Abstract
Background
Combined therapy with vedolizumab and corticosteroids may improve clinical response or remission in Crohn’s disease. The aim of this study is to assess efficacy and safety/tolerability of vedolizumab plus stable doses of corticosteroids at baseline during induction therapy in moderately to severely active Crohn’s disease.
Methods
A post hoc exploratory analysis was performed on data from GEMINI 2 (NCT00783692) and GEMINI 3 (NCT01224171), which assessed outcomes following induction therapy over 6- and 10-week periods, respectively. Patients receiving vedolizumab or placebo were stratified by corticosteroid use at baseline. Efficacy endpoints were clinical remission (CR; Crohn’s Disease Activity Index [CDAI] score ≤150 points) and enhanced clinical response (ECR; decrease of ≥100 points in CDAI score from baseline), assessed at week 6 (GEMINI 2 and GEMINI 3) and week 10 (GEMINI 3). Safety endpoints included the incidence of adverse events.
Results
Vedolizumab plus corticosteroids resulted in higher CR rates than placebo plus corticosteroids at week 6 in GEMINI 2 and at week 6 and week 10 in GEMINI 3. More patients receiving vedolizumab plus corticosteroids achieved CR at week 6 in GEMINI 2 and at week 10 in GEMINI 3 than patients receiving vedolizumab alone. Vedolizumab plus corticosteroids also resulted in significantly higher ECR rates than placebo plus corticosteroids at all timepoints in both studies. More patients receiving vedolizumab plus corticosteroids achieved higher ECR rates at week 6 in GEMINI 2 and at week 10 in GEMINI 3 than patients receiving vedolizumab alone. Adverse event incidence was similar across groups.
Conclusions
Vedolizumab in combination with stable doses of corticosteroids at baseline may improve induction of clinical response or remission in moderately to severely active Crohn’s disease.
Trial registration numbers
Keywords: Crohn’s disease, corticosteroids, vedolizumab, induction
INTRODUCTION
Crohn’s disease (CD) is an inflammatory bowel disease (IBD) characterized by diarrhea and abdominal pain.1 During the initial treatment phase, the aim is to induce clinical remission as quickly as possible and to prevent symptom recurrence.2–4 Because chronic inflammation is the hallmark of the disease, anti-inflammatory therapies using corticosteroids have long been used as induction agents in patients with moderately to severely active CD.2, 5 Indeed, corticosteroid-containing therapies are effective at inducing clinical remission.2, 6 However, long-term corticosteroid use has adverse safety implications, including myopathy, susceptibility to infection, and hypertension.2, 7
Two decades ago, biologic agents targeting tumor necrosis factor alpha (anti-TNFα agents) were successfully implemented in the treatment of IBD.8, 9 More recently, vedolizumab (ENTYVIO, Takeda Pharmaceuticals North America, Inc., USA), a gut-selective, humanized, α4β7 integrin antagonist,10 was approved for the treatment of moderately to severely active CD or ulcerative colitis in patients with inadequate response, loss of response, or intolerance to a corticosteroid, immunomodulator, or anti-TNFα agent.11, 12 Vedolizumab approval for CD was based on evidence from the phase 3, placebo-controlled, randomized GEMINI 2 and GEMINI 3 studies.13, 14 Study results showed that vedolizumab was significantly more efficacious than placebo at inducing clinical remission by week 6 in GEMINI 213 and by week 10 (but not by week 6) in GEMINI 3.14 In both studies, approximately 50% of patients continued prior corticosteroid therapy during vedolizumab induction.
International treatment guidelines suggest that an anti-TNFα–based approach may improve clinical outcomes during induction in patients refractory to or intolerant of corticosteroid therapy.2, 15 Treatment guidelines also suggest that it is possible that the combination of corticosteroids with an anti-TNFα agent and an immunomodulator may improve clinical outcomes in steroid-refractory CD.2 However, these guidelines provide only weak recommendations regarding combination induction therapy for CD; given the limited evidence available to date, further data are needed about therapies that provide benefits when combined with conventional corticosteroid treatment.2, 15
This post hoc analysis of data from the GEMINI 2 and GEMINI 3 studies assessed the efficacy and safety/tolerability of vedolizumab plus corticosteroids compared with vedolizumab alone or corticosteroids alone during induction in patients with moderately to severely active CD. Importantly, corticosteroids were not initiated at baseline; patients who were already using corticosteroids at stable doses before enrolling in these studies continued to receive corticosteroids during induction.
MATERIALS AND METHODS
Study Design
This was a post hoc exploratory analysis of patients randomized to double-blind vedolizumab or placebo in the GEMINI 2 study (ClinicalTrials.gov identifier: NCT00783692; 6-week induction phase comprising 2 doses of study drug before entering a 46-week maintenance phase) and the GEMINI 3 study (ClinicalTrials.gov identifier: NCT01224171; 10-week induction study comprising 3 doses of study drug) (details published elsewhere).13, 14 Patients who were already receiving oral corticosteroids before enrollment were eligible to enroll in the studies if they had stable corticosteroid dosing (ie, doses consistent for the 4 weeks before enrollment if corticosteroid therapy was recently initiated or for the 2 weeks before enrollment if corticosteroids were being tapered). Corticosteroid doses remained stable throughout the 6-week induction phase of GEMINI 2 and the entire 10 weeks of GEMINI 3, unless there were safety concerns.13, 14 Both GEMINI 2 and GEMINI 3 enrolled anti-TNFα treatment-naïve and treatment-failure patients; however, GEMINI 3 was specifically designed to enroll a higher number of patients who had failed previous anti-TNFα therapy.13, 14 Corticosteroid use and anti-TNFα history were recorded at screening and enrollment by the patient answering an interactive voice response system (IVRS) and at study baseline based on the case report form (CRF) (physician assessment).
Ethical Considerations
The GEMINI 2 and GEMINI 3 studies were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. Procedures were approved by local institutional review boards/ethics committees, and all patients provided written informed consent.
Patient Groups
This analysis evaluated patients in the vedolizumab and placebo arms stratified by baseline corticosteroid use. The corticosteroid subgroup included patients who received stable doses of ≤30 mg/day of prednisone or ≤9 mg/day of budesonide or equivalent dosing with another corticosteroid during the induction phase.13, 14
Study Endpoints
Efficacy endpoints for this post hoc analysis were consistent with prespecified endpoints in the primary studies and were as follows: (1) the proportion of patients in clinical remission (defined by a Crohn’s Disease Activity Index [CDAI] score of ≤150 points) or (2) the proportion of patients achieving enhanced clinical response (defined by a decrease from baseline in CDAI score of ≥100 points), both assessed at week 6 (GEMINI 2) and weeks 6 and 10 (GEMINI 3). Safety and tolerability were assessed as the incidence, severity, and type of adverse events (AEs) over the 6-week induction phase of GEMINI 2 and the entire 10 weeks of GEMINI 3.13, 14
Statistical Analyses
Descriptive statistics were used to assess baseline demographics and disease characteristics. Efficacy endpoints of clinical remission and enhanced clinical response were reported as percentages and 95% confidence intervals (CIs). The normal approximation to the binomial was used to calculate the CIs. Percentage-point differences (95% CIs) between groups were used to assess the statistical significance of the following comparisons: vedolizumab plus corticosteroid versus placebo plus corticosteroid and vedolizumab alone versus placebo alone (ie, comparing treatment arms within the corticosteroid subgroups); and vedolizumab plus corticosteroid versus vedolizumab alone and placebo plus corticosteroid versus placebo alone (ie, comparing corticosteroid subgroups within the vedolizumab or placebo arms). Descriptive statistics were also used to assess safety endpoints.
RESULTS
Baseline Demographics and Disease Characteristics
In GEMINI 2, 165 of 368 (44.8%) patients received corticosteroid therapy: 100 of 220 (45.5%) received vedolizumab plus corticosteroid, and 65 of 148 (43.9%) received placebo plus corticosteroid (Table 1). In GEMINI 3, 219 of 416 (52.6%) patients received corticosteroid therapy: 111 of 209 (53.1%) received vedolizumab plus corticosteroid, and 108 of 207 (52.2%) received placebo plus corticosteroid. Baseline demographics and clinical characteristics were generally similar across treatment arms and corticosteroid subgroups in each study. However, the 2 studies differed in terms of the patient’s previous anti-TNFα history. Approximately half of the patients enrolled in GEMINI 2 were anti-TNFα–naïve, whereas the other half had experienced previous anti-TNFα failure. In contrast, approximately one-quarter of patients enrolled in GEMINI 3 were anti-TNFα–naïve, and approximately three quarters had experienced previous anti-TNFα failure.
TABLE 1.
GEMINI 2 and GEMINI 3 Baseline Demographics and Disease Characteristics of Placebo and Vedolizumab Treatment Arms Stratified by Corticosteroid Use
| GEMINI 2 | GEMINI 3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 148) | Vedolizumab (n = 220) | Placebo (n = 207) | Vedolizumab (n = 209) | |||||
| Corticosteroid (n = 65) | No CS (n = 81) | Corticosteroid (n = 100) | No CS (n = 119) | Corticosteroid (n = 108) | No CS (n = 97) | Corticosteroid (n = 111) | No CS (n = 97) | |
| Baseline characteristics | ||||||||
| Male sex, n (%) | 30 (46) | 38 (47) | 52 (52) | 52 (44) | 51 (47) | 37 (38) | 51 (46) | 39 (40) |
| Mean age, years (SD) | 39.9 (13.8) | 37.7 (12.6) | 35.9 (11.2) | 36.6 (11.9) | 37.7 (13.8) | 36.6 (12.5) | 38.2 (12.1) | 39.0 (12.3) |
| Mean BMI, kg/m2 (SD) | 23.7 (4.9) | 23.8 (6.5) | 23.3 (6.4) | 22.9 (4.9) | 24.8 (6.6) | 24.4 (5.6) | 23.8 (5.0) | 24.3 (5.4) |
| Mean disease duration, years (SD) | 8.6 (8.1) | 8.1 (7.6) | 9.0 (8.3) | 9.3 (8.2) | 8.9 (7.4) | 11.1 (8.5) | 10.0 (7.9) | 11.3 (9.7) |
| Mean CDAI score (SD) | 308.0 (64.7) | 339.4 (85.8) | 327.0 (69.6) | 327.1 (72.0) | 308.6 (58.5) | 293.8 (50.3) | 312.7 (54.6) | 315.3 (52.0) |
| Prior therapy | ||||||||
| Any prior therapy, n (%) | 65 (100) | 81 (100) | 100 (100) | 119 (100) | 108 (100) | 97 (100) | 111 (100) | 97 (100) |
| Any systemic corticosteroid, n (%) | 64 (98) | 75 (93) | 94 (94) | 105 (88) | 102 (94) | 84 (87) | 107 (96) | 82 (85) |
| Any IM, n (%) | 47 (72) | 64 (79) | 78 (78) | 95 (80) | 100 (93) | 91 (94) | 95 (86) | 80 (82) |
| Any anti-TNFα, n (%) | 33 (51) | 41 (51) | 52 (52) | 64 (54) | 83 (77) | 74 (76) | 86 (77) | 69 (71) |
| Baseline treatment | ||||||||
| Concomitant aminosalicylic acid use, n (%) | 27 (42) | 39 (48) | 53 (53) | 48 (40) | 33 (31) | 27 (28) | 38 (34) | 30 (31) |
| Anti-TNFα-naïve, n (%)a | 33 (51) | 42 (52) | 50 (50) | 59 (50) | 24 (22) | 25 (26) | 24 (22) | 27 (28) |
| Anti-TNFα-failure, n (%)a | 29 (45) | 40 (49) | 47 (47) | 57 (48) | 82 (76) | 73 (75) | 86 (77) | 68 (70) |
| Concomitant corticosteroid only, n (%) | 42 (65) | 3 (4)b | 64 (64) | 3 (3)b | 70 (65) | 0 (0) | 71 (64) | 1 (1)b |
| Concomitant corticosteroid and IM, n (%) | 22 (34) | 3 (4)b | 35 (35) | 3 (3)b | 35 (32) | 1 (1)b | 37 (33) | 0 (0) |
Note: further details in Supplementary Table 1.
aDiscrepancies in patient numbers compared with the overall group occurred because some patients had previously received anti-TNFα therapy and did not fail and due to double counting (anti-TNFα history was recorded during screening and enrollment by the patient [IVRS] and based on CRF [by the physician] at study baseline).
bDiscrepancies in reporting of concomitant corticosteroid use (subgroups of patients “corticosteroid” and “no-corticosteroid” are based on actual data, whereas row summaries that refer to corticosteroid usage are based on an IVRS).
BMI, body mass index; CS, corticosteroid; IM, immunomodulator; SD, standard deviation; TNFα, necrosis factor alpha.
Efficacy—Clinical Remission
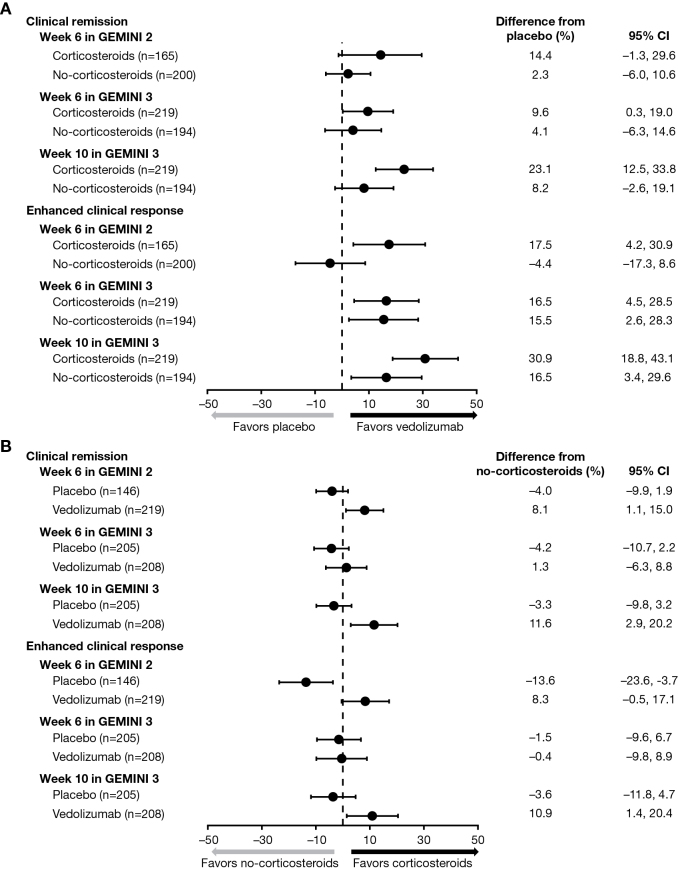
In GEMINI 2, vedolizumab plus corticosteroid treatment was associated with numerically higher rates of clinical remission than placebo plus corticosteroid treatment at week 6 (19.0% vs 4.6%, 14.4% difference; 95% CI, –1.3 to 29.6), whereas clinical remission rates at week 6 were similar between vedolizumab alone and placebo alone. In GEMINI 3, vedolizumab plus corticosteroid was associated with significantly higher rates of clinical remission than placebo plus corticosteroid at week 6 (19.8% vs 10.2%, 9.6% difference; 95% CI, 0.3–19.0) and at week 10 (34.2% vs 11.1%, 23.1% difference; 95% CI, 12.5–33.8) (Table 2, Fig. 1A). Clinical remission rates were similar for vedolizumab alone and placebo alone at week 6 but were numerically higher for vedolizumab alone than placebo alone at week 10 (22.7% vs 14.4%, 8.2% difference; 95% CI, –2.6 to 19.1).
TABLE 2.
Efficacy Endpoints After Induction Therapy in GEMINI 2 and GEMINI 3: Clinical Remission and Enhanced Clinical Response in Placebo and Vedolizumab Treatment Arms Stratified by Corticosteroid Use
| GEMINI 2 At week 6 | GEMINI 3 At week 6 | GEMINI 3 At week 10 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PLA | VDZ | Difference VDZ vs PLA, % [95% CI] | PLA | VDZ | Difference VDZ vs PLA, % [95% CI] | PLA | VDZ | Difference VDZ vs PLA, % [95% CI] | |
| Corticosteroid | n = 65 | n = 100 | n = 108 | n = 111 | n = 108 | n = 111 | |||
| Clinical remission, n (%)a | 3 (4.6) | 19 (19.0) | 14.4 | 11 (10.2) | 22 (19.8) | 9.6 | 12 (11.1) | 38 (34.2) | 23.1 |
| [95% CI] | [1.0–12.9] | [11.8–28.1] | [–1.3 to 29.6] | [4.5–15.9] | [12.4–27.2] | [0.3–19.0]b | [5.2–17.0] | [25.4–43.1] | [12.5–33.8]b |
| Enhanced clinical response, n (%)c | 12 (18.5) | 36 (36.0) | 17.5 | 24 (22.2) | 43 (38.7) | 16.5 | 24 (22.2) | 59 (53.2) | 30.9 |
| [95% CI] | [9.0–27.9] | [26.6–45.4] | [4.2–30.9]b | [14.4–30.1] | [29.7–47.8] | [4.5–28.5]b | [14.4–30.1] | [43.9–62.4] | [18.8–43.1]b |
| No corticosteroid | n = 81 | n = 119 | n = 97 | n = 97 | n = 97 | n = 97 | |||
| Clinical remission, n (%)a | 7 (8.6) | 13 (10.9) | 2.3 | 14 (14.4) | 18 (18.6) | 4.1 | 14 (14.4) | 22 (22.7) | 8.2 |
| [95% CI] | [2.5–14.8] | [5.3–16.5] | [–6.0 to 10.6] | [7.4–21.4] | [10.8–26.3] | [–6.3 to 14.6] | [7.4–21.4] | [14.3–31.0] | [–2.6 to 19.1] |
| Enhanced clinical response, n (%)c | 26 (32.1) | 33 (27.7) | –4.4 | 23 (23.7) | 38 (39.2) | 15.5 | 25 (25.8) | 41 (42.3) | 16.5 |
| [95% CI] | [21.9–42.3] | [19.7–35.8] | [–17.3 to 8.6] | [15.2–32.2] | [29.5–48.9] | [2.6–28.3]b | [17.1–34.5] | [32.4–52.1] | [3.4–29.6]b |
| Difference CS vs no CS | Difference CS vs no CS | Difference CS vs no CS | |||||||
| Clinical remission, %; [95% CI]a | –4.0 [–9.9 to 1.9] | 8.1 [1.1–15.0]b | –4.2 [–10.7 to 2.2] | 1.3 [–6.3 to 8.8] | –3.3 [–9.8 to 3.2] | 11.6 [2.9–20.2]b | |||
| Enhanced clinical response, %; [95% CI]c | –13.6 [–23.6 to –3.7]b | 8.3 [–0.5 to 17.1] | −1.5 [–9.6 to 6.7] | –0.4 [–9.8 to 8.9] | –3.6 [–11.8 to 4.7] | 10.9 [1.4–20.4]b | |||
aCDAI score of ≤150 points.
bIndicates significant difference (see text for details).
cDecrease of ≥100 points in Crohn’s Disease Activity Index score from baseline.
CS, corticosteroid; PLA, placebo; VDZ, vedolizumab.
FIGURE 1.
Clinical remission and enhanced clinical response after induction treatment in GEMINI 2 and GEMINI 3 comparing (A) vedolizumab and placebo treatment arms (stratified by corticosteroid subgroup) and (B) corticosteroid and no-corticosteroid subgroups (stratified by treatment arm).
In GEMINI 2, vedolizumab plus corticosteroid was associated with a significantly higher rate of clinical remission than vedolizumab alone at week 6 (19.0% vs 10.9%, 8.1% difference; 95% CI, 1.1–15.0); similar rates of clinical remission were reported for placebo plus corticosteroid and placebo alone. In GEMINI 3, vedolizumab plus corticosteroid and vedolizumab alone were associated with similar rates of clinical remission at week 6; however, vedolizumab plus corticosteroid was associated with a significantly higher rate of clinical remission than vedolizumab alone at week 10 (34.2% vs 22.7%, 11.6% difference; 95% CI, 2.9–20.2) (Table 2, Fig. 1B). Clinical remission rates were similar for placebo plus corticosteroid and placebo alone at weeks 6 and 10.
Efficacy—Enhanced Clinical Response
In GEMINI 2, vedolizumab plus corticosteroid was associated with a significantly greater proportion of patients achieving enhanced clinical response than placebo plus corticosteroid at week 6 (36.0% vs 18.5%, 17.5% difference; 95% CI, 4.2–30.9) (Table 2, Fig. 1A); similar enhanced clinical response rates were reported for vedolizumab alone and placebo alone at week 6. In GEMINI 3, vedolizumab plus corticosteroid was also associated with a significantly higher rate of enhanced clinical response than placebo plus corticosteroid at week 6 (38.7% vs 22.2%, 16.5% difference; 95% CI, 4.5–28.5) and at week 10 (53.2% vs 22.2%, 30.9% difference; 95% CI, 18.8–43.1); enhanced clinical response rates were also significantly higher for vedolizumab alone compared with placebo alone at week 6 (39.2% vs 23.7%, 15.5% difference; 95% CI, 2.6–28.3) and at week 10 (42.3% vs 25.8%, 16.5% difference; 95% CI, 3.4–29.6).
In GEMINI 2, vedolizumab plus corticosteroid was associated with numerically higher rates of enhanced clinical response compared with vedolizumab alone at week 6 (36.0% vs 27.7%, 8.3% difference; 95% CI, –0.5 to 17.1); enhanced clinical response rates were similar for placebo plus corticosteroid and placebo alone (Table 2, Fig. 1B). In GEMINI 3, vedolizumab plus corticosteroid and vedolizumab alone were associated with similar rates of enhanced clinical response at week 6; however, vedolizumab plus corticosteroid was associated with a significantly higher rate of enhanced clinical response than vedolizumab alone at week 10 (53.2% vs 42.3%, 10.9% difference; 95% CI, 1.4–20.4). Enhanced clinical response rates were similar for placebo plus corticosteroid and placebo alone at weeks 6 and 10.
Safety/Tolerability
Vedolizumab was safe and tolerated well among patients with moderately to severely active CD. The overall incidences of any AEs, any drug-related AEs, any AEs resulting in treatment discontinuation, and any serious AEs were similar in the vedolizumab and placebo arms of GEMINI 2 and GEMINI 3, independent of corticosteroid use (Table 3). Furthermore, incidences of AEs of special interest in relation to corticosteroid use were similar across treatment arms and corticosteroid subgroups in both studies (Table 4).
TABLE 3.
Safety Endpoints During Induction of the GEMINI 2 and GEMINI 3 Studies With Placebo and Vedolizumab Stratified by Corticosteroid Use
| GEMINI 2 | GEMINI 3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 148) | Vedolizumab (n = 220) | Placebo (n = 207) | Vedolizumab (n = 209) | |||||
| CS | No CS | CS | No CS | CS | No CS | CS | No CS | |
| Total population | n = 65 | n = 81 | n = 100 | n = 119 | n = 108 | n = 97 | n = 111 | n = 97 |
| Any AEs,a n (%) | 41 (63) | 45 (56) | 59 (59) | 64 (54) | 62 (57) | 62 (64) | 61 (55) | 57 (59) |
| Drug-related AEs, n (%) | 10 (15) | 19 (23) | 24 (24) | 26 (22) | 17 (16) | 17 (18) | 21 (19) | 13 (13) |
| AEs resulting in discontinuation, n (%) | 4 (6) | 5 (6) | 4 (4) | 5 (4) | 6 (6) | 2 (2) | 2 (2) | 2 (2) |
| Serious AEs,b n (%) | 5 (8) | 4 (5) | 11 (11) | 8 (7) | 3 (3) | 13 (13) | 6 (5) | 8 (8) |
aThere were no deaths during the studies.
bIn GEMINI 2, serious AEs suspected to be drug-related within the vedolizumab treatment arm included worsening of CD, pneumonia (resulting in study discontinuation in both cases), and blurred vision (study continued), which were experienced by 1 patient in the corticosteroid subgroup and 2 patients in the no-corticosteroid subgroup. In GEMINI 3, 1 serious drug-related AE (gastric ulcer [study had completed at time of AE]) occurred in the corticosteroid subgroup of the vedolizumab arm. There were no serious drug-related AEs in the placebo arm of GEMINI 2, and there was 1 serious drug-related AE in the corticosteroid subgroup of the placebo arm of GEMINI 3.
CS, corticosteroid.
TABLE 4.
Safety Endpoints During Induction: AEs of Special Interest in Relation to Corticosteroid Use in the Overall Population in GEMINI 2 and GEMINI 3 With Placebo and Vedolizumab Stratified by Corticosteroid Use
| GEMINI 2 | GEMINI 3 | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 148) | Vedolizumab (n = 220) | Placebo (n = 207) | Vedolizumab (n = 209) | |||||
| MedDRA organ class | CS n = 65 | No CS n = 81 | CS n = 100 | No CS n = 119 | CS n = 108 | No CS n = 97 | CS n = 111 | No CS n = 97 |
| Patients with ≥1 AE, n (%) | 41 (63) | 45 (56) | 59 (59) | 64 (54) | 62 (57) | 62 (64) | 61 (55) | 57 (59) |
| Gastrointestinal disorders | 12 (18) | 22 (27) | 24 (24) | 29 (24) | 20 (19) | 29 (30) | 16 (14) | 22 (23) |
| Infections and infestations | 13 (20) | 13 (16) | 20 (20) | 14 (12) | 16 (15) | 20 (21) | 23 (21) | 16 (16) |
| Musculoskeletal and connective tissue disorders | 8 (12) | 8 (10) | 7 (7) | 17 (14) | 15 (14) | 9 (9) | 11 (10) | 15 (15) |
| Skin and subcutaneous tissue disorders | 3 (5) | 6 (7) | 6 (6) | 8 (7) | 4 (4) | 9 (9) | 9 (8) | 10 (10) |
| Blood and lymphatic system disorders | 4 (6) | 5 (6) | 6 (6) | 3 (3) | 2 (2) | 1 (1) | 5 (5) | 4 (4) |
| Metabolism and nutrition disorders | 4 (6) | 1 (1) | 3 (3) | 3 (3) | 0 (0) | 3 (3) | 6 (5) | 2 (2) |
| Psychiatric disorders | 3 (5) | 1 (1) | 2 (2) | 2 (2) | 7 (6) | 6 (6) | 1 (<1) | 2 (2) |
| Immune system disorders (atopic disorders and seasonal allergy) | 1 (2) | 0 (0) | 0 (0) | 1 (<1) | 1 (<1) | 0 (0) | 1 (<1) | 0 (0) |
| Endocrine disorders (cushingoid) | 0 (0) | 0 (0) | 1 (1) | 1 (<1) | 1 (<1) | 0 (0) | 1 (<1) | 0 (0) |
Note: No direct relation is attributed to corticosteroid use because this was not recorded during the trial (only AEs deemed related to study treatment were specifically recorded).
MedDRA, Medical Dictionary for Regulatory Activities; CS, corticosteroid.
DISCUSSION
In this post hoc analysis, vedolizumab plus ongoing corticosteroid therapy more effectively induced clinical remission and enhanced clinical response in moderately to severely active CD than either placebo plus corticosteroids or vedolizumab alone. Similar to observations in routine clinical practice, approximately half of the patients enrolled in GEMINI 2 and GEMINI 3 were already receiving corticosteroids at baseline.13, 14 In addition, patients receiving induction treatment with the combination of vedolizumab plus stable doses of corticosteroids at baseline demonstrated a safety/tolerability profile similar to that of vedolizumab alone.
Previous studies in active CD have also demonstrated some comparable effects regarding the combination of corticosteroids with anti-TNFα agents. The GAIN study reported that induction therapy with 2 doses of adalimumab plus corticosteroid treatment induced higher rates of clinical remission (defined as a CDAI score <150 points) at week 4 of treatment compared with placebo plus corticosteroid in CD patients who were refractory to or intolerant of infliximab (33% vs 4%; P = 0.01).3 The results of our post hoc analysis suggest that CD patients treated with vedolizumab plus a corticosteroid more frequently achieved clinical remission than those treated with placebo plus a corticosteroid or vedolizumab alone. This difference was evident after 2 doses of vedolizumab in a mixed population of anti-TNFα–naïve and anti-TNFα–failure patients (GEMINI 2) and after a full 3-dose induction therapy among predominantly anti-TNFα–failure patients (GEMINI 3).13, 14
After combined induction therapy with either an anti-TNFα agent or an anti-integrin plus a corticosteroid, patients may then be able to taper their corticosteroid dose to achieve steroid-free clinical remission. Although the absence of symptoms at the end of induction therapy in patients on corticosteroids at baseline does not ensure that long-term, steroid-free clinical outcomes are sustained, there are reports to suggest that this circumstance may be possible for some patients. The ENACT study reported that natalizumab plus corticosteroids achieved a better rate of steroid-free clinical remission than placebo plus corticosteroids at week 60 (42% vs 15%; P = 0.001).16 Likewise, it has been previously reported in GEMINI 2 that vedolizumab plus corticosteroids (29% and 32% for vedolizumab every 4 or 8 weeks, respectively) were more effective than placebo plus corticosteroids (16%) in achieving steroid-free clinical remission at week 52 (P = 0.02 and 0.04, respectively).13 The incidences of overall and serious AEs in this post hoc analysis were similar across treatment arms and subgroups and comparable with those reported in previous vedolizumab studies, indicating that induction with vedolizumab plus corticosteroids did not lead to an increase in AEs compared with vedolizumab alone.13, 14, 17 A recent systematic review of 15 pivotal and post-marketing studies confirmed that vedolizumab treatment is associated with low rates of AEs, including infections, in patients with IBD.18 Both anti-TNFα–naïve patients and patients previously exposed to anti-TNFα therapy were included in this systematic review of vedolizumab treatment. Concomitant corticosteroid use was associated with an increased risk of serious infections among vedolizumab-treated patients (43% [88 of 207]; hazard ratio [HR] 1.72; 95% CI, 1.30–2.28; P = 0.0002).17, 18 Rates of serious AEs among patients receiving concomitant corticosteroids during the 2- and 4-week induction trials with adalimumab ranged from 23%–43% in CLASSIC-119 and from 35%–44% in GAIN.3 Serious AEs during 6-week induction therapy with certolizumab pegol were reported in 22%–23% of patients receiving concomitant corticosteroids in PRECISE-1.20
Limitations of this post hoc analysis should be considered when interpreting the results. First, the small number of patients per group due to subgroup stratification limited the statistical power of this exploratory analysis. Also, although trends seemed apparent for vedolizumab over placebo across subgroups, not all differences reached statistical significance, except where the treatment effect was at its greatest in the combined corticosteroid subgroup (vedolizumab plus corticosteroid). Second, the differences in prior anti-TNFα treatment and anti-TNFα failure history between the patient populations in GEMINI 2 (48% prior failure) and GEMINI 3 (76% prior failure) may have influenced clinical outcomes with vedolizumab plus corticosteroids versus placebo plus corticosteroids or vedolizumab alone. However, this possibility could not be further investigated because of low patient numbers per subgroup after stratification by anti-TNFα exposure status. Third, differences between studies in assessment timepoints were also a limiting factor in interpreting the results and may have introduced bias to the analysis. Finally, as combination therapy was only followed for the induction phases of 6 weeks and 10 weeks in GEMINI 2 and GEMINI 3, respectively, low numbers of AEs did not allow statistical testing.
CONCLUSION
In conclusion, the present analysis suggests that vedolizumab in combination with stable doses of corticosteroids at baseline may improve induction of clinical response or remission without any notable additional safety and tolerability concerns compared with placebo plus corticosteroids or vedolizumab alone in patients with moderately to severely active CD. In patients who have responded to treatment with vedolizumab, corticosteroids may then be reduced and/or discontinued in accordance with the standard of care. Additional data could confirm whether the favorable outcomes observed during induction continue after patients have tapered corticosteroids and are receiving maintenance therapy. Further insights are expected from an ongoing phase 4 study about the long-term benefits of combined induction therapy with vedolizumab plus corticosteroids versus corticosteroid monotherapy in patients with CD (ClinicalTrials.gov identifier: NCT02324699).21
Supplementary Material
ACKNOWLEDGMENTS
The primary study and this post hoc analysis were sponsored by Takeda. Medical writing support was provided by Claudia Wiedemann, PhD, of Chameleon Medical Communications and Ann Marie Fitzmaurice, PhD, of ProEd Communications, Inc., and funded by Takeda.
Conflicts of Interest: GAU is affiliated with Arena Pharmaceuticals, Zug, Switzerland. RIC is affiliated with Monpazier Medical Ltd, Buckinghamshire, United Kingdom. BES has received consulting fees and research grants from 4D Pharma, AbbVie, Allergan, Amgen, Arena Pharmaceuticals, Boehringer-Ingelheim, Capella Biosciences, Celgene, EnGene, Ferring, Gilead, Janssen, Lilly, Lyndra, MedImmune, Oppilan Pharma, Otsuka, Palatin Technologies, Pfizer, Progenity, Rheos Pharmaceuticals, Seres Therapeutics, Synergy Pharmaceuticals, Takeda, Target PharmaSolutions, Theravance Biopharma R&D, TiGenix, and Vivelix Pharmaceuticals; consulting fees and research grants from Celgene, Janssen, Takeda; and honoraria for speaking in a CME program from Vindico Medical, WebMD. GVA reports serving as a Speaker for Takeda, Janssen, AbbVie, Pfizer, Merck; reports serving on advisory boards for Takeda, Janssen, AbbVie, Pfizer; and reports receiving research support from Pfizer and AbbVie. DT, GAU, RIC, and TT were employees of Takeda Pharmaceutical Company Ltd. while the study was conducted and during manuscript development.
Supported by: The preparation of this article was funded by Takeda.
REFERENCES
- 1. Peyrin-Biroulet L, Panés J, Sandborn WJ, et al. . Defining disease severity in inflammatory bowel diseases: current and future directions. Clin Gastroenterol Hepatol. 2016;14:348–354.e17. [DOI] [PubMed] [Google Scholar]
- 2. Gomollón F, Dignass A, Annese V, et al. ; ECCO 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 3. Sandborn WJ, Rutgeerts P, Enns R, et al. . Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–838. [DOI] [PubMed] [Google Scholar]
- 4. Isaacs KL. How rapidly should remission be achieved? Dig Dis. 2010;28:548–555. [DOI] [PubMed] [Google Scholar]
- 5. Ford AC, Bernstein CN, Khan KJ, et al. . Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:590–599; quiz 600. [DOI] [PubMed] [Google Scholar]
- 6. Rezaie A, Kuenzig ME, Benchimol EI, et al. . Budesonide for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2015:CD000296. doi:10.1002/14651858.CD000296.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Irving PM, Gearry RB, Sparrow MP, et al. . Review article: appropriate use of corticosteroids in Crohn’s disease. Aliment Pharmacol Ther. 2007;26:313–329. [DOI] [PubMed] [Google Scholar]
- 8. Feagan BG, Panaccione R, Sandborn WJ, et al. . Effects of adalimumab therapy on incidence of hospitalization and surgery in Crohn’s disease: results from the CHARM study. Gastroenterology. 2008;135:1493–1499. [DOI] [PubMed] [Google Scholar]
- 9. Sands BE, Anderson FH, Bernstein CN, et al. . Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885. [DOI] [PubMed] [Google Scholar]
- 10. Soler D, Chapman T, Yang LL, et al. . The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330:864–875. [DOI] [PubMed] [Google Scholar]
- 11. Entyvio [prescribing information]. Deerfield, IL: Takeda Pharmaceuticals America, Inc; 2014. https://general.takedapharm.com/ENTYVIOPI. Accessed January 2, 2018. [Google Scholar]
- 12. Entyvio [Summary of Product Characteristics]. Taastrup, Denmark: Takeda Pharma A/S; 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002782/WC500168528.pdf Accessed January 2, 2018. [Google Scholar]
- 13. Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721. [DOI] [PubMed] [Google Scholar]
- 14. Sands BE, Feagan BG, Rutgeerts P, et al. . Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618–627.e3. [DOI] [PubMed] [Google Scholar]
- 15. Terdiman JP, Gruss CB, Heidelbaugh JJ, et al. ; AGA Institute Clinical Practice and Quality Management Committee American Gastroenterological Association Institute guideline on the use of thiopurines, methotrexate, and anti-TNF-α biologic drugs for the induction and maintenance of remission in inflammatory Crohn’s disease. Gastroenterology. 2013;145:1459–1463. [DOI] [PubMed] [Google Scholar]
- 16. Sandborn WJ, Colombel JF, Enns R, et al. ; International Efficacy of Natalizumab as Active Crohn’s Therapy (ENACT-1) Trial Group; Evaluation of Natalizumab as Continuous Therapy (ENACT-2) Trial Group Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353:1912–1925. [DOI] [PubMed] [Google Scholar]
- 17. Colombel JF, Sands BE, Rutgeerts P, et al. . The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3–15. [DOI] [PubMed] [Google Scholar]
- 19. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. . Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–33; quiz 591. [DOI] [PubMed] [Google Scholar]
- 20. Sandborn WJ, Feagan BG, Stoinov S, et al. ; PRECISE 1 Study Investigators Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357:228–238. [DOI] [PubMed] [Google Scholar]
- 21. US Department of Health and Human Services. Corticosteroids With Vedolizumab in Crohn’s Disease. NCT02324699.https://clinicaltrials.gov/ct2/show/NCT02324699 Accessed February 14, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.