Key Points
Question
What is the relation between time to treatment and outcome from endovascular-recanalization therapy for acute ischemic stroke (AIS)?
Findings
In this retrospective cohort study of 6756 patients with AIS in a US nationwide clinical registry, earlier onset to treatment was associated with improved outcomes, including, for every 15 minutes faster treatment: higher rates of independent ambulation (absolute increase, 1.14%), functional independence at discharge (absolute increase, 0.91%), and lower mortality/hospice discharge (absolute decrease, −0.77%).
Meaning
Among patients with AIS treated in routine clinical practice, shorter time to endovascular-recanalization therapy was associated with better outcomes.
Abstract
Importance
Randomized clinical trials suggest benefit of endovascular-reperfusion therapy for large vessel occlusion in acute ischemic stroke (AIS) is time dependent, but the extent to which it influences outcome and generalizability to routine clinical practice remains uncertain.
Objective
To characterize the association of speed of treatment with outcome among patients with AIS undergoing endovascular-reperfusion therapy.
Design, Setting, and Participants
Retrospective cohort study using data prospectively collected from January 2015 to December 2016 in the Get With The Guidelines-Stroke nationwide US quality registry, with final follow-up through April 15, 2017. Participants were 6756 patients with anterior circulation large vessel occlusion AIS treated with endovascular-reperfusion therapy with onset-to-puncture time of 8 hours or less.
Exposures
Onset (last-known well time) to arterial puncture, and hospital arrival to arterial puncture (door-to-puncture time).
Main Outcomes and Measures
Substantial reperfusion (modified Thrombolysis in Cerebral Infarction score 2b-3), ambulatory status, global disability (modified Rankin Scale [mRS]) and destination at discharge, symptomatic intracranial hemorrhage (sICH), and in-hospital mortality/hospice discharge.
Results
Among 6756 patients, the mean (SD) age was 69.5 (14.8) years, 51.2% (3460/6756) were women, and median pretreatment score on the National Institutes of Health Stroke Scale was 17 (IQR, 12-22). Median onset-to-puncture time was 230 minutes (IQR, 170-305) and median door-to-puncture time was 87 minutes (IQR, 62-116), with substantial reperfusion in 85.9% (5433/6324) of patients. Adverse events were sICH in 6.7% (449/6693) of patients and in-hospital mortality/hospice discharge in 19.6% (1326/6756) of patients. At discharge, 36.9% (2132/5783) ambulated independently and 23.0% (1225/5334) had functional independence (mRS 0-2). In onset-to-puncture adjusted analysis, time-outcome relationships were nonlinear with steeper slopes between 30 to 270 minutes than 271 to 480 minutes. In the 30- to 270-minute time frame, faster onset to puncture in 15-minute increments was associated with higher likelihood of achieving independent ambulation at discharge (absolute increase, 1.14% [95% CI, 0.75%-1.53%]), lower in-hospital mortality/hospice discharge (absolute decrease, −0.77% [95% CI, −1.07% to −0.47%]), and lower risk of sICH (absolute decrease, −0.22% [95% CI, −0.40% to −0.03%]). Faster door-to-puncture times were similarly associated with improved outcomes, including in the 30- to 120-minute window, higher likelihood of achieving discharge to home (absolute increase, 2.13% [95% CI, 0.81%-3.44%]) and lower in-hospital mortality/hospice discharge (absolute decrease, −1.48% [95% CI, −2.60% to −0.36%]) for each 15-minute increment.
Conclusions and Relevance
Among patients with AIS due to large vessel occlusion treated in routine clinical practice, shorter time to endovascular-reperfusion therapy was significantly associated with better outcomes. These findings support efforts to reduce time to hospital and endovascular treatment in patients with stroke.
This cohort study uses data from the Get With the Guidelines-Stroke registry to report associations between time to treatment and functional outcomes among patients in clinical practice with acute ischemic stroke treated with endovascular recanalization.
Introduction
Randomized clinical trials (RCTs) have demonstrated the benefit of endovascular-reperfusion therapy over medical therapy among patients with large vessel occlusion in acute ischemic stroke (AIS).1,2,3,4,5,6 Several studies suggest a strong time dependency of greater benefit with earlier treatment.7,8,9,10 Existing data regarding the relation of onset-to-treatment time and outcome, however, is limited in precision and representativeness. The pooled analysis of RCTs was of modest size (536 patients undergoing endovascular-reperfusion therapy in 5 trials),8 and RCT findings may not be directly generalizable to routine clinical practice. Observational studies similarly have been underpowered to delineate time effects with high precision.1,7,10,11 To address the need for analysis of a large, practice-based data set, the US nationwide Get With the Guidelines-Stroke (GWTG-Stroke) registry was analyzed to determine the association of time to treatment with outcomes from endovascular-reperfusion therapy.
Methods
GWTG-Stroke is a nationwide registry maintained by the American Heart Association and American Stroke Association to support continuous quality improvement of hospital systems providing care for patients with stroke and transient ischemic attack (TIA).12,13 Details of the design and conduct of the program have been previously described.13,14 GWTG-Stroke uses a web-based patient management tool (IQVIA) to collect clinical data on consecutively admitted patients.13 Hospitals received either approval to enroll patients without individual patient consent under the common rule or a waiver of authorization and exemption from subsequent review by their institutional review board (IRB). The IRB of the data analysis center at Duke University approved the study.
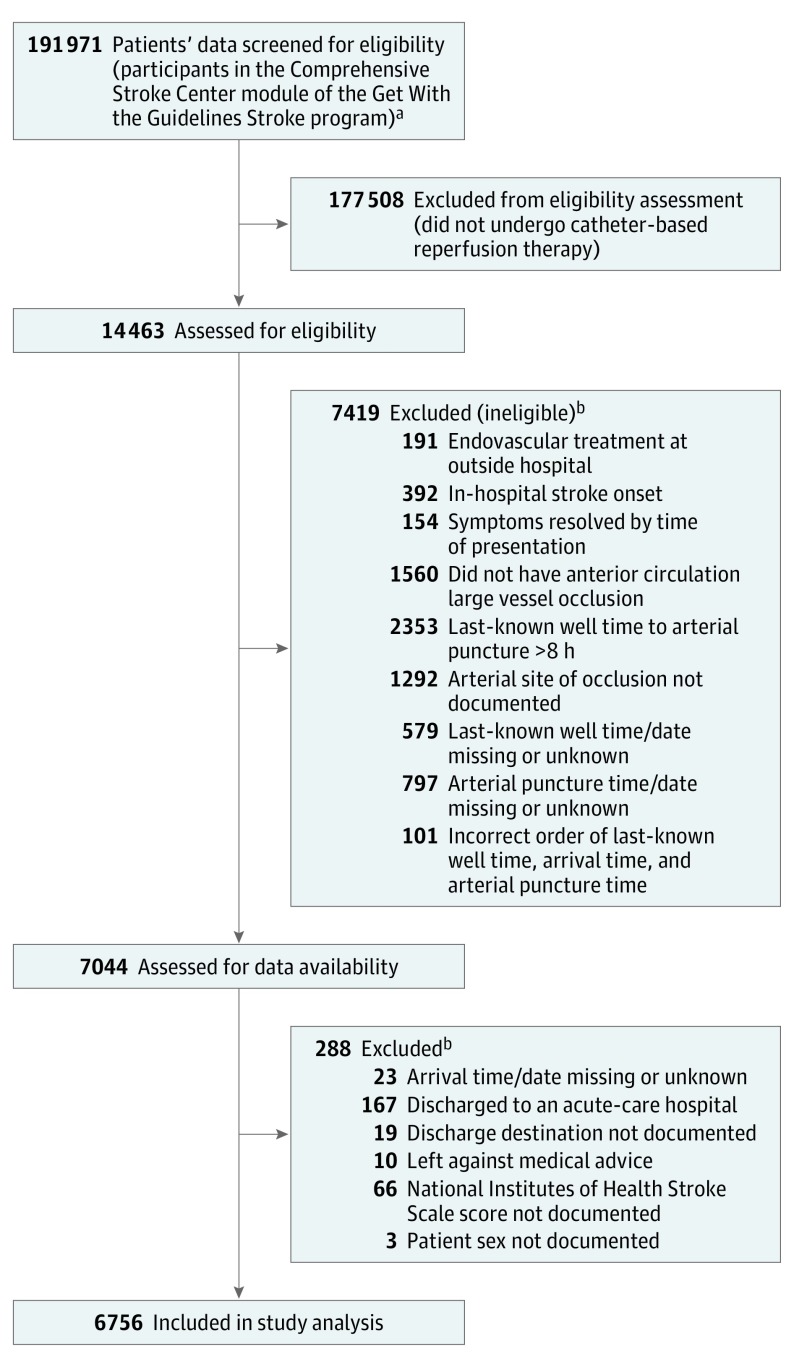
We selected patients with documented anterior circulation large vessel occlusion and interval from last-known well time to arterial puncture time of 8 hours or less. Eligibility criteria are shown in Figure 1. The study time period, January 1, 2015, to December 31, 2016, was selected to reflect care occurring after publication of the first positive RCT of endovascular thrombectomy in December 2014.15 Throughout this time period, endovascular mechanical thrombectomy devices were cleared by the US Food and Drug Administration for use up to 8 hours after onset. In addition, in June 2015, US national practice guidelines recommended endovascular thrombectomy up to 6 hours (high-grade recommendation) and 8 hours (medium-grade recommendation) after onset.16 Accordingly, the onset-to-puncture criterion of 8 hours or less identified patients treated in accordance with prevailing regulatory and expert consensus guidance.
Figure 1. Flow Diagram Showing Study Population Screening, Eligibility, and Inclusion.
aData were for patients with ischemic stroke receiving care between January 1, 2015, and December 31, 2016, with at least 75% of medical history field completion.
bCriteria are listed in the order in which applied.
Analysis was conducted using data from the Comprehensive Stroke Center (CSC) module of the GWTG-Stroke program regarding patients treated between January 1, 2015, and December 31, 2016, with final follow-up through April 15, 2017, (eTable 1 in Supplement 1). Admission or medical staff recorded the patient’s self-reported race/ethnicity, based on open-ended questions, which was analyzed because prior studies have suggested differences in AIS outcome may be associated with race/ethnicity status. Data on hospital-level characteristics were obtained from the American Hospital Association database.
The main clinical outcomes were as follows: (1) discharge to home (vs acute rehabilitation, skilled nursing facility, hospice, death, other); (2) independent ambulation at discharge; (3) freedom from disability (modified Rankin Scale [mRS] score, 0-1) at discharge; and (4) functional independence (mRS score, 0-2) at discharge. The functional independence and freedom from disability outcomes were derived from the mRS, an ordinal measure of global disability with 7 levels ranging from 0 (no symptoms, best) to 5 (severe disability-bedridden) and 6 (dead). Other clinical outcomes were discharge to home or acute rehabilitation, ambulatory with or without assistance at discharge, freedom from disability (mRS score, 0-1) at 3 months, and functional independence (mRS score, 0-2) at 3 months. The main technical outcome was substantial reperfusion, defined as having a modified Thrombolysis in Cerebral Infarction (mTICI) score of 2b to 3 (50%-100% reperfusion).17 The main adverse event outcomes analyzed were (1) in-hospital mortality/hospice discharge, and (2) symptomatic intracranial hemorrhage (sICH) within 36 hours. Another adverse event outcome analyzed was in-hospital mortality (without discharge to hospice).
Two time intervals were evaluated for relation to each of these outcomes: (1) onset (last-known well) to arterial puncture; and (2) hospital arrival to arterial puncture (door-to-puncture time) (further details in eMethods 1 in Supplement 1).
Statistical Analysis
Percentages were reported for categorical variables and medians and interquartile ranges (IQRs) for continuous variables. The Pearson χ2 test and Kruskal-Wallis tests were used to compare variables in the onset-to-puncture and door-to-puncture time epochs. Multivariable logistic regression analysis was performed to assess the association of onset-to-puncture times and door-to-puncture times with clinical and adverse event outcomes. Generalized estimating equations were used in all regression models to account for within-hospital clustering. The multivariable models, detailed in eMethods 2 in Supplement 1, adjusted for 28 patient-level and 9 hospital-level characteristics (eTable 2 in Supplement 1). The logistic regression model assumes that independent variables have a linear relationship with respect to the prevalence of the dependent variable (ie, the outcome) on the logit scale. All the continuous variables included for adjustment were evaluated for nonlinearity with the outcome, and linear splines were used for those that violated the linearity assumption. The linear splines were placed at the point at which the slope of the straight lines approximating the relationship curve changed.
Rates of missingness of baseline patient characteristics data were low, and for the preponderance of patient-level baseline variables, missing values were imputed to the mode or median as detailed in eResults 1 in Supplement 1. Outcomes with low (0%-15%) data missingness were analyzed with complete case analysis, and outcomes with higher data missingness were analyzed using inverse probability weighting (detailed in eMethods 2 in Supplement 1).18
The relationships between onset-to-puncture and door-to-puncture times and the binary outcomes were assessed using logistic regression models with restricted cubic splines of onset-to-puncture or door-to-puncture times with knots at the 5th, 35th, 65th, 95th percentiles (eMethods 2 in Supplement 1). To generate time-benefit curves, outcome-specific predicted probabilities for each value of onset-to-puncture or door-to-puncture time within the observed range were computed while setting all other variables in the model to mean values. Visual assessment and Wald χ2 tests were used to assess the linearity of the relationship.
SAS (version 9.4; SAS Institute Inc) software was used for all statistical analyses. All P values were 2-sided and statistical significance was defined as a P value of less than .05. Adjustments were not made for multiplicity; accordingly, all analyses were considered exploratory. Statistical analyses were not performed on some auxiliary outcomes due to funding constraints. These are reported as not performed (eTable 6 and eTable 8 in Supplement 1).
Results
During the study period, 191 971 patients with ischemic stroke were entered into the GWTG-Stroke CSC module at hospitals with less than 25% of missing data in medical history items, among whom 14 463 (7.5%) underwent endovascular-reperfusion therapy. Among the 7044 patients meeting target vessel site, treatment time window, and other eligibility criteria for this study, 6756 patients (95.9%) from 231 hospitals had documentation of all study baseline covariates (Figure 1; eTable 2 in Supplement 1) and constituted the study population. The patient- and hospital-level characteristics of the 288 patients excluded for missing documentation of 1 or more key baseline covariates is included in eTable 3 in Supplement 1. Standardized difference scores indicated the included and excluded groups did not differ in many prognostic variables, including age and baseline score on the National Institutes of Health Stroke Scale (NIHSS), but did differ in others, with the excluded patients less frequently being ambulatory prior to the index stroke and receiving care more often at lower-volume thrombectomy hospitals.
Patient mean (SD) age was 69.5 (14.8) years, 51.2% (3460/6756) were women, and median presenting NIHSS score was 17 (IQR, 12-22). Mode of arrival was by emergency medical services (EMS) transport for 51.1% (3454/6756) of patients, private vehicle for 3.0% (201/6756), and interfacility transfer for 45.7% (3088/6756). Intravenous (IV) recombinant tissue plasminogen activator (rtPA) was administered prior to endovascular-reperfusion therapy in 68.2% (4610/6756) of patients, and the target occlusion site was the internal carotid artery (cervical or intracranial) in 17.0% (1150/6756) and M1 or M2 segments of the middle cerebral artery in 83.0% (5606/6756). Types of endovascular-reperfusion therapy intervention are shown in eResults 2 in Supplement 1. Symptom onset was witnessed in 67.7% (4572/6756) of patients and unwitnessed in 32.3% (2184/6756) (further details in eFigure 1, eResults 3, eTable 4, and eFigure 2 in Supplement 1).
The median onset-to-puncture time was 230 minutes (IQR, 170-305) (eFigure 1, Supplement 1). Across broad onset-to-puncture windows, 6.9% (463/6756) of patients had onset-to-puncture times of 30 to 120 minutes, 47.5% (3207/6756) had 121 to 240 minutes, 33.1% (2235/6756) had 241 of 360 minutes, and 12.6% (851/6756) had onset-to-puncture times of 361 to 480 minutes. Patient-level factors associated with longer onset-to-puncture times included unwitnessed symptom onset (median, 262 minutes [IQR, 200-340]) vs 216 minutes (IQR, 161-286 [P < .001]), lower NIHSS score, absence of limb weakness, arrival during off hours (holiday, weekend, or before 7 am or after 6 pm on Monday-Friday), arrival by interfacility transfer, not having received IV rtPA, and histories of hypertension and of diabetes (Table 1). Hospital-level factors associated with longer onset-to-puncture time included certification as a CSC, serving as a teaching hospital, and location in the Northeast (Table 1).
Table 1. Patient- and Hospital-Level Characteristics of Patients Treated With Endovascular Reperfusion Therapy, Overall and in Different Onset-to-Puncture Time Windows.
| Onset-to-Puncture Interval Minutes, No. (%) | P Valuea | |||||
|---|---|---|---|---|---|---|
| Overall | 0-120 | 121-240 | 241-360 | 361-480 | ||
| No. of patients | 6756 | 463 | 3207 | 2235 | 851 | |
| Age, mean (SD), y | 69.5 (14.8) | 69.4 (15.3) | 69.9 (14.7) | 69.3 (14.8) | 68.5 (14.8) | .07 |
| Men | 3296 (48.8) | 239 (51.6) | 1557 (48.6) | 1080 (48.3) | 420 (49.4) | .60 |
| Women | 3460 (51.2) | 224 (48.4) | 1650 (51.4) | 1155 (51.7) | 431 (50.6) | |
| Race/ethnicity | ||||||
| White, non-Hispanic | 4667 (69.1) | 287 (62.0) | 2237 (69.7) | 1562 (69.9) | 581 (68.3) | .13 |
| Black, non-Hispanic | 1049 (15.5) | 86 (18.6) | 473 (14.7) | 341 (15.3) | 149 (17.5) | |
| Hispanic (all races) | 433 (6.4) | 36 (7.8) | 211 (6.6) | 138 (6.2) | 48 (5.6) | |
| Asian, non-Hispanic | 178 (2.6) | 17 (3.7) | 80 (2.5) | 57 (2.6) | 24 (2.8) | |
| Other, non-Hispanicb | 429 (6.35) | 37 (7.99) | 206 (6.42) | 137 (6.13) | 49 (5.76) | |
| Arrival at off hoursc | 3427 (50.7) | 107 (23.1) | 1540 (48.0) | 1271 (56.9) | 509 (59.8) | <.001 |
| Arrival by EMS | 3454 (51.1) | 394 (85.1) | 2047 (63.8) | 727 (32.5) | 286 (33.6) | <.001 |
| Last-known well-to-arrival time, median (IQR), min | 141 (62-218) | 35 (25-45) | 83 (51-139) | 208 (159-248) | 309 (255-348) | <.001 |
| Received rtPA (at ERT or outside hospital) | 4610 (68.2) | 350 (75.6) | 2430 (75.8) | 1495 (66.9) | 335 (39.4) | <.0001 |
| Door-to-rtPA (at the ERT hospital) time | ||||||
| No. of patients | 2553 | 333 | 1671 | 495 | 54 | |
| Median (IQR), min | 41 (30-55) | 30 (22-40) | 41 (31-54) | 48 (34-65) | 50 (33-60) | <.001 |
| NIHSS score, median (IQR)d | 17 (12-22) | 18 (14-23) | 17 (13-22) | 17 (12-22) | 16 (10-21) | <.001 |
| Severe stroke, NIHSS score >16 | 4020 (59.5) | 303 (65.4) | 1972 (61.5) | 1297 (58.0) | 448 (52.6) | <.001 |
| Absence of limb weakness | 388 (5.7) | 16 (3.5) | 156 (4.9) | 164 (7.3) | 52 (6.1) | <.001 |
| Medical history | ||||||
| Hypertension | 4850 (71.8) | 311 (67.3) | 2289 (71.4) | 1610 (72.1) | 640 (75.2) | .02 |
| Dyslipidemia | 2856 (42.3) | 203 (44.0) | 1356 (42.3) | 922 (41.3) | 375 (44.1) | .46 |
| Atrial fibrillation/flutter | 2393 (35.4) | 161 (34.8) | 1161 (36.2) | 790 (35.4) | 281 (33.0) | .38 |
| CAD or prior MI | 1665 (24.6) | 117 (25.3) | 814 (25.4) | 520 (23.3) | 214 (25.1) | .33 |
| Obesity | 1652 (24.5) | 103 (22.3) | 763 (23.8) | 570 (25.5) | 216 (25.4) | .30 |
| Diabetes mellitus | 1645 (24.4) | 97 (21.0) | 723 (22.5) | 575 (25.7) | 250 (29.4) | <.001 |
| Previous stroke/TIA | 1517 (22.5) | 94 (20.3) | 744 (23.2) | 494 (22.1) | 185 (21.7) | .46 |
| Smoker | 1124 (18.1) | 72 (15.6) | 559 (17.4) | 422 (18.9) | 171 (20.1) | .10 |
| Heart failure | 944 (14.0) | 50 (10.9) | 462 (14.4) | 311 (13.9) | 121 (14.2) | .22 |
| Depression | 582 (8.6) | 44 (9.5) | 265 (8.3) | 194 (8.7) | 79 (9.3) | .69 |
| Drug/alcohol abuse | 465 (6.9) | 18 (3.9) | 237 (7.4) | 154 (6.9) | 56 (6.6) | .05 |
| Renal insufficiency | 393 (5.8) | 28 (6.1) | 190 (5.9) | 121 (5.4) | 54 (6.3) | .75 |
| Sleep apnea | 263 (3.9) | 12 (2.6) | 117 (3.6) | 90 (4.0) | 44 (5.2) | .09 |
| PVD | 251 (3.7) | 17 (3.7) | 118 (3.7) | 88 (3.9) | 28 (3.3) | .86 |
| Carotid stenosis | 186 (2.7) | 13 (2.8) | 85 (2.6) | 59 (2.6) | 29 (3.4) | .66 |
| Prosthetic heart valve | 144 (2.1) | 8 (1.7) | 73 (2.3) | 40 (1.8) | 23 (2.7) | .35 |
| Medication before admission | ||||||
| Anticoagulant/antiplatelet | 3498 (51.8) | 236 (51.0) | 1661 (51.8) | 1170 (52.3) | 431 (50.6) | .86 |
| Antihypertensive | 3952 (58.5) | 233 (50.3) | 1872 (58.4) | 1338 (59.9) | 509 (59.8) | .19 |
| Cholesterol reducer | 2803 (41.5) | 189 (40.8) | 1334 (41.6) | 940 (42.1) | 340 (40.0) | .75 |
| Antidiabetic | 976 (14.4) | 54 (11.7) | 421 (13.1) | 350 (15.7) | 151 (17.7) | .005 |
| Hospital size, No. of beds, median (IQR) | 572 (425-762) | 572 (438-746) | 572 (424-739) | 595 (429-798) | 572 (424-759) | <.001 |
| Hospital region | ||||||
| West | 843 (12.5) | 65 (14.0) | 380 (11.8) | 302 (13.5) | 96 (11.3) | .001 |
| South | 2943 (43.6) | 223 (48.2) | 1386 (43.2) | 936 (41.9) | 398 (46.8) | |
| Midwest | 1526 (22.6) | 100 (21.6) | 751 (23.4) | 520 (23.3) | 155 (18.2) | |
| Northeast | 1444 (21.4) | 75 (16.2) | 690 (21.5) | 477 (21.3) | 202 (23.7) | |
| Academic hospital | 5922 (87.7) | 374 (80.1) | 2762 (86.1) | 2009 (89.9) | 777 (91.3) | <.001 |
| Primary stroke center | 4714 (69.8) | 335 (72.3) | 2212 (69.0) | 1571 (70.3) | 596 (70.0) | .43 |
| Comprehensive stroke center | 3334 (49.3) | 191 (41.3) | 1560 (48.6) | 1164 (52.1) | 419 (49.2) | <.001 |
| Urban location (vs rural location) | 6756 (100) | 463 (100) | 3207 (100) | 2235 (100) | 851 (100) | |
| Ischemic stroke discharges, median (IQR), /ye | 407 (288-494) | 405 (287-513) | 407 (287-510) | 407 (289-490) | 407 (280-482) | .48 |
| rtPA administration, median (IQR), /yf | 36 (27-50) | 36 (27-51) | 36 (27-56) | 35 (27-50) | 36 (27-50) | .008 |
| ERT cases, median (IQR), /yg | 41 (27-62) | 45 (28-61) | 41 (27-61) | 41 (28-61) | 43 (28-69) | .005 |
Abbreviations: AIS, acute ischemic stroke; Antiplat, antiplatelets; CAD, coronary artery disease; EMS, emergency medical service; ERT, endovascular reperfusion therapy.; IQR, interquartile range; MI, myocardial infarction; NIHSS, National Institutes of Health Stroke Scale; PVD, peripheral vascular disease; rtPA, recombinant tissue plasminogen activator; TIA, transient ischemic attack.
P values are based on Pearson χ2 tests for categorical variables and χ2 rank-based group means score statistics (Kruskal-Wallis tests) for continuous/ordinal variables.
Other category indicates American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, and unable to determine (due to patient inability because of stroke and unavailability of family members).
Off hours indicate holidays, weekends, or times before 7 am and after 6 pm Monday through Friday.
The NIHSS score ranges from 0 to 42 (higher scores indicate greater stroke severity).
Annual volume of ischemic stroke discharges: number of admissions for AIS per year during study period.
Annual volume of rtPA administration: number of admissions in which intravenous rtPA was administered for AIS per year during study period.
Annual volume of ERT cases: number of admissions in which endovascular reperfusion therapy was provided for AIS per year during study period.
Among all patients, median door-to-puncture time was 87 minutes (IQR, 62-116). Door-to-puncture times were longer in EMS direct-arriving patients than in interfacility transfer patients (100 minutes [IQR, 78-127] vs 65 minutes [IQR, 47-92]; P < .001) (eFigure 3 in Supplement 1). Among EMS-arriving patients, patient-level factors associated with longer door-to-puncture times included lower NIHSS score, absence of limb weakness, arrival time during off hours, black or Hispanic race/ethnicity, prior stroke or TIA, and history of hypertension (eTable 5 in Supplement 1). Hospital-level factors associated with longer door-to-puncture times included smaller facility size, lower annual volume of ischemic stroke admissions, fewer annual performances of endovascular-reperfusion therapy, and fewer annual IV rtPA cases.
Data availability and missingness for baseline covariates and outcomes in study population are detailed in eResults 1 in Supplement 1. For outcomes, complete data were available for in-hospital mortality. Rates of missingness were low for discharge destination (0.3% [18/6756]) and sICH (0.9% [63/6756]), and moderate for substantial reperfusion (6.4% [432/6756]) (defined as modified Thrombolysis in Cerebral Infarction 2b-3; 50%-100% reperfusion)17 and ambulatory status at discharge (14.4% [973/6756]). The analyses of these outcomes used complete cases. Rates of missingness were higher for discharge mRS (21.1% [1422/6756]) and were substantial for 3-month mRS (44.1% [2976/6756]). Analyses of these outcomes used inverse probability weighting to compensate for patients with missing data on the outcome.18
Table 2 and eTable 6 in Supplement 1 show unadjusted event rates and adjusted odds ratios (ORs) for clinical and adverse event outcomes in all patients and those in the 4 onset-to-puncture time windows. Some auxiliary outcomes in eTable 6 (Supplement 1) did not have statistical analyses performed. Overall, among patients with documented outcomes at discharge, substantial reperfusion was achieved in 85.9% (5433/6324), 27.8% (1876/6756) were discharged to home, 36.9% (2132/5783) were ambulating independently, 23.0% (1225/5334) had functional independence (mRS, 0-2), sICH occurred in 6.7% (449/6693), and 19.6% (1326/6756) had in-hospital mortality/hospice discharge. In the adjusted analyses, compared with the 6- to 8-hour onset-to-puncture window, patients treated in the 0- to 2-hour time window had significantly better outcomes on 8 of 8 clinical and 2 of 3 adverse event end points. For example, patients in the 0- to 2-hour onset-to-puncture category had higher rates of discharge to home (OR, 2.43 [95% CI, 1.81-3.27]) and functional independence at discharge (OR, 3.45 [95% CI, 2.37-5.02]), and had lower mortality/hospice discharge (OR, 0.51 [95% CI, 0.34-0.75]). Patients treated in the 2- to 4-hour onset-to-puncture window had significantly better outcomes on 6 of 8 clinical end points and 1 of 3 adverse event end points, including higher rates of discharge to home (OR, 1.39 [95% CI, 1.12-1.72]) and functional independence at discharge (OR, 1.69 [95% CI, 1.32-2.17]), but no statistically significant difference in mortality/hospice discharge (OR, 0.92 [95% CI, 0.75-1.12]). Patients treated in the 4- to 6-hour onset-to-puncture window had significantly better outcomes on 1 of 8 clinical end points (functional independence at discharge) and 0 of 3 adverse event end points. eTables 7 and 8 in Supplement 1 show unadjusted rates and adjusted ORs for clinical outcomes and adverse events in EMS-arriving patients in the 5 door-to-puncture time windows, also showing improvement in clinical and adverse event outcomes in earlier time windows. Some auxiliary outcomes in eTable 8 (Supplement 1) did not have statistical analyses performed.
Table 2. Main Clinical Outcomes and Adverse Events of Patients Treated With Endovascular Reperfusion Therapy, Overall and in Different Onset-to-Puncture Time Windows.
| Outcomes | Unadjusted, No. of Patients/Total No. (%) by Onset-to-Puncture Interval Minutes | P Valuea | Adjusted Odds Ratios Comparing Onset-to-Puncture Interval Minutes, (95% CI),b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | 0-120 | 121-240 | 241-360 | 361-480 | 0-120 vs 361-480 | 121-240 vs 361-480 | 241-360 vs 361-480 | ||
| Clinical outcome at discharge | |||||||||
| Discharge to homec | 1876/6756 (27.8) | 186/463 (40.2) | 923/3207 (28.8) | 559/2235 (25.0) | 208/851 (24.4) | <.001 | 2.43 (1.81-3.27) | 1.39 (1.12-1.72) | 1.12 (0.91-1.39) |
| Independent ambulation | 2132/5783 (36.9) | 206/416 (49.5) | 1035/2735 (37.8) | 644/1897 (34.0) | 247/735 (33.6) | <.001 | 2.39 (1.76-3.26) | 1.40 (1.10-1.78) | 1.13 (0.93-1.39) |
| Freedom from disabilityd | 847/5334 (15.9) | 101/368 (27.4) | 419/2520 (16.6) | 235/1755 (13.4) | 92/691 (13.3) | <.001 | 3.18 (2.15-4.71) | 1.64 (1.24-2.17) | 1.19 (0.90-1.59) |
| Functional independencee | 1225/5334 (23.0) | 130/368 (35.3) | 585/2520 (23.2) | 376/1755 (21.4) | 134/691 (19.4) | <.001 | 3.45 (2.37-5.02) | 1.69 (1.32-2.17) | 1.39 (1.09-1.79) |
| Technical outcome | |||||||||
| Substantial reperfusionf | 5433/6324 (85.9) | 384/435 (88.3) | 2607/3025 (86.2) | 1758/2073 (84.8) | 684/791 (86.5) | .21 | 1.14 (0.78-1.68) | 1.02 (0.81-1.29) | 0.90 (0.73-1.10) |
| Adverse event outcome | |||||||||
| Symptomatic intracranial hemorrhage | 449/6693 (6.7) | 14/460 (3.0) | 204/3181 (6.4) | 160/2207 (7.2) | 71/845 (8.4) | .002 | 0.35 (0.19-0.67) | 0.69 (0.51-0.92) | 0.81 (0.59-1.10) |
| In-hospital mortality/hospice discharge | 1326/6756 (19.6) | 61/463 (13.2) | 643/3207 (20.1) | 458/2235 (20.5) | 164/851 (19.3) | .003 | 0.51 (0.34-0.75) | 0.92 (0.75-1.12) | 1.05 (0.87-1.28) |
Calculated using the Pearson χ2 test.
Adjustment variables are listed in eTable 2 in Supplement 1.
Indicates discharge to a private residence (vs discharge to acute rehabilitation, skilled nursing facility, hospice, or or dead).
Freedom from disability (modified Rankin Scale, 0-1).
Functional independence (modified Rankin Scale, 0-2).
Substantial reperfusion (modified Thrombolysis in Cerebral Infarction 2b-3).17
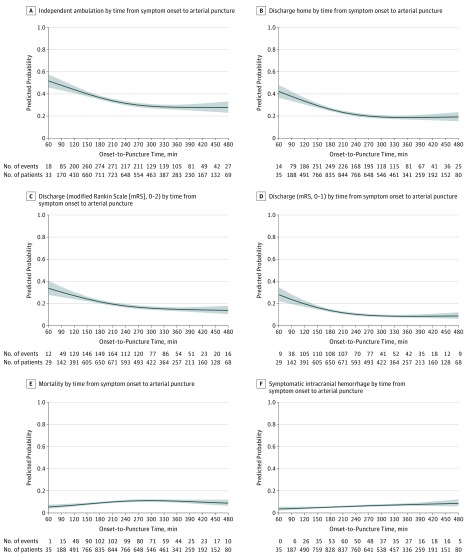
Continuous time-benefit predicted probability curves, showing the relationship with and without adjustment for baseline characteristics between onset-to-puncture and clinical outcomes and adverse events, are shown in Figure 2 (and eFigure 4, eFigure 5, eTable 9A and eTable 9B in Supplement 1). For onset-to-puncture, the time-benefit relationship changed around the 240- to the 270-minute time frame. With placement of a spline at 270 minutes, the time-benefit relationships before and after could be modeled as 2 different linear relations, with a steep time-benefit slope in the 0- to 4.5-hour time window and minimal slope in the greater than 4.5- to 8-hour period. Within 270 minutes, all clinical and adverse event outcomes were better with faster treatment. Among every 1000 patients treated, every 15-minute decrease in onset-to-puncture time was associated with 11 (95% CI, 8-15) more patients ambulating independently at discharge (absolute increased likelihood, 1.14% [95% CI, 0.75%-1.53%]), 12 (95% CI, 8-15) more being discharged to home (absolute increased likelihood, 1.15% [95% CI, 0.78%-1.52%]), 10 (95% CI, 6-14) more having freedom from disability at discharge (absolute increased likelihood, 0.98% [95% CI, 0.57%-1.39%]), and 9 (95% CI, 5-14) more having functional independence at discharge(absolute increased likelihood, 0.91% [95% CI, 0.45%-1.36%]) (eTable 9B in Supplement 1). For adverse events, among every 1000 patients treated, every 15-minute decrease in onset-to-puncture time was associated with 2 (95% CI, 0-4) fewer sICHs (absolute decreased likelihood, −0.22% [95% CI, −0.40% to −0.03%]) and 8 (95% CI, 5-11) fewer deaths prior to discharge or discharge to hospice (absolute decreased likelihood, −0.77% [95% CI, −1.07% to −0.47%]). Time-benefit relationships in analyses confined to patients with witnessed stroke onset and confined to patients with unwitnessed stroke onset are shown in eFigure 6 and eTable 10 in Supplement 1.
Figure 2. Changes in Main Clinical Outcomes and Adverse Events With Continuous Variation in Onset-to-Puncture Time, Adjusted Analysis.
Onset time was defined as last-known well time. Relationships between onset-to-puncture times and the binary outcomes were assessed using logistic regression models with restricted cubic splines with knots at the 5th, 35th, 65th, and 95th percentiles. Curves (blue shading indicates 95% CIs) show the adjusted predicted outcome rate for a hypothetical patient with mean values for baseline characteristics, for symptom onset to arterial puncture times as a continuous variable, and 6 main clinical outcomes: (A), independent ambulation at discharge; (B), discharge to home; (C), functional independence (mRS, 0-2) at discharge; (D), freedom from disability (mRS, 0-1) at discharge; (E), in-hospital mortality; and (F), symptomatic intracranial hemorrhage.
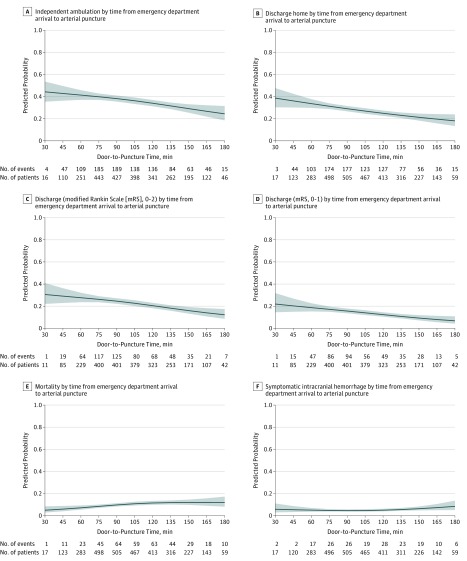
Continuous time-benefit curves for door-to-puncture time and clinical outcomes and adverse events are shown in Figure 3 (and eFigure 7, eFigure 8, eTable 11A, and eTable 11B in Supplement 1). For door-to-puncture time, there were minor changes in time-benefit relationships for 3 of the 6 clinical and adverse event outcomes around 120 minutes. With placement of a spline at 120 minutes, the time-benefit relationships before and after could be modeled as 2 different linear relations for all outcomes. Within 120 minutes, 5 of 6 clinical and adverse event outcomes significantly improved with treatment acceleration. Among every 1000 patients treated, every 15-minute decrease in door-to-puncture time was associated with 17 (95% CI, 1-34) more patients ambulating independently at discharge (absolute increased likelihood, 1.72% [95% CI, 0.08%-3.37%]), 21 (95% CI, 8-34) more being discharged to home (absolute increased likelihood, 2.13% [95% CI, 0.81%-3.44%]), 18 (95% CI, 4-31) more having freedom from disability at discharge (absolute increased likelihood, 1.78% [95% CI, 0.43%-3.14%]), and 22 (95% CI, 7-37) more having functional independence at discharge (absolute increased likelihood, 2.19% [95% CI, 0.71%-3.66%]). For adverse events, among every 1000 patients treated, every 15-minute faster door-to-puncture time was associated with 15 (95% CI, 4-26) fewer patients dying prior to discharge or discharge to hospice (absolute decreased likelihood, −1.48% [95% CI, −2.60% to −0.36%]), without significant changes in sICH (eTable 11B in Supplement 1).
Figure 3. Changes in Main Clinical Outcomes and Adverse Events With Continuous Variation in Door-to-Puncture Time, Adjusted Analysis.
Relationships between door-to-puncture times and the binary outcomes were assessed using logistic regression models with restricted cubic splines with knots at the 5th, 35th, 65th, and 95th percentiles. These door-to-puncture analyses indicate patients who arrived direct via emergency medical services transport, not interfacility transfer. Curves (blue shading indicates 95% CIs) show the adjusted predicted outcome rate for a hypothetical patient with mean values for baseline characteristics, for door-to-puncture times as a continuous variable, and 6 main clinical outcomes: (A), independent ambulation at discharge; (B), discharge to home; (C), functional independence (mRS, 0-2) at discharge; (D), freedom from disability (mRS, 0-1) at discharge; (E), in-hospital mortality; and (F), symptomatic intracranial hemorrhage.
Discussion
In this exploratory study of 6756 patients, earlier endovascular-reperfusion therapy was significantly associated with better outcomes, including independent ambulation at discharge, discharge to home, functional independence and freedom from disability at discharge and at 3 months, and with lower complications, including in-hospital mortality and sICH. In addition, the pace of the reduction in benefit associated with longer onset-to-puncture time intervals was nonlinear for the preponderance of outcomes, with a more rapid benefit loss in the first 30 to 270 minutes and a slower decline between 271 and 480 minutes after witnessed stroke onset.
These findings are consonant with, and extend, prior investigations of the relation between treatment time and functional outcome from endovascular-reperfusion therapy. Prior studies have shown that earlier treatment was associated with better outcomes but generally have been limited due to restricted entry criteria, modest sample sizes, lack of enrollment of consecutive patients, admixture of treated and untreated patients in the intention-to-treat group, and uncertainty about generalizability of findings to routine practice.7,8,9,10 The population treated in the current study was substantially larger than prior studies and reflects data from a diverse range of hospitals, including majority of certified CSCs in the United States.19 The magnitude of the onset-to-puncture time-benefit relationship in this study broadly accords with that reported in a smaller nationwide registry study in the Netherlands.11 With a larger data set, the current study was able to explore nonlinear relationships with the outcomes rather than only linear relationships. The present study also reports the relation of door-to-puncture times and outcomes.
The magnitude of the time-benefit relation observed in this study, while requiring validation in an external data set, is clinically meaningful and emphasizes the importance of policies to accelerate treatment start. The magnitude of the association of faster treatment with improved outcomes exceeds that for start of IV rtPA,20,21 especially among patients with large vessel occlusion,22 and supports the adoption by regional systems of acute stroke care of direct routing of likely large vessel–occlusion patients to thrombectomy-capable stroke centers, provided endovascular hospitals are only modestly more distant than primary stroke centers.23
The findings provide novel information regarding the time-benefit curve for endovascular thrombectomy. Prior studies generally assumed a linear decline of benefit, but with the larger cohort in this study, the time-benefit curve was derived in a data-driven manner and showed a nonlinear relationship with rapid loss of benefit from 0.5 to 4 hours, transitioning to slower loss of benefit in the 4.5- to 8-hour onset-to-puncture time window. This shape of the time-benefit curve likely arises, in part, as a result of imaging selection for treatment. The pace of infarct growth varies widely among individual patients.24,25,26,27 Early after onset, both “fast progressors” and “slow progressors” will have small to moderate volumes of infarct core (irreversibly injured tissue), and therefore be judged appropriate for intervention.28 But later, after onset, fast progressors will have large infarcts and be excluded from intervention. Therefore, the later time windows will have few patients with faster paces of infarct expansion and show an attenuated relation of onset-to-puncture time with outcomes.29 Recent RCTs in imaging-selected patients, up to 24 hours after last-known well time, have confirmed benefit from endovascular-reperfusion therapy in slowly progressing patients.6,29 A question for clinicians is the following: at what chronologic time point, after last-known well time, do a substantial proportion of fast progressors reach large cores that limit excellent outcomes, as that would be the demarcation point at which to consider switching from a time-based to a tissue-based patient selection strategy. The current study’s findings suggest that time point may begin as early as 240 to 270 minutes after last-known well time.
Findings from the current study can help inform the selection of treatment speed metrics for quality-improvement programs.16,30,31,32 The current study reinforces RCT findings indicating there is no single early door-to-puncture time point at which there is a sudden drop in benefit; rather, there is a continuous decline in benefit throughout the first 180 minutes. However, quality measures are generally constructed as the proportion of patients in whom a target is achieved. A successful precedent is the door-to-treatment time for IV rtPA in AIS, for which an initial national target was set at increasing achievement within 60 minutes from 25% to 50% of patients.33 Based on the 25th percentiles in the current study, potential national quality target door-to-puncture times could be selected to be within 75 minutes in EMS direct-arriving patients and within 45 minutes in transfer patients.
Faster endovascular-reperfusion therapy treatment requires faster activation of EMS by witnesses, by prehospital personnel efficiently routing patients to thrombectomy-capable hospitals and with rapid triage and treatment of patients within systems of care. These results identify several targets to reduce treatment delays.34 Patients arriving at the hospital in off hours had delayed treatment times; improved staffing during these periods may reduce this disparity. Expanding availability of endovascular thrombectomy to more hospitals is advantageous to provide rapid access for more patients, though higher case-volume hospitals have more efficient door-to-puncture times,34 suggesting the desirability of avoiding duplicate hospital services when not mandated by geographic distribution. Certification as a stroke center was associated with shorter treatment times, suggesting beneficial effect of continuous quality improvement required by certifying bodies.
Limitations
This study has several limitations. First, the data reported depends on the accuracy and completeness of abstraction from the medical record. To optimize data quality, the GWTG-Stroke program includes detailed training of site chart abstractors, standardized case definitions and coding instructions, predefined logic and range checks on data fields at data entry, audit trails, and regular data quality reports for all sites.
Second, data missingness was present for some outcomes, particularly for the mRS at discharge and at 3 months poststroke. However, complete or only minimal missing data were present for the preponderance of outcomes. Propensity weighting was employed to mitigate potential bias due to missing data, and indeed, time-benefit patterns for outcomes with low and higher missing data were very similar. In addition, prior studies have demonstrated that functional outcomes at discharge correlate highly with 3-month outcomes.35,36 Data missingness for some key baseline covariates led to exclusion of some otherwise eligible patients (4.1%) from the analysis. The included and excluded groups did not differ in many prognostic variables, including the 2 most important for stroke, age, and baseline NIHSS score. The included and excluded groups did differ in other baseline prognostic variables, including the excluded patients less frequently being ambulatory prior to the index stroke and receiving care more often at lower-volume thrombectomy hospitals.
Third, this study analyzed time until arterial puncture rather than time until achievement of substantial reperfusion. The latter time point corresponds more closely with total ischemia time, the underlying determinant of outcome. Arterial puncture time was analyzed because pilot field testing by the Joint Commission determined that treating teams were not documenting reperfusion times with high reliability. In the future, with intensified quality-improvement efforts, reperfusion times may be better documented and analyzable. However, prior studies have found that puncture-to-reperfusion times for AIS account for a small and predictable proportion of onset-to-reperfusion times, so event-to-puncture times track very closely with event-to-reperfusion times.37
Fourth, the GWTG-Stroke database did not collect information regarding which patients had or did not have advanced physiological imaging (perfusion computed tomography [CT], dynamic 3D CT angiography, perfusion-diffusion magnetic resonance imaging) performed and treatment decisions based on tissue state rather than chronologic time. Physiological imaging, if performed, is unlikely to have influenced time-benefit curves substantially in the 30- to 270-minute onset-to-puncture window, when it shows favorable penumbral profiles in the great preponderance of patients, but it may have attenuated the time-benefit relationship in the 271- to 480-minute window.24
Fifth, the time-benefit outcome curves presented were derived from the full study population and analyzed in an exploratory manner without adjustment for multiplicity. They require validation in an external population before reaching conclusions about robustness. Sixth, residual measured and unmeasured confounding may have influenced study findings.
Conclusions
Among patients with large vessel occlusion AIS treated in routine clinical practice, shorter time to endovascular-reperfusion therapy was significantly associated with better outcomes. These findings support efforts to reduce time to hospital and endovascular treatment in patients with stroke.
eMethods 1.
eMethods 2.
eResults 1.
eResults 2.
eResults 3.
eTable 1. Patient baseline characteristics and outcomes analyzed from the comprehensive stroke center module of the GWTG-Stroke program
eTable 2. Baseline patient and hospital characteristics adjusted in the multivariable models
eTable 3. Patient- and hospital-level characteristics for included patients compared with patients excluded for missing documentation of 1 or more key baseline covariates
eTable 4. Patient- and hospital-level characteristics for patients with witnessed versus unwitnessed stroke onset
eTable 5. Patient- and hospital-level characteristics of EMS-arriving patients treated with endovascular reperfusion therapy, overall and in different door to puncture time windows
eTable 6. Additional effectiveness outcomes of patients treated with endovascular reperfusion therapy, overall, and in different onset to puncture time windows
eTable 7. Clincial and adverse event outcomes of EMS-arriving patients treated with endovascular reperfusion therapy, overall and in different door to puncture time intervals
eTable 8. Additional clinical outcomes of EMS-arriving patients treated with endovascular reperfusion therapy, overall and in different door to puncture time intervals
eTable 9A. Comparison of time-benefit relationships in all patients in the 30-270 minute onset-to-puncture time window versus the 271-480 minute onset-to-puncture window, in relation to onset-to-puncture time as a continuous curve, adjusted analysis
eTable 9B. Absolute change in clinical and adverse event outcomes of all patients treated with endovascular reperfusion therapy in relation to onset-to-puncture time as a continuous curve, adjusted analysis, in the 30-270 minute onset-to-puncture window
eTable 10. Comparison of time-benefit relationships in the 30-270 minute onset-to-puncture time window in witnessed versus unwitnessed onset patients, in relation to onset-to-puncture time as a continuous curve, adjusted analysis
eTable 11A. Comparison of time-benefit relationships in EMS-arriving patients in the 30-120 minute door-to-puncture time window versus the 121-180 minute onset-to-puncture window, in relation to door-to-puncture time as a continuous curve, adjusted analysis
eTable 11B. Clinical and adverse event outcomes of EMS-arriving patients treated with endovascular reperfusion therapy in relation to door-to-puncture as a continuous curve, in adjusted analysis, in the 30-120 minute window
eFigure 1. Distribution of time intervals in endovascular reperfusion therapy patients. A) Onset (last known well) to arterial puncture times, in all patients, patients with witnessed onset, and patients with unwitnessed onset; B) Door (ED arrival time) to arterial puncture times in EMS arriving patients
eFigure 2. Onset to Door Times (OTD) among patients with witnessed stroke onset, and unwitnessed stroke onset
eFigure 3. Door to puncture times among patients arriving directly via EMS and patients arriving by interfacility transfer to the endovascular reperfusion therapy hospital
eFigure 4. Changes in main clinical and adverse event outcomes with continuous variation in onset-to-puncture time, unadjusted analysis
eFigure 5. Changes in additional clinical and adverse event outcomes with continuous variation in onset-to-puncture time, adjusted analysis
eFigure 6. Changes in main clinical and adverse event outcomes with continuous variation in onset-to-puncture time, in witnessed and unwitnessed onset patients, adjusted analysis
eFigure 7. Time benefit curves for changes in clinical and adverse event outcomes with continuous variation in door-to-puncture time, unadjusted analysis
eFigure 8. Time benefit curves for changes in clinical and adverse event outcomes with continuous variation in door-to-puncture time, adjusted analysis
Initial Statistical Analysis Plan
Initial Proposal for Study
References
- 1.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES Collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2.Bracard S, Ducrocq X, Mas JL, et al. ; THRACE Investigators . Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15(11):1138-1147. doi: 10.1016/S1474-4422(16)30177-6 [DOI] [PubMed] [Google Scholar]
- 3.Mocco J, Zaidat OO, von Kummer R, et al. ; THERAPY Trial Investigators . Aspiration thrombectomy after intravenous alteplase versus intravenous alteplase alone. Stroke. 2016;47(9):2331-2338. doi: 10.1161/STROKEAHA.116.013372 [DOI] [PubMed] [Google Scholar]
- 4.Muir KW, Ford GA, Messow CM, et al. ; PISTE Investigators . Endovascular therapy for acute ischaemic stroke: the Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE) randomised, controlled trial. J Neurol Neurosurg Psychiatry. 2017;88(1):38-44. doi: 10.1136/jnnp-2016-314117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 7.Mueller-Kronast NH, Zaidat OO, Froehler MT, et al. ; STRATIS Investigators . Systematic evaluation of patients treated with neurothrombectomy devices for acute ischemic stroke: primary results of the STRATIS registry. Stroke. 2017;48(10):2760-2768. doi: 10.1161/STROKEAHA.117.016456 [DOI] [PubMed] [Google Scholar]
- 8.Saver JL, Goyal M, van der Lugt A, et al. ; HERMES Collaborators . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279-1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 9.Sheth SA, Jahan R, Gralla J, et al. ; SWIFT-STAR Trialists . Time to endovascular reperfusion and degree of disability in acute stroke. Ann Neurol. 2015;78(4):584-593. doi: 10.1002/ana.24474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaidat OO, Castonguay AC, Nogueira RG, et al. TREVO stent-retriever mechanical thrombectomy for acute ischemic stroke secondary to large vessel occlusion registry. J Neurointerv Surg. 2018;10(6):516-524. doi: 10.1136/neurintsurg-2017-013328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulder MJHL, Jansen IGH, Goldhoorn RB, et al. ; MR CLEAN Registry Investigators . Time to endovascular treatment and outcome in acute ischemic stroke: MR CLEAN registry results. Circulation. 2018;138(3):232-240. doi: 10.1161/CIRCULATIONAHA.117.032600 [DOI] [PubMed] [Google Scholar]
- 12.LaBresh KA, Reeves MJ, Frankel MR, Albright D, Schwamm LH. Hospital treatment of patients with ischemic stroke or transient ischemic attack using the “Get With The Guidelines” program. Arch Intern Med. 2008;168(4):411-417. doi: 10.1001/archinternmed.2007.101 [DOI] [PubMed] [Google Scholar]
- 13.Schwamm LH, Fonarow GC, Reeves MJ, et al. Get With the Guidelines-Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation. 2009;119(1):107-115. doi: 10.1161/CIRCULATIONAHA.108.783688 [DOI] [PubMed] [Google Scholar]
- 14.Ormseth CH, Sheth KN, Saver JL, Fonarow GC, Schwamm LH. The American Heart Association’s Get With the Guidelines (GWTG)-Stroke development and impact on stroke care. Stroke Vasc Neurol. 2017;2(2):94-105. doi: 10.1136/svn-2017-000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berkhemer OA, Fransen PS, Beumer D, et al. ; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 16.Powers WJ, Rabinstein AA, Ackerson T, et al. ; American Heart Association Stroke Council . 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 17.Zaidat OO, Yoo AJ, Khatri P, et al. ; Cerebral Angiographic Revascularization Grading (CARG) Collaborators; STIR Revascularization Working Group; STIR Thrombolysis in Cerebral Infarction (TICI) Task Force . Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650-2663. doi: 10.1161/STROKEAHA.113.001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278-295. doi: 10.1177/0962280210395740 [DOI] [PubMed] [Google Scholar]
- 19.Schieb LJ, Casper ML, George MG. Mapping primary and comprehensive stroke centers by certification organization. Circ Cardiovasc Qual Outcomes. 2015;8(6)(suppl 3):S193-S194. doi: 10.1161/CIRCOUTCOMES.115.002082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309(23):2480-2488. doi: 10.1001/jama.2013.6959 [DOI] [PubMed] [Google Scholar]
- 21.Kim JT, Fonarow GC, Smith EE, et al. Treatment with tissue plasminogen activator in the golden hour and the shape of the 4.5-hour time-benefit curve in the national United States Get With The Guidelines-Stroke population. Circulation. 2017;135(2):128-139. doi: 10.1161/CIRCULATIONAHA.116.023336 [DOI] [PubMed] [Google Scholar]
- 22.Bhatia R, Hill MD, Shobha N, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010;41(10):2254-2258. doi: 10.1161/STROKEAHA.110.592535 [DOI] [PubMed] [Google Scholar]
- 23.American Heart Association; American Stroke Association . Severity-based stroke triage algorithm for EMS: 2017. http://www.heart.org/idc/groups/ahaecc-public/@wcm/@gwtg/documents/downloadable/ucm_498615.pdf. Accessed July 2, 2018.
- 24.Wheeler HM, Mlynash M, Inoue M, et al. The growth rate of early DWI lesions is highly variable and associated with penumbral salvage and clinical outcomes following endovascular reperfusion. Int J Stroke. 2015;10(5):723-729. doi: 10.1111/ijs.12436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bang OY, Saver JL, Buck BH, et al. ; UCLA Collateral Investigators . Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2008;79(6):625-629. doi: 10.1136/jnnp.2007.132100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bristow MS, Simon JE, Brown RA, et al. MR perfusion and diffusion in acute ischemic stroke: human gray and white matter have different thresholds for infarction. J Cereb Blood Flow Metab. 2005;25(10):1280-1287. doi: 10.1038/sj.jcbfm.9600135 [DOI] [PubMed] [Google Scholar]
- 27.Wegener S, Gottschalk B, Jovanovic V, et al. ; MRI in Acute Stroke Study Group of the German Competence Network Stroke . Transient ischemic attacks before ischemic stroke: preconditioning the human brain? a multicenter magnetic resonance imaging study. Stroke. 2004;35(3):616-621. doi: 10.1161/01.STR.0000115767.17923.6A [DOI] [PubMed] [Google Scholar]
- 28.Rocha M, Jovin TG. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke. 2017;48(9):2621-2627. doi: 10.1161/STROKEAHA.117.017673 [DOI] [PubMed] [Google Scholar]
- 29.Albers GW. Late window paradox. Stroke. 2018;49(3):768-771. doi: 10.1161/STROKEAHA.117.020200 [DOI] [PubMed] [Google Scholar]
- 30.Hebert D, Lindsay MP, McIntyre A, et al. Canadian stroke best practice recommendations: Stroke rehabilitation practice guidelines, update 2015. Int J Stroke. 2016;11(4):459-484. doi: 10.1177/1747493016643553 [DOI] [PubMed] [Google Scholar]
- 31.McTaggart RA, Ansari SA, Goyal M, et al. ; Standards and Guidelines Committee of the Society of NeuroInterventional Surgery (SNIS) . Initial hospital management of patients with emergent large vessel occlusion (ELVO): report of the standards and guidelines committee of the Society of NeuroInterventional Surgery. J Neurointerv Surg. 2017;9(3):316-323. doi: 10.1136/neurintsurg-2015-011984 [DOI] [PubMed] [Google Scholar]
- 32.Sacks D, Black CM, Cognard C, et al. ; American Society of Neuroradiology; Canadian Interventional Radiology Association; Cardiovascular and Interventional Radiological Society of Europe; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society of NeuroInterventional Surgery; European Society of Minimally Invasive Neurological Therapy; Society of Vascular and Interventional Neurology . Multisociety consensus quality improvement guidelines for intraarterial catheter-directed treatment of acute ischemic stroke, from the American Society of Neuroradiology, Canadian Interventional Radiology Association, Cardiovascular and Interventional Radiological Society of Europe, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, European Society of Minimally Invasive Neurological Therapy, and Society of Vascular and Interventional Neurology. J Vasc Interv Radiol. 2013;24(2):151-163. doi: 10.1016/j.jvir.2012.11.028 [DOI] [PubMed] [Google Scholar]
- 33.Fonarow GC, Zhao X, Smith EE, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA. 2014;311(16):1632-1640. doi: 10.1001/jama.2014.3203 [DOI] [PubMed] [Google Scholar]
- 34.Menon BK, Xu H, Cox M, et al. Components and trends in door to treatment times for endovascular therapy in Get With the Guidelines-Stroke hospitals. Circulation. 2019;139(2):169-179. doi: 10.1161/CIRCULATIONAHA.118.036701 [DOI] [PubMed] [Google Scholar]
- 35.Ovbiagele B, Saver JL. Day-90 acute ischemic stroke outcomes can be derived from early functional activity level. Cerebrovasc Dis. 2010;29(1):50-56. doi: 10.1159/000255974 [DOI] [PubMed] [Google Scholar]
- 36.Zhang Q, Yang Y, Saver JL. Discharge destination after acute hospitalization strongly predicts three month disability outcome in ischemic stroke. Restor Neurol Neurosci. 2015;33(5):771-775. [DOI] [PubMed] [Google Scholar]
- 37.Goyal M, Jadhav AP, Bonafe A, et al. ; SWIFT PRIME Investigators . Analysis of workflow and time to treatment and the effects on outcome in endovascular treatment of acute ischemic stroke: results from the SWIFT PRIME randomized controlled trial. Radiology. 2016;279(3):888-897. doi: 10.1148/radiol.2016160204 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1.
eMethods 2.
eResults 1.
eResults 2.
eResults 3.
eTable 1. Patient baseline characteristics and outcomes analyzed from the comprehensive stroke center module of the GWTG-Stroke program
eTable 2. Baseline patient and hospital characteristics adjusted in the multivariable models
eTable 3. Patient- and hospital-level characteristics for included patients compared with patients excluded for missing documentation of 1 or more key baseline covariates
eTable 4. Patient- and hospital-level characteristics for patients with witnessed versus unwitnessed stroke onset
eTable 5. Patient- and hospital-level characteristics of EMS-arriving patients treated with endovascular reperfusion therapy, overall and in different door to puncture time windows
eTable 6. Additional effectiveness outcomes of patients treated with endovascular reperfusion therapy, overall, and in different onset to puncture time windows
eTable 7. Clincial and adverse event outcomes of EMS-arriving patients treated with endovascular reperfusion therapy, overall and in different door to puncture time intervals
eTable 8. Additional clinical outcomes of EMS-arriving patients treated with endovascular reperfusion therapy, overall and in different door to puncture time intervals
eTable 9A. Comparison of time-benefit relationships in all patients in the 30-270 minute onset-to-puncture time window versus the 271-480 minute onset-to-puncture window, in relation to onset-to-puncture time as a continuous curve, adjusted analysis
eTable 9B. Absolute change in clinical and adverse event outcomes of all patients treated with endovascular reperfusion therapy in relation to onset-to-puncture time as a continuous curve, adjusted analysis, in the 30-270 minute onset-to-puncture window
eTable 10. Comparison of time-benefit relationships in the 30-270 minute onset-to-puncture time window in witnessed versus unwitnessed onset patients, in relation to onset-to-puncture time as a continuous curve, adjusted analysis
eTable 11A. Comparison of time-benefit relationships in EMS-arriving patients in the 30-120 minute door-to-puncture time window versus the 121-180 minute onset-to-puncture window, in relation to door-to-puncture time as a continuous curve, adjusted analysis
eTable 11B. Clinical and adverse event outcomes of EMS-arriving patients treated with endovascular reperfusion therapy in relation to door-to-puncture as a continuous curve, in adjusted analysis, in the 30-120 minute window
eFigure 1. Distribution of time intervals in endovascular reperfusion therapy patients. A) Onset (last known well) to arterial puncture times, in all patients, patients with witnessed onset, and patients with unwitnessed onset; B) Door (ED arrival time) to arterial puncture times in EMS arriving patients
eFigure 2. Onset to Door Times (OTD) among patients with witnessed stroke onset, and unwitnessed stroke onset
eFigure 3. Door to puncture times among patients arriving directly via EMS and patients arriving by interfacility transfer to the endovascular reperfusion therapy hospital
eFigure 4. Changes in main clinical and adverse event outcomes with continuous variation in onset-to-puncture time, unadjusted analysis
eFigure 5. Changes in additional clinical and adverse event outcomes with continuous variation in onset-to-puncture time, adjusted analysis
eFigure 6. Changes in main clinical and adverse event outcomes with continuous variation in onset-to-puncture time, in witnessed and unwitnessed onset patients, adjusted analysis
eFigure 7. Time benefit curves for changes in clinical and adverse event outcomes with continuous variation in door-to-puncture time, unadjusted analysis
eFigure 8. Time benefit curves for changes in clinical and adverse event outcomes with continuous variation in door-to-puncture time, adjusted analysis
Initial Statistical Analysis Plan
Initial Proposal for Study