduct. Drug Saf. 2009;32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devarbhavi H. An update on drug-induced liver injury. J. Clin. Exp. Hepatol. 2012;2:247–259. doi: 10.1016/j.jceh.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dayakar K., Mahesh G., Priyanka V., Kishore V. Metronidazole induced liver injury: A rare immune-mediated drug reaction. Case Rep. Gastrointest. Med. 2013;20:1–4. doi: 10.1155/2013/568193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjornsson E.S., Bergmann O.M., Bjornsson H.K., Kvaran R.B., Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of iceland. Gastroenterology. 2013;144:1419–1425. doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Li L., Jiang W., Wang J.Y. Clinical analysis of 275 cases of acute drug-induced liver disese. Front. Med. China. 2007;1:58–61. doi: 10.1007/s11684-007-0012-8. [DOI] [PubMed] [Google Scholar]
- 9.Dağ M.S., Aydinli M., Oztürk Z.A., Türkbeyler I.H., Koruk I., Savaş M.C., Koruk M., Kadayifci A. Drug and herb-induced liver injury: A case series from a single center. Turk. J. Gastroenterol. 2014;25:41–42. doi: 10.5152/tjg.2014.4486. [DOI] [PubMed] [Google Scholar]
- 10.Teschke R., Danan G. Prospective indian study of dili with confirmed causality using the roussel uclaf causality assessment method (rucam): A report of excellence. Ann. Hepatol. 2017;16:324–325. doi: 10.5604/16652681.1235471. [DOI] [PubMed] [Google Scholar]
- 11.Dannan G., Benichou C. Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J. Clin. Epidemiol. 1993;46:1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 12.Teschke R., Schulze J., Schwarzenboeck A., Eickhoff A., Frenzel C. Herbal hepatotoxicity: suspected cases assessed for alternative causes. Eur. J. Gastroenterol. Hepatol. 2013;25:1093–1098. doi: 10.1097/MEG.0b013e3283603e89. [DOI] [PubMed] [Google Scholar]
- 13.Chalasani N.P., Hayashi P.H., Bonkovsky H.L., Navarro V.J., Lee W.M., Fontana R.J. ACG clinical guideline: The diagnosis and management of idiosyncratic drug-induced liver injury. Am. J. Gastroenterol. 2014;109:950–966. doi: 10.1038/ajg.2014.131. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Y., Niu M., Chen J., Zou Z.S., Ma Z.J., Liu S.H., Wang R.L., He T.T., Song H.B., Wang Z.X. Comparison between Chinese herbal medicine and western medicine-induced liver injury of 1985 patients. J. Gastroenterol. Hepatol. 2016;31:1476–1482. doi: 10.1111/jgh.13323. [DOI] [PubMed] [Google Scholar]
- 15.Tiwari S.V., Seijas J.A., Vazquez-Tato M.P., Sarkate A.P., Karnik K.S., Nikalje A.P. Facile synthesis of novel coumarin derivatives, antimicrobial analysis, enzyme assay, docking study, ADMET prediction and toxicity study. Molecules. 2017;22:1–7. doi: 10.3390/molecules22071172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X., Lou Y.J., Wang M.X., Shi Y.W., Xu H.X., Kong L.D. Furocoumarins affect hepatic cytochrome P450 and renal organic ion transporters in mice. Toxicol. Lett. 2012;209:67–77. doi: 10.1016/j.toxlet.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 17.Koenigs L.L., Trager W.F. Mechanism-based inactivation of cytochrome P450 2A6 by furanocoumarins. Biochemistry. 1998;37:13184–13193. doi: 10.1021/bi981198r. [DOI] [PubMed] [Google Scholar]
- 18.Ji L., Lu D., Cao J., Zheng L., Peng Y., Zheng J. Psoralen, a mechanism-based inactivator of CYP2B6. Chem. Biol. Interact. 2015;240:346–352. doi: 10.1016/j.cbi.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Lu D., Ji L., Zheng L., Cao J., Peng Y., Zheng J. Mechanismbased inactivation of cytochrome P450 2B6 by isopsoralen. Chem. Biol. Interact. 2015;226:1–7. doi: 10.1016/j.cbi.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Cao J., Zheng L., Ji L., Lu D., Peng Y., Zheng J. Mechanismbased inactivation of cytochrome P450 2B6 by isoimperatorin. Chem. Biol. Interact. 2015;226:23–29. doi: 10.1016/j.cbi.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Zheng L., Cao J., Lu D., Ji L., Peng Y., Zheng J. Imperatorin is a mechanism-based inactivator of CYP2B6. Drug Metab. Dispos. 2015;43:82–88. doi: 10.1124/dmd.114.060558. [DOI] [PubMed] [Google Scholar]
- 22.Dai J., Zhang F., Zheng J. Retrorsine, but not monocrotaline, is a mechanism-based inactivator of P450 3A4. Chem. Biol. Interact. 2010;183:49–56. doi: 10.1016/j.cbi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Tu M., Li L., Lei H., Ma Z., Chen Z., Sun S., Xu S., Zhou H., Zeng S., Jiang H. Involvement of organic cation transporter 1 and CYP3A4 in retrorsine-induced toxicity. Toxicology. 2014;322:34–42. doi: 10.1016/j.tox.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Fashe M.M., Juvonen R.O., Petsalo A., Rahnastorilla M., Auriola S., Soininen P., Vepsäläinen J., Pasanen M. Recognization of a new reactive metabolite of pyrrolizidine alkaloid retrorsine: (3H-pyrrolizin-7-yl) methanol. Chem. Res. Toxicol. 2014;27:1950–1957. doi: 10.1021/tx5002964. [DOI] [PubMed] [Google Scholar]
- 25.Tang W., Lu A.Y. Metabolic bioactivation and drug-related adverse effects: current status and future directions from a pharmaceutical research perspective. Drug Metab. Rev. 2010;42:225–249. doi: 10.3109/03602530903401658. [DOI] [PubMed] [Google Scholar]
- 26.Amacher D.E. The primary role of hepatic metabolism in idiosyncratic drug-induced liver injury. Expert Opin. Drug Metab. Toxicol. 2012;8:335–347. doi: 10.1517/17425255.2012.658041. [DOI] [PubMed] [Google Scholar]
- 27.Xu J.J., Henstock P.V., Dunn M.C. Cellular imaging predictions of clinical drug-induced liver injury. Toxicol. Sci. 2008;105:97–105. doi: 10.1093/toxsci/kfn109. [DOI] [PubMed] [Google Scholar]
- 28.Fontana E., Dansette P.M., Poli S.M. Cytochrome P450 enzymes mechanism based inhibitors: Common sub-structures and reactivity. Curr. Drug Metab. 2005;6:413–454. doi: 10.2174/138920005774330639. [DOI] [PubMed] [Google Scholar]
- 29.Feng S., He X. Mechanism-based inhibition of CYP450: An indicator of drug-induced hepatotoxicity. Curr. Drug Metab. 2013;14:1–25. doi: 10.2174/138920021131400114. [DOI] [PubMed] [Google Scholar]
- 30.He L.N., Yang A.H., Cui T.Y., Zhai Y.R., Zhang F.L., Chen J.X., Jin C.H., Fan Y.W., Wu Z.J., Wang L.L., He X. Reactive metabolite activation by CYP2C19-mediated rhein hepatotoxicity. Xenobiotica. 2015;45:361–372. doi: 10.3109/00498254.2014.984794. [DOI] [PubMed] [Google Scholar]
- 31.Zhang F.L., He X., Zhai Y.R., He L.N., Zhang S.C., Wang L.L., Yang A.H., An L.J. Mechanism-based inhibition of CYPs and RMs induced hepatoxicity by rutaecarpine. Xenobiotica. 2015;45:978–989. doi: 10.3109/00498254.2015.1038742. [DOI] [PubMed] [Google Scholar]
- 32.Wang L.L., He X., Jin C.H., Ondieki G. Mechanism-based inhibitors from phytomedicine: risks of hepatotoxicity and their potential hepatotoxic substructures. Curr. Drug Metab. 2016;17:971–991. doi: 10.2174/1389200218666161123124253. [DOI] [PubMed] [Google Scholar]
- 33.Wang J.B., Ma Z.J., Niu M., Zhu Y., Liang Q.S., Zhao Y.L., Song J.Y., Bai Z.F., Zhang Y.M., Zhang P., Li N., Meng Y.K., Li Q., Qin L.S., Teng G.J., Cao J.L., Li B.S., Chen S.L., Li Y.G., Zou Z.S., Zhou H.H., Xiao X.H. Evidence chain-based causality recognization in herb-induced liver injury: exemplification of a well-known liver-restorative herb polygonum multiflorum. Front. Med. 2015;9:457–467. doi: 10.1007/s11684-015-0417-8. [DOI] [PubMed] [Google Scholar]
- 34.Danan G., Teschke R. RUCAM in drug and herb induced liver injury: The update. Int. J. Mol. Sci. 2016;17:14. doi: 10.3390/ijms17010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaby D., Rolf T. Drug-induced liver injury: Why is the Roussel Uclaf Causality Assessment Method (RUCAM) still used 25 years after its launch. Drug Saf. 2018;10:1–7. doi: 10.1007/s40264-018-0654-2. [DOI] [PubMed] [Google Scholar]
- 36.Rockey D.C., Seeff L.B., Rochon J., Freston J., Chalasani N., Bonacini M., Fontana R.J., Hayashi P.H. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the roussel-uclaf causality assessment method. Hepatology. 2010;51:2117–2126. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fontana R.J., Seeff L.B., Andrade R.J., Björnsson E., Day C.P., Serrano J., Jay H.H. Standardization of nomenclature and causality assessment in drug-induced liver injury: Summary of a clinical research workshop. Hepatology. 2010;52:730–742. doi: 10.1002/hep.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liss G., Rattan S., Lewis J.H. Predicting and preventing acute drug-induced liver injury: What’s new in 2010? Expert Opin. Drug Metab. Toxicol. 2010;6:1047–1061. doi: 10.1517/17425255.2010.503706. [DOI] [PubMed] [Google Scholar]
- 39.Biour M., Ben S.C., Chazouillères O., Grangé J.D., Serfaty L., Poupon R. Drug-induced liver injury; fourteenth updated edition of the bibliographic database of liver injuries and related drugs. Gastroenterol. Clin. Biol. 2004;28:720. doi: 10.1016/s0399-8320(04)95062-2. [DOI] [PubMed] [Google Scholar]
- 40.Biour M., Poupon R., Grangé J.D., Chazouillères O. Drug-induced hepatotoxicity; thirteenth updated edition of the bibliographic database of drug-related liver injuries and responsible drugs. Gastroenterol. Clin. Biol. 2000;24:1052. [PubMed] [Google Scholar]
- 41.Biour M., Poupon R., Grangé J.D., Chazouillères O., Jaillon P. Drug-induced liver injury; twelfth updated edition of the bibliographic database of liver injuries and related drugs. Gastroenterol. Clin. Biol. 1999;23:1310–1311. [PubMed] [Google Scholar]
- 42.Biour M., Poupon R., Grangé J.D., Chazouillères O., Jaillon P. Drug-induced hepatotoxicity; eleventh update of the bibliographic database on liver injuries and responsible drugs. Gastroenterol. Clin. Biol. 1998;22:1004. [PubMed] [Google Scholar]
- 43.Biour M., Poupon R., Grange J.D., Chazouillères O., Lévy V.G., Jaillon P. Hepatotoxicity of drugs; tenth update of the bibliographic database of hepatic involvements and responsible drugs. Gastroenterol. Clin. Biol. 1997;21:660. [PubMed] [Google Scholar]
- 44.Biour M., Poupon R., Grange J.D., Chazouillères O., Lévy V.G., Bodin F., Cheymol G. Hepatotoxicity of drugs; ninth update of the bibliographic database of hepatic involvements and related drugs. Gastroenterol. Clin. Biol. 1996;20:744. [PubMed] [Google Scholar]
- 45.Biour M., Poupon R., Grangé J.D., Chazouillères O., Lévy V.G., Bodin F., Cheymol G. Hepatotoxicity of drugs; eighth updated bibliographic database of hepatic lesions and responsible drugs. Gastroenterol. Clin. Biol. 1995;19:756. [PubMed] [Google Scholar]
- 46.Biour M., Poupon R., Grangé J.D., Chazouillères O., Levy V.G., Bodin F., Cheymol G. Hepatotoxicity of drugs; seventh update of the bibliographic database of liver lesions and related drugs. Gastroenterol. Clin. Biol. 1994;18:574. [PubMed] [Google Scholar]
- 47.Biour M., Poupon R., Grangé J.D., Chazouillères O., Levy V.G., Bodin F., Cheymol G. Hepatotoxicity of drugs; an updated bibliographic database of liver disorders and responsible drugs. Gastroenterol. Clin. Biol. 1993;17:86–115. [PubMed] [Google Scholar]
- 48.Bourgeois A.L., Auriche P., Palmaro A., Montastruc J.L., Bagheri H. Risk of hormonotherapy in transgender people: Literature review and data from the french database of pharmacovigilance. Ann. Endocrinol. (Paris) 2016;77:14–21. doi: 10.1016/j.ando.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Fontana E., Dansette P.M., Poli S.M. Cytochrome P450 enzymes mechanism based inhibitors: Common sub-structure and reactivity. Curr. Drug Metab. 2005;6:413–454. doi: 10.2174/138920005774330639. [DOI] [PubMed] [Google Scholar]
- 50.Feng S., He X. Mechanism-based inhibition of CYP450: an indicator of drug-induced hepatotoxicity. Curr. Drug Metab. 2013;14:921–945. doi: 10.2174/138920021131400114. [DOI] [PubMed] [Google Scholar]
- 51.Silverman R.B. Methods in enzymology. Biol. Mass. Spectrum. 1995;249:240–283. doi: 10.1016/0076-6879(95)49038-8. [DOI] [PubMed] [Google Scholar]
- 52.Guhlin J., Silverstein K., Zhou P., Tiffin P., Young N.D. ODG: Omics database generator-a tool for generating, querying, and analyzing multi-omics comparative databases to facilitate biological understanding. BMC Bioinformatics. 2017;18:367. doi: 10.1186/s12859-017-1777-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vinel J.P., Pascal J.P. Database on hepatotoxic drugs available through minitel. Gastroenterol. Clin. Biol. 1993;17:121–122. [PubMed] [Google Scholar]
- 54.Ruedazárate H.A., Imazrosshandler I., Cárdenasovando R.A., Castillofernández J.E., Noguezmonroy J., Rangelescareño C. A computational toxicogenomics approach identifies a list of highly hepatotoxic compounds from a large microarray database. PLoS One. 2017;12:1–11. doi: 10.1371/journal.pone.0176284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shantakumar S., Nordstrom B.L., Djousse L., Hall S.A., Gagnon D.R., Fraeman K.H., Herk-Sukel M., Chagin K., Nelson J. Occurrence of hepatotoxicity with pazopanib and other anti-VEGF treatments for renal cell carcinoma: An observational study utilizing a distributed database network. Cancer Chemother. Pharmacol. 2016;78:559–566. doi: 10.1007/s00280-016-3112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hebels D.G., Jetten M.J., Aerts H.J., Herwig R., Theunissen D.H., Gaj S., Delft J.H., Kleinjans J.C. Evaluation of database-derived pathway development for enabling biomarker discovery for hepatotoxicity. Biomarkers Med. 2014;8:185–200. doi: 10.2217/bmm.13.154. [DOI] [PubMed] [Google Scholar]
- 57.Watkins P.B. How to diagnose and exclude drug-induced liver injury. Dig. Dis. 2015;33:472–474. doi: 10.1159/000374091. [DOI] [PubMed] [Google Scholar]
- 58.Jin R., Gu H.Y., Li L.L., Sun L.L. Current status of Chinese herbal preparations included in Livertox database. China. J. Hepatol. 2016;24:817–823. doi: 10.3760/cma.j.issn.1007-3418.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 59.Ming Y.N., Liu X.L., Mao Y.M. A brief introuduction of ACG clinical guideline: The diagnosis and management of idiosyncratic drug-induced liver injury. Chin. Hepatol. 2014;19:564–569. [Google Scholar]
- 60.Hu B. The diagnosis and management guideline of herb-induced liver injury released. China News T. C.M. 2016;3:18. [Google Scholar]
- 61.Martin B.K., Rada R. Building a relational database for a physician document index. Med. Inform. (Lond.) 1987;12:187–201. doi: 10.3109/14639238709044553. [DOI] [PubMed] [Google Scholar]
- 62.Gupta P., Koushal V., Narayan C., Anand A. Building genetic database at medical institutes: Implement patient cost audit and improve biomedical research. Ann. Neurosci. 2017;24:3–4. doi: 10.1159/000464416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imatoh T., Sai K., Fukazawa C., Hinomura Y., Nakamura R., Okamoto-Uchida Y., Segawa K., Saito Y. Association between infection and severe drug adverse reactions: An analysis using data from the Japanese adverse drug event report database. Eur. J. Clin. Pharmacol. 2017;73:1643–1653. doi: 10.1007/s00228-017-2320-5. [DOI] [PubMed] [Google Scholar]
- 64.Sobhonslidsuk A., Poovorawan K., Soonthornworasiri N., Pan-Ngum W., Phaosawasdi K. The incidence, presentation, outcomes, risk of mortality and economic data of drug-induced liver injury from a national database in Thailand: a population-base study. BMC Gastroenterol. 2016;16:135. doi: 10.1186/s12876-016-0550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Udo R. Maitland-van.; Zee, A.H.; Egberts, T.C.; Breeijen, J.H.; Leufkens, H.G.; Solinge, W.W.; Bruin, M.L. Validity of diagnostic codes and laboratory measurements to identify patients with idiopathic acute liver injury in a hospital database. Pharmacoepidemiol. Drug Saf. 2016;25:21–28. doi: 10.1002/pds.3824. [DOI] [PubMed] [Google Scholar]
- 66.Hunt C.M., Yuen N.A., Stirnadel-Farrant H.A., Suzuki A. Age-related differences in reporting of drug-associated liver injury: data-mining of WHO safety report database. Regul. Toxicol. Pharmacol. 2014;70:519–526. doi: 10.1016/j.yrtph.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 67.Brinker A.D., Lyndly J., Tonning J., Money D., Levine J.G., Avigan M.I. Profiling cumulative proportional reporting ratios of drug-induced liver injury in the FDA adverse event reporting system (FAERS) database. Drug Saf. 2013;36:1169–1178. doi: 10.1007/s40264-013-0116-9. [DOI] [PubMed] [Google Scholar]
- 68.Quinton A., Latry P., Biour M. Hepatox: Database on hepatotoxic drugs. Gastroenterol. Clin. Biol. 1993;17:116–120. [PubMed] [Google Scholar]
- 69.Biour M., Poupon R., Calmus Y., Grange J.D., Levy V.G., Cheymol G. Hepatox: A microcomputer database of drug-induced hepatic injury. Indian J. Gastroenterol. 1989;8:175–182. [PubMed] [Google Scholar]
- 70.Nippold M.A., Vigeland L.M., Frantz-Kaspar M.W., Ward-Lonergan J.M. Language sampling with adolescents: Building a normative database with fables. Am. J. Speech Lang. Pathol. 2017;26:908–920. doi: 10.1044/2017_AJSLP-16-0181. [DOI] [PubMed] [Google Scholar]
- 71.Klungel O.H., Kurz X., Groot M.C., Schlienger R.G., Tcherny-Lessenot S., Grimaldi L., Groenwold R.H., Reynolds R.F. Multi-centre, multi-database studies with common protocols: Lessons learnt from the imi protect project. Pharmacoepidemiol. Drug Saf. 2016;25:156–165. doi: 10.1002/pds.3968. [DOI] [PubMed] [Google Scholar]
- 72.Zhu X., Kruhlak N.L. Construction and analysis of a human hepatotoxicity database suitable for qsar modeling using post-market safety data. Toxicology. 2014;321:62–72. doi: 10.1016/j.tox.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 73.Yan G., Wang X., Chen Z., Wu X., Pan J., Huang Y., Wan G., Yang Z. In silico ADME studies for new drug discovery: From chemical compounds to chinese herbal medicines. Curr. Drug Metab. 2017;18:535–539. doi: 10.2174/1389200218666170316094104. [DOI] [PubMed] [Google Scholar]
- 74.Li H., Wang X., Yu H., Zhu J., Jin H., Wang A., Yang Z. Combining in vitro and in silico approaches to find new drugs targeting the pathological proteins related to the Alzheimer’s disease. Curr. Neuropharmacol. 2017;10:2174–1570. doi: 10.2174/1570159X15666171030142108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liao W., Zhang T.T., Gao L., Lee S.S., Xu J., Zhang H., Yang Z., Liu Z., Li W. Integration of novel materials and advanced ‘omics’ technologies into new vaccine design. Curr. Top. Med. Chem. 2017;17:2286–2301. doi: 10.2174/1568026617666170224122117. [DOI] [PubMed] [Google Scholar]
- 76.Zheng Y., Qing T., Song Y., Zhu J., Yu Y., Shi W., Pusztai L., Shi L. Standardization efforts enabling next-generation sequencing and microarray basedbiomarkers for precision medicine. Biomarkers Med. 2015;9:1265–1272. doi: 10.2217/bmm.15.99. [DOI] [PubMed] [Google Scholar]
- 77.Langreth R., Waldholz M. New era of personalized medicine: Targeting drugs for each unique genetic profile. Oncologist. 1999;4:426–427. [PubMed] [Google Scholar]


