Abstract
The metabolic hormone adiponectin is secreted into the circulation by adipocytes and mediates key biological functions, including insulin sensitivity, adipocyte development, and fatty acid oxidation. Adiponectin is also abundant in saliva, where its functions are poorly understood. Here we report that murine taste receptor cells (TRCs) express specific adiponectin receptors and may be a target for salivary adiponectin. This is supported by the presence of all three known adiponectin receptors in transcriptomic data obtained by RNA-seq analysis of purified circumvallate (CV) taste buds. As well, immunohistochemical analysis of murine CV papillae showed that two adiponectin receptors, ADIPOR1 and T-cadherin, are localized to subsets of TRCs. Immunofluorescence for T-cadherin was primarily co-localized with the Type 2 TRC marker phospholipase C β2, suggesting that adiponectin signaling could impact sweet, bitter, or umami taste signaling. However, adiponectin null mice showed no differences in behavioral lick responsiveness compared with wild-type controls in brief-access lick testing. AAV-mediated overexpression of adiponectin in the salivary glands of adiponectin null mice did result in a small but significant increase in behavioral lick responsiveness to the fat emulsion Intralipid. Together, these results suggest that salivary adiponectin can affect TRC function, although its impact on taste responsiveness and peripheral taste coding remains unclear.
Keywords: behavior, gustation, lipids, saliva, taste buds, T-cadherin
Introduction
Recently, numerous peptides that can function as metabolic hormones, or their cognate receptors, have been detected in saliva and/or in taste receptor cells (TRCs) (Zolotukhin 2013). Of the many peptides present in the oral cavity, several appear to modulate taste-evoked behavioral responses (Dotson et al. 2013). For example, both glucagon-like peptide-1 (GLP-1) and glucagon signaling impact behavioral taste responsiveness to sweet stimuli (Shin et al. 2008; Elson et al. 2010; Takai et al. 2015), angiotensin-2 impacts salt taste (Shigemura et al. 2013), and peptide YY (PYY) signaling is implicated in the modulation of orosensory responses to lipids (La Sala et al. 2013). However, the full impact of peptide signaling on taste transduction remains poorly understood.
The anatomical proximity of salivary-expressed peptides with the peripheral gustatory system provides an opportunity for salivary peptides to impact peripheral taste function. Indeed, we previously reported that salivary PYY can modulate behavioral responsiveness to oral lipid stimuli (La Sala et al. 2013). Adiponectin is an anti-inflammatory adipokine primarily secreted from adipocytes into the circulation, where it affects many biological functions such as insulin sensitivity and fatty acid oxidation (Yamauchi et al. 2002; Yoon et al. 2006; Awazawa et al. 2011; Villarreal-Molina and Antuna-Puente 2012). In both plasma and saliva, adiponectin is present in multiple oligomeric forms referred to as low, medium, high, and super high molecular weight, the latter found only in saliva (Bobbert et al. 2005; Lin et al. 2014). The origin of salivary adiponectin is not entirely clear; while it has been shown in humans to be synthesized in salivary gland ductile cells (Katsiougiannis et al. 2006), it is likely that adiponectin is also transferred to saliva from the circulation as occurs with numerous circulating hormones (Pfaffe et al. 2011; Wang et al. 2013). Although the presence of adiponectin in saliva has been demonstrated extensively (Gröschl et al. 2001; Toda et al. 2007; Akuailou et al. 2013; Lin et al. 2014; Nigro et al. 2015), there is limited information regarding its function in saliva. Of note, two studies have proposed functional roles for salivary adiponectin, suggesting that it influences saliva secretion (Ding et al. 2013) or plays an anti-inflammatory role in the oral cavity (Katsiougiannis et al. 2010). To the best of our knowledge, no role in gustation has been shown for adiponectin.
By querying a previously generated murine circumvallate (CV) taste bud transcriptome database (Crosson et al. 2018) for peptide/hormone receptors with known salivary expressed ligands, we identified transcripts for three adiponectin receptors—Adipor1, Adipor2, and Cdh13—that are potentially expressed in taste buds. Using immunohistochemistry (IHC) in this tissue, we were able to validate these findings, and identified two canonical adiponectin receptors—T-cadherin and ADIPOR1—expressed in mouse TRCs. The localization of these receptors to functional subsets of taste cells, along with changes in licking to lipid stimuli upon perturbation of oral adiponectin signaling, suggests a role for salivary adiponectin in the modulation of gustatory function.
Materials and Methods
Mice
This study was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Florida. All procedures were done in accordance with the principles of the National Research Council’s guide for the Care and Use of Laboratory Animals. Mice had ad libitum access to food and water, except where otherwise noted, and were housed at 22–24 °C with a 14/10 h light/dark cycle. Wild-type (WT) C57BL/6J mice were bred in-house and B6;129-Adipoqtm1Chan/J (APN KO, which contains a null allele of the gene encoding adiponectin) mice were purchased from Jackson Labs. In some experiments, APN KO mice each received a total of 1 × 1012 vector genomes (vg) of recombinant Adeno-associated virus (AAV) vector either bilaterally in each submandibular salivary gland or via the tail vein prior to brief-access lick testing (for details, see Katano et al. 2006). DNA was isolated from ear punches of all APN KO mice for genotyping to confirm exon-2 deletion in the Adipoq gene. Genotyping primers are reported in Supplementary Table S1.
Tissue collection
Mice were deeply anesthetized by i.p. injection of a ketamine–xylazine mixture (200 and 10 mg/kg, respectively), then perfused intracardially with 4% paraformaldehyde in phosphate-buffered saline (PBS, pH ~7.4), followed by tissue dissection. Tissues were fixed overnight in 4% paraformaldehyde in PBS (pH ~7.4), cryoprotected by incubation with 30% sucrose in PBS (pH ~7.4) overnight, and frozen in O.C.T. mounting medium prior to cryosectioning. Mice used for 5-HT tissue staining were injected intravascularly with 5-HTP in lactated Ringers solution (200 mg/kg) 1 h before euthanasia to increase the amount of 5-HT in Type 3 TRCs.
Immunohistochemistry
Adiponectin receptor immunofluorescence
Specific information regarding antibody sources, dilutions, and host species are located in Table 1. ADIPOR1 and ADIPOR2 antibodies were graciously provided by Dr. Xia-Rong Peng, and have been previously validated in knockout mouse models of Adipor1 and Adipor2, respectively, by immunoblotting (Bjursell et al. 2007). Antibodies against T-cadherin (encoded by Cdh13) (R&D Systems; AF3264) have been previously validated in a T-cadherin knockout mouse line by IHC staining (Denzel et al. 2010). OCT-imbedded tongues were sectioned in 10–20 μm coronal slices using a cryostat (Leica CM3050 S; Leica Microsystems, Nussloch GmbH, Germany) and mounted on Fisher Superfrost Plus slides. IHC was conducted using traditional indirect immunofluorescence. All washing steps were done using TBST (50 mM Tris–HCl, 0.9% NaCl, and 0.5% Tween 20, pH ~7.6). Tissues were blocked for 1 h at room temperature with in-house blocking buffer (5% normal donkey serum in TBST with 0.05% Triton-X) to reduce nonspecific antibody binding. Sections were then incubated overnight at 4 °C with primary antibody diluted into 5% normal donkey serum in TBST, followed by secondary antibody incubation with either a Donkey-anti-Rabbit IgG Alexa488 conjugate or a Donkey-anti-Goat IgG Alexa649 conjugate (1:1000 dilution in 5% normal donkey serum in TBST, 1 h at room temperature). All sections were counterstained with 4′,6-diaminidino-2-phenylindole (DAPI) and visualized by confocal microscopy (Leica SP5).
Table 1.
Host species, dilution, and supplier information for primary antibodies used in IHC experiments
| 1° Antibody | Host | Supplier | Dilution | Specificity/control |
|---|---|---|---|---|
| ADIPOR1 | Rabbit | Dr. Xiao-Rong Peng | 1:200 | Staining absent in western blot of Adipor1 KO mouse brain tissue (Bjursell et al. 2007) |
| ADIPOR2 | Rabbit | Dr. Xiao-Rong Peng | 1:200 | Staining absent western blot of Adipor2 KO mouse brain tissue (Bjursell et al. 2007) |
| T-cadherin | Goat | R&D systems (Minneapolis, MA, U.S.A. AF3264) RRID: AB_2077121 | 1:500 | Signal absent in IHC staining of T-Cad KO mouse cardiac tissue (Denzel et al. 2010) |
| NTPDase2 | Rabbit | J. Sévigny, (Université Laval, Quebec, Canada. #mN2-36I6) RRID: AB_2314986 | 1:500 | Specificity demonstrated in COS-7 cells and staining patterns coincide with RNA patterns in taste tissue (Bartel et al. 2006) |
| PLCβ2 | Rabbit | Santa Cruz Biotechnologies (Dallas, TX, U.S.A. cat No. sc-206) RRID: AB_632197 | 1:500 | Staining absent when primary or secondary omitted, validated in taste tissue (Yang et al. 2007) |
| Gα gustducin | Rabbit | Santa Cruz Biotechnologies (Dallas, TX, U.S.A. cat No. sc-395) RRID: AB_673678 | 1:500 | Staining absent when primary or secondary omitted, validated in taste tissue (Yang et al. 2007) |
| NCAM | Rabbit | Millipore (Temecula, CA, USA; cat. No. AB5032) RRID: AB_2291692 | 1:500 | Specificity confirmed by western blot of mouse brain homogenates and validated in taste tissue (Wang et al. 2009) |
| 5-HT | Rat | Millipore (Temecula, CA, USA; cat. No. MAB352 clone YC5/45) RRID: AB_11213564 | 1:500 | Staining absent when primary or secondary omitted. Staining pattern matches that of another well- validated 5-HT antibody (Kaya et al. 2004; Ma et al. 2007) and has been used previously (Mansouri-Guilani et al. 2019) |
| KRT8 | Rat | University of Iowa Developmental Studies Hybridoma Bank (RRID: AB_531826) | 1:500 | Extensively used antibody, displays staining pattern consistent with that observed in taste tissues (Biggs et al. 2016) |
Double Labeling Immunofluorescence
Double-labeling techniques were used to co-localize T-cadherin with established taste bud markers to characterize expression in specific taste bud subpopulations. Taste bud marker information is located in Table 1 along with other primary antibody information. Double-labeling experiments used primary antibodies from different host species, and thus utilized a standard indirect dual immunofluorescence staining protocol. Specifically, tissues were incubated simultaneously with both primary antibodies at 4 °C overnight, followed by simultaneous incubation with two secondary antibodies for 1 h at room temperature. Slides were washed with TBST between each incubation to remove excess antibody. Donkey-anti-Rabbit Alexa488 (1:1000), Donkey-anti-Rat Cy3 (1:200) and Donkey-anti-Goat Alexa649 (1:1000) were used as secondary antibodies to detect antisera from each of the three host species used.
Plasmid construction
To generate the different mouse models presented here, three plasmid transgene constructs were packaged into individual AAV vectors. All vector expression cassettes are driven by the small chicken β-actin promoter and contain a bovine growth hormone polyadenylation sequence. To produce the AAV8-APN vector, we used a previously published transgene cassette which was assembled by ligating mouse adiponectin cDNA into the pTR-UF backbone (Zolotukhin et al. 1996), creating the plasmid pTR-Acrp30 (referred to here as pTR-APN), described in detail by Shklyaev et al. (2003). To produce the vector AAV5-GFP-miR, we cloned the transgene cassette pTR-GFP-miR, which expresses GFP (green fluorescent protein) and contains triplicate miR122 and miR206 target sites in the 3′-UTR of the construct. Micro RNA oligos were commercially synthesized and incorporated into an inverted terminal repeat (ITR)-containing plasmid backbone using standard cloning techniques. We created pTR-APN-miR, used to produce AAV5-APN-miR, by swapping out the GFP cDNA with mouse adiponectin cDNA amplified from pTR-APN. All plasmid constructs were confirmed by Sanger sequencing prior to the production of AAV vectors. Primers used for cloning are reported in Supplementary Table S1.
AAV vector production and administration
Recombinant AAV vectors were produced in HEK 293 cells using a triple (AAV5) or double (AAV8) plasmid-based transfection method, and purified via Iodixanol density centrifugation as described previously (Zolotukhin et al. 1999). For AAV5 preps, pHelper (Agilent cat no. 240071-52) was used to supply the adenoviral helper genes and pACG2R5C (Zolotukhin et al. 2002) was used to supply the AAV2 rep and AAV5 cap genes. For AAV8 production, a single helper plasmid (pDG8) containing both the adenoviral genes as well as the AAV2 rep and AAV8 cap genes was used (Grimm et al. 1998). Table 2 shows the plasmids used in the transfection to produce each recombinant AAV vector. Vectors were titered using a PicoGreen-based assay described by Piedra et al. (2015), and were sterile filtered before administration to animals.
Table 2.
Plasmid constructs used in the production of recombinant AAV vectors
| Vector | AAV plasmid | Adenoviral helper plasmid | Transgene plasmid | Gene expressed |
|---|---|---|---|---|
| AAV5-GFP-miR | pACG2R5C | pHelper | pTR-GFP-miR | Green fluorescent protein |
| AAV5-APN-miR | pACG2R5C | pHelper | pTR-APN-miR | Adiponectin |
| AAV8-APN | *pDG8 | *pDG8 | pTR-Acrp30 | Adiponectin |
*Contains both Adenovirus helper genes and AAV rep/cap genes.
Behavior
Animals
For behavioral taste testing of APN KO mice and WT mixed background controls (B6129SF2/J), adult mice (10–12 weeks old) were ordered from Jackson Labs, and allowed to acclimate to their new housing environment for 2 weeks prior to brief-access lick testing. During this 2-week acclimation period, mice were given ad libitum access to food and water until the start of training/testing, and housed individually. In the second set of behavioral experiments, APN KO mice (10–12 weeks old) were administered either AAV8-APN, AAV5-APN-miR, or AAV5-GFP-miR 1 month before the first day of training. AAV was administered either by tail vein injection (AAV8-APN) or submandibular salivary gland cannulation (AAV5-APN-miR and AAV5-GFP-miR) as described previously (Katano et al. 2006). After vector administration, mice were single housed and given ad libitum access to food and water until the start of the training/testing sessions.
Taste stimuli
All tastants were prepared in 18.2 MΩ ultrapure water and dilutions were prepared fresh before each testing session. Tastants and concentrations used are listed as follows: citric acid (CA; 0.3, 1, 3, 10, 30, and 100 mM; Sigma–Aldrich), NaCl (30, 100, 200, 300, 600, and 1000 mM; Sigma–Aldrich), quinine hydrochloride (QHCl; 0.03, 0.1, 0.3, and 1.3 mM; Sigma–Aldrich), sucrose (25, 50, 100, 200, and 400 mM; Fisher Scientific), Intralipid (1.25, 2.5, 5, 10, and 20%; Sigma–Aldrich). Each solution was presented at room temperature and water was used as a “no stimulus” control for each tastant.
Procedure
Training and testing procedures were done in a Davis Rig lickometer (Davis MS-160; DiLog Instruments, Tallahassee, FL, USA). The lickometer allows mice access to a sipper bottle containing the stimulus, and uses AC current to record each lick. The lickometer utilizes a motorized table and shutter to restrict mice to 5 s trials for each sipper tube. Total session times were 25 min, during which mice could initiate as many trials as they wanted. Mice were tested according to previously published protocols (Glendinning et al. 2002; Elson et al. 2010; La Sala et al. 2013). Two protocols were used; one for appetitive stimuli (sucrose and Intralipid) and one for aversive stimuli (NaCl, CA, and QHCl). For the appetitive stimuli, mice were food and water restricted (1 g food and 2 mL water) for the 23.5 h period prior to testing. After each testing period, mice were given a 24 h recovery period where they had ad libitum access to food and water. For aversive stimuli, mice were put on a 23.5 h water restriction schedule throughout the training/testing period and given ad libitum access to food. During aversive stimuli testing, a water rinse was presented in between each stimulus presentation to control for potential carryover effects. All mice were weighed daily and given 24 h supplementary ad libitum access to food and water if at any time their weight dropped below 85% of their pre-testing weight.
Data analysis and statistics
For aversive stimuli, tastant/water lick ratios were obtained by dividing the average number of licks per trial for each stimulus concentration, by the average number of licks per trial to water. This controls for motivation to lick, individual lick rate, and any potential genotypic differences in lick rate. For appetitive stimuli, a standardized lick ratio (SLR) was used, which controls for individual lick rate and potential genotypic differences in lick rate. However, the use of an SLR for appetitive stimuli allows us to eliminate the large impact of small changes in water licks, although this removes control for motivation to lick. The SLR is calculated by dividing the average number of licks per trial for each stimulus concentration, by the maximum potential lick rate for that animal as determined by the mean interlick interval distribution during water spout training (Glendinning et al. 2002). All ratio scores were analyzed pairwise between groups using a two-way repeat-measure analysis of variance (ANOVA). If a significant interaction was observed (identified by values of P ≤ 0.05), a post hoc Holm–Sidak t-test was used to determine whether behavioral responses were significantly different between groups for each individual concentration (identified by values of P ≤ 0.05). Only mice that initiated at least one trial for every concentration were used in the analysis of a given stimulus. For presentation of behavioral data, curves were fit to the mean data for each group using a 2- or 3-parameter logistic function as described previously (Elson et al. 2010).
Plasma collection
Approximately 200 µL of blood was collected from each animal by facial vein puncture upon completion of taste response testing. Blood was collected at a 9:1 ratio into 3.8% trisodium citrate and immediately placed on ice to avoid clotting. Blood samples were centrifuged at 10,000 rpm for 10 min at 4 °C to pellet red blood cells. Plasma was then transferred to new microfuge tubes and frozen at −80 °C prior to assaying with the Mouse Adiponectin ELISA (R&D systems cat no. MRP300).
Saliva collection
Salivation was induced in mice by an i.p. injection of 100 µL of 50 µg/mL pilocarpine in PBS (pH 7.4). One minute after injection, saliva was collected for 10 min from the oral cavity using a p20 pipette. Over this 10 min period, saliva was continuously transferred into ice-cold microfuge tubes containing 20 μL of 10 mg/mL aprotinin (Peptides International cat no. IAT-3830-PI). Saliva samples were frozen at −80 °C prior to assaying with the Mouse Adiponectin ELISA (R&D systems). Before ELISAs were conducted, saliva samples were thawed, then centrifuged at 15,000 rpm for 20 min at 4 °C to pellet mucous and saliva debris. Supernatants were transferred to fresh microfuge tubes and the mucous pellet was discarded.
Adiponectin ELISA
Sample Preparation
To quantify the levels of full-length adiponectin in both blood and saliva, we used the Mouse Adiponectin ELISA Kit from R&D Systems. Per manufacturer’s instructions, plasma samples were diluted into “Calibrator Diluent” (provided with ELISA kit) to ensure that adiponectin levels were within the detection range of the assay. Plasma samples from mice were diluted at the following ratios: 1:2000 for WT C57BL/6J, 1:100,000 for APN KO mice treated with AAV8-APN vector (systemic adiponectin supplementation), and 1:4 for APN KO mice treated with either AAV5-APN-miR of AAV5-GFP-miR vectors (salivary adiponectin supplementation or adiponectin knockout, respectively). Plasma samples were diluted differently to ensure that the adiponectin levels of each sample fell within the detection range of the ELISA. Animals with supraphysiological levels of circulating adiponectin required high dilutions (1:100,000), while animals with low or no circulating adiponectin required low dilutions (1:4). Saliva samples from all mice were diluted 1:4.
Statistical analysis
A Grubb’s outlier test (α = 0.05) was used to identify and remove significant outliers from the ELISA results, prior to further statistical analysis. After outliers had been removed, a two-tailed Student’s t-test (α = 0.05) was used to compare the levels of plasma and salivary adiponectin pairwise, between each group of mice.
Results
Numerous reports have shown that taste responsiveness can be modulated by peptide signaling in taste buds. In previous studies from our group, we found that receptors for the peptide PYY are expressed in subsets of TRCs (Hurtado et al. 2012; La Sala et al. 2013). We thus asked if taste buds express receptors for other peptides enriched in saliva. To initially address this question, we queried the transcriptome recently generated by us, using RNA-seq of purified CV taste buds obtained from C57BL/6J mice (Crosson et al. 2018). Specifically, we wanted to determine the expression levels of cognate receptors for salivary expressed peptides whose general functions are associated with metabolic homeostasis (Zolotukhin 2013).
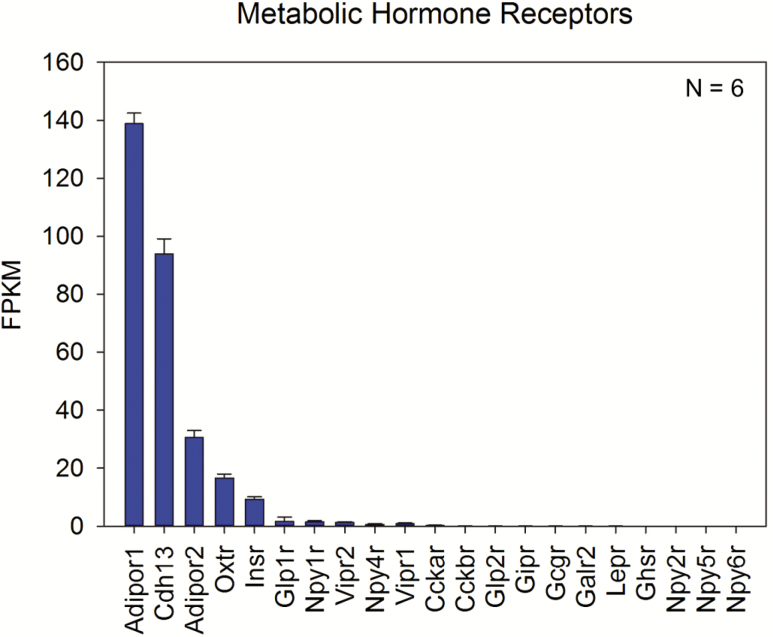
Of the genes queried, transcripts encoding three adiponectin receptors—Adipor1, Cdh13, and Adipor2—were the most highly expressed (Figure 1). Average Adipor1 expression was the highest (138.83 ± 8.89 fragments per kilobase of transcript per million reads mapped (fpkm)), followed by Cdh13 (93.93 ± 12.69 fpkm), and Adipor2 (30.62 ± 5.84 fpkm). Adiponectin receptor expression levels are comparable with those observed for taste bud-enriched genes like PLCβ2, Tas1r1, and Car4 (Crosson et al. 2018, GEO Ascension GSE114624). Since our studies were initiated, expression of Adipor1, Adipor2, and Cdh13 transcripts in Type II and Type III TRCs have been observed by RNA-seq by others (Sukumaran et al. 2017). Several other peptide receptors that have been previously reported in taste buds were also present in the transcriptome dataset, albeit at lower expression levels than those seen for the adiponectin receptors; these include the insulin receptor Insr (Baquero and Gilbertson 2011), oxytocin receptor Oxtr (Sinclair et al. 2010), GLP-1 receptor Glpr1 (Shin et al. 2008; Martin et al. 2009), and neuropeptide Y receptor Npyr1 (Zhao et al. 2005; Hurtado et al. 2012). A full list of the queried receptors and their associated peptide ligands are listed in Supplementary Table S2.
Figure 1.
Gene expression levels of metabolic hormone and peptide receptors in WT TRCs. Expression levels for select receptors as determined by RNA-seq of WT murine CV taste buds. The three highest expressing transcripts in the queried list—Adipor1, Cdh13, and Adipor2—are all receptors for adiponectin. A total of six biological replicates were used for analysis. Supplementary Table S2 contains a key and brief description of the queried receptors.
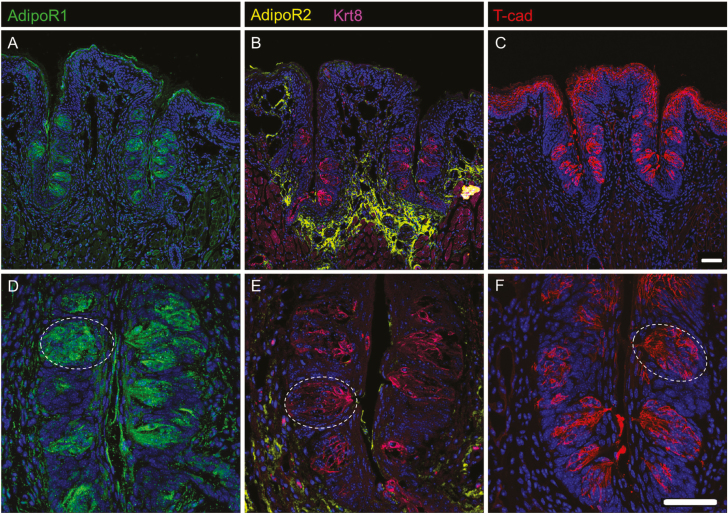
To validate the expression of Adipor1, Adipor2, and Cdh13 in mouse TRCs, we performed IHC on cryosections containing CV papillae from C57BL/6J mice, using polyclonal antibodies against ADIPOR1, ADIPOR2, and T-cadherin. Each adiponectin receptor antisera had been previously validated in a respective knockout mouse strain (Bjursell et al. 2007; Denzel et al. 2010). Both ADIPOR1 and T-cadherin immunolocalize to taste buds (Figure 2A,C). ADIPOR2, however, does not immunolocalize to taste buds; rather, it is found only in the surrounding tissues (Figure 2B). Co-labeling sections for ADIPOR2 and cytokeratin 8 (KRT8), a general TRC marker (Mbiene and Roberts 2003), confirmed that taste buds do not express ADIPOR2 and suggest that the presence of ADIPOR2 in the RNA-seq database was due to contamination of the taste bud samples with surrounding nontaste tissue.
Figure 2.
Expression of ADIPOR1 and T-cadherin in CV TRCs. IHC staining of WT murine CV sections for all three adiponectin receptors, ADIPOR1 (A, D), ADIPOR2 (B, E), and T-cadherin (C, F). ADIPOR1 (A, D) and T-cadherin (C, F) are expressed in CV taste buds (e.g., white dotted oval) while ADIPOR2 (B, E) is expressed in surrounding tissue. ADIPOR2 sections contain KRT8 (B, E), a general taste cell marker. Scale bar is 20 μm.
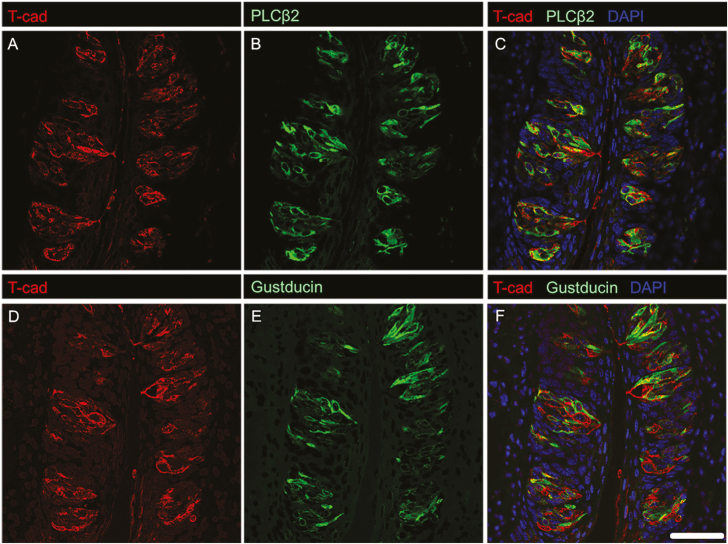
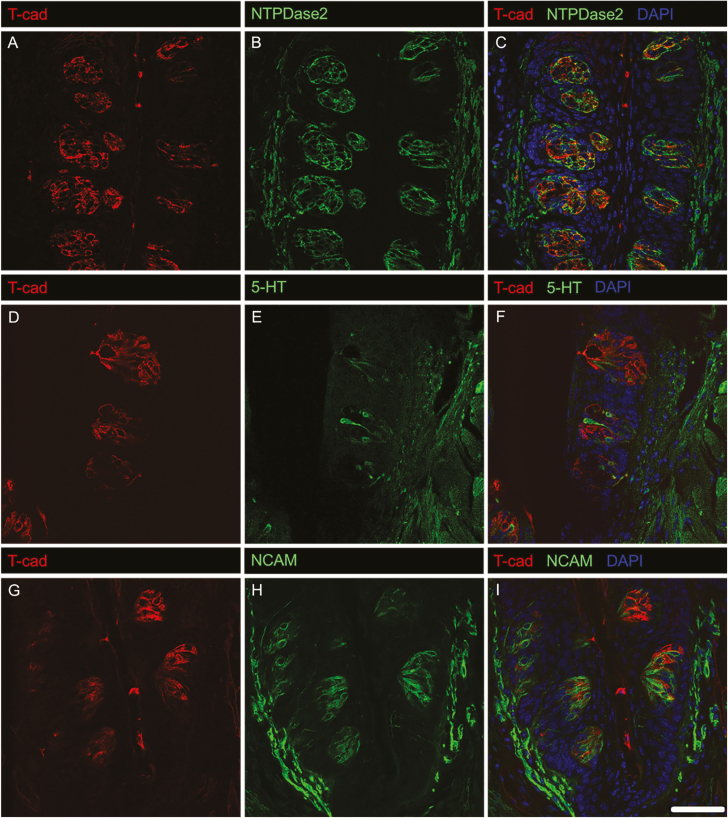
Mammalian taste buds are composed of multiple TRC types, each of which plays different roles in the detection and transmission of taste information (Chaudhari and Roper 2010). To gain insight into the roles of adiponectin signaling in TRCs, we co-localized T-cadherin with established markers for the three main subtypes of taste bud cells (Figures 3 and 4): NTPDase2 (Type 1 TRCs, which are thought to play a supporting role; Vandenbeuch et al. 2013), PLCβ2 and the G protein α-subunit gustducin (sweet, bitter, and/or umami-responsive TRCs; (Ming et al. 1999; Miyoshi et al. 2001), and 5-HT and NCAM (sour-sensitive Type 3 TRCs; (Yee et al. 2001; Huang et al. 2008)). Host species antibody constraints made dual staining difficult for ADIPOR1. T-cadherin immunostaining largely colocalized with both PLCβ2 and gustducin, suggesting expression of this adiponectin receptor primarily in a major subset of Type 2 TRCs (Figure 3). T-cadherin was not co-expressed with Type 3 TRC markers 5-HT or NCAM, although a small subset of NTPDase2-expressing Type 1 TRCs showed some T-cadherin staining (Figure 4). We also measured the co-localization of T-cadherin and these TRC markers by correlation analysis (Costes et al. 2004). Consistent with the visual analysis of the IHC co-staining, calculated Pearson’s correlation coefficients (Table 3) indicate that T-cadherin is primarily expressed in Type 2 TRCs.
Figure 3.
IHC staining of T-cadherin with established markers for sweet-, bitter-, and/or umami-responsive TRCs. T-cadherin localizes to cells expressing PLCβ2 (C), and cells expressing gustducin (F). Single channel images of T-cadherin (A) and PLCβ2 (B) as well as T-cadherin (D) and gustducin (E) are shown for reference. Scale bar is 20 μm.
Figure 4.
IHC staining of T-cadherin with established markers for supporting and sour-responsive taste bud cells. T-cadherin does not localize to sour-responsive TRCs (F, I) and has minimal localization to supporting taste bud cells (C). Single channel images of T-cadherin (A) and NTPDase2 (B), T-cadherin (D) and 5-HT (E), and T-cadherin (G) and NCAM (H) are shown for reference. Scale bar is 20 μm.
Table 3.
Co-localization analysis of T-cadherin and TRC markers assessed by Pearson’s correlation
| Adiponectin receptor | TRC marker | Taste buds counted | Pearson’s correlation coefficient |
|---|---|---|---|
| T-cadherin | NTPDase2 | 12 | 0.136 |
| T-cadherin | PLCβ2 | 11 | 0.461 |
| T-cadherin | Gustducin | 8 | 0.451 |
| T-cadherin | 5-HT | 2 | −0.012 |
| T-cadherin | NCAM | 4 | 0.052 |
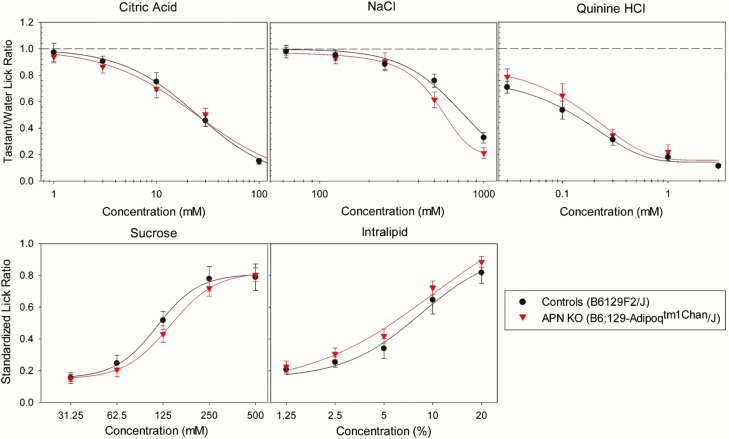
We next asked whether adiponectin signaling impacts taste behaviors. We first assessed taste responses in APN KO mice, and their WT controls (B6:129 SF2/J mice) using brief-access lick testing. No significant differences in lick responses to sucrose, QHCL, NaCl, CA, or Intralipid were observed between APN KO and control mice (Figure 5). We suspect the lack of observed change in response may be due to an inherent compensatory mechanism supplementing for the complete loss of adiponectin, a theory proposed by the creators of this mouse line (Ma et al. 2002). Because the specifics of this potential compensatory pathway are unknown, and no global inducible APN KO mouse lines are available, we decided to use recombinant AAV vectors to modulate the levels of salivary and circulating adiponectin in adult APN KO mice.
Figure 5.
Brief-access lick response testing of APN KO (red) and WT control (black) mice for citric acid, NaCl, quinine hydrochloride, sucrose, and Intralipid. No significant difference (P > 0.05) was observed between groups for any of the stimuli tested as determined by a two-way repeat-measure ANOVA with a significance threshold of α = 0.05. A total of 8 mice were used in each group.
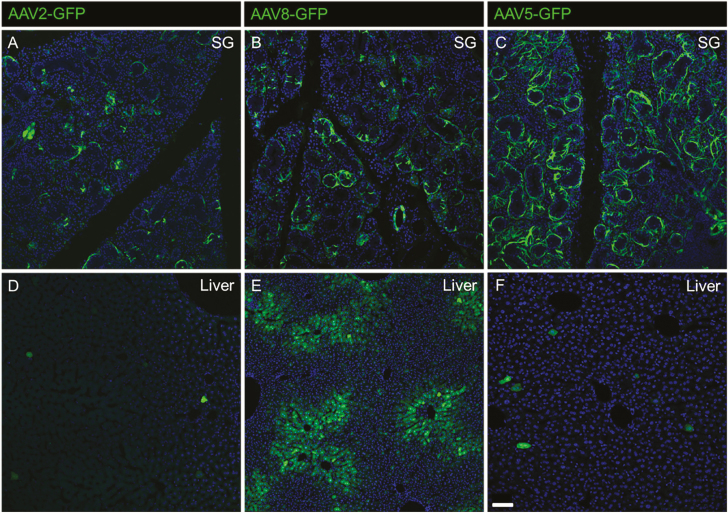
To test the effects of both salivary and circulating adiponectin on tastant lick response, we developed two mouse models with supplemented adiponectin expression: one expressing adiponectin solely in the saliva and the other expressing adiponectin throughout the periphery. We used AAV as our gene delivery vector because it confers robust, long-term gene expression in a variety of tissues types and has been used previously to ectopically express adiponectin in the liver of rats (Shklyaev et al. 2003). Since the tissue tropism and transduction of AAV serotypes is highly dependent on the promoter, vector dose and delivery pathway, we performed several pilot studies to determine the optimal AAV vectors for our purpose. We chose to focus on AAV serotypes 2, 5, and 8 because AAV2 and AAV5 reportedly transduce the salivary gland (Katano et al. 2006; Voutetakis et al. 2010; Gao et al. 2011; Di Pasquale et al. 2012), and AAV8 is a robust, liver tropic vector (Zincarelli et al. 2008; Sands 2011; Markusic and Herzog 2012). To assess the transduction efficiency of vectors in the salivary gland, mice received a total of 1 × 1012 vg of either AAV 2, 5, or 8 (each expressing a GFP reporter under the chicken β-actin promoter) bilaterally in each submandibular gland (Figure 6).
Figure 6.
Salivary gland tropism of AAVs 2, 8, and 5 when administered directly to the submandibular salivary gland. Transduction of WT murine salivary glands (A, B, C) represented by GFP expression 1 month after AAV2 (A), AAV8 (B), or AAV5 (C) administration. Off-target liver expression (D, E, F) was observed for AAV2 (D), AAV8 (E), and AAV5 (F) vectors, with AAV8 being the most liver tropic. Scale bar (bottom left corner F) is 50 μm.
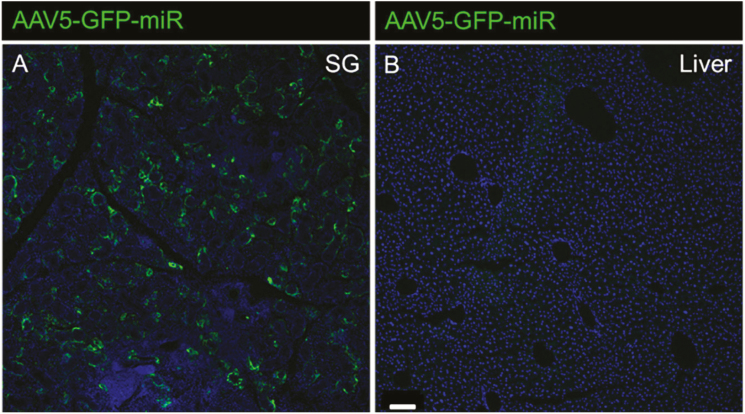
Both AAV5 and AAV8 displayed high salivary gland transduction (Figure 6B,C). However, as anticipated because of its known tropism (Zincarelli et al. 2008; Chen et al. 2013), AAV8 had high transduction in the liver, even when delivered directly to the salivary gland (Figure 6E). Because GFP expression was largely restricted to the salivary gland using AAV5 vectors, we determined this to be the ideal serotype to supplement adiponectin in the saliva. To restore adiponectin expression to the circulation, AAV8 vectors are more suitable because of their general widespread transduction in mice (Zincarelli et al. 2008; Chen et al. 2013). To further increase the specificity of AAV5 transduction in the salivary gland, we included micro-RNA target sites for miR122 and miR206, which are liver and skeletal muscle-specific, respectively (Geisler et al. 2013). Using this microRNA target site containing AAV5 vector, we were able to abolish the already minimal off-target liver transduction (Figure 7).
Figure 7.
Ablation of off-target AAV5 liver tropism achieved by inclusion of miR122 and miR206 target sites into vector construct. Transduction of WT murine salivary glands (A), represented by GFP expression, is unaffected by the inclusion of miR122/miR206 TS in the vector. However, off-target GFP expression in the liver (B) is completely abolished. Transduction was measured 1 month after vector administration and the scale bar (bottom left B) is 100 μm.
Salivary adiponectin expression in APN KO mice was supplemented by administering 1 × 1012 vg of AAV5-APN-miR directly to each submandibular salivary gland of APN KO mice via ductile cannulation. This allows for adiponectin to be expressed in and secreted from salivary gland cells (most likely ductile cells), which endogenously express adiponectin in WT animals (Katsiougiannis et al. 2006; Ding et al. 2013). AAV5-GFP-miR was administered to the salivary glands of APN KO mice as a negative control. Systemic adiponectin expression was supplemented in APN KO mice by administering 1 × 1012 vg of AAV8-APN via tail vein injection. This allows for adiponectin to be expressed ectopically throughout the periphery, primarily in the liver where it is secreted into the bloodstream, a method demonstrated with adiponectin (Shklyaev et al. 2003) and other proteins previously (Conlon et al. 2005; Manno et al. 2006; Puzzo et al. 2017).
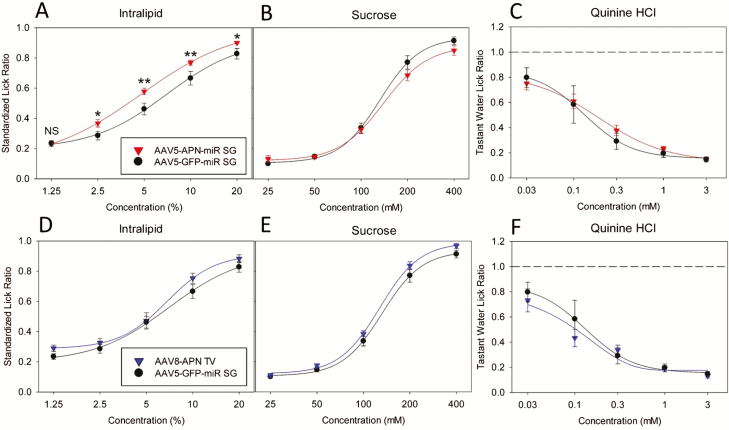
One month after vector administration, we performed brief-access lick response testing for Intralipid, sucrose, and QHCL (Figure 8; see legend for statistics). We observed a modest, yet significant increase in the behavioral responses to Intralipid (Figure 8A), but not sucrose (Figure 8B), or QHCL (Figure 8C), in mice with supplemented salivary adiponectin compared with APN KO control mice. Interestingly, the response of mice with systemic adiponectin supplementation was not significantly different from APN KO mice for Intralipid (Figure 8D), sucrose (Figure 8E), or QHCL (Figure 8F). A post hoc Holm–Sidak t-test was used to compare the mean SLR values of each group if the two-way repeat-measure ANOVA was significant. Mean SLR values were significantly increased in salivary adiponectin supplemented mice relative to APN KO controls (Figure 8A) at 2.5%, 5%, 10%, and 20% Intralipid emulsion. Mean SLR values were not significantly different at the lowest Intralipid dose.
Figure 8.
Brief-access lick response testing of APN KO mice with supplemented salivary or systemic adiponectin relative to untreated APN KO littermates. Brief-access lick response testing results of APN KO mice with supplemented salivary adiponectin (AAV5-APN-miR SG, red) compared with untreated APN KO (AAV5-GFP-miR SG, black) mice are shown in panels A, B, and C. Of the tastants tested, mice with supplemented salivary APN had a significantly increased (F1,22 = 8.247; P = 0.009) response to Intralipid (A) relative to APN KO mice, as determined by two-way repeat-measure ANOVA (α = 0.05). In the case of a significant ANOVA, a post hoc Holm–Sidak t-test (α = 0.05) was applied to compare the significance of the lick response for each tastant concentration (NSP > 0.05, *P ≤ 0.05, **P < 0.01). Mice with supplemented salivary adiponectin (A, red) had a significantly increased lick response relative to APN KO mice (A, black) at each concentration of Intralipid except the lowest (1.25% Intralipid, P > 0.05). Salivary adiponectin supplementation mice did not display significantly altered lick behavior from APN KO mice for sucrose (F1,22 = 0.872; P = 0.361; B) or QHCL (F1,22 = 0.134; P=0.718; C). APN KO mice with systemically supplemented adiponectin (AAV8-APN TV, blue) did not display a significant difference in lick behavior from APN KO mice (black) for Intralipid (F1,15 = 1.722; P = 0.209; D), sucrose (F1,15 = 3.452; P=0.083; E), or QHCL (F1,16 = 0.295; P = 0.595; F).
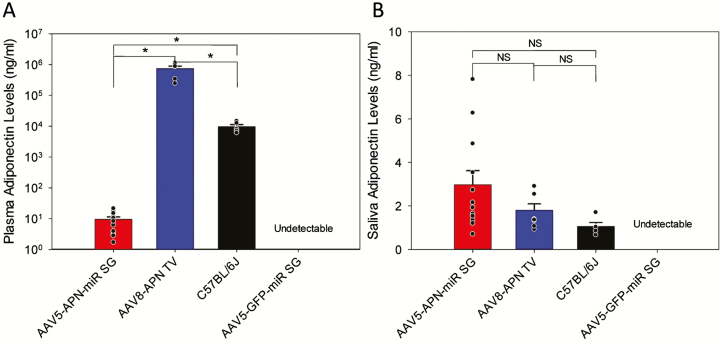
Upon completion of brief-access lick testing, saliva and blood samples were drawn for quantification of adiponectin by ELISA (Figure 9). A two-tailed Student’s t-test was used to determine statistical differences in adiponectin levels between groups. As expected, plasma and saliva samples from APN KO mice which received the AAV5-GFP-miR vector were negative for adiponectin (Figure 9A,B; see legend for statistics). Mice which received the AAV5-APN-miR vector did indeed have levels of salivary adiponectin (2.98 ± 2.23 ng/mL; Figure 9B) comparable with those of WT C57BL/6J mice (1.06 ± 0.39 ng/mL; Figure 9B). These AAV5-APN-miR injected mice also expressed minor amounts of circulating adiponectin (9.50 ± 6.67 ng/mL; Figure 9A) though this is 1,000-fold lower than the adiponectin levels of WT C57BL/6J mice (9,675.89 ± 3742.28 ng/mL; Figure 9A) and less likely to have a biological impact (Frühbeck et al. 2017). In contrast, mice which received systemic AAV8-APN had levels of circulating adiponectin (744,519.61 ± 365,670.59 ng/mL; Figure 9A) significantly higher than those of both WT mice and salivary adiponectin supplemented mice (1.80 ± 0.83 ng/mL; Figure 9B) which were not significantly different from WT.
Figure 9.
Quantification of adiponectin levels in plasma and saliva of recombinant AAV-treated APN KO mice. After completion of brief-access lick testing, plasma (A) and saliva (B) adiponectin levels for each treatment group were measured by ELISA. Mean adiponectin levels were statistically compared in a pairwise fashion using a Student’s t-test (α = 0.05), significance is indicated in the figure by an *. Salivary adiponectin supplemented mice (red; A) had significantly lower levels of circulating adiponectin (P < 0.001) compared with C57BL/6J mice (black; A). Systemic adiponectin supplemented mice (blue; A) expressed significantly higher (P < 0.001), supraphysiological levels of circulating adiponectin relative to C57BL/6J mice (black; A). In the saliva however, neither the salivary (red; P = 0.08; B) nor systemically (blue; P = 0.09; B) supplemented mice had significantly different levels of salivary adiponectin than C57BL/6J mice (black; B). As expected, APN KO mice had no detectable levels of adiponectin in either the saliva or plasma.
Discussion
TRCs and associated taste nerves express a diversity of receptors for peptide hormones related to the control of metabolism and satiety. The expression of two adiponectin receptors—ADIPOR1 and T-cadherin—in TRCs suggests an additional degree of complexity for modulation of TRC function by peptides acting as autocrine, paracrine, and/or endocrine factors. The expression patterns of different peptide receptors (as well as their peptide ligands) can vary significantly. For example, glucagon receptors are expressed in PLCβ2-positive Type 2 TRCs (Elson et al. 2010); oxytocin receptors are found in glial-like Type 1 cells (Sinclair et al. 2010); and the receptor for GLP-1 is localized to afferent nerve terminals innervating taste buds (Shin et al. 2008). We immunolocalized T-cadherin to a subset of PLCβ2-positive, Gα-gustducin-positive TRCs, suggesting that adiponectin might affect responses to taste stimuli transduced by these signaling proteins. We also noted some T-cadherin-expressing cells that lacked immunostaining for PLCβ2, but also for markers of Type 1 and Type 3 TRCs. One possibility is that PLCβ2-negative, T-cadherin-positive TRCs represent an earlier stage of Type 2 cell differentiation and have not yet begun to express PLCβ2. Due to multiple antibody constraints (e.g., species compatibility), we were not able to fully resolve the exact expression patterns of each adiponectin receptor in taste buds using IHC alone. Transcriptomic analyses of individual TRCs would be very useful for fully elucidating the expression profile of adiponectin receptors in TRCs.
While transcriptomic analysis of CV taste buds indicated that all three genes encoding canonical adiponectin receptors—Adipor1, Adipor2, and Cdh13—are expressed in TRCs, IHC staining showed that ADIPOR2 is excluded from TRCs and is instead localized to surrounding nontaste tissue. The discrepancy between the two techniques is not wholly surprising, as the taste buds used in the RNA-seq study were collected by manual dissection making low-level contamination with nontaste tissue likely. Furthermore, differential localization of ADIPOR1 and ADIPOR2 in lingual tissue is consistent with observations in other tissues (Beylot et al. 2006).
Several peptides that affect blood glucose homeostasis, satiation, gastric emptying and secretion of digestive enzymes, including PYY, GLP-1, and glucagon (Kieffer and Habener 1999; Batterham et al. 2002, 2006; Hellström et al. 2004; Nadkarni et al. 2014), are produced in the oral cavity and impact taste responsiveness (Shin et al. 2008; Martin et al. 2009; Elson et al. 2010; Dotson et al. 2013; La Sala et al. 2013; Takai et al. 2015). While the majority of these peptides are produced in taste buds (Dotson et al. 2013), a few including leptin (Kawai et al. 2000), PYY (Acosta et al. 2011; La Sala et al. 2013), and oxytocin (Sinclair et al. 2010) are produced in distant tissues and likely reach the taste buds through saliva or the bloodstream. Adiponectin appears to fit this category as well. This peptide has been widely studied because it plays critical roles in adipocyte metabolism, fatty acid oxidation, and insulin sensitivity (Lihn et al. 2005). Animal studies have shown that exogenous adiponectin administration leads to weight loss and insulin sensitization, and low levels of circulating adiponectin are correlated with metabolic syndrome in obese humans (Shklyaev et al. 2003; Lin et al. 2007; Matafome et al. 2014). However, adiponectin was not previously known to target the gustatory system.
Because the primary function of TRCs is to detect tastants and transduce this information to afferent gustatory nerve fibers, we reasoned that adiponectin may modulate taste responsiveness. Surprisingly, APN KO mice and their WT controls showed equivalent behavior responses to prototypical taste stimuli in a brief-access lick response paradigm. Brief-access lick tests assess gustatory responsiveness and minimize post-ingestive contributions (Nelson et al. 2003), but we cannot eliminate the potential influence of olfactory or nongustatory orosensory factors. It is also possible that ablation of adiponectin could alter gustatory responses to lower tastant concentrations than those tested here. It would be interesting to explore the impact of adiponectin signaling on other sensory systems or on threshold gustatory responses in future studies.
We also cannot exclude the possibility that unknown compensatory mechanisms are able to supplement for the lack of adiponectin (Ma et al. 2002). To address this issue, we performed the same brief-access lick testing in APN KO mice that had been supplemented with recombinant AAV-mediated expression of adiponectin in the saliva or circulation. Mice with supplemented salivary adiponectin (but not supplemented systemic adiponectin or control APN KO mice) showed a modest but significant increase in behavioral responsiveness to Intralipid. Whether lipids elicit a distinct taste perceptual quality remains controversial, but they clearly can impact gustatory responses (Ozdener et al. 2014). Several putative “fat taste” receptors have been suggested in rodent taste buds, including the fatty acid translocase CD36 and the fatty acid-sensitive G protein-coupled receptor GPR120 (Cartoni et al. 2010). Interestingly, adiponectin has been reported to upregulate CD36 expression in cardiomyocytes via activation of the AMPK pathway (Chabowski et al. 2006; Fang et al. 2010). It is unclear whether a similar response may be present in TRCs.
Viral-mediated expression of peptide hormones may be a useful strategy for modulating taste in a clinical context. By targeting expression to just the salivary glands, salivary adiponectin expression reached WT levels while circulating adiponectin levels were 1,000-fold less than those found in WT mice (Frühbeck et al. 2017). Despite this, we were unable to completely limit adiponectin expression to either blood or saliva in either supplementation mouse model. Since pilocarpine was used to stimulate saliva secretion for analysis, it is possible that steady-state or food evoked salivations of animals may have varying levels of adiponectin expression throughout the day (Dawes 1966). However, pilocarpine-induced salivation was necessary to collect sufficient amounts of saliva for adiponectin quantification. To limit off-target expression in AAV tropic tissues with supplementation of salivary adiponectin, we both directly administered AAV vectors into the salivary gland and included microRNAs for miR122 and miR206 which are liver and skeletal muscle specific, respectively. Even so, it was obvious that viral particles were still entering the circulation. Circulating adiponectin seen in this model could also be due to limited off-target transduction of nonsalivary tissue, or the transduced salivary cells themselves may secrete adiponectin nonspecifically into both the blood and the saliva. In the systemic adiponectin supplementation model, circulating adiponectin is likely transferred into saliva (Wang et al. 2013). Altogether, however, the salivary supplementation model provided an impressive degree of expression control.
In summary, we have shown that adiponectin receptors ADIPOR1 and T-cadherin are expressed in subpopulations of TRCs and that saliva-derived adiponectin can positively modulate lick behavioral responsiveness to Intralipid under certain conditions. A clearer understanding of the mechanisms by which adiponectin impacts TRC function awaits further studies of both oral lipid sensing and adiponectin-dependent signaling in the peripheral gustatory system.
Funding
This work was supported by grants F31 DC015751 (S.M.C.) and R01 DC012819 (S.D.M.) from the National Institute on Deafness and Other Communication Disorders.
Conflicts of Interest
Author C.D. Dotson, an employee of the University of Florida throughout the study, is currently employed by the Coca Cola Company. Dotson’s current employment by the Coca Cola Company did not influence any experimental results or financial gain associated with this publication.
Supplementary Material
Acknowledgments
Thanks to the UF Center for Smell and Taste Chemosensory Behavior core for access to behavioral testing instruments. Thanks to Dr. Xiao-Rong Peng for their generous donation of both ADIPOR1 and ADIPOR2 antibodies.
References
- Acosta A, Hurtado MD, Gorbatyuk O, La Sala M, Duncan D, Aslanidi G, Campbell-Thompson M, Zhang L, Herzog H, Voutetakis A, et al. 2011. Salivary PYY: a putative bypass to satiety. PLoS One. 6:e26137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akuailou EN, Vijayagopal P, Imrhan V, Prasad C. 2013. Measurement and validation of the nature of salivary adiponectin. Acta Diabetol. 50:727–730. [DOI] [PubMed] [Google Scholar]
- Awazawa M, Ueki K, Inabe K, Yamauchi T, Kubota N, Kaneko K, Kobayashi M, Iwane A, Sasako T, Okazaki Y, et al. 2011. Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab. 13:401–412. [DOI] [PubMed] [Google Scholar]
- Baquero AF, Gilbertson TA. 2011. Insulin activates epithelial sodium channel (ENaC) via phosphoinositide 3-kinase in mammalian taste receptor cells. Am J Physiol Cell Physiol. 300:C860–C871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DL, Sullivan SL, Lavoie EG, Sévigny J, Finger TE. 2006. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 497:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, et al. 2002. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 418:650–654. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ. 2006. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 4:223–233. [DOI] [PubMed] [Google Scholar]
- Beylot M, Pinteur C, Peroni O. 2006. Expression of the adiponectin receptors AdipoR1 and AdipoR2 in lean rats and in obese Zucker rats. Metabolism. 55:396–401. [DOI] [PubMed] [Google Scholar]
- Biggs BT, Tang T, Krimm RF. 2016. Insulin-like growth factors are expressed in the taste system, but do not maintain adult taste buds. PLoS One. 11:e0148315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjursell M, Ahnmark A, Bohlooly-Y M, William-Olsson L, Rhedin M, Peng XR, Ploj K, Gerdin AK, Arnerup G, Elmgren A, et al. 2007. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes. 56:583–593. [DOI] [PubMed] [Google Scholar]
- Bobbert T, Rochlitz H, Wegewitz U, Akpulat S, Mai K, Weickert MO, Möhlig M, Pfeiffer AF, Spranger J. 2005. Changes of adiponectin oligomer composition by moderate weight reduction. Diabetes. 54:2712–2719. [DOI] [PubMed] [Google Scholar]
- Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S. 2010. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 30:8376–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabowski A, Momken I, Coort SL, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Bonen A. 2006. Prolonged AMPK activation increases the expression of fatty acid transporters in cardiac myocytes and perfused hearts. Mol Cell Biochem. 288:201–212. [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Roper SD. 2010. The cell biology of taste. J Cell Biol. 190:285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SJ, Sanmiguel J, Lock M, McMenamin D, Draper C, Limberis MP, Kassim SH, Somanathan S, Bell P, Johnston JC, et al. 2013. Biodistribution of AAV8 vectors expressing human low-density lipoprotein receptor in a mouse model of homozygous familial hypercholesterolemia. Hum Gene Ther Clin Dev. 24:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon TJ, Cossette T, Erger K, Choi YK, Clarke T, Scott-Jorgensen M, Song S, Campbell-Thompson M, Crawford J, Flotte TR. 2005. Efficient hepatic delivery and expression from a recombinant adeno-associated virus 8 pseudotyped alpha1-antitrypsin vector. Mol Ther. 12:867–875. [DOI] [PubMed] [Google Scholar]
- Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S. 2004. Automatic and quantitative measurement of protein–protein colocalization in live cells. Biophys J. 86:3993–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson SM, Currlin S, Moskalenko O, Yegorova S, Dotson CD, Zolotukhin S. 2018. Differential expression of immune related genes in taste buds of fed and fasted mice. bioRxiv. doi: 10.1101/339911 [Google Scholar]
- Dawes C. 1966. The composition of human saliva secreted in response to a gustatory stimulus and to pilocaprine. J Physiol. 183:360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. 2010. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 120:4342–4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale G, Dicembrini I, Raimondi L, Pagano C, Egan JM, Cozzi A, Cinci L, Loreto A, Manni ME, Berretti S, et al. 2012. Sustained exendin-4 secretion through gene therapy targeting salivary glands in two different rodent models of obesity/type 2 diabetes. PLoS One. 7:e40074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, Li L, Su YC, Xiang RL, Cong X, Yu HK, Li SL, Wu LL, Yu GY. 2013. Adiponectin increases secretion of rat submandibular gland via adiponectin receptors-mediated AMPK signaling. PLoS One. 8:e63878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson CD, Geraedts MC, Munger SD. 2013. Peptide regulators of peripheral taste function. Semin Cell Dev Biol. 24:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson AE, Dotson CD, Egan JM, Munger SD. 2010. Glucagon signaling modulates sweet taste responsiveness. FASEB J. 24:3960–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Palanivel R, Cresser J, Schram K, Ganguly R, Thong FS, Tuinei J, Xu A, Abel ED, Sweeney G. 2010. An APPL1-AMPK signaling axis mediates beneficial metabolic effects of adiponectin in the heart. Am J Physiol Endocrinol Metab 299:E721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühbeck G, Catalán V, Rodríguez A, Ramírez B, Becerril S, Portincasa P, Gómez-Ambrosi J. 2017. Normalization of adiponectin concentrations by leptin replacement in ob/ob mice is accompanied by reductions in systemic oxidative stress and inflammation. Sci Rep. 7:2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Yan X, Zheng C, Goldsmith CM, Afione S, Hai B, Xu J, Zhou J, Zhang C, Chiorini JA, et al. 2011. AAV2-mediated transfer of the human aquaporin-1 cDNA restores fluid secretion from irradiated miniature pig parotid glands. Gene Ther. 18:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler A, Schön C, Größl T, Pinkert S, Stein EA, Kurreck J, Vetter R, Fechner H. 2013. Application of mutated miR-206 target sites enables skeletal muscle-specific silencing of transgene expression of cardiotropic AAV9 vectors. Mol Ther. 21:924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning JI, Gresack J, Spector AC. 2002. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem Senses. 27:461–474. [DOI] [PubMed] [Google Scholar]
- Grimm D, Kern A, Rittner K, Kleinschmidt JA. 1998. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum Gene Ther. 9:2745–2760. [DOI] [PubMed] [Google Scholar]
- Gröschl M, Rauh M, Wagner R, Neuhuber W, Metzler M, Tamgüney G, Zenk J, Schoof E, Dörr HG, Blum WF, et al. 2001. Identification of leptin in human saliva. J Clin Endocrinol Metab. 86:5234–5239. [DOI] [PubMed] [Google Scholar]
- Hellström PM, Geliebter A, Näslund E, Schmidt PT, Yahav EK, Hashim SA, Yeomans MR. 2004. Peripheral and central signals in the control of eating in normal, obese and binge-eating human subjects. Br J Nutr. 92(Suppl 1):S47–S57. [DOI] [PubMed] [Google Scholar]
- Huang YA, Maruyama Y, Stimac R, Roper SD. 2008. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J Physiol. 586:2903–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado MD, Acosta A, Riveros PP, Baum BJ, Ukhanov K, Brown AR, Dotson CD, Herzog H, Zolotukhin S. 2012. Distribution of Y-receptors in murine lingual epithelia. PLoS One. 7:e46358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano H, Kok MR, Cotrim AP, Yamano S, Schmidt M, Afione S, Baum BJ, Chiorini JA. 2006. Enhanced transduction of mouse salivary glands with AAV5-based vectors. Gene Ther. 13:594–601. [DOI] [PubMed] [Google Scholar]
- Katsiougiannis S, Kapsogeorgou EK, Manoussakis MN, Skopouli FN. 2006. Salivary gland epithelial cells: a new source of the immunoregulatory hormone adiponectin. Arthritis Rheum. 54:2295–2299. [DOI] [PubMed] [Google Scholar]
- Katsiougiannis S, Tenta R, Skopouli FN. 2010. Activation of AMP-activated protein kinase by adiponectin rescues salivary gland epithelial cells from spontaneous and interferon-gamma-induced apoptosis. Arthritis Rheum. 62:414–419. [DOI] [PubMed] [Google Scholar]
- Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. 2000. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci USA. 97:11044–11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya N, Shen T, Lu SG, Zhao FL, Herness S. 2004. A paracrine signaling role for serotonin in rat taste buds: expression and localization of serotonin receptor subtypes. Am J Physiol Regul Integr Comp Physiol. 286:R649–R658. [DOI] [PubMed] [Google Scholar]
- Kieffer TJ, Habener JF. 1999. The glucagon-like peptides. Endocr Rev. 20:876–913. [DOI] [PubMed] [Google Scholar]
- La Sala MS, Hurtado MD, Brown AR, Bohórquez DV, Liddle RA, Herzog H, Zolotukhin S, Dotson CD. 2013. Modulation of taste responsiveness by the satiation hormone peptide YY. FASEB J. 27:5022–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lihn AS, Pedersen SB, Richelsen B. 2005. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 6:13–21. [DOI] [PubMed] [Google Scholar]
- Lin HV, Kim JY, Pocai A, Rossetti L, Shapiro L, Scherer PE, Accili D. 2007. Adiponectin resistance exacerbates insulin resistance in insulin receptor transgenic/knockout mice. Diabetes. 56:1969–1976. [DOI] [PubMed] [Google Scholar]
- Lin H, Maeda K, Fukuhara A, Shimomura I, Ito T. 2014. Molecular expression of adiponectin in human saliva. Biochem Biophys Res Commun. 445:294–298. [DOI] [PubMed] [Google Scholar]
- Ma K, Cabrero A, Saha PK, Kojima H, Li L, Chang BH, Paul A, Chan L. 2002. Increased beta-oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J Biol Chem. 277:34658–34661. [DOI] [PubMed] [Google Scholar]
- Ma H, Yang R, Thomas SM, Kinnamon JC. 2007. Qualitative and quantitative differences between taste buds of the rat and mouse. BMC Neurosci. 8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Rasko J, Ozelo MC, Hoots K, Blatt P, et al. 2006. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 12:342–347. [DOI] [PubMed] [Google Scholar]
- Mansouri-Guilani N, Bernard V, Vigneault E, Vialou V, Daumas S, El Mestikawy S, Gangarossa G. 2019. VGLUT3 gates psychomotor effects induced by amphetamine. J Neurochem. 148:779–795. [DOI] [PubMed] [Google Scholar]
- Markusic DM, Herzog RW. 2012. Liver-directed adeno-associated viral gene therapy for hemophilia. J Genet Syndr Gene Ther. 1:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Dotson CD, Shin YK, Ji S, Drucker DJ, Maudsley S, Munger SD. 2009. Modulation of taste sensitivity by GLP-1 signaling in taste buds. Ann N Y Acad Sci. 1170:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matafome P, Rodrigues T, Pereira A, Letra L, Azevedo H, Paixão A, Silvério M, Almeida A, Sena C, Seiça R. 2014. Long-term globular adiponectin administration improves adipose tissue dysmetabolism in high-fat diet-fed Wistar rats. Arch Physiol Biochem. 120:147–157. [DOI] [PubMed] [Google Scholar]
- Mbiene JP, Roberts JD. 2003. Distribution of keratin 8-containing cell clusters in mouse embryonic tongue: evidence for a prepattern for taste bud development. J Comp Neurol. 457:111–122. [DOI] [PubMed] [Google Scholar]
- Ming D, Ninomiya Y, Margolskee RF. 1999. Blocking taste receptor activation of gustducin inhibits gustatory responses to bitter compounds. Proc Natl Acad Sci USA. 96:9903–9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi MA, Abe K, Emori Y. 2001. IP(3) receptor type 3 and PLCbeta2 are co-expressed with taste receptors T1R and T2R in rat taste bud cells. Chem Senses. 26:259–265. [DOI] [PubMed] [Google Scholar]
- Nadkarni P, Chepurny OG, Holz GG. 2014. Regulation of glucose homeostasis by GLP-1. Prog Mol Biol Transl Sci. 121:23–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TM, Munger SD, Boughter JD Jr. 2003. Taste sensitivities to PROP and PTC vary independently in mice. Chem Senses. 28:695–704. [DOI] [PubMed] [Google Scholar]
- Nigro E, Piombino P, Scudiero O, Monaco ML, Schettino P, Chambery A, Daniele A. 2015. Evaluation of salivary adiponectin profile in obese patients. Peptides. 63:150–155. [DOI] [PubMed] [Google Scholar]
- Ozdener MH, Subramaniam S, Sundaresan S, Sery O, Hashimoto T, Asakawa Y, Besnard P, Abumrad NA, Khan NA. 2014. CD36- and GPR120-mediated Ca2+ signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology. 146:995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffe T, Cooper-White J, Beyerlein P, Kostner K, Punyadeera C. 2011. Diagnostic potential of saliva: current state and future applications. Clin Chem. 57:675–687. [DOI] [PubMed] [Google Scholar]
- Piedra J, Ontiveros M, Miravet S, Penalva C, Monfar M, Chillon M. 2015. Development of a rapid, robust, and universal picogreen-based method to titer adeno-associated vectors. Hum Gene Ther Methods. 26:35–42. [DOI] [PubMed] [Google Scholar]
- Puzzo F, Colella P, Biferi MG, Bali D, Paulk NK, Vidal P, Collaud F, Simon-Sola M, Charles S, Hardet R, et al. 2017. Rescue of Pompe disease in mice by AAV-mediated liver delivery of secretable acid α-glucosidase. Sci Transl Med 9. doi: 10.1126/scitranslmed.aam6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands MS. 2011. AAV-mediated liver-directed gene therapy. Methods Mol Biol. 807:141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemura N, Iwata S, Yasumatsu K, Ohkuri T, Horio N, Sanematsu K, Yoshida R, Margolskee RF, Ninomiya Y. 2013. Angiotensin II modulates salty and sweet taste sensitivities. J Neurosci. 33:6267–6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YK, Martin B, Golden E, Dotson CD, Maudsley S, Kim W, Jang HJ, Mattson MP, Drucker DJ, Egan JM, et al. 2008. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem. 106:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shklyaev S, Aslanidi G, Tennant M, Prima V, Kohlbrenner E, Kroutov V, Campbell-Thompson M, Crawford J, Shek EW, Scarpace PJ, et al. 2003. Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc Natl Acad Sci USA. 100:14217–14222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair MS, Perea-Martinez I, Dvoryanchikov G, Yoshida M, Nishimori K, Roper SD, Chaudhari N. 2010. Oxytocin signaling in mouse taste buds. PLoS One. 5:e11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumaran SK, Lewandowski BC, Qin Y, Kotha R, Bachmanov AA, Margolskee RF. 2017. Whole transcriptome profiling of taste bud cells. Sci Rep. 7:7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S, Yasumatsu K, Inoue M, Iwata S, Yoshida R, Shigemura N, Yanagawa Y, Drucker DJ, Margolskee RF, Ninomiya Y. 2015. Glucagon-like peptide-1 is specifically involved in sweet taste transmission. FASEB J. 29:2268–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda M, Tsukinoki R, Morimoto K. 2007. Measurement of salivary adiponectin levels. Acta Diabetol. 44:20–22. [DOI] [PubMed] [Google Scholar]
- Vandenbeuch A, Anderson CB, Parnes J, Enjyoji K, Robson SC, Finger TE, Kinnamon SC. 2013. Role of the ectonucleotidase NTPDase2 in taste bud function. Proc Natl Acad Sci USA. 110:14789–14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal-Molina MT, Antuna-Puente B. 2012. Adiponectin: anti-inflammatory and cardioprotective effects. Biochimie. 94:2143–2149. [DOI] [PubMed] [Google Scholar]
- Voutetakis A, Zheng C, Cotrim AP, Mineshiba F, Afione S, Roescher N, Swaim WD, Metzger M, Eckhaus MA, Donahue RE, et al. 2010. AAV5-mediated gene transfer to the parotid glands of non-human primates. Gene Ther. 17:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Iguchi N, Rong Q, Zhou M, Ogunkorode M, Inoue M, Pribitkin EA, Bachmanov AA, Margolskee RF, Pfeifer K, et al. 2009. Expression of the voltage-gated potassium channel KCNQ1 in mammalian taste bud cells and the effect of its null-mutation on taste preferences. J Comp Neurol. 512:384–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liang Y, Wang Y, Cui J, Liu M, Du W, Xu Y. 2013. Computational prediction of human salivary proteins from blood circulation and application to diagnostic biomarker identification. PLoS One. 8:e80211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. 2002. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 8:1288–1295. [DOI] [PubMed] [Google Scholar]
- Yang R, Ma H, Thomas SM, Kinnamon JC. 2007. Immunocytochemical analysis of syntaxin-1 in rat circumvallate taste buds. J Comp Neurol. 502:883–893. [DOI] [PubMed] [Google Scholar]
- Yee CL, Yang R, Böttger B, Finger TE, Kinnamon JC. 2001. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol. 440:97–108. [DOI] [PubMed] [Google Scholar]
- Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. 2006. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 55:2562–2570. [DOI] [PubMed] [Google Scholar]
- Zhao FL, Shen T, Kaya N, Lu SG, Cao Y, Herness S. 2005. Expression, physiological action, and coexpression patterns of neuropeptide Y in rat taste-bud cells. Proc Natl Acad Sci USA. 102:11100–11105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. 2008. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 16:1073–1080. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S. 2013. Metabolic hormones in saliva: origins and functions. Oral Dis. 19:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ, Muzyczka N. 1999. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 6:973–985. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Potter M, Hauswirth WW, Guy J, Muzyczka N. 1996. A “humanized” green fluorescent protein cDNA adapted for high-level expression in mammalian cells. J Virol. 70:4646–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ Jr, Chiodo VA, Phillipsberg T, Muzyczka N, Hauswirth WW, et al. 2002. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 28:158–167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.