Abstract
Intrinsically disordered stress proteins have been shown to act as chaperones, protecting proteins from damage caused by stresses such as freezing and thawing. Dehydration proteins (dehydrins) are intrinsically disordered stress proteins that are found in almost all land plants. They consist of a variable number of the short, semi‐conserved, Y‐, S‐, and K‐segments, with longer stretches of poorly conserved sequences in between. Previous studies have provided conflicting views on the details of the dehydrin cryoprotective mechanism of enzymes. Experiments with polyethylene glycol (PEG) have shown that PEG cryoprotective efficiency is the same as dehydrins of the same hydrodynamic radius, suggesting that the protein's disordered and polar nature is important, rather than the specific order of the residues. To further elucidate the mechanism, we created scrambled variants of the wild grape dehydrins K2 and YSK2 and tested their ability to protect lactate dehydrogenase and yeast frataxin homolog‐1 from freeze/thaw damage. The results show that for preventing aggregation, it is the sequence composition and the size of the dehydrin that is the most important factor in protection, while for freeze/thaw damage causing loss of secondary structure, it is the sequence composition that is most significant.
Keywords: intrinsically disordered proteins, cryoprotection, dehydrins, lactate dehydrogenase, yeast frataxin homolog 1, scrambling, circular dichroism
Introduction
Plants, being sessile organisms, are continuously exposed to environmental stresses, which can be detrimental to their growth and development.1 Dehydration induced by drought, low temperatures, or salt has many effects, causing changes in turgor pressure and loss of cell integrity,1 and also disrupting biomolecular structures by increasing the concentration of radical oxygen species.2 In response to these stresses, many plants have evolved systems, which allow them to acclimate by stress avoidance and/or stress tolerance.2, 3 Stress tolerance involves increasing the concentration of diverse compounds with cryoprotective properties to stabilize and maintain protein structure and function, prevent membrane disruption and maintain fluidity, and scavenge radical oxidative species to reduce oxidative stress. These compounds include the late embryogenesis abundant (LEA) proteins.
LEA proteins are small, thermostable proteins, which are produced in large amounts in plants under cold and dehydration stress.4, 5, 6 LEA proteins were initially discovered by Dure's group7 in the embryonal tissue of cottonseed during late embryogenesis, a time when natural dehydration occurs. LEA proteins are found ubiquitously across land plant species, including angiosperms, gymnosperms, and bryophytes.5 The expression of LEA proteins or the accumulation of LEA mRNAs has been consistently positively correlated with stress tolerance in transgenic and gene knockout studies.8, 9, 10 LEA proteins can be categorized into seven groups based on sequence similarity, where the most extensively studied family is the Group 2 dehydration proteins (dehydrins).11
Members of the dehydrin family range from about 9 to 70 kDa in size, and are characterized by the presence of three semi‐conserved sequence motifs.12, 13 By definition, all dehydrins must contain at least one K‐segment, which is a 15‐residue motif rich in lysine residues.14 In a recent analysis of multiple vascular plant genomes by Malik et al., the K‐segment consensus sequence was described as [XKXGXX(D/E)KIK(D/E)KXPG], where X represents any amino acid.15 Another motif is the Y‐segment,14 which is named after the Tyr in the motif, although His or Phe may be present at this position.15 The Y‐segment consensus motif can be written as [D(D/E)(Y/H/F)GNPX],15 where the C‐terminal X is a hydrophobic residue. Lastly, the S‐segment14 contains a poly‐serine “stutter” of 4–6 Ser residues.16 This segment often starts with a Leu and two positively charged residues and ends with four negatively charged residues, resulting in the consensus sequence [LHR(S/T)GS4‐6(S/D/E)(D/E)3].15, 17 Regions outside of these motifs are denoted as φ‐segments, and are rich in Gly, Thr, and Glu residues, and also contain other amino acids that are small, polar, or charged.
With the exception of the K‐segment, the Y‐ and S‐segments are not present in all dehydrins, resulting in variable architectures.14 When present, the Y‐segment is located near the N‐terminus in 1–3 tandem copies, followed by the S‐segment, which may be present once or not at all. At the C‐terminus, the K‐segment occurs in 1–11 copies, where 1 or 2 repeats are the most common. 12 A small number of dehydrins have an S‐segment located after a K‐segment. The various combinations of segments give rise to five architectures, written as Kn, SKn, KnS, YnKn, and YnSKn,12 where n represents the number of times the segment repeats. The roles of the various segments are still under active investigation; for more information, see recent reviews.13, 18, 19, 20
Dehydrins are extremely hydrophilic, containing a high number of glycine residues (6%–25%),5, 15 a high density of uncompensated charged amino acids, and only a few hydrophobic residues, resulting in intra‐residue repulsion and lack of a hydrophobic core, creating a flexible, extended conformation. They therefore lack a well‐defined structure and possess a large hydrodynamic radius. As a result, they are floppy in solution, resembling “cooked spaghetti”21 or “protein clouds,”22 and are classified as intrinsically disordered proteins.
One of the key proposed functions of dehydrins is the protection of proteins from damage caused by dehydrative stress, including that caused by freezing and thawing. The objective of this work is to investigate the mechanism of dehydrin cryoprotective function and to probe whether protein sequence composition, rather than specific residue order, is important in their cryoprotective behavior. Our motivation for this approach comes from the studies on the cryoprotective behavior of polyethylene glycol (PEG). Enzyme cryoprotection by PEG was essentially identical to dehydrins,23 suggesting that it is simply the polar nature of dehydrins (i.e., amino acid composition) and their hydrodynamic radius that contributes to their mechanism. In contrast, other studies have suggested that the K‐segments are directly involved in enzyme protection.24, 25, 26 The issue is that these deletions and peptide studies fail to take the hydrodynamic radius into account, which we have shown is a key element in dehydrin cryoprotective efficiency.23, 27
Our previous work showed that PEG molecules of similar hydrodynamic radius to dehydrins provide a very similar cryoprotective effect on the model enzyme lactate dehydrogenase (LDH);23 this suggested that the polar nature of dehydrins is a critical property for protection, rather than the specific order of residues. Here we use the K2 and YSK2 dehydrins from Vitis riparia (wild grape) as model dehydrins, and scramble their sequence to understand how important sequence composition is in their function. The cryoprotective efficiencies of LDH with these scrambled constructs were compared to that of wild‐type K2 and YSK2. To further elucidate the molecular mechanism of dehydrin protection, the effects of these dehydrins on the conformational stability of a cold‐sensitive protein, yeast frataxin homolog 1 (Yfh1), were also studied.
Results
Sequence construction
The sequences of K2 and YSK2 were scrambled such that the altered dehydrin would have residues in places where they are not found in the wild‐type protein [Fig. 1(a,b)]. In the wild‐type dehydrins, most of the Lys residues are in or close to the K‐segments; in the 48‐residue V. riparia K2 protein, the Lys residues are localized in the first and last 15 residues of its sequence (i.e., the two K‐segments), whereas in the 130‐residue V. riparia YSK2, most of the Lys residues are located in the last 51 residues (again in the two K‐segments). Five shuffled K2 and YSK2 sequences were generated, and then visually inspected to determine which one least resembled the Y‐, S‐, or K‐segments (data not shown). The appropriate sequences were chosen for this study and the final constructs were named scrK2 and scrYSK2 [Fig. 1(a,b)].
Figure 1.

Wild type and scrambled dehydrin constructs to probe the importance of residue position. (a) Wild‐type (K2) and scrambled (scrK2) sequences. (b) Wild‐type (YSK2) and scrambled (scrYSK2) sequences. (c) Disorder of K2 and mutated K2 constructs as predicted by MetasdisorderMD2 algorithm. K2, solid black line; scrK2, solid red line. (d) Disorder of YSK2 and scrYSK2 as predicted by the MetadisorderMD2 algorithm. YSK2, dashed black line; scrYSK2, dashed red line.
To ensure that structural order was not accidentally introduced into the scrK2 and scrYSK2 proteins, the sequences were submitted to the MetaDisorder server for an in silico disorder prediction analysis.28 This service uses the weighted consensus of 13 disorder predictors and eight protein fold recognition methods. The value of 0.5 is the chosen threshold that separates ordered residues (<0.5) from disordered ones (>0.5). As shown in Figure 1(c,d), the predictions suggested that all of the proteins are similarly disordered.
Construct structure
To experimentally confirm that the scrK2 and scrYSK2 dehydrins are actually disordered, their secondary structures were examined by circular dichroism (CD) and compared to wild‐type K2 and YSK2 [Fig. 2(a,b)]. Typical characteristics of a disordered protein include a strong minimum near 200 nm and a weak minimum at 222 nm.29 In contrast, globular proteins with predominantly α‐helical structures would display minima near 208 and 222 nm, and a positive band near 193 nm, whereas proteins with β‐sheets would show a minimum at 218 nm and a maximum near 195 nm.30 The wild‐type K2 CD spectrum [Fig. 2(a), black lines] and the spectra of YSK2 and scrYSK2 [Fig. 2(b), black and red dashed lines, respectively] confirm that these proteins are disordered. All profiles are very similar, showing that scrambling the YSK2 sequence had not significantly altered the structure, with all three spectra having molar elliptical minima at 198 nm and near zero values at 222 nm.
Figure 2.

Circular dichroism data of scrambled constructs compared to the wild‐type proteins. (a) CD spectra of K2 (solid black line) and scrK2 (solid red line). (b) CD spectra of YSK2 (dashed black line) and scrYSK2 (dashed red line).
Interestingly, the scrK2 had a slightly different spectrum [Fig. 2(a), red line]; the minimum has shifted to 201 nm and is considerably less negative than the other dehydrins. The value at 222 nm is slightly lower, suggesting weak helicity. While the scrK2 is still mostly disordered, it may be slightly more helical than the wild‐type K2.
Lactate dehydrogenase cryoprotection
LDH is a tetrameric enzyme, which has been found to aggregate and unfold below 4°C, and its activity decreases to nearly zero after freeze/thaw treatment.31 A number of dehydrins have been previously shown to protect LDH from activity loss due to freezing and thawing.23, 24, 25, 26, 32, 33, 34 When plotted as a log scale of additive concentration versus percent LDH recovered activity, the data show a sigmoidal pattern which can be fitted using Eq. 1 (see Materials and Methods). The efficiency of additives can be compared by calculating their PD50 value, which is the concentration of added compound required to recover 50% of LDH activity after treatment. A lower PD50 value therefore represents a more efficient cryoprotectant.
The ability of K2 and scrK2 to protect LDH from freeze/thaw damage is shown in Figure 3(a). K2 (black lines) demonstrated highly efficient recovery of LDH activity, with a PD50 value of 14 μM, while scrK2 (red lines) could not restore 100% activity, even at a high concentration (>100 μM), but instead maximized at ~70% recovery. The negative control, lysozyme, was even worse, achieving only a maximum recovery of ~40%. The effect of YSK2 and scrYSK2 on LDH activity is examined in Figure 3(b) (black and red dashed lines, respectively). Here, scrYSK2 has essentially the same activity as the wild‐type protein (PD50 values of 6.1 and 6.2 μM, respectively), while the β‐casein, a negative control, is worse at protecting the LDH from damage (PD50 of 17.5 μM) and could only restore maximally 75% LDH activity at its highest concentrations.
Figure 3.
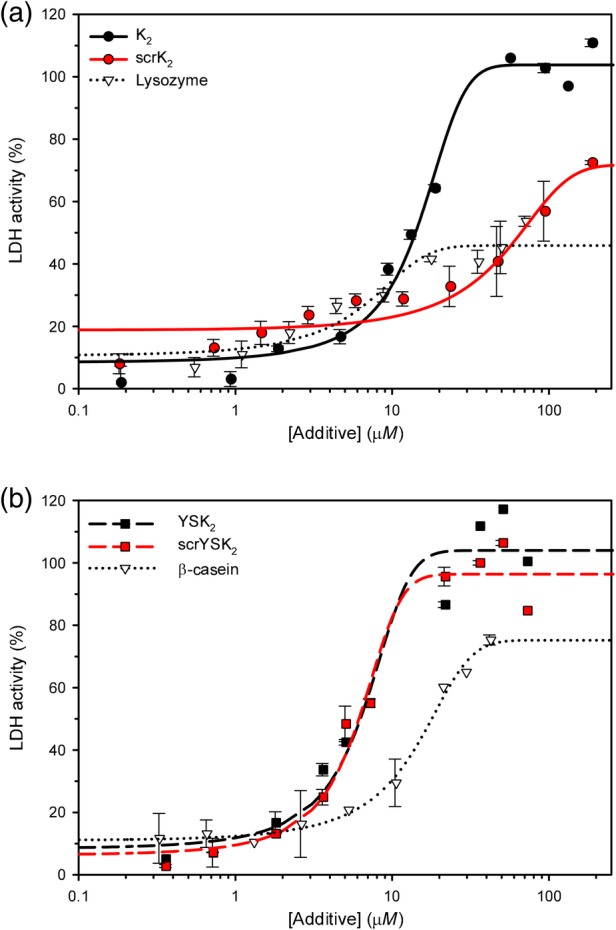
Cryoprotective level of lactate dehydrogenase varies by protein. The recovered activity of the enzyme was measured after the freeze/thaw treatment in the presence of several different proteins. (a) Protection in the presence of K2, its scrambled form, and a control. K2, solid black line and black circles; scrK2, solid red line and red circles; lysozyme, dotted black line and triangles. (b) Protection in the presence of YSK2, its scrambled form, and a control. YSK2, dashed black line and black squares; scrYSK2, dashed red line and red squares; and β‐casein, dotted black line and triangles. Error bars show the standard deviation of n = 6 replicates.
Yeast frataxin 1 homolog room temperature stabilization
We also used yeast frataxin homolog 1 (Yfh1) as a model protein that undergoes loss of structure at low temperatures35 by examining it in a freeze/thaw damage assay. Changes in Yfh1 helical content were monitored in the presence and absence of K2, scrK2, YSK2, and scrYSK2 using CD spectroscopy. Before freezing the samples, we observed that the room temperature Yfh1 samples had different amounts of helical structure in the presence of the different dehydrins. To further explore this effect, and provide a baseline for latter cryoprotective experiments, we performed a dehydrin titration experiment at room temperature.
The CD spectrum of 10 μM Yfh1 alone at room temperature displays the characteristics of an ordered protein with defined α‐helical structure, as indicated by the minimum ellipticities at 207 and 222 nm (Fig. 4, black lines). We found that a K2 concentration of 5 μM was sufficient to considerably affect the structure of Yfh1 (Fig. 4). In the presence of this dehydrin, the CD spectra showed a simultaneous larger minimum intensity and a shift in the minimum wavelength (from 206 to 208 nm), showing that Yfh1 is gaining more helicity. The addition of more K2 resulted in greater increases in the minimum, but did not shift the wavelength significantly. The titration of scrK2 resulted in spectra with similar shapes to K2, though the minimum near 208 nm decreased more and that near 222 nm less.
Figure 4.
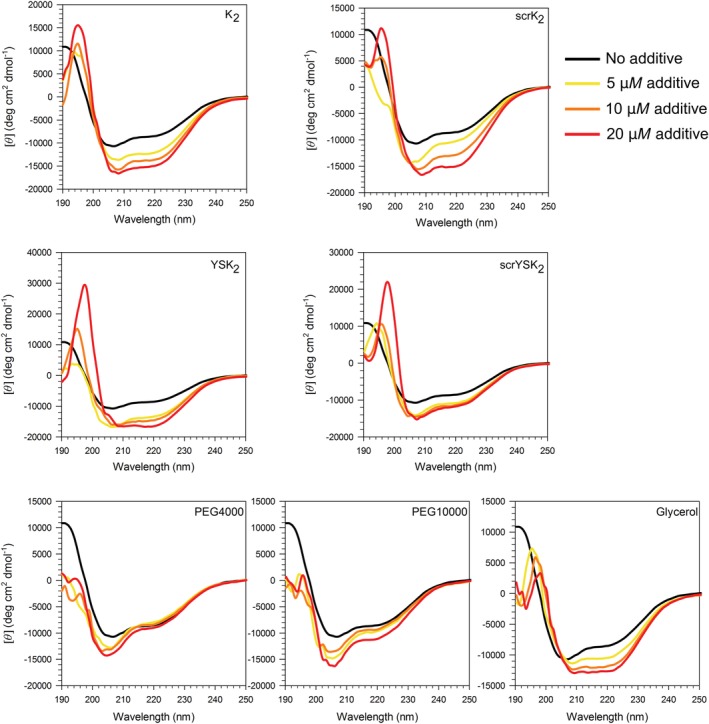
Dehydrins induce helicity in Yfh1 at room temperature. CD spectra of 10 μM Yfh1 in the presence of wild‐type and scrambled protein dehydrins, and in the presence of PEG 4000, PEG 10,000, and glycerol, as labeled. No additive, black line; 5 μM additive, yellow line; 10 μM additive, orange line; 20 μM additive, red line. For glycerol—no glycerol, black line; 10% (v/v) glycerol, yellow line; 20% (v/v) glycerol, orange line; and 30% (v/v) glycerol, red line.
The addition of 5 μM of YSK2 also caused a significant increase in the helical signal. The addition of 10 μM YSK2 did not further increase the intensity of the Yfh1 minima, whereas 20 μM YSK2 caused the minima to shift to a longer wavelength and a further negative increase at 222 nm. The addition of 5 μM scrYSK2 to Yfh1 caused an increase in the intensity of the Yfh1 minima, like YSK2. However, the influence of scrYSK2 on the structure of Yfh1 reached its maximal effect at 5 μM, such that adding more protein caused little further change in the CD spectra (Fig. 4). The observation that K2 and YSK2 have a similar effect on Yfh1 suggests that size is not a factor, unlike the importance of hydrodynamic radii in LDH protection.23
The changes in the CD spectra of Yfh1 were also monitored in the presence of three nonprotein compounds [PEG 4000, PEG 10,000, and glycerol (Fig. 4)]. These are functionally similar compounds to dehydrins in that they are used as protein stabilizers and cryoprotectants, yet their mechanisms of cryoprotection are thought to differ.36, 37, 38 The CD spectra of Yfh1 titrated with PEG 4000 showed an increase in the minima at 205 nm, but had little effect on the signal at 222 nm. When Yfh1 was titrated with increasing concentrations of PEG 10,000, there was some increase in the signal intensity at both minima, but with only a very small change at 222 nm. At a concentration of 20 μM, PEG 10,000 affected the structure of Yfh1 more than the 5 and 10 μM concentrations. In contrast, glycerol had a notable effect on decreasing both minima. The minimum at 206 nm shifted to 208 nm, while the minimum at 218 nm shifted to 222 nm. The glycerol titration effect on Yfh1 more closely resembled that of the dehydrin constructs, where the intensity of both minima increased and shifted to longer wavelengths with increasing amounts of additive.
Quantification of the effect of these three compounds and the dehydrin constructs on Yfh1 is shown in Figure 5(a,b). The change in the helical content of Yfh1 corresponds to the same trends as described above. The α‐helical content of Yfh1 in the presence of 20 μM K2, scrK2, and YSK2 is increased by ~60%, and the 30% glycerol (v/v) induced a ~40% increase, while 20 μM scrYSK2 increased Yfh1 helicity by ~30%. PEG 4000 had essentially no effect on Yfh1 helicity (<5%), while PEG 10,000 had some effect (~20%), and only at its highest concentration.
Figure 5.

Yfh1 helical content at room temperature as a function of additive concentration. (a) Percent helical content of Yfh1 as a function of the molar concentration of several additives. (b) Percent helical content of Yfh1 as a function of glycerol concentration on a percent (v/v) basis. Graph legends are shown as insets.
Yeast frataxin homolog 1 cryoprotection
Having established a baseline for the effects of the dehydrins on the helical structure of Yfh1 at room temperature, we next examined what happens to this protein after freeze/thaw treatment. The α‐helical content of Yfh1 was monitored after three and six freeze/thaw treatments in the presence and absence of the various dehydrins that had been added in a 1:1 molar ratio [Fig. 6(a)]. With Yfh1 alone, the position of the minima near 208 and 222 nm did not shift appreciably. However, the spectra showed a decreasing CD signal, reflecting an increasing loss of structure with each freeze–thaw treatment. With K2 and scrK2, the Yfh1 spectra did not change appreciably at all before and after freezing. For both YSK2 and scrYSK2 samples, Yfh1 showed some structure loss in the minimum near 222 nm, with scrYSK2 showing slightly more loss. Figure 6(b,c) shows that the freeze/thaw treatment of all of the dehydrin constructs themselves did not result in a significant change in structure.
Figure 6.
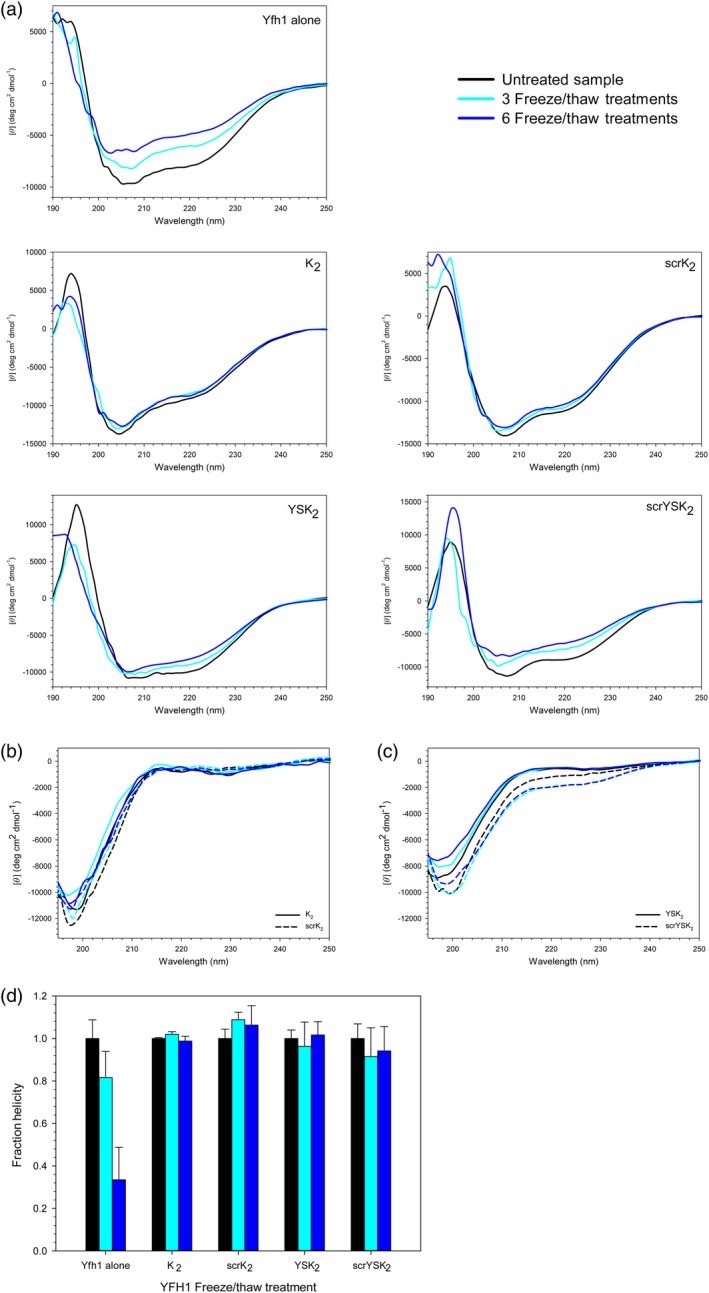
Dehydrins protect Yfh1 from loss of helicity after freeze/thaw treatment. (a) CD spectra of 10 μM Yfh1 in the presence and absence of 10 μM wild‐type and scrambled dehydrins. Untreated, black line; after three freeze/thaw treatments, light blue line; after six freeze/thaw treatments, dark blue line. (b) The CD spectra of K2 (solid line) and scrK2 (dashed line) alone, before and after the freeze/thaw treatment. (c) The CD spectra of YSK2 (solid line) and scrYSK2 (dashed line) alone, before and after the freeze/thaw treatment. (d) Fraction helical content of Yfh1 in the presence and absence of various dehydrin constructs relative to the unfrozen control. Unfrozen sample, black bars; after three freeze/thaw treatments, light blue bars; after six freeze/thaw treatments, dark blue bars. Error bars represent the standard deviation of n = 5.
We quantified the α‐helical content of Yfh1 after the freeze/thaw treatment, the results of which were plotted in Figure 6(d). Note that the α‐helicity is represented as fractional helicity relative to the unfrozen sample (black lines in the presence of dehydrins) to account for their different effects on Yfh1 at room temperature. As reflected in the spectra, Yfh1 alone lost helicity after three (~20% loss) and six (~70% loss) freeze/thaw cycles. In the presence of K2, scrK2, YSK2, and scrYSK2, Yfh1 maintained the same helical content despite the freeze/thaw stress, not showing any significant differences, suggesting that for the most part Yfh1 has maintained most of its structure, and that scrambling the wild‐type dehydrins did not change their ability to protect this protein.
Discussion
Dehydrin constructs and structure
The aim of this study was to further dissect the mechanism behind dehydrin's ability to protect proteins from damage caused by freezing and thawing. Previous studies on dehydrins and LEA proteins have presented somewhat contradictory views. Our studies suggested that the protection is a general effect, mainly driven by the polar and disordered nature of dehydrins.23, 32 The work with dehydrins and PEG molecules of a similar molecular weight shows that the hydrodynamic radius and polar nature of the compound are important for LDH cryoprotection.23 Other studies suggested that it is the sequence composition of the K‐segment that is a factor.26, 39 It is also possible that dehydrins possess functionally important residues or stretches of residues that have not yet been identified.
To resolve several of these questions, we created scrambled sequences of the wild‐type K2 and YSK2 dehydrins in order to understand the importance of sequence composition compared to sequence order in their cryoprotective functions. The changes would alter the location of the Lys residues that typically make up the K‐segment, which has been suggested to be important in enzyme cryoprotection.26, 40 Detailed structural studies have shown that there is very weak helicity in the K‐segments when alone in solution, and relatively strong helicity, possibly amphipathic in nature, in the presence of membranes.32, 41, 42 This would allow us to determine if the protein cryoprotective function of K2 and YSK2 is influenced by their ability to form helices, even weak ones, in the K‐segments.
The CD spectrum of scrK2 [Fig. 2(a), red line] did suggest that the scrambled sequence had a change in the amount of coil structure. This is somewhat surprising, since there was no evidence from the disordered prediction of any change on either a per residue basis [Fig. 1(c)] or with the global disordered parameter (0.777 for scrK2 vs. 0.795 for K2). Also, an inspection of each algorithm used in MetadisorderMD2 did not hint at one of the individual algorithms predicting a loss in disorder; if anything, they all suggested that scrK2 would be more disordered than K2. Nevertheless, we used scrK2 in the cryoprotection assays to see what effect this partial loss of disorder may have on a dehydrin's protective function.
Effect of scrambled sequences on LDH cryoprotection
Our laboratory has previously shown that the K2 and YSK2 dehydrins, as well as artificial K2 concatemers (K4, K6, K8, and K10), peptides (K and KK), and several natural dehydrins (PCA60, Dhn5, TsDHN‐2, and OpsDHN‐1), all have the ability to protect LDH activity when it was frozen and thawed multiple times, and their efficiency (PD50 value) depended on their hydrodynamic radius.23 Since the sequences of dehydrin K‐segments are not absolutely conserved, and the φ‐segment is fairly poorly conserved but makes up a fair part of the sequence,15 one can question the importance of their sequence specificity in protein cryoprotection. Arabidopsis thaliana has 10 dehydrin genes, which gives rise to 24 different K‐segments, all of which display similar cryoprotective efficiency on LDH.26 Supporting this idea is our observation here that scrYSK2 is as effective as the wild‐type YSK2 at protecting LDH, despite the scrambling of the sequence [Fig. 3(b), black and red dashed lines]. As well, in another plant stress protein study the protective behavior of a scrambled LEA3 was comparable to that of the wild‐type peptide sequence.39 Similar results were seen with other LEA proteins, which do not contain K‐segments.43, 44 We can therefore conclude that for dehydrins it is the polar amino acid composition that is important, and not the number and sequence of the K‐segments.
Interestingly, scrK2 provided less protection than K2 [Fig. 3(b), dashed red line]. Part of the reason for this loss is likely the decreased coil structure, since ordered proteins are generally poor at protection LDH.23 This may also explain the results seen in the K‐segment mutated peptides.26 We suggest that the conversion of hydrophobic residues to polar or charged ones resulted in a loss of cryoprotective efficiency due to the peptides gaining structure, such as α‐helicity, and therefore having a shorter hydrodynamic radius.
Another potential contributor to the loss of activity of scrK2 is the density of charged residues. An inspection of the scrK2 sequence [Fig. 1(b)] shows that in residues 16–27 there are seven positively charged residues (both Lys and Arg); we suggest that they are disrupting LDH structure rather than protecting it. A similar issue occurs with the highly charged, disordered protein known as FROST, which was also shown to be a poor protectant of LDH,45 lending further support to this proposal. However, we cannot exclude the possibility that a different protective effect is at play for this scrambled dehydrin variant compared to the wild‐type dehydrins.
Effect of scrambled sequences on Yfh1 structure
We planned to dissect the ability of dehydrins to protect LDH from freeze/thaw damage by examining changes in the structure of LDH. Unfortunately, aggregation of LDH during freeze/thaw treatment prevented us from using CD (Hughes and Graether, unpublished results), and we therefore sought to use another system. Yfh1 is a natural protein that denatures at cold temperatures without the need for destabilization agents,46, 47 making it an ideal candidate for study in this system. Our first observation was that even before the samples were frozen and thawed, all dehydrins increased the amount of structure of Yfh1 (Figs. 4 and 5). We therefore first explored this phenomenon to understand this effect.
The room temperature Yfh1 experiments were repeated in the presence of two common classes of stabilization agents (PEG and glycerol). As seen in Figures 4 and 5, both PEG 4000 and PEG 10,000 are poor stabilizers with only minimal effects, showing that neither volume exclusion nor the presence of a polar polymer plays a role in this cryoprotection. In contrast, the glycerol‐induced changes in the CD spectrum of Yfh1 were very similar to dehydrins (compare panels in Fig. 4). Glycerol is a small, hydrophilic and simple molecule that has been found to strongly induce α‐helical structure, increase structurally compact protein conformations,48 reduce protein flexibility,36 and stabilize a partially unfolded protein, decreasing the tendency for a protein to aggregate.48 Given the high hydration capacity of dehydrins49 and their large number of polar residues, it is possible that dehydrins interact with Yfh1 via a similar mechanism to glycerol, so that by interacting with protein surface via polar interactions, dehydrins may promote the formation of a compact secondary structure, which reduces the protein conformational flexibility. PEG, in contrast, is thought to preferentially bind to positively charged residues and interact with the hydrophobic cleft.50 This may also provide some explanation as to why the K‐segments are rich in positively charged Lys rather than negatively charged amino acids, since they would be repelled from positively charged surface residues.
Lastly, the stabilization effects of the dehydrins on frozen and thawed Yfh1 were probed (Fig. 6). Quantification of the α‐helical content under all conditions [Fig. 6(d)] agrees with what the CD spectra showed—that Yfh1 alone loses ~20% helicity after three freeze/thaws and ~70% after six freeze thaws, while in the presence of dehydrins, Yfh1 essentially loses no structure. These results suggest that the polar nature of dehydrins is important for protecting Yfh1, and that as with the room temperature stabilization, the size of the dehydrin and the specific order of the residues are not important.
However, we cannot completely exclude the idea that the dehydrins are changing their structure in the presence of Yfh1, a possibility that will be addressed in future experiments.
Conclusions
The experiments performed here show that cryoprotection of proteins by dehydrins depends on numerous factors, including the protein assay used. For a freeze/thaw assay that causes aggregation, such as that with LDH, the hydrodynamic radius is a major factor, and possibly many charges clustered together can contribute to poor protection. For a freeze/thaw assay that causes a loss of structure, such as that with Yfh1, it appears that the size is unimportant, and that any of the dehydrin constructs are able to effectively prevent the loss of secondary structure in Yfh1.
Materials and Methods
Dehydrin sequence construction
The EMBOSS program shuffleseq51 was used to generate scrambled sequences of K2 and YSK2 (named scrK2 and scrYSK2, respectively). The sequences pertaining to all of these dehydrins are summarized in Figure 1(a,b), and the disordered nature of these constructs, predicted using the GeneSilico MetaDisorder web server,28 are shown in Figure 1(c,d). Following disorder prediction, the protein sequences for scrK2 and scrYSK2 were submitted for DNA sequence optimization for expression in E. coli. Constructs were subcloned into the pET‐22b(+) expression vector using routine molecular biological methods, and all positive clones were confirmed by sequencing. K2, scrK2, YSK2, and scrYSK2 proteins were expressed and purified as previously described.32, 52 In brief, the purification was performed by first boiling samples to remove many folded proteins, and then running cell lysates through a 5 mL HiTrap S column. Samples containing the dehydrin were pooled, and subsequently purified by reverse‐phased C18 HPLC. For storage, the dehydrin constructs were lyophilized. Purity was typically in the 90–95% range (data not shown). Protein concentration was determined by weighing out the protein and dissolving it in a defined volume.
Yfh1 protein
The pETYF‐1 plasmid, encoding 52–174 of the mature yeast frataxin homolog protein (Yfh1), was a generous gift from Dr. Grazia Isaya (Mayo Clinic, Rochester, MN). The purification protocol for Yfh1 was performed as described by Adamec et al.53 Briefly, cells containing the plasmid were grown at 37°C with shaking at 180 rpm until an optical density of 0.6–0.7 was reached; Yfh1 protein expression was induced by the addition of 0.4 mM IPTG, followed by incubation for 2 h at 37°C. Cells were harvested by centrifugation at 6000g for 15 min, and the pellet was resuspended in 10 mL of 20 mM Tris HCl pH 8 (buffer A) with 50 mM NaCl and 1 mM phenylmethanesulfonyl fluoride. Cells were lysed by sonication and subsequently centrifuged at 20,000g for 20 min at 4°C to remove cell debris. The supernatant was filtered through a 0.22 μm polyvinylidene fluoride (PVDF) membrane prior to further purification.
The supernatant was loaded onto a 5 mL HiTrap Q FF anion‐exchange column. Yfh1 was eluted using a linear NaCl gradient from 0 to 600 mM in 20 mM Tris HCl pH 8 (Buffer B). Fractions containing Yfh1 were identified by 12% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and pooled. Yfh1 protein was concentrated to 1 mL with an Amicon Ultra‐15 concentrator with 3 kDa cutoff, and then loaded onto a Superdex 75 10/300 GL size‐exclusion column that had been equilibrated with 10 mM Tris pH 7.4, 100 mM NaCl. Fractions were pooled and desalted into 10 mM Tris pH 7.4 buffer using a HiPrep 26/10 desalting column. All steps were performed at 4°C. Purified Yfh1 protein was stored in snap‐frozen aliquots of 200 μL at −20°C until use.
Enzyme cryoprotection
To measure the ability of the dehydrin constructs (scrK2 and scrYSK2) to protect LDH from freeze–thaw damage in comparison to wild‐type K2 and YSK2, the method of Hughes et al. was used.23 In brief, LDH (100 μg/mL) in 10 mM Na2HPO4 pH 7.4 was dialyzed for 1 h against 2 L of 10 mM Na2HPO4, pH 7.4 at 4°C prior to dilution to 50 μg/mL. A mixture of 8 μL LDH and 8 μL additive (K2, scrK2, YSK2, scrYSK2, BSA, β‐casein, or lysozyme at a concentration of 5–1000 μg/mL) was frozen in liquid nitrogen for 30 s and thawed for 5 min in a 16°C water bath. This treatment was performed a total of five times. The 16 μL mixture was then added to 724 μL of reaction mix (where 724 μL of reaction mix contained 680.6 μL of 30 mM Na2HPO4 pH 7.4, 21.7 μL of 2 mM reduced nicotinamide adenine dinucleotide (NADH), and 21.7 μL of 10 mM pyruvic acid). The oxidation of NADH was monitored over 2.5 min by measuring the absorbance at 340 nm. The data are plotted as percent recovery of LDH activity versus the log molar concentration of the additive. To show the data trend lines, the sigmoidal patterns of the resultant data were fitted to the equation 32:
| (1) |
where x is the additive concentration, x 0 is the percent recovery in the absence of the additive, e is Euler's number, and a and b are fitted variables.
CD analysis of dehydrin constructs and Yfh1
All CD measurements were performed using a JASCO‐815 spectropolarimeter (Easton, MD) at 25°C in a quartz cuvette with 0.2 cm path length. The spectra were collected from 250 to 190 nm as six accumulations using a bandwidth of 1 nm and a scan speed of 50 nm/min unless otherwise specified. All samples were prepared in 10 mM Tris HCl pH 7.4 and filtered through a 0.22 μm PVDF membrane prior to use. Protein concentrations were 10 μM unless stated otherwise. The CD spectra of scrK2 and scrYSK2 were collected and compared to the spectra of wild‐type K2 and YSK2 dehydrins. Spectra were corrected for signal contributions from the buffer.
Dehydrin titration studies were performed to investigate their effect on the structure of room temperature and freeze/thaw‐treated Yfh1. For the room temperature studies, Yfh1 was incubated with 0, 5, 10, and 20 μM of each of the dehydrins, and the helical content of Yfh1 was calculated using Dichroweb using CD data from 250 to 190 nm with CONTIN analysis54 with the dataset from Sreerama and Woody.55 Five accumulations were collected, and the spectra were corrected for signal contributions from the dehydrin and buffer.
To analyze the effect of dehydrins on the cold denaturation of Yfh1, the dehydrin constructs (K2, scrK2, YSK2, and scrYSK2) were incubated with and without Yfh1 in a 1:1 molar ratio prior to freezing in liquid nitrogen for 30 s and thawing for 5 min at 16°C. Samples were allowed to recover at 25°C for 15 min prior to collecting the CD spectra. Data were collected after three and six freeze/thaw treatments. Spectra were corrected for signal contributions from the buffer and the dehydrins. The helical content of Yfh1 was calculated as described above.
Conflict of Interest
The authors declare that they have no conflict of interest with the contents of this article.
Acknowledgments
The authors thank Dr. Rod Merrill (University of Guelph, Guelph, ON) for the use of the CD spectropolarimeter. They also thank Dr. Grazia Isaya (Mayo Clinic, Rochester, MN) for providing the pETYF‐1 plasmid containing Yfh1. This work was supported by an NSERC Discovery Grant to S.P.G. Infrastructure support to S.P.G. comes from the Canadian Foundation for Innovation, Ontario Innovation Trust, and NSERC Research Tools, and Instrument Grant.
Significance Statement: Disordered proteins can function as chaperones to protect proteins from damage caused by cold. One such protein is dehydration protein (dehydrin) found in plants. We show that dehydrins are able to protect an enzyme and a protein from freeze/thaw damage. The scrambling of the dehydrin sequence did not strongly affect its protective ability, suggesting that it is its polar amino acid composition, and not its sequence order, that is essential for its cryoprotective function.
References
- 1. Amara I, Zaidi I, Masmoudi K, Ludevid MD, Pagès M, Goday A, Brini F (2014) Insights into late embryogenesis abundant (LEA) proteins in plants: from structure to the functions. Am J Plant Sci 5:3440–3455. [Google Scholar]
- 2. Preston JC, Sandve SR (2013) Adaptation to seasonality and the winter freeze. Front Plant Sci 4:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Janmohammadi M, Zolla L, Rinalducci S (2015) Low temperature tolerance in plants: changes at the protein level. Phytochemistry 117:76–89. [DOI] [PubMed] [Google Scholar]
- 4. Tunnacliffe A, Wise MJ (2007) The continuing conundrum of the LEA proteins. Naturwissenschaften 94:791–812. [DOI] [PubMed] [Google Scholar]
- 5. Battaglia M, Olvera‐Carrillo Y, Garciarrubio A, Campos F, Covarrubias AA (2008) The enigmatic LEA proteins and other hydrophilins. Plant Physiol 148:6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hincha DK, Thalhammer A (2012) LEA proteins: IDPs with versatile functions in cellular dehydration tolerance. Biochem Soc Trans 40:1000–1003. [DOI] [PubMed] [Google Scholar]
- 7. Galau GA, Hughes DW, Dure L III (1986) Abscisic acid induction of cloned cotton late embryogenesis‐abundant (Lea) mRNAs. Plant Mol Biol 7:155–170. [DOI] [PubMed] [Google Scholar]
- 8. Houde M, Dallaire S, N'Dong D, Sarhan F (2004) Overexpression of the acidic dehydrin WCOR410 improves freezing tolerance in transgenic strawberry leaves. Plant Biotechnol J 2:381–387. [DOI] [PubMed] [Google Scholar]
- 9. Saibi W, Feki K, Ben Mahmoud R, Brini F (2015) Durum wheat dehydrin (DHN‐5) confers salinity tolerance to transgenic Arabidopsis plants through the regulation of proline metabolism and ROS scavenging system. Planta 242:1187–1194. [DOI] [PubMed] [Google Scholar]
- 10. Muñoz‐Mayor A, Pineda B, Garcia‐Abellán JO, Antón T, Garcia‐Sogo B, Sanchez‐Bel P, Flores FB, Atarés A, Angosto T, Pintor‐Toro JA, Moreno V, Bolarin MC (2012) Overexpression of dehydrin tas14 gene improves the osmotic stress imposed by drought and salinity in tomato. J Plant Physiol 169:459–468. [DOI] [PubMed] [Google Scholar]
- 11. Close TJ, Kortt AA, Chandler PM (1989) A cDNA‐based comparison of dehydration‐induced proteins (dehydrins) in barley and corn. Plant Mol Biol 13:95–108. [DOI] [PubMed] [Google Scholar]
- 12. Close TJ (1996) Dehydrins: emergence of a biochemical role of a family of plant dehydration proteins. Physiol Plant 97:795–803. [Google Scholar]
- 13. Graether SP, Boddington KF (2014) Disorder and function: a review of the dehydrin protein family. Front Plant Sci 5:e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Close TJ (1997) Dehydrins: a commonalty in the response of plants to dehydration and low temperature. Physiol Plant 100:291–296. [Google Scholar]
- 15. Malik AA, Veltri M, Boddington KF, Singh KK, Graether SP (2017) Genome analysis of conserved dehydrin motifs in vascular plants. Front Plant Sci 8:40882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wise MJ (2003) LEAping to conclusions: a computational reanalysis of late embryogenesis abundant proteins and their possible roles. BMC Bioinformatics 4:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Svensson J, Ismail AM, Palva ET. Dehydrins, 2002. Sensing, signaling and cell adaptation. Volume 3 Amsterdam, The Netherlands: Elsevier; p. 155–171. [Google Scholar]
- 18. Kosová K, Liscovitch M, Prášil IT (2014) Wheat and barley dehydrins under cold, drought, and salinity ‐ what can LEA‐II proteins tell us about plant stress response? Front Plant Sci 5:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hanin M, Brini F, Ebel C, Toda Y, Takeda S, Masmoudi K (2011) Plant dehydrins and stress tolerance: versatile proteins for complex mechanisms. Plant Signal Behav 6:1503–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y, Song Q, Li D, Yang X, Li D (2017) Multifunctional roles of plant dehydrins in response to environmental stresses. Front Plant Sci 8:40882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uversky VN (2013) A decade and a half of protein intrinsic disorder: biology still waits for physics. Protein Sci 22:693–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uversky VN (2016) Dancing protein clouds: the strange biology and chaotic physics of intrinsically disordered proteins. J Biol Chem 291:6681–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hughes SL, Schart V, Malcolmson J, Hogarth KA, Martynowicz DM, Tralman‐Baker E, Patel SN, Graether SP (2013) The importance of size and disorder in the cryoprotective effects of dehydrins. Plant Physiol 163:1376–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reyes JL, Campos F, Wei H, Arora R, Yang Y, Karlson DT, Covarrubias AA (2008) Functional dissection of hydrophilins during in vitro freeze protection. Plant Cell Environ 31:1781–1790. [DOI] [PubMed] [Google Scholar]
- 25. Drira M, Saibi W, Brini F, Gargouri A, Masmoudi K, Hanin M (2013) The K‐segments of the wheat dehydrin DHN‐5 are essential for the protection of lactate dehydrogenase and β‐glucosidase activities in vitro. Mol Biotechnol 54:643–650. [DOI] [PubMed] [Google Scholar]
- 26. Hara M, Endo T, Kamiya K, Kameyama A (2017) The role of hydrophobic amino acids of K‐segments in the cryoprotection of lactate dehydrogenase by dehydrins. J Plant Physiol 210:18–23. [DOI] [PubMed] [Google Scholar]
- 27. Ferreira LA, Walczyk Mooradally A, Zaslavsky B, Uversky VN, Graether SP (2018) Effect of an intrinsically disordered plant stress protein on the properties of water. Biophys J 115:1696–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kozlowski LP, Bujnicki JM (2012) MetaDisorder: a meta‐server for the prediction of intrinsic disorder in proteins. BMC Bioinformatics 13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Uversky VN (2002) Natively unfolded proteins: a point where biology waits for physics. Protein Sci 11:739–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greenfield NJ (2006) Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc 1:2876–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin C, Thomashow MF (1992) A cold‐regulated Arabidopsis gene encodes a polypeptide having potent cryoprotective activity. Biochem Biophys Res Commun 183:1103–1108. [DOI] [PubMed] [Google Scholar]
- 32. Hughes S, Graether SP (2011) Cryoprotective mechanism of a small intrinsically disordered dehydrin protein. Protein Sci 20:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wisniewski M, Webb R, Balsamo R, Close TJ, Yu X‐M, Griffith M (1999) Purification, immunolocalization, cryoprotective, and antifreeze activity of PCA60: a dehydrin from peach (Prunus persica). Physiol Plant 105:600–608. [Google Scholar]
- 34. Kovacs D, Kalmar E, Torok Z, Tompa P (2008) Chaperone activity of ERD10 and ERD14, two disordered stress‐related plant proteins. Plant Physiol 147:381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pastore A, Martin SR, Politou A, Kondapalli KC, Stemmler T, Temussi PA (2007) Unbiased cold denaturation: low‐ and high‐temperature unfolding of yeast frataxin under physiological conditions. J Am Chem Soc 129:5374–5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sousa R (1995) Use of glycerol, polyols and other protein structure stabilizing agents in protein crystallization. Acta Crystallogr D51:271–277. [DOI] [PubMed] [Google Scholar]
- 37. Zaroog MS, Abdul Kadir H, Tayyab S (2013) Stabilizing effect of various polyols on the native and the denatured states of glucoamylase. Sci World J 2013:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Simon LM, Kotormán M, Garab G, Laczkó I (2002) Effects of polyhydroxy compounds on the structure and activity of α‐chymotrypsin. Biochem Biophys Res Commun 293:416–420. [DOI] [PubMed] [Google Scholar]
- 39. Furuki T, Shimizu T, Chakrabortee S, Yamakawa K, Hatanaka R, Takahashi T, Kikawada T, Okuda T, Mihara H, Tunnacliffe A, Sakurai M (2012) Effects of group 3 LEA protein model peptides on desiccation‐induced protein aggregation. Biochim Biophys Acta 1824:891–897. [DOI] [PubMed] [Google Scholar]
- 40. Rosales R, Romero I, Escribano MI, Merodio C, Sanchez‐Ballesta MT (2014) The crucial role of Φ‐ and K‐segments in the in vitro functionality of Vitis vinifera dehydrin DHN1a. Phytochemistry 108:17–25. [DOI] [PubMed] [Google Scholar]
- 41. Clarke MW, Boddington KF, Warnica JM, Atkinson J, McKenna S, Madge J, Barker CH, Graether SP (2015) Structural and functional insights into the cryoprotection of membranes by the intrinsically disordered dehydrins. J Biol Chem 290:26900–26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Atkinson J, Clarke MW, Warnica JM, Boddington KF, Graether SP (2016) Structure of an intrinsically disordered stress protein alone and bound to a membrane surface. Biophys J 111:480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dang NX, Popova AV, Hundertmark M, Hincha DK (2014) Functional characterization of selected LEA proteins from Arabidopsis thaliana in yeast and in vitro. Planta 240:325–336. [DOI] [PubMed] [Google Scholar]
- 44. Liu Y, Wang L, Xing X, Sun L, Pan J, Kong X, Zhang M, Li D (2013) ZmLEA3, a multifunctional group 3 LEA protein from maize (Zea mays L.), is involved in biotic and abiotic stresses. Plant Cell Physiol 54:944–959. [DOI] [PubMed] [Google Scholar]
- 45. Newman CE, Toxopeus J, Udaka H, Ahn S, Martynowicz DM, Graether SP, Sinclair BJ, Percival‐Smith A (2017) CRISPR‐induced null alleles show that Frostprotects Drosophila melanogasterreproduction after cold exposure. J Exp Biol 220:3344–3354. [DOI] [PubMed] [Google Scholar]
- 46. Adrover M, Esposito V, Martorell G, Pastore A, Temussi PA (2010) Understanding cold denaturation: the case study of Yfh1. J Am Chem Soc 132:16240–16246. [DOI] [PubMed] [Google Scholar]
- 47. Breydo L, Reddy KD, Piai A, Felli IC, Pierattelli R, Uversky VN (2014) The crowd you're in with: effects of different types of crowding agents on protein aggregation. Biochim Biophys Acta Proteins Proteomics 1844:346–357. [DOI] [PubMed] [Google Scholar]
- 48. Vagenende V, Yap MGS, Trout BL (2009) Mechanisms of protein stabilization and prevention of protein aggregation by glycerol. Biochemistry 48:11084–11096. [DOI] [PubMed] [Google Scholar]
- 49. Tompa P, Bánki P, Bokor M, Kamasa P, Kovacs D, Lasanda G, Tompa K (2006) Protein‐water and protein‐buffer interactions in the aqueous solution of an intrinsically unstructured plant dehydrin: NMR intensity and DSC aspects. Biophys J 91:2243–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hašek J (2006) Poly(ethylene glycol) interactions with proteins. Z Kristallogr Suppl 23:613–618. [Google Scholar]
- 51. Rice P, Longden I, Bleasby A (2000) EMBOSS: the European molecular biology open software suite. Trends Genet 16:276–277. [DOI] [PubMed] [Google Scholar]
- 52. Livernois AM, Hnatchuk DJ, Findlater EE, Graether SP (2009) Obtaining highly purified intrinsically disordered protein by boiling lysis and single step ion exchange. Anal Biochem 392:70–76. [DOI] [PubMed] [Google Scholar]
- 53. Adamec J, Rusnak F, Owen WG, Naylor S, Benson LM, Gacy AM, Isaya G (2000) Iron‐dependent self‐assembly of recombinant yeast frataxin: implications for Friedreich ataxia. Am J Hum Genet 67:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Provencher SW, Glockner J (1981) Estimation of globular protein secondary structure from circular‐dichroism. Biochemistry 20:33–37. [DOI] [PubMed] [Google Scholar]
- 55. Sreerama N, Woody RW (2000) Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal Biochem 287:252–260. [DOI] [PubMed] [Google Scholar]


