Abstract
Rationale: Interstitial lung abnormalities (ILA) are radiologic abnormalities on chest computed tomography scans that have been associated with an early or mild form of pulmonary fibrosis. Although ILA have been associated with radiologic progression, it is not known if specific imaging patterns are associated with progression or risk of mortality.
Objectives: To determine the role of imaging patterns on the risk of death and ILA progression.
Methods: ILA (and imaging pattern) were assessed in 5,320 participants from the AGES-Reykjavik Study, and ILA progression was assessed in 3,167 participants. Multivariable logistic regression was used to assess factors associated with ILA progression, and Cox proportional hazards models were used to assess time to mortality.
Measurements and Main Results: Over 5 years, 327 (10%) had ILA on at least one computed tomography, and 1,435 (45%) did not have ILA on either computed tomography. Of those with ILA, 238 (73%) had imaging progression, whereas 89 (27%) had stable to improved imaging; increasing age and copies of MUC5B genotype were associated with imaging progression. The definite fibrosis pattern was associated with the highest risk of progression (odds ratio, 8.4; 95% confidence interval, 2.7–25; P = 0.0003). Specific imaging patterns were also associated with an increased risk of death. After adjustment, both a probable usual interstitial pneumonia and usual interstitial pneumonia pattern were associated with an increased risk of death when compared with those indeterminate for usual interstitial pneumonia (hazard ratio, 1.7; 95% confidence interval, 1.2–2.4; P = 0.001; hazard ratio, 3.9; 95% confidence interval, 2.3–6.8;P < 0.0001), respectively.
Conclusions: In those with ILA, imaging patterns can be used to help predict who is at the greatest risk of progression and early death.
Keywords: progression, interstitial lung abnormalities, idiopathic pulmonary fibrosis, mortality, imaging pattern
At a Glance Commentary
Scientific Knowledge on the Subject
Previous cross-sectional studies have demonstrated multiple associations with interstitial lung abnormalities (ILA), including increased respiratory symptoms, pulmonary function decrements, an increased rate of mortality, and an association with the most common genetic risk locus in idiopathic pulmonary fibrosis (IPF), the MUC5B promoter polymorphism (rs35705950). Longitudinal studies have also demonstrated that ILA imaging progression is fairly common and associated with an increased risk of death. Because ILA encompasses a range of imaging features, little is known about the role of imaging patterns on imaging progression and risk of death.
What This Study Adds to the Field
To our knowledge, this study is the first to present the importance of imaging patterns in ILA progression and risk of death. We present evidence that specific imaging patterns are associated with an increased likelihood of imaging progression and an increased risk of death. In addition, we present the first evidence that the recently published Fleischner Society and the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association guidelines for the diagnosis of IPF can be used to stratify the risk of imaging progression and death in undiagnosed research participants. These data suggest that easily identifiable imaging patterns can help refine the ILA phenotype and stratify those at the greatest risk of progression and most likely to die early.
Evidence demonstrates that interstitial lung abnormalities (ILA), imaging abnormalities on chest computed tomography (CT) scans in research participants without a clinical diagnosis of interstitial lung disease (1–3), can represent an early and/or mild form of pulmonary fibrosis in some cases (4–6). This evidence includes assessments of serial chest CT scans (7–9) that have demonstrated imaging progression to be common (7–9), to be associated with accelerated lung function decline (9), and an increased rate of mortality (9). Despite these findings, the discrepancy between the rates of ILA progression (7–9) and the reported prevalence of the most common and severe form of pulmonary fibrosis, idiopathic pulmonary fibrosis (IPF) (10, 11), suggests that more refined assessments of those with ILA may be needed to determine those at the greatest risk of developing more advanced forms of pulmonary fibrosis and experiencing adverse outcomes.
Because ILA is defined by various imaging features (e.g., reticular markings, ground glass) and patterns, we hypothesized that some specific imaging features and patterns would help to define those with the greatest risk of imaging progression. To test these hypotheses, we evaluated serial chest CT scans for the development and/or progression of ILA from 3,167 participants from the Age Gene/Environment Susceptibility (AGES)-Reykjavik follow-up study. Based on our results we additionally sought to determine if specific imaging patterns would influence mortality.
Methods
Study Population
Protocols for participant enrolment in the AGES-Reykjavik study have been previously reported (12). The AGES-Reykjavik study is a longitudinal birth cohort derived from the Reykjavik Study, which was established in 1967 and includes men and women that were born in Reykjavik, Iceland from 1907 to 1935 and are now followed by the Icelandic Heart Association (12). We have previously published genetic and epidemiologic associations with ILA assessed from the chest CT scans from the baseline examination in this cohort (4, 13). Of the 5,764 participants recruited from 2002 to 2006, a total of 3,167 participants had an additional chest CT scan from 2007 to 2011 (approximately 5 yr apart), which were used for this analysis. Mortality was ascertained as of December 2016. Written informed consent was obtained from all participants, including consent for genetic studies. The Icelandic Bioethics Committee (VSN: 00–063) and the institutional review boards of the Brigham and Women’s Hospital and participating centers approved this study.
Genotyping
All genotyping of the MUC5B promoter polymorphism (rs35705950) was done using TaqMan Genotyping Assays (Applied Biosystems), as previously described (1, 14).
ILA Evaluation
Chest CT scans were evaluated in multiple stages. First, the chest CT scans were evaluated for ILA using a sequential reading method, as previously described (1, 2, 15) by up to three readers (radiologists and pulmonologists on a Canon Medical Inc., Japan workstation), who were blind to all participant specific information, prior radiologic interpretations of the same participant, and radiologic interpretations of other readers. ILA on chest CT scans were defined as nondependent changes affecting greater than 5% of any lung zone. These abnormalities include ground glass, reticular abnormalities, diffuse centrilobular nodularity, nonemphysematous cysts, traction bronchiectasis, or honeycombing. Chest CT scans with either focal or unilateral ground-glass attenuation, focal or unilateral reticulation, or patchy ground-glass abnormalities were indeterminate for ILA (1, 2).
To further characterize the types of radiologic changes present in participants with ILA, first the pattern of ILA was identified, specifically classified into four categories based on the type and predominant location of changes: 1) centrilobular, 2) subpleural, 3) mixed, and 4) radiologic evidence of interstitial lung disease (2, 13). In addition to identifying the pattern of ILA, the following information was recorded for each chest CT: the presence of any ground-glass opacities, subpleural reticulation, centrilobular nodularity, nonemphysematous cysts, traction bronchiectasis, and honeycombing; and the location (subpleural, centrilobular, mixed) and distribution (lower lobe, upper lobe, diffuse, or multifocal) of these features. Then an additional subset was created, defined by the presence of pulmonary parenchymal architectural distortion (e.g., traction bronchiectasis, honeycombing) consistent with a fibrotic lung disease (definite fibrosis), which is not limited to those whose imaging pattern is consistent with a usual interstitial pneumonia (UIP) or probable UIP pattern (1, 16).
Next, all scans with ILA were characterized into those with and without a UIP pattern, according to the diagnostic criteria for IPF from both the Fleischner Society (17) and the official American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association clinical practice guidelines (18). To characterize the scans with ILA according to the new diagnostic criteria, no cases of UIP were reclassified, the cases that were previously classified as possible UIP were subset into a probable UIP pattern and a pattern indeterminate for UIP. Cases were classified as a probable UIP pattern based on the presence of traction bronchiectasis in the appropriate distribution, the remainder were classified as a CT pattern indeterminate for UIP (17, 18). All ILA subtyping was performed by a consensus of at least three readers who were blind to all participant-specific information and to prior CT interpretation.
Finally, after completing ILA phenotyping and subtyping, all participants with ILA present on at least one of either the initial or the subsequent CT scans had both sets of images simultaneously compared. The paired CT scans were scored on a five-point scale (definite regression, probable regression, no change, probable progression, and definite progression), as previously described (19). Progressive change was defined as an increase in lung areas affected with nondependent ground glass, reticular abnormalities, diffuse centrilobular nodularity, nonemphysematous cysts, honeycombing, or traction bronchiectasis, or a new appearance of at least one such abnormality. All paired CT evaluations were performed by a consensus of at least three readers blind to any additional participant-specific information.
Statistical Analysis
All analyses with ILA progression as the outcome variable were performed using logistic regression, where ILA progression was dichotomized with progression defined as probable or definite progression, and regression defined as no change, probable regression, and definite regression. The multivariable analyses were adjusted for age, sex, body mass index, pack-years smoking, and current smoking status. To evaluate the association among ILA progression, radiologic patterns of ILA, and mortality we used Cox proportional hazards models for models evaluating time-to-mortality. In Cox models, all variables were assessed, and none violated the proportional hazards assumption. All genetic analyses performed used an additive genetic model (14). Reported P values were two-sided and those less than 0.05 were considered statistically significant; SAS version 9.4 (SAS Institute Inc.) was used for analyses.
Results
For analyses of progression, 3,167 participants with serial CT scans (median time between CT scans, 5.1 yr; interquartile range, 4.99–5.26 yr) are included. For analyses of mortality, an additional 2,153 participants (5,320 participants in total) who had completed the baseline CT are included. Of the participants completing the second CT, similar to previously published reports on the characterization of the first CT (4, 13), 284 (9%) had ILA, 1,792 (57%) did not have ILA, and 1,091 (34%) were indeterminate for ILA. Of the cases reviewed by at least two readers, there was concordance of ILA scoring in 81% of those cases.
Of the 3,167 participants with two serial CTs, 327 (10%) had ILA on at least one CT scan, 1,435 (45%) did not have ILA on either CT scan, and 1,405 (44%) did not have ILA on either CT scan but were found to be indeterminate for ILA on at least one CT scan. Of the 327 participants with ILA noted on at least one CT scan, 132 (40%) had definitely progressed, 106 (32%) had probably progressed, 49 (15%) had stayed the same, 21 (6%) had probably improved, and 19 (6%) had definitely improved (Figure 1). To provide meaningful comparison (9), those who developed or had progression of ILA (238 of 3,167 participants; 8%) were compared with participants with stable or improving ILA (89 of 3,167 participants; 3%), and with those without ILA on either CT (1,435 of 3,167; 45%).
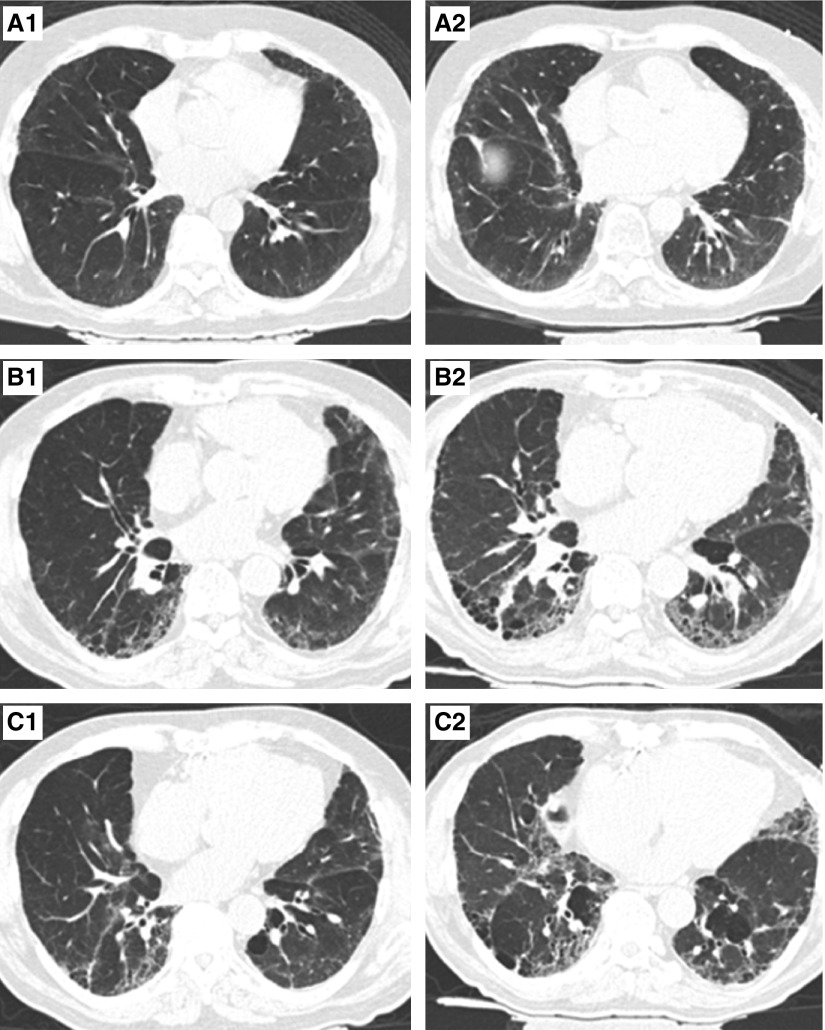
Figure 1.
Serial chest computed tomography scans from three participants with interstitial lung abnormalities. (A1–C1) Representative axial images from computed tomography scan 1. (A2–C2) Representative axial images from computed tomography scan 2. Participant A is indeterminate for usual interstitial pneumonia (UIP) on scan 1 (A1) and progressed to a probable UIP pattern (A2). Participant B has a probable UIP pattern on scan 1 (B1) and progressed to a UIP pattern (B2). Participant C had a UIP pattern on scan 1 (C1) and had imaging progression that remained consistent with a UIP pattern (C2).
Factors Associated with ILA Progression
Baseline characteristics of participants stratified by ILA progression are presented in Table 1. Baseline characteristics at the second CT scan are in Table E1 in the online supplement, and from the first CT scan have been previously published (4, 13). Our interest was in assessing the relationship with progression.
Table 1.
Baseline Characteristics of Participants, at the Time of the Second Computed Tomography Scan, Stratified by ILA Status and ILA Progression Status*
| No ILA (n = 1,777; 56%) | ILA without Progression (n = 89; 3%) | ILA with Progression (n = 238; 8%) |
P Value |
||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | All | 0 vs. 1 | 0 vs. 2 | 1 vs. 2 | |
| Age, yr | 74 ± 5 | 75 ± 5 | 76 ± 5 | <0.0001 | 0.4 | <0.0001 | 0.02 |
| Sex, n (%) | <0.0001 | 0.2 | <0.0001 | 0.2 | |||
| M | 704 (40) | 42 (47) | 131 (55) | ||||
| F | 1,073 (60) | 47 (53) | 107 (45) | ||||
| Body mass index, kg/m2 | 27 ± 4 | 27 ± 5 | 28 ± 4 | 0.06 | 0.8 | 0.02 | 0.4 |
| Pack-years smoking, median, IQR | 0 (15) | 11 (30) | 11 (30) | <0.0001 | 0.0001 | <0.0001 | 0.7 |
| Smoking status, n (%) | <0.0001 | 0.001 | <0.0001 | 0.5 | |||
| Never | 843 (48) | 25 (28) | 70 (29) | ||||
| Former | 751 (42) | 48 (55) | 138 (58) | ||||
| Current | 183 (10) | 15 (17) | 30 (13) | ||||
| MUC5B, n (%) | <0.0001 | 0.3 | <0.0001 | 0.0005 | |||
| GG | 1,426 (80) | 67 (76) | 131 (55) | ||||
| GT | 335 (19) | 20 (23) | 98 (41) | ||||
| TT | 15 (1) | 1 (1) | 9 (4) | ||||
Definition of abbreviations: 0 = no ILA; 1 = ILA without progression; 2 = ILA with progression; ILA = interstitial lung abnormalities; IQR = interquartile range; MUC5B = genotype at the MUC5B promoter polymorphism (rs35705950).
Values are n (%) or mean ± SD.
Comparison of categorical variables was done using Fisher exact tests, continuous variables with two-tailed Student’s t test, and across all three categories comparisons were made using ANOVA.
Participants with ILA that progressed were older and had increasing copies of the MUC5B promoter polymorphism when compared with both those with ILA without progression, and without ILA. When compared with those without ILA, and with those with ILA without progression, in multivariable models, only age and MUC5B genotype were significantly associated with ILA progression (Table 2). For example, for each additional year older the odds of progressive imaging abnormalities increased by 8% (odds ratio [OR], 1.08; 95% confidence interval [CI], 1.02–1.14; P = 0.01), and for each copy of the MUC5B promoter polymorphism, there was a 260% increase in the odds of progressive imaging findings (OR, 2.6; 95% CI, 1.5–4.4; P = 0.0004), when compared with those with ILA without progressive imaging changes. Results of analyses limited to those with an imaging pattern indeterminate for IPF can be found in Table E2.
Table 2.
Multivariable Logistic Regression to Assess Factors Associated with ILA Progression, Comparing Those with Imaging Progression with Those without Imaging Progression and Comparing Those with Imaging Progression with Those without ILA on Either Computed Tomography Scan
| |
Comparison of ILA with Progression with ILA without Progression |
Comparison of ILA with Progression with No ILA |
||
|---|---|---|---|---|
| Covariate | OR (95% CI) | P Value | OR (95% CI) | P Value |
| MUC5B genotype* | 2.6 (1.5–4.4) | 0.0004 | 2.9 (2.2–3.8) | <0.0001 |
| Age† | 1.08 (1.02–1.1) | 0.01 | 1.08 (1.05–1.11) | <0.0001 |
| Sex‡ | 0.6 (0.4–1.1) | 0.1 | 0.6 (0.4–0.8) | 0.0002 |
| Body mass index§ | 1.05 (0.99–1.1) | 0.1 | 1.06 (1.02–1.09) | 0.001 |
| Pack-years smoking|| | 0.99 (0.98–1.01) | 0.3 | 1.01 (1.01–1.02) | <0.0001 |
| Current smoking status¶ | 1.1 (0.5–2.4) | 0.8 | 1.1 (0.7–1.8) | 0.6 |
Definition of abbreviations: CI = confidence interval; ILA = interstitial lung abnormalities; OR = odds ratio.
OR is per copy of MUC5B minor allele.
OR is for each additional year older.
OR is for comparison of female with male.
OR is for 1-kg/m2 increase in body mass index.
OR is for each additional pack-year smoked.
OR is for the comparison of current with former/never.
Imaging Features and ILA Progression
The unadjusted and adjusted analyses of association between specific imaging features noted on a participant’s first CT and ILA progression are presented in Table 2 (see Tables E3–E7 for full multivariable models). Some imaging features helping to define the presence of ILA increased the likelihood of progression on subsequent chest CT imaging (e.g., the presence of subpleural reticular markings increased the odds of progression more than sixfold [OR, 6.6; 95% CI, 2.3–19; P = 0.0004]), whereas other imaging features decreased the likelihood of progression (e.g., the presence of centrilobular nodules decreased the likelihood of progression [OR, 0.2; 95% CI, 0.1–0.5; P = 0.0002]) (Table 3). Some imaging features defining ILA were present in all scans (e.g., ground-glass abnormalities) and some imaging features were always associated with progression (e.g., of the 16 participants with ILA with honeycombing on their first CT [see Table E8] only five [31%] had a subsequent chest CT and all had progressed).
Table 3.
Association between Imaging Features and ILA Progression
| Unadjusted Analysis |
Adjusted Analysis* |
|||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Centrilobular nodules | 0.2 (0.1–0.4) | <0.0001 | 0.2 (0.1–0.5) | 0.0002 |
| Ground glass† | — | — | — | — |
| Subpleural reticular markings | 5.9 (2.3–15) | 0.0002 | 6.6 (2.3–19) | 0.0004 |
| Nonemphysematous cysts | 3.1 (1.6–5.9) | 0.0005 | 2.5 (1.3–5.1) | 0.009 |
| Lower lobe predominant changes | 5.2 (1.8–15) | 0.002 | 6.7 (1.8–25) | 0.004 |
| Traction bronchiectasis | 5.9 (2.3–14.9) | 0.0002 | 6.6 (2.3–19) | 0.0004 |
| Honeycombing‡ | — | — | — | — |
For definition of abbreviations, see Table 2.
Adjusted for age, sex, body mass index, pack-years smoking, current smoking status, and MUC5B genotype.
Odds of progression cannot be calculated for ground glass because all participants with ILA had ground glass on computed tomography scan.
Odds of progression cannot be calculated because all participants with honeycombing had evidence of imaging progression.
Imaging Patterns and ILA Progression
To determine the overall effect of imaging features on ILA progression, imaging features were combined into previously described (1, 17) and easily characterizable patterns. Some imaging patterns were strongly associated with ILA progression (see Table E9). For example, of the 46 participants with definite fibrosis (those with pulmonary parenchymal architectural distortion in any distribution) (1), 41 (89%) had evidence of progression on the second CT scan (e.g., the presence of definite fibrosis increased the odds of progression more than eightfold [OR, 8.4; 95% CI, 2.7–25; P = 0.0003] when compared with those with ILA without definite fibrosis). Of the five participants with definite fibrosis who did not progress, all had a CT pattern inconsistent with UIP and suggestive of an alternate diagnosis, three were stable over the follow-up period, and two had probably improved (see Table E10).
To assess whether consistency with a UIP pattern was associated with progressive imaging abnormalities additional analyses were performed based on the diagnostic criteria for IPF from the Fleischner Society and American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association guidelines (17, 18). When compared with those who were consistent with an alternate non-IPF diagnosis overall, there was no evidence that those indeterminate for a UIP pattern were more likely to progress (e.g., the presence of an indeterminate for a UIP pattern was not associated with an increased odds of progression [OR, 1.9; 95% CI, 0.9–4.3; P = 0.9]); however, this comparison was dependent on the presence of fibrosis (e.g., the presence of an indeterminate for a UIP pattern was associated with an increased odds of progression [OR, 2.3; 95% CI, 1.04–5.1; P = 0.04] when compared with those consistent with a non-IPF diagnosis without fibrosis but, not when compared with those consistent with a non-IPF diagnosis with fibrosis [OR, 1.5; 95% CI, 0.4–6.32; P = 0.6]). All participants with probable (30/30) or UIP pattern (five/five) had imaging progression over the follow-up period.
ILA Progression, Imaging Patterns, and Mortality
Over the median follow-up period of 11 years, (interquartile range, 7–13 yr), based on ILA classification from the first CT scan (baseline), 277 of the 378 (73%) participants with ILA died compared with 1,562 of the 3,216 (49%) participants without ILA (1,038 of the 1,726 [60%] participants indeterminate for ILA had died). In analyses limited to those who completed both rounds of chest CT imaging, 143 of 238 (60%) participants with ILA with progressive imaging changes had died, compared with 35 of the 89 (39%) participants with ILA without progressive imaging findings. ILA progression was associated with an increased risk of death when compared with those without ILA (hazard ratio [HR], 1.4; 95% CI, 1.3–1.5; P < 0.0001) or with those with ILA but without progression (HR, 1.9; 95% CI, 1.3–2.8; P = 0.0009). Results related to imaging features and mortality are also presented in Table 4.
Table 4.
Association between Imaging Pattern and Features and Mortality*
| |
Unadjusted Analysis |
Adjusted Analysis† |
||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Reticular markings | 2.0 (1.3–3.1) | 0.002 | 1.6 (1.0–2.5) | 0.049 |
| Centrilobular nodules | 0.7 (0.6–0.9) | 0.01 | 0.9 (0.7–1.1) | 0.3 |
| Nonemphysematous cysts | 1.7 (1.3–2.2) | <0.0001 | 1.4 (1.1–1.8) | 0.02 |
| Traction bronchiectasis | 2.0 (1.6–2.6) | <0.0001 | 1.6 (1.3–2.1) | 0.0001 |
| Lower lobe‡ predominance | 1.5 (0.95–2.5) | 0.08 | 1.1 (0.6–1.7) | 0.8 |
| Subpleural location§ | 2.0 (1.3–3.2) | 0.003 | 1.6 (1.0–2.7) | 0.050 |
| ILA without fibrosis | 1.3 (1.2–1.4) | <0.0001 | 1.2 (1.1–1.3) | 0.0004 |
| Definite fibrosis | 1.9 (1.7–2.1) | <0.0001 | 1.5 (1.3–1.6) | <0.0001 |
| Indeterminate for UIP | 1.6 (1.3–2.0) | <0.0001 | 1.2 (0.98–1.5) | 0.07 |
| Probable UIP pattern | 3.3 (2.6–4.2) | <0.0001 | 1.9 (1.5–2.5) | <0.0001 |
| UIP pattern | 6.9 (4.2–11) | <0.0001 | 4.5 (2.8–7.2) | <0.0001 |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio; ILA = interstitial lung abnormalities; UIP = usual interstitial pneumonia.
All comparisons are with participants without ILA.
Adjusted analyses are adjusted for age, sex, pack-years smoking, current smoking status, and body mass index.
Comparison is with upper lobe predominance.
Comparison is with centrilobular location.
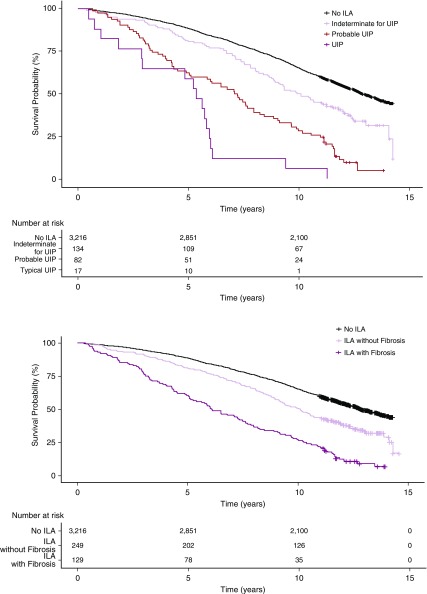
When compared with those without ILA at baseline, specific ILA imaging patterns were associated with a variable increase in the rate of mortality (Figure 2 and Table 4). In addition, there was evidence that ILA imaging pattern influenced the rate of mortality among those with ILA. For example, during follow-up, 115 of the 129 (89%) participants with definite fibrosis had died compared with 162 of the 249 (65%) participants without definite fibrosis (compared with those without definite fibrosis, ILA with definite fibrosis was associated with a 70% increase in the risk of death [HR, 1.7; 95% CI, 1.3–2.1; P < 0.0001]). During follow-up, all 17 participants with a UIP pattern died, 73 of the 82 (89%) participants with a probable UIP pattern died, and 88 of the 134 (66%) participants with a CT pattern indeterminate for UIP had died (whereas 99 of the 145 [68%] participants with a CT pattern consistent with a non-IPF diagnosis had died, the rate was higher among those with definite fibrosis [25 of 30; 83%] compared with those without definite fibrosis [74 of 115; 64%] [see Table E11]). Participants with either a CT pattern of UIP and probable for UIP had an increased risk of death when compared with participants indeterminate for UIP (HR, 3.9; 95% CI, 2.3–6.8; P < 0.0001; HR, 1.7; 95% CI, 1.2–2.4; P = 0.001), respectively.
Figure 2.
Kaplan-Meier survival curves showing percent survival, comparing participants without interstitial lung abnormalities (ILA) with those with ILA, first with ILA subset based on consistency with an idiopathic pulmonary fibrosis diagnosis, and then by the presence of definite fibrosis (pulmonary parenchymal architectural distortion). UIP = usual interstitial pneumonia.
Discussion
To date, this study represents the largest longitudinal visual assessment of chest CTs for the purposes of characterizing ILA development and progression and presents several significant findings. Confirming prior reports (9), this study demonstrates that ILA progression is relatively common and is associated with increased age, increasing copies of the MUC5B promotor polymorphism (rs35705950) risk allele, and an increased rate of mortality. Most importantly, for the first time, this study demonstrates that specific imaging features (e.g., traction bronchiectasis) and patterns (e.g., a probable UIP pattern) (20) are strongly associated with ILA progression. Finally, this study demonstrates that the same ILA patterns associated progression are those most closely tied to an increased rate of mortality. These findings present evidence to support a rational refinement of the ILA imaging phenotype to help best determine those at the greatest risk to progress and to experience adverse outcomes.
This study confirms many prior findings of studies that have included serial visual assessments of chest CTs for the purposes of assessing ILA progression (7–9, 21). First, comparable with prior characterizations of ILA progression in the Framingham Heart Study and from lung cancer screening populations (7, 8, 21), the prevalence of ILA (∼7% at baseline and ∼9% at follow-up in the AGES-Reykjavik cohort) was similar (noted to be between 3% and 10% in prior reports) (7–9, 21). Second, we confirm the prior reports of the association between ILA progression and increased age and copies of the MUC5B promoter polymorphism risk allele (9). Finally, this study demonstrates a consistent association between ILA progression (similar to associations with ILA occurrence) (4, 9, 22–25) and an accelerated rate of mortality (9). Of note, the rate of ILA progression in the AGES-Reykjavik cohort (73% at 5 yr) was higher than the rates of ILA progression previously noted in the Framingham Heart Study (43% at 6 yr) (9) and in some lung cancer screening cohorts (20% at 2 yr [21], 46% at 4 yr [7], respectively). This difference is likely, in part, explained by the advanced baseline age (and the more advanced imaging patterns correlated with advanced age) of the AGES-Reykjavik cohort when compared with other populations.
For the first time, this study provides statistical evidence that some imaging features and patterns are more strongly associated with ILA progression than others. Although prior reports have provided some descriptive statistics on the imaging follow-up of various imaging features and patterns (7–9, 21), statistical power limited prior comparisons. As expected, imaging features noted on the AGES-Reykjavik scans that were more closely associated with pulmonary fibrosis in general, and IPF specifically (10, 17, 26), were more likely to progress on subsequent CT imaging. For example, although centrilobular nodules were likely to improve over time, subpleural reticular markings and traction bronchiectasis were associated with imaging progression. These imaging features could be summarized into easily definable imaging patterns that were associated with ILA progression. For example, approximately one-quarter of those with ILA had definite fibrosis on their first chest CT scan and these participants had a greater than 800% increase in their odds of having progression in an assessment of their subsequent CT scan 5 years later. All participants with UIP or probable UIP patterns had progressed in their subsequent imaging.
The same imaging patterns associated with ILA progression in the AGES-Reykjavik cohort were also associated with an increased risk of death. For example, those with definite fibrosis had a 70% increase in their risk of death when compared with those with ILA without definite fibrosis. In addition, this study provides evidence that chest CT-based diagnostic imaging criteria for IPF (17) can be used to stratify the risk of death in this population. For example, over the follow-up period in this cohort of older adults, there was an increase in the risk of death associated with both a probable UIP and UIP pattern when compared with those with a CT pattern indeterminate for UIP, with a UIP pattern having the highest risk of death.
Our study has several limitations. First, although this study provides evidence that specific imaging features and patterns are associated with imaging progression and an increased rate of mortality among this cohort of older adults, we urge caution in extrapolating these findings to younger populations. It is likely that some of the associations between specific imaging patterns and mortality would be attenuated in cohorts with younger, or more variable, ages at recruitment. Second, even though both blinded and sequential comparisons of serial chest CT scans were performed, we cannot rule out the possibility that some amount of misclassification of ILA could have occurred in our serial comparisons. Third, even though the initial ILA scoring and subtyping was blinded, and during the sequential comparison for progression readers were blinded to prior ILA scores, readers were not blind to the sequence of the CT scans which could have introduced bias.
Fourth, when subtyping ILA to definite fibrosis, defined by the presence of pulmonary parenchymal architectural distortion, we recognize that the lack of a consensus nomenclature can create confusion for readers and difficulty creating comparisons across studies by different groups. In addition, we also note that there is an imperfect correlation between fibrosis on imaging and on histopathology, and that fibrosis is often present on pathologic evaluation with less advanced imaging findings, such as subpleural reticulation (5). Fifth, small sample size limits our ability to determine if additional imaging features predict ILA progression among those with specific imaging patterns. In addition, although ILA progression has been associated with an accelerated decline in FVC in the Framingham Heart Study (9), lack of repeated measures of pulmonary function in the AGES-Reykjavik cohort prevents this assessment. Also, because imaging progression could only be assessed in those participants who completed follow-up imaging, we cannot rule out that early mortality could have led to some selection bias. Finally, we cannot rule out the possibility that additional imaging assessments including quantitative metrics (6, 27) could also be helpful in stratifying those at the greatest risk to progress and to experience an accelerated rate of mortality.
In conclusion, our study demonstrates that ILA progression is relatively common over a 5-year period and is associated with increasing age, copies of the MUC5B genotype risk allele, and the risk of death. This study demonstrates that easily definable (1, 20) imaging patterns may be important in determining those who are at the greatest risk to progress and to die early. This study adds to the data supporting the idea that early pulmonary fibrosis is associated with important clinical outcomes (4, 6, 9), and suggests a path toward refining the ILA phenotype by stratifying those at the greatest risk to experience adverse clinical outcomes. This study also brings to the forefront the need to develop standards for reporting imaging patterns among those with likely early stages of pulmonary fibrosis and should begin a discussion about what imaging abnormalities should be considered sufficient to diagnose a disease.
Supplementary Material
Footnotes
Supported by NIH grants K08 HL140087 (R.K.P.); R01 CA203636 (M.N.); U01 HL133232 and R01 HL130974 (I.O.R.); R01 HL116473 and R01 HL122464 (G.R.W.); R01 HL135142, R01 HL113264, and R01 HL137927 (M.H.C.); and P01 HL092870, R01 HL097163, and R33 HL120770 (D.A.S.). Supported by Oddur Olafsson Fund, project grant 141513-051 from the Icelandic Research Fund and Landspitali Scientific Fund A-2015-030 and A-2016-023 (G.G.). The Age, Gene/Environment Susceptibility-Reykjavik Study was supported by NIH contracts N01-AG-1-2100 and HHSN27120120022C, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). NIA grant 27120120022C and project grant 141513-051 from the Icelandic Research Fund (V.G.). G.M.H. and this work were supported by NIH grants R01 HL111024, R01 HL130974, R01 135142, and project grant 141513-051 from the Icelandic Research Fund.
Author Contributions: Study design, R.K.P., G.G., I.O.R., G.R.W., D.A.S., V.G., H. Hatabu, and G.M.H. Acquisition, analysis, or interpretation of the data, R.K.P., T.H., O.H., T.A., M.N., H. Hatabu, and G.M.H. Critical revision of the manuscript for important intellectual content, R.K.P., G.G., G.T.A., T.H., O.H., T.A., M.Y., M.N., E.R.M., G.E., E.F.G., N.T., H. Honda, I.O.R., G.R.W., M.H.C., D.A.S., V.G., H. Hatabu, and G.M.H. Statistical analysis, R.K.P. and G.M.H. Obtained funding, G.G., M.H.C., V.G., and G.M.H.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201809-1652OC on January 23, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368:2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. COPDGene Investigators. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med. 2009;180:407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Putman RK, Hatabu H, Araki T, Gudmundsson G, Gao W, Nishino M, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators; COPDGene Investigators. Association between interstitial lung abnormalities and all-cause mortality. JAMA. 2016;315:672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller ER, Putman RK, Vivero M, Hung Y, Araki T, Nishino M, et al. Interstitial lung abnormalities and histopathologic correlates in patients undergoing lung nodule resection [abstract] Am J Respir Crit Care Med. 2017;195:A1120. [Google Scholar]
- 6.Podolanczuk AJ, Oelsner EC, Barr RG, Bernstein EJ, Hoffman EA, Easthausen IJ, et al. High-attenuation areas on chest computed tomography and clinical respiratory outcomes in community-dwelling adults. Am J Respir Crit Care Med. 2017;196:1434–1442. doi: 10.1164/rccm.201703-0555OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsushima K, Sone S, Yoshikawa S, Yokoyama T, Suzuki T, Kubo K. The radiological patterns of interstitial change at an early phase: over a 4-year follow-up. Respir Med. 2010;104:1712–1721. doi: 10.1016/j.rmed.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Sverzellati N, Guerci L, Randi G, Calabrò E, La Vecchia C, Marchianò A, et al. Interstitial lung diseases in a lung cancer screening trial. Eur Respir J. 2011;38:392–400. doi: 10.1183/09031936.00201809. [DOI] [PubMed] [Google Scholar]
- 9.Araki T, Putman RK, Hatabu H, Gao W, Dupuis J, Latourelle JC, et al. Development and progression of interstitial lung abnormalities in the Framingham Heart Study. Am J Respir Crit Care Med. 2016;194:1514–1522. doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis. Evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, et al. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001-11. Lancet Respir Med. 2014;2:566–572. doi: 10.1016/S2213-2600(14)70101-8. [DOI] [PubMed] [Google Scholar]
- 12.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–1087. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putman RK, Gudmundsson G, Araki T, Nishino M, Sigurdsson S, Gudmundsson EF, et al. The MUC5B promoter polymorphism is associated with specific interstitial lung abnormality subtypes. Eur Respir J. 2017;50:1700537. doi: 10.1183/13993003.00537-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu JF, Washko GR, Nakahira K, Hatabu H, Patel AS, Fernandez IE, et al. COPDGene Investigators. Statins and pulmonary fibrosis: the potential role of NLRP3 inflammasome activation. Am J Respir Crit Care Med. 2012;185:547–556. doi: 10.1164/rccm.201108-1574OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HY, Seo JB, Steele MP, Schwarz MI, Brown KK, Loyd JE, et al. The high-resolution CT scan findings in familial interstitial pneumonia do not conform to those of idiopathic interstitial pneumonia. Chest. 2012;142:1577–1583. doi: 10.1378/chest.11-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch DA, Sverzellati N, Travis WD, Brown KK, Colby TV, Galvin JR, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med. 2018;6:138–153. doi: 10.1016/S2213-2600(17)30433-2. [DOI] [PubMed] [Google Scholar]
- 18.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. American Thoracic Society; European Respiratory Society; Japanese Respiratory Society; Latin American Thoracic Society. Diagnosis of idiopathic pulmonary fibrosis: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 19.Araki T, Nishino M, Gao W, Dupuis J, Hunninghake GM, Murakami T, et al. Normal thymus in adults: appearance on CT and associations with age, sex, BMI and smoking. Eur Radiol. 2016;26:15–24. doi: 10.1007/s00330-015-3796-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch DA, Sverzellati N, Travis WD, Brown KK, Colby TV, Galvin JR, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med. 2018;6:138–153. doi: 10.1016/S2213-2600(17)30433-2. [DOI] [PubMed] [Google Scholar]
- 21.Jin GY, Lynch D, Chawla A, Garg K, Tammemagi MC, Sahin H, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology. 2013;268:563–571. doi: 10.1148/radiol.13120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Podolanczuk AJ, Oelsner EC, Barr RG, Hoffman EA, Armstrong HF, Austin JH, et al. High attenuation areas on chest computed tomography in community-dwelling adults: the MESA study. Eur Respir J. 2016;48:1442–1452. doi: 10.1183/13993003.00129-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Putman RK, Hunninghake GM, Dieffenbach PB, Barragan-Bradford D, Serhan K, Adams U, et al. Interstitial lung abnormalities are associated with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195:138–141. doi: 10.1164/rccm.201604-0818LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pompe E, de Jong PA, Lynch DA, Lessmann N, Išgum I, van Ginneken B, et al. Computed tomographic findings in subjects who died from respiratory disease in the National Lung Screening Trial. Eur Respir J. 2017;49:1601814. doi: 10.1183/13993003.01814-2016. [DOI] [PubMed] [Google Scholar]
- 25.Nishino M, Cardarella S, Dahlberg SE, Araki T, Lydon C, Jackman DM, et al. Interstitial lung abnormalities in treatment-naïve advanced non-small-cell lung cancer patients are associated with shorter survival. Eur J Radiol. 2015;84:998–1004. doi: 10.1016/j.ejrad.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias: this joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 27.Hansell DM, Goldin JG, King TE, Jr, Lynch DA, Richeldi L, Wells AU. CT staging and monitoring of fibrotic interstitial lung diseases in clinical practice and treatment trials: a position paper from the Fleischner Society. Lancet Respir Med. 2015;3:483–496. doi: 10.1016/S2213-2600(15)00096-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.