To the Editor:
Excessive collagen deposition is a hallmark of idiopathic pulmonary fibrosis (IPF). Although conventional imaging modalities, such as computed tomography (CT), can visualize the end results of collagen deposition, including reticular opacities and honeycombing, direct collagen visualization currently requires histopathology. We developed a positron emission tomography (PET) radiotracer, termed 68Ga-CBP8, that binds specifically to type I collagen (1). In a mouse model of bleomycin-induced lung injury, we showed that 68Ga-CBP8 can detect and quantify the degree of pulmonary fibrosis, and in a second mouse model, we showed that 68Ga-CBP8 PET can measure treatment response with an anti-αvβ6 antibody. Additionally, 68Ga-CBP8 signal was highly correlated with the amount of collagen in explanted human IPF lungs as determined by the percentage of Sirius Red staining. Here, we present the results of the first-in-human studies using 68Ga-CBP8 to assess safety and tracer distribution in healthy volunteers, and the ability to noninvasively measure increased lung collagen in subjects with IPF. Some of the results of these studies have been previously reported in the form of an abstract (2).
Methods
This study was approved by the Partners Institutional Review Board and registered at clinicaltrials.gov (identifier: NCT03535545). All of the subjects provided informed consent. Healthy volunteers were without pulmonary disease, and all but one were nonsmokers (after completion of the study, one subject was found to have a history of tobacco use). Subjects with IPF had not used tobacco within the past 6 months. All subjects were excluded for contraindications to undergoing magnetic resonance imaging, respiratory infection within the prior 6 weeks, and prior radiation therapy to the thorax.
68Ga-CBP8 PET and magnetic resonance imaging were performed simultaneously using the Siemens Biograph mMR scanner (Siemens Healthineers). Healthy volunteers underwent whole-body scanning, and subjects with IPF underwent scanning of the thorax. The subjects were monitored continuously during the imaging study and contacted the following day to assess for any adverse effects. Approximately 250 MBq (range 156–433 MBq) of 68Ga-CBP8 was administered. Emission data were acquired for up to 2 hours in listmode format. Attenuation correction was performed using the manufacturer’s MR-based method. Images corresponding to dynamic frames were reconstructed using the standard reconstruction algorithm (OSEM, 21 subsets, three iterations, 256 × 256 matrix size, 127 slices) and smoothed with a 4-mm full width at half maximum Gaussian filter. Standardized uptake values (SUVs) were obtained from regions of interest defined by segmenting each lung into three equal parts in the axial direction and dividing each part into subpleural (the outer 1.8 cm of the lung) and central regions. We averaged SUVs from the left and right lungs at each measurement. Statistical analysis was performed using the Mann-Whitney U test (Prism 6.0, GraphPad Software), with P value < 0.05 considered significant. Data are reported as mean ± SD or median (range) as appropriate. PET images were constructed for visualization of collagen tracer signal intensity and compared with CT of the chest performed for clinical indications in the subjects with IPF.
Results
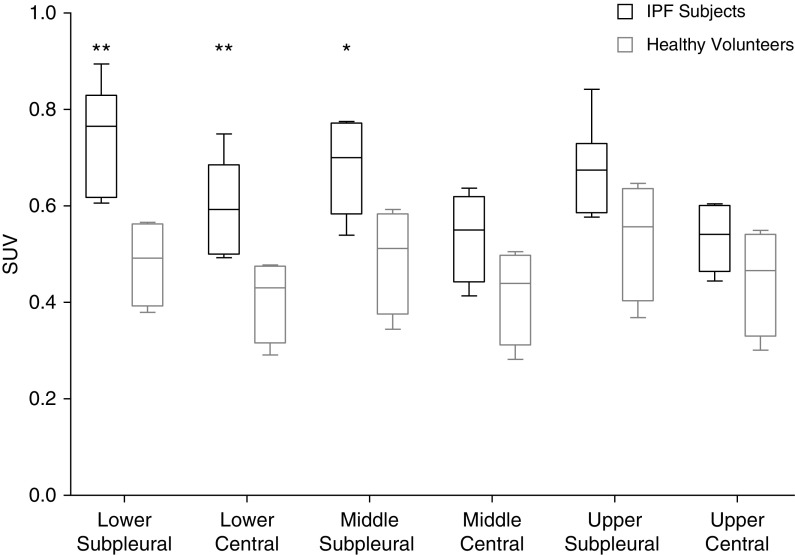
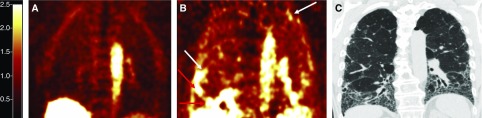
We imaged five healthy volunteers (three males and two females, age 62.2 ± 7.8 yr) and nine subjects with IPF (six males and three females, age 72.7 ± 6.1 yr). Eight of the nine subjects with IPF were on antifibrotic therapy (pirfenidone or nintedanib). All of the subjects tolerated 68Ga-CBP8 without difficulty and with no unexpected adverse effects. One healthy volunteer (intravenous extravasation) and one subject with IPF (MR-based attenuation correction map failed) were excluded from SUV analyses. 68Ga-CBP8 displayed rapid renal clearance with low background uptake in the lungs of healthy volunteers. Whole-lung SUVs were increased in subjects with IPF compared with healthy volunteers when measured 1 hour after injection (0.65 [0.51–0.72] vs. 0.48 [0.33–0.56], P = 0.048). Assessment for regional differences showed that SUVs for subjects with IPF were significantly increased in the middle subpleural and lower lung regions (Figure 1). In contrast to healthy volunteers, there was a trend toward the collagen tracer signal being more heterogeneous in subjects with IPF (coefficient of variation 15.4% [12.6–20.4] vs. 11.2% [9.3–17.3], P = 0.1). High collagen tracer signal was seen in fibrotic lung regions determined by chest CT and also in regions where the lung appeared to be normal on CT (Figure 2).
Figure 1.
68Ga-CBP8 standardized uptake values (SUVs). Data are reported as box-and-whisker plots. *P < 0.05 and **P < 0.01 for subjects with idiopathic pulmonary fibrosis (IPF) compared with healthy volunteers.
Figure 2.
Collagen-targeted molecular imaging using 68Ga-CBP8 positron emission tomography (PET). (A) Coronal PET image of a healthy volunteer, demonstrating low collagen tracer signal in the lungs. (B) PET image of a subject with idiopathic pulmonary fibrosis rendered on the same scale as in A, demonstrating areas of high collagen tracer signal predominantly in subpleural and basilar regions in the lungs. (C) High-resolution computed tomography (CT) of the same subject with idiopathic pulmonary fibrosis obtained within 8 weeks of PET–magnetic resonance imaging. Compared with the PET imaging (B), increased collagen tracer signal is not confined to areas of fibrosis visualized on CT. White arrows highlight regions of high tracer signal within areas of lung without apparent fibrosis on CT. Red arrows highlight regions of high tracer signal within areas of established fibrosis on CT.
Discussion
We successfully performed the first noninvasive direct visualization of type I collagen in humans using 68Ga-CBP8 PET. 68Ga-CBP8 is safe and well tolerated, with favorable tracer characteristics. 68Ga-CBP8 signal was increased in the lungs of subjects with IPF compared with healthy volunteers. The increased collagen tracer signal in IPF displayed a lower lung and subpleural predominance consistent with the anatomic distribution of IPF. Our findings add to a growing literature on the use of molecular imaging for noninvasive characterization and anatomic localization of fibrosis-related processes (3–6) and the use of PET for pulmonary fibrosis imaging (7–10).
Type I collagen is a key structural protein of the extracellular matrix. Excessive extracellular matrix accumulation is a pathogenic feature of fibrosis (11). 68Ga-CBP8 binds type I human collagen with a Kd of 2.1 μM (1). Preclinical data suggest that 68Ga-CBP8 may be more sensitive to recently synthesized collagen than to established collagen. Although the 68Ga-CBP8 signal linearly correlated with lung collagen in pulmonary fibrosis models, it was much lower in skin and bone even though both tissues had an abundance of type I collagen. Furthermore, within explanted IPF lung tissue, there was less 68Ga-CBP8 uptake in end-stage honeycomb cysts than in less advanced fibrotic areas. As collagen becomes more organized into fibers, it provides fewer binding sites for 68Ga-CBP8 than freshly synthesized and disorganized collagen.
We detected increased collagen tracer signal within areas of known fibrosis and regions where fibrosis was not apparent by CT. These preliminary results suggest that areas of high collagen tracer signal represent active or recent collagen synthesis and that 68Ga-CBP8 PET may detect active collagen deposition that is not yet visible, thus serving as a viable disease activity measure. Although we did not validate our results histologically or confirm 68Ga-CBP8’s specificity for collagen in this study, we have strong data from two mouse models of pulmonary fibrosis and explanted IPF lungs demonstrating that 68Ga-CBP8 accurately and specifically detects the presence of collagen (1). Additional studies are needed to determine whether 68Ga-CBP8 can predict IPF disease progression and detect early response to antifibrotic therapies, and how 68Ga-CBP8 compares to other PET tracers, such as 18F-fluorodeoxyglucose, when applied to pulmonary fibrosis. Given that type I collagen is a hallmark of fibrosis across organ systems, 68Ga-CBP8 may have wide-ranging clinical applicability for fibrosis imaging.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Dr. Leo Ginns, Stephanie Koo, Sunakshi Paul, and the entire PET and MRI team of the Athinoula A. Martinos Center for Biomedical Imaging for their assistance.
Footnotes
Supported by NIH grant R01HL131907 (P.C. and M.L.). S.B.M. is supported by grants from the Francis Family Foundation and the Scleroderma Foundation.
Author Contributions: S.B.M., P.D., M.L., P.C., and C.C. designed the study. S.B.M., D.I.-G., E.A., L.L.L., S.D., R.S., P.C., and C.C. performed data collection, analysis, and/or interpretation. S.B.M. and C.C. performed statistical analyses, had full access to all of the data, and were responsible for the decision to submit for publication. S.B.M. drafted the manuscript. All authors contributed to a critical review of the manuscript and provided final approval for submission.
Originally Published in Press as DOI: 10.1164/rccm.201903-0503LE on June 4, 2019
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Désogère P, Tapias LF, Hariri LP, Rotile NJ, Rietz TA, Probst CK, et al. Type I collagen-targeted PET probe for pulmonary fibrosis detection and staging in preclinical models. Sci Transl Med. 2017;9:eaaf4696. doi: 10.1126/scitranslmed.aaf4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montesi SB, Izquierdo-Garcia D, Abston E, Désogère P, Digumarthy SR, Seethamraju R, et al. Collagen-targeted PET imaging in idiopathic pulmonary fibrosis: first-in-human studies [abstract] Am J Respir Crit Care Med. 2019;199:A7349. doi: 10.1164/rccm.201903-0503LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montesi SB, Rao R, Liang LL, Goulart HE, Sharma A, Digumarthy SR, et al. Gadofosveset-enhanced lung magnetic resonance imaging to detect ongoing vascular leak in pulmonary fibrosis. Eur Respir J. 2018;51:1800171. doi: 10.1183/13993003.00171-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montesi SB, Désogère P, Fuchs BC, Caravan P. Molecular imaging of fibrosis: recent advances and future directions. J Clin Invest. 2019;129:24–33. doi: 10.1172/JCI122132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Désogère P, Montesi SB, Caravan P. Molecular probes for imaging fibrosis and fibrogenesis. Chemistry. 2019;25:1128–1141. doi: 10.1002/chem.201801578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.John AE, Luckett JC, Tatler AL, Awais RO, Desai A, Habgood A, et al. Preclinical SPECT/CT imaging of αvβ6 integrins for molecular stratification of idiopathic pulmonary fibrosis. J Nucl Med. 2013;54:2146–2152. doi: 10.2967/jnumed.113.120592. [DOI] [PubMed] [Google Scholar]
- 7.Ambrosini V, Zompatori M, De Luca F, Antonia D, Allegri V, Nanni C, et al. 68Ga-DOTANOC PET/CT allows somatostatin receptor imaging in idiopathic pulmonary fibrosis: preliminary results. J Nucl Med. 2010;51:1950–1955. doi: 10.2967/jnumed.110.079962. [DOI] [PubMed] [Google Scholar]
- 8.Win T, Thomas BA, Lambrou T, Hutton BF, Screaton NJ, Porter JC, et al. Areas of normal pulmonary parenchyma on HRCT exhibit increased FDG PET signal in IPF patients. Eur J Nucl Med Mol Imaging. 2014;41:337–342. doi: 10.1007/s00259-013-2514-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Withana NP, Ma X, McGuire HM, Verdoes M, van der Linden WA, Ofori LO, et al. Non-invasive imaging of idiopathic pulmonary fibrosis using cathepsin protease probes. Sci Rep. 2016;6:19755. doi: 10.1038/srep19755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Win T, Screaton NJ, Porter JC, Ganeshan B, Maher TM, Fraioli F, et al. Pulmonary 18F-FDG uptake helps refine current risk stratification in idiopathic pulmonary fibrosis (IPF) Eur J Nucl Med Mol Imaging. 2018;45:806–815. doi: 10.1007/s00259-017-3917-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.