Abstract
Background: CD19-directed chimeric antigen receptor (CAR) T cells have substantial benefit in the treatment of patients with B-cell malignancies. However, despite encouraging therapeutic efficiency, there is limited overall response rate when anti-CD19 CAR-T cells are used to treat patients with relapsed and refractory (R/R) B cell lymphomas. Therefore, it further investigation is urgently needed to improve treatment efficacy.
Method: A combined treatment protocol of CAR-T cell with decitabine (DAC) to treat B cell lymphoma was developed and tested on lymphoma cell lines first, and then efficacy and the underlying mechanism were investigated. After ethical approval was granted, the combined treatment protocol was applied to treat two patients with R/R B-cell lymphomas.
Results: CAR-T cells were prepared successfully, and they recognized CD19 antigen expressed on lymphoma cell lines specifically. Cell-line studies also showed that CD19 antigen expression was increased by DAC pretreatment, and the function of CAR-T cells was not compromised. The cell-line study further demonstrated that lymphoma cells pretreated by DAC responded more to the treatment of CAR-T cells. Two patients with R/R B cell lymphoma were pretreated with DAC then treated with CAR-T, and both achieved complete remission (CR).
Conclusions: The epigenetic modifying drug DAC increases expression of the surface antigen CD19 on lymphoma cells. The DAC pretreatment protocol may lead patients with B cell lymphoma to be more susceptible to adoptive transfer of anti-CD19 CAR-T cells treKeywordsatment.
Keywords: CD19, B cell lymphoma, decitabine (DAC), complete remission, chimeric antigen receptor (CAR) T cells
Background
Despite some treatment success with chemotherapy and hematopoietic stem cell transplantation, long-term survival rates in the majority of B cell lymphoma patients remain unsatisfactory, especially for those with refractory and relapsed (R/R) B cell malignancies. Patients with B cell-derived acute lymphocytic leukemia (ALL) present clinically a far more aggressive disease and usually have a very poor prognosis. Clinical research demonstrates that if patients are able to be treated by allogeneic hematopoietic stem cell transplantation (allo-HSCT), they may have a long-term survival; especially for those with no minimal residual disease (MRD) detectable.1,2 The problem, however, is that after chemotherapy, most patients hardly present clinical conditions suitable for receiving the allo-HSCT treatment.1 Therefore, novel therapeutic regimens are urgently needed for R/R B cell malignancies. Increased evidence shows that by using chimeric antigen receptor (CAR) T cell therapy to treat patients with refractory and relapsed B-ALL and lymphoma, the clinical outcomes are improved significantly.3–6
CARs consist of a single-chain variable fragment (scFv) of a monoclonal antibody that recognizes a tumor antigen, an extracellular spacer region (termed hinge), a transmembrane domain, CD3ζ signaling domain, and usually costimulatory domain(s). CARs are transfected into T cells to express tumor antigen receptors, to enhance T cell activation and function.7–10 CAR-T cells that target the CD19 antigen on the tumor cell surface have been successfully applied to treat patients by us and other researchers.2,5,11 There are many literature reviews and meta-analyses of published clinical trials to demonstrate the efficacy and safety of CD19 and CD20 CAR-T in treatment of B-cell hematologic malignancies. From a review of at least 16 clinic studies (including 195 patients) published so far, the complete remission (CR) rate for B-ALL and non-Hodgkin’s lymphoma (NHL) patients was higher significantly than for other diseases. Accordingly, CAR-T therapy outcome is superior in ALL patients compared with B-cell lymphoma patients with a marginal significance. The literature review also indicates that the effectiveness of CAR-T therapy variable depending on the type of B-cell malignancy. Therefore, improving the efficacy of CAR-T therapy on ALL, lymphoma and chronic lymphocytic leukemia (CLL) will be a long-term effort faced by the field.2,12–17
Decitabine (DAC) is a nucleoside analog. The phosphor-group of DAC can covalently bind with DNA methyltransferase (DNMT) to inhibit its activity. Consequently, DAC shows a role in de-methylation as a hypomethylating reagent (HMA). DNMT usually plays a role in cell differentiation; inhibition of its activity induces apoptosis of tumor cells and activates tumor suppressor gene activities.18–20 Higher dosage of HMA exerts direct toxicities towards lymphoma cells; lower dosage of HMA, however, can modulate gene expression. DAC also upregulates the expression of NK-activating molecules such as NKG2D ligands in tumor cells through epigenetic modulation.21–24 In turn, epigenetic modification may modulate target antigen expression. After treatment by using CD20-targeting mAb rituximab, CD20 expression was induced on lymphoma cells following treatment with the anti-methylation drug, azacytidine (5-AZA).25 However, it is unclear whether CD19 expression can also be induced by DAC treatment on lymphoma cells, to increase the efficacy of CAR-T cell-mediated anti-tumor responses. We therefore hypothesize that combining DAC treatment with CAR-T targeting CD19 may improve treatment outcomes for the disease of B-cell lymphoma.
We undertook this in vitro study to evaluate the impact of DAC on CAR-T cell viability and functionalities, as well as their capacity to potentiate the activity of CAR-T cells towards B cell malignancies. Intriguingly, we demonstrated that DAC is able to augment CD19 expression on surfaces of lymphoma cells (Ramos and Raji), and then the lymphoma cells became more sensitive towards the cytotoxic effect of CAR-T after treatment. With the in vitro data in hand, we further tested the procedure in two patients with relapsed and refractory B cell lymphoma, and as expected both patients achieved CR. Further investigation of more patients is required for further validation.
Methods
Reagents and cell lines
Fluorochrome-conjugated isotype controls against CD19 and 7-AAD were obtained from BD Biosciences (San Jose, CA, USA). CFSE was diluted in dimethyl sulfoxide (DMSO) at a final concentration of 10 μm (kept at −2°C). DAC was purchased from CTTQ Pharmaceutical Co., Ltd, Lianyungang, China. For in vitro studies, DAC was dissolved in PBS to 0.1–1 mM, aliquoted for single use and stored at −20°C. Drugs were used immediately after thawing and treatment of the cell cultures was performed with limited light exposure. All reagents were used according to the manufacturer’s instructions. The percentage of cells in a region of flow cytometric analysis was calculated by FlowJo 7.6. The B cell-derived lymphoma cell lines Ramos and Raji were purchased from the American Type Culture Collection (Manassas, VA, USA). All cell lines were cultured in RPMI-1640 medium (Sigma-Aldrich Co., St Louis, MO, USA) supplemented with 10% heat-inactivated fetal calf serum (Gibco-Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) at 37°C in 5% CO2.
Generation of CAR modified T cells
Peripheral blood mononuclear cells (PBMCs) were obtained from volunteer donors by leukapheresis, and CAR-modified T cells were prepared as described.26 T cells from leukapheresis product were isolated and activated using Miltenyi T cell transact (Miltenyi Biotec, Bergisch Gladbach, Germany), and transduced with PA-019 Lentivirus.
Measurement of transduction efficiency of CD19 CAR transduced T cells
Transduced and non-transduced T cells were harvested, and antibody against CAR was added and incubated at 37°C for 10 min. The cells were then washed 1× with ice-cold 1×PBS and stained with 7-AAD antibody (10 min, light protected). Afterward, the cells were washed 1× with ice-cold 1×PBS and centrifugated. The supernatant was discarded. Transduction efficiency was subjected to fluorescence-activated cell sorting (FACS) analysis (Miltenyi Biotec GmbH, MACSQuant).
Cytotoxicity assays
Lymphoma cells (Ramos and Raji) were labeled and stained by CFSE (37°C, 15 min); cells were washed and centrifuged to eliminate the effect of residual CFSE in the medium. Lymphoma cells were counted, and CD19-specific CAR-T cells (E) were then co-cultured with lymphoma cells (T) in 48-well plastic plates at different E:T ratios (0,5:1,1:1,1:5). In the meantime, activated T cell as effector was co-cultured with targeted cells (Ramos, Raji) as a negative control group. After 24 h, cells were collected from chambers and stained with 7-AAD according to the manufacturer’s instructions. Concomitantly, flow cytometric analysis was performed for evaluation of the cytotoxicity of CD19-specific T cells against targeted cells. The percentage of CAR-T cells cytotoxicity was calculated as: cytotoxicity%=(CFSE positive-7-AAD Percep negative)/(CFSE FITC positive)×100 by FACS analysis.
Cytokine detection assays
Cytokines were quantified using the Human Soluble Protein Master Buffer Kit and analyzed using flow cytometry on MACSQuant.
In vitro treatment of cultured cells with DAC
Lymphoma cells (Ramos and Raji) mixed with different concentrations of DAC were incubated at 37°C in a 5% CO2 incubator. After 24 h, cells were stained with antibody against CD19 and 7-AAD, the expression level of CD19 on cell surface and survival of lymphoma cells were analyzed by flow cytometry. In addition, the same procedure was followed for CAR-T cells treated by DAC. Following treatment, the survival and cytolytic activity of CAR-T cells were analyzed.
RNA extraction and preparation of cDNA
RNA was extracted using the RNA isolater (Vazyme Biotech Co., Ltd, Nanjing, China). The concentration and purity were evaluated using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). RNA was reverse transcribed using the HiScript II Q RT SuperMix for qPCR (Vazyme Biotech Co., Ltd). All reagants were used according to the manufacturer’s instructions. The cDNA was either used for RT-qPCR or stored at −80°C.
Quantitative RT-PCR
Quantitative RT-PCR was performed on the ABIPRISM1 7000 thermal cycler (Applied Biosystems, Foster City, CA, USA). The mixture (20 μL) consisted of 8.5 μL water, 0.7 μL cDNA, 0.8 μL primers (TissF_5′-TGTGGTAATGGAGACGGGT-3′; TissR_5′-AGTGCCATAGTACTGGCCG-3′) and 10 μL AceQ qPCR SYBR Green Master Mix. Each sample was tested in three independent experiments and loaded in triplicates for each of the qRT-PCR experiments.
Immunohistochemistry (IHC) staining
Monoclonal mouse CD19 antibody (LE-CD19) NB100-65672 was from Agilent Technologies (Santa Clara, CA, USA). All reagants were used according to the manufacturer’s instructions. All tissue microarrays were scanned at 40× magnification (Aperio, Leica Microsystems, Buffalo Grove, IL, USA). CD19 immunoreactivity was evaluated in a modified semiquantitative graded criteria based on membrane staining intensity and distribution. CD19ining intensity and distribution modified semiquantitby two authors using below scoring system. The percentage of positive tumor cells was graded as follows: 0, none; 1, 1–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%. Immunostaining intensity was rated as follows: 0, none; 1, weak; 2, moderate; and 3, intense. In this scoring system, each component of the tumor was scored independently and the results were summed. Final score = (sum of (percent positive rating) x (intensity rating)) for each component of the tumor.27
Combined treatment of DAC with anti-CD19 CAR-T cells therapy in relapsed and refractory (R/R) B-NHL patients
Two patients with R/R B-NHL enrolled in the clinical trial (NCT02851589) were recruited in the First Affiliated Hospital of USTC, Hefei, China. The protocol was reviewed and approved by the ethics committee of the First Affiliated Hospital of USTC. All patients submitted written informed consent prior to enrolling in the study. CD19 expression by malignancies was confirmed by immunohistochemistry. A DAC (15 mg/m2/d, day −9 to −5) plus fludarabine (30 mg/m2/d, day −4 to −2) and cyclophosphamide (300 mg/m2/d, day −4 to −2) chemotherapy regimen was administered before anti-CD19 CAR-T cells infusions to enhance the activity of adoptively transferred T cells (Figure 5A). Treatment responses were defined according to standard international criteria.
Figure 5.
Immunohistochemistry analysis of CD19 expression on lymphoma cell lines. The immunohistochemistry pictures of the lymphoma cell lines are shown without (-) or after (+) DAC (150 nmol/L) treatment for 48 h. (A) Illustrations of CD19 expression in RAJI cell surface (×400). (B) Illustrations of CD19 expression in RAMOS cell surface (×400).
Abbreviation: DAC, decitabine.
Statistical analysis
All results are reported as mean ± SEM. Statistical analysis was performed with GraphPad Prism 4.0 (Graph Pad Software, La Jolla, CA, USA). The statistical analyses involved the Student’s t-test to determine the significance of differences between treatment groups. Values of p<0.05 were considered statistically significant.
Results
CD19 CAR-transduced primary human T cells kill CD19+lymphoma cell lines
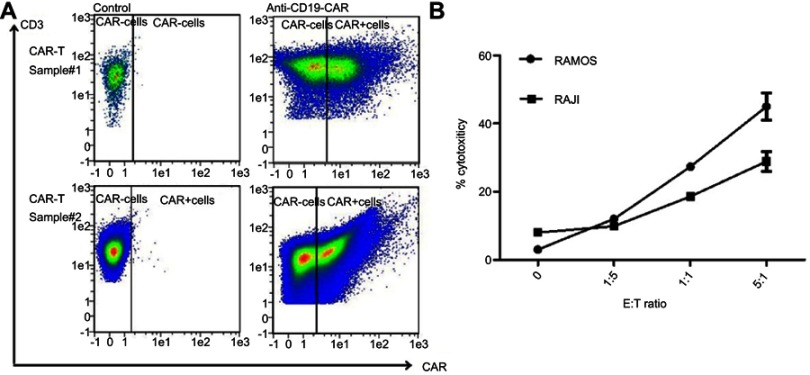
CD19 CAR-T cells were prepared successfully after human peripheral blood CD3+T cells were transduced with high dosage of a retroviral vector encoding CD19 CAR (Figure 1A). The CD19 CAR-T cells recognized and killed CD19+Lymphoma cells of Ramos and Raji in the culture (Figure 1B).
Figure 1.
Human primary T cells transduced by CD19 chimeric antigen receptor (CAR) are able to kill CD19+ human lymphoma cell lines. (A) The transduction efficiency of T cells was measured by fluorescence-activated cell sorting (FACS) analysis as described in Materials and Methods. Two independent experiment results are presented here. (B) The sensitivity of CD19+ NHL lines (Ramos and Raji) to CD19 CAR-T cells at different E:T ratios.
Abbreviation: NHL, non-Hodgkin’s lymphoma.
DAC augments CAR-T cell efficacy against CD19 positive B cell derived malignancies in vitro
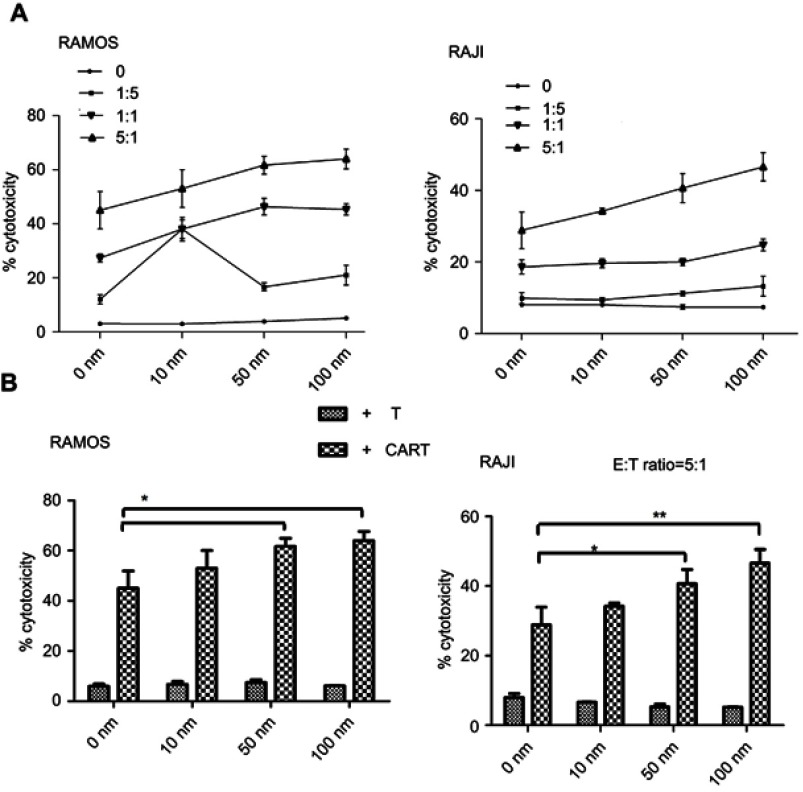
To investigate the possibility of combining CAR-T cell therapy with DAC therapy, we tested whether pretreated lymphoma cells with DAC would influence their susceptibility to CAR-T cell-mediated killing. We pretreated lymphoma cell (T) with DAC and then co-cultured with CAR-T cells (E) or T cells at different E:T ratios. Interestingly, combined treatment demonstrated that sustained killing of lymphoma cells by CAR-T cells was observed at different E:T ratio (Figure 2A), and E:T ratio equal to 5:1 offered the most obvious killing efficiency (Figure 2B). No specific cell lysis was observed when the CD19 negative cell line K562 was treated with the CD19 CAR-T cells (data not shown). The results demonstrated that pretreatment with DAC made lymphoma cells more susceptible to CAR-T killing compared to CAR-T treatment alone. Importantly, our results indicate that there is a potential effect cooperated between CAR-T and DAC against CD19+B cell-derived malignancies.
Figure 2.
The cytotoxicity of CAR-T cells in combination with DAC pretreatment enhanced lymphoma cell killing in vitro. (A) Lymphoma cells were cultured in the presence of DAC using the indicated concentrations and harvested as targets with or without drug wash-out. CAR-T cells were then added at different E:T ratios and co-cultured for another 24 h. The cytotoxicity of CAR-T cell to lymphoma cells was determined by FACS. (B) CAR-T cell (E) mixed with lymphoma cells (T) treated with or without DAC at E:T ratio 5:1 shows the best efficacy for DAC treatment (*p<0.05, **p<0.01, compared with cells without DAC treatment).
Abbreviations: CAR, chimeric antigen receptor; DAC, decitabine.
Exposure to low dose of DAC does not impair lymphoma cell viability
We studied the effect of DAC on the viability of two lymphoma cell lines. Ramos and Raji cells were treated with DAC at different concentrations. As expected, low concentrations of DAC (10, 50, 100 nmol/L) have little effect on the number of viable lymphoma cells compared to untreated cells (data not shown). DAC concentrations of 10, 50 and 100 nmol/L were selected for subsequent studies.
Exposure to low dose of DAC does not impair CAR-T cell cytolytic activity in vitro
We then aimed to determine whether low concentrations of DAC impaired CAR-T cell function. CAR-T cells were cultured in the presence of DAC at 10, 50, 100 nmol/L, respectively, for 24 h. We found that DAC treatment did not affect CAR-T cells viability (data not shown). We then analyzed the cytolytic activity of DAC-treated CAR-T cells against RAJI cells at 1:1 of E:T ratio (Figure 3A), and there is no cytotoxicity difference between CAR-T cells pretreated with the tested dosages of DAC. Similar results were also observed indirectly at the single cell level using IFN-γ and Granzyme B staining (data not shown). As depicted in Figure 3B, C, the cytokine-release experiment showed that all tested concentrations of DAC had no impact on the killing ability of CAR-T cells compared to CAR-T cells without pretreatment. There was no significant difference for IFN-γ and Granzyme B responses between DAC treated and untreated CAR-T cells. Our results strongly suggest that CAR-T cells maintain potent anti-lymphoma activity after DAC exposure.
Figure 3.
Low dose of DAC does not impair CAR-T cell viability, proliferation, and cytolytic functions. RAJI cells were co-cultured with DAC as described in the text, and the drug was then washed out. The same numbers of viable CAR-T cells (E) or T cells (E) were plated with RAJI cells (T) in each experimental well at E:T ratio 1:1, and cultured overnight. Data were analyzed by FACS and depicted as mean ± SEM. MACSQuant was used for statistical analysis. (A) Specific killing of RAJI cells by CAR-T cells and T cells. (B and C) The production capacity of IFNγ or Granzyme B by CAR-T cells and T cells, respectively, after RAJI cells treated with or without DAC.
Abbreviations: CAR, chimeric antigen receptor; DAC, decitabine; FACS, fluorescence-activated cell sorting.
Modulation of CD19 protein and mRNA expression in DAC-treated lymphoma cells
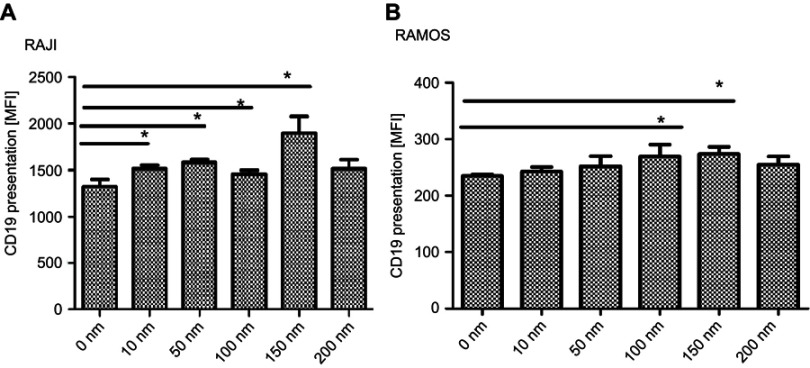
To understand the potential underlying mechanism of the synergistic effect of DAC and CAR-T cells on enhanced lymphoma cell killing, we assessed the CD19 antigen presentation on tumor cells under treatment of different DAC concentration (0, 10, 50, 100, 150, 200 nmol/L) on cultured cancer cells by flow cytometric analysis. The DAC concentration ranging from 10 to 150 nmol/L showed a slight but consistent increase in antigen presentation on Ramos and Raji cells after 48 h co-culture. Surprisingly, DAC doses of 200 nmol/L reduced antigen expression. Furthermore, DAC exposure for 24 h did not induce higher antigen expression when compared to the basal level of untreated (data not shown). Antigen expression on Ramos and Raji cells was already significantly increased after stimulated by DAC for 48 h when compared to untreated cells (Figure 4).
Figure 4.
Analysis of CD19 antigen expression in DAC-treated lymphoma cells. RAJI cells (A) or RAMOS cells (B) were treated with DAC at five different concentrations (10 to 200 nm) for 48 h (n=6). Concentration of DAC at 0 nm was used as the negative control. Data were analyzed using unpaired, two-tailed t-test (*p<0.05).
Abbreviation: DAC, decitabine.
In addition to flow cytometry, we determined the CD19 antigen expression on lymphoma cells using immunohistochemistry. After the treatment of DAC (150 nmol/L) on lymphoma cells lines (RAMOS, RAJI) for 48 h, expression was higher post-DAC than pre-DAC for CD19 antigen (Figure 5). According to the semiquantitative graded criteria (Methods), there was a significant increase in CD19 scores with decitabine in RAMOS cells (post- versus pre-DAC scores 4.67±0.94 versus 2.33±0.47, p<0.01) and RAJI cells (post- versus pre-DAC scores 10.67±1.25 versus 8.33±1.25, p<0.05).
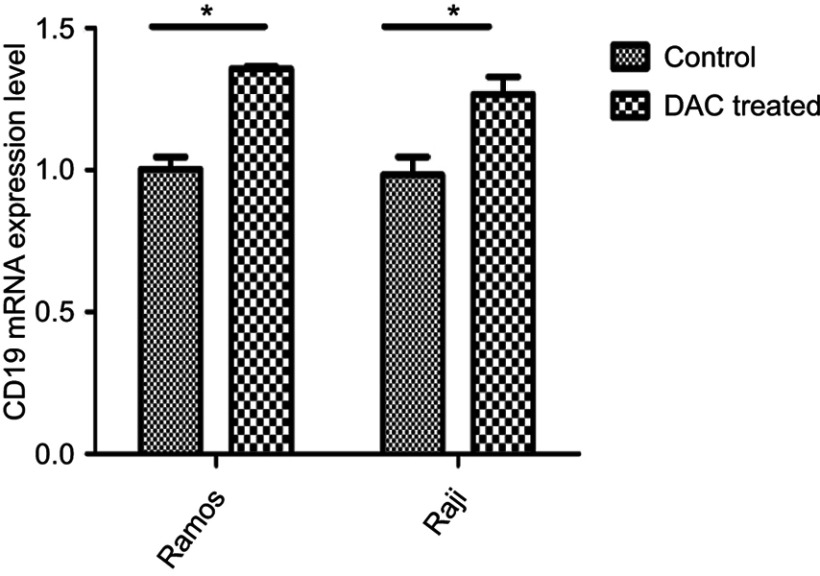
To determine whether the treatment of those tumor cells with DAC would affect the expression of CD19 mRNA level, cells were treated with 150 nmol/L of DAC for 48 h and qRT-PCR was performed. We found that exposure to DAC invariably increased the expression of CD19 in both lymphoma cell lines (RAMOS, RAJI) (Figure 6).
Figure 6.
Effects of DAC treatment on CD19 mRNA in lymphoma cell lines. After lymphoma cell lines (RAJI and RAMOS) pretreated by DAC (150 nmol/L) 48 h, quantified CD19-specific mRNA by qRT-PCR. All data are representative of three independent experiments performed in triplicate. Data were analyzed using unpaired, two-tailed t-test (*p<0.05).
Abbreviation: DAC, decitabine.
Combining decitabine with anti-CD19 CAR-T cells therapy is a feasible and potentially effective immunotherapeutic approach to treating relapsed B-NHL
To test the effect of combined therapy and evaluate the feasibility of DAC with anti-CD19 CAR-T cells in relapsed and refractory (R/R) B-NHL, one patient with R/R mantle cell lymphoma (MCL) and one patient with R/R diffuse large B cell lymphoma (DLBCL) were enrolled for the test. After infusion of CAR-T cells, both patients presented high fever (Figure 7A) with elevated serum cytokine (Figure 7B). The patient with R/R developed severe cytokine release syndrome (CRS), and the patient with R/R DLBCL developed CRS and CAR-related encephalopathy syndrome (CRES). Both patients received Tocilizumab (8 mg/kg) and high-dose glucocorticoid treatment, and then the clinical symptoms were relieved. After treatment procedure, a PET/CT scan showed that patients achieved a second CR and have remained disease-free for 4 months (Figure 7D) and 2 months (Figure 7E), respectively, until now.
Figure 7.
Combining decitabine with anti-CD19 CAR-T cells eradicated large masses of chemotherapy-refractory lymphoma. (A) The temperature curve and treatment of the two patients. Patient 1 was R/R DLBCL, and patient 2 was R/R MCL. (B) The expressions of serum cytokine after CAR infusion. (C) Copy number of CAR DNA after CAR infusion. (D) Positron emission tomography (PET)/computed tomography (CT) scans show CR of R/R DLBCL in patient 1 with extensive abdominal involvement. (E) PET/CT scans demonstrate CR of lymphoma in patient 2 who had R/R MCL with stomach and intestinal involvement.
Abbreviations: CAR, chimeric antigen receptor; CR, complete remission; DLBCL, diffuse large B cell lymphoma; MCL, mantle cell lymphoma; R/R, relapsed and refractory.
Discussion
Patients with relapsed B cell-derived malignancies have an overall poor prognosis. Almost all patients undertake traditional therapy on salvage chemotherapy protocols and allo-HSCT. However, many patients are ineligible for additional transplantation therapy and consequently fail to achieve a second CR.1 A novel therapy – CAR-T cells specifically targeting the CD19 antigen – leads to hopes for a higher rate of CR.2 However, there are serious problems regarding CAR-T applications. In some cases, the tumor relapsed after treatment with CAR-T cell therapy, and tumor burden was increased heavily due to tumor antigen escape.28,29 For instance, in the 2015 American Society of Clinical Oncology annual meeting, Memorial Sloan Kettering Cancer Center (MSKCC) reported that 2 of 14 patients who experienced disease relapse had an outcome of antigen-negative tumor cells.30 Antigen loss renders CAR-T cells unable to defend against B-cell malignancies, which may cause limited success of the broader CAR-T cell therapy. Increasing the CR rate and reducing the recurrence rate are essential problems that need to be addressed in order to deploy CAR-T cell treatment in clinical care.
The first objective of the present study was to develop a protocol that can improve CAR-T cell function to achieve durable tumor remissions. CD19 CAR-T cells exhibit efficient anti-CD19 positive hematologic malignancy activity in vivo as previously described.2,5,31,32 To the best of our knowledge, our study was the first evidence that DAC is able to promote CAR-T cell-mediated anti-leukemic activity in vitro. Consistent with other studies, abnormal hypermethylation occurs in lymphoma patients and their cell lines,33 often in the CpG island within the promoter region of tumor suppressor gene (such as P15INK4B, SHP l, P53), and leading to the silencing of the gene.34,35 Research shows that DAC can inhibit tumor cell growth, through demethylation of tumor suppressor genes that are silenced by methylation of CpG islands on their promoters.36 In line with previous reports, we showed that DAC exerts direct effects on lymphoma cell viability and proliferation. In this study, we demonstrated that DAC, in addition to its demethylation effect directly on tumor cells, can enhance the CAR-T cell-specific killing function. Consequently, we observed that T cells transduced with anti-CD19-CAR combined with DAC presented marked cytotoxicity against lymphoma cells.
Based on these data, we then investigated the possible mechanism of this combined effect. A previous study has reported that epigenetic modulators such as DNA methyltransferase inhibitors, and azacytidine (5-AZA), can re-induce CD20 expression on lymphoma cells after using CD20-targeting mAb rituximab.37 Inducing expression of NY-ESO-1 following the treatment with DAC has also been demonstrated to trigger a functional recognition by CAR-T cells.25 Using the optimized time- and dose-dependent DAC protocol, we observed that DAC treatment clearly induced or upregulated CD19 expression on lymphoma cells (Raji, Ramos). CAR-T cells combined with DAC pretreatment demonstrated that DAC has a broader antitumor repertoire against lymphoma cells, which is consistent with increased antigen stimulation hypothesis.
In our study, we demonstrated that exposure to DAC has minimal impact on CAR-T cell viability, proliferation, and cytolytic functions. Our findings provide a strong rationale to investigate DAC therapy in patients not only prior to adoptive CAR-T cell immunotherapy, but also concomitantly with CAR-T cell infusion to exploit superior efficacy of the combined regimens. Potential clinical applications are broad. For B cell malignancy, current induction remission and consolidation program could be complemented with adoptive CAR-T cells combined with certain doses DAC as an adjuvant consolidation or bridge-to-transplant strategy.
Conclusion
In summary, the sensibility of DAC-treated tumor cell lines (Ramos, Raji) to antigen-CD19 specific CAR-T cell recognition strongly verifies DAC as a pharmacologic agent for future clinical trials in combination with specific T cell immunotherapies for the treatment of patients with relapsed CD19+ B cell-derived malignancies. The combination could further eradicate minimal residual disease (MRD), and ultimately improve the outcome of allogeneic stem cell transplantation.
Acknowledgments
The authors would like to thank all members of the study team, the patient and their family. This work was supported by the Science and Technology Planning Project of Anhui Province, China (Grant No. 1604a0802071).
Abbreviation list
ALL, acute lymphoma leukemia; allo-HSCT, allogeneic hematopoietic stem cell transplantation; AZA, azacytidine; CAR, chimeric antigen receptor; CR, complete remission; CLL, chronic lymphocytic leukemia; CRES, CAR-related encephalopathy syndrome; CT, computed tomography; DNMTs, DNA methyltransferase; DMSO, dimethylsulfoxide; DLBCL, diffuse large B cell lymphoma; FACS, fluorescence-activated cell sorting; HMA, hypomethylating reagent; IFN-γ, interferon-γ; MRD, minimal residual disease; MCL, mantle cell lymphoma; NHL, non-Hodgkin’s lymphoma; PBMCs, peripheral blood mononuclear cells; PET, positron emission tomography; R/R, relapsed and refractory; scFv, single-chain variable fragment; TAA, tumor-associated antigen.
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of USTC, Hefei, People's Republic of China (2016-101). The patient and donor gave their written informed consent in accordance with the Declaration of Helsinki. The clinical trial is registered at https://www.clinicaltrials.gov/ as NCT02851589.
Data sharing statement
On reasonable request, the subject level analysis data sets for the research presented in the publication are available by contacting Xingbing Wang. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations.
Author contributions
All authors made substantial contributions to conception and design,acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Dr Lin Yang reports grants from PersonGen BioTherapeutics, during the conduct of the study. Min Wang, Wenyao Kang, Fengtao You, Hanying Xu, Yu Wang and Lin Yang are employees of PersonGen-Anke Cellular Therapeutics Co., Ltd. The authors report no other conflicts on interest in this work.
References
- 1.Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944–950. doi: 10.1182/blood-2006-05-018192 [DOI] [PubMed] [Google Scholar]
- 2.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38. doi: 10.1126/scitranslmed.3005930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kochenderfer JN, Yu Z, Frasheri D, Restifo NP, Rosenberg SA. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116(19):3875–3886. doi: 10.1182/blood-2010-01-265041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9(3):279–286. doi: 10.1038/nm827 [DOI] [PubMed] [Google Scholar]
- 5.Kenderian SS, Ruella M, Gill S, Kalos M. Chimeric antigen receptor T-cell therapy to target hematologic malignancies. Cancer Res. 2014;74(22):6383–6389. doi: 10.1158/0008-5472.CAN-14-1530 [DOI] [PubMed] [Google Scholar]
- 6.Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112(6):2261–2271. doi: 10.1182/blood-2007-12-128843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudecek M, Sommermeyer D, Kosasih PL, et al. The non-signaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res. 2015;3(2):125–135. doi: 10.1158/2326-6066.CIR-14-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17(8):1453–1464. doi: 10.1038/mt.2009.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Z, Condomines M, van der Stegen SJ, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28(4):415–428. doi: 10.1016/j.ccell.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20(1):70–75. doi: 10.1038/nbt0102-70 [DOI] [PubMed] [Google Scholar]
- 11.Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol. 2013;10(5):267–276. doi: 10.1038/nrclinonc.2013.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Naranjo A, Brown CE, et al. Phenotypic and functional attributes of lentivirus-modified CD19-specific human CD8+central memory T cells manufactured at clinical scale. J Immunother. 2012;35(9):689–701. doi: 10.1097/CJI.0b013e318270dec7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–549. doi: 10.1200/JCO.2014.56.2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–2720. doi: 10.1182/blood-2011-10-384388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Zhang WY, Han QW, et al. Effective response and delayed toxicities of refractory advanced diffuse large B-cell lymphoma treated by CD20-directed chimeric antigen receptor-modified T cells. Clin Immunol. 2014;155(2):160–175. doi: 10.1016/j.clim.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 16.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones P, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juttermann R, Li E, Jaenisch R. Toxicity of 5-aza-2′-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc Natl Acad Sci USA. 1994;91(25):11797–11801. doi: 10.1073/pnas.91.25.11797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruijsen M, Lubbert M, Wijermans P, Huls G. Clinical Results of Hypomethylating Agents in AML Treatment. J Clin Med. 2014;4(1):1–17. doi: 10.3390/jcm4010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohner A, Langenkamp U, Siegler U, Kalberer CP, Wodnar Filipowicz A. Differentiation-promoting drugs up-regulate NKG2D ligand expression and enhance the susceptibility of acute myeloid leukemia cells to natural killer cell-mediated lysis. Leuk Res. 2007;31(10):1393–1402. doi: 10.1016/j.leukres.2007.02.020 [DOI] [PubMed] [Google Scholar]
- 22.Schmiedel BJ, Arelin V, Gruenebach F, Krusch M, Schmidt SM, Salih HR. Azacytidine impairs NK cell reactivity while decitabine augments NK cell responsiveness toward stimulation. Int J Cancer. 2011;128(12):2911–2922. doi: 10.1002/ijc.25635 [DOI] [PubMed] [Google Scholar]
- 23.Zhu Z, Lu X, Jiang L, et al. STAT3 signaling pathway is involved in decitabine induced biological phenotype regulation of acute myeloid leukemia cells. Am J Transl Res. 2015;7(10):1896–1907. [PMC free article] [PubMed] [Google Scholar]
- 24.Baragano Raneros A, Martin-Palanco V, Fernandez AF, et al. Methylation of NKG2D ligands contributes to immune system evasion in acute myeloid leukemia. Genes Immun. 2015;16(1):71–82. doi: 10.1038/gene.2014.58 [DOI] [PubMed] [Google Scholar]
- 25.Hiraga J, Tomita A, Suzuki N, Takagi Y, Narita M, Kagami Y. Partial restoration of CD20 protein expression and rituximab sensitivity after treatment with azacitidine in CD20-negative transformed diffuse large B cell lymphoma after using rituximab. Ann Hematol. 2018;97(11):2253–2255. doi: 10.1007/s00277-018-3354-1 [DOI] [PubMed] [Google Scholar]
- 26.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR–T cells of defined CD4+: CD8+composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–2138. doi: 10.1172/JCI85309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krajewska M, Krajewski S, Epstein JI, et al. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol. 1996;148:1567–1576. [PMC free article] [PubMed] [Google Scholar]
- 28.Dai H, Zhang W, Li X, et al. Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. Oncoimmunology. 2015;4(11):e1027469. doi: 10.1080/2162402X.2015.1027469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park JH, Riviere I, Wang X, et al. Efficacy and safety of CD19-targeted 19-28z CAR modified T cells in adult patients with relapsed or refractory B-ALL. J Clin Oncol. 2015;33(15_suppl):7010. doi: 10.1200/jco.2015.33.15_suppl.7010 [DOI] [Google Scholar]
- 31.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. doi: 10.1126/scitranslmed.3008226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esteller M. Epigenetic gene silencing in cancer: the DNA hypermethylome. Hum Mol Genet. 2007;16:R50–9. doi: 10.1093/hmg/ddm018 [DOI] [PubMed] [Google Scholar]
- 34.Ferreira HJ, Esteller M. CpG islands in cancer: heads, Tails, and Sides. Methods Mol Biol. 2018;1766:49–80. doi: 10.1007/978-1-4939-7768-0_4 [DOI] [PubMed] [Google Scholar]
- 35.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349(21):2042–2054. doi: 10.1056/NEJMra023075 [DOI] [PubMed] [Google Scholar]
- 36.Negrotto S, Ng KP, Jankowska AM, et al. CpG methylation patterns and decitabine treatment response in acute myeloid leukemia cells and normal hematopoietic precursors. Leukemia. 2012;26(2):244–254. doi: 10.1038/leu.2011.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klar AS, Gopinadh J, Kleber S, et al. Treatment with 5-Aza-2ʹ-DEOXYCYTIDINE INDUCES Expression of NY-ESO-1 and facilitates cytotoxic T lymphocyte-mediated tumor cell killing. PLoS One. 2015;10(10):e0139221. doi: 10.1371/journal.pone.0139221 [DOI] [PMC free article] [PubMed] [Google Scholar]