Abstract
Background
Malnutrition, commonly observed in inflammatory bowel disease (IBD), is associated with increased morbidity and mortality and is attributed to multiple causes. The added energy costs of growth in the child and adolescent with IBD are an additional risk factor.
Methods
The aim of the study was to perform a cross-sectional comparison of nutritional parameters in IBD between pediatric and adult cases.
Results
We found that prevalence of undernutrition (low body mass index) and hypoalbuminemia was not different in pediatric, compared with adult patients. Anemia and iron deficiency were more often observed in pediatric subjects, compared with adults (59.1% vs 36.9%, respectively, P < 0.0001; and 37.9% vs 25.3%, P < 0.002). Vitamin B12 deficiency was significantly less common in the pediatric than in the adult group (5.4% vs 19.4%, P < 0.0001). Elevated C-reactive protein was more frequent in pediatric compared with adult cases (49.8% vs 38.4%, P < 0.01).
Conclusions
Patients with active Crohn’s disease were more likely to be undernourished in both pediatric and adult populations. In both groups, predicators of undernutrition included low albumin levels (odds ratio [OR], 2.53; P < 0.006) and active disease (OR, 1.99; P < 0.03). Our results call for close surveillance of nutritional status for IBD patients, regardless of age.
Keywords: inflammatory bowel disease, malnutrition, anemia, Crohn’s disease, ulcerative colitis
INTRODUCTION
Inflammatory bowel diseases (IBDs), comprising Crohn’s disease (CD), ulcerative colitis (UC), and unclassified colitis (IBDU), are chronic relapsing-remitting, immune-mediated disorders of the gastrointestinal tract. Malnutrition is observed in patients with IBD and is associated with increased morbidity and mortality.1 It has been reported that malnutrition is present in up to 85% of patients with IBD and that weight loss occurs in up to 80% of CD patients and 18%–62% of UC patients.2 In CD, the prevalence and degree of malnutrition are influenced by the extent and severity of the disease.3 Although it has been often reported that patients with CD are at higher risk of malnutrition than those with UC, other studies have demonstrated that nutritional deficits occur to a similar degree.4
The most important factor associated with malnutrition in IBD is inadequate dietary intake, which can be secondary to avoidance due to exacerbation of symptoms when eating, exclusion of certain foods, or an impaired sense of taste.4, 5 Excessive levels of inflammatory mediators, such as tumor necrosis factor–α, interleukin-1, and interleukin-6, increase catabolism and lead to anorexia.6 Malnutrition in IBD can also be the result of increased energy requirements,7 malabsorption of nutrients, gastrointestinal losses,8 dietary intake,9 food avoidance,5,10 and drug–nutrient interactions.11
One of the most common complications of IBD is anemia, with a prevalence of up to 74%,12 often due to the combination of iron (Fe) deficiency and anemia from chronic disease. In some cases, anemia can be drug-induced (mesalazine, sulfasalazine, thiopurines) or due to folate or vitamin B12 deficiency. Fe deficiency in IBD is primarily caused by chronic gastrointestinal tract blood loss. However, decreased absorption may also play a role in patients with active CD involving the proximal small bowel.13 The prevalence of Fe deficiency ranges from 36% to 90%, depending on the criteria used for the definition and cohort selection.14, 15
Inflammatory bowel disease patients may also present a variety of vitamin deficiencies as a consequence of the disease per se or of reduced dietary intake.16, 17 Vitamin B12 and folate deficiency are also relatively common in CD, especially in patients with active disease.18 This can be caused by malabsorption or surgery, low-fiber diets, or antifolate drugs such as methotrexate and sulfasalazine.18, 19 Cobalamin deficiency is more common in CD patients who have undergone ileal resections of 20 cm or more.20
Malnutrition may have serious consequences for IBD patients. Calorie–protein malnutrition is associated with impaired humoral and cellular immunity, which adversely affects the mucosal barrier. It has also been associated with deterioration in muscle and respiratory and immune functions, delayed wound healing, and postoperative complications.21 The added energy costs of growth in the child and adolescent with IBD are an additional risk factor. Weight loss is present at diagnosis in up to 90% of children,22 and growth failure is one of the most frequent extraintestinal manifestations seen in pediatric-onset disease.23 Indeed, growth retardation at diagnosis has been reported in 23%–88% of CD children and may precede the gastrointestinal manifestations by years.24
Several studies have evaluated nutritional status in IBD patients, but few have compared malnutrition between children and adults. The aim of this study was to perform a comparative analysis of nutritional parameters between pediatric and adult patients with IBD.
METHODS
Patient Cohorts
We performed a cross-sectional comparison of nutritional status in pediatric and adult patients with IBD, grouped as CD, UC, or IBDU, according to the Montreal classification.25 Subjects aged 16 years and under were classified as pediatric IBD.25 In cases where the age of a subject overlapped the 2 categories over different visits, the average age was used to classify the patient as pediatric or adult. Patients were recruited from the 4 principal tertiary medical centers of the McGill Faculty of Medicine (Montreal Children’s Hospital, Montreal General Hospital, Royal Victoria Hospital, Jewish General Hospital) over a 2-year period (2008–2010). Clinical and biochemical parameters at each visit were collected using the McGill IBD database. Parameters surveyed included body mass index (BMI), hemoglobin (Hb), serum Fe, vitamin B12, folate, albumin, and C-reactive protein (CRP). The average BMI of each visit was calculated. Disease activity was determined at each clinical visit by the Harvey-Bradshaw Index (HBI) for CD26 or the Lichtiger Index (LI) for UC and IBDU.27 The disease was considered active when the HBI was >4 or the LI was >2. Data on medications taken and usage of enteral nutritional therapy, nutritional supplements, and vitamins were also collected.
Assessment of Undernutrition and Height Deficit
Body mass index was assessed at each visit as weight (kg) divided by squared height (m2). Severe undernutrition was defined based on World Health Organization (WHO) criteria: BMI z-score for age >2 SDs below the mean in patients <20 years of age,28 or a BMI <18.5 in adults age 20–64 years,28 and a BMI <20 in adults >65.29 Mild undernutrition was defined by BMI z-score for age <1.5 SDs in patients <20 years of age,28 or a BMI <19.5 in adults age 20–64 years,30 and a BMI <22 in adults >65.29 Patients meeting these criteria at a minimum of 1 visit during their follow-up were classified with severe or mild undernutrition.
In parallel, we determined the height deficit of children by computing the z-scores for height-for-age (5 to 19 years old) and for weight-for-height (<5 years old) using the WHO charts.28 Growth was classified as normal (>1.5 SDs), with moderate deficit (≤1.5 SDs and >2 SDs), and severe deficit (≤2 SDs). Children with abnormal criteria at a minimum of 1 visit during their follow-up visit were classified as having moderate or severe growth retardation.
Assessment of Nutritional Status Using Biochemical Parameters
Abnormal micronutrient status was defined by age-adjusted values below normal cutoffs. Levels of serum albumin, Hb, Fe, vitamin B12, folate, and CRP were determined at each visit. Hypoalbuminemia was defined according to age: 0–1 year: <30 g/L; 1–10 years: <39 g/L; 10–18 years: <41 g/L; 18 y and over: <38 g/L.4,31 Hb levels were evaluated according to age and sex. The following cutoff values were used: 1–5 years: <110 g/L; 6–12 years: <120 g/L; male 12–17 years: <130 g/L; female 12–17 years: <120 g/L; male 18 and over: <140 g/L; female 18 and over: <120 g/L.32 For all subjects, serum Fe concentrations were classified as low when <10 μmol/L.33 Vitamin B12 levels <148 pmol/L34 and/or the need for ongoing B12 supplementation indicated B12 deficiency. Folate levels were characterized as low when inferior to 7 nmol/L in serum, or 317 nmol/L in red blood cells.35, 36 CRP levels were elevated when >5 mg/L in children37 and >10 mg/L in adults.38 Patients meeting these criteria at a minimum of 1 visit during their follow-up were classified as having abnormal levels of the specific biochemical parameter.
Measurement of Antioxidant Vitamins
To examine the levels of plasma antioxidant vitamins (retinol, β-carotene, α-tocopherol, and γ-tocopherol), blood samples were collected in tubes containing 1 g EDTA/L. Plasma was separated immediately by centrifugation (700g for 20 minutes at 4°C), and the vitamin levels of 48 CD adult patients were determined using an improved method previously described by our team.39
Essential Fatty Acid Deficiency
To detect essential fatty acid (EFA) deficiencies in 48 adult CD patients, blood samples were collected in tubes containing 1 g EDTA/L, and plasma was separated immediately by centrifugation (700g for 20 minutes at 4°C). For trans-esterification, an internal standard consisting of 14.952 µg of nonadecenoic acid (C19:1) was precisely weighed, dissolved in 2 mL of methanol-hexane (4:1, v:v), and mixed with 100 µL of cell homogenate. Then 200 µL of acetyl chloride was added, and each tube was subjected to methanolysis at 100°C for 1 hour. After tubes had been cooled, 5 mL of 6% K2CO3 solution was added to stop the reaction and neutralize the mixture. The tubes were then shaken and centrifuged, and an aliquot of the hexane upper phase was injected into the chromatograph, as previously described,40 using the Varian 8400 GC Autosampler system (Cole-Parmer, Vernon Hills, IL). The fatty acids were identified by comparison with the expected retention times of known standards and were analyzed with Galaxie Chromatography Data System software (Varian Inc., Palo Alto, CA, USA). To assess EFA status, we used the 16:1ω-7/18:1ω-6 and 20:ω-9/20:4ω-6 ratios.41
Statistical Analysis
We used Prism 5 for Mac OS X (GraphPad Software, Inc, La Jolla, CA, USA) and SPSS (version 24, IBM Analytics, Armonk, NY, USA) for the statistical analyses. Results were considered statistically different at P < 0.05. Data are expressed as mean ± SD, median, prevalence (No.), or percentage. For patients’ descriptive characteristics (continuous variables), differences in means between groups were assessed using the Mann-Whitney t test. Differences in the prevalence of undernutrition and abnormal biochemical parameters assessing nutritional status between groups (categorical variables) were analyzed using the chi-square (χ2) test. If criteria for the use of the χ2 test were not met, the Fisher exact test was used. Comparisons were made between the pediatric and adult cohorts, and, in each subgroup, between CD, UC, and IBDU, and between males and females. To determine predictors of undernutrition in CD, we used a logistic regression model including all variables. Odds ratios (ORs) and corresponding 95% confidence intervals (95% CIs) were estimated.
Ethical Considerations
Consent was obtained from the participants, and the ethics committee of the McGill University Health Center approved the study.
RESULTS
Characteristics of Patients
In total, 590 patients with IBD (203 pediatric and 387 adult) were evaluated over the study period. The cohort was composed of patients seen for follow-up outpatient visits. Descriptive characteristics are presented in Table 1. The mean age at visit was 13.9 ± 2.8 years and 28.7 ± 13.2 years for pediatric and adult cases, respectively. Overall, there was no difference in the proportion of males and females in the pediatric and adult cohorts. Because patients were grouped according to their mean age at visit over the study period, some started the study as pediatric subjects but were, in the end, classified as adults. A total of 11 men (all CD) and 14 women (13 CD and 1 IBDU) overlapped the 2 categories.
TABLE 1.
Characteristics of Patients
|
All Children |
All Adults | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Total | CD | UC | IBDU | UC+IBDU | Total | CD | UC | IBDU | UC+IBDU |
| Mean ± SD | Mean ± SD | |||||||||
| Subjects, No. (%) | 203 | 179 (88.2) | 4 (2.0) | 20 (9.9) | 24 (11.8) | 387 | 274 (70.8)c | 97 (25.1)c | 16 (4.1)a | 113 (29.2)c |
| Visits, No. | 5.6 ± 2.8 | 5.9 ± 2.7 | 2.8 ± 2.2g | 3.7 ± 2.3h | 3.5 ± 2.3g | 3.5 ± 3.0c | 4.0 ± 3.0c | 2.3 ± 2.7i | 2.9 ± 2.8 | 2.4 ± 2.7i |
| Age at visit, y | 13.9 ± 2.8 | 13.9 ± 2.7 | 14.9 ± 2.7 | 13.8 ± 3.9 | 13.9 ± 3.8 | 28.7 ± 13.2c | 27.4 ± 12.4c | 35.8 ± 15.3a,i | 27.3 ± 10.9c,j | 34.3 ± 15.0c,i |
| Age at diagnosis, y | 11.4 ± 3.5 | 11.6 ± 3.2 | 12.9 ± 3.6 | 9.2 ± 4.8h | 10.0 ± 4.8g | 23.4 ± 13.1c | 20.9 ± 11.3c | 30.7 ± 15.5a,i | 21.9 ± 10.7c,j | 29.4 ± 15.2c,i |
| Follow-up duration, y | 2.8 ± 2.2 | 2.8 ± 2.2 | 1.4 ± 1.1 | 3.83 ± 2.78g | 3.3 ± 2.7 | 9.8 ± 8.7c | 9.8 ± 8.5c | 10.0 ± 9.7 | 7.0 ± 6.7 | 9.6 ± 9.4a |
| BMI, kg/m2 | 19.9 ± 4.0 | 19.8 ± 3.9 | 23.0 ± 5.6 | 21.6 ± 4.8 | 21.8 ± 4.9g | 23.1 ± 4.3c | 23.0 ± 4.3 | 23.5 ± 4.7 | 22.8 ± 2.6 | 23.6 ± 4.4 |
| Male Children | Male Adults | |||||||||
| Total | CD | UC | IBDU | UC+IBDU | Total | CD | UC | IBDU | UC+IBDU | |
| Mean ± SD | Mean ± SD | |||||||||
| Subjects, No. (%) | 114 (56.2) | 105 (92.1) | 0 (0) | 9 (7.9) | 9 (7.9) | 195 (50.4) | 141 (72.3)c | 51 (26.2)c | 3 (1.5)a | 54 (27.7)c |
| Visits, No. | 5.6 ± 2.7 | 5.9 ± 2.6 | N/A | 2.2 ± 1.0i | 2.2 ± 1.0i | 3.4 ± 2.9c | 3.9 ± 3.0c | 1.8 ± 1.5i | 4.0 ± 4.4 | 1.9 ± 1.7i |
| Age at visit, y | 14.1 ± 2.5 | 14.2 ± 2.3 | N/A | 9.7 ± 4.7i | 9.7 ± 4.7i | 28.9 ± 14.4c | 27.1 ± 12.9c | 41.1 ± 17.2i | 20.9 ± 7.2a,j | 38.8 ± 17.9c,i |
| Age at diagnosis, y | 11.3 ± 3.6 | 11.8 ± 3.2 | N/A | 3.6 ± 1.5i | 3.6 ± 1.5i | 24.4 ± 13.7c | 21.2 ± 11.5c | 33.6 ± 15.4i | 14.5 ± 5.5b,j | 32.6 ± 15.9c,i |
| Follow-up duration, y | 3.0 ± 2.3 | 3.0 ± 2.3 | N/A | 3.3 ± 3.9 | 3.3 ± 3.9 | 9.2 ± 8.0c | 9.2 ± 8.1c | 9.1 ± 8.0 | 10.0 ± 7.9 | 9.2 ± 7.9a |
| BMI, kg/m2 | 20.1 ± 3.6 | 20.2 ± 3.6 | N/A | 19.3 ± 3.9 | 19.3 ± 3.9 | 23.6 ± 4.6c | 23.2 ± 4.3c | 25.9 ± 6.0h | 24.00 ± 1.8 | 25.6 ± 5.7a,h |
| Female Children | Female Adults | |||||||||
| Total | CD | UC | IBDU | UC+IBDU | Total | CD | UC | IBDU | UC+IBDU | |
| Mean ± SD | Mean ± SD | |||||||||
| Subjects, No. (%) | 89 (43.8) | 74 (83.2) | 4 (4.5) | 11 (12.4) | 15 (16.9) | 192 (49.6) | 133 (69.3)c | 46 (24.0)c | 13 (6.8)d | 59 (30.7)a |
| Visits, No. | 5.6 ± 2.9 | 5.9 ± 2.9 | 2.8 ± 2.2g | 4.8 ± 2.4e | 4.3 ± 2.5g,d | 3.7 ± 3.1c | 4.1 ± 2.9c | 2.9 ± 3.6g,d | 2.7 ± 2.5a | 2.8 ± 3.3h |
| Age at visit, y | 13.6 ± 3.0 | 13.3 ± 3.0 | 14.9 ± 2.7 | 15.3 ± 2.1d,g | 15.2 ± 2.2e,g | 28.6 ± 12.1c | 27.6 ± 11.8c | 32.1 ± 12.7a,d,g | 29.4 ± 11.2b | 30.6 ± 12.4c,d |
| Age at diagnosis, y | 11.5 ± 3.3 | 11.39 ± 3.42 | 12.9 ± 3.6 | 11.8 ± 3.5f | 12.1 ± 3.3f | 22.5 ± 12.4c | 20.7 ± 11.0c | 27.4 ± 15.2d,h | 23.6 ± 11.0a | 26.5 ± 14.4b,d,g |
| Follow-up duration, y | 2.6 ± 2.1 | 2.4 ± 2.0 | 1.4 ± 1.1 | 4.1 ± 2.3g,j | 3.4 ± 2.4 | 10.4 ± 9.3c | 10.5 ± 8.7c | 10.9 ± 11.4 | 6.4 ± 6.5 | 9.9 ± 10.6a |
| BMI, kg/m2 | 19.7 ± 4.4 | 19.3 ± 4.2 | 23.0 ± 5.9 | 22.4 ± 4.9g | 22.5 ± 4.9g | 22.5 ± 3.9c | 22.7 ± 4.2c | 21.8 ± 2.4f | 22.3 ± 2.7 | 22.0 ± 4.5e |
Characteristics of IBD patients grouped as CD, UC, or IBDU according to the Montreal classification. Subjects age 16 years and younger were classified as pediatric and 17 years and older as adults. The average of BMI captured at each visit is presented.
a P < 0.05, bP < 0.001, cP < 0.0001 vs children.
d P < 0.05, eP < 0.001, fP < 0.0001 vs male.
g P < 0.05, hP < 0.001, iP < 0.0001 vs CD.
j P < 0.05 vs UC.
Crohn’s disease was the most prevalent form of IBD in both age groups (88.2% in pediatric and 70.8% in adults). In adults, IBDU was less frequent (4.1% vs 9.8%, P < 0.01) and UC was more common (25.1% vs 2.0%, P < 0.0001). In the adult group, the prevalence of IBDU was lower among males compared with females (1.5% vs 6.8%, P < 0.01). The mean number of visits was greater in the pediatric compared with the adult population (5.6 ± 2.8 vs 3.5 ± 3.0, P < 0.0001) and in the CD compared with the UC group (in children: 5.9 ± 2.7 vs 2.8 ± 2.2, P < 0.02; in adults: 4.0 ± 2.9 vs 2.3 ± 2.7, P < 0.0001). Age at diagnosis was significantly lower in adults with CD than with UC (20.9 ± 11.3 vs 30.7 ± 15.5 years, P < 0.0001), both in males and females. As expected, the duration of follow-up was shorter in pediatric compared with adult patients (2.8 ± 2.2 vs 9.8 ± 8.7 years, P < 0.0001). In both pediatric and adult cohorts, there was no significant difference in BMI between males and females, or between CD, UC, and IBDU. However, the average BMI was lower in children than in adults (19.9 ± 4.0 vs 23.1 ± 4.3, P < 0.0001) (Table 1).
The population was mainly of Caucasian origin (65.8%), followed by Jewish Ashkenazi (19.0%), Middle Eastern (4.6%), Asian (3.9%), African American (3.0%), Jewish Sepharadic (2.7%), Latin American (0.8%), and mixed (0.2%). In the pediatric cohort, 37 cases (18.2%) were included in the database at the time of diagnosis (16 boys, 14.0%; 21 girls, 23.6%). Most of them had a diagnosis of CD (16 boys, 100%; 19 girls, 90.4%), 1 girl was diagnosed with UC (4.8%), and another had IBDU (4.8%). The proportion of newly diagnosed adults was significantly lower: only 10 patients (2.6%, P < 0.0001 vs children; 4 men, 2.1%; 6 women, 3.1%) were included in the database at the time of first visit. This included 6 CD (2.2%; 2 men, 1.4%; 4 women, 3.0%), 2 UC (2 men, 3.9%), and 2 IBDU patients (2 women, 3.4%).
In keeping with the Montreal Classification, subjects age 16 years and under were classified as pediatric and those 17 and over as adult IBD. However, to elucidate the influence of age classifications on results, all analyses were repeated using 18 years as the cutoff for the pediatric age group. The results obtained from this additional analysis did not yield to significantly different findings from the primary analysis.
Prevalence of Undernutrition Based on BMI in Children and Adults
The data on prevalence of undernutrition, based on BMI, for pediatric and adult IBD cohorts are illustrated in Table 2. The overall prevalence of undernutrition was similar in pediatric compared with adult patients (14.3% vs 13.7%), as was the proportion of cases with severe or mild undernutrition. There was a trend toward a higher proportion of mild undernutrition in female pediatric cases compared with female adult cases (12.4% vs 5.7%, P < 0.06) and compared with male children (P < 0.06). We did not observe differences in the prevalence of undernutrition between the CD, UC, and IBDU groups. The prevalence of severe undernutrition, defined according to WHO criteria, was similar between the 2 age groups (6.4% in children and 9.0% in adults).
TABLE 2.
Prevalence of Undernutrition According to BMI
| All Children, No. (%) | All Adults, No. (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Degree of Undernutrition | Total | CD | UC | IBDU | UC+IBDU | Total | CD | UC | IBDU | UC+IBDU |
| (n = 203) | (n = 179) | (n = 4) | (n = 20) | (n = 24) | (n = 387) | (n = 274) | (n = 97) | (n = 16) | (n = 113) | |
| Severe | 13 (6.4) | 13 (7.3) | 0 (0) | 0 (0) | 0 (0) | 35 (9.0) | 25 (9.1) | 8 (8.3) | 2 (12.5) | 10 (8.9) |
| Mild | 16 (7.9) | 15 (8.4) | 1 (25.0) | 0 (0) | 1 (4.2) | 20 (5.2) | 18 (6.6) | 2 (2.1) | 0 (0) | 2 (1.8) |
| Combined | 29 (14.3) | 28 (15.6) | 1 (25.0) | 0 (0) | 1 (4.2) | 53 (13.7) | 41 (15.0) | 10 (10.3) | 2 (12.5) | 12 (10.6) |
| Male Children, No. (%) | Male Adults, No. (%) | |||||||||
| Degree of Undernutrition | Total | CD | UC | IBDU | UC+IBDU | Total | CD | UC | IBDU | UC+IBDU |
| (n = 114) | (n = 105) | (n = 0) | (n = 9) | (n = 9) | (n = 195) | (n = 141) | (n = 51) | (n = 3) | (n = 54) | |
| Severe | 8 (7.0) | 8 (7.6) | N/A | 0 (0) | 0 (0) | 16 (8.2) | 13 (9.2) | 3 (5.9) | 0 (0) | 3 (5.6) |
| Mild | 5 (4.4) | 5 (4.8) | N/A | 0 (0) | 0 (0) | 9 (4.6) | 9 (6.4) | 0 (0) | 0 (0) | 0 (0) |
| Combined | 13 (11.4) | 13 (12.4) | N/A | 0 (0) | 0 (0) | 25 (12.8) | 22 (15.6) | 3 (5.9) | 0 (0) | 3 (5.6) |
| Female Children, No. (%) | Female Adults, No. (%) | |||||||||
| Degree of Undernutrition | Total | CD | UC | IBDU | UC+IBDU | Total | CD | UC | IBDU | UC+IBDU |
| (n = 89) | (n = 74) | (n = 4) | (n = 11) | (n = 15) | (n = 192) | (n = 133) | (n = 46) | (n = 13) | (n = 59) | |
| Severe | 5 (5.6) | 5 (6.8) | 0 (0) | 0 (0) | 0 (0) | 19 (9.9) | 12 (9.0) | 5 (10.9) | 2 (15.4) | 7 (11.9) |
| Mild | 11 (12.4)b | 10 (13.5)c | 1 (25.0) | 0 (0) | 1 (6.7) | 11 (5.7)a | 9 (6.8) | 2 (4.4) | 0 (0) | 2 (3.4) |
| Combined | 16 (18.0) | 15 (20.3) | 1 (25.0) | 0 (0) | 1 (6.7) | 30 (15.6) | 21 (15.8) | 7 (15.2) | 2 (15.4) | 9 (15.3) |
Prevalence of severe, mild, and total undernutrition in pediatric and adult cohorts.
a P < 0.06 vs children.
b P < 0.06, cP < 0.05 vs male.
We carried out further analyses to determine if undernutrition was associated with disease activity. Subjects with active CD were more likely to be undernourished, in both the pediatric (26.3% vs 11.5% with inactive disease, P < 0.016) and adult (21.2% vs 11.8% with inactive disease, P < 0.043) cohorts. This was also observed in adult UC (22.2% of those with active disease vs 5.7% with inactive disease, P < 0.026), but the low number of pediatric UC cases does not allow for drawing conclusions in children. When analyses were performed separately for sex, the significant relationship between disease activity and undernutrition was maintained in female children and in male adults.
In CD, 87 children (48.6%) and 155 adults (56.6%) were receiving treatment with a biological and/or steroid at the time of a visit. The proportion of patients on a steroid alone or both steroid and a biological was not different between children and adults (31.3% vs 26.3% and 13.4% vs 17.1%, respectively). However, the proportion of children treated only with a biological was significantly lower than adults (3.9% vs 13.1%, P < 0.0009). Overall, the number of patients treated with enteral nutrition was low and only comprised of CD patients (children: 2 boys and 1 girl; adults: 2 men).
As the usage of medication can affect BMI, undernutrition data were analyzed according to the administration of steroids and/or biologicals (Table 3). Undernourished CD children were more likely to be treated with biologicals and/or steroids than undernourished CD adults (78.6% vs 53.7%, P < 0.05). Among females with CD, a larger proportion of children than adults were receiving steroid treatment (66.7% vs 19.0%, P < 0.006). Interestingly, in children, the proportion of subjects on medication was higher in the undernourished group (78.6% in undernourished vs 43.0% in well-nourished, P < 0.0008), whereas the percentages of patients with and without undernutrition taking medication were similar in adults (57.1% vs 53.7%). No significant differences were noted between males and females in both the pediatric and adult groups or in UC and IBDU (Supplementary Tables 1–3).
TABLE 3.
Medications in CD Patients According to Nutritional Status
| Medication | All CD Children, No. (%) | All CD Adults, No. (%) | ||
|---|---|---|---|---|
| Undernutrition | Undernutrition | |||
| No (n = 151) | Yes (n = 28) | No (n = 233) | Yes (n = 41) | |
| Biologicals or steroids | 65 (43.0) | 22 (78.6)c | 133 (57.1) | 22 (53.7)a |
| Biologicals | 6 (4.0) | 1 (3.6) | 35 (15.0) | 1 (2.4) |
| Steroids+immunomodulators | 38 (25.2) | 14 (50.0) | 52 (22.3) | 10 (24.4) |
| Steroids only | 4 (2.7) | 0 (0) | 7 (3.0) | 3 (7.3) |
| Biologicals+steroids | 17 (11.3) | 7 (25.0) | 39 (16.7) | 8 (19.5) |
| Medication | Male CD Children, No. (%) | Male CD Adults, No. (%) | ||
| Undernutrition | Undernutrition | |||
| No (n = 92) | Yes (n = 13) | No (n = 119) | Yes (n = 22) | |
| Biologicals or steroids | 42 (45.6) | 9 (69.2) | 61 (51.2) | 14 (63.6) |
| Biologicals | 4 (4.3) | 1 (7.7) | 15 (12.6) | 0 (0) |
| Steroids+immunomodulators | 25 (27.2) | 4 (30.8) | 21 (17.6) | 8 (36.4) |
| Steroids only | 3 (3.3) | 0 (0) | 5 (4.2) | 1 (4.5) |
| Biologicals+steroids | 10 (10.9) | 4 (30.8) | 20 (16.8) | 5 (22.7) |
| Medication | Female CD Children, No. (%) | Female CD Adults, No. (%) | ||
| Undernutrition | Undernutrition | |||
| No (n = 59) | Yes (n = 15) | No (n = 112) | Yes (n = 21) | |
| Biologicals or steroids | 23 (39.0) | 13 (86.7) | 72 (64.3) | 8 (38.1)b |
| Biologicals | 2 (3.4) | 0 (0) | 20 (17.9) | 1 (4.8) |
| Steroids+immunomodulators | 13 (22.0) | 10 (66.7)a | 31 (27.7) | 2 (9.5)b |
| Steroids only | 1 (1.7) | 0 (0) | 2 (1.8) | 2 (9.5) |
| Biologicals+steroids | 7 (11.9) | 3 (20.0) | 19 (17.0) | 3 (14.3) |
Prevalence of CD patients on steroids and/or biological treatments according to their nutritional status.
a P < 0.05, bP < 0.01 vs children.
c P < 0.0008 vs well-nourished.
Prevalence of Growth Retardation in Children
We found that 14.3% of children presented with growth retardation (moderate and severe), which was the same prevalence as undernutrition. Although most patients had both conditions, 4.4% had either growth retardation or undernutrition. However, no statistical differences were observed between the prevalence of growth retardation and undernutrition when groups were analyzed according to sex and disease (data not shown).
Biochemical Markers
Data on biochemical markers of malnutrition are shown in Table 4. As seen for BMI, the prevalence of hypoalbuminemia was not different between the pediatric and adult IBD groups. The frequency of hypoalbuminemia was higher in CD, compared with subjects with UC and IBDU, for both the pediatric and adult cohorts (in children: 30.7% vs 8.3%, respectively, P < 0.05; in adults: 29.2% vs 9.7%, P < 0.0001). In the adult group, men with UC were less likely to have low levels of serum albumin than women (3.9% vs 17.4%, P < 0.05).
TABLE 4.
Prevalence of Abnormal Biochemical Parameters in Adult and Pediatric IBD
| All Children, No. (%) | All Adults, No. (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | CD | UC | IBDU | UC+IBDU | Total | CD | UC | IBDU | UC+IBDU | |
| (n = 203) | (n = 179) | (n = 4) | (n = 20) | (n = 24) | (n = 387) | (n = 274) | (n = 97) | (n = 16) | (n = 113) | |
| Albumin | 57 (28.1) | 55 (30.7) | 0 (0) | 2 (10.0) | 2 (8.3)e | 91 (23.5) | 80 (29.2) | 10 (10.3)g | 1 (6.2)e | 11 (9.7)g |
| Hemoglobin | 120 (59.1) | 109 (60.9) | 3 (75.0) | 8 (40.0) | 11 (45.8) | 143 (37.0)c | 122 (44.5)b | 18 (18.6)a,i | 3 (18.8) | 21 (18.6)a,g |
| Iron | 77 (37.9) | 72 (40.2) | 0 (0) | 5 (25.0) | 5 (20.8) | 98 (25.3)a | 85 (31.0) | 13 (13.4)f | 0 (0) | 13 (11.5)g |
| B12 | 11 (5.4) | 10 (5.6) | 0 (0) | 1 (5.0) | 1 (4.2) | 75 (19.4)c | 72 (26.3)c | 3 (3.1)g | 0 (0)e | 3 (2.7)g |
| Folate | 6 (3.0) | 6 (3.4) | 0 (0) | 0 (0) | 0 (0) | 10 (2.6) | 9 (3.3) | 1 (1.0) | 0 (0) | 1 (0.9) |
| CRP | 101 (49.8) | 99 (55.3) | 0 (0) | 2 (10.0)f | 2 (8.3)g | 149 (38.5)a | 130 (47.4) | 15 (15.5)g | 4 (25.0) | 19 (16.8)g |
| Male Children, No. (%) | Male Adults, No. (%) | |||||||||
| Total | CD | UC | IBDU | UC+IBDU | Total | CD | UC | IBDU | UC+IBDU | |
| (n = 114) | (n = 105) | (n = 0) | (n = 9) | (n = 9) | (n = 195) | (n = 141) | (n = 51) | (n = 3) | (n = 54) | |
| Albumin | 32 (28.1) | 32 (30.5) | 0 (0) | 0 (0) | 0 (0) | 41 (21.0) | 39 (27.7) | 2 (3.9)f | 0 (0) | 2 (3.7)g |
| Hemoglobin | 68 (59.7) | 65 (61.9) | 0 (0) | 3 (33.3) | 3 (33.3) | 78 (40.0)b | 66 (46.8)a | 11 (21.6)e | 1 (33.3) | 12 (22.2)e |
| Iron | 43 (37.7) | 41 (39.1) | 0 (0) | 2 (22.2) | 2 (22.2) | 48 (24.6)a | 41 (29.1) | 7 (13.7)e | 0 (0) | 7 (13.0)e |
| B12 | 6 (5.3) | 6 (5.7) | 0 (0) | 0 (0) | 0 (0) | 32 (16.4)a | 29 (20.57)b | 3 (5.9)e | 0 (0) | 3 (5.6)e |
| Folate | 5 (4.4) | 5 (4.8) | 0 (0) | 0 (0) | 0 (0) | 5 (2.6) | 5 (3.6) | 0 (0) | 0 (0) | 0 (0) |
| CRP | 59 (51.8) | 58 (55.2) | 0 (0) | 1 (11.1)e | 1 (11.1)e | 74 (38.0)a | 66 (46.8) | 6 (11.8)g | 2 (66.7) | 8 (14.8)f |
| Female Children, No. (%) | Female Adults, No. (%) | |||||||||
| Total | CD | UC | IBDU | UC+IBDU | Total | CD | UC | IBDU | UC+IBDU | |
| (n = 89) | (n = 74) | (n = 4) | (n = 11) | (n = 15) | (n = 192) | (n = 133) | (n = 46) | (n = 13) | (n = 59) | |
| Albumin | 25 (28.1) | 23 (31.1) | 0 (0) | 2 (18.2) | 2 (13.3) | 50 (26.0) | 41 (30.8) | 8 (17.4)d | 1 (7.7) | 9 (15.3)e |
| Hemoglobin | 52 (58.4) | 44 (59.5) | 3 (75.0) | 5 (45.5) | 8 (53.3) | 65 (33.9)c | 56 (42.1)a | 7 (15.2)e | 2 (15.4) | 9 (15.3)a,f |
| Iron | 34 (38.2) | 31 (41.9) | 0 (0) | 3 (27.3) | 3 (20.0) | 50 (26.0)a | 44 (33.1) | 6 (13.0)e | 0 (0) | 6 (10.2)f |
| B12 | 5 (5.6) | 4 (5.4) | 0 (0) | 1 (9.1) | 1 (6.7) | 43 (22.4)b | 43 (32.3)c,d | 0 (0)g | 0 (0)e | 0 (0)g |
| Folate | 1 (1.1) | 1 (1.4) | 0 (0) | 0 (0) | 0 (0) | 5 (2.6) | 4 (3.01) | 1 (2.2) | 0 (0) | 1 (1.7) |
| CRP | 42 (47.2) | 41 (55.4) | 0 (0) | 1 (9.1)e | 1 (6.7)f | 75 (39.1) | 64 (48.1) | 9 (19.6)e | 2 (15.4) | 11 (18.6)f |
Prevalence of abnormal biochemical markers in our IBD cohort. The numbers of patients with low albumin, hemoglobin, iron, vitamin B12, and folate, and with high CRP levels, are shown.
a P < 0.05, bP < 0.001, cP < 0.0001 vs children.
d P < 0.05 vs male.
e P < 0.05, fP < 0.0002, gP < 0.0001 vs CD.
Anemia was observed more frequently in pediatric compared with adult IBD patients (59.1% vs 36.9%, P < 0.0001). This significant difference was also observed for the CD, UC, and UC+IBDU subgroups and in both the male and female cohorts. In adults, the prevalence of anemia was higher in subjects with CD compared with those with UC or UC+IBDU (44.5%, 18.6%, and 18.5%, respectively, P < 0.0001). This was also significant in the adult male and female cohorts but was not observed in the pediatric population. Also, we found that a large proportion of undernourished patients had anemia (92% of children and 80% of adults). However, a considerable proportion of patients who had normal BMI also presented with anemia (41% of children and 61% of adults).
The frequency of low blood Fe was higher in children compared with adults for the total IBD groups (37.9% vs 25.3%, P < 0.0017). This remained significant when the male and female groups were analyzed independently. In adults only, low Fe was more frequent in the CD group compared with the UC and UC+IBDU groups (31.0%, 13.4%, and 11.5%, respectively, P < 0.0007).
We further analyzed the prevalence of anemia based on age at diagnosis (pediatric vs adult) rather than chronological age at the time of testing. Adults with onset of IBD in the pediatric age group were more often anemic than those diagnosed after age 17 (45.3% vs 30.9%, P < 0.005; data not shown). The same outcome was found with the evaluation of low blood Fe levels according to age at diagnosis (31.1% vs 21.2%, P < 0.033; data not shown). When analyzed according to sex, the difference in frequencies for low albumin and Fe according to age at diagnosis remained significant for women only.
Next, we assessed anemia status in relationship with iron supplementation. In CD, the proportion of anemic children taking Fe supplements was 60.6% (53.8% in boys and 70.5% in girls), which was not significantly different from adults (proportion of 45.9%, 42.4% in men and 50.0% in women). Also, most of the subjects taking Fe supplements were diagnosed with low Hb levels at least at 1 visit during their follow-up (85.4% of boys, 79.5% of girls, 75.7% of men, and 90.3% of women).
Vitamin B12 deficiency was less common in the pediatric than the adult groups for CD (5.6% vs 26.3%, P < 0.0001), but not in the UC or IBDU groups. In adults, the proportion of subjects with low B12 was higher in CD compared with UC and IBDU (P < 0.0001). Adult men had a lower prevalence of B12 deficiency than women (20.6% vs 32.3%, P < 0.05). In children with CD, 36.4% of subjects with low B12 levels were receiving B12 supplementation (33.3% in boys and 40.0% in girls), whereas in adults with CD, 52.7% of subjects with low B12 were taking B12 supplements (52.0% men and 53.4% women). These proportions were not significantly different. Among the 3 UC male adult patients who had B12 deficiency, 1 was taking B12 supplements. Relatively few subjects had low folate levels (3.0% in children and 2.7% in adults).
We evaluated the prevalence of high CRP levels as a biomarker of inflammation. Pediatric IBD patients more often had an elevated CRP (49.8% vs 38.4%, P < 0.01). The proportion of patients with an elevated CRP was greater in CD compared with UC and UC+IBDU in children (P < 0.001) and in adults (P < 0.0001). No differences were observed based on sex.
Prevalence of Active Disease
We assessed the proportion of children and adults presenting at a visit with active disease. Among all IBD types, this proportion was not different between children and adults. However, when data were analyzed for CD, there were more adults than children with active disease (41.2% vs 31.8%, P < 0.05). In both groups, males were less likely than females to present with active disease, but the difference was only significant in adults (30.8% vs 44.3%, P < 0.0065). This finding between men and women was also found in CD only (31.9% vs 51.1%, P < 0.0014).
To assess the correlation between disease activity and undernutrition, we calculated, for each group, the percentage of both the presence of undernutrition and high disease activity score at each visit. Among male children with CD, when severe undernutrition was recorded at a visit, there was a high HBI score 25.0% of the time. In this group, the number was 20% for mild undernutrition. These proportions were more elevated in female children, as a high disease activity score was recorded 100% of the time in cases with severe undernutrition, but only 30.0% of the time in cases with mild undernutrition. In male adults with CD, an HBI >4 and undernutrition were observed simultaneously at 61.5% of visits for severe and 11.0% for mild undernutrition. In women with CD, these percentages were 66.7% for severe and 44.4% for mild undernutrition. In male adults with UC, there was severe undernutrition and an LI score >2 66.7% of the time. As for female adults with UC, the proportions were 60.0% for severe and 50.0% for mild undernutrition. The 2 women with IBDU and severe undernutrition did not have a high LI score at visits. None of these differences were statistically significant.
We have also analyzed the relationship between serum albumin and CRP with disease activity in CD. Seventy-seven percent of children and 67% of adults with active disease had high CRP, compared with 48% and 43% with low albumin, showing that CRP is a better surrogate of disease activity than serum albumin. However, CRP was found to be very unspecific as 45% of children and 38% of adults without active disease presented with high CRP, compared with 23% (children) and 21% (adults) for low albumin. Differences between children and adults were not statistically significant.
Disease Location and Behavior and Undernutrition in CD
We next assessed the association between variables relative to nutritional status and undernutrition. Because of the low number of patients with undernutrition in the UC and IBDU groups, analyses were only conducted in CD patients. Data for disease location were available for 188 children and 366 adults, whereas data for disease behavior were available for 200 pediatric and 376 adult patients. Most children had ileocolonic (83.5%) and luminal disease (84.5%). A majority of adults also presented with ileocolonic disease (54.9%), but unique colonic involvement was more prevalent (25.7%) than in children. To assess if certain variables were associated with undernutrition, we first compared prevalence using χ2 tests (Table 5). These independent analyses revealed that hypoalbuminemia (P < 0.0001), low Hb (P < 0.0001), low-serum Fe (P < 0.027), high CRP (P < 0.037), and ileocolonic disease location (P < 0.004) were associated with undernutrition. When all variables were included in a logistic regression model, the associations with hypoalbuminemia (OR, 2.530; P < 0.006) and high disease activity (OR, 1.988; P < 0.03) remained significant, whereas the association with ileocolonic disease failed to reach significance (OR, 1.514; P < 0.06). When separate models were fit for pediatric and adult patients, similar associations with undernutrition were observed.
TABLE 5.
Factors Associated With Undernutrition in Pediatric and Adult CD
| Logistic Regression | Chi-Square | |||
|---|---|---|---|---|
| Odds Ratio | 95% CI | P | P | |
| Age | 0.920 | 0.493–1.714 | 0.792 | 0.975 |
| Sex | 0.946 | 0.530–1.689 | 0.852 | 0.366 |
| Low albumin | 2.530 | 1.304–4.909 | 0.006 | 0.0001 |
| Low hemoglobin | 1.730 | 0.853–3.506 | 0.128 | 0.0001 |
| Low iron | 1.078 | 0.562–2.067 | 0.820 | 0.027 |
| Low B12 | 0.819 | 0.384–1.746 | 0.605 | 0.512 |
| Low folate | 1.184 | 0.291–4.821 | 0.813 | 0.629 |
| High CRP | 0.637 | 0.309–1.313 | 0.222 | 0.037 |
| High disease activity | 1.988 | 1.068–3.701 | 0.030 | 0.002 |
| Disease location (ileocolonic) | 1.514 | 0.983–2.333 | 0.060 | 0.004 |
| Disease behavior | 0.989 | 0.655–1.493 | 0.958 | 0.260 |
Predicators of undernutrition in pediatric and adult CD patients, as assessed by logistic regression and by individual χ2 tests.
Antioxidant Vitamins
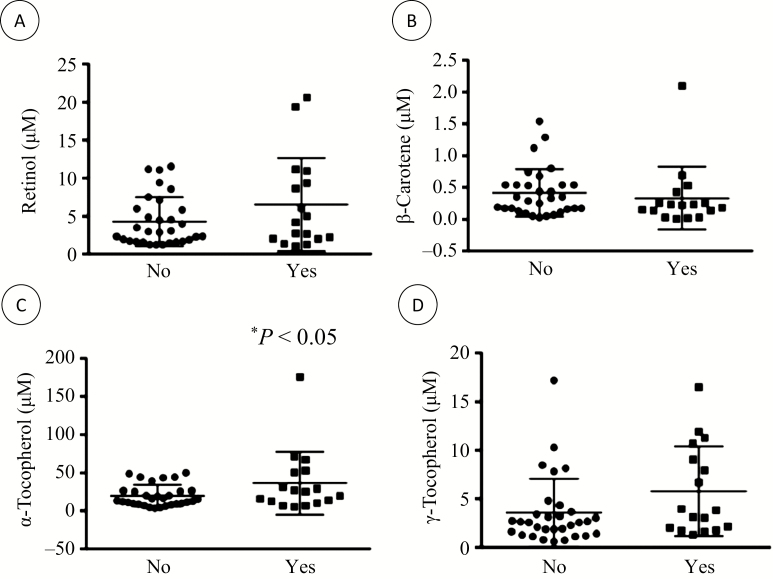
We examined the correlation between the plasma levels of antioxidant vitamins and BMI. Results show a weak but significant correlation (r2 = 0.103; P < 0.026) with plasma levels of retinol. When analyzed by sex, the correlation between retinol and BMI was stronger in females (r2 = 0.213; P < 0.027) but was not existent in males (r2 = 0.071). No significant correlation was found with other vitamins. Also, no correlation was observed between antioxidant vitamin levels and HBI score. Next, we analyzed antioxidant vitamin levels according to undernutrition status (no/yes), active disease (no/yes), high CRP (no/yes), and low levels of albumin, Hb, Fe, vitamin B12, and folates (no/yes). Levels of antioxidant vitamins were not different in patients with low albumin, Hb, vitamin B12, or folates. However, as shown in Figure 1, compared with subjects with normal blood Fe values, patients suffering from Fe deficiency had higher levels of α-tocopherol (19.8 ± 14.2 μM and 36.5 ± 41.4 μM, respectively, P < 0.05), nonsignificant elevations of retinol (4.3 ± 3.2 μM and 6.5 ± 6.1 μM, respectively, P < 0.10) and γ-tocopherol (3.6 ± 3.5 μM and 5.8 ± 4.6 μM, respectively, P < 0.07), and a tendency toward reduction in β-carotene levels (0.42 ± 0.37 μM and 0.33 ± 0.49 μM, respectively, P < 0.06).
FIGURE 1.
Antioxidant vitamin levels according to Fe deficiency. The levels of plasma antioxidant vitamins were analyzed according to Fe deficiency (no/yes). Plasma levels of (A) retinol, (B) β-carotene, (C) α-tocopherol, and (D) γ-tocopherol were determined in 48 CD adult patients according to their Fe deficiency status. *P < 0.05 vs no Fe deficiency.
Compared with subjects who did not consume supplements, patients who reported taking vitamins displayed higher levels of β-carotene (0.31 ± 0.34 μM and 0.59 ± 0.53 μM, respectively, P < 0.05) and α-tocopherol (20.8 ± 17.2 μM and 38.9 ± 44.2 μM, respectively, P < 0.05), whereas no significant differences were observed for retinol (5.0 ± 4.9 μM and 5.3 ± 3.4 μM, respectively) or γ-tocopherol (4.3 ± 4.3 μM and 4.5 ± 3.4 μM, respectively).
Essential Fatty Acid Deficiency
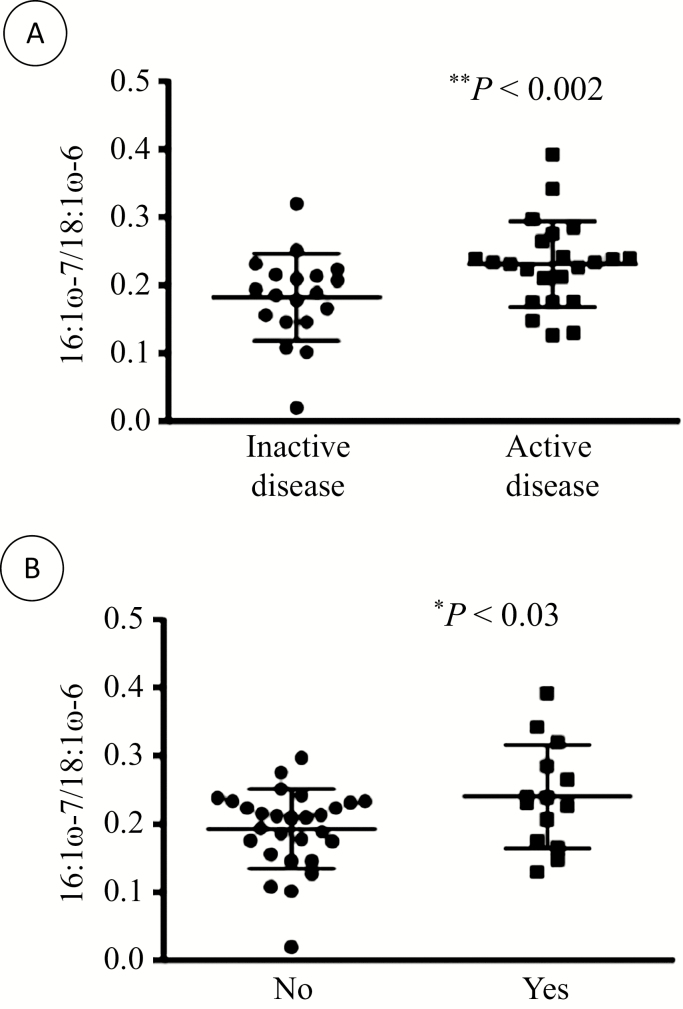
Reduced ratios of 16:1ω-7/18:1ω-6 and 20:ω-9/20:4ω-6 are indicators of EFA deficiency. We analyzed them according to undernutrition status, active disease, high CRP, and low levels of albumin, Hb, Fe, vitamin B12, and folates. No significant differences were found for the 20:ω-9/20:4ω-6 ratio. However, subjects with active disease had a lower 16:1ω-7/18:1ω-6 ratio compared with those with inactive disease (0.23 ± 0.06 and 0.18 ± 0.6, respectively, P < 0.002) (Fig. 2A). Patients with Fe deficiencies also had a reduced 16:1ω-7/18:1ω-6 ratio (0.24 ± 0.08 and 0.19 ± 0.06, respectively, P < 0.03) (Fig. 2B). When the analyses were performed for sex, these findings remained significant for women (0.30 ± 0.06 and 0.20 ± 0.07, respectively, P < 0.01) but not for men (0.20 ± 0.05 and 0.18 ± 0.05, respectively).
FIGURE 2.
Essential fatty acid deficiency according to disease activity and Fe deficiency. Plasma fatty acid levels were determined in 48 CD adult patients, and the ratio between 16:1ω-7 and 18:1ω-6 was computed in (A) patients with inactive and active disease and (B) patients without and with Fe deficiency. *P < 0.03 and **P < 0.002 vs inactive disease.
DISCUSSION
In this cross-sectional study, we aimed to compare the prevalence of malnutrition in children and adults with IBD. When evaluated with BMI or albumin levels, malnutrition was not more common in children compared with adults. However, the measurement of several biochemical parameters reflecting nutritional status revealed that the prevalence of low Hb, low Fe, and high CRP was higher in children, whereas vitamin B12 deficiency was more frequent in adults.
In our cohort, composed of outpatients mainly visiting for routine follow-up appointments, undernutrition was observed in 14.3% of pediatric patients and 13.7% of adults, including both severe and mild cases. Some studies reported striking differences in the nutritional status between IBD patients and controls,42, 43 whereas others did not.16, 44, 45 The reported prevalence of undernutrition varies according to the method used for its evaluation and the type of patients examined. Studies that focused on hospitalized IBD patients with severe disease activity reported a high incidence of undernutrition.46, 47 A study examining nutritional status in outpatients in remission observed undernutrition in less than 5% of CD patients according to Subjective Global Assessment (SGA) and BMI, substantially lower than previous studies.48 Ladd et al. found that 32.4% of pediatric CD patients were malnourished, including 4.1% with severe malnutrition.49 In a report including both CD and UC patients in remission, CD patients had significantly lower body weights as compared with UC or controls.43 Studies found no difference in weight or BMI between CD patients and UC patients or controls.9, 44, 45 However, Geerling et al. reported lower body fat mass in male patients with CD, and, despite the lack of normally recognized indicators of malnutrition, patients with CD had less power and endurance in their flexor muscles.44, 45 Using criteria based on BMI, we did not observe a difference in the frequency of undernutrition between CD and colitis (UC and IBDU), or between males or females. Other measures of nutritional status, such as skin fold thickness, could have led to different results. Malnutrition, defined with BMI, was found in 14.0% of subjects with CD and 5.7% of UC patients, whereas muscle mass depletion, measured by triceps plus subscapular skin fold thickness and by arm muscle area, was detected in more than half of the CD and UC patients.50 Nonetheless, as BMI is a simple and useful tool in clinical practice and comparisons between anthropometric parameters have already been performed in IBD, it was selected as our indicator for undernutrition.
It has been demonstrated that treatment choice can impact nutritional status in IBD patients. For example, infliximab,30 steroids,51 and enteral nutrition therapy51 can improve BMI. However, our results show that there was no difference in the proportion of undernourished and well-nourished adults treated with steroids and/or a biological. On the contrary, we observed that steroids and biologicals were more frequently used in undernourished children compared with those who were well-nourished. This difference might reflect more severe disease in children taking these medications.
It was reported that a high proportion of hospitalized CD (25%–80%) and UC patients (25%–50%) have hypoalbuminemia.52 We found that 28.1% of children and 23.5% of adults, mostly outpatients, had hypoalbuminemia. The prevalence of hypoalbuminemia was higher in CD compared with UC and IBDU patients, in both pediatric and adult cohorts. Circulating albumin levels, along with BMI, are often used as a nutritional assessment in IBD patients,53 although low albumin levels better reflect the status of inflammation.54 In our study, even when including cases with mild undernutrition (using BMI), the prevalence was almost half that of low-serum albumin. In line with our results, it has been pointed out that BMI underestimates malnutrition and should be used with caution, especially in IBD patients.55 Some patients classified as overweight or obese with BMI were found to be malnourished based on triceps skin folds and handgrip strength.55 In that study, the prevalence of malnutrition based on BMI was only 6.0%, but was found to be as high as 73.3% when assessed with handgrip strength.55 As BMI is such a simple clinical tool to use, we believe that determining its correlation with other markers of nutritional status is useful for proper assessment, in keeping with a recent position paper on nutritional status in pediatric IBD.56
The specific considerations of pediatric-onset IBD in terms of their nutritional needs are well recognized.56, 57 Growth during childhood and adolescence increases nutritional requirements, which can be difficult to meet in a patient with IBD. Growth failure is seen in about 30% of children with CD from 9 years of age to adolescence and in 5%–10% of children with UC.58 Permanent short stature in patients with pediatric-onset CD has been reported in 19%–35% of patients,24, 59 whereas it is relatively rare in pediatric-onset UC.59 Given the additional nutritional challenge of growth in children, we were surprised by the similar prevalence of malnutrition found in pediatric and adult groups. These results uphold the need for adequate nutritional assessment and follow-up in IBD at any age.
Anemia is a frequent complication of IBD, with reported prevalence varying between 19% and 60%.60, 61 Anemia has significant impact on quality of life, contributing to chronic fatigue. Both Fe deficiency and anemia of chronic disease contribute most to the development of anemia in IBD.62 Our study revealed that, although both children and adults had a high prevalence of low Hb, especially when undernourished, children were at higher risk of anemia. In adults, CD patients were at higher risk of low Hb compared with those with UC or IBDU. Low Fe levels were also more prevalent in children than adults, and in CD compared with UC or IBDU. Fe deficiency is the most common micronutrient deficiency in IBD and may be a result of malabsorption, impaired dietary intake, inflammation, or chronic intestinal blood loss.61 Fe deficiency anemia in adults with IBD was found in 6%–73%, depending on disease type and diagnostic criteria.63, 64 In a study in pediatric IBD, the reported prevalence was 17%.65 This discrepancy in frequency may be partially caused by differences in diagnostic criteria and the populations studied. A higher prevalence of anemia is seen in hospitalized patients. As most studies were performed at referral centers, one can speculate that they may have overestimated the actual prevalence in a community-based setting. However, despite being focused on a population largely comprised of outpatients, our study underlines the high prevalence of anemia and low Fe in IBD, especially in children and particularly in CD.
It has been reported that, in spite of appropriate energy and macronutrient intakes, CD patients in remission have lower plasma concentrations of several vitamins and minerals.61, 66, 67 In particular, vitamin B12 and folate deficiencies are common in IBD. We found that the prevalence of vitamin B12 deficiency was significantly higher in adults (19.4%) compared with children (5.4%). Women with CD were particularly at risk for vitamin B12 deficiency, with a prevalence of 32.3%. Because absorption of vitamin B12 requires an intact and functional ileum, it has been assumed that patients with CD are at high risk for B12 deficiency.68 We found that vitamin B12 deficiency was greater in adults with CD than in those with UC or IBDU (26.3% vs 2.6%). Similarly, two studies found a higher prevalence of low serum B12 in CD compared to UC: 18.4% vs 5%68 and 22% vs 7.5%.53 Oostenbrug and collaborators found that 41.9% of patients with ileal CD had low-serum cobalamin levels, compared with 20.7% in patients with disease confined to the colon.69 We recently reported that ileal resection exceeding 20 cm is a risk factor for B12 deficiency.20
The prevalence of folate deficiency was relatively low in our study (3.0% in children, 2.6% in adults). This can be explained by the widespread consumption of folate supplements among IBD patients. In line with our findings, folate concentrations have been reported to be normal or low compared with those in non-IBD controls in adult61, 70 and pediatric71 IBD patients. Studies have even observed higher serum, leukocyte, and red blood cell folate concentrations in pediatric patients with newly diagnosed IBD than in controls.72 However, other studies have reported higher folate deficiencies in adults with IBD, 10%–29% in CD patients and 8.6% in UC patients.53, 70 A meta-analysis found that folate concentrations in patients with UC but not CD were lower than those in controls.73
CRP is produced mainly in hepatocytes in response to acute phase stimuli such as inflammatory cytokines and is commonly used to monitor disease activity in IBD.37 CRP levels are known to correlate with clinical disease activity, endoscopic inflammation, and active histological inflammation.74 In our study, the prevalence of high CRP levels was elevated in both age groups, although it was more frequent in children than in adults (49.8% vs 38.5%) and in adult CD compared with UC and IBDU (47.4% vs 16.8%). Others have also observed that CD patients have higher CRP levels than those with UC.75 One study showed that 75% of CD and 29% of UC adult patients had elevated CRP levels at diagnosis,38 which is higher than our findings. In a pediatric study, CRP was detectable in 36% of patients, but serum levels were independent of disease activity.37
Searching for variables that can predict undernutrition revealed that hypoalbuminemia and high disease activity had the strongest associations, for both the pediatric and adult groups. Low Hb, low Fe, high CRP, and ileocolonic disease location were also associated with undernutrition. Similarly, it was found that BMI and serum albumin were the best predicators of nutritional status in IBD patients with active disease.53 In children, tructuring disease turned out to be the disease behavior most often associated with impaired nutritional status.76 In hospitalized CD patients, malnutrition on admission was more frequent in those with active fistulizing disease than in those without this complication.1 Our data did not reveal any predicative value of disease behavior.
Our results showed that retinol levels correlated with BMI but only in women. A meta-analysis of 19 case-control studies revealed that levels of fat-soluble vitamins, including vitamin A, were lower in patients with IBD.77 In the EUREYE study, which included 4753 participants, BMI was one of the factors positively associated with plasma retinol levels.78 The reasons why this was only significant in women in our study are not clear, but might be explained by the small sample size. Interestingly, levels of retinol, α-tocopherol, and γ-tocopherol were elevated in patients with low blood Fe. As Fe is known to promote oxidative stress,79 it is rational to presume that, as less antioxidant protection is needed in these patients, levels of antioxidant vitamins are higher.
Aberrations in plasma fatty acid profiles of CD patients have been reported previously,39 and altered EFA profiles have been correlated with disease activity in children.80 Our data found EFA deficiency in adult CD patients with active disease and revealed its relation to blood Fe status, implicating elevated oxidative stress.
To enhance the feasibility of the McGill IBD Database, only values outside normal cutoffs were registered. Consequently, the lack of normal values for biological parameters is a limitation of this study. Moreover, we did not systematically collect data on caloric and nutrient intake, preventing us exploring the specific factors causing malnutrition, such as decreased intake, increased loss, or malabsorption.
Furthermore, disease activity was calculated using HBI (CD) and LI (UC and IBDU) for both pediatric and adult populations. These indexes, which do not require endoscopy score or laboratory results, were used for practicality and efficiency. The Pediatric Crohn’s Disease Activity Index (PCDAI) and Pediatric Ulcerative Colitis Activity Index (PUCAI) have become the standard indices for measuring disease activity in pediatric CD and UC, respectively, but the PCDAI necessitates endoscopy and laboratory testing. However, the HBI and LI have been validated and employed in several pediatric studies. As we only needed to discriminate between active and inactive disease, without gradation based on severity, we believe that the HBI and LI adequately captured active disease in our pediatric population.
Another limitation is that vitamin B12 deficiency may have been poorly diagnosed or misdiagnosed in the IBD population, as blood levels were found insufficient to identify deficiency in asymptomatic patients.18 Also, we did not collect data on total iron-binding capacity, ferritin, or transferrin, which could have been used to assess iron deficiency anemia.
In conclusion, data from our McGill University Health Center IBD database show that malnutrition, as measured by undernutrition using BMI and hypoalbuminemia, is seen in adult IBD patients as often as in children. Additionally, high disease activity and low albumin levels are strong predicators of malnutrition in CD, both in pediatric and adult patients. Anemia, low-serum Fe, and high CRP are more frequently observed in pediatric CD patients, compared with adults. Vitamin B12 deficiency is more common in adult compared with pediatric CD patients. Participants with active disease and Fe deficiency had a lower 16:1ω-7/18:1ω-6 ratio, an indicator of EFA deficiency. These results justify close follow-up of nutritional status for IBD patients regardless of age.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Mrs. Melissa Diamond for her technical assistance.
Conflicts of interest: The authors declare that they have no competing interests.
Supported by: This study was supported in part by a Canadian Institutes of Health Research Team Grant (CTP-82942; E.G.S., E.L.) and the McGill IBD Research Group. Salary support was provided by the J.A. DeSève Research Chair in Nutrition (E.L.), the Canada Research Chair in Immune Mediated Gastrointestinal Disorders (E.G.S.), the Bruce Kaufman Endowed Chair in IBD at McGill (E.G.S.), a Canadian Institutes of Health Research Fellowship Award, and The Richard and Edith Strauss Postdoctoral Fellowships Award in Medicine, McGill University (V.M.).
Author contributions: V.M. was involved in the study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, technical support, statistical analysis, and study supervision; E.G.S. contributed to the study concept and design, acquisition of data, analysis, interpretation of data, and study supervision; E.L., D.A., A.B., A.M.S., A.S., D.S., and E.G.S. contributed to critical revision of the manuscript for important intellectual content, administrative support, technical support, material support, study supervision, and obtaining funding; D.S. (both) and F.B. were involved in the study concept, acquisition, and analysis of data. All the authors have approved the final draft submitted.
REFERENCES
- 1. Nguyen GC, Munsell M, Harris ML. Nationwide prevalence and prognostic significance of clinically diagnosable protein-calorie malnutrition in hospitalized inflammatory bowel disease patients. Inflamm Bowel Dis. 2008;14:1105–1111. [DOI] [PubMed] [Google Scholar]
- 2. Gassull MA, Cabré E. Nutrition in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. 2001;4:561–569. [DOI] [PubMed] [Google Scholar]
- 3. Lanfranchi GA, Brignola C, Campieri M, et al. Assessment of nutritional status in Crohn’s disease in remission or low activity. Hepatogastroenterology. 1984;31:129–132. [PubMed] [Google Scholar]
- 4. Valentini L, Schaper L, Buning C, et al. Malnutrition and impaired muscle strength in patients with Crohn’s disease and ulcerative colitis in remission. Nutrition. 2008;24:694–702. [DOI] [PubMed] [Google Scholar]
- 5. Bergeron F, Bouin M, D’Aoust L, et al. Food avoidance in patients with inflammatory bowel disease: what, when and who? Clin Nutr. 2018;37:884–889. [DOI] [PubMed] [Google Scholar]
- 6. Hermann GE, Rogers RC. TNF activates astrocytes and catecholaminergic neurons in the solitary nucleus: implications for autonomic control. Brain Res. 2009;1273:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al-Jaouni R, Hébuterne X, Pouget I, et al. Energy metabolism and substrate oxidation in patients with Crohn’s disease. Nutrition. 2000;16:173–178. [DOI] [PubMed] [Google Scholar]
- 8. Vaisman N, Dotan I, Halack A, et al. Malabsorption is a major contributor to underweight in Crohn’s disease patients in remission. Nutrition. 2006;22:855–859. [DOI] [PubMed] [Google Scholar]
- 9. Diederen K, Krom H, Koole JCD, et al. Diet and anthropometrics of children with inflammatory bowel disease: acomparison with the general population. Inflamm Bowel Dis. 2018;24:1632–1640. [DOI] [PubMed] [Google Scholar]
- 10. Lim HS, Kim SK, Hong SJ. Food elimination diet and nutritional deficiency in patients with inflammatory bowel disease. Clin Nutr Res. 2018;7:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ling SC, Griffiths AM. Nutrition in inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. 2000;3:339–344. [DOI] [PubMed] [Google Scholar]
- 12. Kulnigg S, Stoinov S, Simanenkov V, et al. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol. 2008;103:1182–1192. [DOI] [PubMed] [Google Scholar]
- 13. Semrin G, Fishman DS, Bousvaros A, et al. Impaired intestinal iron absorption in Crohn’s disease correlates with disease activity and markers of inflammation. Inflamm Bowel Dis. 2006;12:1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gasché C, Reinisch W, Lochs H, et al. Anemia in Crohn’s disease. Importance of inadequate erythropoietin production and iron deficiency. Dig Dis Sci. 1994;39:1930–1934. [DOI] [PubMed] [Google Scholar]
- 15. Gomollón F, Gisbert JP. Anemia and inflammatory bowel diseases. World J Gastroenterol. 2009;15:4659–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Felipez LM, Gokhale R, Tierney MP, et al. Thalidomide use and outcomes in pediatric patients with Crohn disease refractory to infliximab and adalimumab. J Pediatr Gastroenterol Nutr. 2012;54:28–33. [DOI] [PubMed] [Google Scholar]
- 17. Reimund JM, Hirth C, Koehl C, et al. Antioxidant and immune status in active Crohn’s disease. A possible relationship. Clin Nutr. 2000;19:43–48. [DOI] [PubMed] [Google Scholar]
- 18. Battat R, Kopylov U, Szilagyi A, et al. Vitamin B12 deficiency in inflammatory bowel disease: prevalence, risk factors, evaluation, and management. Inflamm Bowel Dis. 2014;20:1120–1128. [DOI] [PubMed] [Google Scholar]
- 19. Morgenstern I, Raijmakers MT, Peters WH, et al. Homocysteine, cysteine, and glutathione in human colonic mucosa: elevated levels of homocysteine in patients with inflammatory bowel disease. Dig Dis Sci. 2003;48:2083–2090. [DOI] [PubMed] [Google Scholar]
- 20. Battat R, Kopylov U, Byer J, et al. Vitamin B12 deficiency in inflammatory bowel disease: a prospective observational pilot study. Eur J Gastroenterol Hepatol. 2017;29:1361–1367. [DOI] [PubMed] [Google Scholar]
- 21. O’Sullivan MA, O’Morain CA. Nutritional therapy in Crohn’s disease. Inflamm Bowel Dis. 1998;4:45–53. [PubMed] [Google Scholar]
- 22. Seidman E, LeLeiko N, Ament M, et al. Nutritional issues in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1991;12:424–438. [DOI] [PubMed] [Google Scholar]
- 23. Motil KJ, Grand RJ, Davis-Kraft L, et al. Growth failure in children with inflammatory bowel disease: a prospective study. Gastroenterology. 1993;105:681–691. [DOI] [PubMed] [Google Scholar]
- 24. Sentongo TA, Semeao EJ, Piccoli DA, et al. Growth, body composition, and nutritional status in children and adolescents with Crohn’s disease. J Pediatr Gastroenterol Nutr. 2000;31:33–40. [DOI] [PubMed] [Google Scholar]
- 25. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5A–36A. [DOI] [PubMed] [Google Scholar]
- 26. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 27. Lichtiger S, Present DH, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841–1845. [DOI] [PubMed] [Google Scholar]
- 28. WHO. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 29. Lipschitz DA. Screening for nutritional status in the elderly. Prim Care. 1994;21:55–67. [PubMed] [Google Scholar]
- 30. Vadan R, Gheorghe LS, Constantinescu A, et al. The prevalence of malnutrition and the evolution of nutritional status in patients with moderate to severe forms of Crohn’s disease treated with infliximab. Clin Nutr. 2011;30:86–91. [DOI] [PubMed] [Google Scholar]
- 31. Secker DJ, Jeejeebhoy KN. Subjective global nutritional assessment for children. Am J Clin Nutr. 2007;85:1083–1089. [DOI] [PubMed] [Google Scholar]
- 32. Mack DR, Langton C, Markowitz J, et al. ; Pediatric Inflammatory Bowel Disease Collaborative Research Group Laboratory values for children with newly diagnosed inflammatory bowel disease. Pediatrics. 2007;119:1113–1119. [DOI] [PubMed] [Google Scholar]
- 33. Gisbert JP, Bermejo F, Pajares R, et al. Oral and intravenous iron treatment in inflammatory bowel disease: hematological response and quality of life improvement. Inflamm Bowel Dis. 2009;15:1485–1491. [DOI] [PubMed] [Google Scholar]
- 34. Yetley EA, Pfeiffer CM, Phinney KW, et al. Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am J Clin Nutr. 2011;94:313S–321S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hercberg S, Galan P. Nutritional anaemias. Baillieres Clin Haematol. 1992;5:143–168. [DOI] [PubMed] [Google Scholar]
- 36. Yakut M, Ustün Y, Kabaçam G, et al. Serum vitamin B12 and folate status in patients with inflammatory bowel diseases. Eur J Intern Med. 2010;21:320–323. [DOI] [PubMed] [Google Scholar]
- 37. Sidoroff M, Karikoski R, Raivio T, et al. High-sensitivity C-reactive protein in paediatric inflammatory bowel disease. World J Gastroenterol. 2010;16:2901–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Henriksen M, Jahnsen J, Lygren I, et al. ; IBSEN Study Group C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut. 2008;57:1518–1523. [DOI] [PubMed] [Google Scholar]
- 39. Levy E, Rizwan Y, Thibault L, et al. Altered lipid profile, lipoprotein composition, and oxidant and antioxidant status in pediatric Crohn disease. Am J Clin Nutr. 2000;71:807–815. [DOI] [PubMed] [Google Scholar]
- 40. Lepage G, Levy E, Ronco N, et al. Direct transesterification of plasma fatty acids for the diagnosis of essential fatty acid deficiency in cystic fibrosis. J Lipid Res. 1989;30:1483–1490. [PubMed] [Google Scholar]
- 41. Spahis S, Vanasse M, Bélanger SA, et al. Lipid profile, fatty acid composition and pro- and anti-oxidant status in pediatric patients with attention-deficit/hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids. 2008;79:47–53. [DOI] [PubMed] [Google Scholar]
- 42. Jahnsen J, Falch JA, Mowinckel P, et al. Body composition in patients with inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2003;98:1556–1562. [DOI] [PubMed] [Google Scholar]
- 43. Capristo E, Addolorato G, Mingrone G, et al. Effect of disease localization on the anthropometric and metabolic features of Crohn’s disease. Am J Gastroenterol. 1998;93:2411–2419. [DOI] [PubMed] [Google Scholar]
- 44. Geerling BJ, Badart-Smook A, Stockbrügger RW, et al. Comprehensive nutritional status in recently diagnosed patients with inflammatory bowel disease compared with population controls. Eur J Clin Nutr. 2000;54:514–521. [DOI] [PubMed] [Google Scholar]
- 45. Geerling BJ, Badart-Smook A, Stockbrügger RW, et al. Comprehensive nutritional status in patients with long-standing Crohn disease currently in remission. Am J Clin Nutr. 1998;67:919–926. [DOI] [PubMed] [Google Scholar]
- 46. Fernandez-Banares F, Abad-Lacruz A, Xiol X, et al. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol. 1989;84:744–748. [PubMed] [Google Scholar]
- 47. Fernández-Bañares F, Mingorance MD, Esteve M, et al. Serum zinc, copper, and selenium levels in inflammatory bowel disease: effect of total enteral nutrition on trace element status. Am J Gastroenterol. 1990;85:1584–1589. [PubMed] [Google Scholar]
- 48. Sousa Guerreiro C, Cravo M, Costa AR, et al. A comprehensive approach to evaluate nutritional status in Crohn’s patients in the era of biologic therapy: a case-control study. Am J Gastroenterol. 2007;102:2551–2556. [DOI] [PubMed] [Google Scholar]
- 49. Ladd MR, Garcia AV, Leeds IL, et al. Malnutrition increases the risk of 30-day complications after surgery in pediatric patients with Crohn disease. J Pediatr Surg. 2018;53:2336–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rocha R, Santana GO, Almeida N, et al. Analysis of fat and muscle mass in patients with inflammatory bowel disease during remission and active phase. Br J Nutr. 2009;101:676–679. [DOI] [PubMed] [Google Scholar]
- 51. Nielsen AA, Nielsen JN, Grønbaek H, et al. Impact of enteral supplements enriched with omega-3 fatty acids and/or omega-6 fatty acids, arginine and ribonucleic acid compounds on leptin levels and nutritional status in active Crohn’s disease treated with prednisolone. Digestion. 2007;75:10–16. [DOI] [PubMed] [Google Scholar]
- 52. Goh J, O’Morain CA. Review article: nutrition and adult inflammatory bowel disease. Aliment Pharmacol Ther. 2003;17:307–320. [DOI] [PubMed] [Google Scholar]
- 53. Mijac DD, Janković GL, Jorga J, et al. Nutritional status in patients with active inflammatory bowel disease: prevalence of malnutrition and methods for routine nutritional assessment. Eur J Intern Med. 2010;21:315–319. [DOI] [PubMed] [Google Scholar]
- 54. Seres DS. Surrogate nutrition markers, malnutrition, and adequacy of nutrition support. Nutr Clin Pract. 2005;20:308–313. [DOI] [PubMed] [Google Scholar]
- 55. Bin CM, Flores C, Alvares-da-Silva MR, et al. Comparison between handgrip strength, subjective global assessment, anthropometry, and biochemical markers in assessing nutritional status of patients with Crohn’s disease in clinical remission. Dig Dis Sci. 2010;55:137–144. [DOI] [PubMed] [Google Scholar]
- 56. Miele E, Shamir R, Aloi M, et al. Nutrition in pediatric inflammatory bowel disease: a position paper on behalf of the Porto Inflammatory Bowel Disease Group of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:687–708. [DOI] [PubMed] [Google Scholar]
- 57. Kim SC, Ferry GD. Inflammatory bowel diseases in pediatric and adolescent patients: clinical, therapeutic, and psychosocial considerations. Gastroenterology. 2004;126:1550–1560. [DOI] [PubMed] [Google Scholar]
- 58. Mallon DP, Suskind DL. Nutrition in pediatric inflammatory bowel disease. Nutr Clin Pract. 2010;25:335–339. [DOI] [PubMed] [Google Scholar]
- 59. Markowitz J, Grancher K, Rosa J, et al. Growth failure in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1993;16:373–380. [DOI] [PubMed] [Google Scholar]
- 60. Eriksson C, Henriksson I, Brus O, et al. Incidence, prevalence and clinical outcome of anaemia in inflammatory bowel disease: a population-based cohort study. Aliment Pharmacol Ther. 2018;48:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311–319. [DOI] [PubMed] [Google Scholar]
- 62. Gasche C, Lomer MC, Cavill I, et al. Iron, anaemia, and inflammatory bowel diseases. Gut. 2004;53:1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ebinger M, Leidl R, Thomas S, et al. Cost of outpatient care in patients with inflammatory bowel disease in a German university hospital. J Gastroenterol Hepatol. 2004;19:192–199. [DOI] [PubMed] [Google Scholar]
- 64. Kulnigg S, Gasche C. Systematic review: managing anaemia in Crohn’s disease. Aliment Pharmacol Ther. 2006;24:1507–1523. [DOI] [PubMed] [Google Scholar]
- 65. Revel-Vilk S, Tamary H, Broide E, et al. Serum transferrin receptor in children and adolescents with inflammatory bowel disease. Eur J Pediatr. 2000;159:585–589. [DOI] [PubMed] [Google Scholar]
- 66. Aghdassi E, Wendland BE, Stapleton M, et al. Adequacy of nutritional intake in a Canadian population of patients with Crohn’s disease. J Am Diet Assoc. 2007;107:1575–1580. [DOI] [PubMed] [Google Scholar]
- 67. Schneider SM, Al-Jaouni R, Filippi J, et al. Sarcopenia is prevalent in patients with Crohn’s disease in clinical remission. Inflamm Bowel Dis. 2008;14:1562–1568. [DOI] [PubMed] [Google Scholar]
- 68. Headstrom PD, Rulyak SJ, Lee SD. Prevalence of and risk factors for vitamin B(12) deficiency in patients with Crohn’s disease. Inflamm Bowel Dis. 2008;14:217–223. [DOI] [PubMed] [Google Scholar]
- 69. Oostenbrug LE, van Dullemen HM, te Meerman GJ, et al. Clinical outcome of Crohn’s disease according to the Vienna classification: disease location is a useful predictor of disease course. Eur J Gastroenterol Hepatol. 2006;18:255–261. [DOI] [PubMed] [Google Scholar]
- 70. Chowers Y, Sela BA, Holland R, et al. Increased levels of homocysteine in patients with Crohn’s disease are related to folate levels. Am J Gastroenterol. 2000;95:3498–3502. [DOI] [PubMed] [Google Scholar]
- 71. Holland N, Harmatz P, Golden D, et al. Cytogenetic damage in blood lymphocytes and exfoliated epithelial cells of children with inflammatory bowel disease. Pediatr Res. 2007;61:209–214. [DOI] [PubMed] [Google Scholar]
- 72. Heyman MB, Garnett EA, Shaikh N, et al. Folate concentrations in pediatric patients with newly diagnosed inflammatory bowel disease. Am J Clin Nutr. 2009;89:545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pan Y, Liu Y, Guo H, et al. Associations between folate and vitamin B12 levels and inflammatory bowel disease: a meta-analysis. Nutrients. 2017;9:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Solem CA, Loftus EV Jr, Tremaine WJ, et al. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707–712. [DOI] [PubMed] [Google Scholar]
- 75. Fagan EA, Dyck RF, Maton PN, et al. Serum levels of C-reactive protein in Crohn’s disease and ulcerative colitis. Eur J Clin Invest. 1982;12:351–359. [DOI] [PubMed] [Google Scholar]
- 76. Vasseur F, Gower-Rousseau C, Vernier-Massouille G, et al. Nutritional status and growth in pediatric Crohn’s disease: a population-based study. Am J Gastroenterol. 2010;105:1893–1900. [DOI] [PubMed] [Google Scholar]
- 77. Fabisiak N, Fabisiak A, Watala C, et al. Fat-soluble vitamin deficiencies and inflammatory bowel disease: systematic review and meta-analysis. J Clin Gastroenterol. 2017;51:878–889. [DOI] [PubMed] [Google Scholar]
- 78. Woodside JV, Young IS, Gilchrist SE, et al. Factors associated with serum/plasma concentrations of vitamins A, C, E and carotenoids in older people throughout Europe: the EUREYE study. Eur J Nutr. 2013;52:1493–1501. [DOI] [PubMed] [Google Scholar]
- 79. Leiva E, Mujica V, Sepúlveda P, et al. High levels of iron status and oxidative stress in patients with metabolic syndrome. Biol Trace Elem Res. 2013;151:1–8. [DOI] [PubMed] [Google Scholar]
- 80. Trebble TM, Wootton SA, May A, et al. Essential fatty acid status in paediatric Crohn’s disease: relationship with disease activity and nutritional status. Aliment Pharmacol Ther. 2003;18:433–442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.