A common gain-of-function promoter polymorphism rs35705950 (G major allele, T minor allele) in the airway mucin MUC5B is the strongest and most replicated genetic risk factor for idiopathic pulmonary fibrosis (IPF) (1). It has been demonstrated that the MUC5B promoter variant affects MUC5B expression in the distal airways in IPF (2), and that Muc5b overexpression causes mucociliary dysfunction and enhances lung fibrosis in mice (3). However, less is known about the role this variant, located in a highly conserved region of the MUC5B promoter −3.5 kb upstream of the transcription start site (1), plays in transcriptional regulation of MUC5B. Publically available data through the Encyclopedia of DNA Elements (ENCODE) suggest this is a complex area of the genome with many transcription factors showing evidence of binding in the −3.5 kb region of the MUC5B promoter, in addition to the −0.1 kb proximal promoter (4). Another more general question in the field of mucin biology is that of selective mechanisms that differentially regulate MUC5B and its close neighbor and relative MUC5AC. Studies in recent years have shown that the transcription factors SPDEF (SAM pointed domain-containing ETS transcription factor) (5), NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) (6, 7), and FOXA2 (Forkhead box protein A2) (4, 8) bind to both MUC5B and MUC5AC promoters, regulate their gene expression, and hence, lack the specificity needed to differentially regulate these two mucins (although FOXA3 is known to regulate MUC5AC specifically in Th2-dependent manner).
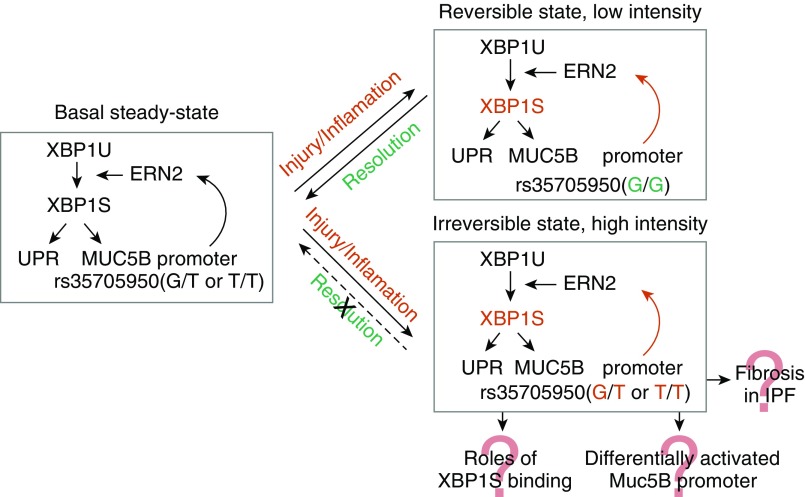
In this issue of the Journal, Chen and colleagues (pp. 220–234) identified a novel pathway that selectively regulates MUC5B, but not MUC5AC, expression in the distal airways (9). In their elegant and comprehensive report, they demonstrated three important findings. First, the endoplasmic reticulum to nucleus signaling 2 (ERN2) selectively promotes expression of the MUC5B mucin in distal airways via its downstream effector, the spliced form of the XBP1S (X-box–binding protein 1) transcription factor. In a series of meticulous experiments, the authors used human IPF tissue, in vivo animal models, and primary cells to elucidate the role of the spliced form of XBP1 in regulation of MUC5B, but not MUC5AC, expression in response to stimulation with cytokine IL1β. Among other findings, they show that there is a strong correlation of XBP1S and MUC5B mRNA on IL1β treatment, but not at baseline, whereas correlation of MUC5AC and XBP1S is weak at baseline and after treatment with IL1β. Second, XBP1S differentially regulates MUC5B promoter variant activity. Chen and colleagues report that induction of MUC5B(T) by XBP1S is greater than MUC5B(G) at all times tested by luciferase reporter activity. Finally, importantly, they also showed that pharmacologic inhibition and genetic deletion of ERN2-XBP1S reduced MUC5B expression. Inhibiting the ERN kinase had a moderate inhibitory effect, and deletion of XBP1 had a strong MUC5B inhibitory effect on expression levels. Higher levels of ERN2 and XBP1S were also observed in patients with IPF, and the results open potential avenues for novel therapeutic strategies using these observations. Using all data they collected, Chen and colleagues propose a “bistable model,” which is a positive feedback loop by ERN2-XBP1S that explains accumulation of mucus in IPF (Figure 1). This model exhibits both a reversible state (low stimulus) and an irreversible state (high stimulus). In response to insults that produce injury/or inflammation that accelerates MUC5B transcription, ER stress is induced, ERN2 is activated, and spliced XBP1 increases UPR gene and MUC5B transcription rates. This response is reversible on removal of the injury/cytokine stimulus. However, the presence of the MUC5B promoter minor allele amplifies XBP1S-induced MUC5B transcription, producing an irreversible positive feedback state that may be sufficient to trigger impaired host defense and accelerate cell senescence and/or damage.
Figure 1.
A bistable model of ERN2 (endoplasmic reticulum to nucleus signaling 2)/spliced form of XBP1S (X-box–binding protein 1)-mediated regulation of MUC5B and its promoter variant in distal airway epithelia of idiopathic pulmonary fibrosis (IPF). Adapted from Chen and colleagues (9).
The report by Chen and colleagues is a major step forward in understanding the selective regulation of MUC5B expression levels in distal airways. The direct role of the spliced XBP1 on the MUC5B promoter, and especially the IPF-associated variant, further suggests that the described pathway is closely linked to disease pathogenesis. This is especially significant, as it highlights, for the first time, the role for ER stress in the airway epithelium in pathogenesis of IPF, in addition to previous reports in the alveolar epithelium (10). Overall, there is strong evidence presented for selective regulation of MUC5B, but not MUC5AC, as well as evidence for localization of the effect to the distal airway, which is of special importance in the context of IPF.
Naturally, the exciting results by Chen and colleagues raise many more questions. Although the evidence for selective regulation of MUC5B in the distal airways presented in the report is overwhelming, experimental evidence for the specific role of the rs35705950 variant is limited. In fact, the authors show that XBP1S binds predominantly to the proximal −0.1 kb promoter, and to a much lesser extent to the −3.5 kb region of the MUC5B promoter. Future work is needed to delineate respective roles of XBP1S and other transcription factors in binding this area of the promoter, as well as the effect of the variant on this binding. Furthermore, other regulatory mechanisms such as DNA methylation and histone modification will need to be taken into account to fully understand regulation of MUC5B in the distal airway. Another important area of future investigation will be understanding the role of XBP1S in pathogenesis of IPF by using animal and cell models of lung fibrosis. Importantly, the seminal study by Chen and colleagues opens up an entire line of investigation that should bring us closer to understanding regulation of MUC5B expression in IPF lung, fully elucidate the link of MUC5B overexpression and ER stress, and provide novel therapeutic options for this devastating disease with limited treatment options.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201904-0809ED on May 2, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakano Y, Yang IV, Walts AD, Watson AM, Helling BA, Fletcher AA, et al. Muc5b promoter variant rs35705950 affects muc5b expression in the distal airways in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016;193:464–466. doi: 10.1164/rccm.201509-1872LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hancock LA, Hennessy CE, Solomon GM, Dobrinskikh E, Estrella A, Hara N, et al. Muc5b overexpression causes mucociliary dysfunction and enhances lung fibrosis in mice. Nat Commun. 2018;9:5363. doi: 10.1038/s41467-018-07768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helling BA, Gerber AN, Kadiyala V, Sasse SK, Pedersen BS, Sparks L, et al. Regulation of muc5b expression in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2017;57:91–99. doi: 10.1165/rcmb.2017-0046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo M, Tomoshige K, Meister M, Muley T, Fukazawa T, Tsuchiya T, et al. Gene signature driving invasive mucinous adenocarcinoma of the lung. EMBO Mol Med. 2017;9:462–481. doi: 10.15252/emmm.201606711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujisawa T, Chang MM, Velichko S, Thai P, Hung LY, Huang F, et al. NF-κB mediates IL-1β- and IL-17A-induced MUC5B expression in airway epithelial cells. Am J Respir Cell Mol Biol. 2011;45:246–252. doi: 10.1165/rcmb.2009-0313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujisawa T, Velichko S, Thai P, Hung LY, Huang F, Wu R. Regulation of airway MUC5AC expression by IL-1beta and IL-17A; the NF-kappaB paradigm. J Immunol. 2009;183:6236–6243. doi: 10.4049/jimmunol.0900614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young HW, Williams OW, Chandra D, Bellinghausen LK, Pérez G, Suárez A, et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am J Respir Cell Mol Biol. 2007;37:273–290. doi: 10.1165/rcmb.2005-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G, Ribeiro CMP, Sun L, Okuda K, Kato T, Gilmore RC, et al. XBP1S regulates MUC5B in a promoter variant–dependent pathway in idiopathic pulmonary fibrosis airway epithelia. Am J Respir Crit Care Med. 2019;200:220–234. doi: 10.1164/rccm.201810-1972OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanjore H, Cheng DS, Degryse AL, Zoz DF, Abdolrasulnia R, Lawson WE, et al. Alveolar epithelial cells undergo epithelial-to-mesenchymal transition in response to endoplasmic reticulum stress. J Biol Chem. 2011;286:30972–30980. doi: 10.1074/jbc.M110.181164. [DOI] [PMC free article] [PubMed] [Google Scholar]