Abstract
Objective
We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to evaluate the efficacy of cognitive stimulation therapy (CST) of different durations for Alzheimer’s disease (AD).
Methods
A comprehensive search was carried out in three databases. The primary outcome was Mini-Mental State Examination (MMSE) score. We conducted a meta-analysis with Review Manager, version 5.3 and assessed the methodological quality of the included studies using the Cochrane Collaboration Recommendations assessment tool.
Results
Treatment effects from the meta-analysis showed that CST plus acetylcholinesterase inhibitors (ChEIs) was better than the control assessed by MMSE. In addition, the meta-analysis indicated that long-term CST was better than short-term or maintenance CST.
Conclusion
Our study confirmed that the combination of CST and drug treatment for AD is effective in AD, regardless of whether short-term CST, maintenance CST, or long-term CST is used. The long-term CST appears to be more effective.
Keywords: cognitive stimulation therapy, Alzheimer’s disease, cognitive symptom, meta-analysis
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease which is also the most common type of dementia. The major clinical symptoms of AD are impairment in memory, language, judgement and observational ability, as well as behavioral and personality change.1 These symptoms seriously affect daily life.
The main drugs used for the treatment of AD are cholinesterase inhibitors, such as donepezil, and excitatory amino acid receptor antagonists, such as memantine hydrochloric acid. In addition, many ongoing studies are developing new drugs. However, recently, several drug experiments have been terminated in phase 3 or 4, because they did not meet any of their co-primary efficacy endpoints.2,3 Thus, pharmacotherapy research on AD is at a bottleneck. This presents a good opportunity to test non-drug treatments in clinical practice.
Despite decades of research, options for treating AD are various among the non-pharmacological therapies,4 especially complementary & alternative therapies5 and non-invasive brain stimulation techniques,6 but the effects of the current therapies remain unclear. In recent years, there has been growing interest in cognitive stimulation therapy for people with AD, starting with increasing evidence of improving memory, cognitive skills and quality of life.7 Alzheimer’s Research UK advocates the use of cognitive stimulation. Cognitive stimulation therapy (CST), is a short-term treatment for those with mild to moderate dementia, including dementia caused by AD.8 Cognitive stimulation was first introduced in 1994 and was designed through systematically review.9 Group CST treatment involves 14 or more sessions of themed activities, which typically run twice weekly for 7 weeks. Longer-term, or “maintenance CST,” has been outlined in a published treatment manual.10 Both the CST and maintenance CST trials found that CST is a more cost-effective therapy than placebos, in terms of cognition and quality of life. Meanwhile, a long-term RCT has indicated that CST has a better effect on AD than did a control group.
Although many studies on CST have been conducted in varying treatment periods, there is still a lack of systematic review and meta-analysis which could be instructive in the treatment of AD. Therefore, we conducted a systematic meta-analysis to review the efficacy of cognitive stimulation therapy, with standard usual care, in adults with Alzheimer’s Disease.
Methods
This systematic review and meta-analysis was conducted in accordance with the Cochrane Handbook for the Systematic Review of Interventions11 and the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines.12
Search strategy
For this systematic review and meta-analysis, two authors (JC, YD) implemented computerized searches of the MEDLINE, EMBASE and PsycINFO through the OVID database and CENTRAL through the Cochrane Library. Sources were searched from their inception dates to May 25, 2019, using search terms for cognitive stimulation therapy (eg, “cognitive stimulation” OR “CST” OR “stimulations, cognitive”) and Alzheimer’s disease (eg, “disease, Alzheimer” OR “Alzheimer dementia” OR “Alzheimer type dementia”). Detailed search strategies used in all databases are presented in the Supplementary material. These searches have been complemented by examining review articles.
Study selection
Two reviewers (JC, YD) independently screened the titles, abstracts, and selected full texts of articles for inclusion using a study eligibility form based on items from the inclusion/exclusion criteria. Disagreements were resolved by discussion and consultation with a third reviewer (CT). Only articles published in English, available in full text, and reporting the results of randomized controlled trials were included. There was no restriction on the age or gender of participants.
We included studies investigating participants with AD, as diagnosed by the National Institute of Neurological and Communication Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA) criteria or the Diagnostic and Statistical Manual of Mental Disorders (DSM). We excluded studies investigating participants with mild cognitive impairment (MCI) or other types of non-AD dementia. Only studies on cognitive stimulation therapy or combinations of cognitive stimulation therapy and ChEIs were included. There was no restriction on the intervention parameters (eg duration, course). According to existed studies, Short-term CST is less than 3 months. Maintenance CST is 3 months to 6 months, and Long-term CST is more than one year.13 Studies with no comparison intervention were excluded. Control interventions included usual care or CHEIs. Studies were included if they assessed cognitive functioning. We included only randomized trials and excluded single-arm trials (ie those with no comparison group), case series, and case reports.
Methodological quality
Two reviewers (HL, JL) independently assessed the methodological quality of the included studies using the Cochrane Collaboration Recommendations assessment tool.11 This is a tool for assessing 7 domains, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding (or masking) of outcome assessors, incomplete outcome data, selective reporting and other bias. Based on these items, studies in which the key domains were all low-risk were considered low-risk. The remainder were deemed either high-risk or unclear-risk, depending on the number of key domains of high- or unclear- risk. Disagreements between the review authors over the risk of bias were resolved by discussion with another author (CT).
Data extraction
Two authors (JC, YD) devised a pre-determined data extraction sheet and extracted the data from the selected studies. Another author (LL) reviewed the data. The extracted information included study design, year, first author, sample size, demographic characteristics of the participants, details of the intervention and control groups, outcomes, and information for assessing the risk of bias.
Statistical analysis
We conducted a meta-analysis using Review Manager, version 5.3 (Cochrane, London, UK). For the meta-analyses, mean differences (MD) and 95% CIs were used for continuous data. All outcome measures were estimated based on the change from baseline to follow-up.
We examined heterogeneity among the studies using the Higgins I2 test. We considered low to be where I2 < 25%, moderate where I2 ≥ 50%, and high where I2 ≥ 75%.14 Fixed-effects models were used when I2 < 50%, and random-effects models were used when I2 ≥ 50%.15 If there were enough studies to further classify the CST or control groups, we did additional subgroup analysis
Combining studies providing only positive and negative results with studies of different treatment courses could have led to biased results. Therefore, we stratified several pre-defined subgroup analyses to assess the effects.
Patient and public involvement
Patient and Public Involvement are not involved as this study is a meta-analysis based on published studies.
Results
Study description
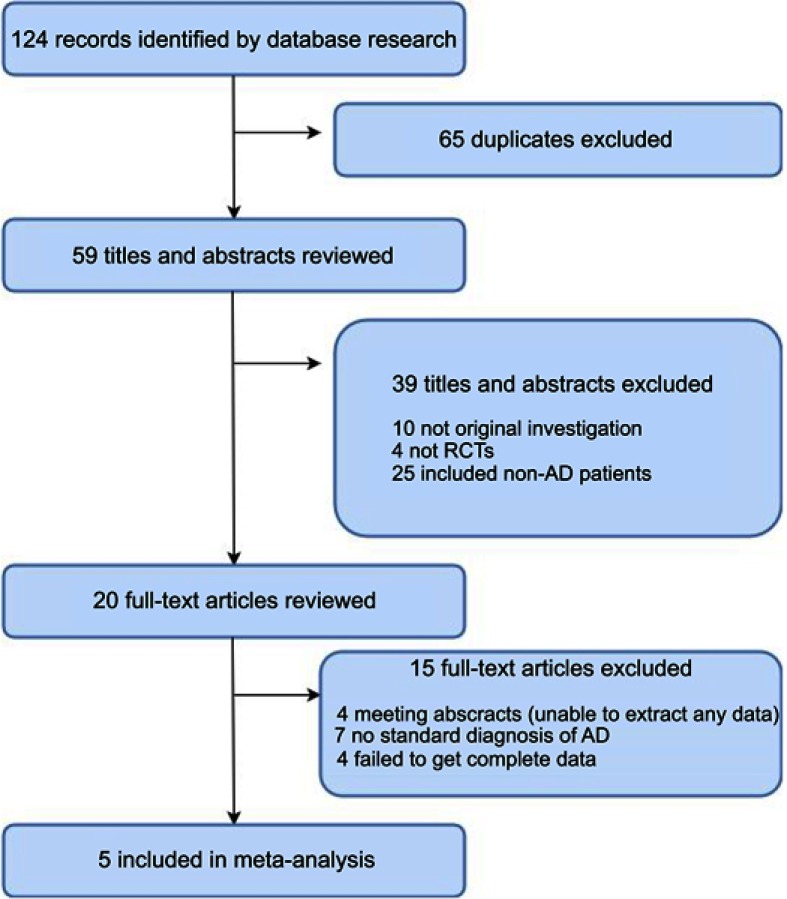
The systematic search first identified 124 records in all databases. After removing duplicates, 59 records remained. A review of the titles and abstracts excluded 39 records. After assessing the full text of the remaining 20 articles, five studies that met all eligibility criteria were included in our analysis (Figure 1). Table 1 shows the detailed characteristics of the included studies.16–20
Figure 1.
PRISMA flow chart of the literature screening and selection process.
Abbreviations: AD, Alzheimer's disease; RCTS, randomized controlled trials.
Table 1.
Baseline of included studies
| Year | First author | Study design | Principle health problem |
Mean age in years | SEX | MMSE Inclusion criteria |
Final sample size | Intervention | Duration | Control | Main outcomes | Main findings | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | Domingo J | Double-blind RCT |
AD | No mention | M/F (54/66) | ≥18 | 120 | MBAS+ChEIs; CST+ChEIs;PMR+ChEIs |
2 years | ChEIs | MMSE; CAMCOG | The mindfulness group showed significant scores compared with the control and muscle relaxation groups. Group cognitive stimulation evolved better than the control group but not better than the muscle relaxation group. | low |
| 2015 | D’Onofrio | single-blind RCT | AD | 78.19 | M/F (42/48) | ≥10 | 90 | CST+ChEIs | 6-month | ChEIs | MMSE;CDR;HAMD;NPI | The study showed that the integrated treatment of RTP with CS in AD patients for 6 months improved significantly cognition, depressive and neuropsychiatric symptoms, fuctional status, and mortality risk in comparison with a group of AD patients receiving only RTP. | low |
| 2006 | L Ta´rraga | Single-blind, pilot RCT | AD | 76.7 | F (84.78) | 18–24 | 43 | CST+ChEIs | 24 weeks | ChEIs | ADAS-Cog;MMSE | Cognitive stimulation treatment improved cognition in patients who were treated with a stable dose of ChEI, compared with those who were treated only with ChEIs. | Unclear |
| 2006 | Osamu Matsuda | A single-blind RCT | AD | 70.77 | M/F (10/20) | No mention | 30 | CST+ChEIs | 1 year | ChEIs | MMSE | The results of this study suggest that CST may be an important component of therapy for mild AD treated with acetylcholinesterase inhibitors. | Unclear |
| 2010 | Yi-Xuan Niu | Rater-blind RCT | Mild to moderate AD | 79.85 | M/F (25/7) | 10–24 | 32 | CST+ChEIs | 10 weeks | ChEIs | NPI; MMSE | The study showed that cognitive stimulation therapy has significant efficacy in lowering apathy and depression symptomatology and in the Mini Mental State Examination in patients with mild to moderate AD. | Low |
Abbreviations: AD, Alzheimer’s disease; CST, cognitive stimulation therapy; ChEIs, acetylcholinesterase inhibitor; MMSE, Mini-Mental State Examination; RCT, randomized controlled trials; ADAS-Cog, Alzheimer’s Disease Assessment Scale-cognitive; NPI, neuropsychiatric inventory; MBAS, The mindfulness-based Alzheimer's stimulation; PMR, progressive muscle relaxation; CAMCOG, Cambridge Cognitive Examination; CDR, Clinical Dementia Rating; HAMD, Hamilton Rating Scale for Depression; RTP, rivastigmine transdermal patch.
In total, 315 patients were included in this meta-analysis. The included studies were published between 1994 and 2017, inclusive. Experimental interventions involved cognitive stimulation therapy in all the included studies. Among 5 trials, 1 study used CST for less than three months’ duration, while 2 studies used CST between 3 months and 6 months’ duration. Two studies used CST for more than one year’s duration. Moreover, 1 mid-term study reported short-term data, and 1 long-term study reported mid-term data. As outcome measures, MMSE was evaluated in 5 trials; NPI was evaluated in 2 trials.
Quality assessment
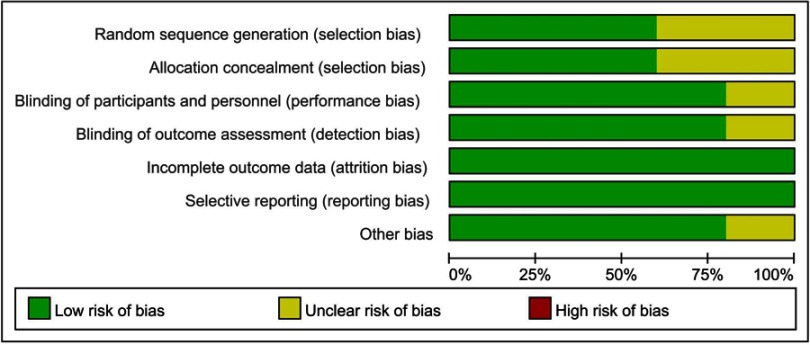
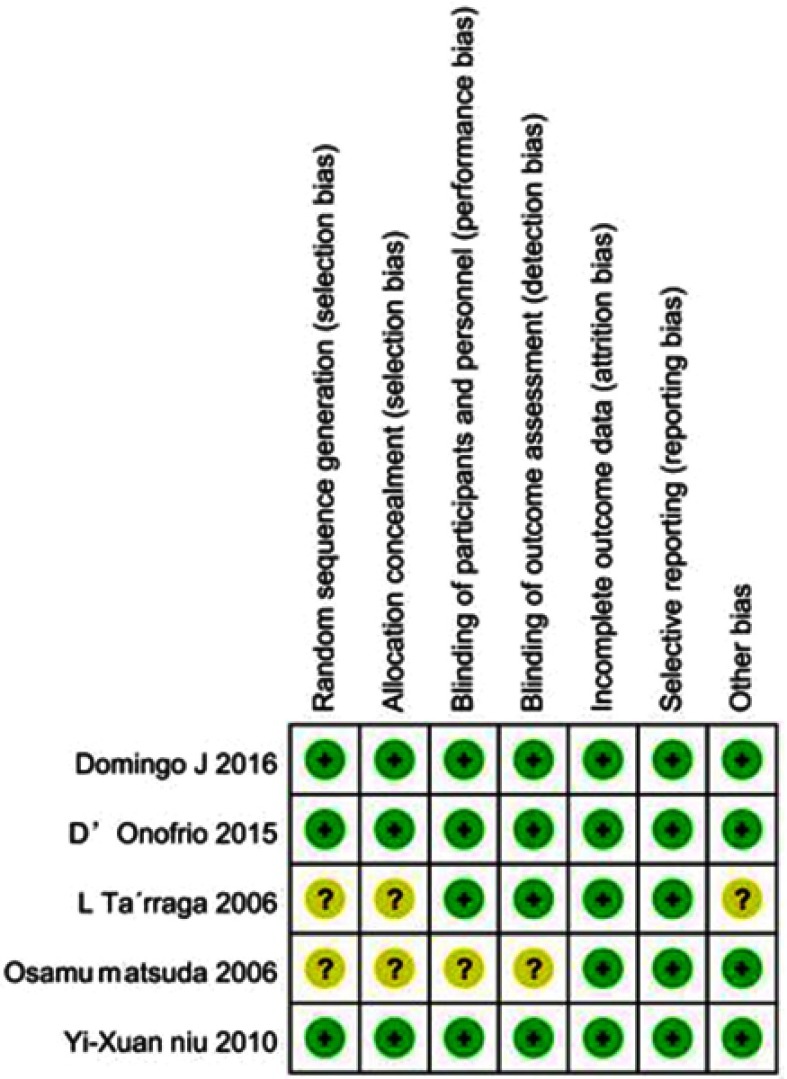
We evaluated the quality of included studies with the Cochrane Collaboration Recommendations assessment tools. Of the 5 trials included, only 3 had used a computer-generated random number or a random number table and had been rated as having “low-risk” of bias in random sequence generation. Allocation concealment was performed using an appropriately sealed method in three studies. One study was evaluated as “unclear” due to lack of information. Blinding of participants and outcome assessments were reported in 4 trials. All studies had “low-risk” of attrition bias and reporting bias, as they had reported all scheduled results. Except for one study with possible baseline imbalances among participants in the experimental and control groups, the remaining four were evaluated as being “low-risk” in the other bias domain. Overall, 3 studies were considered low-risk, while 2 were considered unclear-risk. Methodological assessments for each included study are presented in Figures 2 and 3.
Figure 2.
Risk of bias graph.
Figure 3.
Risk of bias summary.
Efficacy of cognitive stimulation therapy
Cognitive function
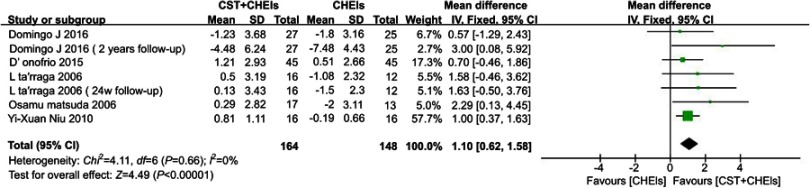
Five studies assessed cognition function by using the Mini-Mental State Examination (MMSE). It was a significant effect for cognitive stimulation therapy plus phamacotheray in AD compared to the control group (MD 1.10, 95% CI 0.62 to 1.58, I2 = 0%, P<0.0001) (Figure 4).
Figure 4.
Forests plots for comparison of CST plus ChEIs versus ChEIs alone assessed by MMSE.
Abbreviations: CST, cognitive stimulation therapy; ChEIs, acetylcholinesterase inhibitor; MMSE, Mini-Mental State Examination.
Behaviours
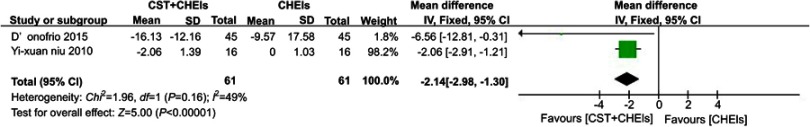
Two studies used neuropsychiatric inventory (NPI) to assess behaviours that commonly occur in dementia. They indicated that the comparison of CST and CHEIs was significantly more effective than CHEIs alone (MD −2.14, 95% CI −2.98 to −1.30, I2 =49%, P<0.0001) (Figure 5).
Figure 5.
Forests plots for comparison of CST plus ChEIs versus ChEIs alone assessed by NPI.
Abbreviations: CST, cognitive stimulation therapy; ChEIs, acetylcholinesterase inhibitor; NPI, neuropsychiatric inventory.
Subgroup analysis of different courses of cognitive stimulation therapy
Cognitive function
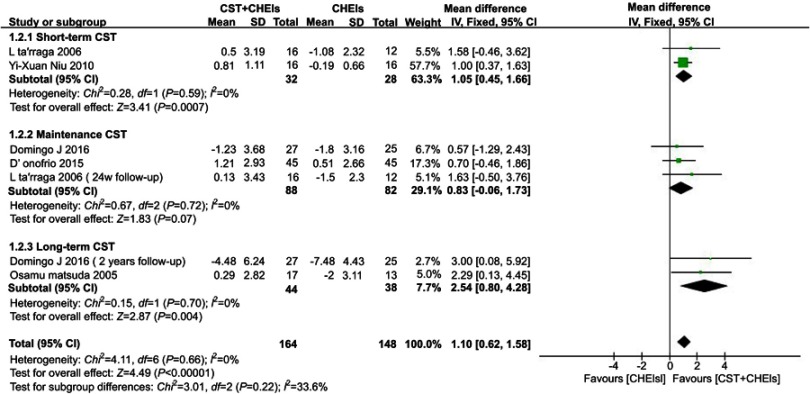
Studies which measured cognitive function using MMSE showed differences in favour of the combined treatment of short-term maintenance and long-term CST and drugs (short-term CST:MD 1.05, 95% CI 0.45 to 1.66, I2 = 12%, P=0.0007; Maintenance CST:MD 0.83, 95% CI −0.06 to 1.73, I2 = 0%, P=0.07; long-term CST:MD 2.54, 95% CI 0.80 to 4.28, I2 = 0%, P=0.004) (Figure 6).
Figure 6.
Forest plots for the comparison of short-term/maintenance/long-term CST plus ChEIs and ChEIs alone in MMSE.
Abbreviations: CST, cognitive stimulation therapy; ChEIs, acetylcholinesterase inhibitor; MMSE, Mini-Mental State Examination.
Discussion
We have provided a systematic review and meta-analysis of cognitive stimulation therapy for people suffering from AD. We have pointed out their main characteristics and goals, and the scientific evidence in support of cognitive and behavioral improvement. The main findings were that (a) cognitive function and neuropsychiatric symptoms can be improved through cognitive stimulation therapy in people with AD; (b) long-term CST showed a better effect on the rehabilitation of cognitive function; and (c) CST showed that it is effective as a complementary therapy for AD pharmacotherapy. These findings highlight a new rehabilitation opportunity for people living with Alzheimer’s disease, and they have implications for improving cognitive function.
The World Alzheimer’s Report21 published in 2011 stated that CST should be routinely given to people with early stage dementia. A number of studies have shown that CST has an effect on cognitive function and quality of life in different outcome measures.22,23 MMSE, ADAS-Cog (Alzheimer’s Disease Assessment Scale-cognitive), QoL-AD (Quality of Life – Alzheimer’s Disease). They have also shown that NPI are specific outcome measures for AD. Fewer studies were included in the analysis using ADAS-Cog and QoL, and therefore the outcome assessments of our review were MMSE and NPI. Based on our research, we have supplemented the evidence that CST combined with drugs is an effective treatment for AD. Our review found that the combined treatment had a significantly different effect on MMSE than did drug treatment alone, regardless of the CST course. In addition, in order to further analyze which course of CST was more effective, we conducted a subgroup analysis. The benefits of cognitive stimulation therapy for patients with AD were found mostly in the long-term follow-up period for cognitive outcomes. Moreover, our study provides evidence of the effects of cognitive stimulation intervention combined with cholinesterase inhibitor therapy on neuropsychiatric outcomes. However, the number of trials did not satisfy our pre-defined inclusion criteria.
While synapse changes, especially presynaptic changes, and cognitive decline in AD is well documented,24,25 very little is known about the possible mechanisms of the effects of CST. It is commonly accepted that exercise can promote synaptic plasticity, which believed to be crucial to cognitive process.26,27 Moreover, an increasing amount of studies showed deep brain stimulation can modulate neuronal activity within memory circuits for AD.28 In general, the research hotspot of AD regulation mechanism is mostly focused on neuronal loss, neuroplastic cortical phenomena29 and neural plasticity, similarly to other neurological and psychiatric diseases like depression.30 Accordingly, a recent longitudinal study demonstrated that an activity which stimulated the brain might be a preventive factor for Alzheimer’s disease and related dementias as theorized in cognitive reserve research.31 Therefore, it may be likely that cognitive stimulation may be leading to multiple modulation liked above. More work is warranted to identify the mechanisms of the effect of CST.
This review has several limitations which warrant consideration. First, the low methodological quality of some studies remains a challenge. When evaluating these studies, we found that many lacked details on randomization. The low methodological quality of some studies could have led to over-estimation of the effects of CST on AD. Second, although we evaluated the studies according to the tool, any evaluation of bias is subjective. There is no quantitative index that can evaluate only artificial risk of bias. Third, because we used strict inclusion and exclusion criteria, the number of included studies was less than it could have been otherwise. This may have influenced the strength of the evidence. Additionally, although we reviewed the articles carefully and extracted data from different nodes, some of the included studies did not provide data on the CST durations. Furthermore, only one outcome measure was used in all included studies. MMSE have some major limitations, especially when it comes to sensitivity and test-retest reliability. MMSE is only a brief screening tool, so there may be a test-retest practice effect, which may lead to a non-comprehensive analysis of the efficacy of the intervention group. But in general, MMSE is still the most commonly used assessment tool in clinical practice.
The inclusion of new studies allowed for both a larger population and a greater number of studies. It objectively revealed heterogeneity and low quality of the evidence. This suggests the need for meta-analysis of both more expansive and more sophisticated studies with more specific populations, interventions, co-interventions or outcome measures.
This study also has implications for future research. Firstly, we found that CST was used as a complement to drug therapy in most of the studies. The UK Government NICE guidelines recommend the use of group Cognitive Stimulation instead of drug treatments for individuals with mild to moderate dementia.7 AD is a type of dementia. Thus, we recommend that CST alone should be compared with drug therapy in future studies. This would allow for testing whether CST can be used as an alternative therapy for dementia caused by AD. Furthermore, due to a lack of standardization, there were variations in the types of CST. Therefore, we also suggest that future research standardize and generalize cognitive stimulation treatment for AD. Finally, dementia caregiving is challenging because of the stressful work and adverse effects on the physical and mental health of the caregiver.32,33 An extensive study has indicated that caregivers are more likely to suffer from depression and poorer physical health than are non-caregivers.34 Therefore, we suggest that the quality of life for caregivers of patients with AD should be considered as an additional important outcome measure in future clinical trials.
Conclusion
This review has confirmed the effectiveness of cognitive stimulation therapy in improving the cognitive function of individuals with AD. This is the case, regardless of whether long-term CST, maintenance CST, or long-term CST is used. Nevertheless, the long-term CST appeared to be more effective. However, due to heterogeneity among the trials, as well as the low methodological quality of several of the included studies, additional rigorous, well-designed RCTs with larger sample sizes should be performed.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Project No. 81873375). Juexuan Chen and Yuting Duan should be considered joint senior authors for this study.
Data sharing statement
All data are freely available within the supplementary materials.
Abbreviations list
AD, Alzheimer’s disease; CST, cognitive stimulation therapy; ChEIs, acetylcholinesterase inhibitor; MMSE, Mini–Mental State Examination; ADAS-Cog, Alzheimer’s Disease Assessment Scale-cognitive; QoL-AD, Quality of Life – Alzheimer’s Disease; NPI, neuropsychiatric inventory; RevMan, Review Manager; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analyses; RCTs, randomized controlled trials.
Supplementary materials
Search strategy
MEDLINE (OVID) search strategy, May 25, 2019
exp Dementia.mp.
(Dementias OR Amentia OR Amentias OR Senile Paranoid Dementia OR Dementias, Senile Paranoid OR Paranoid Dementia, Senile OR Paranoid Dementias, Senile OR Senile Paranoid Dementias OR Familial Dementia OR Dementia, Familial
OR Dementias, Familial OR Familial Dementias) .tw.
3. 1 OR 2
4. exp Alzheimer Disease.mp.
5. (Disease, Alzheimer OR Alzheimer Sclerosis OR Sclerosis, Alzheimer OR Alzheimer Syndrome OR Syndrome, Alzheimer OR Alzheimer Dementia OR Dementia, Alzheimer OR Alzheimer-Type Dementia OR Alzheimer Type Dementia OR Dementia, Alzheimer-Type OR Primary Senile Degenerative Dementia OR Dementia, Senile OR Senile Dementia OR Dementia, Alzheimer Type OR Alzheimer Type Dementia OR Senile Dementia, Alzheimer Type OR Alzheimer Type Senile Dementia OR Dementia, Primary Senile Degenerative OR Alzheimer’s Disease OR Disease, Alzheimer’s OR Acute Confusional Senile Dementia OR Senile Dementia, Acute Confusional OR Dementia, Presenile OR Presenile Dementia OR Alzheimer Disease, Late Onset OR Late Onset Alzheimer Disease OR Alzheimer’s Disease, Focal Onset OR Focal Onset Alzheimer’s Disease
OR Familial Alzheimer Disease OR Alzheimer Disease, Early Onset Early Onset Alzheimer Disease OR Presenile Alzheimer Dementia).tw.
6. 4 OR 5
7. exp Cognitive stimulation Therapy.mp.
8. (Therapy, Cognitive stimulation OR cognitive stimulation Therapies OR Therapies, Cognitive stimulation OR CST).tw.
9. 7 OR 8
10. Randomized controlled trial.pt.
11. controlled clinical trial.pt.
12. randomized.ti,ab.
13. placebo.ti,ab.
14. randomly.ti,ab.
15. trial.ti,ab.
16. groups.ti,ab.
17. OR/10-16
18. 3 AND 6 AND 9 AND 17
EMBASE (OVID) search strategy, May 25, 2019
1. exp Dementia.mp.
2. (Dementias OR Amentia OR Amentias OR Senile Paranoid Dementia OR Dementias, Senile Paranoid OR Paranoid Dementia, Senile OR Paranoid Dementias, Senile OR Senile Paranoid Dementias OR Familial Dementia OR Dementia, Familial
OR Dementias, Familial OR Familial Dementias) .tw.
3. 1 OR 2
4. exp Alzheimer Disease.mp.
5. (Disease, Alzheimer OR Alzheimer Sclerosis OR Sclerosis, Alzheimer OR Alzheimer Syndrome OR Syndrome, Alzheimer OR Alzheimer Dementia OR Dementia, Alzheimer OR Alzheimer-Type Dementia OR Alzheimer Type Dementia OR Dementia, Alzheimer-Type OR Primary Senile Degenerative Dementia OR Dementia, Senile OR Senile Dementia OR Dementia, Alzheimer Type OR Alzheimer Type Dementia OR Senile Dementia, Alzheimer Type OR Alzheimer Type Senile Dementia OR Dementia, Primary Senile Degenerative OR Alzheimer’s Disease OR Disease, Alzheimer’s OR Acute Confusional Senile Dementia OR Senile Dementia, Acute Confusional OR Dementia, Presenile OR Presenile Dementia OR Alzheimer Disease, Late Onset OR Late Onset Alzheimer Disease OR Alzheimer’s Disease, Focal Onset OR Focal Onset Alzheimer’s Disease
OR Familial Alzheimer Disease OR Alzheimer Disease, Early Onset Early Onset Alzheimer Disease OR Presenile Alzheimer Dementia).tw.
6. 4 OR 5
7. exp Cognitive stimulation Therapy.mp.
8. (Therapy, Cognitive stimulation OR cognitive stimulation Therapies OR Therapies, Cognitive stimulation OR CST).tw.
9. 7 OR 8
10. Randomized controlled trial.pt.
11. controlled clinical trial.pt.
12. randomized.ti,ab.
13. placebo.ti,ab.
14. randomly.ti,ab.
15. trial.ti,ab.
16. groups.ti,ab.
17. OR/10-16
18. 3 AND 6 AND 9 AND 17
PsycINFO (OVID) search strategy, May 25, 2019
1. exp Dementia.mp.
2. (Dementias OR Amentia OR Amentias OR Senile Paranoid Dementia OR Dementias, Senile Paranoid OR Paranoid Dementia, Senile OR Paranoid Dementias, Senile OR Senile Paranoid Dementias OR Familial Dementia OR Dementia, Familial
OR Dementias, Familial OR Familial Dementias) .tw.
3. 1 OR 2
4. exp Alzheimer Disease.mp.
5. (Disease, Alzheimer OR Alzheimer Sclerosis OR Sclerosis, Alzheimer OR Alzheimer Syndrome OR Syndrome, Alzheimer OR Alzheimer Dementia OR Dementia, Alzheimer OR Alzheimer-Type Dementia OR Alzheimer Type Dementia OR Dementia, Alzheimer-Type OR Primary Senile Degenerative Dementia OR Dementia, Senile OR Senile Dementia OR Dementia, Alzheimer Type OR Alzheimer Type Dementia OR Senile Dementia, Alzheimer Type OR Alzheimer Type Senile Dementia OR Dementia, Primary Senile Degenerative OR Alzheimer’s Disease OR Disease, Alzheimer’s OR Acute Confusional Senile Dementia OR Senile Dementia, Acute Confusional OR Dementia, Presenile OR Presenile Dementia OR Alzheimer Disease, Late Onset OR Late Onset Alzheimer Disease OR Alzheimer’s Disease, Focal Onset OR Focal Onset Alzheimer’s Disease
OR Familial Alzheimer Disease OR Alzheimer Disease, Early Onset Early Onset Alzheimer Disease OR Presenile Alzheimer Dementia).tw.
6. 4 OR 5
7. exp Cognitive stimulation Therapy.mp.
8. (Therapy, Cognitive stimulation OR cognitive stimulation Therapies OR Therapies, Cognitive stimulation OR CST).tw.
9. 7 OR 8
10. Randomized controlled trial.pt.
11. controlled clinical trial.pt.
12. randomized.ti,ab.
13. placebo.ti,ab.
14. randomly.ti,ab.
15. trial.ti,ab.
16. groups.ti,ab.
17. OR/10-16
18. 3 AND 6 AND 9 AND 17
CENTRAL (Cochrane Library) search strategy, May 25, 2019s
1. MeSH descriptor: [Dementia] explode all trees
2. (Dementias or Amentia or Amentias or Senile Paranoid Dementia or Dementias, Senile Paranoid or Paranoid Dementia, Senile or Paranoid Dementias, Senile or Senile Paranoid Dementias or Familial Dementia or Dementia, Familial or Dementias, Familial or Familial Dementias):ti,ab,kw (Word variations have been searched)
3. #1 or #2
4. MeSH descriptor: [Alzheimer Disease] explode all trees
5. (Disease, Alzheimer or Alzheimer Sclerosis or Sclerosis, Alzheimer or Alzheimer Syndrome or Syndrome, Alzheimer or Alzheimer Dementia or Dementia, Alzheimer or Alzheimer-Type Dementia or Alzheimer Type Dementia or Dementia, Alzheimer-Type or Primary Senile Degenerative Dementia or Dementia, Senile or Senile Dementia or Dementia, Alzheimer Type or Alzheimer Type Dementia or Senile Dementia, Alzheimer Type or Alzheimer Type Senile Dementia or Dementia, Primary Senile Degenerative or Alzheimer’s Disease or Disease, Alzheimer’s or Acute Confusional Senile Dementia or Senile Dementia, Acute Confusional or Dementia, Presenile or Presenile Dementia or Alzheimer Disease, Late Onset or Late Onset Alzheimer Disease or Alzheimer’s Disease, Focal Onset or Focal Onset Alzheimer’s Disease
or Familial Alzheimer Disease or Alzheimer Disease, Early Onset Early Onset Alzheimer Disease or Presenile Alzheimer Dementia):ti,ab,kw (Word variations have been searched)
6. #4 or #5
7. MeSH descriptor: [Cognitive stimulation Therapy] explode all trees
8. (Therapy, Cognitive stimulation OR cognitive stimulation Therapies OR Therapies, Cognitive stimulation OR CST):ti,ab,kw (Word variations have been searched)
9. #7 or #8
10. #3 and #6 and #9
Author contributions
JC, YD, HL, LL, JL and CT conceived and designed the study. JC, YD, and LL selected the articles and JC and YD extracted the data. HL, CT and JL analyzed the data. JC, YD, and LL wrote the first draft of the manuscript. HL, CT and JL interpreted the data and wrote the final version of the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Atri A. The alzheimer’s disease clinical spectrum: diagnosis and management. Med Clin North Am. 2019;103(2):263–293. doi: 10.1016/j.mcna.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 2.Ltd TT. Pharmaceuticals innovation in neurodegeneration. 2016; Available from: http://taurx.com/taurx-phase-3-trial-trx-237-007-in-behavioural-variant-frontotemporal-dementia.pdf. Accessed 22July, 2016.
- 3.Honig LS, Vellas B, Woodward M, et al. Trial of solanezumab for mild dementia due to alzheimer’s disease. N Engl J Med. 2018;378(4):321–330. doi: 10.1056/NEJMoa1705971 [DOI] [PubMed] [Google Scholar]
- 4.Abraha I, Rimland JM, Trotta FM, et al. Systematic review of systematic reviews of non-pharmacological interventions to treat behavioural disturbances in older patients with dementia. The SENATOR-OnTop series. BMJ Open. 2017;7(3):e012759. doi: 10.1136/bmjopen-2016-012759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanza G, Centonze SS, Destro G, et al. Shiatsu as an adjuvant therapy for depression in patients with alzheimer’s disease: a pilot study. Complement Ther Med. 2018;38:74–78. doi: 10.1016/j.ctim.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 6.Bordet R, Ihl R, Korczyn AD, et al. Towards the concept of disease-modifier in post-stroke or vascular cognitive impairment: a consensus report. BMC Med. 2017;15(1):107. doi: 10.1186/s12916-017-0869-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Excellence NIfHaC. Dementia: supporting people with dementia and their carers in health and social care. 2006; Available from: https://www.nice.org.uk/guidance/cg42. Accessed November 2006.
- 8.UK AsR. Treatments available. 2018; http://www.alzheimersresearchuk.org/treatments/. Accessed April, 2018.
- 9.VERONIQUE BREUIL RJ, FORETTE AF, Broca H. INSERM. COGNITIVE STIMULATION OF PATIENTS WITH DEMENTIA: PRELIMINARY RESULTS. Int J Geriatr Psychiatry. 1994;9:211–217. doi: 10.1002/gps.930090306 [DOI] [Google Scholar]
- 10.Aguirre ESA, Streater A, Hoe J, Woods B, Orrell M. Making a Difference 2. UK: Hawker Publications; 2011. [Google Scholar]
- 11.Chris Champion CW, Sambunjak D, Willis O, et al. Cochrane handbook for systematic reviews of interventions: cochrane Training; 2017: Available from:http://training.cochrane.org/handbook. Accessed June 2017.
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguirre E, Spector A, Hoe J, et al. Maintenance Cognitive Stimulation Therapy (CST) for dementia: a single-blind, multi-centre, randomized controlled trial of maintenance CST vs. CST for dementia. Trials. 2010;11:46. doi: 10.1186/1745-6215-11-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. doi: 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 16.D’Onofrio G, Sancarlo D, Addante F, et al. A pilot randomized controlled trial evaluating an integrated treatment of rivastigmine transdermal patch and cognitive stimulation in patients with alzheimer’s disease. Int J Geriatr Psychiatry. 2015;30(9):965–975. doi: 10.1002/gps.4247 [DOI] [PubMed] [Google Scholar]
- 17.Matsuda O. Cognitive stimulation therapy for Alzheimer’s disease: the effect of cognitive stimulation therapy on the progression of mild Alzheimer’s disease in patients treated with donepezil. Int Psychogeriatr. 2007;19(2):241–252. doi: 10.1017/S1041610206004194 [DOI] [PubMed] [Google Scholar]
- 18.Niu YX, Tan JP, Guan JQ, Zhang ZQ, Wang LN. Cognitive stimulation therapy in the treatment of neuropsychiatric symptoms in alzheimer’s disease: a randomized controlled trial. Clin Rehabil. 2010;24(12):1102–1111. doi: 10.1177/0269215510376004 [DOI] [PubMed] [Google Scholar]
- 19.Quintana-Hernandez DJ, Miro-Barrachina MT, Ibanez-Fernandez IJ, et al. Mindfulness in the maintenance of cognitive capacities in alzheimer’s disease: a randomized clinical trial. J Alzheimers Dis. 2016;50(1):217–232. doi: 10.3233/JAD-143009 [DOI] [PubMed] [Google Scholar]
- 20.Tarraga L, Boada M, Modinos G, et al. A randomised pilot study to assess the efficacy of an interactive, multimedia tool of cognitive stimulation in alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77(10):1116–1121. doi: 10.1136/jnnp.2005.086074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International AsD. 2011; Available from: http://www.alz.co.uk/research/WorldAlzheimerReport2011.pdf. Accessed September 12, 2011.
- 22.Capotosto E, Belacchi C, Gardini S, et al. Cognitive stimulation therapy in the Italian context: its efficacy in cognitive and non-cognitive measures in older adults with dementia. Int J Geriatr Psychiatry. 2017;32(3):331–340. doi: 10.1002/gps.4521 [DOI] [PubMed] [Google Scholar]
- 23.Orrell M, Spector A, Thorgrimsen L, Woods B. A pilot study examining the effectiveness of maintenance Cognitive Stimulation Therapy (MCST) for people with dementia. Int J Geriatr Psychiatry. 2005;20(5):446–451. doi: 10.1002/gps.1304 [DOI] [PubMed] [Google Scholar]
- 24.Gylys KH, Fein JA, Yang F, Wiley DJ, Miller CA, Cole GM. Synaptic changes in alzheimer’s disease: increased amyloid-beta and gliosis in surviving terminals is accompanied by decreased PSD-95 fluorescence. Am J Pathol. 2004;165(5):1809–1817. doi: 10.1016/s0002-9440(10)63436-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gajera CR, Fernandez R, Postupna N, et al. Mass synaptometry: high-dimensional multi parametric assay for single synapses. J Neurosci Methods. 2019;312:73–83. doi: 10.1016/j.jneumeth.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christie BR, Eadie BD, Kannangara TS, Robillard JM, Shin J, Titterness AK. Exercising our brains: how physical activity impacts synaptic plasticity in the dentate gyrus. Neuromolecular Med. 2008;10(2):47–58. doi: 10.1007/s12017-008-8033-2 [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Mesa Y, Lopez-Ramos JC, Gimenez-Llort L, et al. Physical exercise protects against alzheimer’s disease in 3xTg-AD mice. J Alzheimers Dis. 2011;24(3):421–454. doi: 10.3233/JAD-2011-101635 [DOI] [PubMed] [Google Scholar]
- 28.Aldehri M, Temel Y, Alnaami I, Jahanshahi A, Hescham S. Deep brain stimulation for alzheimer’s disease: an update. Surg Neurol Int. 2018;9:58. doi: 10.4103/sni.sni_32_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pennisi G, Bella R, Lanza G. Motor cortex plasticity in subcortical ischemic vascular dementia: what can TMS say? Clin Neurophysiol. 2015;126(5):851–852. doi: 10.1016/j.clinph.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 30.Cantone M, Bramanti A, Lanza G, et al. Cortical plasticity in depression. ASN Neuro. 2017;9(3):1759091417711512. doi: 10.1177/1759091417711512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clouston SAP, Smith DM, Mukherjee S, et al. Education and cognitive decline: an integrative analysis of global longitudinal studies of cognitive aging. J Gerontol B Psychol Sci Soc Sci. 2019. doi: 10.1093/geronb/gbz053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roth DL, Fredman L, Haley WE. Informal caregiving and its impact on health: a reappraisal from population-based studies. Gerontologist. 2015;55(2):309–319. doi: 10.1093/geront/gnu177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulz R, Sherwood PR. Physical and mental health effects of family caregiving. Am J Nurs. 2008;108(9 Suppl):23–27. quiz 27. doi: 10.1097/01.NAJ.0000336406.45248.4c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinquart M, Sorensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a meta-analysis. Psychol Aging. 2003;18(2):250–267. [DOI] [PubMed] [Google Scholar]