SUMMARY
OBJECTIVE:
To estimate the incremental cost-effectiveness of universal vs. test-directed treatment of latent tuberculous infection (LTBI) among human immunodeficiency virus (HIV) positive pregnant women in South Africa.
METHODS:
We compared tuberculin skin test (TST) directed isoniazid preventive therapy (IPT) (TST placement with delivery of IPT to women with positive results) against QuantiFERON®-TB Gold In-Tube (QGIT) directed IPT and universal IPT using decision analysis. Costs were measured empirically in six primary care public health clinics in Matlosana, South Africa. The primary outcome was the incremental cost-effectiveness ratio, expressed in 2016 US$ per disability-adjusted life-year (DALY) averted.
RESULTS:
We estimated that 29.2 of every 1000 pregnant women would develop TB over the course of 1 year in the absence of IPT. TST-directed IPT reduced this number to 24.5 vs. 22.6 with QGIT-directed IPT and 21.0 with universal IPT. Universal IPT was estimated to cost $640/DALY averted (95% uncertainty range $44–$3146) relative to TST-directed IPT and was less costly and more effective (i.e., dominant) than QGIT-directed IPT. Cost-effectiveness was most sensitive to the probability of developing TB and LTBI prevalence.
CONCLUSION :
Providing IPT to all eligible women can be a cost-effective strategy to prevent TB among HIV-positive pregnant women in South Africa.
Keywords: isoniazid preventive therapy, cost-effectiveness, HIV-infected pregnant women, South Africa
RÉSUMÉ
OBJECTIF :
Estimer le rendement incrémentiel du traitement universel contre celui consécuétif à une recherche de l’infection tuberculeuse latente (LTBI) parmi des femmes enceintes positives à l’infection au virus de l’immunodficience humaine (VIH) en Afrique du Sud.
MÉTHODE :
Nous avons comparé le traitement préventif par isoniazide (IPT) consécutif à un test cutané à la tuberculine (TST), c’est-à-dire, la mise en place du TST avec fourniture de l’IPT aux femmes dont les résultats sont positifs contre l’IPT consécutif au QuantiFERON®-TB Gold In-Tube (QGIT) et à 1’IPT universel basé sur une analyse de décision. Les coûts ont été mesurés empiriquement dans six centres de soins de santé primaires publics àMatlosana, Afrique du Sud. Le résultat principal a été le rapport couˆt-efficacité incrémentiel, exprimé en $US 2016 par années de vie ajustées sur l’incapacité (DALY) évitées.
RÉSULTATS :
Nous avons estimé que 29,2 sur 1000 femmes enceintes développeraient une tuberculose (TB) dans l’année qui suit en l’absence de l’IPT. L’IPT consécutif à un TST a réduit ce nombre à 24,5 contre 22,6 avec l’IPT consécutif au QGIT et à 21,0 avec l’IPT universel. Le couˆt de l’IPT universel a été estimé à 640$/DALY évitée (fourchette d’incertitude de 95% 44–3146 $) relativement à l’IPT consécutif au TST et a été moins couˆteuse et plus efficace (c’est-à-dire, dominant) que l’IPT consécutif au QGIT. Le rapport couˆt-efficacité a été trés sensible à la probabilité de développer une TB et à la prévalence du LTBI.
CONCLUSION :
Fournir l’IPT à toutes les femmes éligibles peut étre une stratégie rentable de prévention de la TB parmi les femmes enceintes positives à l’infection par le VIH en Afrique du Sud.
RESUMEN
OBJETIVO:
Estimar la costo-efectividad incremental del tratamiento de la infección tuberculosa latente (LTBI) para todos, en lugar de un tratamiento dirigido por las pruebas diagnósticas, en las embarazadas positivas frente al virus de la inmunodeficiencia humana (VIH) en Suráfrica.
MÉTODOS:
Mediante un análisis decisional se comparóel tratamiento preventivo con isoniazida (IPT), orientado con la prueba tuberculínica (TST) (práctica de la TST y suministro del IPT a las mujeres con resultado positivo), con el IPT dirigido con la prueba QuantiFERON®-TB Gold In-Tube (QGIT) y el IPT para todos. Los costos se midieron de manera empírica en seis consultorios de atención primaria de Matlosana, en Suráfrica. El criterio primario de valoración fue el cociente de costo-efectividad incremental expresado en dólares estadounidenses del 2016, por años de vida ajustados por discapacidad (DALY).
RESULTADOS:
Se calculó que 29,2 de cada 1000 embarazadas sufrirían de tuberculosis (TB) durante el curso de 1 año si no se administra el IPT. El IPT orientado con la reacción TST redujo esta cifra a 24,5 embarazadas, contra 22,6 con el IPT dirigido con la prueba QGIT y 21,0 embarazadas cuando se suministra el tratamiento a todas. El costo estimado del IPT universal fue 640 USD por DALY evitado (margen de incertidumbre del 95% 44 USD–3146 USD), con respecto al IPT dirigido con la TST, y era menos costoso y más eficaz (es decir, dominante) al compararlo con el IPT orientado con el resultado de la prueba QGIT. Las variables que modificaban en mayor grado la costo-efectividad fueron la probabilidad de sufrir TB y la prevalencia de la LTBI.
CONCLUSIÓN:
El suministro del IPT a todas las embarazadas es una estrategia costo-efectiva para prevenir la TB en las embarazadas positivas frente al VIH en Suráfrica.
AN ESTIMATED 37 MILLION PEOPLE were living with the human immunodeficiency virus (PLHIV) in 2016, and tuberculosis (TB) is the leading cause of death among HIV-positive individuals.1 The risk of progressing from latent tuberculous infection (LTBI) to active TB after HIV infection can be up to 15% per year without antiretroviral therapy (ART).2 In South Africa, where HIV and TB are major public health challenges, about 37% of women who attended antenatal care visits in 2014 were HIV-positive,3 and the prevalence of LTBI among females aged 15–49 years can be as high as 70%.4
Isoniazid preventive therapy (IPT) has been recommended for PLHIV in TB-endemic settings.1 IPT is most effective among individuals with LTBI—typically diagnosed using the tuberculin skin test (TST) or interferon-gamma release assays (IGRAs); however, testing for LTBI can increase loss to follow-up.5,6 TST in particular poses operational challenges as tuberculin must be refrigerated, and patients must return for TST readings, which may not occur for up to 50% of patients in some settings.5,7 Health care workers might be hesitant, however, to initiate eligible patients on IPT without any LTBI screening.8 In South Africa, TST is recommended only to determine the duration of IPT prescribed to PLHIV,9 however, TST and IGRAs are often used as screening tests, with IPT offered only to those who test positive.
HIV-positive pregnant women are a particularly relevant population for IPT delivery. Maternal TB is a risk factor for maternal mortality and adverse infant health outcomes,7 and antenatal care (ANC) visits represent a unique opportunity to screen for TB and provide IPT.
In the present study, we sought to examine the key drivers of cost-effectiveness in comparing universal IPT and test-driven IPT among HIV-positive pregnant women in South Africa.
METHODS
Overview
This analysis was nested within an ongoing cluster randomized trial of TST vs. QuantiFERON®-TB Gold In-Tube (QGIT) in 14 South African clinics.10 We used costing data from that trial to inform a cost-effectiveness analysis from the health system perspective in a hypothetical cohort of HIV-positive pregnant women in South Africa. We used decision analysis to evaluate three strategies for providing IPT to adult HIV-positive pregnant women presenting for routine ANC visits at public primary health centers (Figure 1): 1) TST-directed IPT (performance of TST with delivery of IPT to women with positive results); 2) QGIT-directed IPT (performance of QGIT with delivery of IPT to women with positive results); and 3) universal IPT (IPT offered to all HIV-positive women without screening for LTBI).
Figure 1.
Model diagram. Where diagrams in panel A) end in chance nodes (circles) rather than terminal nodes (triangles), this denotes additional sub-trees as described in panel B) for IPTor C) for no IPT. All outcomes (e.g., progression to TB) are evaluated over a 12-month time horizon. HIV = human immunodeficiency virus; IPT = isoniazid preventive therapy; TST = tuberculin skin test; QGIT = QuantiFERON®-TB Gold In-Tube; LTBI = latent tuberculous infection; +=positive; ‒=negative; DILI = drug-induced liver injury; TB = tuberculosis.
To minimize assumptions about long-term adherence to, or effectiveness of, IPT in this high-transmission setting, we limited our analysis to a 12-month time horizon,11 during which IPT was assumed to be delivered continuously.6 This short time horizon should provide conservative estimates of the cost-effectiveness of IPT, as it gives a lower-bound estimate of both the effectiveness and averted future costs of TB treatment. The primary outcome of analysis was the incremental cost-effectiveness ratio (ICER), measured in units of 2016 US$ per disability-adjusted life-year (DALY) averted. Future DALYs and costs were discounted at 3% per year.
Empiric costing
Costs of IPT provision under different strategies were empirically measured using an ‘ingredients’ (bottom-up) approach in six primary care public health clinics in Matlosana subdistrict, South Africa, from June 2016 to January 2017. The six study clinics (three clinics in each arm: QGIT vs. TST) were chosen to represent the range of geographic locations and patient volumes in the parent study. We collected the costs of overheads, building space, equipment, staff, and consumables related to TST, QGIT, and IPT initiation and management. Financial reviews were performed at the subdistrict office. Overheads and other shared costs were allocated based on the percentage of staff time devoted to LTBI screening and IPT management. Time-and-motion studies of all the staff involved in LTBI screening and IPT management were used to estimate human resource costs. Costs of building space were estimated from an average public-sector construction cost (per m2) and allocated based on the proportional space required. Costs related to treatment for TB and drug-induced liver injury (DILI) were estimated from the published literature and South African drug costs. The cost of QGIT was estimated from the National Health Laboratory Service (NHLS) and published kit price,12 assuming these reflected market values. All costs were measured in local currency (South African rand), inflated to 2016 using the South African consumer price index,13 and converted to US$ using the 2016 exchange rate (1 US$ = 14.72 rand).14
Epidemiologic, diagnosis and treatment parameters
Parameters related to epidemiologic outcomes were estimated from the literature, giving preference to studies of HIV-infected pregnant women, when available (Table 1). For our base estimate of the proportion of women who would initiate IPT under a universal strategy, we selected 50% based on study estimates and ease of interpretation, but we evaluated a wide range of values (22–77%) in sensitivity analyses. In the base case, we assumed the same probability of initiating IPTwith or without a positive screening test, but we also evaluated scenarios in which health care workers might be more willing to recommend—and patients more willing to initiate— IPT after receiving a positive screening test for LTBI, relative to a universal recommendation for IPT.8
Table 1.
Epidemiologic, diagnostic and treatment parameters
| Input variable | Base value | Low | High | Source |
|---|---|---|---|---|
| TB and IPT | ||||
| Prevalence of LTBI | 0.767 | 0.454 | 0.932 | (4) |
| Annual risk of active TB, LTBI-positive | 0.052 | 0.043 | 0.097 | (5,7,15) |
| RR of active TB, LTBI-negative vs. LTBI-positive | 0.33 | 0.24 | 0.91 | |
| RR of active TB, IPT vs. no IPT | 0.38 | 0.24 | 0.68 | (5,16–18) |
| Proportion of patients initiating IPT under a universal strategy | 0.50 | 0.22 | 0.77 | Study; (19) |
| Additional proportion of patients initiating IPT following a | ||||
| positive test for LTBI | 0 | 0 | 0.5 | Assumption |
| Probability of return for TST reading | 0.7 | 0.5 | 0.9 | (5,7) |
| Proportion of patients completing IPT | 0.645 | 0.47 | 0.88 | (5,19,20) |
| TST accuracy | ||||
| Sensitivity | 0.60 | 0.38 | 0.67 | (7,21,22) |
| Specificity | 0.91 | 0.68 | 0.99 | (21) |
| QGIT accuracy | ||||
| Sensitivity | 0.61 | 0.41 | 0.75 | (23) |
| Specificity | 0.977 | 0.89 | 0.99 | (23,24) |
| DILI | ||||
| Probability of IPT-induced DILI | 0.03 | 0.002 | 0.15 | (11,17,25,26) |
| Proportion of DILI that is severe | 0.014 | 0.00005 | 0.025 | (17,25,26) |
| Probability of death from severe DILI | 0.053 | 0.0001 | 0.1 | (26) |
| TB diagnosis and treatment | ||||
| Probability of anti-tuberculosis treatment | 0.738 | 0.48 | 0.80 | (1,27) |
| Probability of TB mortality if untreated | 0.50 | 0.375 | 0.625 | (1) |
| Probability of TB mortality if treated | 0.083 | 0.064 | 0.107 | (1) |
| Disability weights | ||||
| Mild IPT-induced hepatotoxicity | 0.15 | 0.05 | 0.3 | (28) |
| Severe IPT-induced hepatotoxicity | 0.6 | 0.1 | 0.9 | |
| Active TB treatment | 0.1 | 0.01 | 0.25 | |
| Active TB (HIV+ co-infection) | 0.408 | 0.274 | 0.549 | |
| HIV+ (receiving ART) | 0.078 | 0.052 | 0.111 | |
| Life expectancy at initiation of ART | 33.9 | 31.5 | 36.5 | (29) |
TB = tuberculosis; IPT = isoniazid preventive therapy; LTBI = latent tuberculous infection; RR = relative risk; TST = tuberculin skin test; QGIT = QuantiFERON®-TB Gold In-Tube; DILI = drug-induced liver injury; HIV = human immunodeficiency virus; += positive; ART = antiretroviral therapy.
We estimated DALYs assuming that pregnant women had a mean age of 25 years with a mean additional life expectancy of 33.9 years in the absence of TB.3,29 Future years of life lost were discounted at 3% per year. We assumed that HIV-positive pregnant women experienced disability associated with HIVon ART,28 and that those who initiated IPT had a defined risk of severe hepatotoxicity, which would incur additional costs and risk of mortality.17,25,26 Patients developing active TB were assumed to be diagnosed and treated based on current case detection and treatment rates in South Africa.1 We did not include any additional costs due to HIV or ART associated with IPT initiation, assuming that all women eligible for IPT were already diagnosed with HIV and eligible for ART. The model was built in TreeAge 2017 (TreeAge Software Inc, Williamstown, MA, USA).
Sensitivity and uncertainty analyses
We performed one-way sensitivity analyses in which all model parameters were varied over reasonable ranges, using a range of ±75% of the original parameter value when data were limited. Two-way sensitivity analyses were performed on model parameters to which the outcome was particularly sensitive. A probabilistic sensitivity analysis using 10 000 Monte Carlo simulations was conducted in which all parameters were varied simultaneously across their ranges to generate both 95% uncertainty ranges (URs; defined as the 2.5th and 97.5th percentile of all simulated results) and probabilities of cost-effectiveness at different willingness-to-pay thresholds.
Ethical considerations
The study protocol was approved by the Institutional Review Board of the Johns Hopkins School of Medicine, Baltimore, MD, USA, and the University of the Witwatersrand Human Research Ethics Committee, Johannesburg, South Africa.
RESULTS
Costs
Table 2 presents the unit costs and corresponding breakdowns for costs measured empirically in our study. The average total per-patient health system cost (including TB screening, IPT, and clinical management) under the TST-directed, universal, and QGIT-directed IPT strategies was respectively $11.93, $17.78, and $68.01 (Table 3). Providing IPT to all HIV-positive pregnant women had the highest per-patient IPT cost ($8.29) and IPT-related DILI treatment cost ($7.03), but slightly lower per-patient TB treatment cost ($2.46) than providing IPT only to women with positive QGITor TST. To deliver the intervention to all HIV-infected pregnant women in South Africa (approximately 324 000)3 would therefore cost an estimated $3.9 million for TST-directed IPT, $5.8 million for universal IPT, and $22.0 million for QGIT-directed IPT.
Table 2.
Unit costs and cost decomposition in 2016 US$
| Description | Base US$ | Low US$ | High US$ | Source* |
|---|---|---|---|---|
| Cost of TST | 3.90 | 3.32 | 7.27 | Study; (30) |
| Nursing staff time (application and reading) | 1.55 | |||
| Consumables and materials (gloves, syringes, needles, box for syringes) | 1.51 | |||
| Training unit cost | 0.77 | |||
| Overhead unit cost | 0.07 | |||
| Cost of QGIT | 57.88 | 27.13 | 101.43 | Study |
| Nursing staff time | 1.44 | |||
| Consumables and materials | 12.15 | |||
| Laboratory cost | 38.99 | |||
| Sample transport cost | 5.26 | |||
| Overhead unit cost | 0.04 | |||
| Costs of IPT | ||||
| Isoniazid 300 mg/day (monthly) | 0.90 | Study | ||
| Initial consultation | 0.98 | 0.49 | 2.65 | Study |
| Follow-up out-patient consultation | 0.77 | 0.28 | 1.66 | Study |
| Total cost of IPT treatment (12 months) | 20.25 | 11.97 | 34.11 | Calculated; (31) |
| Costs related to DILI and TB treatment costs | ||||
| Basic laboratory test (blood count) | 4.18 | 1.05 | 7.32 | (32,33) |
| Liver function test | 9.47 | 2.37 | 16.57 | (32,33) |
| Sputum test | 2.13 | 1.08 | 2.33 | (32,33) |
| Chest X-ray | 20.08 | 9.92 | 24.91 | (32,33) |
| Out-patient follow-up at tertiary hospital | 13.32 | 9.80 | 38.93 | (31) |
| Hospitalization per diem | 116.15 | 47.80 | 126.64 | Study; (31) |
| Active TB treatment | 158.74 | 120.61 | 304.06 | (30,32) |
Estimates were derived from data collected at study clinics and local hospitals.
TST = tuberculin skin test; QGIT = QuantiFERON®-TB Gold In-Tube; IPT = isoniazid preventive therapy; DILI = drug-induced liver injury; TB = tuberculosis.
Table 3.
Average cost per patient (2016 US$)
| Universal IPT US$ | IPT with TST US$ | IPT with QGIT US$ | |
|---|---|---|---|
| Diagnostic testing | 0.23 | 3.90 | 58.11 |
| LTBI treatment | 8.29 | 2.79 | 3.92 |
| IPT-related DILI | 7.03 | 2.37 | 3.33 |
| Anti-tuberculosis treatment | 2.46 | 2.87 | 2.65 |
| Total cost per patient | 17.78 | 11.93 | 68.01 |
IPT = isoniazid preventive therapy; TST = tuberculin skin test; QGIT = QuantiFERON®-TB Gold In-Tube; LTBI = latent tuberculous infection; DILI = drug-induced liver injury.
Effectiveness and cost-effectiveness
In the absence of IPT, we estimated that, for every 1000 HIV-positive pregnant women, 767 would have LTBI, 29.2 would develop active TB within 1 year, and 5.6 would die of TB. In the TST-directed IPT strategy, an estimated 168 women would receive IPT (seven of whom would not have LTBI), thereby reducing the number of TB cases to 24.5 and TB deaths to 4.8 (Table 4). An estimated 8.9 women/1000 would experience mild or severe DILI, and 0.1 deaths would occur due to DILI. Under the universal IPT strategy, 500 women would receive IPT (117 of whom would not have LTBI), reducing the number of TB cases to 21.0 and TB deaths to 4.4. An estimated 22.0 women would experience mild or severe DILI, with 0.3 DILI-related deaths. The ICER was $640/DALY averted by universal IPT relative to TST-directed IPT; QGIT-directed IPT was more costly and less effective than (i.e., dominated by) universal IPT.
Table 4.
Expected costs, DALYs averted and incremental cost-effectiveness per 1000 patients (2016 US$)
| Strategy | Total cost US$ | Active TB cases | Deaths from TB or DILI | DALYs | Incremental cost/DALY averted US$ |
|---|---|---|---|---|---|
| TST-directed IPT | 11 928 | 24.5 | 4.8 | 177 | |
| Universal IPT | 17 777 | 21.0 | 4.4 | 168 | |
| Incremental (vs. TST) | 5 849 | ‒2.5 | ‒0.4 | ‒9 | 640 |
| QGIT-directed IPT | 68 012 | 22.6 | 171 |
DALY = disability-adjusted life-year; TB = tuberculosis; DILI = drug-induced liver injury; TST = tuberculin skin test; IPT = isoniazid preventive therapy; QGIT = QuantiFERON®-TB Gold In-Tube.
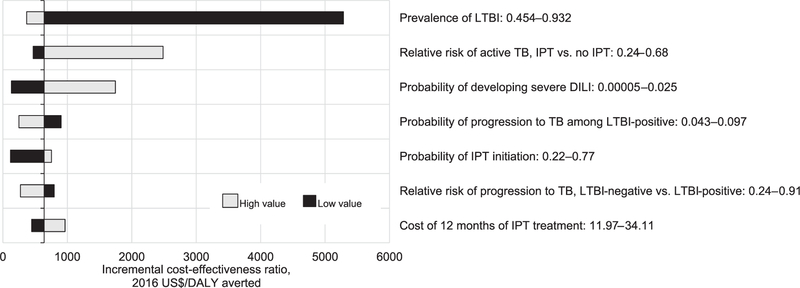
Sensitivity analyses
The incremental cost-effectiveness of universal IPT was most sensitive to LTBI prevalence and the efficacy of IPT (Figure 2). When we assumed a reduced LTBI prevalence of 45%, the incremental cost-effectiveness of universal IPT was estimated at $5683/DALY averted. When we assumed a higher LTBI prevalence of 93%, the cost-effectiveness ratio decreased to $370/DALY averted. When we assumed a lower efficacy of IPT at 32%, universal IPT was estimated to cost $2486/DALY averted relative to TST-directed IPT. On probabilistic sensitivity analysis, the median cost-effectiveness of universal IPT (relative to TST-directed IPT) across all simulations was $496/DALY averted (95% UR $44–$3146). At a willingness-to-pay threshold of $12 860/DALY averted (the per-capita gross national income of South Africa), 99% of simulations favored the universal IPT arm.
Figure 2.
One-way sensitivity analyses comparing universal IPT to TST-directed IPT. Bars represent the incremental cost-effectiveness under the high (light grey line) and low (black) bounds associated with each parameter, holding all other parameter values constant. LTBI = latent tuberculous infection; TB =; IPT = isoniazid preventive therapy; DILI = drug-induced liver injury; DALY = disability-adjusted life-year; TST = tuberculin skin test.
We ran additional sensitivity analyses to investigate the effect of TST-based screening on loss to follow-up (failure to return for TST reading) and IPT initiation (assuming that a positive TST might increase the probability of initiating IPT), as shown in Figure 3. Under most reasonable scenarios of return probabilities and additional probabilities of initiation, the incremental cost-effectiveness of universal IPT remained <$5000/DALY averted, although there were some scenarios of high probabilities of both return and IPT initiation under which TST-directed IPT became more effective than universal IPT (top right of Figure 3, in black).
Figure 3.
Incremental cost-effectiveness of universal IPT among HIV-positive pregnant women in South Africa according to TST implementation. Each shaded region corresponds to a range of incremental cost-effectiveness ratios (in 2016 US$/DALY averted) for universal IPT relative to TST-directed IPT. The x-axis denotes different probabilities of IPT initiation after a positive TST result (assuming that 50% of individuals will initiate IPT under a universal treatment scenario), and the y-axis gives different probabilities of returning for TST reading. The black region in the upper right denotes that TST-directed IPT is more effective than universal IPT. TST = tuberculin skin test; IPT = isoniazid preventive therapy; DALY = disability-adjusted life-year; HIV = human immunodeficiency virus.
DISCUSSION
In most high-incidence settings, screening for LTBI is performed before the initiation of TB preventive therapy, even among high-risk populations such as HIV-positive pregnant women. Our results suggest that universal IPT for 12 months, compared with TST-driven IPT, conservatively costs between $44/DALY and $3146/DALY averted in this population. When considering effects over a longer time horizon than the 12 months considered here, universal IPT may be even more cost-effective. When compared with universal IPT, QGIT-directed IPT was both less effective and more costly.
Our study adds important data to the existing literature on the cost-effectiveness of IPT. First, it is among the first to link the empirical costing of TST and IGRA directly to cost-effectiveness of IPT. Our estimated cost of TST was substantially lower than many previously reported values; this estimate might be higher in low-volume clinics (as each vial of tuberculin contains 10 tests and must be used within a month). Training and implementation of nursing staff for LTBI screening also contributed substantially to variability in the cost of TST and IGRA across sites. We estimated that the high cost of IGRA would likely make this test prohibitive relative to a universal IPT strategy, unless IGRA could provide additional ancillary benefit (for example, increased initiation of IPT among people testing positive).
Second, this study helps to build a strong economic case for universal IPT among HIV-positive pregnant women in South Africa. Our findings are similar to those of another recent analysis in India,34 but extend those findings to a setting of high TB transmission where the long-term effectiveness of IPT cannot be assumed. We demonstrate that, even over a 12-month horizon, universal IPT is likely to be cost-effective for this population. Other studies have examined the cost-effectiveness of IPT among PLHIV in countries with low or moderate TB burdens,30,31 but relatively few economic evaluations have been conducted in settings endemic for both TB and HIV. These findings have policy implications for South Africa, which originally recommended universal IPT in 2011 but later revised their guidelines to re-introduce TST as a mechanism for determining treatment duration. Some have argued that this new recommendation poses a barrier to IPT implementation.35 Our findings support a recommendation for universal IPT to all HIV-positive pregnant women in South Africa, which is consistent with current policy.
One important consideration is that IPT may be more likely to be recommended by health care providers and/or initiated by patients following a positive test result for LTBI (either TST or IGRA). This effect may, however, be counterbalanced by loss to follow-up between the time of testing and the time of receiving results. Our analysis suggests that, for TST-directed IPT to be preferred relative to universal IPT delivery, the effect of a positive test result must be strong, and losses to follow-up minimal. For example, we estimated that TST-directed IPT would be preferred to universal IPT if no losses to follow-up occurred between TST placement and reading, or if 70% of all TSTs placed were read and a positive TST result could increase the proportion of women initiating IPT from 50% to 75%. For IGRA testing, even stronger effects would be required. These results show the importance of changing clinical awareness such that high uptake of IPT can be assured regardless of the outcome of LTBI testing.
Our analysis also had important limitations. Although we found that the intervention was cost-effective compared with conventional thresholds, cost-effectiveness may be better assessed in a country-specific fashion that accounts for local context. We also assumed that every pregnant woman remained on ART for the 12-month period of analysis; unfortunately, ART (and IPT) coverage may decrease in the postpartum period, which may have affected our estimates of IPT cost-effectiveness, particularly as ART and IPT can have synergistic effects.5,18 We also considered IPT delivery in a binary fashion (i.e., we modeled partial adherence to IPT as a weighted average of complete adherence and non-initiation); to the extent that partial adherence is more or less effective per dose than complete adherence, our estimates of cost-effectiveness may also have been biased. Finally, as described above, we evaluated IPT effectiveness only over a 12-month horizon, without consideration of averted secondary transmission, and our estimates of IPT cost-effectiveness may have been conservatively biased as a result. However, even with this conservative assumption, we found IPT met the traditional benchmarks of cost-effectiveness in this setting.
In conclusion, we estimate that universal IPT is likely to be both effective and cost-effective for HIV-positive pregnant women in South Africa, even when conservatively evaluated over a 12-month time horizon. The effectiveness—and thus cost-effectiveness—of universal IPT can be enhanced even further by ensuring high levels of IPTuptake in this important population. These findings strongly support a policy of universal IPT delivery among pregnant women in South Africa and other similar high-burden settings.
Acknowledgements
This work was supported by National Institutes of Health (Bethesda, MD, USA) supplement R01AI095014 02S1 and doctoral dissertation funding from the Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA. The authors thank all study participants for devoting their time to take part in this study; study coordinators, S Chon, C Quomfo, M Malegotsia, J Market, E Rangxa, and T Mmoledi, as well as all study staff who helped in data collection.
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report, 2016. WHO/HTM/TB/2016.13 Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 2.Lawn SD, Churchyard G. Epidemiology of HIV-associated tuberculosis. Curr Opin HIV AIDS 2009; 4: 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Department of Health. The National Antenatal Sentinel HIV Prevalence Survey South Africa Pretoria, South Africa: National Department of Health, 2014. [Google Scholar]
- 4.Wood R, Liang H, Wu H, et al. Changing prevalence of TB infection with increasing age in high TB burden townships in South Africa. Int J Tuberc Lung Dis 2010; 14: 406–412. [PMC free article] [PubMed] [Google Scholar]
- 5.Golub J, Pronyk P, Mohapi L, et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS 2009; 23: 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clouse K, Pettifor A, Shearer K, et al. Loss to follow-up before and after delivery among women testing HIV positive during pregnancy in Johannesburg, South Africa. Trop Med Int Health 2013; 18: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A, Nayak U, Ram M, et al. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002–2005. Clin Infect Dis 2007; 45: 241–249. [DOI] [PubMed] [Google Scholar]
- 8.Getahun H, Granich R, Sculier D, et al. Implementation of isoniazid preventive therapy for people living with HIV worldwide: barriers and solutions. AIDS 2010; 24 (Suppl 5): S57–S65. [DOI] [PubMed] [Google Scholar]
- 9.National Department of Health. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults Pretoria, South Africa: National Department of Health, 2015. [Google Scholar]
- 10.Golub J, Lebina L, Qomfu C, et al. Implementation of QuantiFERON® TB Gold In-Tube test for diagnosing latent tuberculosis among newly diagnosed HIV-infected patients in South Africa. In: 46th World Conference on Lung Health of the International Union Against Tuberculosis and Lung Disease, Cape Town, South Africa, 2015. [Abstract OA-399–05]. [Google Scholar]
- 11.Samandari T, Agizew TB, Nyirenda S, et al. Tuberculosis incidence after 36 months’ isoniazid prophylaxis in HIV-infected adults in Botswana. AIDS 2015; 29: 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiagen. QuantiFERON-TB Gold (QFT) Kit [Internet]. MD, USA; 2017. Available from: https://www.qiagen.com/us/
- 13.Statistics South Africa. Consumer Price Index December 2016 Pretoria, South Africa: SSA, 2016. [Google Scholar]
- 14.Oanda. Historical Exchange Rates—USD: ZAR 01 January 2016 to 31 December 2016 New York, NY, USA: Oanda, 2018. https://www.oanda.com/fx-for-business/historical-rates Accessed August 2018. [Google Scholar]
- 15.Bucher HC, Griffith LE, Guyatt GH, et al. Isoniazid prophylaxis for tuberculosis in HIV infection: a meta-analysis of randomized controlled trials. AIDS 1999; 13: 501–507. [DOI] [PubMed] [Google Scholar]
- 16.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev 2010; 1: CD000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rangaka MX, Wilkinson RJ, Boulle A, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet 2014; 384: 682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS 2007; 21: 1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiam A, Machekano R, Gounder CR, et al. Preventing tuberculosis among HIV-infected pregnant women in Lesotho: the case for rolling out active case finding and isoniazid preventive therapy. J Acquir Immune Defic Syndr 2014; 67: e5–e11. [DOI] [PubMed] [Google Scholar]
- 20.Uyei J, Coetzee D, Macinko J, Guttmacher S. Integrated delivery of HIVand tuberculosis services in sub-Saharan Africa: a systematic review. Lancet Infect Dis 2011; 11: 855–867. [DOI] [PubMed] [Google Scholar]
- 21.Mtei L, Matee M, Herfort O, et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin Infect Dis 2005; 40: 1500–1507. [DOI] [PubMed] [Google Scholar]
- 22.Raby E, Moyo M, Devendra A, et al. The effects of HIV on the sensitivity of a whole blood IFN-gamma release assay in Zambian adults with active tuberculosis. PLOS ONE 2008; 3: e2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cattamanchi A, Smith R, Steingart KR, et al. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J Acquir Immune Defic Syndr 2011; 56: 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 2007; 146: 340–354. [DOI] [PubMed] [Google Scholar]
- 25.Franks AL, Binkin NJ, Snider DE, Rokaw WM, Becker S, Becker S. Isoniazid hepatitis among pregnant and postpartum Hispanic patients. Public Health Rep 1989; 104: 151–155. [PMC free article] [PubMed] [Google Scholar]
- 26.Tedla Z, Nyirenda S, Peeler C, et al. Isoniazid-associated hepatitis and antiretroviral drugs during tuberculosis prophylaxis in HIV-infected adults in Botswana. Am J Respir Crit Care Med 2010; 182: 278–285. [DOI] [PubMed] [Google Scholar]
- 27.Naidoo P, Theron G, Rangaka MX, et al. The South African tuberculosis care cascade: estimated losses and methodological challenges. J Infect Dis 2017; 216 (Suppl 7): S702–S713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salomon JA, Haagsma JA, Davis A, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health 2015; 3: e712–723. [DOI] [PubMed] [Google Scholar]
- 29.Johnson L, Mossong J, Dorrington R. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLOS Med 2013; 10: e1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrestha RK, Mugisha B, Bunnell R, et al. Cost-utility of tuberculosis prevention among HIV-infected adults in Kampala, Uganda. Int J Tuberc Lung Dis 2007; 11: 747–754. [PubMed] [Google Scholar]
- 31.Pho MT, Swaminathan S, Kumarasamy N, et al. The cost-effectiveness of tuberculosis preventive therapy for HIV-infected individuals in southern India: a trial-based analysis. PLOS ONE 2012; 7: e36001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pooran A, Pieterson E, Davids M, Theron G, Dheda K. What is the cost of diagnosis and management of drug resistant tuberculosis in South Africa? PLOS ONE 2013; 8: e54587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer-Rath G, Schnippel K, Long L, et al. The impact and cost of scaling up GeneXpert MTB/RIF in South Africa. PLOS ONE 2012; 7: e36966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapoor S, Gupta A, Shah M. Cost-effectiveness of isoniazid preventive therapy for HIV-infected pregnant women in India. Int J Tuberc Lung Dis 2016; 20: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mindachew M, Deribew A, Memiah P, Biadgilign S. Perceived barriers to the implementation of isoniazid preventive therapy for people living with HIV in resource constrained settings: a qualitative study. Pan Afr Med J 2014; 17: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]