Identifying patients with idiopathic pulmonary fibrosis (IPF) at the earliest opportunity remains one of the most urgent challenges for the effective management of this deadly disease. A theoretical basis for early IPF detection comes from large lung cancer and cardiovascular cohort studies that have reported shared clinical associations between incidentally detected subclinical interstitial lung abnormalities (ILAs) on computed tomography (CT) and IPF (1). ILAs are more prevalent with increasing age (2–4), in smokers (1, 5, 6), and in patients over the age of 50 who exhibit MUC5B promoter polymorphism positivity (4). ILAs are also associated with a reduction in pulmonary function (1, 5) and exercise capacity (7). Importantly, patients with progressive ILAs demonstrate greater serial pulmonary function decline when compared with patients with stable ILAs (8). However, the consistent observation that ILA prevalence exceeds that of IPF by more than an order of magnitude means that refinement of current ILA definitions and identification of progressive ILA subtypes are critical if screening for early IPF is to be successful (1).
In this issue of the Journal, Putman and colleagues (pp. 175–183) extend their impressive portfolio of ILA studies by evaluating the impact of specific ILA features on ILA progression as judged by follow-up CT in a population of adults from the AGES-Reykjavik (Age, Gene/Environment Susceptibility–Reykjavik) study (9). This study is important because it represents the first attempt to identify specific CT features and radiologic patterns linked to the progressive ILA phenotype. ILAs defined as subpleural and reticular, those associated with traction bronchiectasis, and ILAs with a lower-lobe predilection were associated with a greater than sixfold likelihood of progression. Moreover, in 16 patients with ILAs characterized by honeycombing, all five who had follow-up imaging had progressed. The authors also generated a “definite fibrosis” score by amalgamating traction bronchiectasis and honeycombing, which was associated with a greater than eightfold likelihood of progression. Finally, previously reported clinical associations with ILA progression, including increasing age and MUC5B promoter polymorphism positivity, were confirmed.
The importance of standardizing ILA definitions cannot be overstated, and the authors make a considerable effort to maintain consistency and clarity in this regard. However, two issues warrant consideration. First, the separation of fibrotic from nonfibrotic ILA is crucial but challenging in limited or early disease because CT-histologic correlation in this setting is imperfect. In one patient, limited subpleural reticulation may be the sole CT manifestation of advanced fibrosis histologically, but in another patient it may not represent fibrosis at all (10). The authors attempt to address this issue by combining traction bronchiectasis and honeycombing, two reliable CT signs of fibrosis, as one variable (“definite fibrosis”), but this is likely to miss at least some cases of reticular ILA representing real fibrotic disease. It is interesting to note that the latest iteration of the international evidence-based joint clinical practice guideline for IPF diagnosis includes in its definition of “indeterminate” for usual interstitial pneumonia (UIP) on CT “subtle reticulation,” which is also described as an “early UIP” pattern (11). In the study by Putman and colleagues, the prevalence of reticular ILA is more than 80%, but only 34% of these cases were considered to represent “definite fibrosis” (9).
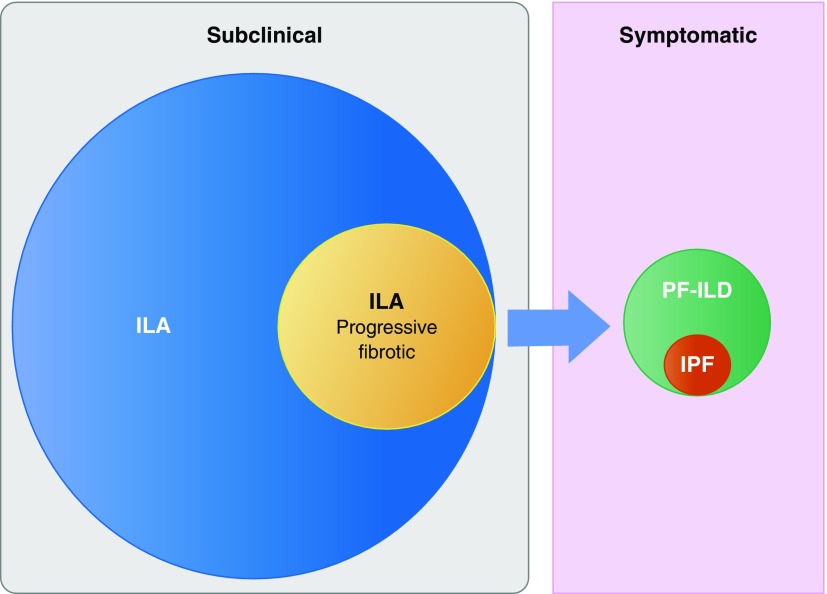
The second, related issue is that if “definite fibrosis” is confined to ILAs in which traction bronchiectasis or honeycombing is present, what defines the boundary between this subtype of fibrotic ILA and the presence of established fibrotic lung disease? In the study by Putman and colleagues, patients whose pattern of ILA met guideline criteria for “probable” or “definite” UIP were included in the analysis and, as expected, all of these patients progressed. Furthermore, 89%/100% of those with a “probable”/“definite” UIP pattern, respectively, died at the end of the follow-up period. The inclusion of these patients raises questions regarding the overarching definition of ILA: “imaging abnormalities on chest CT in research participants without a clinical diagnosis of interstitial lung disease.” Based on current guidelines, the majority of these patients would meet diagnostic criteria for IPF (11). Although there is currently no evidence showing that a UIP pattern on chest CT predicts outcome in research participants who have not received a diagnosis, our understanding of UIP as the prototypic progressive radiologic phenotype is supported by a large body of evidence across idiopathic and nonidiopathic fibrotic lung disease cohorts (12–15). If a screening strategy for detecting early IPF is to be successful, we should probably focus our efforts on determining which of the ILAs that are currently considered nonspecific represent early and clinically relevant progressive fibrotic lung disease (Figure 1).
Figure 1.
Progressive fibrotic interstitial lung abnormalities (ILAs) represent a subtype of ILAs that progress to symptomatic progressive fibrotic interstitial lung disease (PF-ILD), of which idiopathic pulmonary fibrosis (IPF) is one form. The challenge is to determine with accuracy which forms of ILA belong to the progressive fibrotic ILA subtype.
Undoubtedly, a set of standard definitions that would allow consistent ILA reporting and harmonization of cohorts to power larger studies is urgently required. How these definitions should be devised is less clear. Taxonomy can be a double-edged sword that, when wielded indiscriminately, merely replaces understanding with filing—the crux of the well-known “lumping and splitting” debate. Categories that are too rigorously defined lead to distinctions without a difference or impracticable precision, particularly when knowledge is incomplete. Overlapping ILA patterns may exist that by definition cannot be easily placed within a single category. ILA distribution in three dimensions is also likely to be significant based on our knowledge of ILD, but it can be notoriously difficult to classify visually. Finally, pattern recognition on CT is liable to substantial interobserver variability even among experts (16, 17). Thoracic radiologists have difficulties agreeing on a radiologic diagnosis when an established fibrotic lung disease is present; how agreement on ILA definitions will be impacted when the abnormalities are sparse or poorly defined is anyone’s guess. In the current study, the authors report a concordance rate of 81% for the categorization of ILA as present, indeterminate, or absent, but the concordance regarding the classification of ILA subtypes is not clear.
These issues raise tantalizing possibilities for the application of computational image analysis to ILA classification. Over the past decade, there has been a surge in quantitative CT (QCT) research in the ILD setting. Quantitative CT can detect subtle disease progression and prognostic imaging features that elude human assessment (18, 19). These benefits, combined with computer objectivity, are making imaging biomarker exploration in fibrotic lung disease based on visual scoring outdated. More advanced machine-learning methods, and in particular deep learning–based image analysis, will undoubtedly enable further progress in this field, particularly in the domain of new knowledge generation (20, 21). Deep learning is particularly suited to discovering intricate patterns in high-dimensional data, such as images, and mapping them to simple but objective classifications, such as disease progression and mortality. When applied to an appropriately sized cohort, this technology has the potential to facilitate the discovery of radiologic phenotypes representing subclinical fibrotic lung disease that encompass pattern, distribution, and any other predictive CT parameter, including ones imperceptible to human observers. These radiologic phenotypes could be combined with digital lung sound signatures or serum biomarkers to further improve detection of subclinical fibrotic lung disease (22, 23). In the context of the unmet clinical need represented by subclinical IPF detection, the application of computer-based image analysis is undoubtedly a compelling next step.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201901-0180ED on January 30, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators; COPDGene Investigators. Association between interstitial lung abnormalities and all-cause mortality. JAMA. 2016;315:672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. COPDGene Investigators. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sverzellati N, Guerci L, Randi G, Calabrò E, La Vecchia C, Marchianò A, et al. Interstitial lung diseases in a lung cancer screening trial. Eur Respir J. 2011;38:392–400. doi: 10.1183/09031936.00201809. [DOI] [PubMed] [Google Scholar]
- 4.Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368:2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med. 2009;180:407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin GY, Lynch D, Chawla A, Garg K, Tammemagi MC, Sahin H, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology. 2013;268:563–571. doi: 10.1148/radiol.13120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle TJ, Washko GR, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. COPDGene Investigators. Interstitial lung abnormalities and reduced exercise capacity. Am J Respir Crit Care Med. 2012;185:756–762. doi: 10.1164/rccm.201109-1618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araki T, Putman RK, Hatabu H, Gao W, Dupuis J, Latourelle JC, et al. Development and progression of interstitial lung abnormalities in the Framingham Heart Study. Am J Respir Crit Care Med. 2016;194:1514–1522. doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Putman RK, Gudmundsson G, Axelsson GT, Hida T, Honda O, Araki T, et al. Imaging patterns are associated with interstitial lung abnormality progression and mortality. Am J Respir Crit Care Med. 2019;200:175–183. doi: 10.1164/rccm.201809-1652OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller ER, Putman RK, Vivero M, Hung Y, Araki T, Nishino M, et al. Histopathology of interstitial lung abnormalities in the context of lung nodule resections. Am J Respir Crit Care Med. 2018;197:955–958. doi: 10.1164/rccm.201708-1679LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. American Thoracic Society; European Respiratory Society; Japanese Respiratory Society; Latin American Thoracic Society. Diagnosis of idiopathic pulmonary fibrosis: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 12.Sumikawa H, Johkoh T, Colby TV, Ichikado K, Suga M, Taniguchi H, et al. Computed tomography findings in pathological usual interstitial pneumonia: relationship to survival. Am J Respir Crit Care Med. 2008;177:433–439. doi: 10.1164/rccm.200611-1696OC. [DOI] [PubMed] [Google Scholar]
- 13.Walsh SL, Sverzellati N, Devaraj A, Keir GJ, Wells AU, Hansell DM. Connective tissue disease related fibrotic lung disease: high resolution computed tomographic and pulmonary function indices as prognostic determinants. Thorax. 2014;69:216–222. doi: 10.1136/thoraxjnl-2013-203843. [DOI] [PubMed] [Google Scholar]
- 14.Kim EJ, Elicker BM, Maldonado F, Webb WR, Ryu JH, Van Uden JH, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2010;35:1322–1328. doi: 10.1183/09031936.00092309. [DOI] [PubMed] [Google Scholar]
- 15.Padrão E, Santos V, Mota PC, Melo N, Cunha R, Pereira JM, et al. Usual interstitial pneumonia pattern in chronic hypersensitivity pneumonitis. Eur Respir J. 2016;48:PA800. [Google Scholar]
- 16.Walsh SL, Calandriello L, Sverzellati N, Wells AU, Hansell DM UIP Observer Consort. Interobserver agreement for the ATS/ERS/JRS/ALAT criteria for a UIP pattern on CT. Thorax. 2016;71:45–51. doi: 10.1136/thoraxjnl-2015-207252. [DOI] [PubMed] [Google Scholar]
- 17.Watadani T, Sakai F, Johkoh T, Noma S, Akira M, Fujimoto K, et al. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology. 2013;266:936–944. doi: 10.1148/radiol.12112516. [DOI] [PubMed] [Google Scholar]
- 18.Salisbury ML, Lynch DA, van Beek EJ, Kazerooni EA, Guo J, Xia M, et al. IPFnet Investigators. Idiopathic pulmonary fibrosis: the association between the adaptive multiple features method and fibrosis outcomes. Am J Respir Crit Care Med. 2017;195:921–929. doi: 10.1164/rccm.201607-1385OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humphries SM, Swigris JJ, Brown KK, Strand M, Gong Q, Sundy JS, et al. Quantitative high-resolution computed tomography fibrosis score: performance characteristics in idiopathic pulmonary fibrosis. Eur Respir J. 2018;52:1801384. doi: 10.1183/13993003.01384-2018. [DOI] [PubMed] [Google Scholar]
- 20.Silver D, Schrittwieser J, Simonyan K, Antonoglou I, Huang A, Guez A, et al. Mastering the game of Go without human knowledge. Nature. 2017;550:354–359. doi: 10.1038/nature24270. [DOI] [PubMed] [Google Scholar]
- 21.Walsh SLF, Calandriello L, Silva M, Sverzellati N. Deep learning for classifying fibrotic lung disease on high-resolution computed tomography: a case-cohort study. Lancet Respir Med. 2018;6:837–845. doi: 10.1016/S2213-2600(18)30286-8. [DOI] [PubMed] [Google Scholar]
- 22.Sgalla G, Walsh SLF, Sverzellati N, Fletcher S, Cerri S, Dimitrov B, et al. “Velcro-type” crackles predict specific radiologic features of fibrotic interstitial lung disease. BMC Pulm Med. 2018;18:103. doi: 10.1186/s12890-018-0670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maher TM, Oballa E, Simpson JK, Porte J, Habgood A, Fahy WA, et al. An epithelial biomarker signature for idiopathic pulmonary fibrosis: an analysis from the multicentre PROFILE cohort study. Lancet Respir Med. 2017;5:946–955. doi: 10.1016/S2213-2600(17)30430-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.